Abstract
Palladium (Pd) and its alloys, renowned for their good corrosion resistance, catalytic efficiency, and hydrogen affinity, find extensive use in various industrial applications. However, the susceptibility of pure Pd to hydrogen embrittlement necessitates alloying strategies such as Pd-Ag systems. This study investigates the impact of the ordering on the phase stability and elastic properties of Pd-Ag alloys through first-principles calculations. We explore a series of ordered phase structures alongside random solid solutions using Special Quasirandom Structures (SQSs), evaluating their thermodynamic stability and elastic properties. Our findings indicate the possible existence of stable ordered L12 Pd3Ag and PdAg3 and L11 PdAg phases, which are thought to exist only in Cu-Pt alloys. An analysis of the elastic constants and anisotropy indices underscores some pronounced directional dependencies in the mechanical responses between the random solid-solution and ordered phases. This suggests that the ordered phases not only are thermodynamically and mechanically more stable than solid-solution phases, but also display a decrease in anisotropy indices. The results provide a deeper understanding of the atomic behavior of Pd-Ag alloys, and shed light on the design of multiphase Pd-Ag alloys to improve their mechanical properties.
1. Introduction
Palladium and its alloys display high melting points, excellent catalytic performance, and a unique affinity towards hydrogen [1,2,3,4,5], and are widely used as catalysts for various hydrogenation reactions [6,7,8,9,10,11,12,13,14], including but not limited to ethanol steam-reforming reactions, C2H2 semi-hydrogenation to C2H4, and photocatalysis of carbon dioxide. Nevertheless, pure Pd exhibits strong hydrogen embrittlement due to the α ↔ β phase transitions, regardless of its use as a hydrogen permeation material or as a catalyst for hydrogenation reactions [15]. A common solution is to utilize Pd alloys instead of pure Pd to modulate the mechanical properties when subject to a hydrogen environment [16,17,18].
In past few decades, the Pd-Ag system has been considered as one of the most promising choices to improve the hydrogen sustainability and membrane separation properties of Pd-based membranes [16,19,20,21,22,23,24]. Compared to other noble metal elements such as Au and Pt [25,26], which can similarly ameliorate the hydrogen embrittlement of Pd, Ag is much cheaper and more efficient in enhancing the diffusion coefficient of H in Pd. On the other hand, although the more affordable Cu can also play a role [17,27], the addition of Cu elements actually changes the crystal structure of Pd-based membranes. On the contrary, Ag can form substitutional solid solutions with Pd, enabling a consistent and uniform microstructure across the membrane.
Nevertheless, experimental and theoretical investigations suggest the existence of ordered phases in Pd-Ag systems [28,29,30,31,32,33,34], which may overturn the mechanical properties of solid solution. Dating back to 1960s, Savitskii et al. [28] found some abnormities in electrical resistivity measurements below 1400 K for the Pd-Ag solid solution, and suggested the existence of AgPd and Ag2Pd3 ordered phases. Recently, more and more disorder–order transformations in Pd-based alloys have been observed in experiments [35,36,37]. However, due to the fact that the scattering powers of Ag and Pd atoms in most characteristic methods, like X-ray, electron, etc. are almost equal, the atomic ordering and its corresponding crystal structure have yet to be well established [20,38,39,40,41,42]. On the other hand, theoretical calculations have indicated the existence of various ordered phases [29,30,31,32]. For example, Müller et al. [29] predicted the L11 structure for a Pd-Ag ordered phase with a 1:1 composition ratio using the mixed-space cluster expansion method. However, through the screened generalized perturbation method (SGPM), Ruban et al. [31] suggested L11, L12, D022 and D023 ordered structures hardly occur in Pd-Ag alloys due to their low order energy value and low order–disorder transition temperature. In addition, the calculation of Pd-Ag alloys in different compositions or different catalytic surfaces [43,44,45] implies that hydrogen catalysis could be strongly influenced by atomic ordering and crystal structures. It is thus of great importance to understand the effect of atomic ordering on the phase stability and elastic properties of Pd-Ag alloys.
In this work, various ordered phase structures alongside random solid solutions using Special Quasirandom Structures (SQSs) [46] of Pd-Ag alloys are systematically investigated using first-principles calculations. The phase stability of the ordered and solid-solution phases is evaluated by not only the thermodynamic heat of formation but also Born’s mechanical criteria and electronic structure [47]. This work yielded disparate conclusions regarding the stability of the L12 structure in comparison to previous analysis solely focused on energy-related considerations [31]. The ordering range is thus singled out in the Pd-Ag systems, and its role in the transition of mechanical properties is discussed in detail. Moreover, the elastic anisotropies are presented for providing valuable data for the design of better mechanical properties of Pd-Ag systems. In the end, concluding remarks are drawn.
2. Methods and Details
First-principles calculations were performed in the renowned Vienna ab initio simulation package (VASP, V6.4.3) [48]. The computations were carried out utilizing a plane wave basis set with the projector-augmented wave (PAW) method [49]. The exchange-correlation potentials were captured through the generalized gradient approximation (GGA), developed by Perdew and others [50]. After testing within a range from 300 eV to 700 eV, energy cutoffs of 500 eV and 750 eV were selected for the plane wave basis and augmentation, respectively, as they converge at the ~1 meV scale. We adopted the k-mesh of 15 × 15 × 15 after the k-point convergence test [51]. In terms of k-space integration, the temperature smearing technique proposed by Methfessel and Paxton for relaxation calculations is adopted [52], while employing the modified tetrahedron method introduced by Blochl, Jepsen, and Andersen for static calculations [53].
The elastic properties of the alloy were estimated using the Hill averaging method [54], which integrates the Voigt and Reuss approximation. For a cubic crystal system, the Voigt and Reuss approximation are given by
where the GV (BV) and GR (BR) are the shear (bulk) modulus determined by the Voigt and Reuss [55] approximation, respectively. And C11, C12 and C44 are single-crystal elastic constants in the stiffness matrix of solid solutions or ordered phases. Then, the shear modulus G, the bulk modulus B, Young’s modulus E, and the Poisson rate υ in the Hill approximation were expressed by
3. Results and Discussion
3.1. Phase Stability
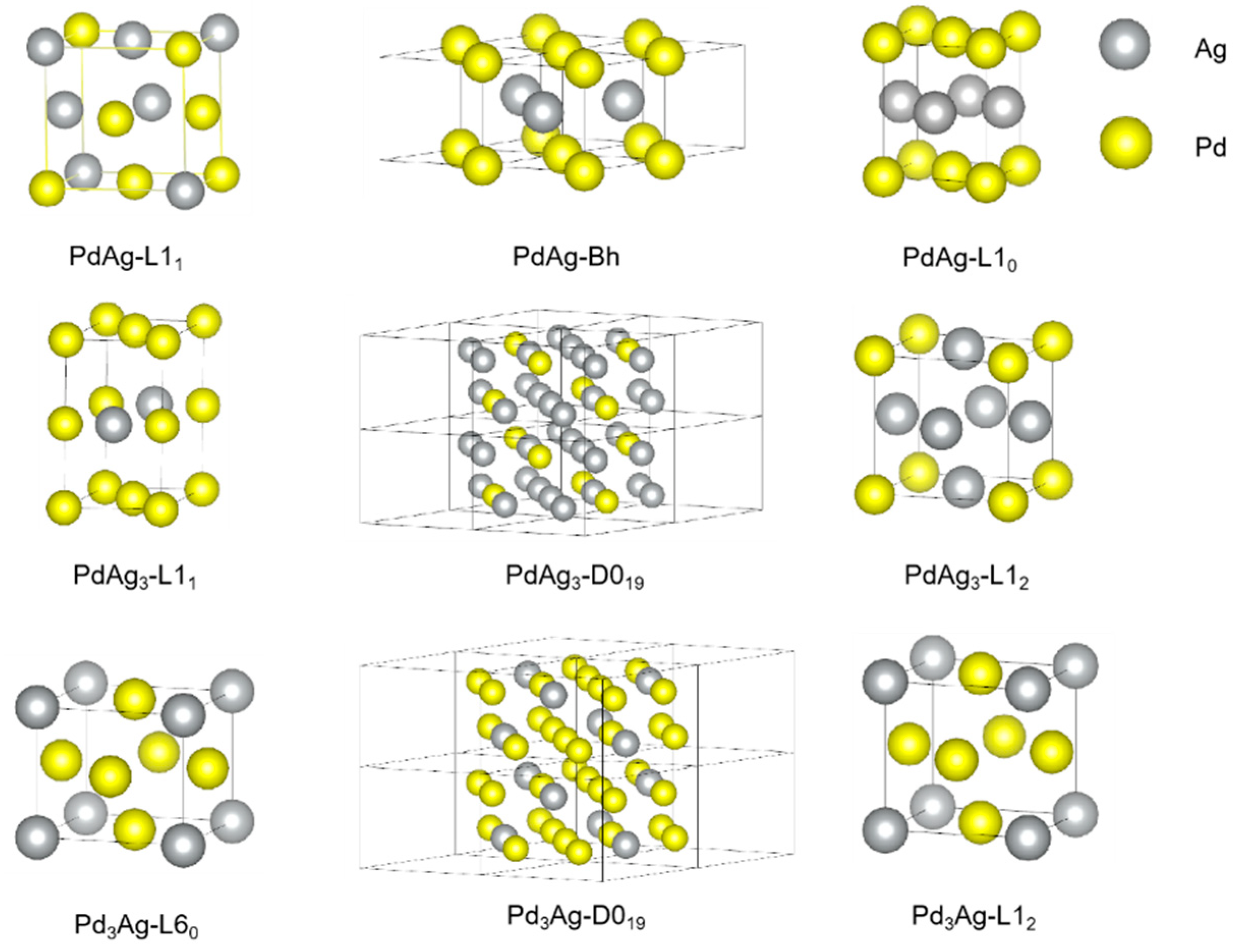
A series of ordered Pd-Ag structures were chosen in this work and depicted in Figure 1 using VESTA software (ver. 3.5.8 64-bit Edition) [56]. The choice of those structures was based on the possible stable phases reported in the literature. For example, the L11 structure, which is an ordered FCC phase, was found to be stable in many precious metal alloys, like Pt-Cu and Pt-Ag for an extremely narrow composition rage [57]. Additionally, it is also predicted to be stable for Pd-Ag through density function theory. Other common ordered FCC structures, like L12, L10, and L60, which have been demonstrated to exist in other Pd-based binary alloys in experimental works were taken into consideration [58,59,60,61,62]. In addition, some ordered hexagonal close-packed (HCP) structures, D019 and Bh, were also considered for comparison. On the other hand, Special Quasirandom Structures (SQSs), constructed by Pezold et al. [63], were used for modeling the solid-solution Pd-Ag phases.
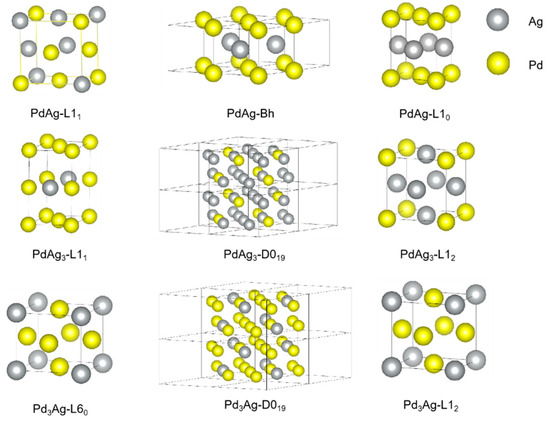
Figure 1.
Crystal structures of the ordered phases in Pd-Ag binary system.
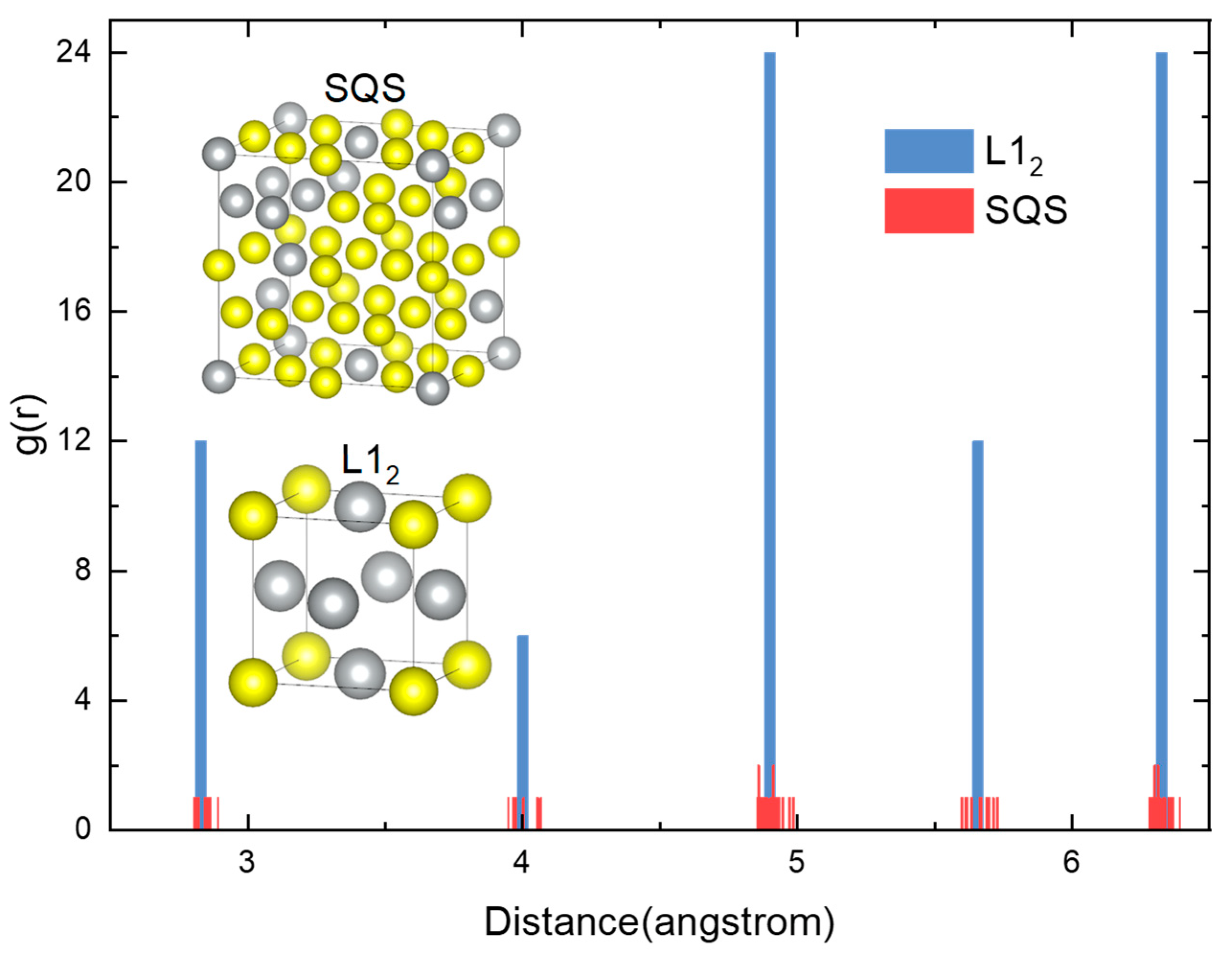
In order to demonstrate the reliability of the SQS method, a radial distribution function between L12 Pd3Ag and SQS Pd32Ag8 after relaxation was demonstrated in Figure 2. The radial distribution function represents the probability of an atom existing within a unit volume from the center, with the function being centered on a selected atom. The blue bars in Figure 2 depict the radial distribution of the L12 Pd3Ag ordered phase. The radial distribution of L12 is similar to that of the FCC structure, which is also evident in the L12 supercell structure on the right side of the figure. The red bar in the figure indicates the radial distribution of the SQS Pd12Ag4 structure after relaxation. As illustrated by the comparison of two distribution functions, the distances of the atoms in the SQS structure are shifted by a minor amount in the original FCC structure. It can be concluded that the SQS method can simulate the random atomic structure of the Pd-Ag solid solution while avoiding deviating from the FCC Bravais lattice.
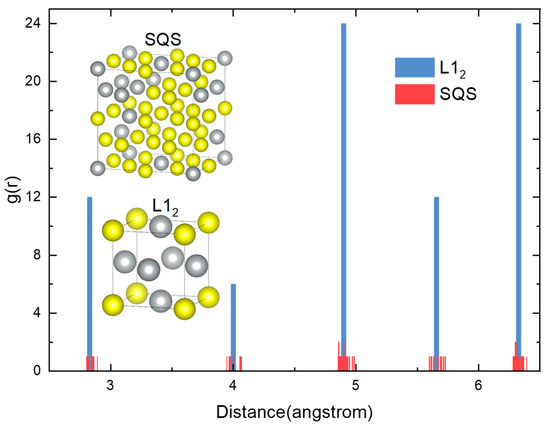
Figure 2.
Radial distribution function between L12 Pd3Ag and SQS Pd12Ag4.
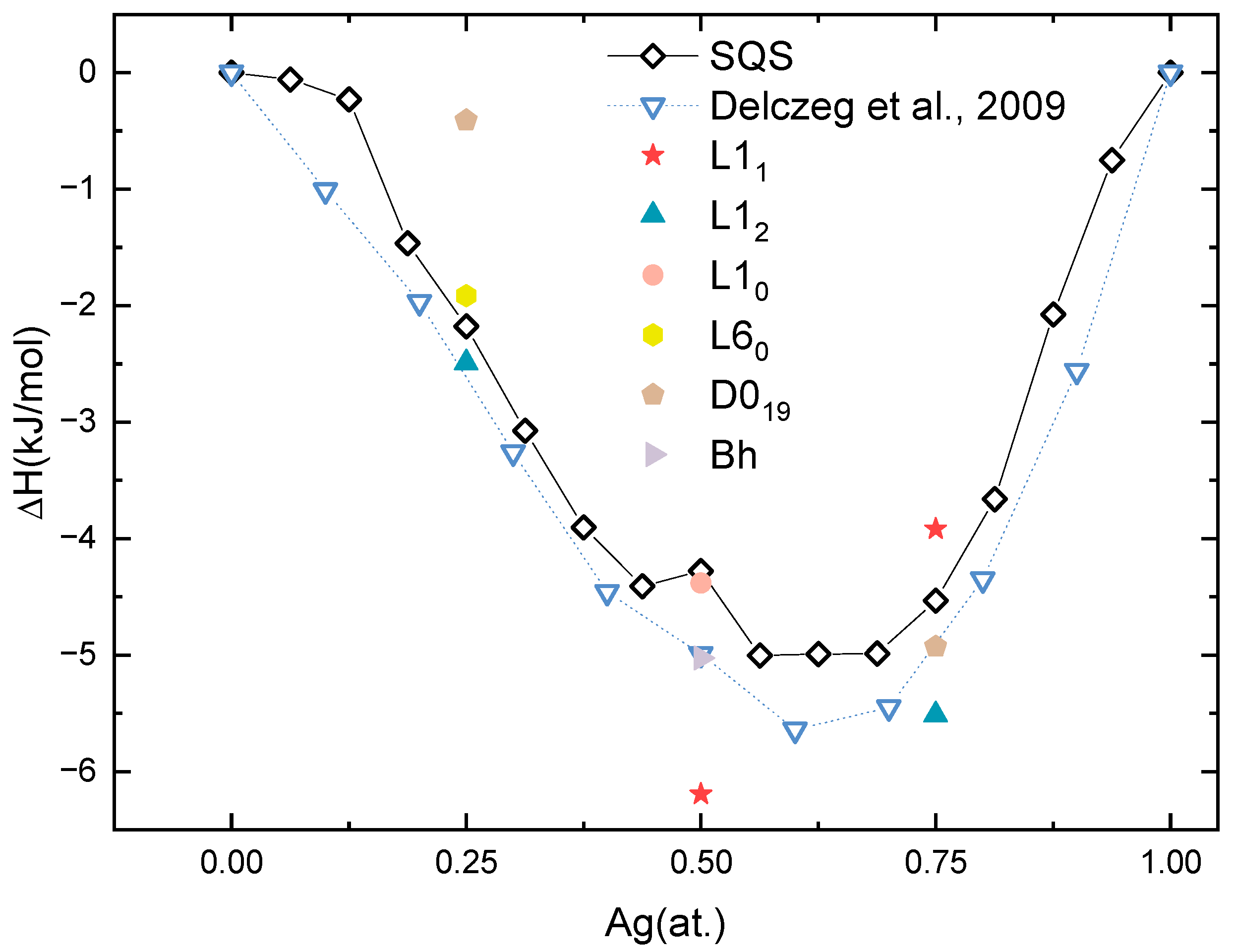
The thermodynamic stability of ordered and solid-solution Pd-Ag structures was determined by their heat of formation (ΔHf). The ΔHf is calculated by
where the , and are the total energy of PdxAgy, pure Pd and pure Ag in the ground state; meanwhile, the x and y are the atom ratios of Pd and Ag. Figure 3 depicts the calculated ΔHf of the aforementioned phases, in addition to the available experimental values of the Pd-Ag system as in reference [64]. Several features can be discerned from this figure.
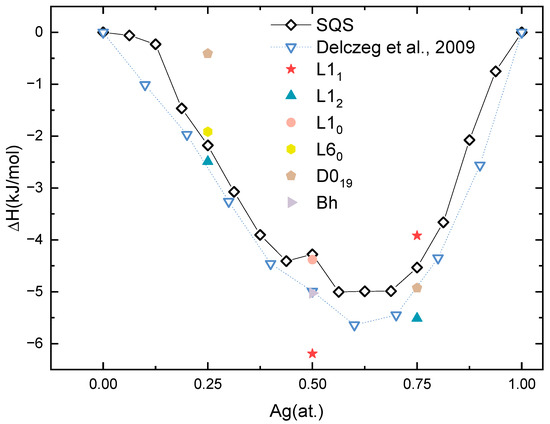
Figure 3.
Heat of formation (ΔH) of solid-solution (SQS) phases and ordered phases as a function of Ag concentration [64].
Firstly, it can be seen from Figure 3 that all the calculated ΔHf values, both of the solid solutions and ordered phases, are negative, implying that these Pd-Ag structures are thermodynamically stable.
Secondly, the ΔHf of the SQSs is in good agreement with that derived from experiments [65]. Even though the experimental values are obtained at 1173 K, the shape of the calculated one is similar to the experimental counterpart. For example, the lowest ΔHf is −5.64 kJ/mol at Pd0.4Ag0.6 from experiments, which is consistent with −5.00 kJ/mol from Pd7Ag9 to Pd6Ag10. Considering the ΔHf will normally decrease as a function of temperature, the SQS model adopted in this work can describe the solid-solution Pd-Ag phases well.
Thirdly, the three ordered phases, L12 Pd3Ag, L11 PdAg, and L12 PdAg3, based on the FCC lattice, display the most thermodynamic stability. Therefore, the ordered phases to be discussed the following sections are L12 Pd3Ag, L11 PdAg and L12 PdAg3 with Ag contents of 0.25, 0.5, and 0.75, respectively. The ΔHf values of L12 Pd3Ag, L11 PdAg, and L12 PdAg3 are −2.49, −6.19, and −5.51 kJ/mol, respectively. They are lower than the HCP ordered counterparts at the same relative concentration. In addition, the calculated ΔHf values of these three ordered phases are also lower than those of the Pd-Ag solid solutions at each composition, indicating that ordering could happen in Pd-Ag systems.
Fourthly, one can discern from Figure 3 that the ΔHf of the SQSs exhibits an anomalously high value of −4.28 kJ/mol for the component Pd8Ag8. Conversely, the ΔHf of the L11 ordered phase at the same Ag concentration exhibits a minimum value among all the compounds. This indicates the Pd8Ag8 solid solution is likely to be in non-equilibrium and more likely to form an L11 ordered phase, a phase observed in other precious metal alloys [57]. This singularity will be discussed in the following sections.
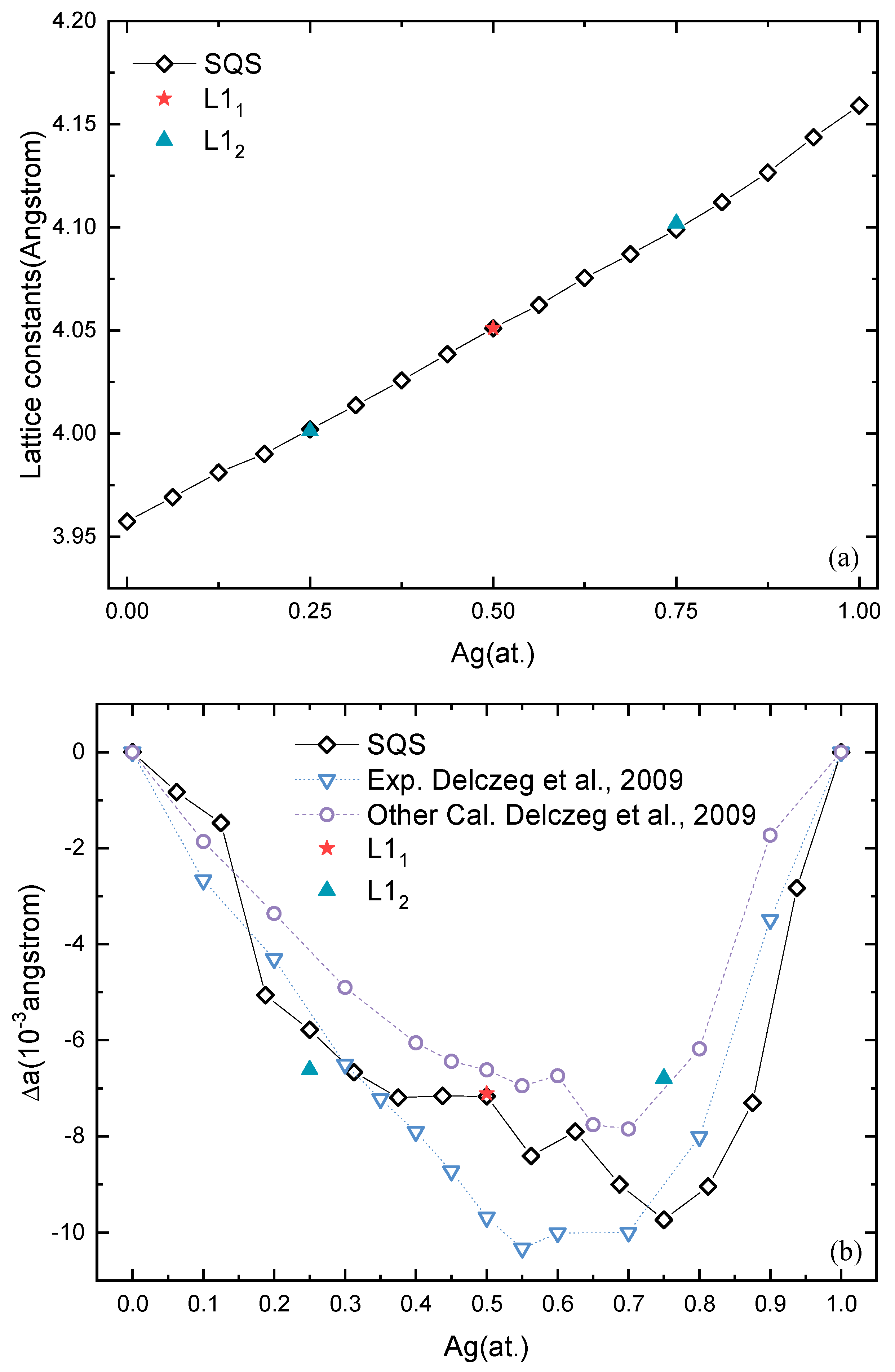
The lattice constants of solid-solution and stable ordered Pd-Ag phases, L12 Pd3Ag, L11 PdAg, and L12 PdAg3, are shown in Figure 4a. The lattice constants almost linearly increase from pure Pd to pure Ag. The lattice constants of ordered L12 Pd3Ag, L11 PdAg, and L12 PdAg3 are close to those of the solid-solution phases. For example, L11 and SQS PdAg have almost the same lattice constant, 4.05 Å.
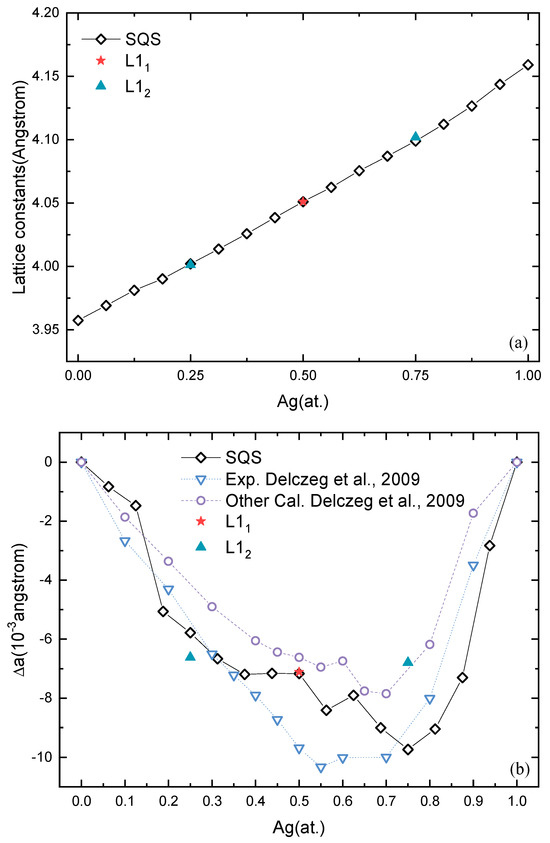
Figure 4.
(a) Lattice constants of solid-solution phases and ordered phases as a function of Ag concentration. (b) Deviations of lattice constants from the Vegard’s law as a function of Ag concentration [64].
In order to further examine the lattice distortion in Pd-Ag systems, the deviations of the lattice constants from Vegard’s law are investigated, and the results are shown in Figure 4b, together with available calculation and experimental values [64]. Unlike the lattice constants shown in Figure 4a, the deviations in the lattice constants in Figure 4b are more pronounced. The negative deviations indicate that the lattice shrinks after forming either solid-solution or ordered structures. It can also be seen that the deviations of the SQSs display a similar trend with ΔHf. That is, the more negative the ΔHf, the larger the deviation. For ordered structures, the situation is different. L12 Pd3Ag presents a larger deviation than the corresponding solid solution, while L12 PdAg3 is smaller. On the other hand, L11 PdAg has the same deviation of −7.17 × 10−3 Å. The lattice distortion is known to reflect the electronic interaction in the alloys, which is not related to the thermodynamic stability but also results in quite distinct mechanical properties. Additionally, we found that the SQSs show better agreement with the experimental values in comparison to the exact muffin-tin orbital (EMTO) method described in the literature [64]. This gives strong support for the accuracy of the first-principles calculation in this work.
3.2. Mechanical Stability
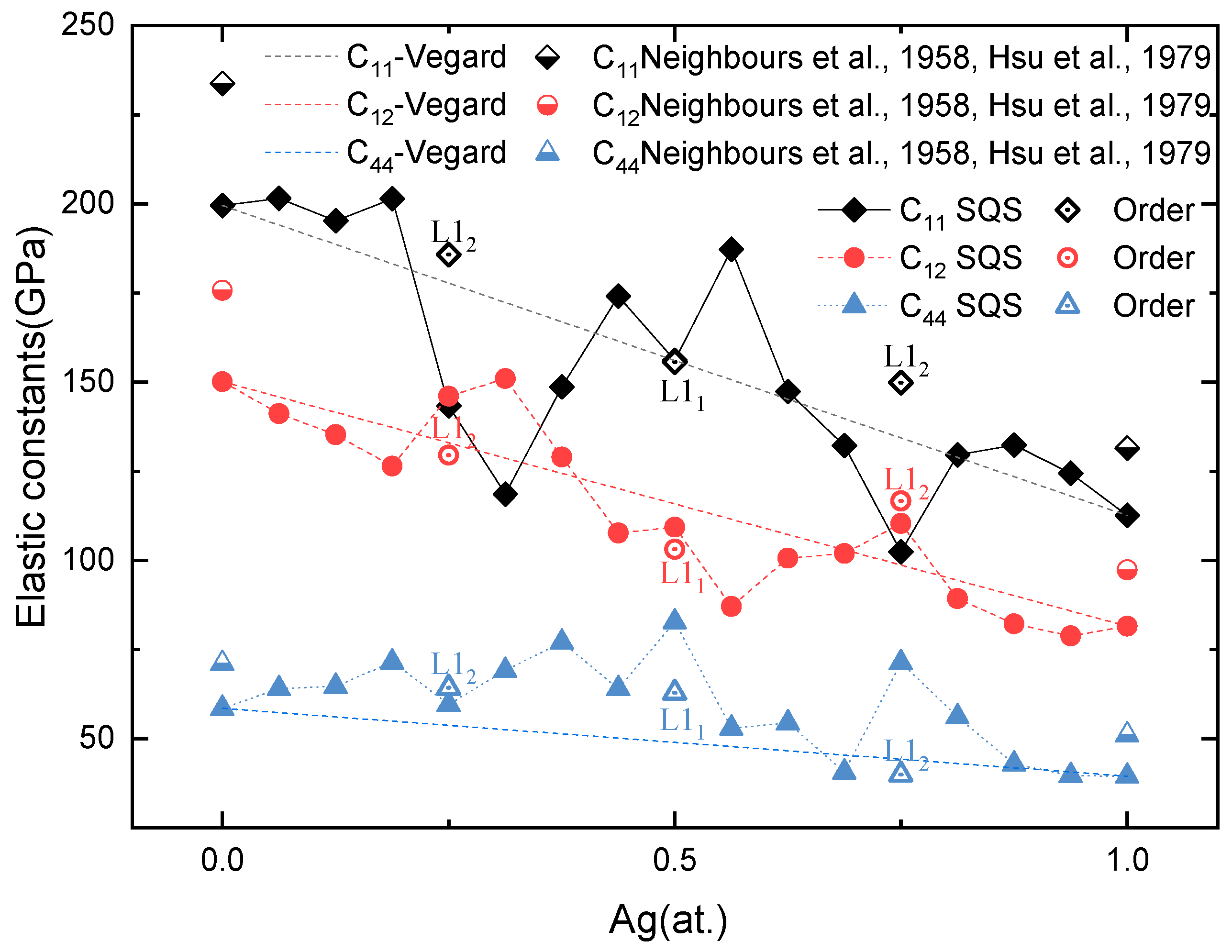
Based on the solid-solution structures from SQSs and the most stable intermetallic structures at Ag concentrations of 0.25, 0.5, and 0.75, a first-principles calculation is conducted to obtain the elastic properties of Pd-Ag alloys and L12 Pd3Ag, L11 PdAg, and L12 PdAg3 ordered phases. The single-crystal elastic constants are calculated through the universal linear-independent coupling strains (ULICS) method [66]. The results are shown in Figure 5, as well as the available experimental values [67,68]. It can be seen that the calculated results agree well with the experimental ones. For instance, the C11, C12, and C44 of pure Ag in this work are 112.61, 81.57, and 39.51 GPa, respectively, which match well with 124, 94, and 46 GPa from the experiments [67]. In addition, the C11, C12, and C44 of pure Pd are also consistent with the experimental values [68].
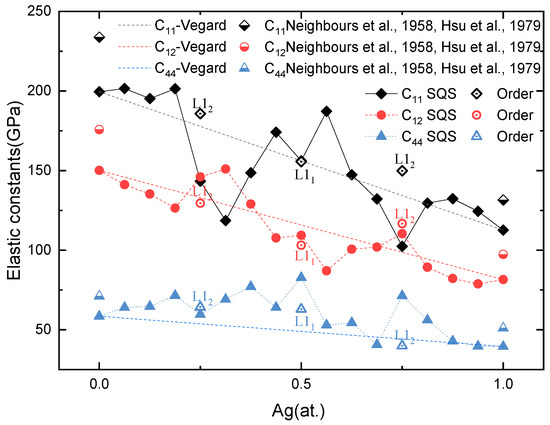
Figure 5.
Calculated single-crystal elastic constants for Pd-Ag alloys as a function of Ag concentration [67,68].
The single-crystal elastic constants show different features with respect to the Ag concentration. For the SQSs, the elastic constants gradually decrease from Pd to Ag, with some abnormal ups and downs at Pd3Ag, PdAg, and PdAg3. For example, C11 abruptly drops at a Ag concentration of 0.25, while C12 sharply increases at this concentration. On the contrary, the single-crystal elastic constants of ordered L12 Pd3Ag, L11 PdAg, and L12 PdAg3 decrease almost linearly and close to the reference Vegard’s law.
In order to explore the intrinsic mechanism related to the elastic properties of ordered and solid-solution phases, Born’s criterion [69] is adopted in the analysis. According to Born’s theory, a solid is mechanically stable when the elastic matrix is contingently positive definite. For cubic crystals, that means that Cij > 0, and C11 > C12. From Figure 6, we can see that all C11, C12, and C44 are positive. However, C11 is less than C12 for the SQS Pd3Ag, Pd12Ag6, and PdAg3. The ordered L12 Pd3Ag and PdAg3, on the contrary, have a C11 larger than C12, and meet Born’s criterion [69]. The single-crystal elastic constants of ordered and solid-solution Pd3Ag and PdAg3 comply with the ΔHf of those phases, that the ordered phases have a more negative ΔHf. For ordered and solid-solution PdAg, the C11 and C12 are similar with each other, with the C44 of the ordered L11 PdAg being less than its solid-solution counterpart. This indicates a softening in the mechanical strength, as the C44 reflects the shear strength.
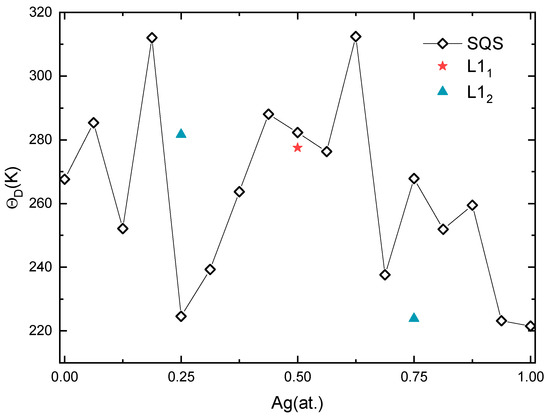
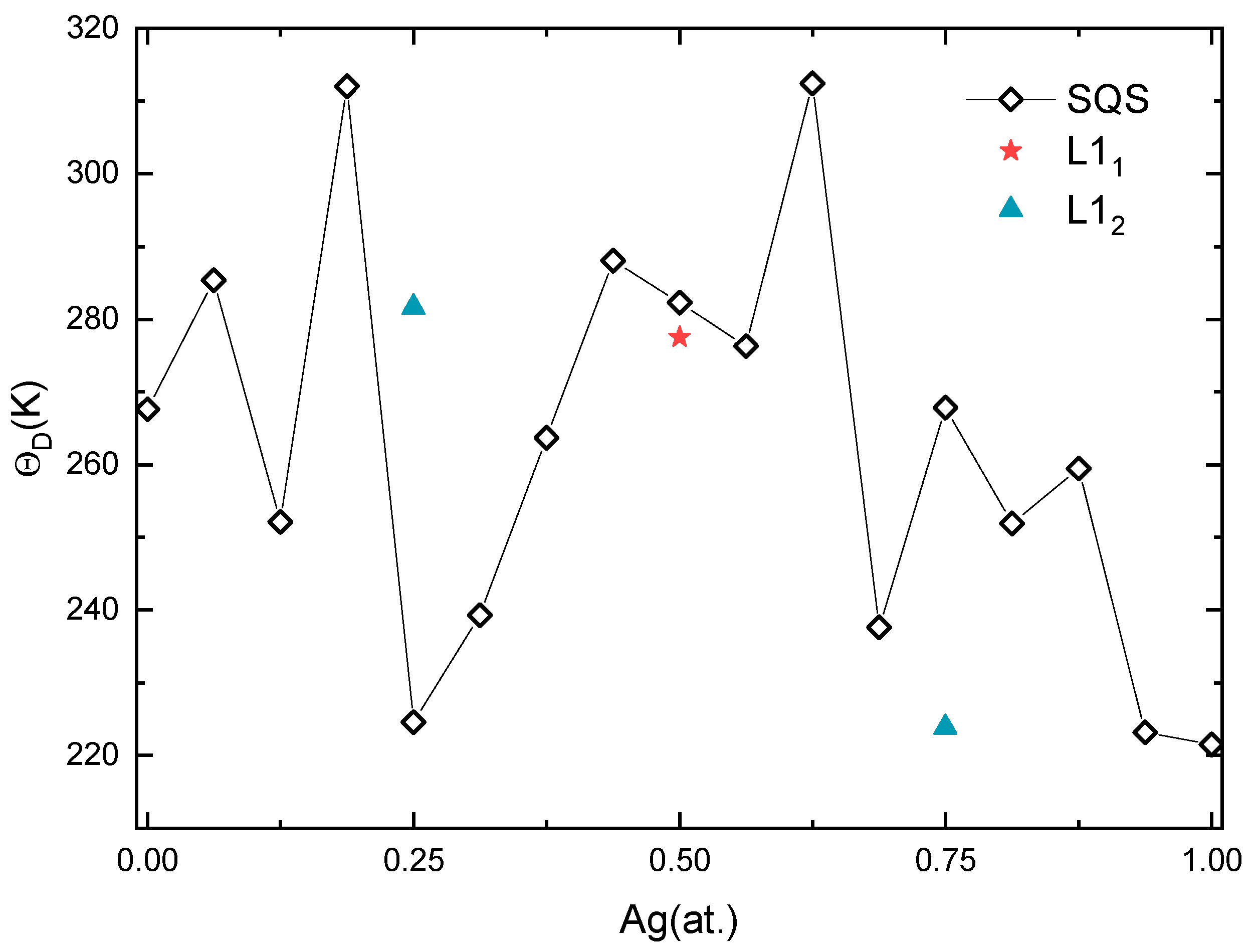
Figure 6.
Calculated Debye temperature of Pd-Ag alloys.
It is known that elastic constants are intrinsic properties of crystals and a direct reflection of electronic bonding [70]. To build the relation between the elastic properties and bonding features, the Debye temperature, , is derived. The Debye temperature is derived from the elastic constants calculated above through the following formula [71]:
where h and k are the Plank and Boltzmann constant, respectively; n, NA, M, and ρ are the number of atoms, Avogadro’s number, mean molecular weight, and the density, respectively. vm is the average velocity that can be calculated by the following equation:
where the vt and vt are the transverse velocity and longitudinal velocity, respectively; B and G are the isothermal bulk modulus and shear modulus, which is calculated by the Voigt–Heuss–Hill average [54] with elastic constants C11, C12, and C44.
After a series of calculations, the for Pd-Ag alloys is plotted in Figure 6. The values of the solid solutions reach a maximum of about 312 K at Ag concentrations of 0.1875 and 0.625, and then decrease to 225 K and 238 K at Ag concentrations of 0.25 and 0.6875, respectively. The sudden drop in the indicates that the alloy is weakly bonded when close to Ag concentrations of 0.25 and 0.6875, which are consistent with the unstable elastic constants around these Ag concentrations. In addition, the value of of L12 intermetallic Pd3Ag is significantly higher while that of L12 PdAg3 is relatively low. Accordingly, the L12 structure is more likely to be formed at a Ag concentration of 0.25. This observation is also supported by experiments on Pd3Ag and PdAg3 nanoparticles. For instance, researchers have successfully synthesized Pd3Ag alloys and found they are stable at the nanoscale [72,73]. On the contrary, the synthesis of PdAg3 nanoparticles seems more difficult, as it is prone to segregation in pure Ag and Pd phases [74]. The of L11 PdAg is slightly lower than the corresponding solid solution, as a reflection of the decline in the C44 value.
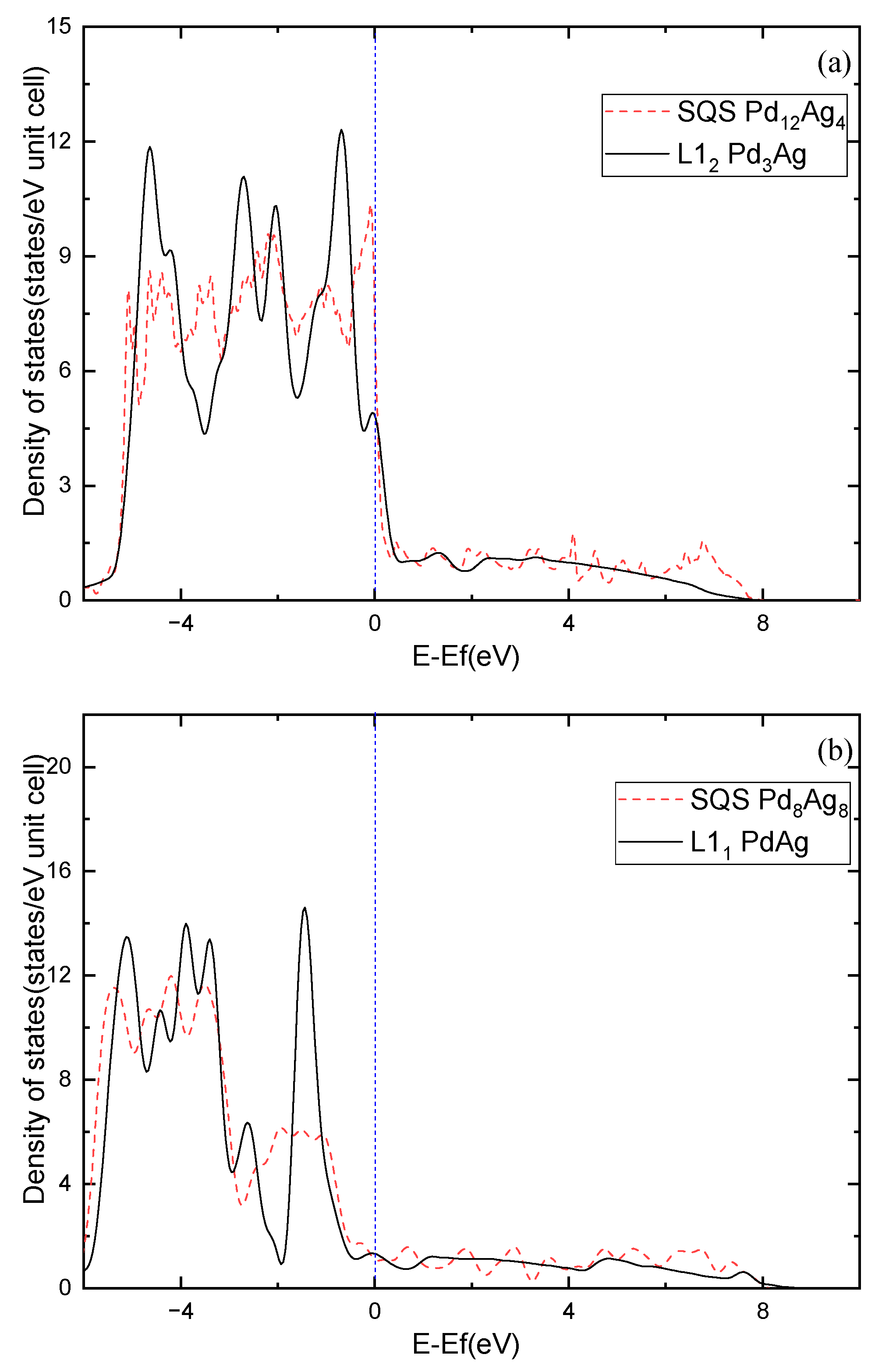
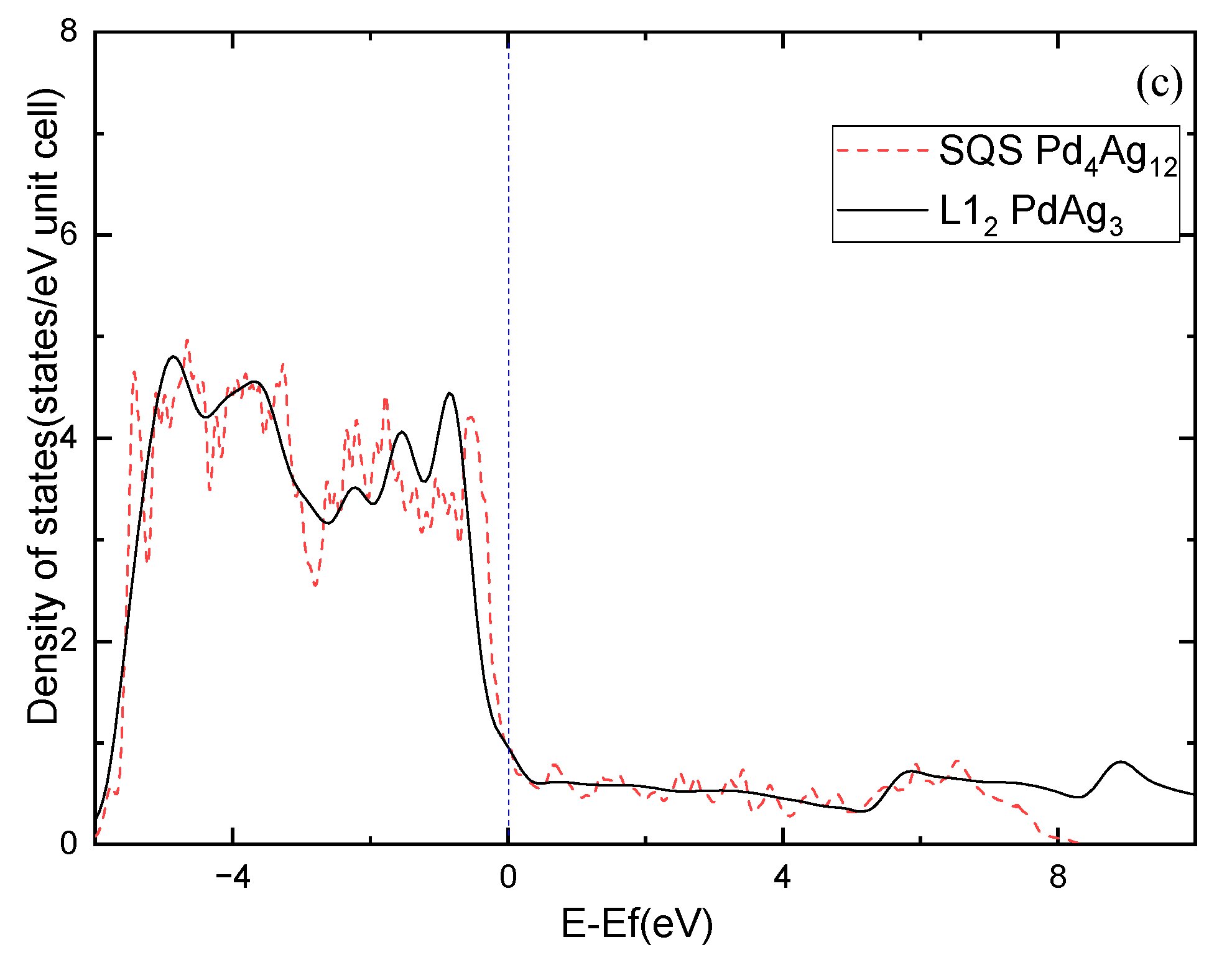
Moreover, the electronic structure of Pd-Ag alloys is further investigated. Figure 7 shows the calculated density of states (DOS) for the ordered and solid-solution Pd3Ag, PdAg, and PdAg3 phases. The ordered phases have much sharper peaks compared to the solid-solution ones, demonstrating partial covalent bonding features. Nevertheless, the DOSs of the ordered phases at the Fermi level show different features in comparison to those of the solid-solution phases. For example, L12 Pd3Ag has a lower DOS at the Fermi level than that of the SQS Pd3Ag, and are 7.3475 and 5.358 states/eV, respectively. L11 PdAg and L12 PdAg3 have smaller DOSs at the Fermi level than their solid-solution counterparts. This is consistent with the calculated of those ordered and solid-solution phases. Interestingly, the peaks of the SQS PdAg3 below the Fermi level are higher than those of the L12 PdAg3, suggesting the local atomic interaction may be stronger than those in the ordered L12 phase. As a result, the of the SQS PdAg3 is much higher than that of the ordered L12 phase.
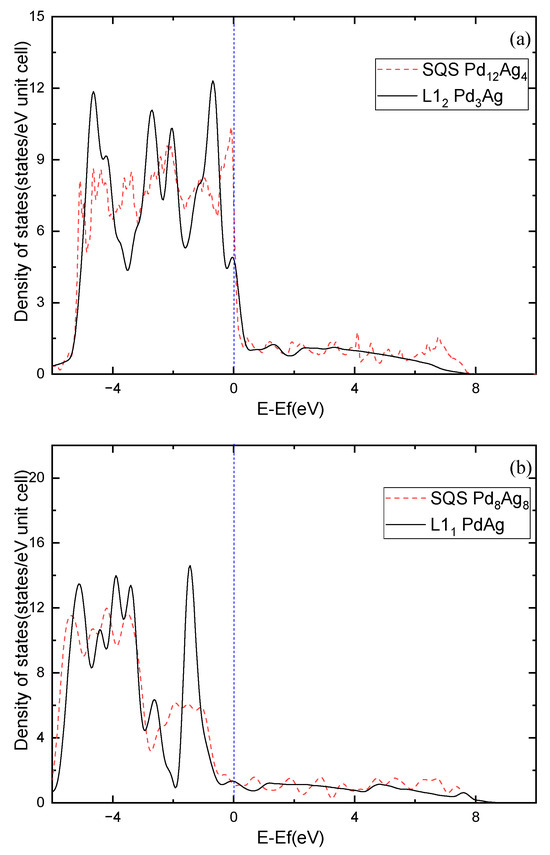
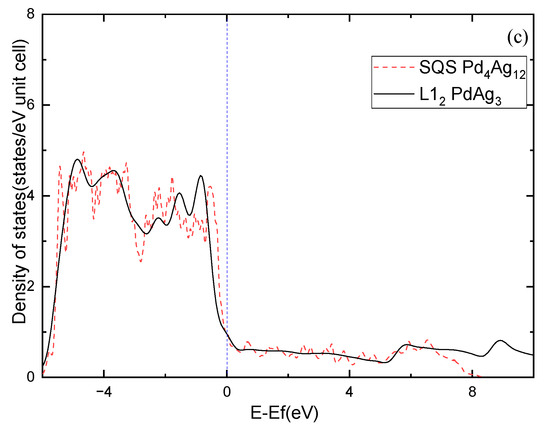
Figure 7.
Total density of states of Pd-Ag alloys: (a) Pd3Ag, (b) PdAg, (c) PdAg3.
3.3. Mechanical Anisotropy
As both structural and functional materials, Pd-based membranes are used in hydrogen separation and catalytic reactions. The durability and selectivity of membranes are dependent upon their mechanical properties and fabrication parameters. For polycrystalline membranes, the thickness of the membrane typically influences the deformation mechanism of the material. Hosseinian et al. [75] studied thin films with 30–100 nm thickness by in situ TEM tests and concluded the mechanism through which the nano-crack forms ahead of the main crack only in thinner films. Additionally, experiments have shown that the hydrogen permeation behavior of membranes increases with membrane thickness [20,76,77]. However, an increase in the film thickness will result in higher costs and potential assembly challenges. Prior research has demonstrated that the incorporation of Ag can effectively enhance the solubility of hydrogen, thereby increasing the permeability of hydrogen [20]. It is therefore crucial to identify Pd-Ag materials with thick conditions (as, in theory, the periodic boundary condition is used to simulate the thick condition).
For monocrystalline membranes, parameters such as geometry and direction might be important because of the mechanical anisotropy, which is expected to be investigated [78]. Generally speaking, the ordered structure will display strong mechanical anisotropy compared to solid solutions. As the and electronic structure show some interesting features for ordered phases, it is of importance to understand the mechanical anisotropy of those ordered and solid-solution phases. Moreover, mechanical anisotropy undermines the workability and limits the application of Pd-Ag alloys as structural materials [79].
To quantitively represent the anisotropy of those ordered and solid-solution Pd-Ag phases, the universal elastic anisotropy index (AU), proposed by Ranganathan and Ostoja-Starzewski [80], was adopted.
In addition, the AU has a value of 0 for the isotropic materials. Additionally, the anisotropy in compressibility is also estimated by the bulk modulus within the Voigt and Reuss approximations, as follows:
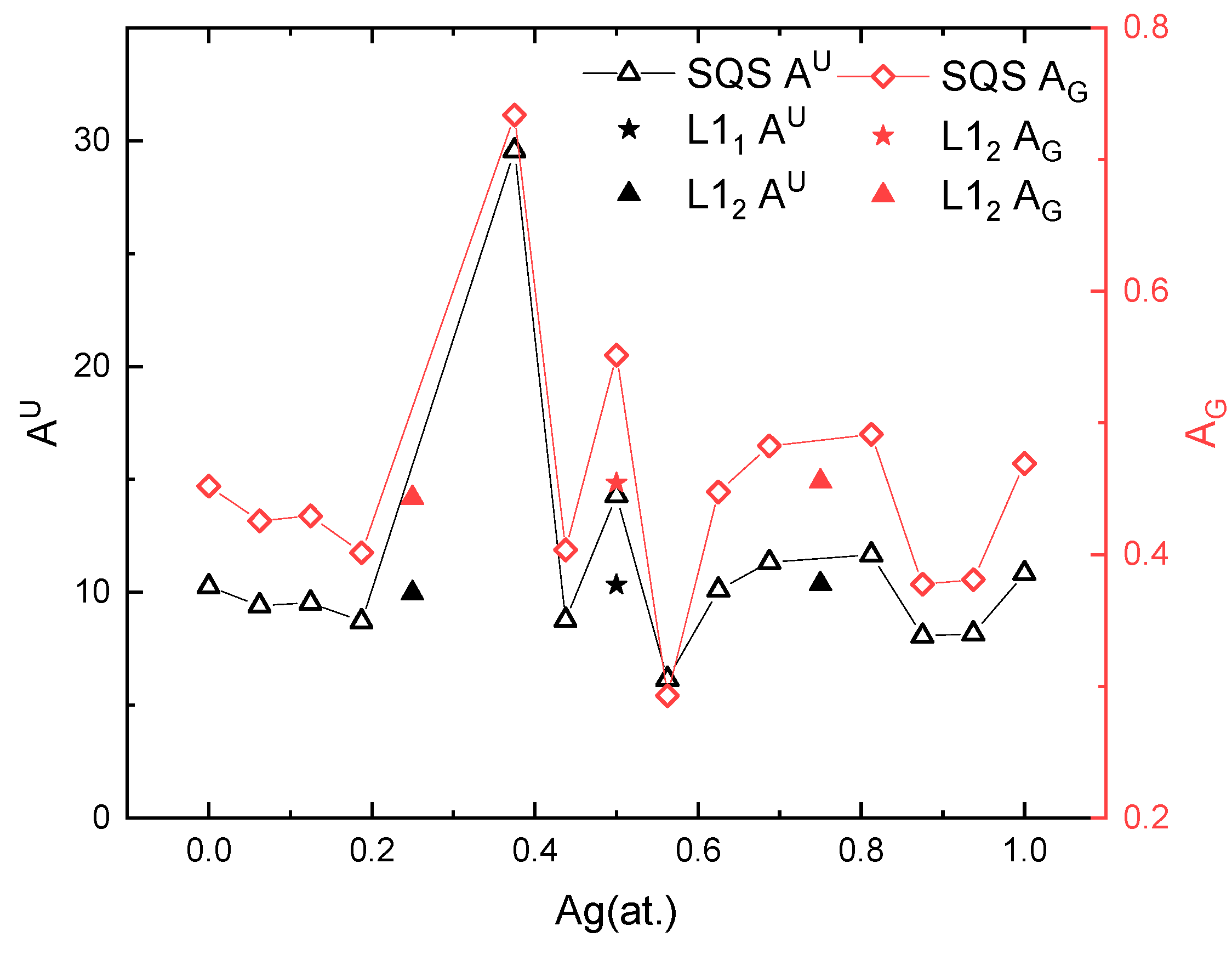
The anisotropy results are summarized in Figure 8. It should be noted that the solid-solution phases which are mechanically unstable in the previous section have been omitted in Figure 8, namely, xAg = 0.25, 0.3125, and 0.75. Regardless of the method used to calculate the anisotropy index, the anisotropic behavior is the same for the Pd-Ag systems. Interestingly, the ordered phases have a lower anisotropy index than the solid-solution phases at the same composition. For example, the of the L11 PdAg is 10, which is smaller than that (15) of the SQS PdAg. Except for their lower anisotropy index, the ordered phases incur abnormally high or low anisotropy for the nearby solid-solution phases. The SQS Pd10Ag8 near L12 Pd3Ag shows an anisotropy index as high as 31 for and 0.73 for , while the SQS Pd7Ag9 and Pd9Ag7 around L11 PdAg shows a lower anisotropy index compared to the ordered phase. This may originate from the fact that the solid-solution phases near the ordered phase are prone to local short-range or long-range ordering, and thus display an abnormal anisotropy index.
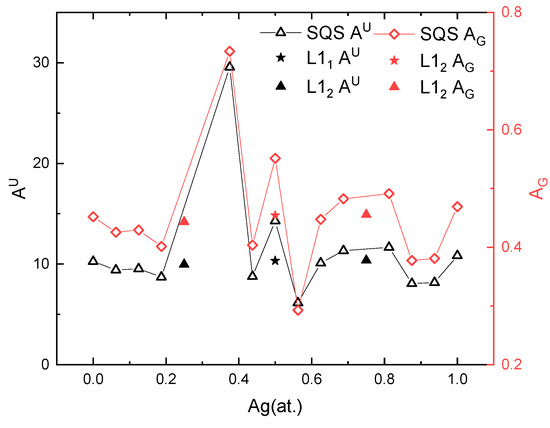
Figure 8.
Calculated anisotropy index of solid-solution and ordered phases at different Ag concentrations.
In order to dig into the details of mechanical anisotropy, the spatial dependence of elastic moduli, like Young’s modulus, the shear modulus, and the Poisson ratio, is investigated. The expression for the spatial dependence of Young’s modulus in cubic crystals is given by the following expression [81]:
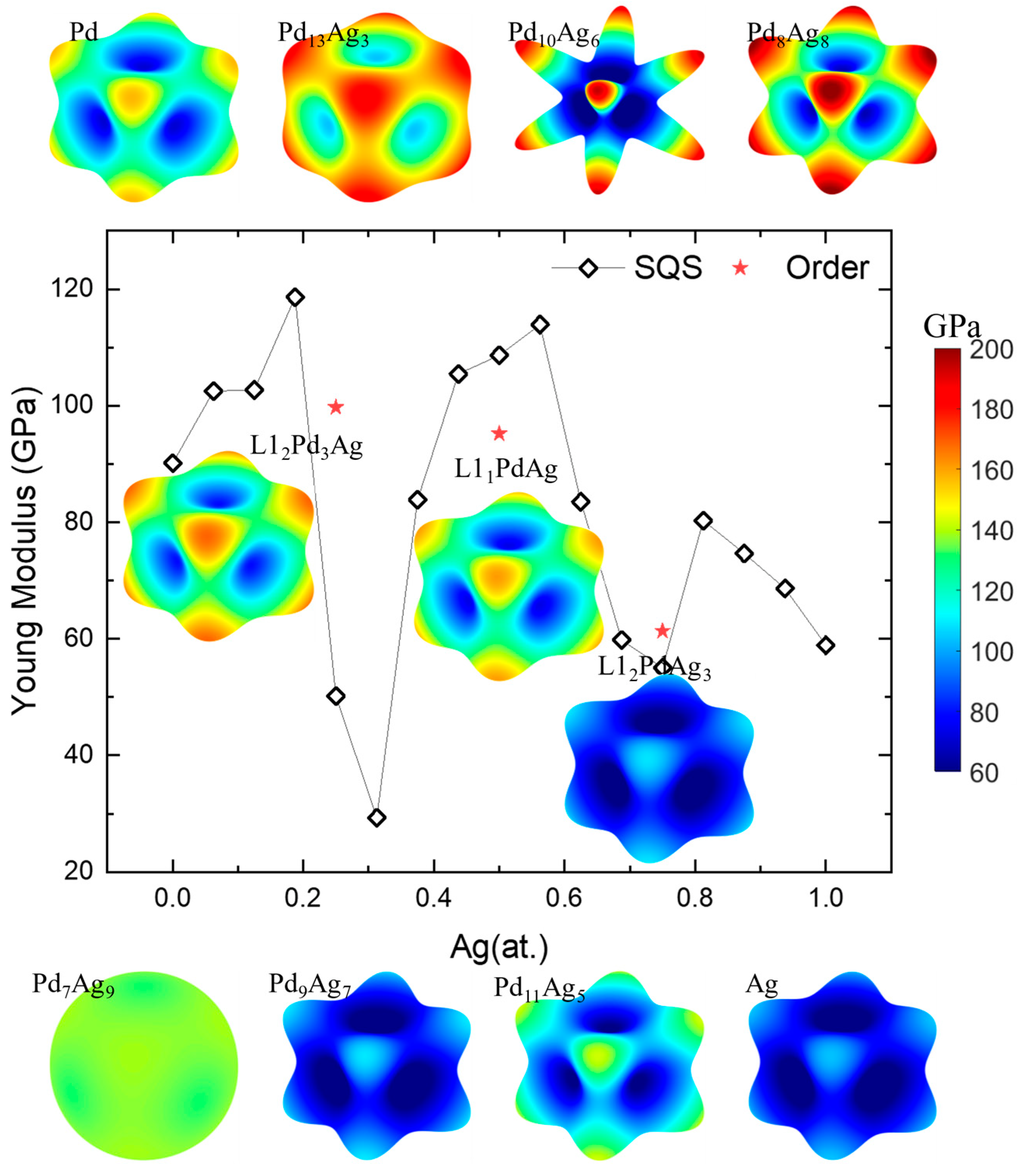
where l1, l2, and l3 are in the cosine direction. Figure 9 shows the calculated average Young’s modulus (E) [54] and its spatial-dependent 3D morphology in Pd-Ag systems. In the figure, the theoretically calculated values of the Young’s moduli of Pd and Ag is 96.16 GPa and 58.92 GPa, respectively, which are a little bit lower than the experimentally measured values of 112 GPa and 76 GPa [82]. This finding is in accordance with the result that the calculated values of the elastic constants are lower than the experimental values illustrated in Figure 5. The shape of the Young’s modulus of Pd-Ag, whether for the solid-solution or ordered phase, are quite similar and look like a cubic (except Pd7Ag9 and Pd10Ag6). The maximal E is along the crystallographic orientation <111> and the minimal E is along the <100> orientation. For Pd10Ag6, the solid solution shows the highest anisotropy of E, presenting as an eight-pointed star. Along the pointed <111> orientation, the E shows a maximum value of nearly 200 GPa, while the minimum value is lower than 80 GPa along the <100> orientation. The results align well with those of other research on the directional effects of Pd monocrystalline (111) oriented films [78]. In contrast, for Pd7Ag9, the Young’s modulus of the Pd-Ag solid solution has a sphere-like morphology, i.e., all the E values are within a narrow range of 130–150 GPa, showing near isotropic features. Moreover, the average Young’s modulus shows a decreasing tendency as a function of the Ag concentration, except for the solid solution with a Ag concentration around 0.25 and 0.75. The spatial-dependent 3D Young’s modulus is consistent with the anisotropy index in Figure 8, and reveals the large fluctuation for solid-solution phases near the ordered L12 Pd3Ag, L11 PdAg, and L12 PdAg3. This suggests that from a mechanical point of view, the design of Pd-Ag alloys should involve the selection of a concentration close to the stoichiometric composition near those ordered phases. Indeed, most experiments in the literature describe the selection of Pd3Ag, PdAg, and PdAg3 compositions and achieve better material performance [43,83,84,85].
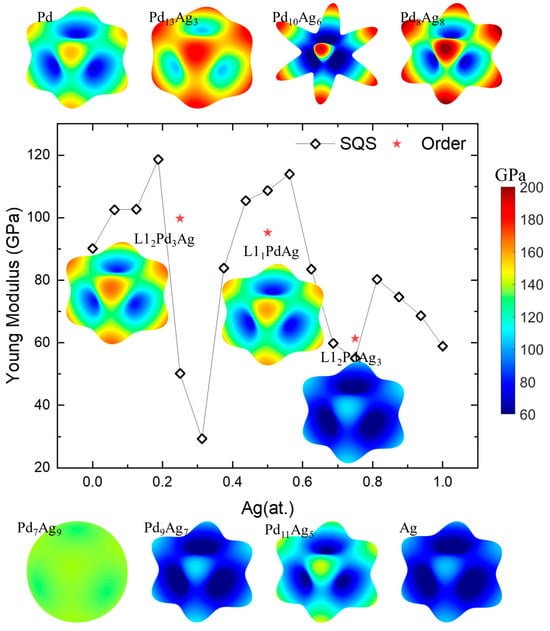
Figure 9.
Young’s modulus and its spatial-dependent 3D morphology of Pd-Ag alloys.
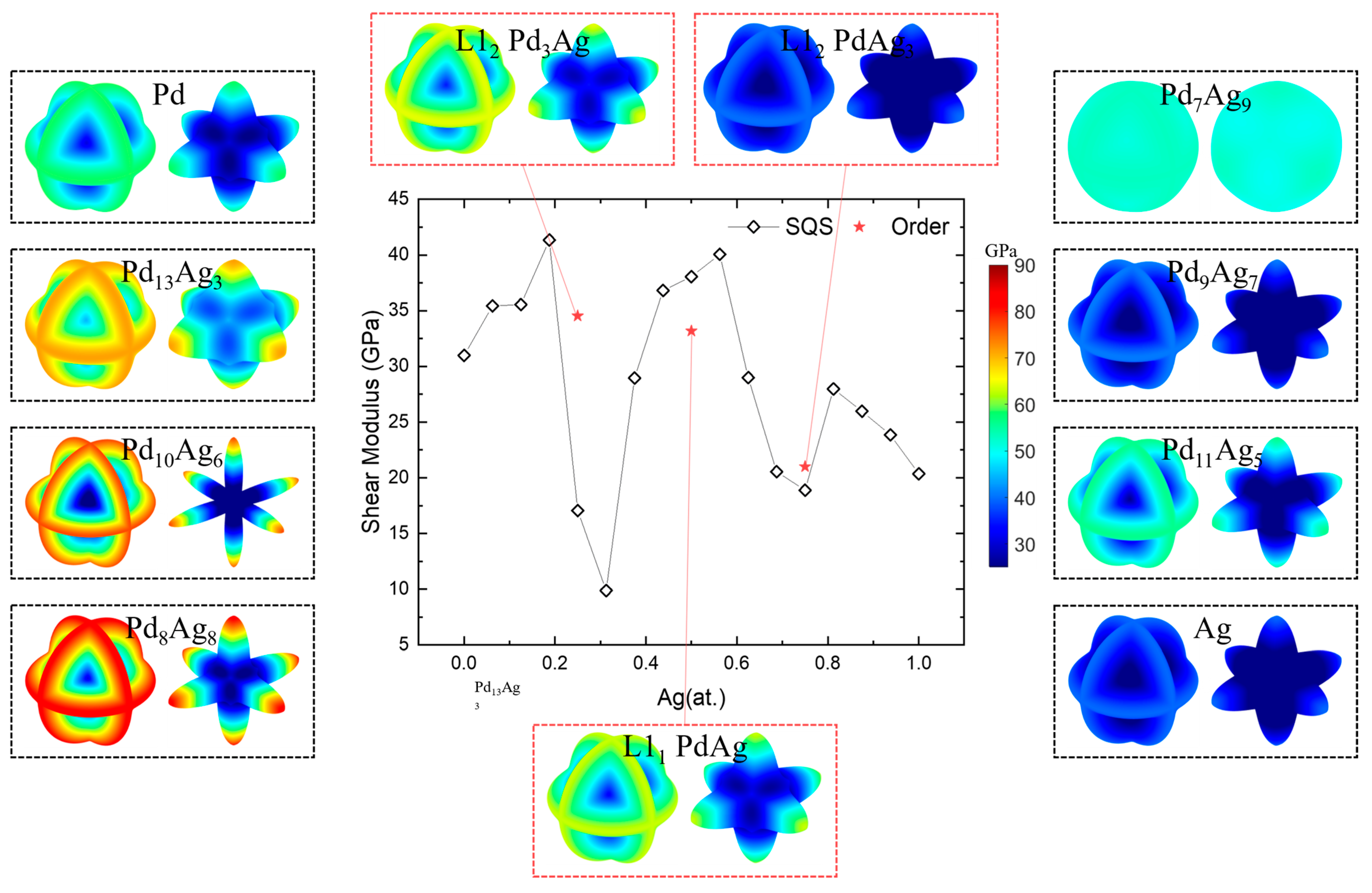
A high shear modulus and low anisotropy reduce the susceptibility of the Pd-Ag membrane to fracture due to lattice expansion caused by hydrogen dissolution under high hydrogen flux and complex temperature conditions [20]. The spatial-dependent and averaged shear modulus (G) of Pd-Ag systems are shown in Figure 10. Note that the left part in the dashed box represents the contours of the shear modulus on the maximal shear plane, while the right part represents those on the minimal shear plane [86]. The shear moduli of pure polycrystalline Pd and Ag, as illustrated in the figure, are 30.98 GPa and 20.36 GPa, respectively. Consistent with the case of the Young’s modulus, the calculated values of the shear modulus are found to be close to the experimental values of 47.50 GPa and 28.76 GPa [87,88]. Except for Pd7Ag9, the max shear planes are located on the three planetary rings of (100), (010), and (001), while the minimal shear planes are like six <100> pointed stars. Similar to the Young’s modulus, solid-solution Pd7Ag9 presents near-isotropic features, either the maximum or the minimum shear planes, like a sphere. Similar to the Young’s modulus and anisotropy index, abnormity appears for solid-solution phases near the ordered ones. This means that except for Pd7Ag9, the better choice is still the stoichiometric composition, for more uniform elastic properties.
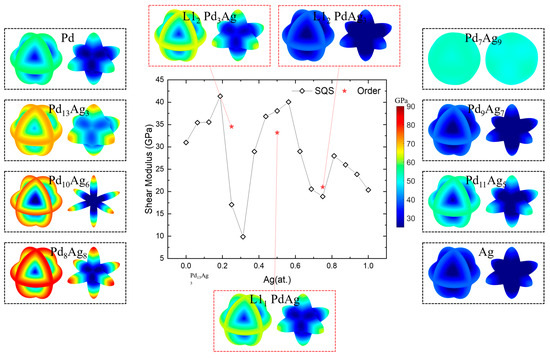
Figure 10.
Shear modulus and spatial-dependent 3D morphology of Pd-Ag alloys.
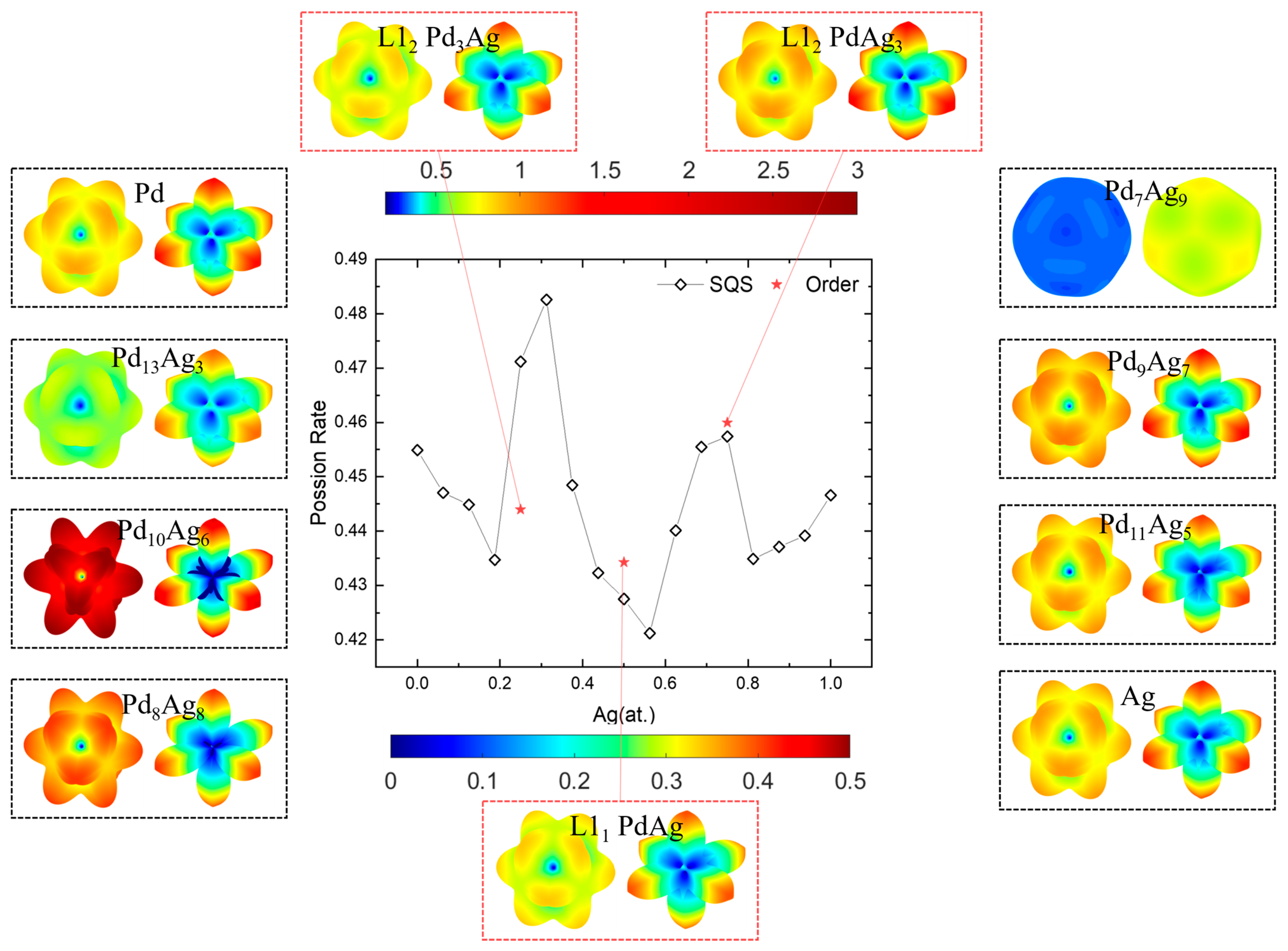
Among the elastic properties with respect to the Ag concentration, the Poisson ratio shows a divergent trend from the Young’s modulus and shear modulus, as indicated in the spatial-dependent and averaged Poisson ratios (υ) in Figure 11. Note that a dual color bar is employed here, where the upper color bar is the scale of υ on the maximal shear plane on the right, and the lower color bar is the scale of υ on the minimal shear plane on the left in the dashed box. The calculated values of the Poisson ratio for Pd and Ag are closer, 0.455 and 0.447, respectively, slightly higher than the experimental values of 0.386 and 0.335 [87,89]. In general, the shapes of υ are like flowers on the right and like six <100> pointed stars on the left. This is a feature caused by the maximum and minimum shear moduli, shown in Figure 10. Likewise, the solid-solution Pd7Ag9 presents near-isotropic features, and those near the ordered phases have abnormally large or small υ values. This again signifies the importance of choosing the correct concentration to achieve a better mechanical performance.
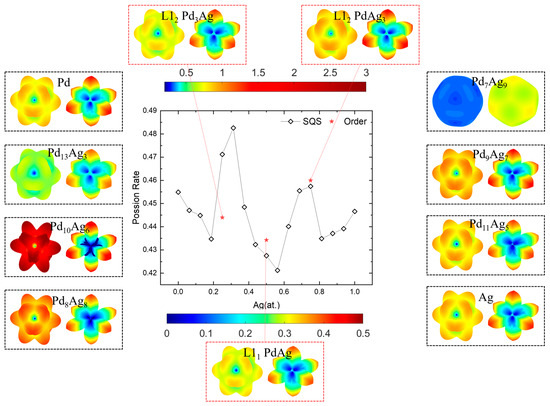
Figure 11.
Poisson’s ratio and spatial-dependent 3D morphology of Pd-Ag alloys.
Based on the results in this section, we propose the following compositions should be chosen for Pd-Ag membranes: Pd7Ag9 solid solution, Pd13Ag3 solid solution, and Pd3Ag L12 ordered phase. The Pd7Ag9 solid solution has exhibited favorable mechanical properties for polycrystalline materials and low anisotropy for monocrystalline materials, as illustrated in Figure 9, Figure 10 and Figure 11 of this manuscript. This suggests the high durability of the membranes under high hydrogen flux and stability under complex stress and temperature conditions. In addition, the high Ag content of Pd7Ag9 provides a greater H solubility along with lower raw material costs [20].
However, the synthesis of the Pd7Ag9 solid solution may be challenging. This is due to the formation energy of Pd7Ag9 solid solution being comparable to that of the solid solution with a higher Ag concentration, such as Pd6Ag10, and is lower than that of the PdAg L11 ordered phase. The alloys with an elemental ratio of Pd/Ag = 7:9 may precipitate out of the Pd7Ag9 ordered phase, thereby forming a mixed phase. Therefore, the Pd13Ag3 solid solution and Pd3Ag ordered phase are selected as substitutive phases of the Pd-Ag membrane material. As these phases with more significant mechanical anisotropy are employed as monocrystalline membranes, it is essential to consider the operational conditions and geometry. This implies that the direction of loading should be aligned with the orientation exhibiting the most robust mechanical properties. In the event that the long-term structural stability of purely Pd-Ag membranes cannot be guaranteed at high hydrogen fluxes, the use of composite membranes may be a viable solution [90,91].
4. Conclusions
In this work, the thermodynamical and mechanical stability of various ordered and random solid-solution phases were systematically studied through first-principles calculations. The ordered phases, L12 Pd3Ag, L11 PdAg, and L12 PdAg3, were found to be more stable than the solid-solution ones in terms of the heat of formation. The Born criterion of the single-crystal elastic constants confirms the improved stability of the ordered phases over their solid solution counterparts. The existence of the ordered phase was analyzed through the Debye temperature and electronic signatures. The mechanical anisotropy of all the solid solutions and the stable ordered phases were determined using the anisotropy index and spatial dependence of elastic moduli, like Young’s modulus, the shear modulus, and Poisson’s ratio. The mechanical anisotropy underlines the importance of maintaining a stoichiometric composition for better mechanical uniformity. Our findings not only align well with relevant experimental results, but also deepen our understanding of the phase structure of Pd-Ag alloys.
Author Contributions
Conceptualization, X.C. and C.L.; methodology, X.C.; software, X.C. and C.L.; validation, X.C., G.L. and Y.C.; formal analysis, X.C. and C.L.; investigation, X.C. and G.L.; resources, C.L.; data curation, X.C. and G.L.; writing—original draft preparation, X.C.; writing—review and editing, X.C., C.L. and Y.C.; visualization, X.C.; supervision, C.L.; project administration, C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 92373208) and Natural Science Foundation of Hunan Province (Grant Nos.: 2020JJ5733). We are grateful for the technical support from the High Performance Computing Center of Central South University.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Betancourt, L.E.; Rojas-Pérez, A.; Orozco, I.; Frenkel, A.I.; Li, Y.; Sasaki, K.; Senanayake, S.D.; Cabrera, C.R. Enhancing ORR Performance of Bimetallic PdAg Electrocatalysts by Designing Interactions between Pd and Ag. ACS Appl. Energy Mater. 2020, 3, 2342–2349. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Ling, L.; Zhang, R.; Fan, M. Probe into the Effects of Surface Composition and Ensemble Effect of Active Sites on the Catalytic Performance of C2H2 Semi-Hydrogenation over the Pd-Ag Bimetallic Catalysts. Chem. Eng. Sci. 2020, 218, 115549. [Google Scholar] [CrossRef]
- Mostashari, S.M.; Dehkharghani, R.A.; Afshar-Taromi, F.; Farsadrooh, M. A Straightforward One-Pot Synthesis of Pd–Ag Supported on Activated Carbon as a Robust Catalyst toward Ethanol Electrooxidation. Int. J. Hydrogen Energy 2021, 46, 9406–9416. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kwon, Y.-H.; Seol, H.-J. Effect of Cooling Rate on Hardness and Phase Transformation of a Pd-Ag-Based Metal–Ceramic Alloy with or without Ice-Quenching. Metals 2021, 11, 680. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhang, P.; Yang, X.; Chen, Y. Evolution of Interface Microstructure and Tensile Properties of AgPd30/CuNi18Zn26 Bilayer Laminated Composite Manufactured by Rolling and Annealing. Metals 2022, 12, 367. [Google Scholar] [CrossRef]
- Cao, X.; Jang, B.W.-L.; Hu, J.; Wang, L.; Zhang, S. Synthetic Strategies of Supported Pd-Based Bimetallic Catalysts for Selective Semi-Hydrogenation of Acetylene: A Review and Perspectives. Molecules 2023, 28, 2572. [Google Scholar] [CrossRef]
- Chen, W.-H.; Biswas, P.P.; Ong, H.C.; Hoang, A.T.; Nguyen, T.-B.; Dong, C.-D. A Critical and Systematic Review of Sustainable Hydrogen Production from Ethanol/Bioethanol: Steam Reforming, Partial Oxidation, and Autothermal Reforming. Fuel 2023, 333, 126526. [Google Scholar] [CrossRef]
- Chen, W.-H.; Li, S.-C.; Lim, S.; Chen, Z.-Y.; Juan, J.C. Reaction and Hydrogen Production Phenomena of Ethanol Steam Reforming in a Catalytic Membrane Reactor. Energy 2021, 220, 119737. [Google Scholar] [CrossRef]
- He, M.; Fang, Z.; Wang, P.; You, Y.; Li, Z. Recent Progress in Photocatalytic Chemical Fixation of Carbon Dioxide. ACS Sustain. Chem. Eng. 2023, 11, 12194–12217. [Google Scholar] [CrossRef]
- Razmgar, K.; Altarawneh, M.; Oluwoye, I.; Senanayake, G. Ceria-Based Catalysts for Selective Hydrogenation Reactions: A Critical Review. Catal. Surv. Asia 2021, 25, 27–47. [Google Scholar] [CrossRef]
- Rusinque, B.; Escobedo, S.; De Lasa, H. Hydrogen Production via Pd-TiO2 Photocatalytic Water Splitting under Near-UV and Visible Light: Analysis of the Reaction Mechanism. Catalysts 2021, 11, 405. [Google Scholar] [CrossRef]
- Wang, M.; Liang, L.; Liu, X.; Sun, Q.; Guo, M.; Bai, S.; Xu, Y. Selective Semi-Hydrogenation of Alkynes on Palladium-Selenium Nanocrystals. J. Catal. 2023, 418, 247–255. [Google Scholar] [CrossRef]
- Yang, J.-J.; Zhang, Y.; Xie, X.-Y.; Fang, W.-H.; Cui, G. Photocatalytic Reduction of Carbon Dioxide to Methane at the Pd-Supported TiO2 Interface: Mechanistic Insights from Theoretical Studies. ACS Catal. 2022, 12, 8558–8571. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, Q.; Ma, Y.; Guan, Q.; Jin, R.; Wang, H.; Yang, B.; Lu, J. Support-Induced Unusual Size Dependence of Pd Catalysts in Chemoselective Hydrogenation of Para-Chloronitrobenzene. J. Catal. 2021, 400, 173–183. [Google Scholar] [CrossRef]
- Tucho, W.M.; Venvik, H.J.; Stange, M.; Walmsley, J.C.; Holmestad, R.; Bredesen, R. Effects of Thermal Activation on Hydrogen Permeation Properties of Thin, Self-Supported Pd/Ag Membranes. Sep. Purif. Technol. 2009, 68, 403–410. [Google Scholar] [CrossRef]
- Grashoff, G.J.; Pilkington, C.E.; Corti, C.W. The Purification of Hydrogen: A Review of the Technology Emphasising the Current Status of Palladium Membrane Diffusion. Platin. Met. Rev. 1983, 27, 157–169. [Google Scholar] [CrossRef]
- Opetubo, O.; Ibitoye, A.I.; Oyinbo, S.T.; Jen, T.-C. Analysis of Hydrogen Embrittlement in Palladium–Copper Alloys Membrane from First Principal Method Using Density Functional Theory. Vacuum 2022, 205, 111439. [Google Scholar] [CrossRef]
- Zhu, K.; Li, X.; Zhang, Y.; Zhao, X.; Liu, Z.; Guo, J. Tailoring the Hydrogen Transport Properties of Highly Permeable Nb51W5Ti23Ni21 Alloy Membrane by Pd Substitution. Int. J. Hydrogen Energy 2022, 47, 6734–6744. [Google Scholar] [CrossRef]
- Lin, W.-H.; Chang, H.-F. Characterizations of Pd–Ag Membrane Prepared by Sequential Electroless Deposition. Surf. Coat. Technol. 2005, 194, 157–166. [Google Scholar] [CrossRef]
- Zeng, G.; Goldbach, A.; Shi, L.; Xu, H. On Alloying and Low-Temperature Stability of Thin, Supported PdAg Membranes. Int. J. Hydrogen Energy 2012, 37, 6012–6019. [Google Scholar] [CrossRef]
- Ververs, W.J.R.; Arratibel Plazaola, A.; Di Felice, L.; Gallucci, F. On the Applicability of PdAg Membranes in Propane Dehydrogenation Processes. Int. J. Hydrogen Energy 2024, 50, 409–419. [Google Scholar] [CrossRef]
- Albano, M.; Madeira, L.M.; Miguel, C.V. Use of Pd-Ag Membrane Reactors for Low-Temperature Dry Reforming of Biogas—A Simulation Study. Membranes 2023, 13, 630. [Google Scholar] [CrossRef]
- Cechetto, V.; Agnolin, S.; Di Felice, L.; Pacheco Tanaka, A.; Llosa Tanco, M.; Gallucci, F. Metallic Supported Pd-Ag Membranes for Simultaneous Ammonia Decomposition and H2 Separation in a Membrane Reactor: Experimental Proof of Concept. Catalysts 2023, 13, 920. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Andreev, G.; Ivanin, S.; Dzhimak, S. High-Performance Hydrogen-Selective Pd-Ag Membranes Modified with Pd-Pt Nanoparticles for Use in Steam Reforming Membrane Reactors. Int. J. Mol. Sci. 2023, 24, 17403. [Google Scholar] [CrossRef]
- Escalante, Y.; Tarditi, A.M. Thermally Stable Membranes Based on PdNiAu Systems with High Nickel Content for Hydrogen Separation. J. Membr. Sci. 2023, 676, 121581. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, P.; Yang, D.; Yang, P.; Liao, N. First-Principles Evaluation of Pd–Pt–Ag and Pd–Pt–Au Ternary Alloys as High Performance Membranes for Hydrogen Separation. Int. J. Hydrogen Energy 2024, 68, 607–613. [Google Scholar] [CrossRef]
- Ryu, S.; Badakhsh, A.; Oh, J.G.; Ham, H.C.; Sohn, H.; Yoon, S.P.; Choi, S.H. Experimental and Numerical Study of Pd/Ta and PdCu/Ta Composites for Thermocatalytic Hydrogen Permeation. Membranes 2022, 13, 23. [Google Scholar] [CrossRef]
- Savitskii, E.M.; Pravoverov, N.L. Kurnakov Phases in the Palladium-Silver System. Russ. J. Inorg. Chem. 1961, 6, 253–254. [Google Scholar]
- Müller, S.; Zunger, A. First-Principles Predictions of Yet-Unobserved Ordered Structures in the Ag-Pd Phase Diagram. Phys. Rev. Lett. 2001, 87, 165502. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Jiang, W.; Gong, H. Hydrogen Solubility in Pd3Ag Phases from First-Principles Calculation. Metals 2019, 9, 121. [Google Scholar] [CrossRef]
- Ruban, A.V.; Simak, S.I.; Korzhavyi, P.A.; Johansson, B. Theoretical Investigation of Bulk Ordering and Surface Segregation in Ag-Pd and Other Isoelectornic Alloys. Phys. Rev. B 2007, 75, 054113. [Google Scholar] [CrossRef]
- Svenum, I.-H.; Herron, J.A.; Mavrikakis, M.; Venvik, H.J. Adsorbate-Induced Segregation in a PdAg Membrane Model System: Pd3Ag(111). Catal. Today 2012, 193, 111–119. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Reuter, K.; Scheffler, M. Alloy Surface Segregation in Reactive Environments: First-Principles Atomistic Thermodynamics Study of Ag3Pd(111) in Oxygen Atmospheres. Phys. Rev. B 2008, 77, 075437. [Google Scholar] [CrossRef]
- Tang, J.; Deng, L.; Deng, H.; Xiao, S.; Zhang, X.; Hu, W. Surface Segregation and Chemical Ordering Patterns of Ag–Pd Nanoalloys: Energetic Factors, Nanoscale Effects, and Catalytic Implication. J. Phys. Chem. C 2014, 118, 27850–27860. [Google Scholar] [CrossRef]
- Calvo, F. Thermodynamics of Nanoalloys. Phys. Chem. Chem. Phys. 2015, 17, 27922–27939. [Google Scholar] [CrossRef]
- Cui, M.; Yang, C.; Hwang, S.; Yang, M.; Overa, S.; Dong, Q.; Yao, Y.; Brozena, A.H.; Cullen, D.A.; Chi, M.; et al. Multi-Principal Elemental Intermetallic Nanoparticles Synthesized via a Disorder-to-Order Transition. Sci. Adv. 2022, 8, eabm4322. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, D.P.; Sang, X.; Unocic, R.R.; Skrabalak, S.E. Size-Dependent Disorder–Order Transformation in the Synthesis of Monodisperse Intermetallic PdCu Nanocatalysts. ACS Nano 2016, 10, 6345–6353. [Google Scholar] [CrossRef] [PubMed]
- Keuler, J.N.; Lorenzen, L. Developing a Heating Procedure to Optimise Hydrogen Permeance through Pd–Ag Membranes of Thickness Less than 2.2 Μm. J. Membr. Sci. 2002, 195, 203–213. [Google Scholar] [CrossRef]
- Wang, D.; Flanagan, T.B.; Shanahan, K. Diffusion of H through Pd-Ag Alloys (423–523 K). J. Phys. Chem. B 2008, 112, 1135–1148. [Google Scholar] [CrossRef]
- Tosti, S.; Borgognoni, F.; Santucci, A. Electrical Resistivity, Strain and Permeability of Pd–Ag Membrane Tubes. Int. J. Hydrogen Energy 2010, 35, 7796–7802. [Google Scholar] [CrossRef]
- Lindau, R.; Möslang, A. Mechanical and Microstructural Properties of Tritium Permeable PdAg Alloy after Helium Implantation. J. Nucl. Mater. 1992, 191, 178–182. [Google Scholar] [CrossRef]
- Luo, H.; Liu, W.; Ma, Y.; Xiao, D.; Liang, C. Unraveling L12Al3X (X = Ti, Zr, Hf) Nano-Precipitate Evolution in Aluminum Alloys via Multi-Scale Diffusion Simulation. J. Mater. Res. Technol. 2024, 30, 7104–7114. [Google Scholar] [CrossRef]
- Othman, P.N.A.M.; Karim, N.A.; Kamarudin, S.K. First Principle Study of the Electronic and Catalytic Properties of Palladium-Silver (PdAg) Alloys Catalyst for Direct Liquid Fuel Cells. Chem. Phys. 2023, 564, 111711. [Google Scholar] [CrossRef]
- Farberow, C.A.; Godinez-Garcia, A.; Peng, G.; Perez-Robles, J.F.; Solorza-Feria, O.; Mavrikakis, M. Mechanistic Studies of Oxygen Reduction by Hydrogen on PdAg(110). ACS Catal. 2013, 3, 1622–1632. [Google Scholar] [CrossRef]
- Li, Q.; Song, L.; Pan, L.; Chen, Y.; Ling, M.; Zhuang, X.; Zhang, X. Density Functional Theory Studies of Electronic Properties of PdAg/Pd Surface Alloys. Appl. Surf. Sci. 2014, 288, 69–75. [Google Scholar] [CrossRef]
- Zunger, A.; Wei, S.-H.; Ferreira, L.G.; Bernard, J.E. Special Quasirandom Structures. Phys. Rev. Lett. 1990, 65, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhu, W.; Ma, Y.; Liu, W.; Huang, Y.; Liang, C. Evaluation of Phase Relationship in W-Fe-C Ternary System through Symmetry Principles and First-Principles Calculation. Mater. Des. 2022, 224, 111376. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, Molecules, Solids, and Surfaces: Applications of the Generalized Gradient Approximation for Exchange and Correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Liu, L.C.; Wang, J.W.; Qian, J.; He, Y.H.; Gong, H.R.; Liang, C.P.; Zhou, S.F. Fundamental Effects of Ag Alloying on Hydrogen Behaviors in PdCu. J. Membr. Sci. 2018, 550, 230–237. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-Precision Sampling for Brillouin-Zone Integration in Metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved Tetrahedron Method for Brillouin-Zone Integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. 1952, 65, 349. [Google Scholar] [CrossRef]
- Reuss, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. Z. Angew. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Hart, G.L.W.; Nelson, L.J.; Vanfleet, R.R.; Campbell, B.J.; Sluiter, M.H.F.; Neethling, J.H.; Olivier, E.J.; Allies, S.; Lang, C.I.; Meredig, B.; et al. Revisiting the Revised Ag-Pt Phase Diagram. Acta Mater. 2017, 124, 325–332. [Google Scholar] [CrossRef]
- Liang, J.; Xia, Y.; Liu, X.; Huang, F.; Liu, J.; Li, S.; Wang, T.; Jiao, S.; Cao, R.; Han, J.; et al. Molybdenum-doped Ordered L10-PdZn Nanosheets for Enhanced Oxygen Reduction Electrocatalysis. SusMat 2022, 2, 347–356. [Google Scholar] [CrossRef]
- Volkova, E.G.; Novikova, O.S.; Volkov, A.Y. Formation of the L12-Type Superstructure in Cu-5.9 at.%Pd and Cu-8 at%Pd Alloys. IOP Conf. Ser. Mater. Sci. Eng. 2018, 447, 012029. [Google Scholar] [CrossRef]
- Yu, G.; Cheng, T.; Zhang, X.; Gong, W. Pressure-Induced Two Magnetic Collapses in the Ferromagnetic L12-Fe3Pd Alloy and Related Elasticity and Lattice Dynamics Anomalies. J. Magn. Magn. Mater. 2021, 538, 168322. [Google Scholar] [CrossRef]
- Zharkov, S.M.; Moiseenko, E.T.; Altunin, R.R. L10 Ordered Phase Formation at Solid State Reactions in Cu/Au and Fe/Pd Thin Films. J. Solid State Chem. 2019, 269, 36–42. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, J.; Ling, C.; Ge, Y.; Chen, B.; Tan, C.; Fan, Z.; Huang, J.; Chen, J.; Liu, Z.; et al. Synthesis of Pd3Sn and PdCuSn Nanorods with L12Phase for Highly Efficient Electrocatalytic Ethanol Oxidation. Adv. Mater. 2022, 34, 2106115. [Google Scholar] [CrossRef]
- Von Pezold, J.; Dick, A.; Friák, M.; Neugebauer, J. Generation and Performance of Special Quasirandom Structures for Studying the Elastic Properties of Random Alloys: Application to Al-Ti. Phys. Rev. B 2010, 81, 094203. [Google Scholar] [CrossRef]
- Delczeg-Czirjak, E.K.; Delczeg, L.; Ropo, M.; Kokko, K.; Punkkinen, M.P.J.; Johansson, B.; Vitos, L. Ab Initio Study of the Elastic Anomalies in Pd-Ag Alloys. Phys. Rev. B 2009, 79, 085107. [Google Scholar] [CrossRef]
- Hultgren, R.; Desai, P.D.; Hawkins, D.T.; Gleiser, M.; Kelley, K.K. Selected Values of the Thermodynamic Properties of the Elements; American Society for Metals: Metals Park, OH, USA, 1973; pp. 264–267. [Google Scholar]
- Yu, R.; Zhu, J.; Ye, H.Q. Calculations of Single-Crystal Elastic Constants Made Simple. Comput. Phys. Commun. 2010, 181, 671–675. [Google Scholar] [CrossRef]
- Neighbours, J.R.; Alers, G.A. Elastic Constants of Silver and Gold. Phys. Rev. 1958, 111, 707–712. [Google Scholar] [CrossRef]
- Hsu, D.K.; Leisure, R.G. Elastic Constants of Palladium and β-Phase Palladium Hydride between 4 and 300 K. Phys. Rev. B 1979, 20, 1339–1344. [Google Scholar] [CrossRef]
- Zener, C. Contributions to the Theory of Beta-Phase Alloys. Phys. Rev. 1947, 71, 846–851. [Google Scholar] [CrossRef]
- Nakashima, P.N.H.; Smith, A.E.; Etheridge, J.; Muddle, B.C. The Bonding Electron Density in Aluminum. Science 2011, 331, 1583–1586. [Google Scholar] [CrossRef]
- Anderson, O.L. A Simplified Method for Calculating the Debye Temperature from Elastic Constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Lin, C.-K.; Lin, Y.-G.; Wu, T.; Barkholtz, H.M.; Lin, Q.; Wei, H.; Brewe, D.L.; Miller, J.T.; Liu, D.-J.; Ren, Y.; et al. Direct Synthesis of Bimetallic Pd3Ag Nanoalloys from Bulk Pd3Ag Alloy. Inorg. Chem. 2012, 51, 13281–13288. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, N.S.; Baeva, G.N.; Markov, P.V.; Mashkovsky, I.S.; Bukhtiyarov, A.V.; Zubavichus, Y.V.; Stakheev, A.Y. In Situ FTIR Study of Surface Site Transformations in Pd3In/α-Al2O3 and Pd3Ag/α-Al2O3 Induced by CO Adsorption. Mendeleev Commun. 2022, 32, 807–809. [Google Scholar] [CrossRef]
- Santoveña-Uribe, A.; Maya-Cornejo, J.; Bahena, D.; Ledesma, J.; Pérez, R.; Esparza, R. Synthesis and Characterization of AgPd Bimetallic Nanoparticles as Efficient Electrocatalysts for Oxygen Reduction Reaction. Electrocatalysis 2020, 11, 536–545. [Google Scholar] [CrossRef]
- Hosseinian, E.; Gupta, S.; Pierron, O.N.; Legros, M. Size Effects on Intergranular Crack Growth Mechanisms in Ultrathin Nanocrystalline Gold Free-Standing Films. Acta Mater. 2018, 143, 77–87. [Google Scholar] [CrossRef]
- Liu, J.; Bellini, S.; De Nooijer, N.C.A.; Sun, Y.; Pacheco Tanaka, D.A.; Tang, C.; Li, H.; Gallucci, F.; Caravella, A. Hydrogen Permeation and Stability in Ultra-Thin Pd Ru Supported Membranes. Int. J. Hydrogen Energy 2020, 45, 7455–7467. [Google Scholar] [CrossRef]
- Wunsch, A.; Gapp, E.; Peters, T.; Pfeifer, P. Impact of Product Gas Impurities from Dehydrogenation of Perhydro-Dibenzyltoluene on the Performance of a 10 Μm PdAg-Membrane. J. Membr. Sci. 2021, 628, 119094. [Google Scholar] [CrossRef]
- Alí, M.L.; Crespo, E.A.; Ruda, M.; Bringa, E.M.; Ramos, S.B. Hydrogen Effects on the Mechanical Properties of Nanocrystalline Free-Standing Palladium Thin Films. Int. J. Hydrogen Energy 2020, 45, 15213–15225. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Tang, S.; Ma, Y.; Liu, W.; Liang, C. Effects of Order-Disorder Transition on Phase Relationship, Elastic Strength, and Mechanical Anisotropy of Al-Li Alloys. Materialia 2022, 24, 101483. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal Elastic Anisotropy Index. Phys. Rev. Lett. 2008, 101, 055504. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, F.; Tang, S.; Ma, Y.; Liu, W.; Liang, C. Unraveling the Leverage Effect in Phases Stability and Mechanical Properties of Al-Zn-Mg-Cu Alloy. J. Alloys Compd. 2024, 982, 173763. [Google Scholar] [CrossRef]
- Wang, S.F.; Dougherty, J.P.; Huebner, W.; Pepin, J.G. Silver-Palladium Thick-Film Conductors. J. Am. Ceram. Soc. 1994, 77, 3051–3072. [Google Scholar] [CrossRef]
- Mandal, K. Time-Dependent Synthesis of Pd0.5Ag0.5 Nano-Catalysts and Their Catalytic Performance in the Decomposition of Formic Acid. J. Indian Chem. Soc. 2024, 101, 101137. [Google Scholar] [CrossRef]
- Liu, H.; Tang, S.; Ma, Y.; Liu, W.; Liang, C. Short-Range Ordering Governs Brittleness and Ductility in W-Ta Solid Solution: Insights from Pugh’s Shear-to-Bulk Modulus Ratio. Scr. Mater. 2021, 204, 114136. [Google Scholar] [CrossRef]
- Yan, Z.; Cui, L.; Shi, K.; Zhang, M.; Pang, Z.; Guo, J.; Gao, R.; Hao, H. Theoretical Study the Influence of Partial Substitute Noble Metal Pd/Ag of PdAg-Based Catalyst by Non-Noble Metal Ni/Cu for 1,3-Butadiene Hydrogenation. Appl. Surf. Sci. 2022, 588, 152897. [Google Scholar] [CrossRef]
- Marmier, A. ElAM: A Computer Program for the Analysis and Representation of Anisotropic Elastic Properties. Comput. Phys. Commun. 2010, 181, 2102–2115. [Google Scholar] [CrossRef]
- Sanders, P.G.; Eastman, J.A.; Weertman, J.R. Elastic and Tensile Behavior of Nanocrystalline Copper and Palladium. Acta Mater. 1997, 45, 4019–4025. [Google Scholar] [CrossRef]
- Trappeniers, N.J.; Biswas, S.N.; Ten Seldam, C.A. Effect of Pressure on the Shear Modulus of Polycrystalline Aluminium, Copper and Silver. Phys. BC 1976, 85, 20–32. [Google Scholar] [CrossRef]
- McCarthy, E.K.; Bellew, A.T.; Sader, J.E.; Boland, J.J. Poisson’s Ratio of Individual Metal Nanowires. Nat. Commun. 2014, 5, 4336. [Google Scholar] [CrossRef] [PubMed]
- Alimov, V.N.; Busnyuk, A.O.; Notkin, M.E.; Livshits, A.I. Pd–V–Pd Composite Membranes: Hydrogen Transport in a Wide Pressure Range and Mechanical Stability. J. Membr. Sci. 2014, 457, 103–112. [Google Scholar] [CrossRef]
- Bhargav, A.; Jackson, G.S.; Ciora, R.J., Jr.; Liu, P.T.K. Model Development and Validation of Hydrogen Transport through Supported Palladium Membranes. J. Membr. Sci. 2010, 356, 123–132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).