Effect of Ball Milling Parameters on Properties of Nano-Sized Tungsten Powder via Mechanochemical Processing
Abstract
1. Introduction
2. Materials and Methods
3. Results
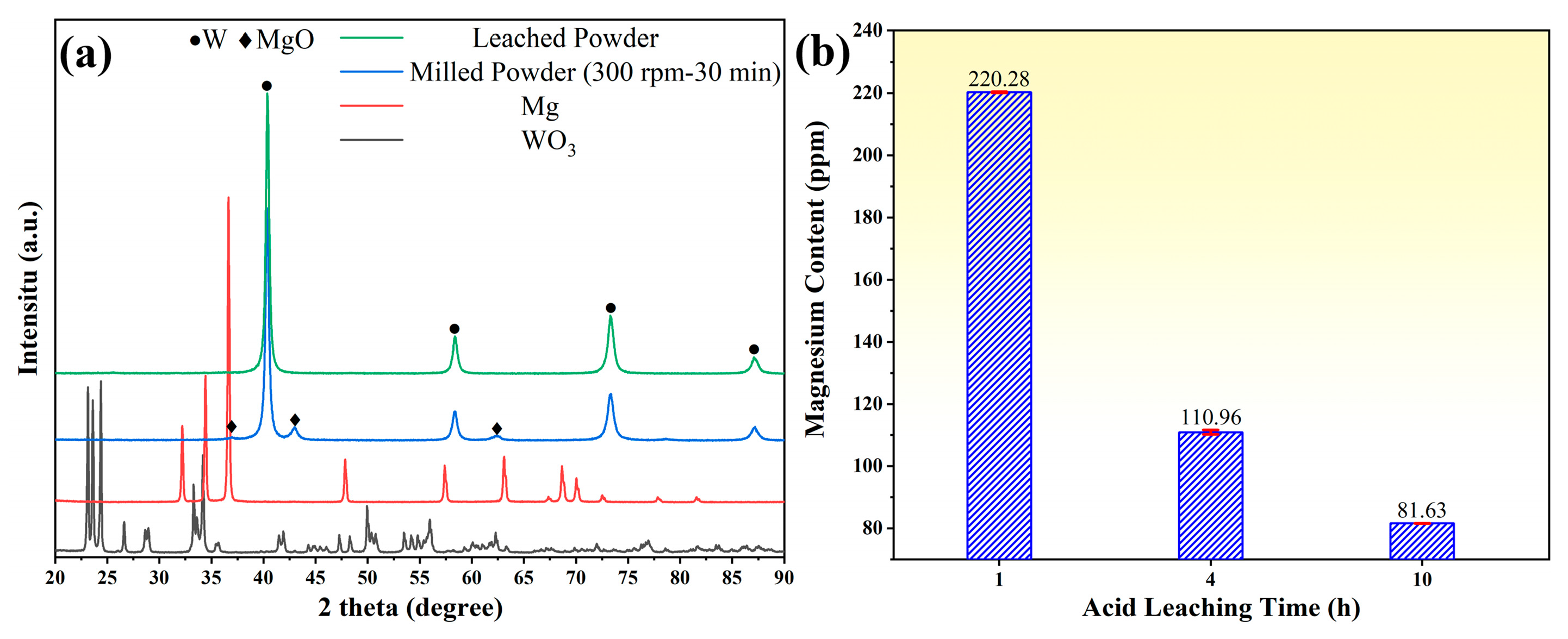
3.1. Phases and Morphology of Tungsten Powder Prepared by Mechanochemical Method
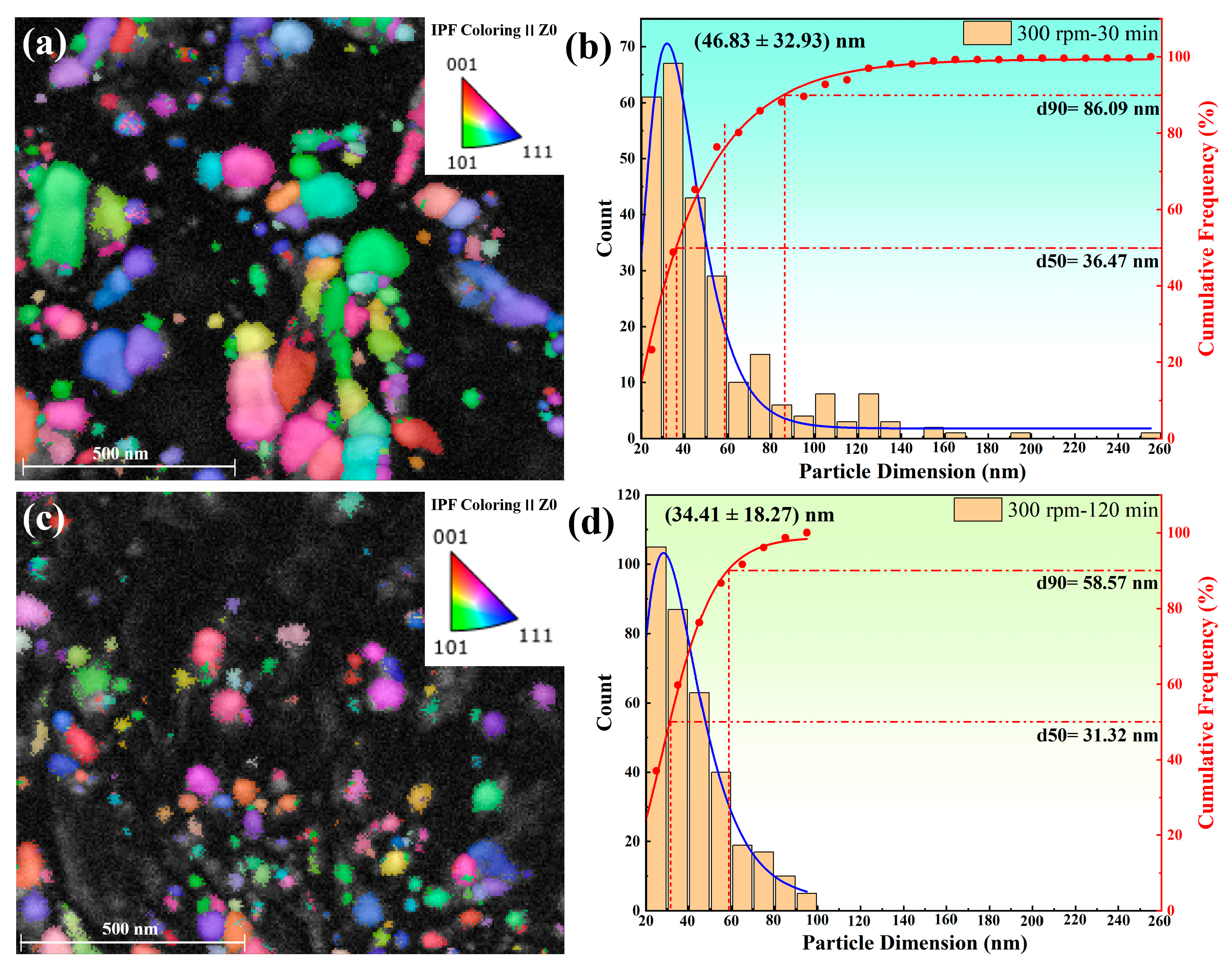
3.2. The Effect of Milling Parameters on Powder Properties
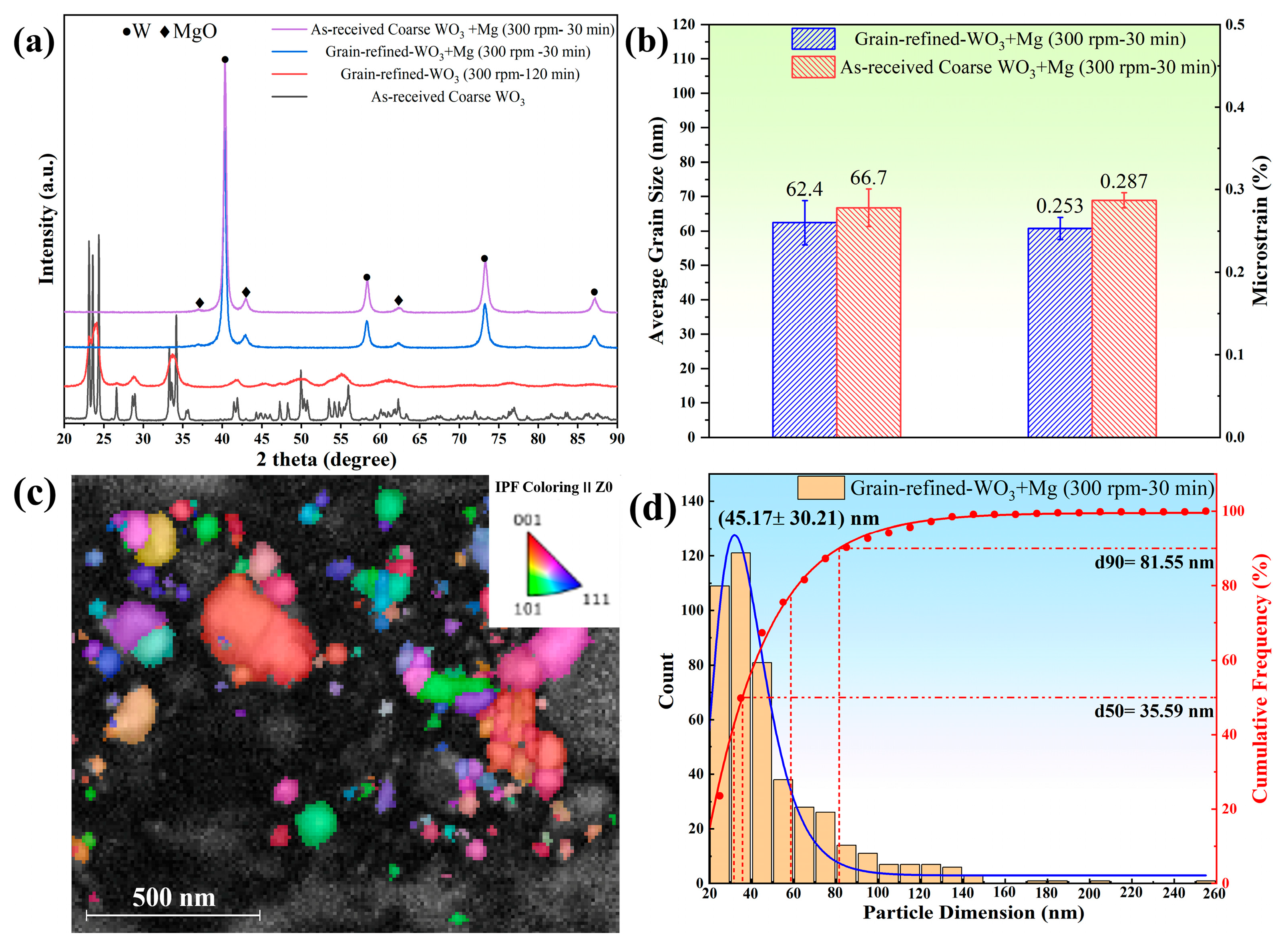
3.3. Effects of the Properties of WO3 Powder and Sieving on the Prepared Tungsten Powder
4. Discussion
5. Conclusions
- (1)
- Highly dispersed nano-sized tungsten powder can be produced by the mechanochemical solid-state reaction of WO3 with Mg at the moderate milling conditions. The obtained nano-sized tungsten powder possesses different average gran size depending on the milling parameters. Moreover, the optimized milling process should be conducted at a milling speed of 300 rpm for a milling duration of 30 to 120 min.
- (2)
- The entire mechanochemical process can be separated into the reaction stage and grain size refinement stage. In the case of 300 rpm ball milling, the reaction stage is almost completed after several minutes of ball milling. The subsequent stage is dominated by the ball milling process, and the refinement degree and uniformity of obtained tungsten powder increase with the milling duration. Although the introduction of WC impurity is inevitable in this stage, the impurity level is acceptable compared to those found in the high-energy ball milling processes.
- (3)
- There is no discernible difference in the average grain size and microstrain of the final tungsten powders between those derived from grain-refined and as-received coarse WO3 powders. Furthermore, the sieving experiments conducted using the centrifugation method can obtain nano-sized tungsten powder with the average grain size of ~19.5 nm.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ozolin, A.V.; Sokolov, E.G.; Golius, D.A. Obtaining of tungsten nanopowders by high energy ball milling. IOP Conf. Ser. Mater. Sci. Eng. 2020, 862, 022057. [Google Scholar] [CrossRef]
- Aaoullar, D.; Balc, Z.; Duman, S. Nanocrystalline tungsten powders synthesized from ULUDAĞ scheelite concentrates via mechanochemical route. Nanosci. Nanotechnol. 2011, 11, 231–234. [Google Scholar]
- Ricceri, R.; Matteazzi, P. A study of formation of nanometric W by room temperature mechanosynthesis. J. Alloys Compd. 2003, 358, 71–75. [Google Scholar] [CrossRef]
- Wu, Z.M.; Liang, Y.X.; Fan, Y.; Wang, P.P.; Du, J.L.; Zhao, Y.B.; Fu, E.G. The ball to powder ratio (BPR) dependent morphology and microstructure of tungsten powder refined by ball milling. Powder 2018, 339, 256–263. [Google Scholar] [CrossRef]
- Ding, X.Y.; Fang, J.H.; Xu, Q.; Zhang, P.P.; Zhang, H.J.; Luo, L.M.; Wu, Y.C.; Yao, J.H. Microstructure evolution and effect on deuterium retention in TiC- and ZrC-doped tungsten under He+ ion irradiation. Metals 2023, 13, 783. [Google Scholar] [CrossRef]
- Shah, V.; Dommelen, J.A.W.V.; Geers, M.G.D. Spatially dependent kinetics of helium in tungsten under fusion conditions. J. Nucl. Mater. 2020, 535, 152104. [Google Scholar] [CrossRef]
- Oda, E.; Ameyama, K.; Yamaguchi, S. Fabrication of nano grain tungsten compact by mechanical milling process and its high temperature properties. Mater. Sci. Forum 2006, 503–504, 573–578. [Google Scholar] [CrossRef]
- Malewar, R.; Kumar, K.S.; Murty, B.S.; Sarma, B.; Pabi, S.K. On sinterability of nanostructured W produced by high-energy ball milling. J. Mater. Res. 2007, 22, 1200–1206. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.Y.; Qu, X.H.; Qin, M.L.; Que, Z.Y.; Wei, Z.C.; Guo, C.G.; Lu, X.; Dong, Y.H. Powder metallurgy route to ultrafine-grained refractory metals. Adv. Mater. 2023, 2205807, 1–25. [Google Scholar] [CrossRef]
- Xu, L.; Yan, Q.Z.; Xia, M.; Zhu, L.X. Preparation of La2O3 doped ultra-fine W powders by hydrothermal-hydrogen reduction process. Int. J. Refract. Met. H. 2013, 36, 238–242. [Google Scholar] [CrossRef]
- Xia, M.; Yan, Q.Z.; Xu, L.; Zhu, L.X.; Guo, H.Y.; Ge, C.C. Synthesis of TiC/W core–shell nanoparticles by precipitate-coating process. J. Nucl. Mater. 2012, 430, 216–220. [Google Scholar] [CrossRef]
- Xia, M.; Yan, Q.Z.; Xu, L.; Guo, H.Y.; Zhu, L.X.; Ge, C.C. Bulk tungsten with uniformly dispersed La2O3 nanoparticles sintered from co-precipitated La2O3/W nanoparticles. J. Nucl. Mater. 2013, 434, 85–89. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Lv, Y.Q.; Zhang, Z.W.; Fan, J.L. Synthesis and characterization of W-Y2O3 composites with core-shell structure via wet chemical method in an acidic solution. Int. J. Refract. Met. Hard Mater. 2024, 122, 106729. [Google Scholar] [CrossRef]
- Xu, W.Z.; Wang, J.; Luo, L.M.; Zan, X.; Wu, Y.C. The preparation process of ultrafine gain W-Re powder by wet chemical method and its effect on alloy properties. Mater. Today Commun. 2023, 34, 104955. [Google Scholar] [CrossRef]
- Chen, J.; Luo, L.; Lin, J.; Zan, X.; Zhu, X.; Luo, G.; Wu, Y. Influence of ball milling processing on the microstructure and characteristic of W-Nb alloy. J. Alloys Compd. 2017, 694, 905–913. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, Z.; Tan, J.; Ding, Q.; Zhong, M. Effects of ball milling parameters on microstructural evolution and mechanical properties of W–3% Y composites. J. Nucl. Mater. 2015, 465, 6–12. [Google Scholar] [CrossRef]
- Debata, M.; Acharya, T.S.; Sengupta, P.; Acharya, P.P.; Baipai, S.; Jayasankkar, K. Effect of high energy ball milling on structure and properties of 95W-3.5Ni-1.5Fe heavy alloys. Int. J. Refract. Met. Hard Mater. 2017, 69, 170–179. [Google Scholar] [CrossRef]
- Kurishita, H.; Kobayashi, S.; Nakai, K.; Arakawa, H.; Matsuo, S.; Takida, T.; Takebe, K.; Kawai, M. Current status of ultra-fine grained W–TiC development for use in irradiation environments. Phys. Scr. 2007, 128, 76–80. [Google Scholar] [CrossRef]
- Wu, Z.M.; Liang, Y.X.; Fu, E.P.; Du, J.L.; Wang, P.P. The process and mechanisms for the transformation of coarse grain to nanoscale grain in tungsten by ball milling. Powder Technol. 2018, 326, 222–227. [Google Scholar] [CrossRef]
- Pohshna, C.; Mailapalli, D.R. Modeling the particle size of nanomaterials synthesized in a planetary ball mill. OpenNano 2023, 14, 100191. [Google Scholar] [CrossRef]
- Wu, Z.M.; Liang, Y.X.; Fu, E.G.; Du, J.L.; Wang, P.P.; Fan, Y.; Zhao, Y.B. Effect of ball milling parameters on the refinement of tungsten powder. Metals 2018, 8, 281. [Google Scholar] [CrossRef]
- Liang, Y.X.; Wu, Z.M.; Fu, E.G.; Du, J.L.; Wang, P.P.; Zhao, Y.B.; Qiu, Y.H.; Hu, Z.Y. Refinement process and mechanisms of tungsten powder by high energy ball milling. Int. J. Refract. Met. Hard Mater. 2017, 67, 1–8. [Google Scholar] [CrossRef]
- Oda, E.; Fujiwara, H.; Ameyama, K. Nano grain formation in tungsten by severe plastic deformation-mechanical milling process. Mater. Trans. 2008, 49, 54–57. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, L.; Dong, Y.H.; Gao, R.; Qin, M.L.; Qu, X.H.; Li, J. Pressureless two-step sintering of ultrafine-grained tungsten. Acta Mater. 2020, 186, 116–123. [Google Scholar] [CrossRef]
- He, Q.Y.; Zhao, B.L.; Sun, S.K. Nanosized tungsten powder synthesized using the nitridation–decomposition method. Ceramics 2024, 7, 680–688. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, Z.Q.; Yu, L.M.; Liu, Y.C. Achieving high strength and ductility in ODS-W alloy by employing oxide@W core-shell nanopowder as precursor. Nat. Commun. 2021, 12, 5052. [Google Scholar] [CrossRef]
- Welham, N.J. Room temperature reduction of scheelite (CaWO4). J. Mater. Res. 1999, 14, 619–627. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.K.; Prisbrey, K.A.; Suryanarayana, C.; Froes, F.H. Ball-milling, a novel extraction process for production of W from WO3 using Mg as a reductant. In Proceedings of the Third International Conference on Tungsten and Refractory Metals, McLean, VA, USA, 15–17 October 1995. [Google Scholar]
- Małgorzata, S.M. High-Energy Ball Milling: Mechanochemical Processing of Nanopowders, 1st ed.; Woodhead Publishing: Abington Hall, Granta Park, Great Abington Cambridge, UK, 2010; pp. 1–6. [Google Scholar]
- Belyakova, D.A.; Udalova, T.A. Synthesis of highly dispersed tungsten by mechanochemical reduction of magnesium tungstate. Mater. Today Proc. 2020, 31, 476–478. [Google Scholar] [CrossRef]
- Zhang, J.H.; Yang, G.J.; Li, Z.H. Discovery of natural tungsten carbide (WC) in China. Acta Mineral. Sinica 1986, 6, 344–349. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Kurlov, A.S.; Gusev, A.I. Model for milling of powders. Tech. Phys. 2011, 56, 975–980. [Google Scholar] [CrossRef]
- Zholdassov, Y.S.; Yuan, L.; Garcia, S.R.; Kwok, R.W.; Boscoboinik, A.; Valles, D.J.; Marianski, M.; Martini, A.; Carpick, R.W.; Braunschweig, A.B. Acceleration of diels-alder reactions by mechanical distortion. Science 2023, 380, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Gonnet, L.; Chamayou, A.; André-Barrès, C.; Micheau, J.C.; Guidetti, B.; Sato, T.; Baron, M.; Baltas, M.; Calvet, R. Elucidation of the diels–alder reaction kinetics between diphenylfulvene and maleimide by mechanochemistry and in solution. ACS Sustainable Chem. Eng. 2021, 9, 4453–4462. [Google Scholar] [CrossRef]



| Powder | Milling Parameters | Average Grain Size/nm | Impurities |
|---|---|---|---|
| WO3 + Mg | 300 rpm—30 min—Sieved | 60.7 | Undetected |
| 19.5 | |||
| WO3 + Mg [2] | 1200 rpm—10 min | 83.33 | Unreacted WO3 |
| WO3 + Mg [3] | Unknown rpm—3 h | <100 | W-Fe compounds |
| CaWO4 + Mg [27] | 165 rpm—100 h | 13.5 ± 4.5 | ~0.6%Fe, ~0.4%Cr |
| WO3 + MgO + Mg [30] | 1000 rpm—8 min | 100 | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zhang, G.; Zheng, P.; Qian, W.; Wei, Y.; Li, B.; Zhang, M.; Zhang, Z.; Che, T. Effect of Ball Milling Parameters on Properties of Nano-Sized Tungsten Powder via Mechanochemical Processing. Metals 2024, 14, 1079. https://doi.org/10.3390/met14091079
Li F, Zhang G, Zheng P, Qian W, Wei Y, Li B, Zhang M, Zhang Z, Che T. Effect of Ball Milling Parameters on Properties of Nano-Sized Tungsten Powder via Mechanochemical Processing. Metals. 2024; 14(9):1079. https://doi.org/10.3390/met14091079
Chicago/Turabian StyleLi, Feng, Guihang Zhang, Pengfei Zheng, Wei Qian, Yaxia Wei, Bingsheng Li, Ming Zhang, Zhijie Zhang, and Tong Che. 2024. "Effect of Ball Milling Parameters on Properties of Nano-Sized Tungsten Powder via Mechanochemical Processing" Metals 14, no. 9: 1079. https://doi.org/10.3390/met14091079
APA StyleLi, F., Zhang, G., Zheng, P., Qian, W., Wei, Y., Li, B., Zhang, M., Zhang, Z., & Che, T. (2024). Effect of Ball Milling Parameters on Properties of Nano-Sized Tungsten Powder via Mechanochemical Processing. Metals, 14(9), 1079. https://doi.org/10.3390/met14091079





