Solving the Solidification Cracking in Maraging Steel during Mass Production by Adjusting High-Temperature Delta Ferrite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Materials
2.2. Experimental Methods
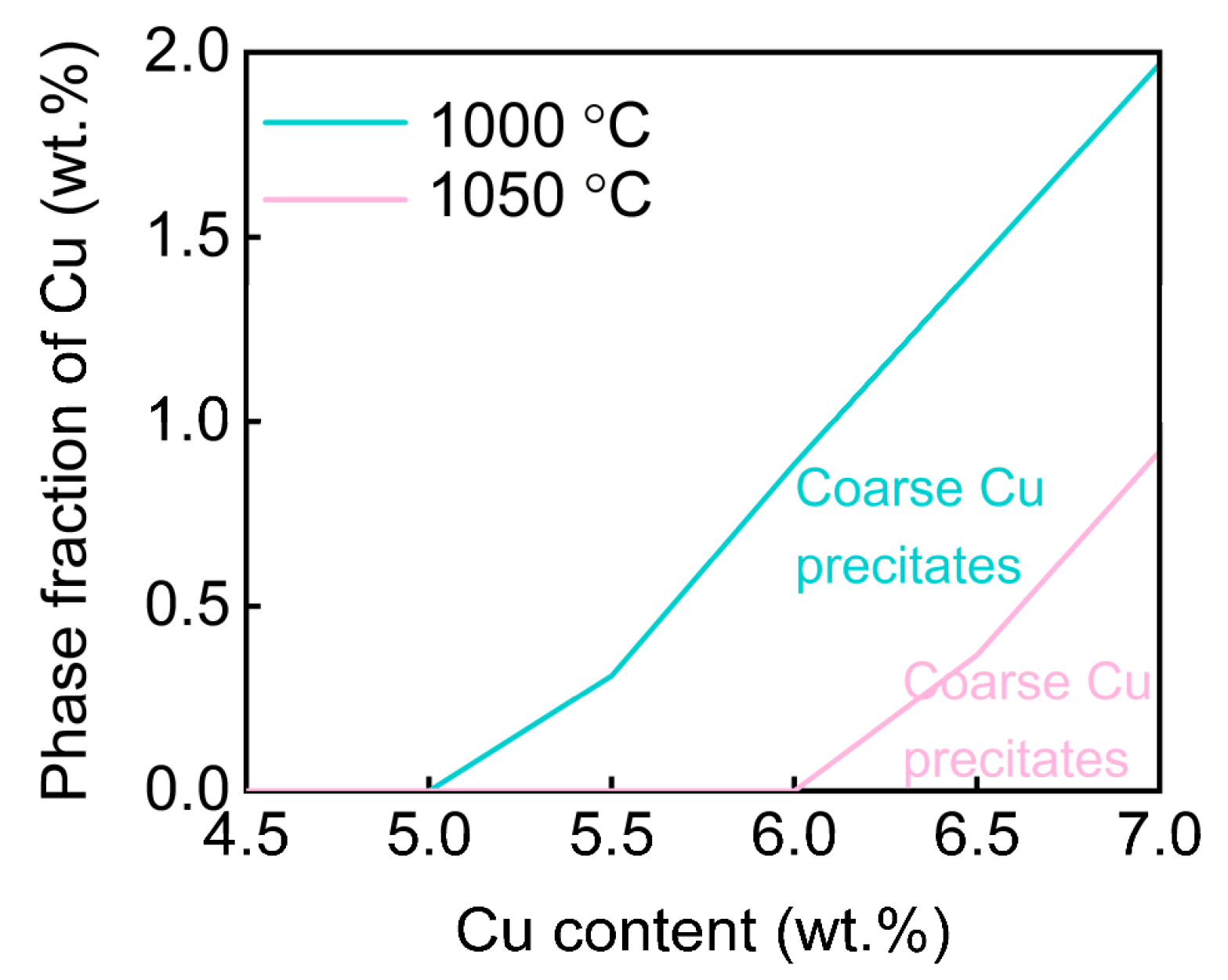
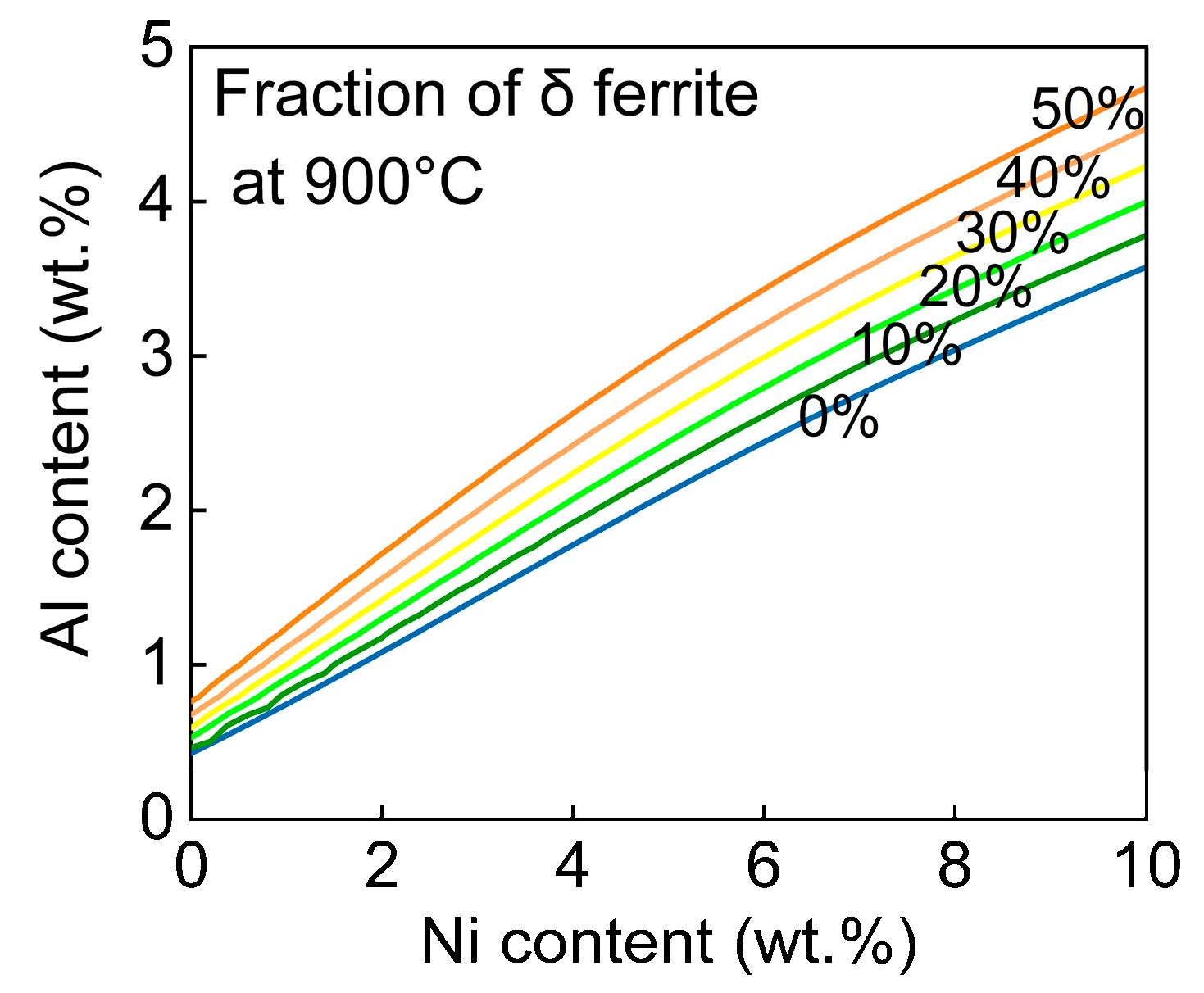
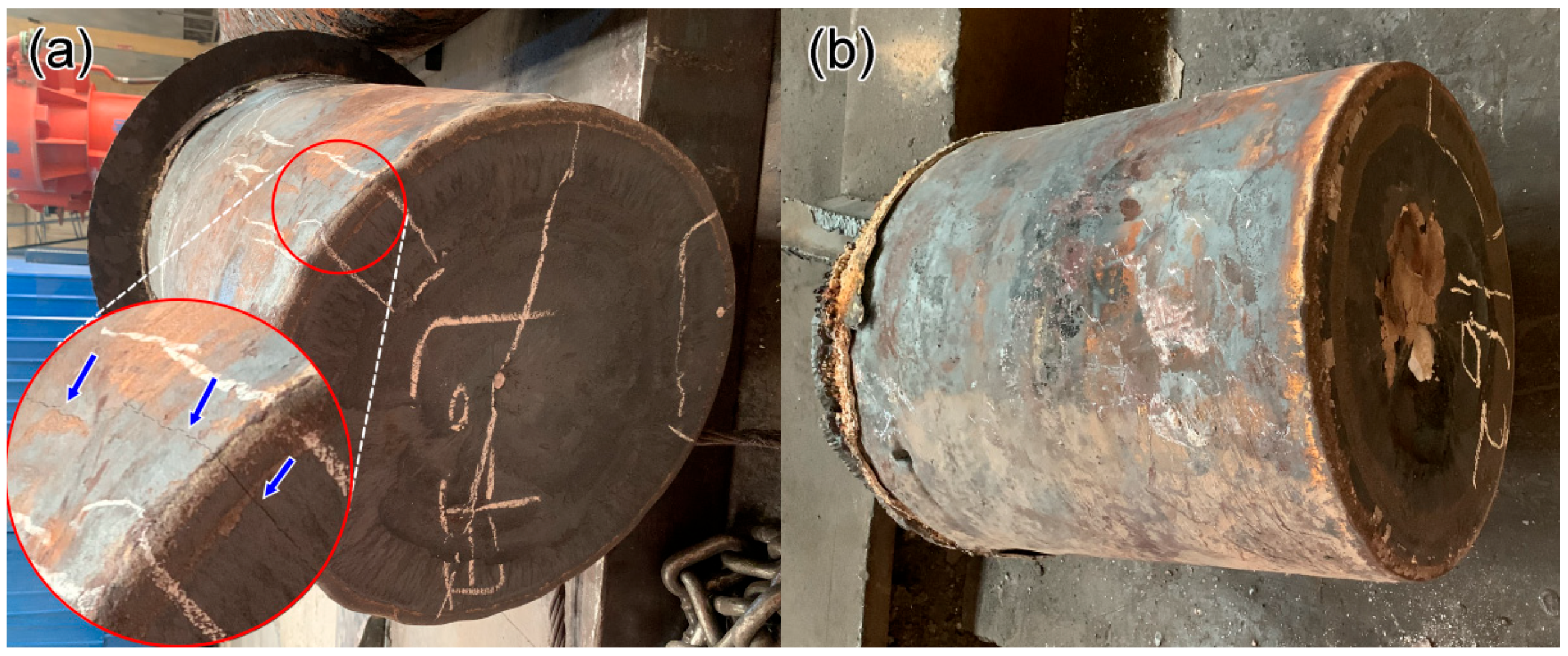
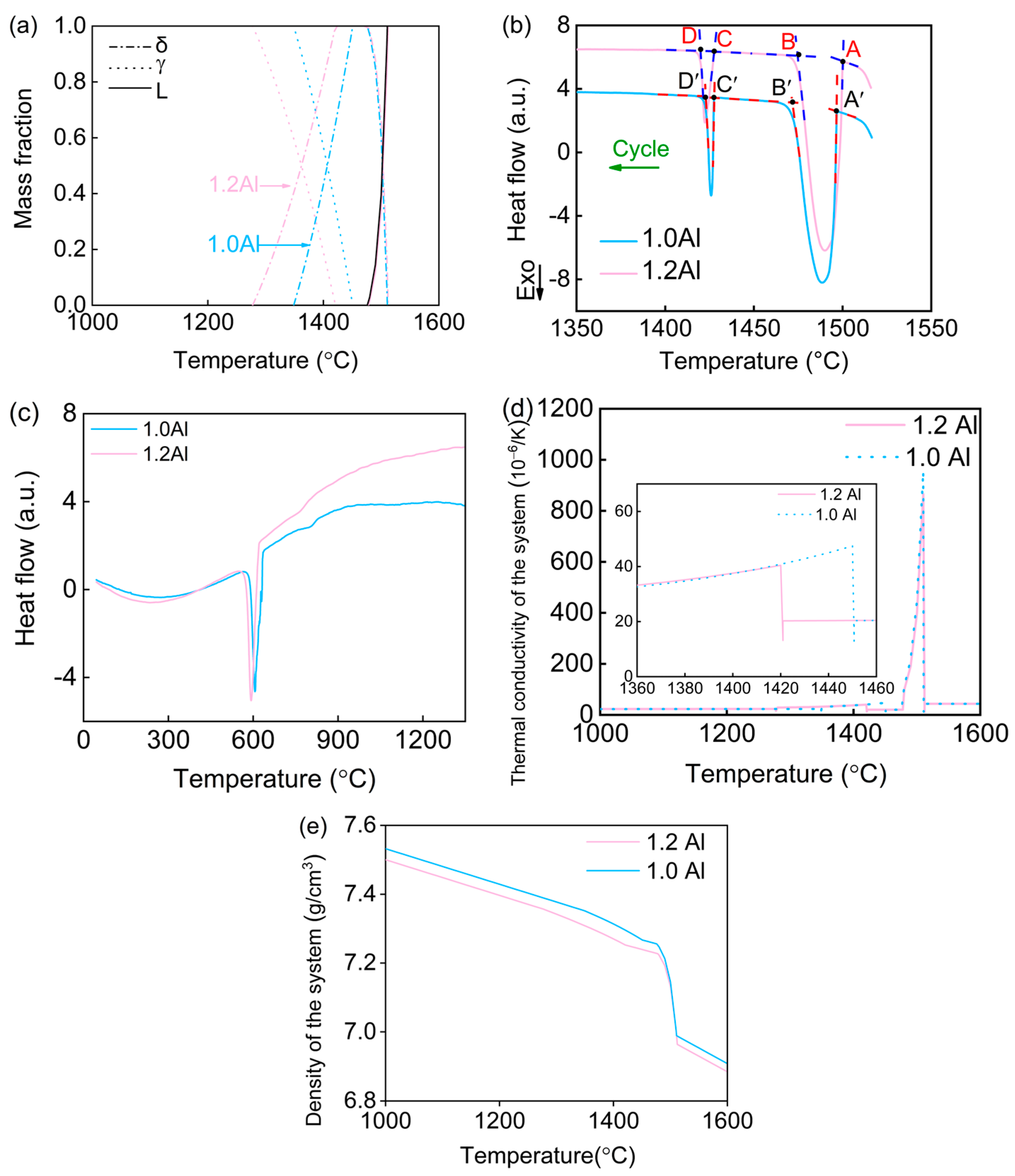
3. Results and Discussion
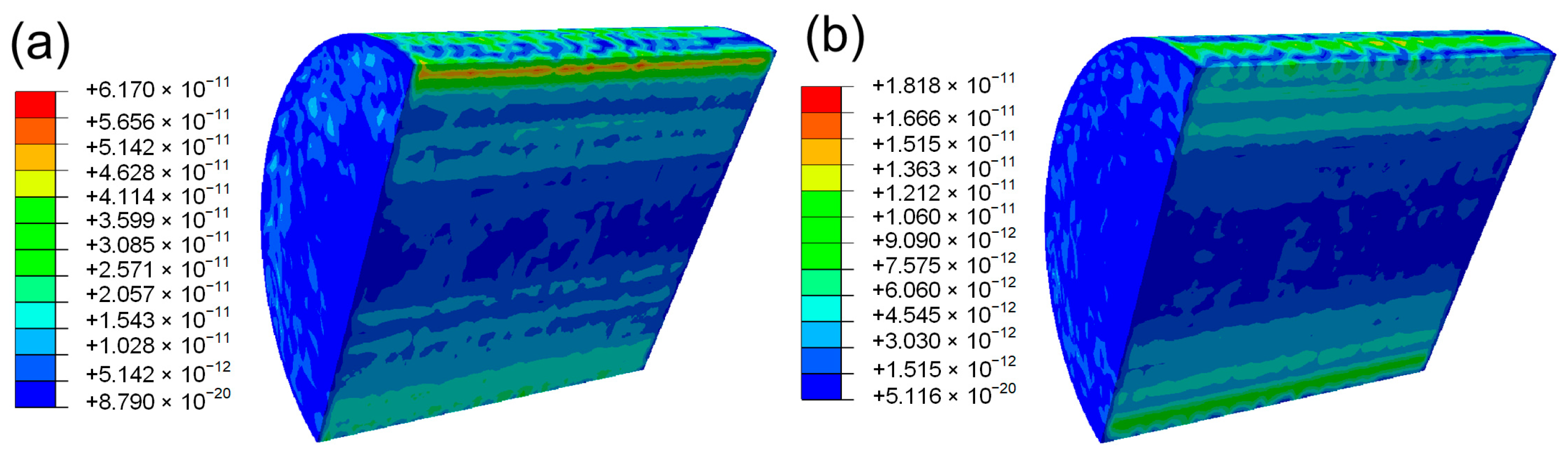
4. Conclusions
- The solidification cracks were observed in 1.0Al steel during the high-temperature solidification process, which subsequently oxidized during the cooling process. This defect was a significant challenge, compromising the structural integrity of the steel.
- By increasing the aluminum content to 1.2 wt.%, the issue of crack formation was effectively mitigated. This is due to the fact that aluminum can expand the δ ferrite region and lower the δ → γ transformation temperature. This microstructural adjustment played a crucial role in addressing the cracking issue.
- The increase in aluminum content led to a reduction in volume shrinkage during the cooling process at high temperatures. This reduction in shrinkage consequently lessened the thermal stress within the material, which was confirmed through finite element method (FEM) simulations. The lower thermal stress was directly linked to the prevention of solidification cracking.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nong, X.D.; Xiong, X.J.; Gu, X.; Wang, L.; Jiang, Y.L.; Yu, L.; Rao, J.H.; Bi, Y.J. A novel low-cost ultra-strong maraging steel by additive manufacturing. Mater. Sci. Eng. A 2023, 887, 145747. [Google Scholar] [CrossRef]
- Brytan, Z.; Król, M.; Benedyk, M.; Pakieła, W.; Tomasz, T.; Dagnaw, M.J.; Snopiński, P.; Pagáč, M.; Czech, A. Microstructural and Mechanical Properties of Novel Co-Free Maraging Steel M789 Prepared by Additive Manufacturing. Materials 2022, 15, 1734. [Google Scholar] [CrossRef]
- Yang, X.C.; Li, C.N.; Han, J.Y.; Yang, Y.K.; Ju, Y.Z.; Ba, L.Z.; Wang, C.; Di, X.J. Effect of welding state on the re-precipitation behavior of Cu-rich and NiAl nanoparticles in HAZ of 1100 MPa grade low carbon ultra-high strength steel. Mater. Sci. Eng. A 2024, 897, 146334. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, X.H.L.X.X.; Liu, S.C.; Zhang, Y.; Chen, L.; Xu, S.S.; Liang, X.; Li, X.Z.; Zhang, Z.W. Microstructure and mechanical properties of a Cu/NiAl nanoprecipitate strengthened dual-phase steel. Mater. Charact. 2023, 196, 112594. [Google Scholar] [CrossRef]
- Yang, X.C.; Di, X.J.; Wang, J.S.; Fang, C.; Fu, W.; Ba, L.Z.; Zhou, X.F.; Zhang, C.Y.; Li, C.N. The co-precipitation evolution of NiAl and Cu nanoparticles and its influence on strengthening and toughening mechanisms in low-carbon ultra-high strength martensite seamless tube steel. Int. J. Plast. 2023, 166, 103654. [Google Scholar] [CrossRef]
- Zhou, B.C.; Yang, T.; Zhou, G.; Wang, H.; Luan, J.H.; Jiao, Z.B. Mechanisms for suppressing discontinuous precipitation and improving mechanical properties of NiAl-strengthened steels through nanoscale Cu partitioning. Acta Mater. 2021, 205, 116561. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, D.Z.; Liu, W.Q.; Li, F.J.; Lu, Q. Effect of Cu content on the precipitation behavior of Cu-rich and NiAl phases in steel. Mater. Charact. 2022, 187, 111849. [Google Scholar] [CrossRef]
- Du, Y.B.; Hu, X.F.; Zhang, S.Q.; Song, Y.Y.; Jiang, H.C.; Rong, L.J. Precipitation behavior of Cu-NiAl nanoscale particles and their effect on mechanical properties in a high strength low alloy steel. Mater. Charact. 2022, 190, 112014. [Google Scholar] [CrossRef]
- Guo, L.L.; Zhang, L.N.; Andersson, J.; Ojo, O. Additive manufacturing of 18% nickel maraging steels: Defect, structure and mechanical properties: A review. Mater. Sci. Technol. 2022, 120, 227–252. [Google Scholar] [CrossRef]
- Kou, S. A criterion for cracking during solidification. Acta Mater. 2015, 88, 366–374. [Google Scholar] [CrossRef]
- Olson, G.B. Computational Design of Hierarchically Structured Materials. Science 1997, 277, 1237–1242. [Google Scholar] [CrossRef]
- Zhao, J.C. Combinatorial approaches as effective tools in the study of phase diagrams and composition–structure–property relationships. Prog. Mater. Sci. 2006, 51, 557–631. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Miller, M.K.; Liu, C.T. Precipitation mechanism and mechanical properties of an ultra-high strength steel hardened by nanoscale NiAl and Cu particles. Acta Mater. 2015, 97, 58–67. [Google Scholar] [CrossRef]
- Czyryca, E.J. Advances in high strength steel technology for naval hull construction. Key Eng. Mater. 1993, 84, 491–520. [Google Scholar] [CrossRef]
- Sahoo, G.; Singh, B.; Saxena, A. Effect of strain rate, soaking time and alloying elements on hot ductility and hot shortness of low alloy steels. Mater. Sci. Eng. A 2018, 718, 292–300. [Google Scholar] [CrossRef]
- Kondo, Y.; Tanei, H. Effect of Oxygen Concentration on Surface Hot Shortness of Steel Induced by Copper. ISIJ Int. 2015, 55, 1044–1047. [Google Scholar] [CrossRef]
- Hydrean, P.P.; Kitchin, A.L.; Schaller, F.W. Hot rolling and heat treatment of Ni−Cu−Cb(Nb) steel. Metall. Trans. 1971, 2, 2541–2548. [Google Scholar] [CrossRef]
- Mintz, B.; Abushosha, R.; Crowther, D.N. Influence of small additions of copper and niekel on hot ductility of steels. Mater. Sci. Technol. 2013, 11, 474–481. [Google Scholar] [CrossRef]
- Schäfer, L. Influence of delta ferrite and dendritic carbides on the impact and tensile properties of a martensitic chromium steel. J. Nucl. Mater. 1998, 258–263, 1336–1339. [Google Scholar] [CrossRef]
- Akram, J.; Kalvala, P.R.; Misra, M.; Charit, I. Creep behavior of dissimilar metal weld joints between P91 and AISI 304. Mater. Sci. Eng. A 2017, 688, 396–406. [Google Scholar] [CrossRef]
- Chen, S.P.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Yang, X.C.; Di, X.J.; Duan, Q.Y.; Fu, W.; Ba, L.Z.; Li, C.N. Effect of precipitation evolution of NiAl and Cu nanoparticles on strengthening mechanism of low carbon ultra-high strength seamless tube steel. Mater. Sci. Eng. A 2023, 872, 144939. [Google Scholar] [CrossRef]
- Niu, M.C.; Yang, K.; Luan, J.H.; Wang, W.; Jiao, Z.B. Cu-assisted austenite reversion and enhanced TRIP effect in maraging stainless steels. J. Mater. Sci. Technol. 2022, 104, 52–58. [Google Scholar] [CrossRef]
- Sha, W.; Leitner, H.; Guo, Z.; Xu, W. Phase transformations in maraging steels. In Phase Transformations in Steels; Pereloma, E., Edmonds, D.V., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 332–362. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Chen, P.; Liu, M.; Wang, G.; Yi, H. Solving the Solidification Cracking in Maraging Steel during Mass Production by Adjusting High-Temperature Delta Ferrite. Metals 2024, 14, 1020. https://doi.org/10.3390/met14091020
Liu L, Chen P, Liu M, Wang G, Yi H. Solving the Solidification Cracking in Maraging Steel during Mass Production by Adjusting High-Temperature Delta Ferrite. Metals. 2024; 14(9):1020. https://doi.org/10.3390/met14091020
Chicago/Turabian StyleLiu, Lianqian, Peng Chen, Ming Liu, Guodong Wang, and Hongliang Yi. 2024. "Solving the Solidification Cracking in Maraging Steel during Mass Production by Adjusting High-Temperature Delta Ferrite" Metals 14, no. 9: 1020. https://doi.org/10.3390/met14091020







