Effective Corrosion Inhibition of Galvanic Corrosion of Cu Coupled to Au by Sodium Dodecyl Sulfate (SDS) and Polyethylene Glycol (PEG) in Acid Solution
Abstract
:1. Introduction
2. Experimental Specimen Preparation
Electrochemical Experiments
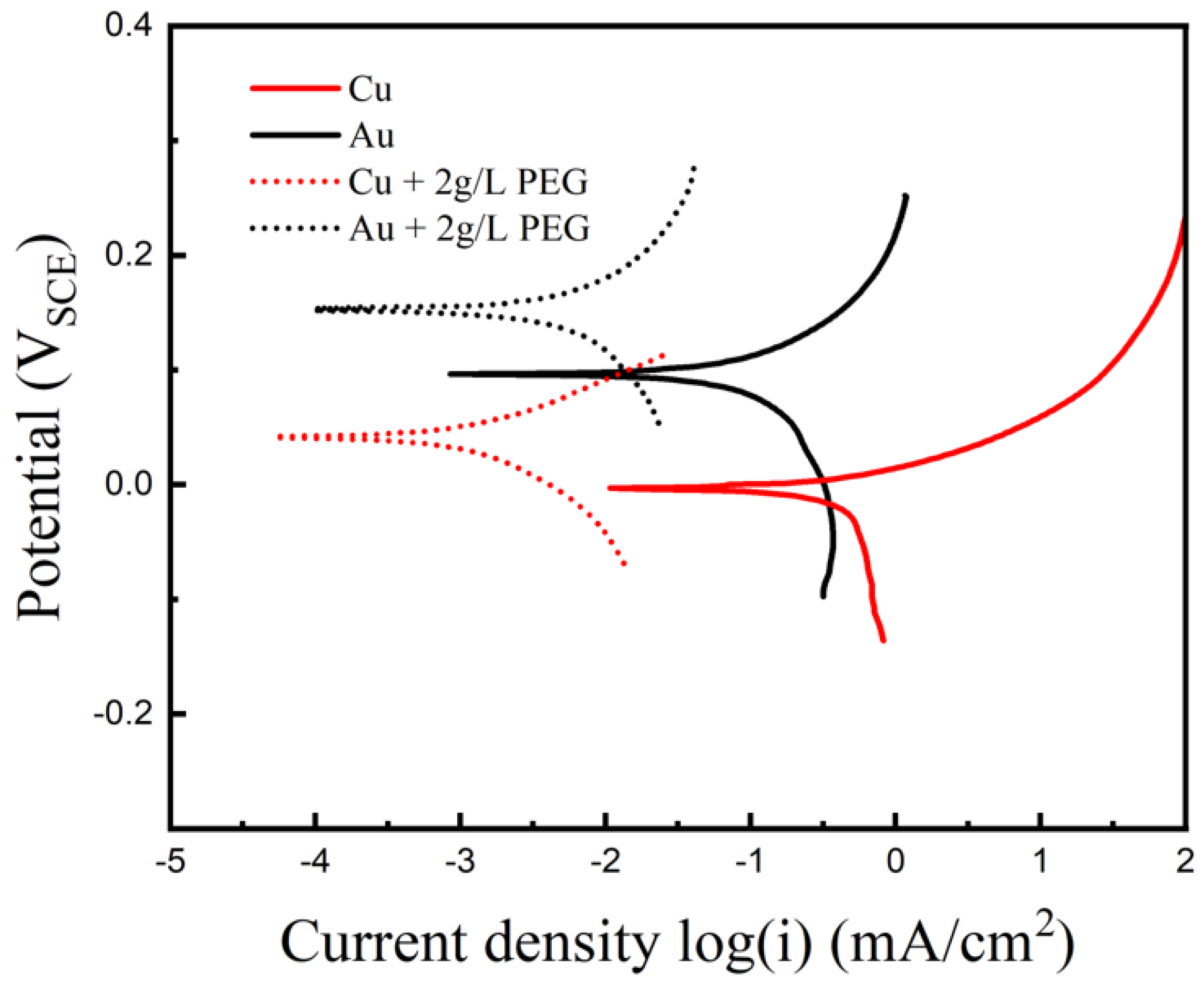
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dorang, S.; Schuldt, M.; Khanal, M. Effect of Stiffening the Printed Circuit Board in the Fatigue Life of the Solder Joint. Materials 2022, 15, 6208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, Z.; Xiao, K.; Gao, X.; Yi, P.; Dong, C.; Wu, J.; Wei, D. Influence of atmospheric particulates on initial corrosion behavior of printed circuit board in pollution environments. Appl. Surf. Sci. 2019, 467, 889. [Google Scholar] [CrossRef]
- Wang, J.; Lim, H.K.; Lew, H.S.; Saw, W.T.; Tan, C.H. A testing method for assessing solder joint reliability of FCBGA packages. Microelectron. Reliab. 2004, 44, 833. [Google Scholar] [CrossRef]
- Julian, Y.; Hon, C.; Cotterell, B.; Chai, T.C. The mechanics of the solder ball shear test and the effect of shear rate. Mater. Sci. Eng. A 2006, 417, 259. [Google Scholar]
- Huh, M.; Yu, Y.; Kahn, H.; Payer, J.H.; Heuer, A.H. Galvanic Corrosion during Processing of Polysilicon Microelectromechanical Systems. J. Electrochem. Soc. 2006, 153, 644. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.; Jung, K.; Kim, J.; Shon, M.; Kwon, H. Effects of Temperature and Operation Parameters on the Galvanic Corrosion of Cu Coupled to Au in Organic Solderability Preservatives Process. Met. Mater. Int. 2017, 23, 290. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.; Jung, K.; Park, M.; Shon, M.; Kwon, H. Galvanic Corrosion Behaviors of Cu Coupled to Au on a Printed Circuit Board in Ammonia Solution. Met. Mater. Int. 2018, 24, 67. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.; Shon, M.; Kwon, H. Galvanic Corrosion of Cu Coupled to Au on a Print Circuit Board: Effects of Pretreatment Solution and Etchant Concentration in Organic Solderability Preservatives Soft Etching Solution. Met. Mater. Int. 2016, 22, 781. [Google Scholar] [CrossRef]
- Idrac, J.; Mankowski, G.; Thompson, G.; Skeldon, P.; Kihn, Y.; Blan, C. Galvanic corrosion of aluminium–copper model alloys. Electrochim. Acta 2007, 52, 7626. [Google Scholar] [CrossRef]
- Murira, M.C.; Punckt, C.; Schniepp, H.C.; Khusid, B.; Aksay, I.A. Inhibition and Promotion of Copper Corrosion by CTAB in a Microreactor System. Langmuir 2008, 24, 14269. [Google Scholar] [CrossRef]
- Arkhipushkin, I.A.; Shikhaliev, K.S.; Potapov, A.Y.; Sapronova, L.V.; Kazansky, L.P. Inhibition of Brass (80/20) by 5-Mercaptopentyl-3-Amino-1,2,4-Triazole in Neutral Solutions. Metals 2017, 488, 3390. [Google Scholar] [CrossRef]
- Koundal, M.; Singh, A.K.; Sharma, C. Study on the effect of imidazolium ionic liquid as a modulator of corrosion inhibition of anionic surfactant sodium dodecyl sulfate (SDS) on mild steel in sodium chloride solution. J. Mol. Liq. 2022, 350, 118561. [Google Scholar] [CrossRef]
- Umorena, S.A.; Solomon, M.M. Synergistic corrosion inhibition effect of metal cations and mixtures of organic compounds: A Review. J. Environ. Chem. Eng. 2017, 5, 246. [Google Scholar] [CrossRef]
- Farahmanda, R.; Sohrabi, B.; Ghaffarinejad, A.; Meymiand, M.R.Z. Synergistic effect of molybdenum coating and SDS surfactant on corrosion inhibition of mild steel in presence of 3.5% NaCl. Corros. Sci. 2018, 136, 393. [Google Scholar] [CrossRef]
- Hussein, S.A.; Khadom, A.A. Okra Leaves Extract as Green Corrosion Inhibitor for Steel in Sulfuric Acid: Gravimetric, Electrochemical, and Surface Morphological Investigations. Results Chem. 2024, 8, 101566. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Radeva, T.; Shipochka, M.; Boshkov, N. Chitosan–Alginate Nanocontainers with Caffeine as Green Corrosion Inhibitors for Protection of Galvanized Steel. Crystals 2024, 14, 660. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Yan, J.; Zhang, J.; Zhang, Q.; Yan, Y. Recent Progress of Polymeric Corrosion Inhibitors: Structure and Application. Materials 2023, 16, 2954. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, X.; Wang, P.; Jiang, J.; Zhang, H.; Slaný, M.; Bian, J. Shielding against Erosion: Exploring the Effectiveness of Pre-Erosion Surface Corrosion Inhibitors. J. Colloid Interface Sci. 2024, 675, 1130. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xu, Z.; Benabou, L.; Yin, Z.; Guan, H.; Ya, H.; Chao, L.; Hu, Z.; Wa, X. Sodium dodecyl sulfate (SDS) as an effective corrosion inhibitor for Mg-8Li-3Al alloy in aqueous NaCl: A combined experimental and theoretical investigation. J. Magnes. Alloy. 2023, 11, 287. [Google Scholar] [CrossRef]
- Yousefi, A.; Javadian, S.; Dalir, N.; Kakemam, J.; Akbari, J. Imidazolium-based ionic liquids as modulators of corrosion inhibition of SDS on mild steel in hydrochloric acid solutions: Experimental and theoretical studies. RSC Adv. 2015, 5, 11697. [Google Scholar] [CrossRef]
- Tan, J.; Guo, L.; Yang, H.; Zhang, F.; Bakri, Y.E. Synergistic effect of potassium iodide and sodium dodecyl sulfonate on the corrosion inhibition of carbon steel in HCl medium: A combined experimental and theoretical investigation. RSC Adv. 2020, 10, 15163. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Li, Y.; Ju, P.; Chen, Y.; Yang, J.; Qiane, K.; Zhanga, T.; Wang, F. Unveiling the inhibition mechanism of an effective inhibitor for AZ91 Mg alloy. Corros. Sci. 2019, 148, 264. [Google Scholar] [CrossRef]
- Fouda, A.S.; El-Dossoki, F.I.; Shady, I.A. Adsorption and corrosion inhibition behavior of polyethylene glycol on α-brass alloy in nitric acid solution. Green Chem. Lett. Rev. 2018, 11, 67. [Google Scholar] [CrossRef]
- Boudellioua, H.; Hamlaoui, Y.; Tifoutia, L.; Pedrazac, F. Effects of polyethylene glycol (PEG) on the corrosion inhibition of mild steel by cerium nitrate in chloride solution. Appl. Surf. Sci. 2019, 473, 449. [Google Scholar] [CrossRef]
- AlGhamdi, J.M.; Haladu, S.A.; Mu’azu, N.D.; Alqahtani, H.A.; Zubair, M.; Manzar, M.S.; Alkhowildi, F.A.; Kuban, R.Z.M.; AlSubaie, N.F. Polyethyleneglycol bisphenol A epichlorohydrin copolymer (PEG-BEC) as a highly efficient inhibitor for mild steel corrosion in 1M HCl solutions. S. Afr. J. Chem. Eng. 2024, 49, 326. [Google Scholar] [CrossRef]
- Vaidya, N.R.; Aklujkar, P.; Rao, A.R. Modification of natural gums for application as corrosion inhibitor: A review. J. Coat. Technol. Res. 2022, 19, 223. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, J.M.; Wang, Q.L.; Shao, H.B.; Zhang, J.Q. The effects of polyethylene glycol(PEG) as an electrolyte additive on the corrosion behavior and electrochemical performances of pure aluminum in an alkaline zincate solution. Mater. Corros. 2011, 62, 1149. [Google Scholar] [CrossRef]
- Umoren, S.A.; Abdullahi, M.T.; Solomon, M.M. An overview on the use of corrosion inhibitors for the corrosion control of Mg and its alloys in diverse media. J. Mater. Res. 2022, 20, 2060. [Google Scholar] [CrossRef]
- Ma, I.A.W.; Ammar, S.; Kumar, S.S.A.; Ramesh, K.; Ramesh, S. A concise review on corrosion inhibitors: Types, mechanisms and electrochemical evaluation studies. J. Coat. Technol. Res. 2022, 19, 241–268. [Google Scholar] [CrossRef]
- Bijapur, K.; Molahalli, V.; Shetty, A.; Toghan, A.; De Padova, P.; Hegde, G. Recent trends and progress in corrosion inhibitors and electrochemical evaluation. Appl. Sci. 2023, 13, 10107. [Google Scholar] [CrossRef]
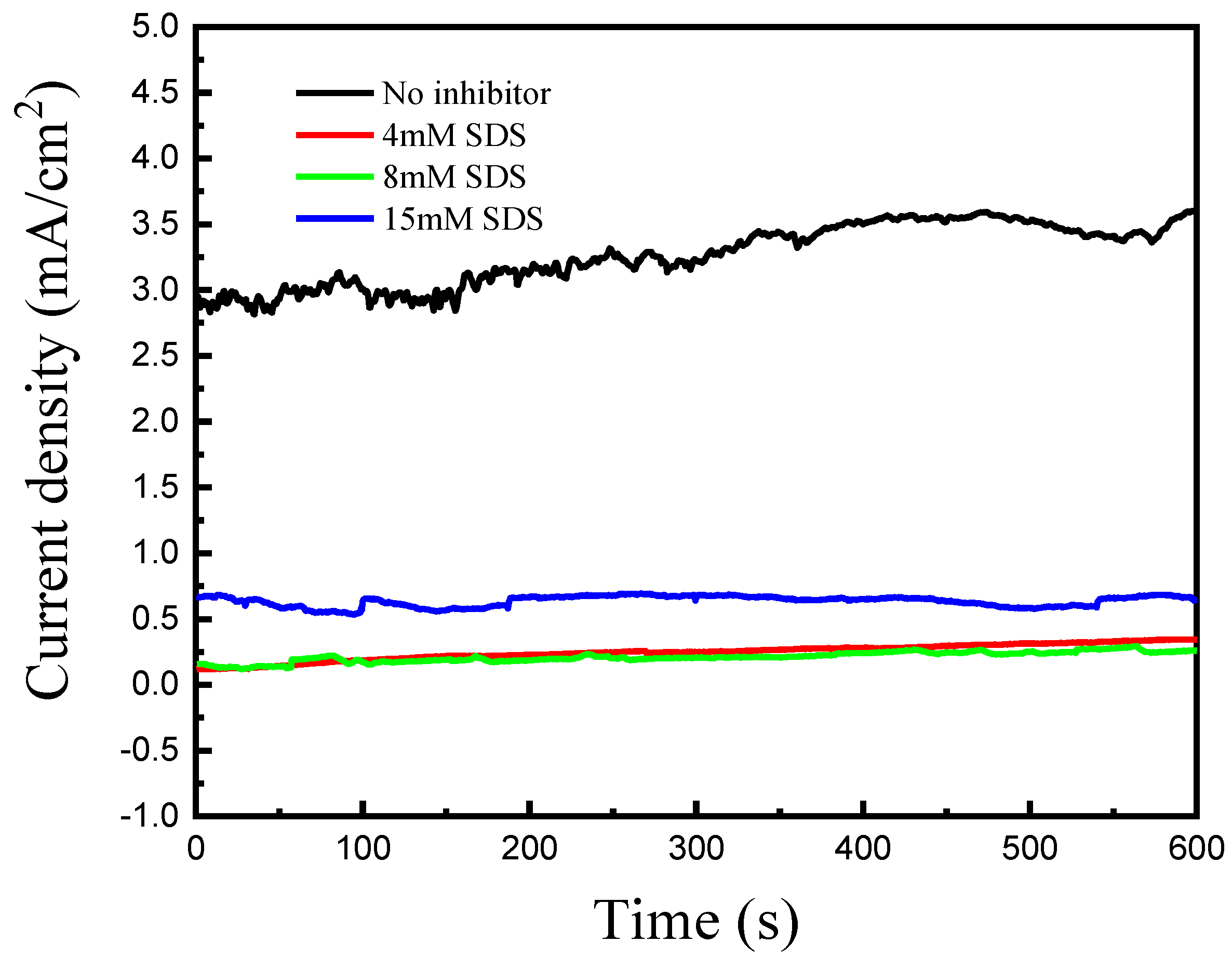
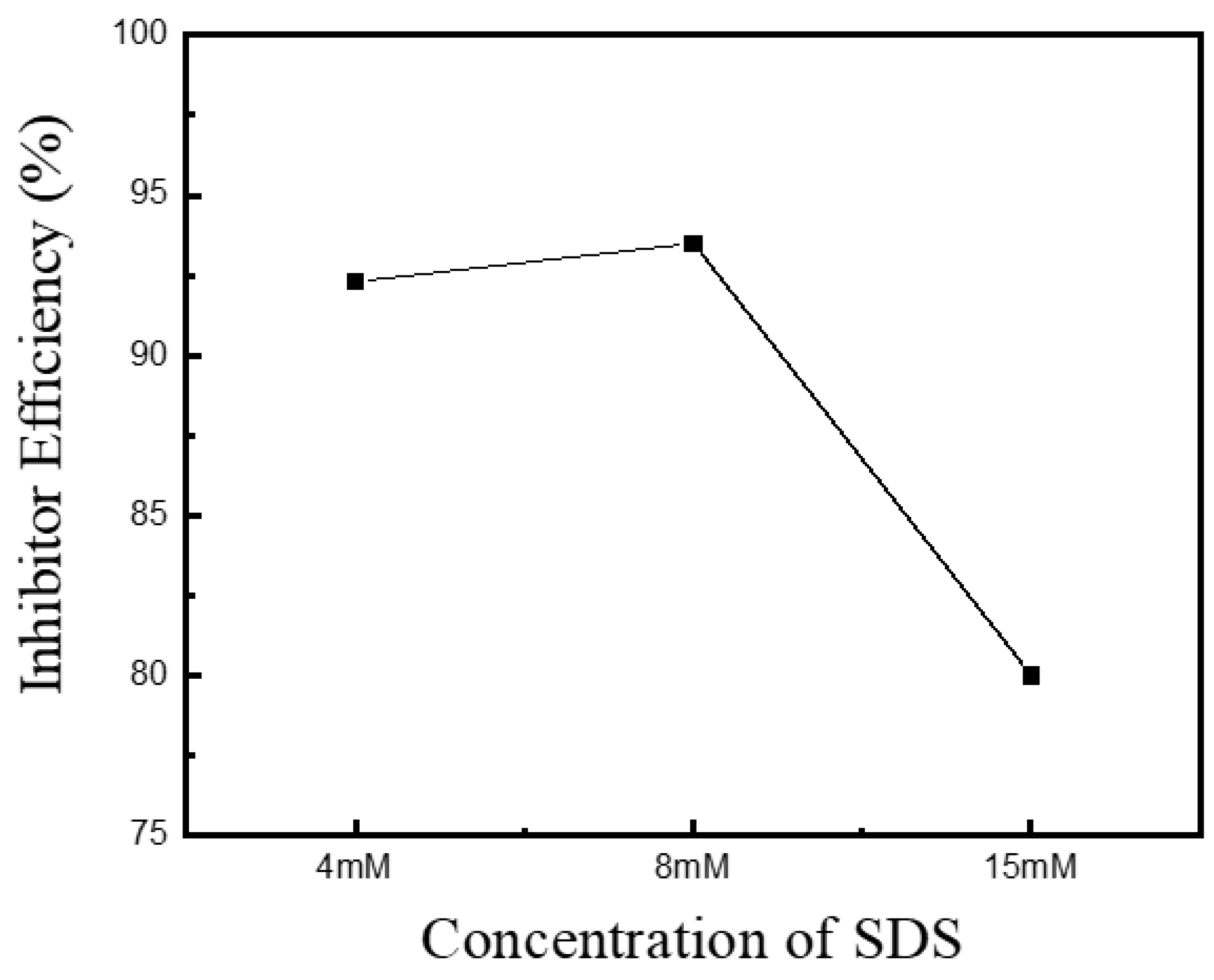
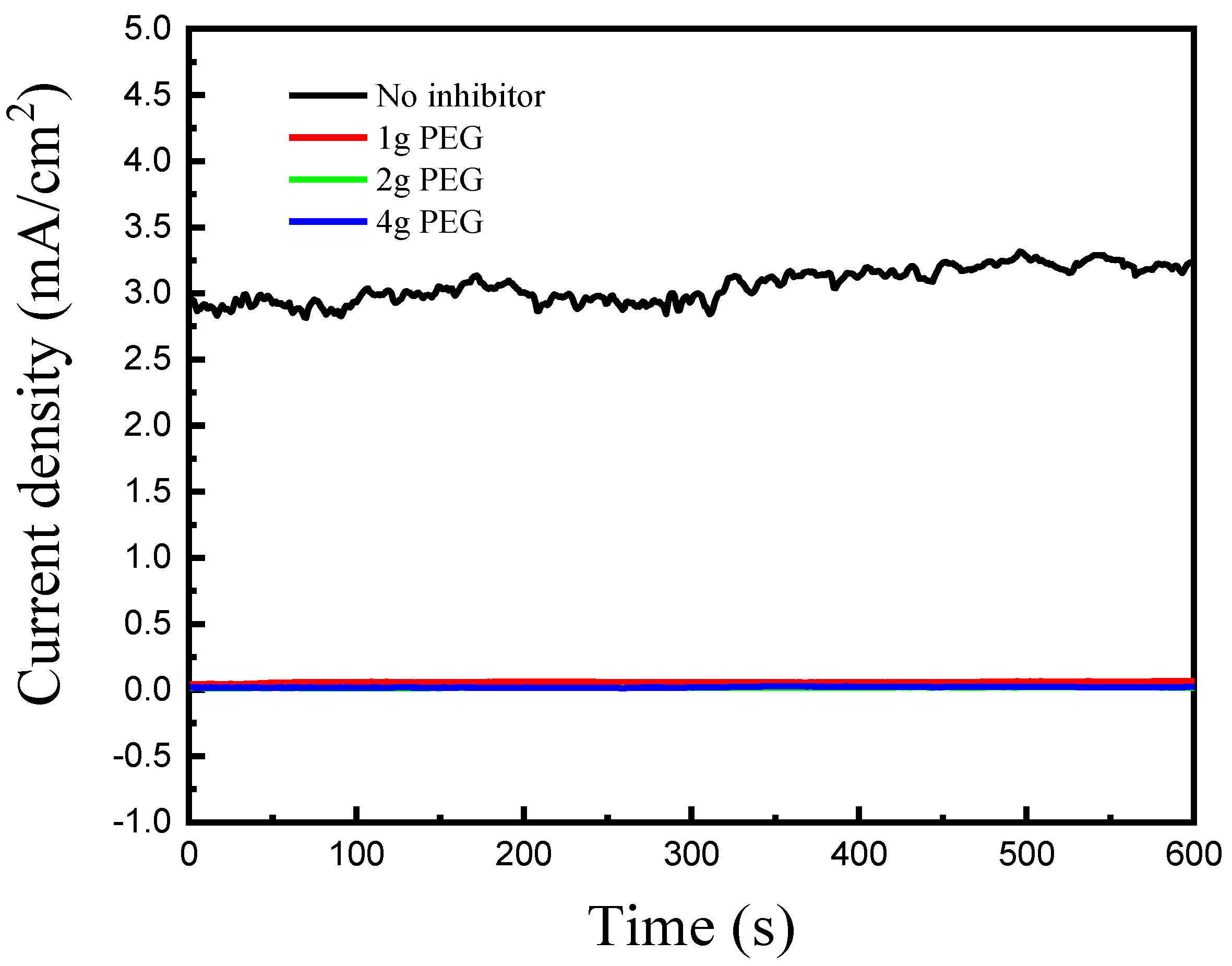

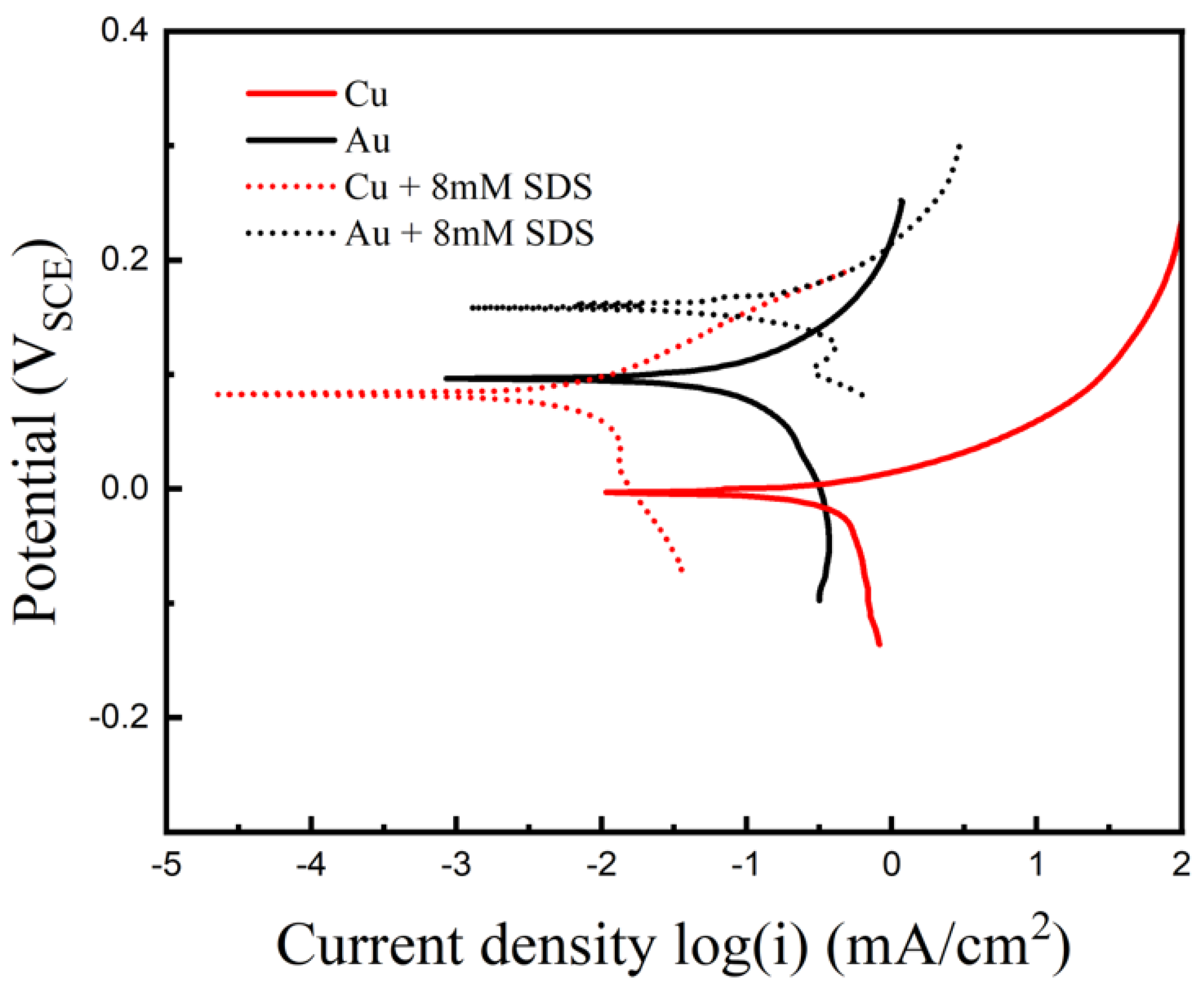
| Concentration (g/L) | icouple, (Cu-Au) (mA/cm2) | Inhibitor Efficiency (%) | |
|---|---|---|---|
| No inhibitor | - | 3.26 | |
| SDS | 4 | 0.248 | 92.3 |
| 8 | 0.211 | 93.5 | |
| 15 | 0.636 | 80.5 | |
| PEG | 1 | 0.06 | 98.1 |
| 2 | 0.017 | 99.5 | |
| 4 | 0.02 | 99.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.; Oh, S. Effective Corrosion Inhibition of Galvanic Corrosion of Cu Coupled to Au by Sodium Dodecyl Sulfate (SDS) and Polyethylene Glycol (PEG) in Acid Solution. Metals 2024, 14, 1080. https://doi.org/10.3390/met14091080
Shin H, Oh S. Effective Corrosion Inhibition of Galvanic Corrosion of Cu Coupled to Au by Sodium Dodecyl Sulfate (SDS) and Polyethylene Glycol (PEG) in Acid Solution. Metals. 2024; 14(9):1080. https://doi.org/10.3390/met14091080
Chicago/Turabian StyleShin, HeeKwon, and SeKwon Oh. 2024. "Effective Corrosion Inhibition of Galvanic Corrosion of Cu Coupled to Au by Sodium Dodecyl Sulfate (SDS) and Polyethylene Glycol (PEG) in Acid Solution" Metals 14, no. 9: 1080. https://doi.org/10.3390/met14091080
APA StyleShin, H., & Oh, S. (2024). Effective Corrosion Inhibition of Galvanic Corrosion of Cu Coupled to Au by Sodium Dodecyl Sulfate (SDS) and Polyethylene Glycol (PEG) in Acid Solution. Metals, 14(9), 1080. https://doi.org/10.3390/met14091080





