Effects of CTAB (Cetyltrimethylammonium Bromide) and Betaine as Corrosion Inhibitors on the Galvanic Corrosion of Cu Coupled with Au on Print Circuit Board in Etching Solution
Abstract
1. Introduction
2. Experimental
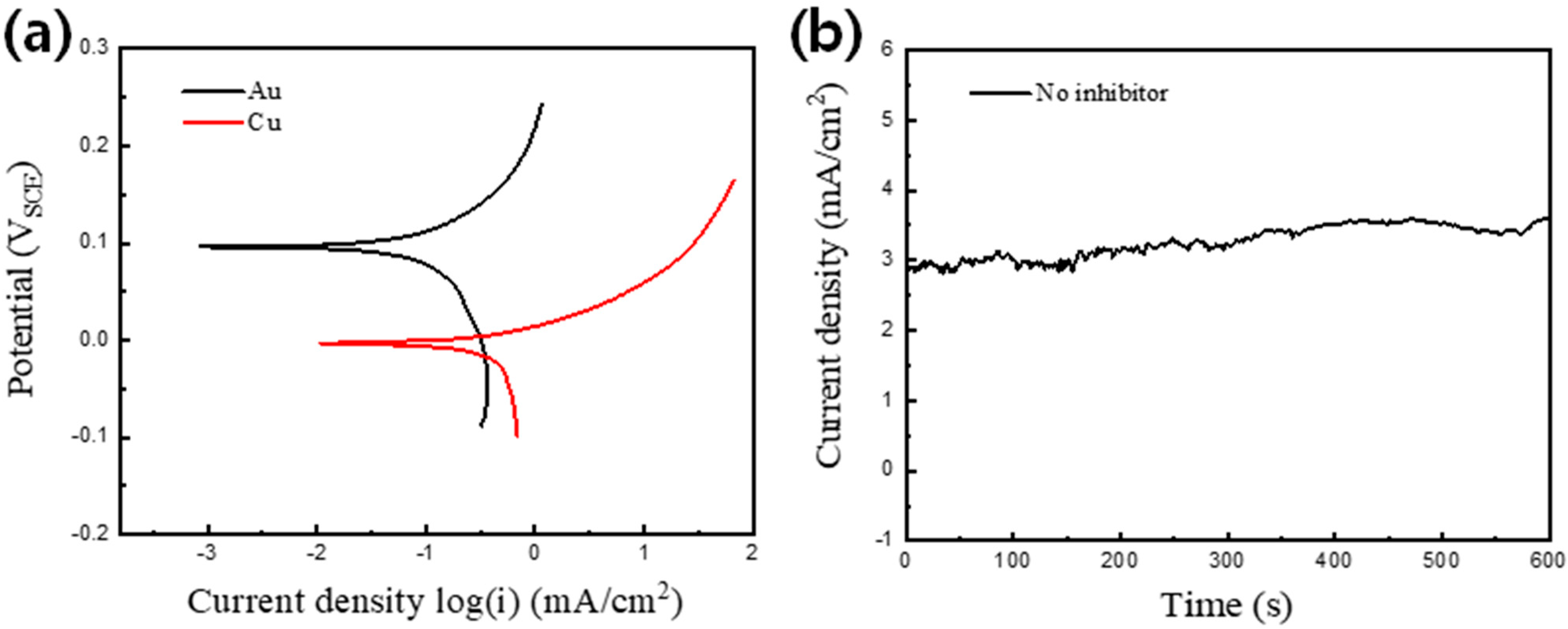
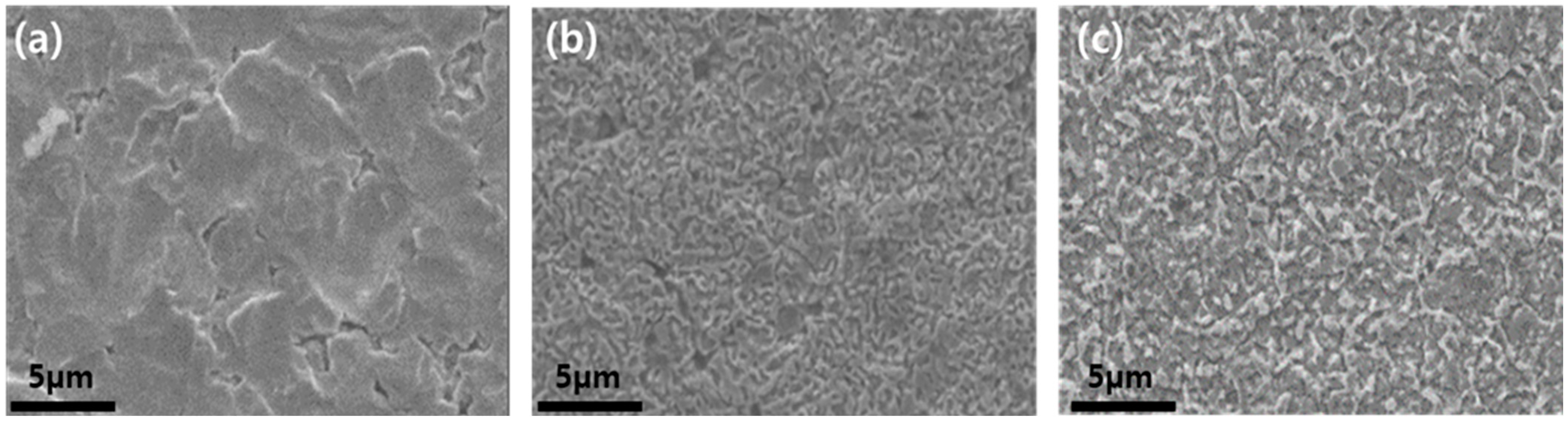
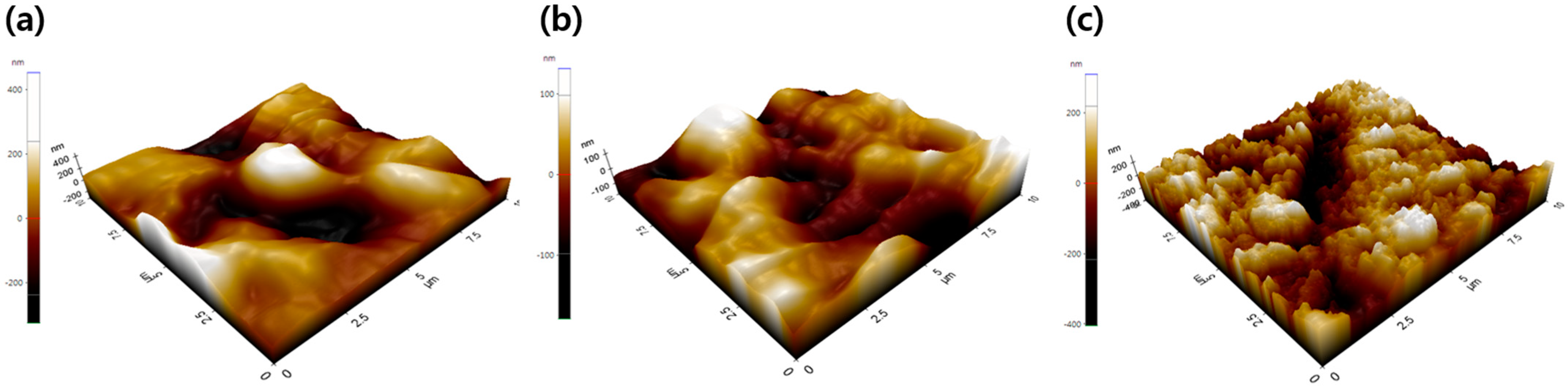
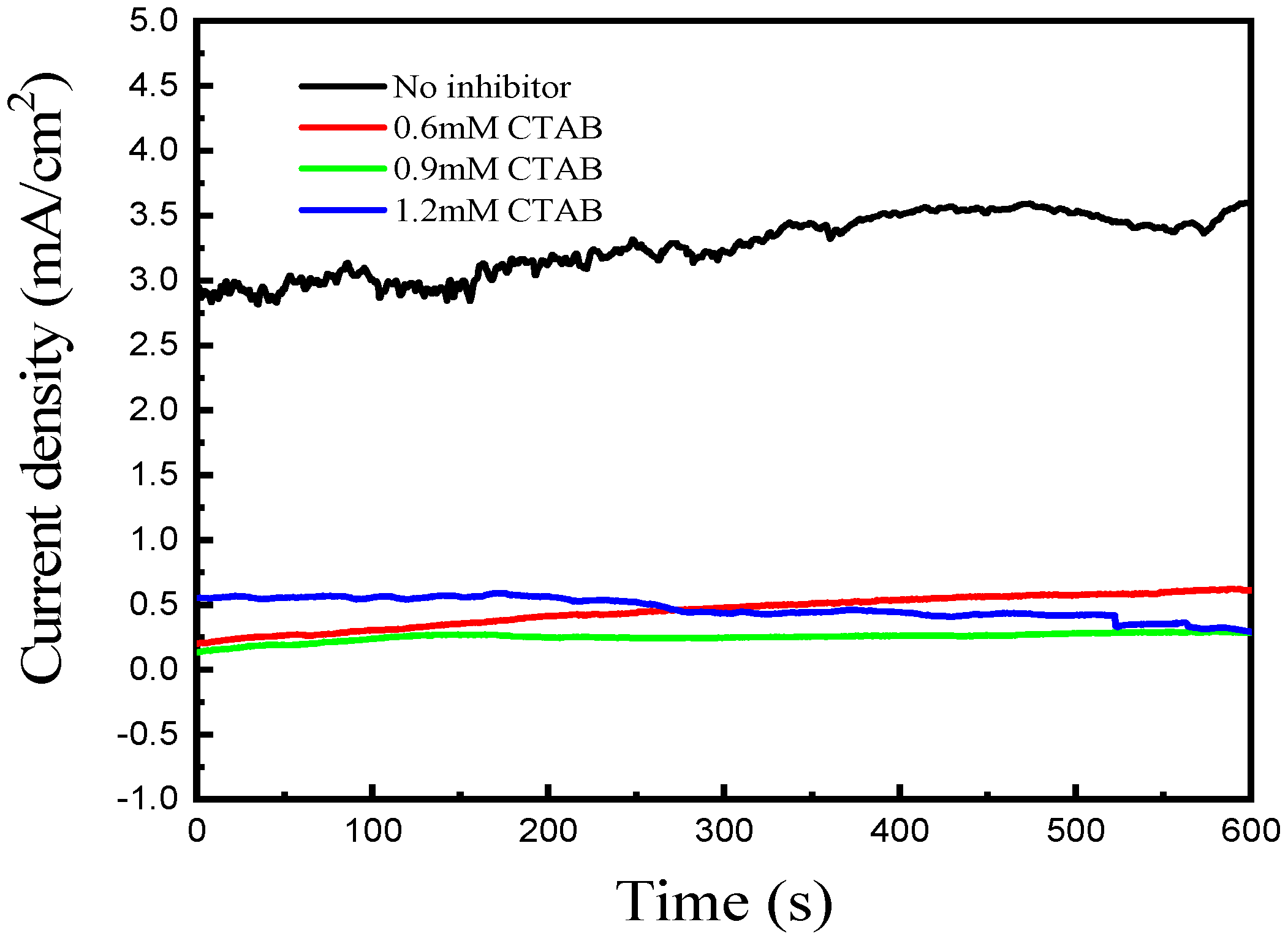
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miura, S.; Honma, H. Advanced copper electroplating for application of electronics. Surf. Coat. Technol. 2003, 169, 91. [Google Scholar] [CrossRef]
- Li, Z.; Chang, S.; Khuje, S.; Ren, S. Recent Advancement of Emerging Nano Copper-Based Printable Flexible Hybrid Electronics. ACS Nano 2021, 15, 6211. [Google Scholar] [CrossRef] [PubMed]
- Julian, Y.; Hon, C.; Cotterell, B.; Chai, T.C. The mechanics of the solder ball shear test and the effect of shear rate. Mater. Sci. Eng. A 2006, 417, 259. [Google Scholar]
- Wang, J.; Lim, H.K.; Lew, H.S.; Saw, W.T.; Tan, C.H. A testing method for assessing solder joint reliability of FCBGA packages. Microelectron. Reliab. 2004, 44, 833. [Google Scholar] [CrossRef]
- Doranga, S.; Schuldt, M.; Khanal, M. Effect of Stiffening the Printed Circuit Board in the Fatigue Life of the Solder Joint. Materials 2022, 15, 6208. [Google Scholar] [CrossRef]
- Murira, M.C.; Punckt, C.; Schniepp, H.C.; Khusid, B.; Aksay, I.A. Inhibition and Promotion of Copper Corrosion by CTAB in a Microreactor System. Langmuir 2008, 24, 14269. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.; Jung, K.; Kim, J.; Shon, M.; Kwon, H. Effects of Temperature and Operation Parameters on the Galvanic Corrosion of Cu Coupled to Au in Organic Solderability Preservatives Process. Met. Mater. Int. 2017, 23, 290. [Google Scholar] [CrossRef]
- Xiao-qing, D.; Yang, Q.; Chen, Y.; Yang, Y.; Zhao, Z. Galvanic corrosion behavior of copper/titanium galvanic couple in artificial seawater. Trans. Nonferrous Met. Soc. China 2014, 24, 570. [Google Scholar]
- Idrac, J.; Mankowski, G.; Thompson, G.; Skeldon, P.; Kihn, Y.; Blan, C. Galvanic corrosion of aluminium–copper model alloys. Electrochim. Acta 2007, 52, 7626. [Google Scholar] [CrossRef]
- Huh, M.; Yu, Y.; Kahn, H.; Payer, J.H.; Heuer, A.H. Galvanic Corrosion during Processing of Polysilicon Microelectromechanical Systems. J. Electrochem. Soc. 2006, 153, 644. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.; Shon, M.; Kwon, H. Galvanic Corrosion of Cu Coupled to Au on a Printed Circuit Board; Effects of Pretreatment Solution and Etchant Concentration in Organic Solderability Preservatives Soft Etching Solution. Met. Mater. Int. 2016, 22, 781. [Google Scholar] [CrossRef]
- Huang, H.; Guo, X.; Zhang, G.; Dong, Z. The effects of temperature and electric field on atmospheric corrosion behaviour of PCB-Cu under absorbed thin electrolyte layer. Corros. Sci. 2011, 53, 1700. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.; Jung, K.; Park, M.; Shon, M.; Kwon, H. Galvanic Corrosion Behaviors of Cu Connected to Au on a Printed Circuit Board in Ammonia Solution. Met. Mater. Int. 2018, 24, 67. [Google Scholar] [CrossRef]
- Khadom, A.A.; Abod, B.M.; Mahood, H.B.; Kadhum, A.A.H. Galvanic Corrosion of Steel–Brass Couple in Petroleum Waste Water in Presence of a Green Corrosion Inhibitor: Electrochemical, Kinetics, and Mathematical View. J. Fail. Anal. Preven. 2018, 18, 1300–1310. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Yan, J.; Zhang, J.; Zhang, Q.; Yan, Y. Recent Progress of Polymeric Corrosion Inhibitors: Structure and Application. Materials 2023, 16, 2954. [Google Scholar] [CrossRef]
- Samarawickrama, C.; Pöhlker, S.; White, P.; Cole, I.; Keil, P. Corrosion Inhibitor Screening for AA6014 Aluminum Alloy Under Different Ambient Conditions Using a Novel Multielectrode Methodology. Mol. Syst. Des. Eng. 2024, 9, 518–531. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Radeva, T.; Shipochka, M.; Boshkov, N. Chitosan–Alginate Nanocontainers with Caffeine as Green Corrosion Inhibitors for Protection of Galvanized Steel. Crystals 2024, 14, 660. [Google Scholar] [CrossRef]
- Shwetha, K.M.; Praveen, B.M.; Devendra, B.K. A Review on Corrosion Inhibitors: Types, Mechanisms, Electrochemical Analysis, Corrosion Rate and Efficiency of Corrosion Inhibitors on Mild Steel in an Acidic Environment. Results Surf. Interfaces 2024, 16, 100258–100277. [Google Scholar]
- Wulandari, M.; Nofrizal, N.; Impey, S.; Georgarakis, K.; Raja, P.B.; Hussin, M.H. The Effect of Corrosion Inhibitor on X-65 Steel Weldment in High Flow Rate Conditions. Case Stud. Chem. Environ. Eng. 2024, 10, 100868–100876. [Google Scholar] [CrossRef]
- Hussein, S.A.; Khadom, A.A. Okra Leaves Extract as Green Corrosion Inhibitor for Steel in Sulfuric Acid: Gravimetric, Electrochemical, and Surface Morphological Investigations. Results Chem. 2024, 8, 101566–101577. [Google Scholar] [CrossRef]
- Farh, H.M.H.; Ben Seghier, M.E.A.; Zayed, T. A Comprehensive Review of Corrosion Protection and Control Techniques for Metallic Pipelines. Eng. Fail. Anal. 2023, 143, 106885–106907. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, X.; Wang, P.; Jiang, J.; Zhang, H.; Slaný, M.; Bian, J. Shielding against Erosion: Exploring the Effectiveness of Pre-Erosion Surface Corrosion Inhibitors. J. Colloid. Interface Sci. 2024, 675, 1130–1148. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bu, F. Correlations between the inhibition performances and the inhibitor structures of some azoles on the galvanic corrosion of copper coupled with silver in artificial seawater. Corros. Sci. 2020, 165, 108413. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, B.; Zhang, Q.; Wang, R.; Yu, X.; Wang, C.; Liu, Y. A Study of Cobalt Galvanic and Pitting Corrosion with combination of BTA and PMP. ECS J. Solid State Sci. Technol. 2019, 8, 416. [Google Scholar] [CrossRef]
- Sargolzaei, B.; Arab, A. Synergism of CTAB and NLS surfactants on the corrosion inhibition of mild steel in sodium chloride solution. Mater. Today Commun. 2021, 29, 102809. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Zhao, S.; Liu, X.; Li, D. A Study of Corrosion Behavior of Copper in Acidic Solutions Containing Cetyltrimethylammonium Bromide. J. Electrochem. Soc. 2001, 148, 482–488. [Google Scholar] [CrossRef]
- El Bakri, M.; Touir, R.; Tazouti, A.; Dkhireche, N.; Ebn Touhami, M.; Rochdi, A.; Zarrouk, A. Corrosion Inhibition Study of Brass in Simulated Cooling Water by Triazole Derivatives, Cetyltrimethylammonium Bromide and Their Mixture. Arab. J. Sci. Eng. 2016, 41, 75–88. [Google Scholar] [CrossRef]
- Wu, C.; Yang, H.; Zhang, S.; Han, P.; Sun, H.; Sheng, Z.; Yu, H.; Ma, X. The inhibition performance of anionic surfactant and zwitterionic surfactant toward the corrosion of carbon steel in NaCl solution. Int. J. Chem. Kinet. 2023, 55, 537. [Google Scholar] [CrossRef]
- Arkhipushkin, I.A.; Shikhaliev, K.S.; Potapov, A.Y.; Sapronova, L.V.; Kazansky, L.P. Inhibition of Brass (80/20) by 5-Mercaptopentyl-3-Amino-1,2,4-Triazole in Neutral Solutions. Metals 2017, 488, 3390. [Google Scholar] [CrossRef]
- Tkacz, J.; Slouková, K.; Minda, J.; Drábiková, J.; Fintová, S.; Doležal, P.; Wasserbauer, J. Influence of the Composition of the Hank’s Balanced Salt Solution on the Corrosion Behavior of AZ31 and AZ61 Magnesium Alloys. Metals 2017, 7, 465. [Google Scholar] [CrossRef]
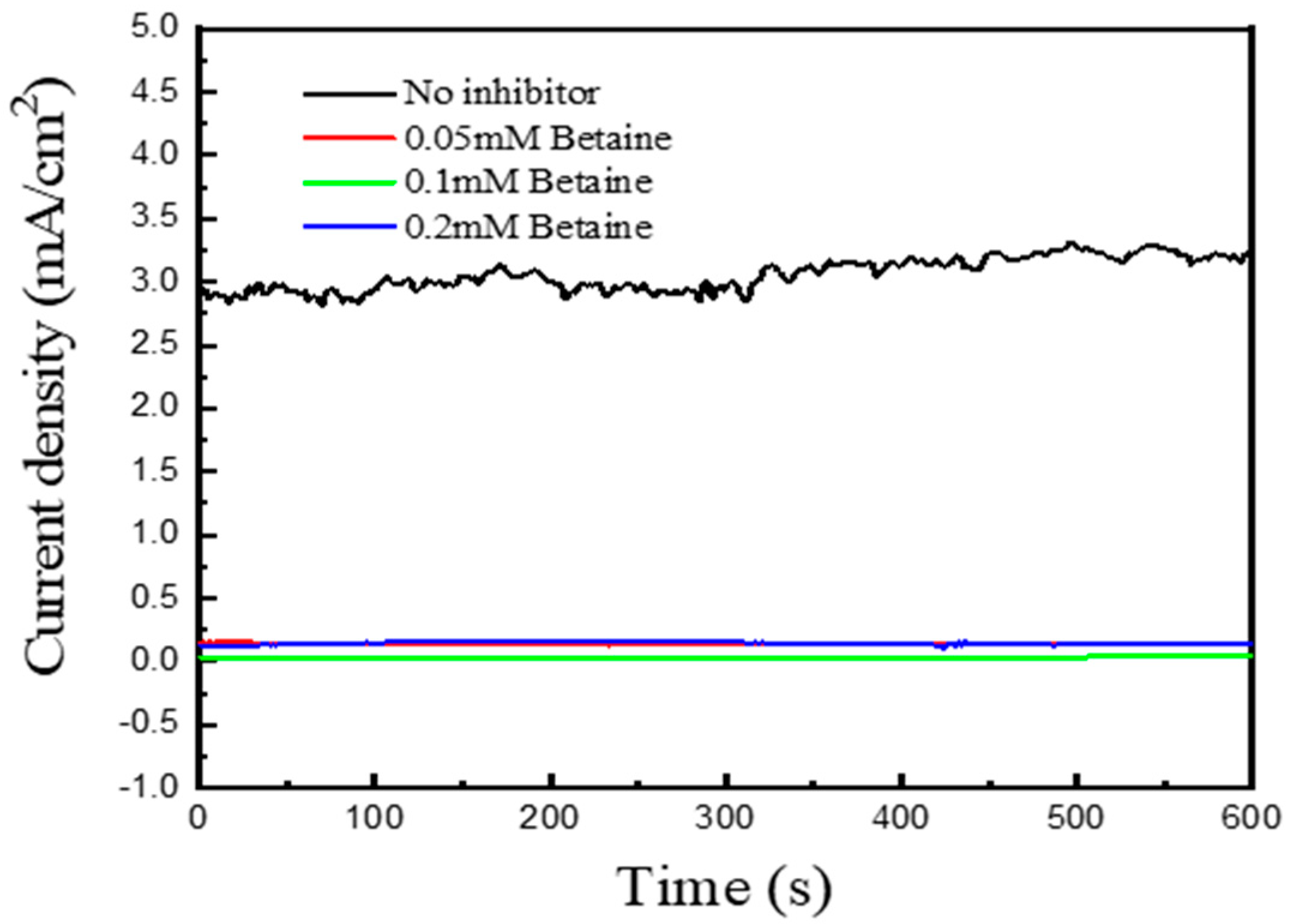
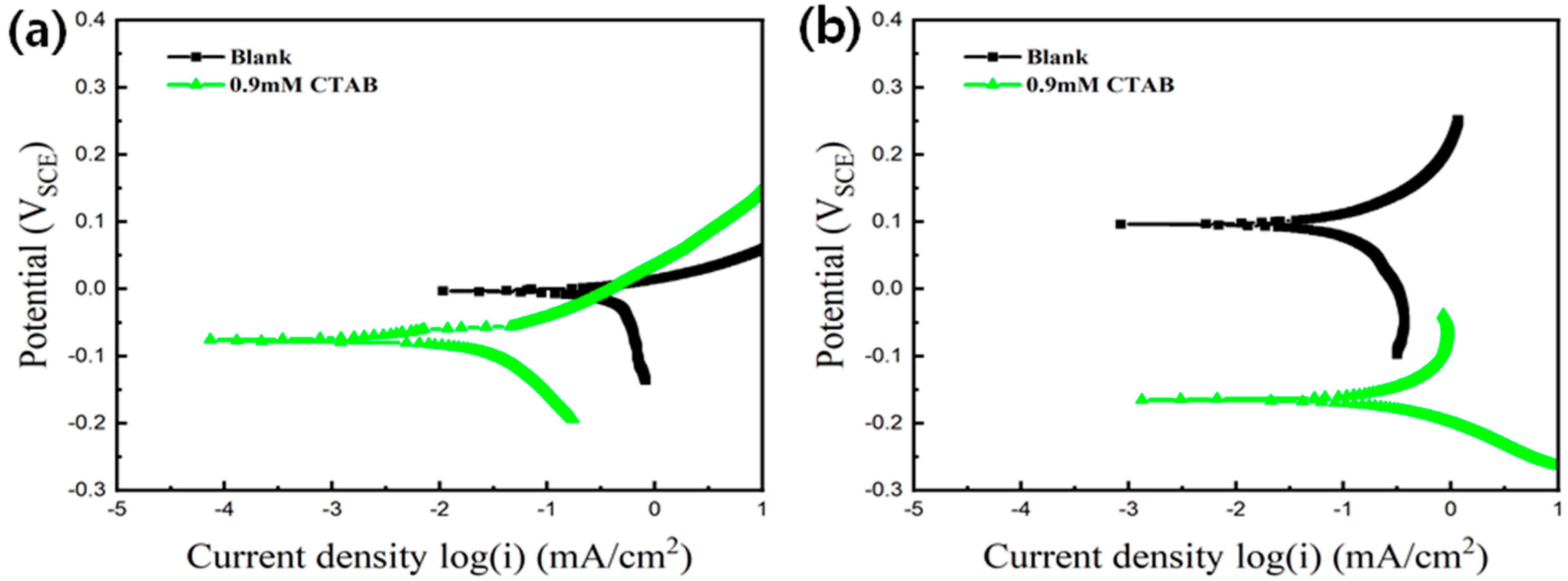
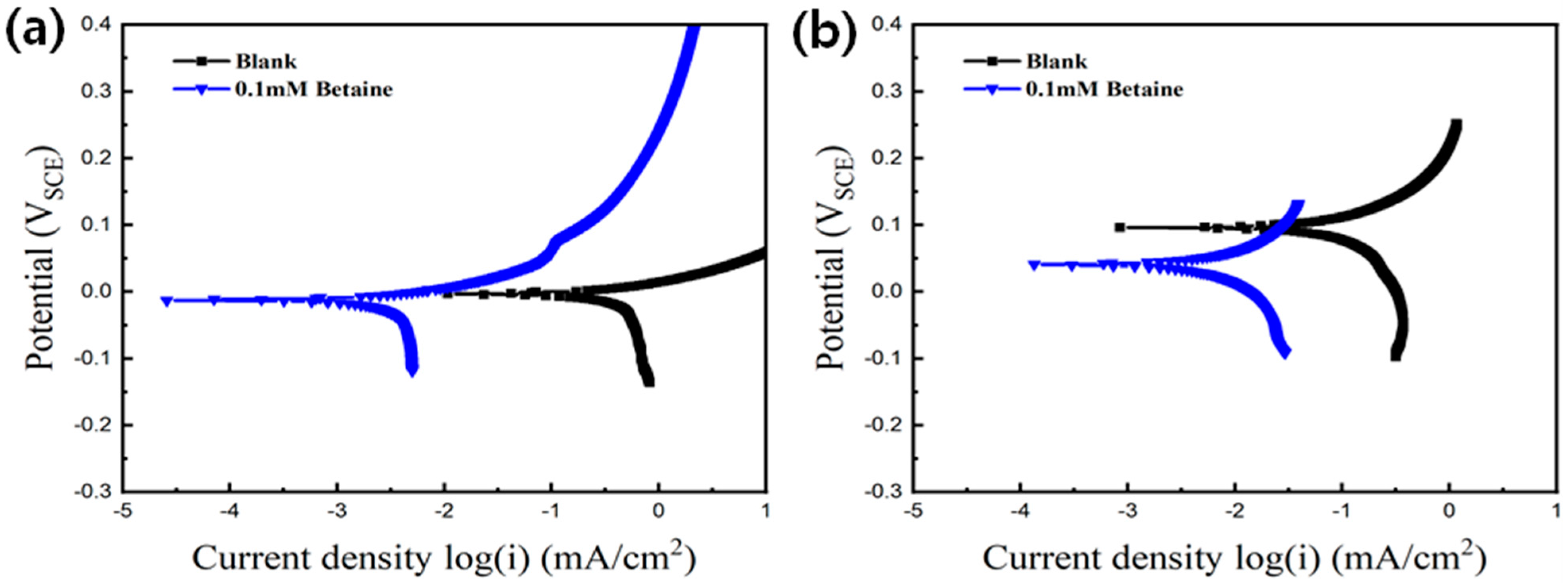
| Concentration of CTAB (mM) | icouple (Cu-Au) (mA/cm2) | Inhibitor Efficiency |
|---|---|---|
| No inhibitor | 3.26 | |
| 0.6 | 0.453 | 86.1% |
| 0.9 | 0.251 | 92.3% |
| 1.2 | 0.474 | 85.5% |
| Concentration of Betaine (mM) | icouple (Cu-Au) (mA/cm2) | Inhibitor Efficiency |
|---|---|---|
| No inhibitor | 3.26 | |
| 0.05 | 0.14 | 95.7% |
| 0.1 | 0.03 | 99.1% |
| 0.2 | 0.146 | 95.5% |
| Cu | Au | |||
|---|---|---|---|---|
| icorr (mA/cm2) | Ecorr (mV) | icorr (mA/cm2) | Ecorr (mV) | |
| Bare | 0.49 | −7.01 | 0.09 | 91 |
| CTAB | 0.028 | −84 | 0.386 | −172 |
| Betaine | 0.0051 | −12 | 0.007 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.; Oh, S. Effects of CTAB (Cetyltrimethylammonium Bromide) and Betaine as Corrosion Inhibitors on the Galvanic Corrosion of Cu Coupled with Au on Print Circuit Board in Etching Solution. Metals 2024, 14, 1090. https://doi.org/10.3390/met14091090
Shin H, Oh S. Effects of CTAB (Cetyltrimethylammonium Bromide) and Betaine as Corrosion Inhibitors on the Galvanic Corrosion of Cu Coupled with Au on Print Circuit Board in Etching Solution. Metals. 2024; 14(9):1090. https://doi.org/10.3390/met14091090
Chicago/Turabian StyleShin, HeeKwon, and SeKwon Oh. 2024. "Effects of CTAB (Cetyltrimethylammonium Bromide) and Betaine as Corrosion Inhibitors on the Galvanic Corrosion of Cu Coupled with Au on Print Circuit Board in Etching Solution" Metals 14, no. 9: 1090. https://doi.org/10.3390/met14091090
APA StyleShin, H., & Oh, S. (2024). Effects of CTAB (Cetyltrimethylammonium Bromide) and Betaine as Corrosion Inhibitors on the Galvanic Corrosion of Cu Coupled with Au on Print Circuit Board in Etching Solution. Metals, 14(9), 1090. https://doi.org/10.3390/met14091090





