Ultrafast Hydrogen Production via Hydrolysis of MgH2-NaH Composite
Abstract
1. Introduction
2. Experiments
2.1. Preparation of MgH2-NaH Composites
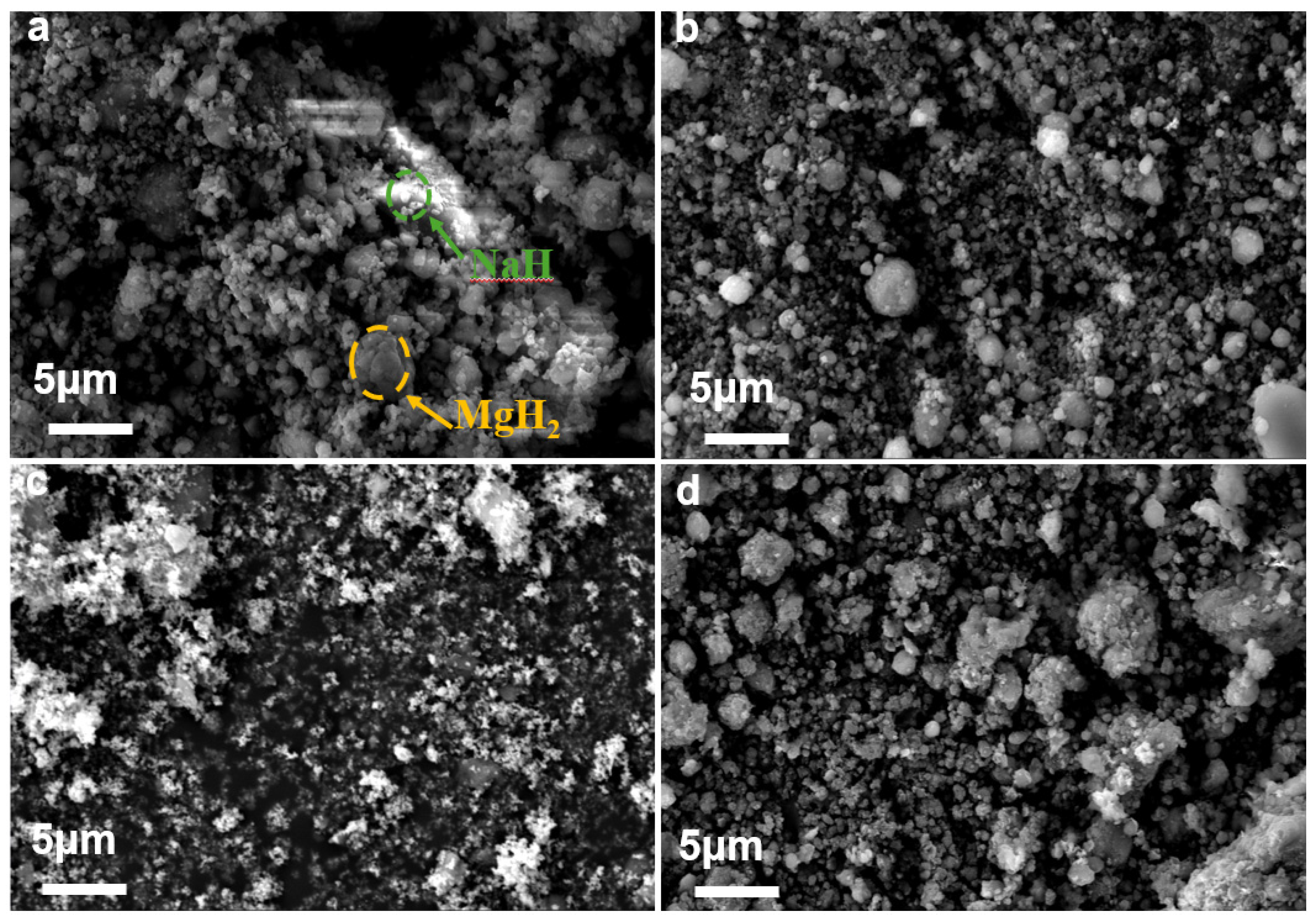
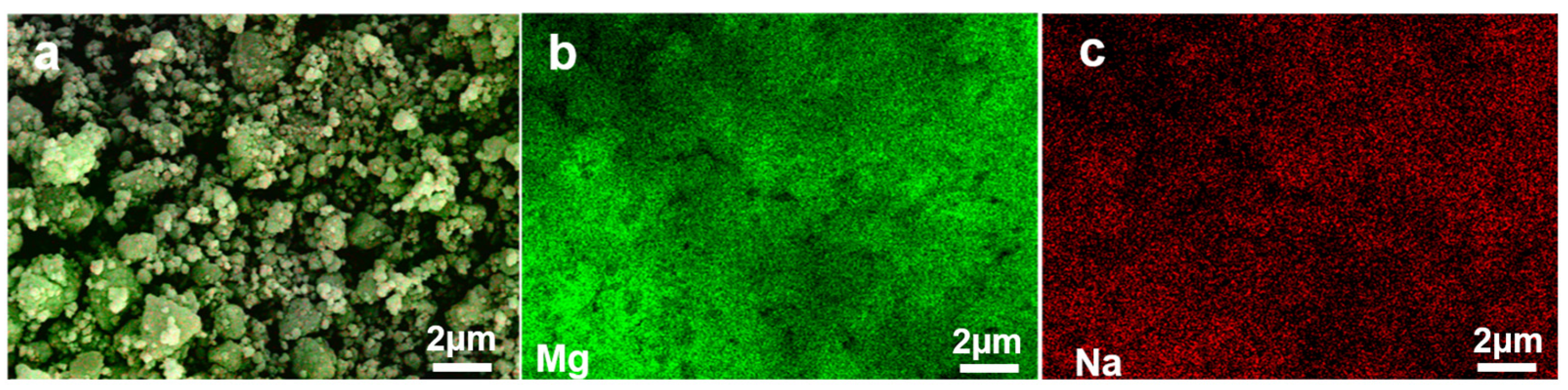
2.2. Structural Characterisation of Materials
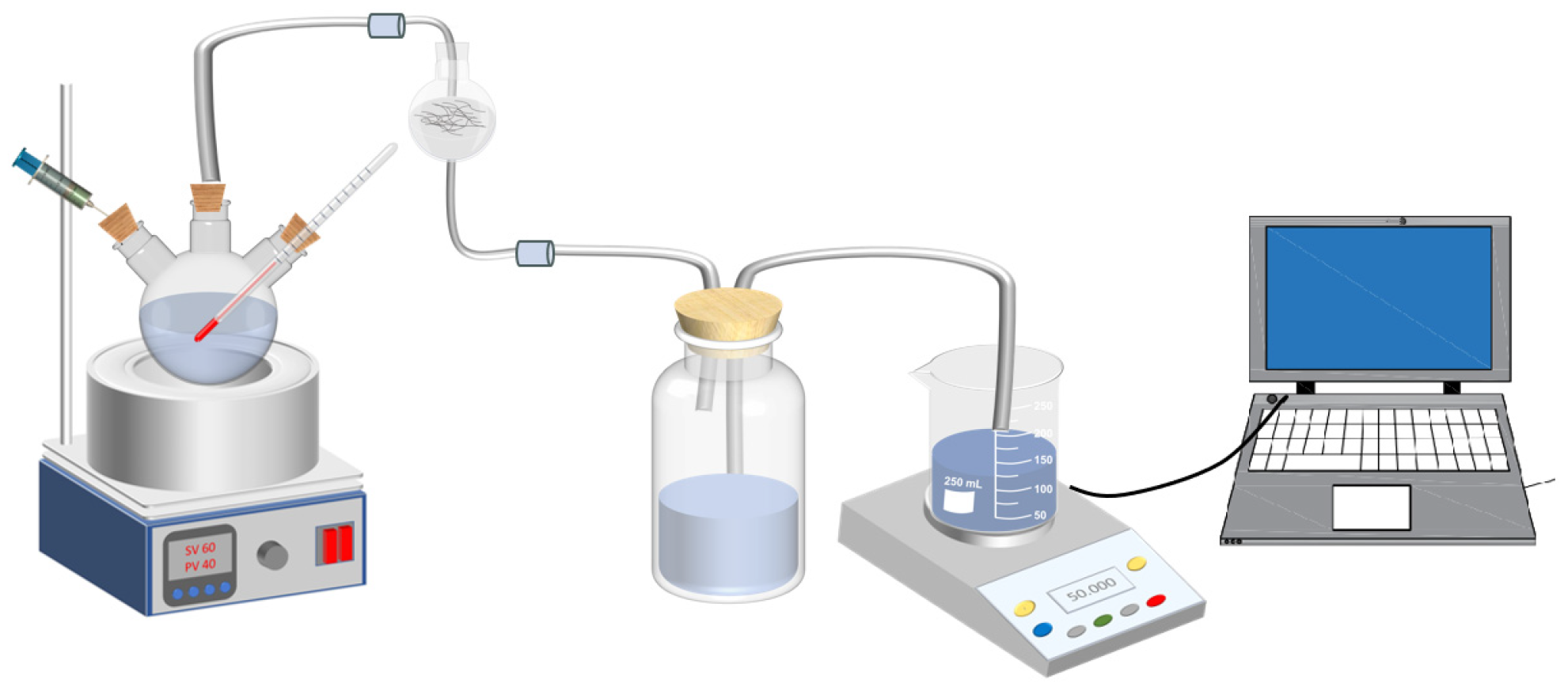
2.3. Hydrogen Hydrolysis Performance Test
3. Experimental Results and Discussion
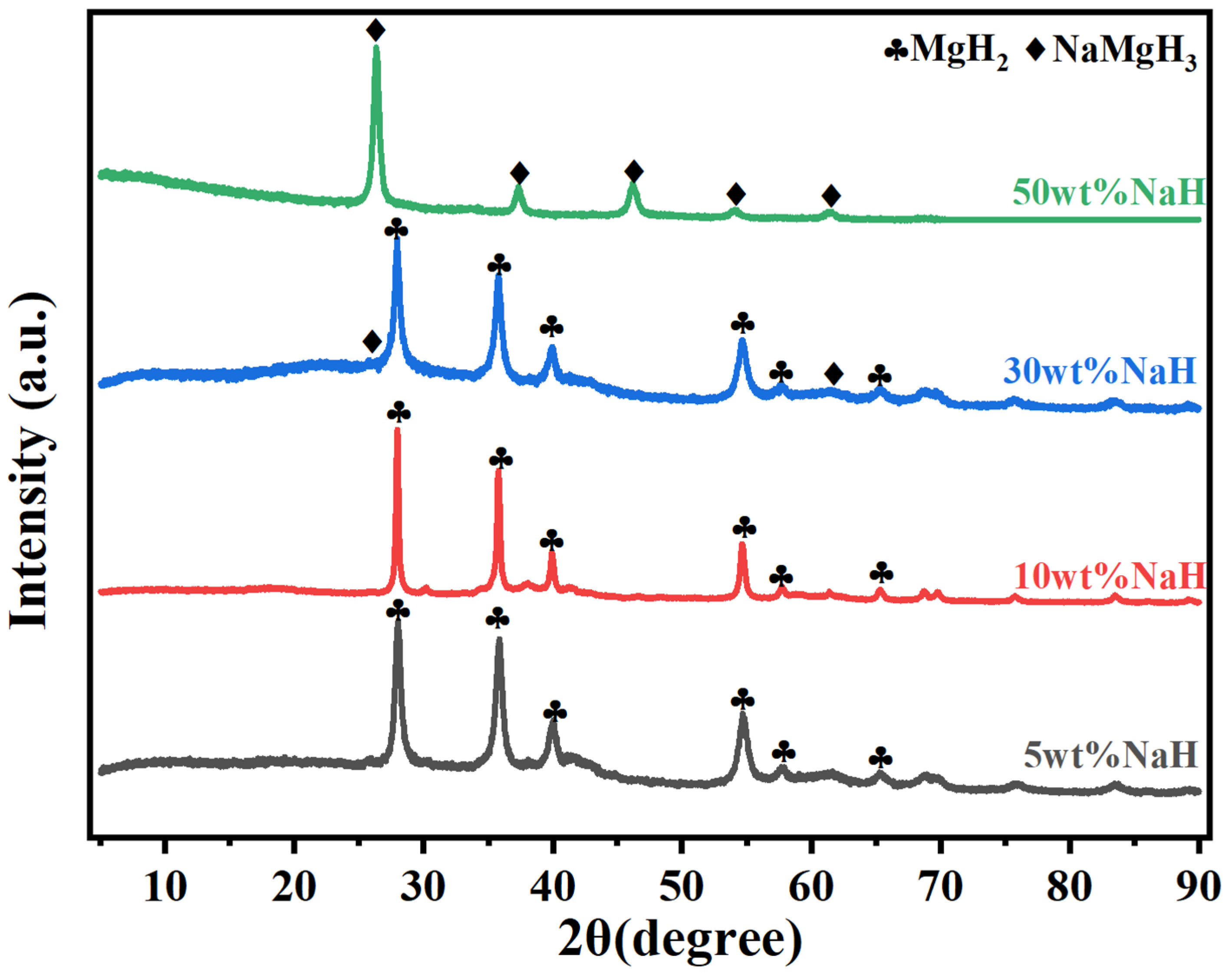
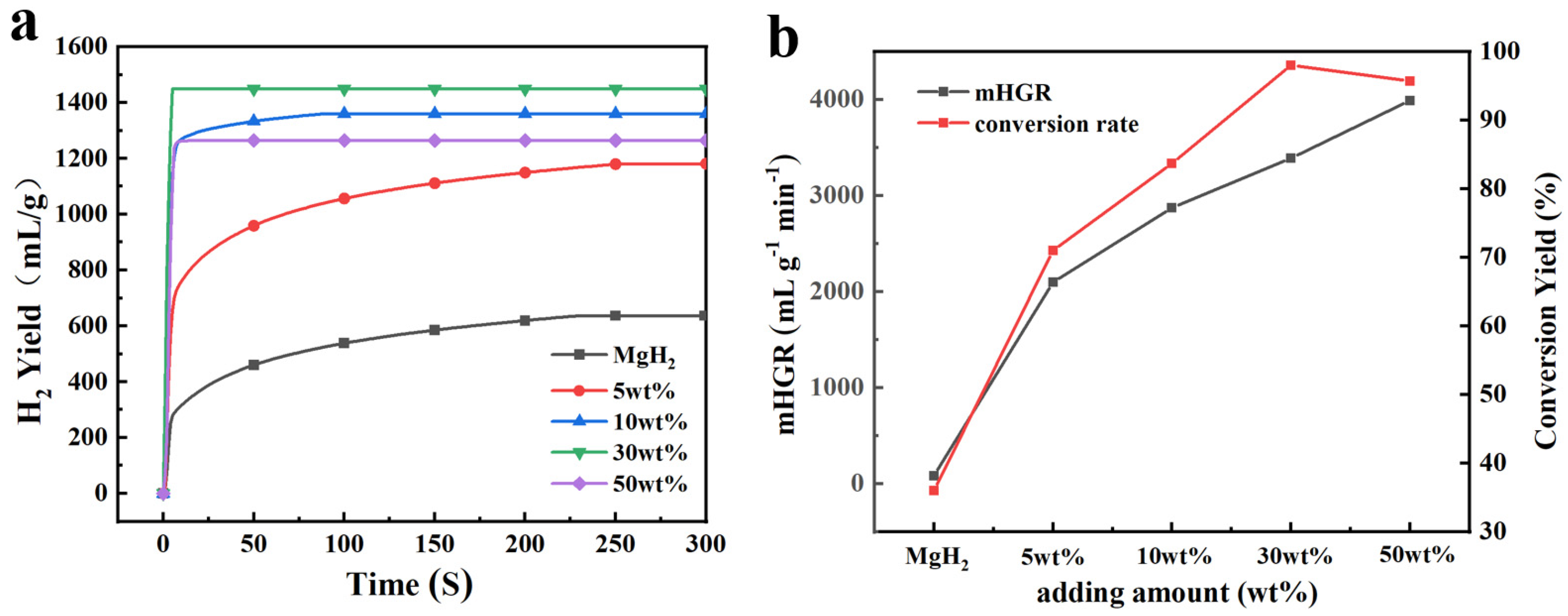
3.1. Effect of NaH Addition Content
3.2. Effect of Temperature on Hydrolysis Properties of MgH2-NaH Composites
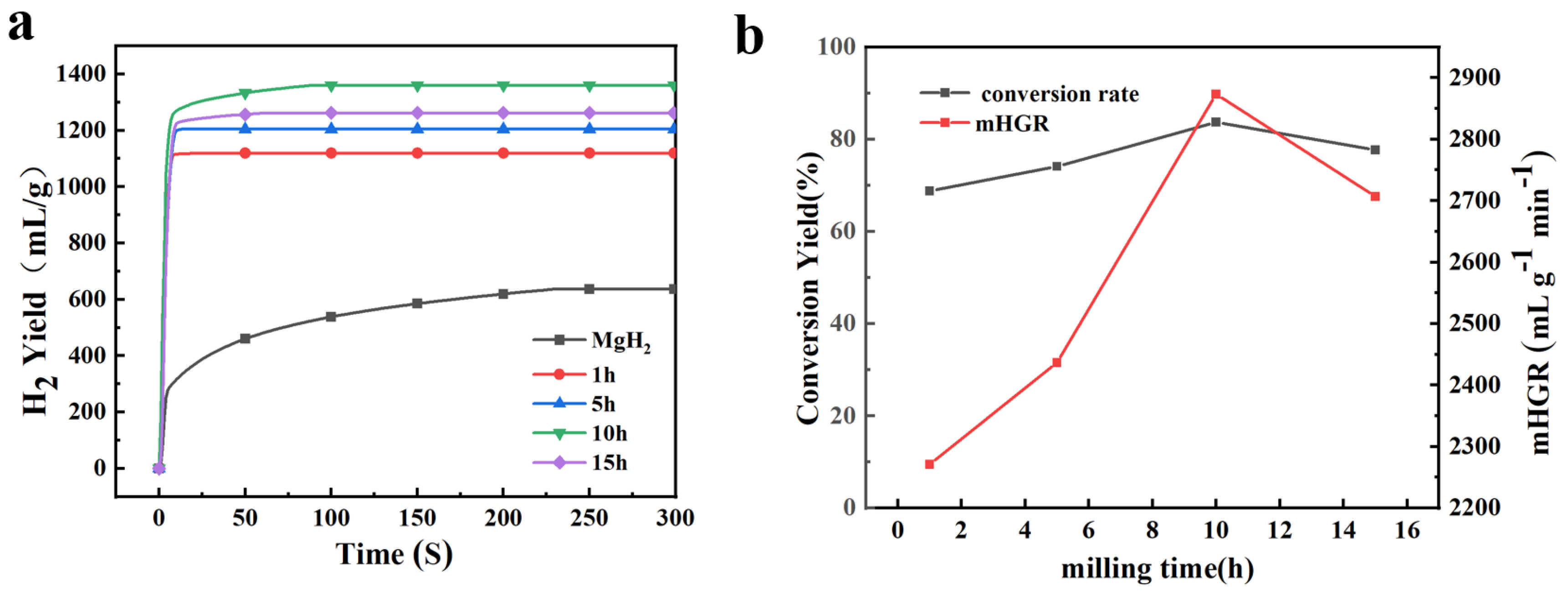
3.3. Effect of Ball Milling Time on Hydrogen Production Performance of MgH2-10 wt% NaH
4. Conclusions
- MgH2-NaH composites prepared by mechanical ball milling have excellent hydrolysis properties. The hydrolysis reaction kinetics of MgH2 can be significantly improved by adding a trace amount of NaH. The hydrogen production of MgH2-5 wt% NaH composites was 830 mL g−1 hydrogen in 20 s, which is 50% of the theoretical hydrogen production, and this can be regarded as almost instantaneous hydrogen production. With the increase in NaH addition content, this instantaneous hydrogen production effect was continuously amplified while ensuring high hydrogen production, which further enhanced the hydrolysis reaction rate and shortened the reaction time.
- The increase in temperature can improve the hydrolysis hydrogen production performance of composites. By analysing hydrolysis curves at different temperatures, the activation energy of the hydrolysis reaction of MgH2-10 wt% NaH was determined to be 17.79 kJ mol−1, indicating a significant reduction compared to pure MgH2. The highest hydrolysis hydrogen production and mHGR were achieved after 10 h of ball milling, with values of 1360 mL g−1 and 2873.5 mL g−1 min−1, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Zhang, B.; Yu, H.; Li, T.; Hu, M.; Yang, J. A novel oxidation-resistible Mg@Ni foam material for safe, efficient, and controllable hydrogen generation. J. Magnes. Alloys 2023, in press. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamaridarkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review☆. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Muir, S.S.; Yao, X. Progress in sodium borohydride as a hydrogen storage material: Development of hydrolysis catalysts and reaction systems. Int. J. Hydrogen Energy 2011, 36, 5983–5997. [Google Scholar] [CrossRef]
- Xingguo, L.I.; Jie, Z.; Xiaojuan, W.U.; Shunpeng, C.; Jifeng, D. Recent Progress on Materials for Hydrogen Generation via Hydrolysis. J. Inorg. Mater. 2021, 36, 1–8. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelghafar, A.A.; Baroutaji, A.; Sayed, E.T.; Alami, A.H.; Rezk, H.; Abdelkareem, M.A. Large-vscale hydrogen production and storage technologies: Current status and future directions. Int. J. Hydrogen Energy 2021, 46, 23498–23528. [Google Scholar] [CrossRef]
- Li, Y.; Lee, L.Q.; Zhao, H.; Zhao, Y.; Gao, P.; Li, H. Alcohol–alkali hydrolysis for high-throughput PET waste electroreforming-assisted green hydrogen generation. J. Mater. Chem. A 2024, 12, 2121–2128. [Google Scholar] [CrossRef]
- Urbonavicius, M.; Varnagiris, S.; Knoks, A.; Mezulis, A.; Kleperis, J.; Richter, C.; Meirbekova, R.; Gunnarsson, G.; Milcius, D. Enhanced Hydrogen Generation through Low-Temperature Plasma Treatment of Waste Aluminum for Hydrolysis Reaction. Materials 2024, 17, 2637. [Google Scholar] [CrossRef] [PubMed]
- Hammad, A.; Ning, F.; Zou, S.; Liu, Y.; Tian, B.; He, C.; Chai, Z.; Wen, Q.; He, L.; Zhou, X. Aluminum hydrolysis for hydrogen generation enhanced by sodium hydride. Int. J. Hydrogen Energy 2024, 77, 138–148. [Google Scholar] [CrossRef]
- Ma, M.; Ouyang, L.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Air-stable hydrogen generation materials and enhanced hydrolysis performance of MgH2-LiNH2 composites. J. Power Sources 2017, 359, 427–434. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Fu, W.; Wei, L.; Zhao, X.; Zhou, X.; Ni, M.; Wang, H. Highly active and durable catalyst for hydrogen generation by the NaBH4 hydrolysis reaction: CoWB/NF nanodendrite with an acicular array structure. J. Alloys Compd. 2020, 836, 155429. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Tang, X.; Liu, Z.; Zhang, J.; Ye, C.; Ye, Y.; Zhang, R. Hydrogen generation by ammonia decomposition over Co/CeO2 catalyst: Influence of support morphologies. Appl. Surf. Sci. 2020, 532, 147335. [Google Scholar] [CrossRef]
- Lei, W.; Jin, H.; Gao, J.; Chen, Y. Efficient hydrogen generation from the NaBH4 hydrolysis by amorphous Co–Mo–B alloy supported on reduced graphene oxide. J. Mater. Res. 2021, 36, 4154–4168. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Guo, Q.; Li, Y.; Ma, X.; Li, B.; Zhu, H.; Yuan, C.-G. Preparation of Ag@PNCMs nanocomposite as an effective catalyst for hydrogen generation from hydrolysis of sodium borohydride. Mater. Lett. 2021, 297, 129828. [Google Scholar] [CrossRef]
- Deng, J.; Sun, B.; Xu, J.; Shi, Y.; Xie, L.; Zheng, J.; Li, X. A monolithic sponge catalyst for hydrogen generation from sodium borohydride solution for portable fuel cells. Inorg. Chem. Front. 2021, 8, 35–40. [Google Scholar] [CrossRef]
- Balbay, A.; Saka, C. Semi-methanolysis reaction of potassium borohydride with phosphoric acid for effective hydrogen production. Int. J. Hydrogen Energy 2018, 43, 21299–21306. [Google Scholar] [CrossRef]
- Qiu, H.; Han, X.; Zang, S.; Liu, W.; Yang, G.; Lv, L.; Wang, X.; Duan, J.; Wang, S. Effect of LiH on the fast hydrolysis and hydrogen generation of MgH2 by ball milling. New J. Chem. 2022, 46, 19900–19908. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Liu, J.W.; Ouyang, L.Z.; Zhu, M. Catalysis and hydrolysis properties of perovskite hydride NaMgH3. J. Alloys Compd. 2013, 580, S197–S201. [Google Scholar] [CrossRef]
- Kushch, S.D.; Kuyunko, N.S.; Nazarov, R.S.; Tarasov, B.P. Hydrogen-generating compositions based on magnesium. Int. J. Hydrogen Energy 2011, 36, 1321–1325. [Google Scholar] [CrossRef]
- Tayeh, T.; Awad, A.S.; Nakhl, M.; Zakhour, M.; Silvain, J.F.; Bobet, J.L. Production of hydrogen from magnesium hydrides hydrolysis. Int. J. Hydrogen Energy 2014, 39, 3109–3117. [Google Scholar] [CrossRef]
- Verbovytskyy, Y.V.; Berezovets, V.V.; Kytsya, A.R.; Zavaliy, I.Y.; Yartys, V.A. Hydrogen Generation by the Hydrolysis of MgH2. Mater. Sci. 2020, 56, 1–14. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen generation by hydrolysis reaction of lithium borohydride. Int. J. Hydrogen Energy 2004, 29, 1213–1217. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, W.; Yang, Z.; Huang, Z.; Yu, X. The effect of various cations/anions for MgH2 hydrolysis reaction. J. Mater. Sci. Technol. 2021, 73, 186–192. [Google Scholar] [CrossRef]
- Chen, J.; Fu, H.; Xiong, Y.; Xu, J.; Zheng, J.; Li, X. MgCl2 promoted hydrolysis of MgH2 nanoparticles for highly efficient H2 generation. Nano Energy 2014, 10, 337–343. [Google Scholar] [CrossRef]
- Gao, H.; Shi, R.; Zhu, J.; Liu, Y.; Shao, Y.; Zhu, Y.; Zhang, J.; Li, L.; Hu, X. Interface effect in sandwich like Ni/Ti3C2 catalysts on hydrogen storage performance of MgH2. Appl. Surf. Sci. 2021, 564, 150302. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Wang, H.; Liu, J.; Zhu, M. Hydrogen generation by hydrolysis of MgH2 and enhanced kinetics performance of ammonium chloride introducing. Int. J. Hydrogen Energy 2015, 40, 6145–6150. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Zhu, Y.; Liu, Y.; Li, L. Controllable hydrogen generation behavior by hydrolysis of MgH2-based materials. J. Power Sources 2021, 494, 229726. [Google Scholar] [CrossRef]
- Liu, P.; Wu, H.; Wu, C.; Chen, Y.; Xu, Y.; Wang, X.; Zhang, Y. Microstructure characteristics and hydrolysis mechanism of Mg–Ca alloy hydrides for hydrogen generation. Int. J. Hydrogen Energy 2015, 40, 3806–3812. [Google Scholar] [CrossRef]
- Al Bacha, S.; Thienpont, A.; Zakhour, M.; Nakhl, M.; Bobet, J.L. Clean hydrogen production by the hydrolysis of magnesium-based material: Effect of the hydrolysis solution. J. Clean. Prod. 2021, 282, 124498. [Google Scholar] [CrossRef]
- Su, M.; Hu, H.; Gan, J.; Ye, W.; Zhang, W.; Wang, H. Thermodynamics, kinetics and reaction mechanism of hydrogen production from a novel Al alloy/NaCl/g-C3N4 composite by low temperature hydrolysis. Energy 2021, 218, 119489. [Google Scholar] [CrossRef]
- Pighin, S.A.; Urretavizcaya, G.; Bobet, J.L.; Castro, F.J. Nanostructured Mg for hydrogen production by hydrolysis obtained by MgH2 milling and dehydriding. J. Alloys Compd. 2020, 827, 154000. [Google Scholar] [CrossRef]
- Oh, S.; Cho, T.; Kim, M.; Lim, J.; Eom, K.; Kim, D.; Cho, E.; Kwon, H. Fabrication of Mg–Ni–Sn alloys for fast hydrogen generation in seawater. Int. J. Hydrogen Energy 2017, 42, 7761–7769. [Google Scholar] [CrossRef]
- Hiraki, T.; Hiroi, S.; Akashi, T.; Okinaka, N.; Akiyama, T. Chemical equilibrium analysis for hydrolysis of magnesium hydride to generate hydrogen. Int. J. Hydrogen Energy 2012, 37, 12114–12119. [Google Scholar] [CrossRef]
- Grosjean, M.; Zidoune, M.; Roue, L.; Huot, J. Hydrogen production via hydrolysis reaction from ball-milled Mg-based materials. Int. J. Hydrogen Energy 2006, 31, 109–119. [Google Scholar] [CrossRef]
- Awad, A.S.; El-Asmar, E.; Tayeh, T.; Mauvy, F.; Nakhl, M.; Zakhour, M.; Bobet, J.L. Effect of carbons (G and CFs), TM (Ni, Fe and Al) and oxides (Nb2O5 and V2O5) on hydrogen generation from ball milled Mg-based hydrolysis reaction for fuel cell. Energy 2016, 95, 175–186. [Google Scholar] [CrossRef]



| Materials | Hydrolysis Solution | Ea (kJ mol−1) | Ref. |
|---|---|---|---|
| MgH2−10 wt% NaH | deionised water | 17.79 | This work |
| MgH2−10 wt% CoCl2 | deionised water | 31.3 | [27] |
| MgH2 (HCS) | 0.1 M AlCl3 solution | 34.68 | [21] |
| MgH2 (HCS) | 0.5 M AlCl3 solution | 21.64 | [21] |
| 16MgH2−LiNH2 | pure water | 37.40 | [10] |
| MgH2 | 0.5 M Fe2 (SO4)3 solution | 19.15 | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, Z.; Zhao, W.; Zhang, Y.; Peng, C.; Liu, C.; Guo, L. Ultrafast Hydrogen Production via Hydrolysis of MgH2-NaH Composite. Metals 2024, 14, 1038. https://doi.org/10.3390/met14091038
Zhang Z, Li Z, Zhao W, Zhang Y, Peng C, Liu C, Guo L. Ultrafast Hydrogen Production via Hydrolysis of MgH2-NaH Composite. Metals. 2024; 14(9):1038. https://doi.org/10.3390/met14091038
Chicago/Turabian StyleZhang, Zhao, Zhenji Li, Wei Zhao, Yushan Zhang, Chong Peng, Changcheng Liu, and Li Guo. 2024. "Ultrafast Hydrogen Production via Hydrolysis of MgH2-NaH Composite" Metals 14, no. 9: 1038. https://doi.org/10.3390/met14091038
APA StyleZhang, Z., Li, Z., Zhao, W., Zhang, Y., Peng, C., Liu, C., & Guo, L. (2024). Ultrafast Hydrogen Production via Hydrolysis of MgH2-NaH Composite. Metals, 14(9), 1038. https://doi.org/10.3390/met14091038







