Influence of Ag Doping on Wide-Emperature Tribological Properties of γ-Fe2O3@SiO2 Nanocomposite Coatings on Steel
Abstract
1. Introduction
2. Experimental Details
2.1. Preparation of γ-Fe2O3@SiO2-Ag Nanocomposite Coatings
2.1.1. Synthesis of the Sol
2.1.2. The Preparation of the Nanocomposite Coatings
2.2. Characterization of the γ-Fe2O3@SiO2-Ag Nanocomposite
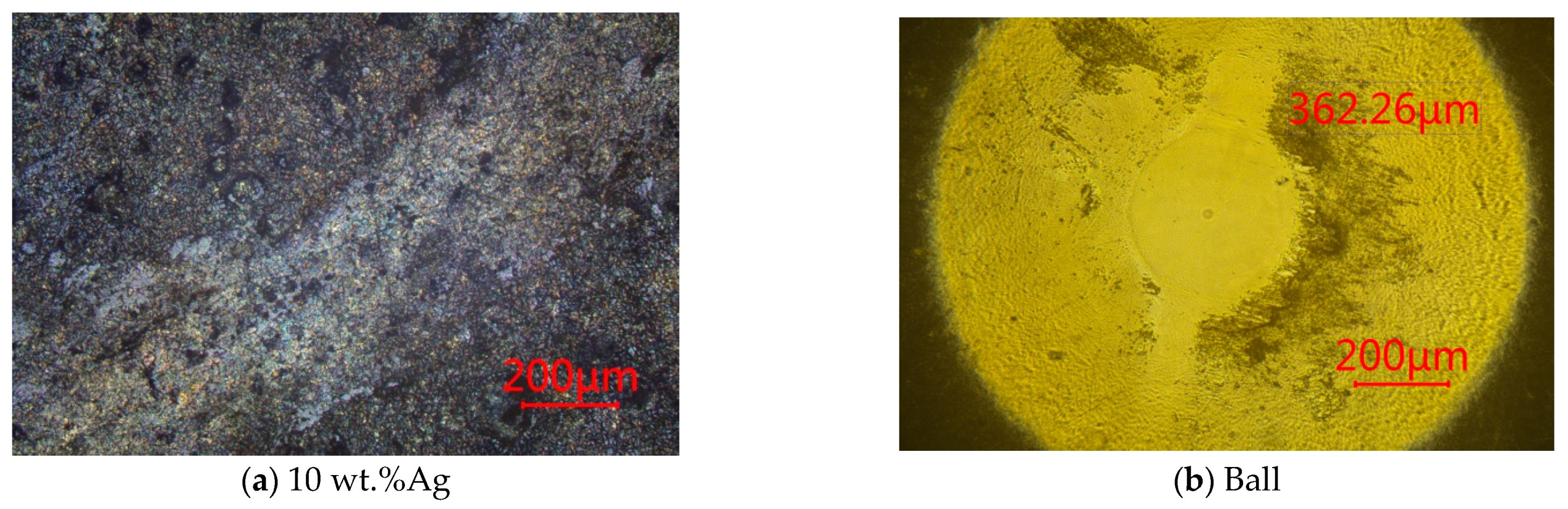
2.3. Tribotest of γ-Fe2O3@SiO2-Ag Nanocomposite Coatings
3. Results and Discussion
3.1. Microstructure of γ-Fe2O3@SiO2-Ag Nanocomposite Coatings
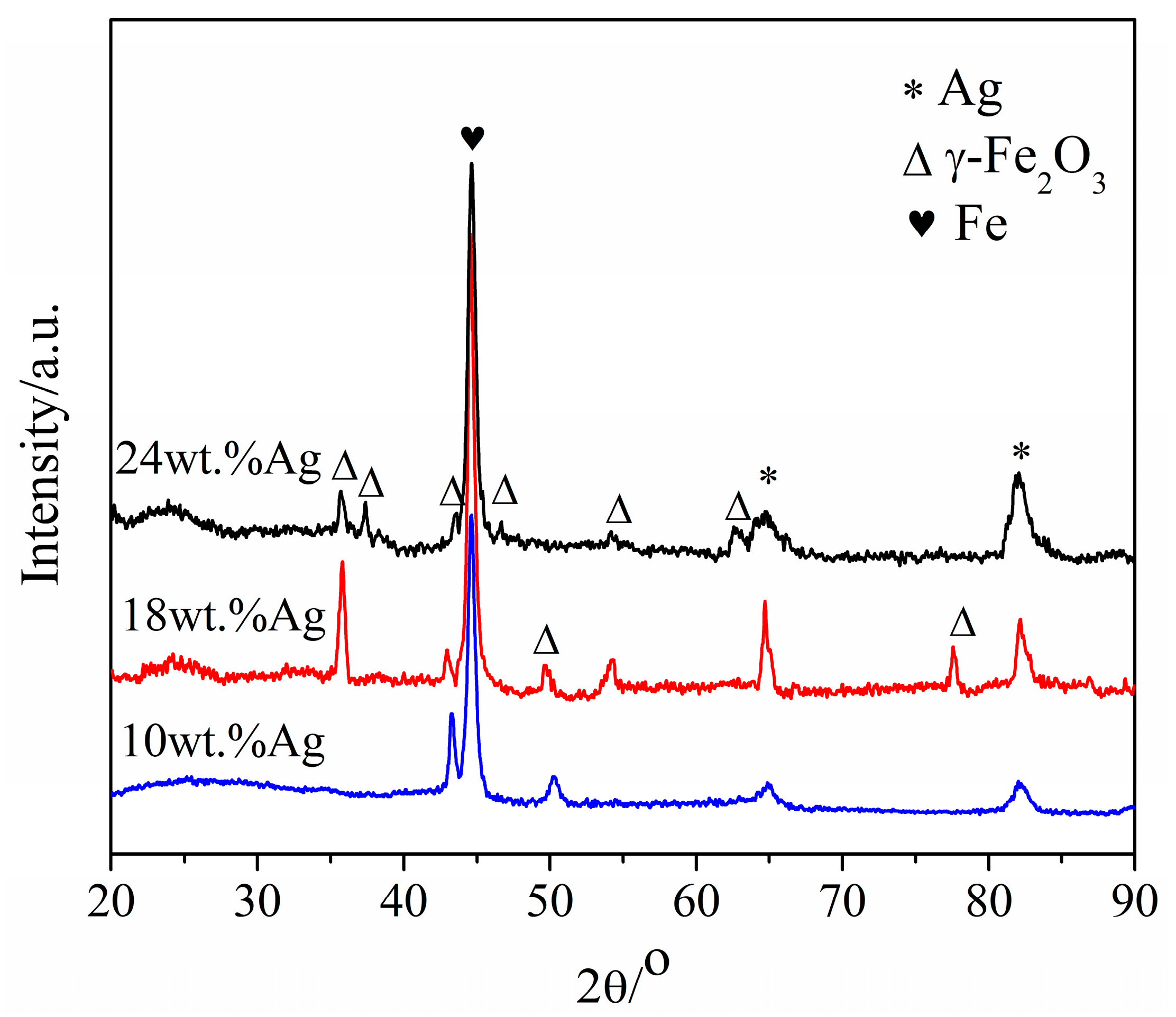
3.1.1. XRD Measurements
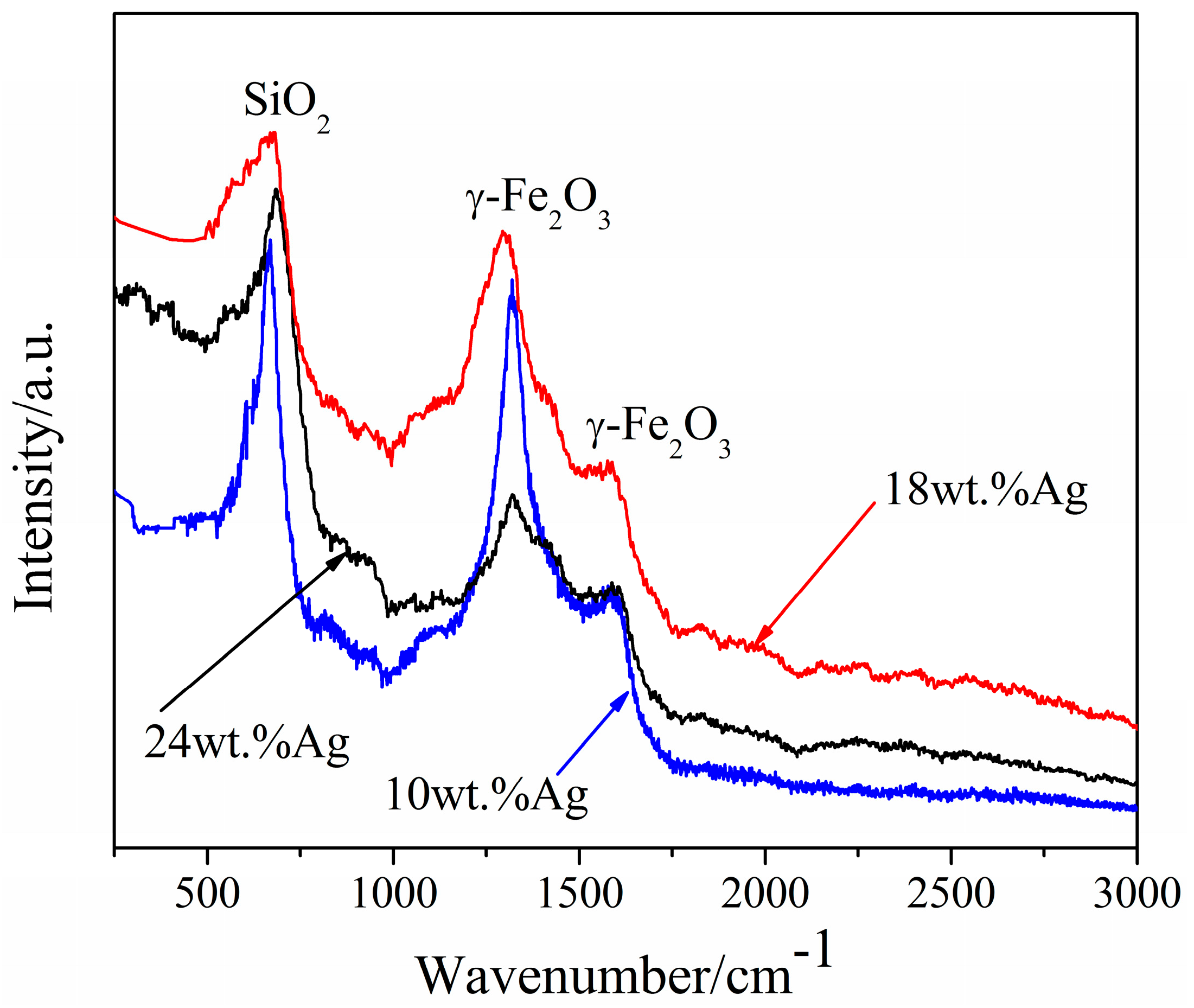
3.1.2. Raman Measurements
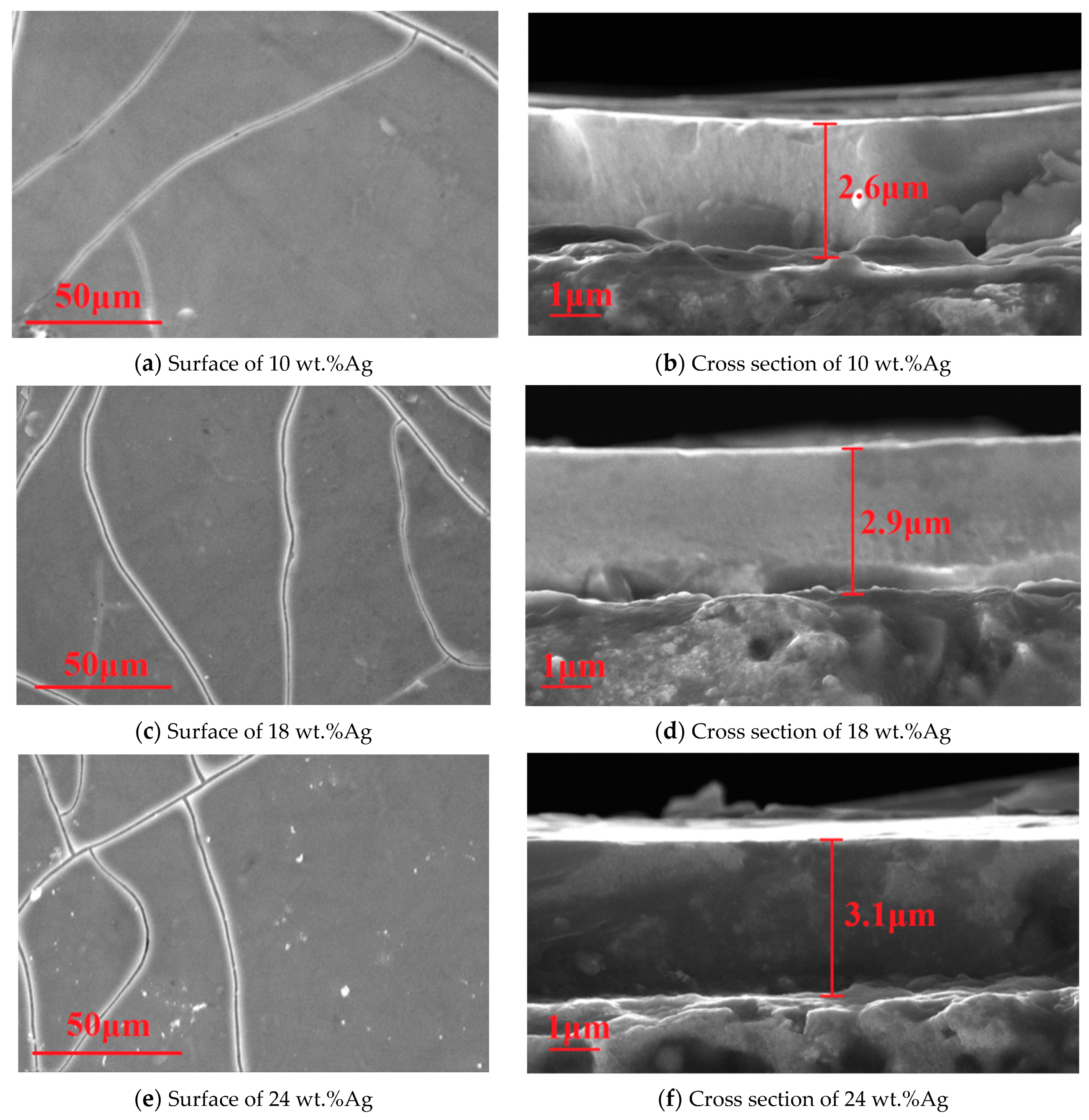
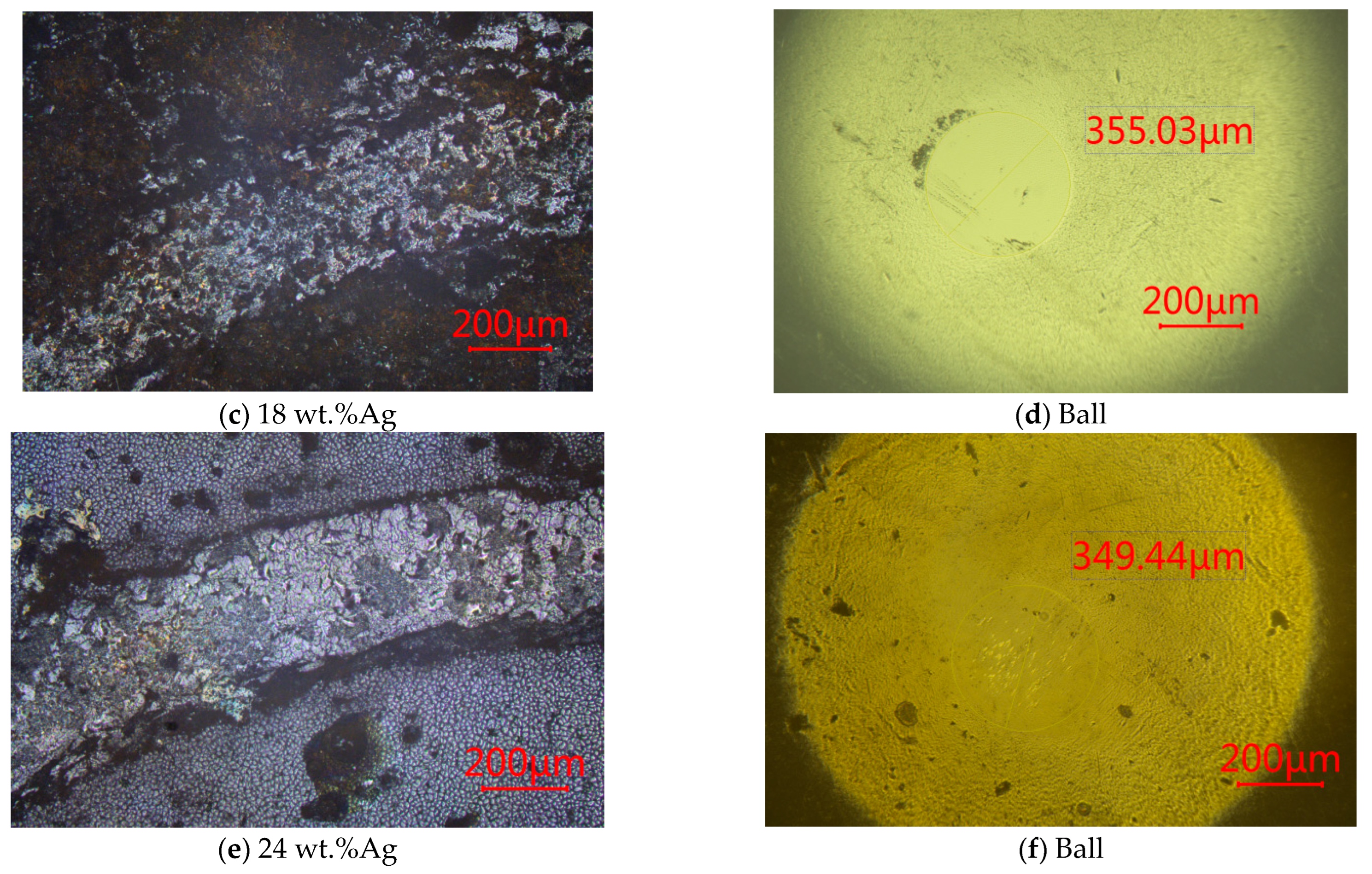
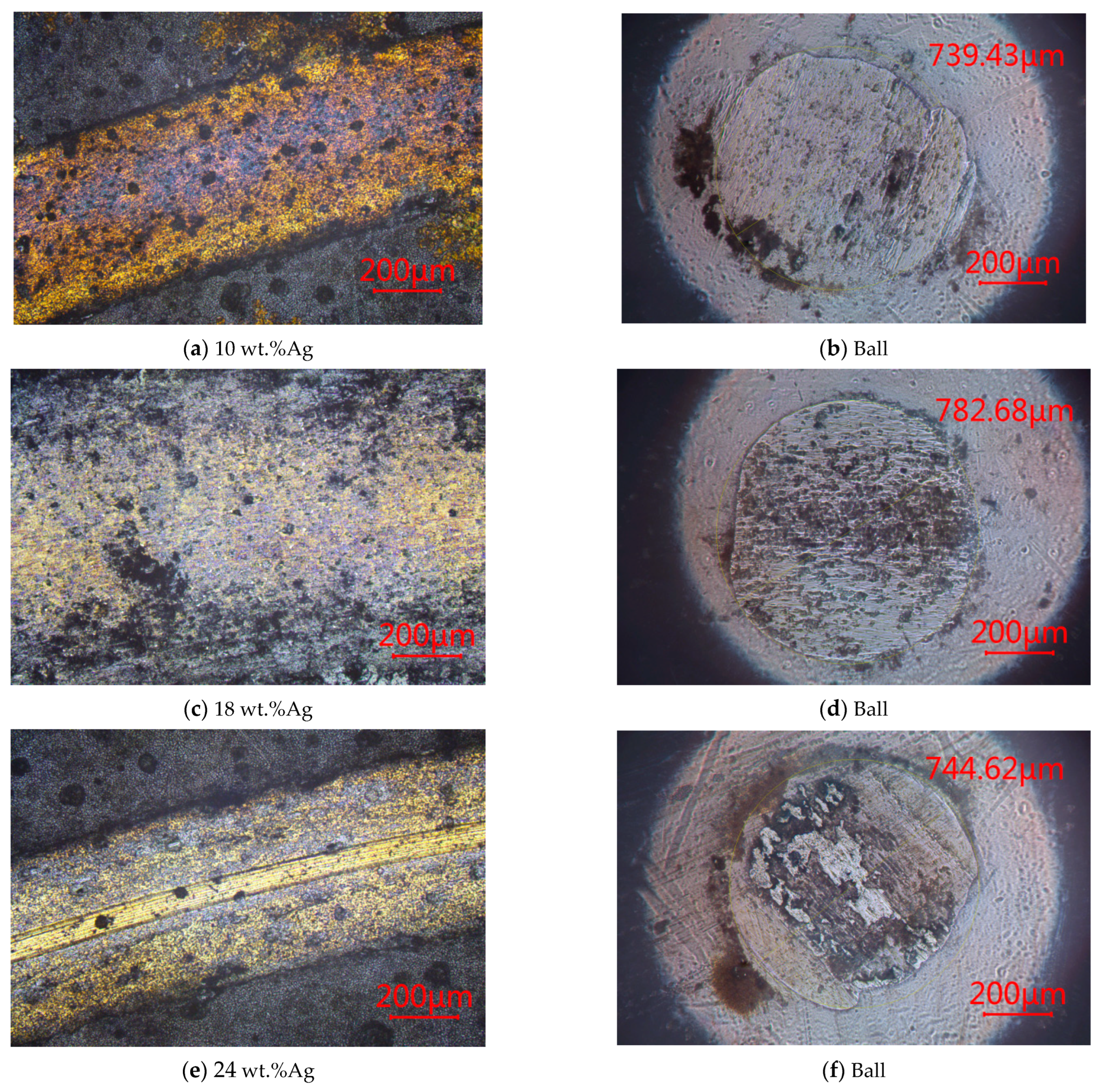
3.1.3. SEM
3.1.4. Hardness of γ-Fe2O3@SiO2-Ag Nanocomposite Coatings
3.2. Tribological Properties of γ-Fe2O3@SiO2-Ag Nanocomposite Coatings
3.2.1. Room Temperature
- CoF
- 2.
- Wear rate
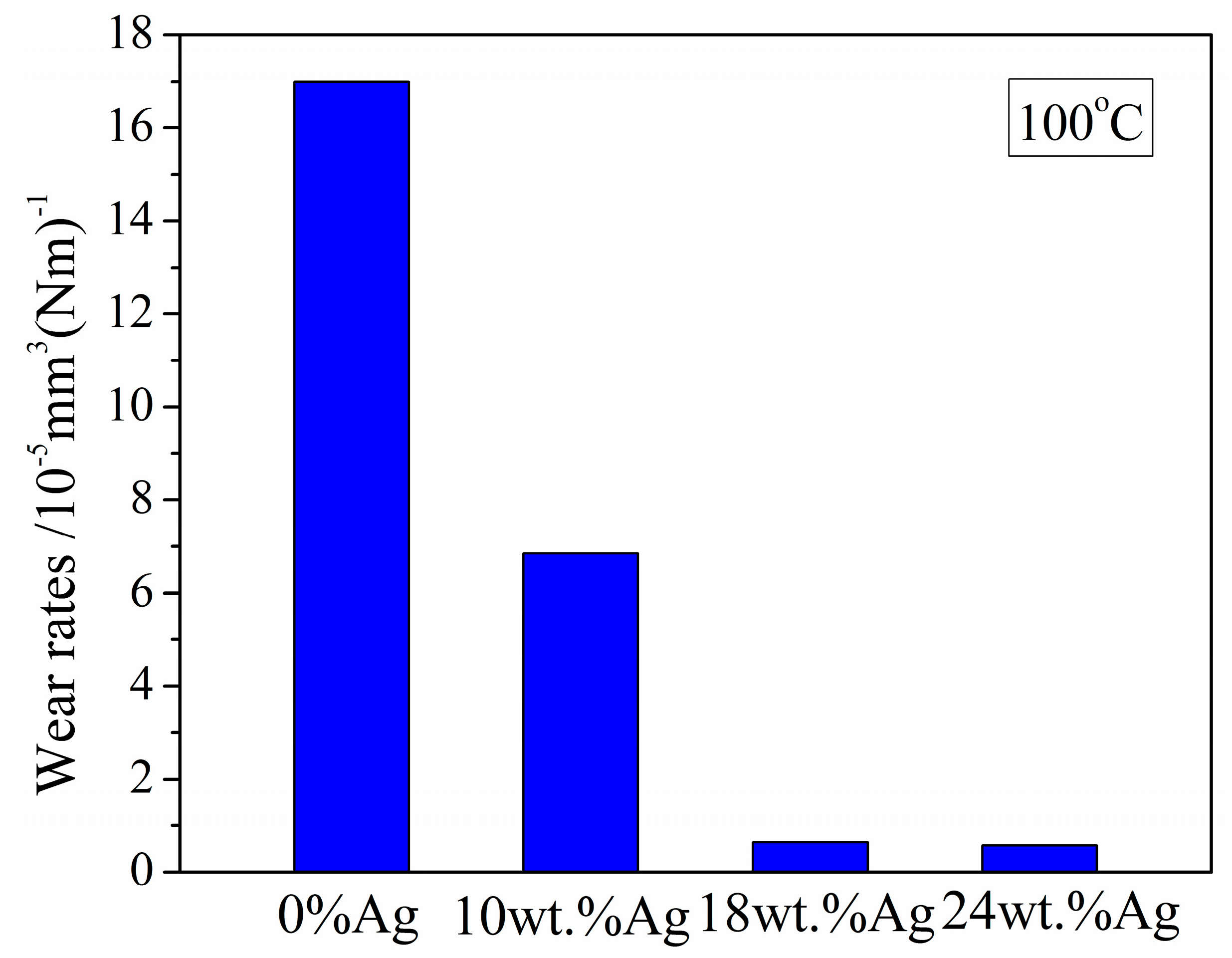
3.2.2. At 100 °C
- CoF
- 2.
- Wear rate
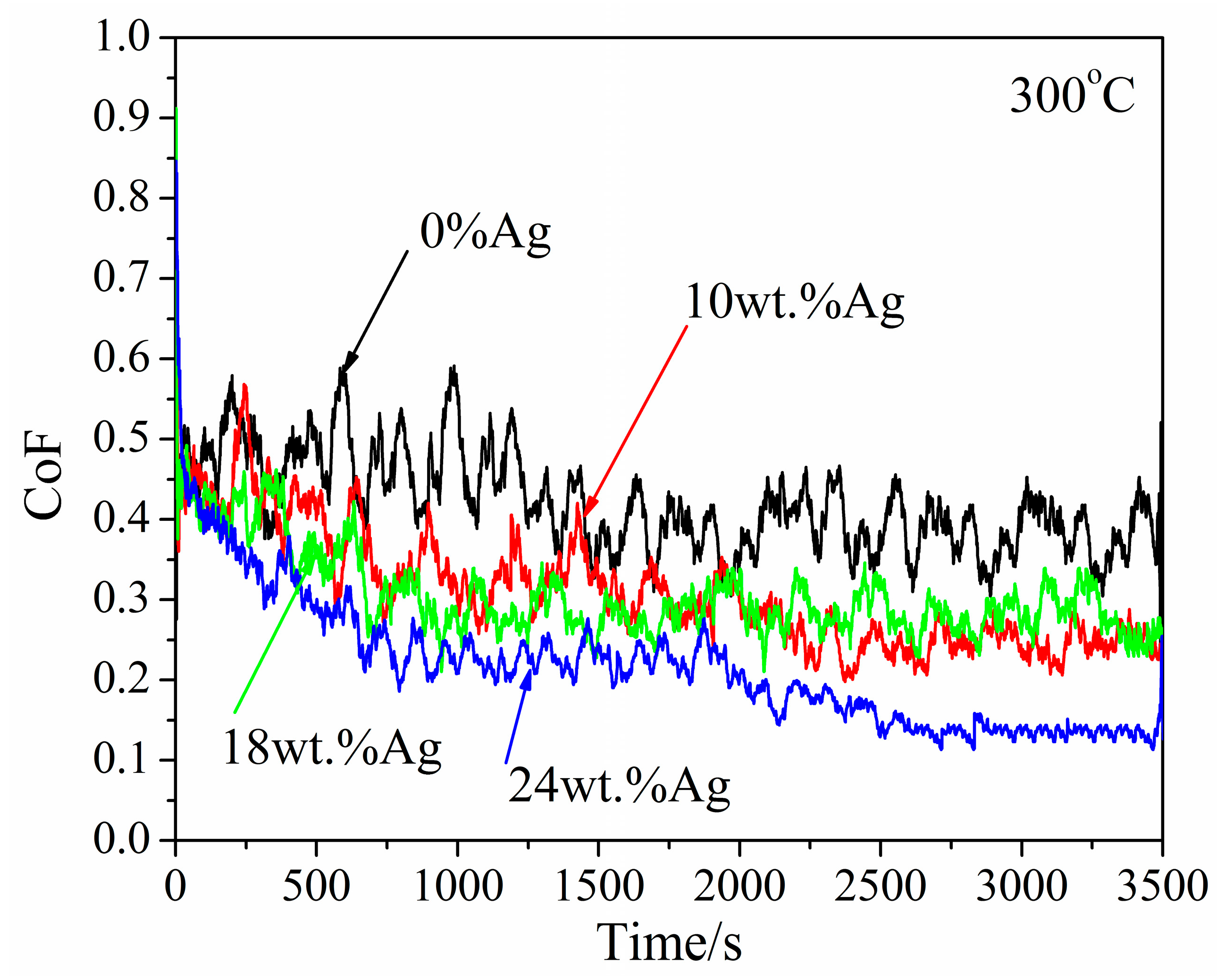
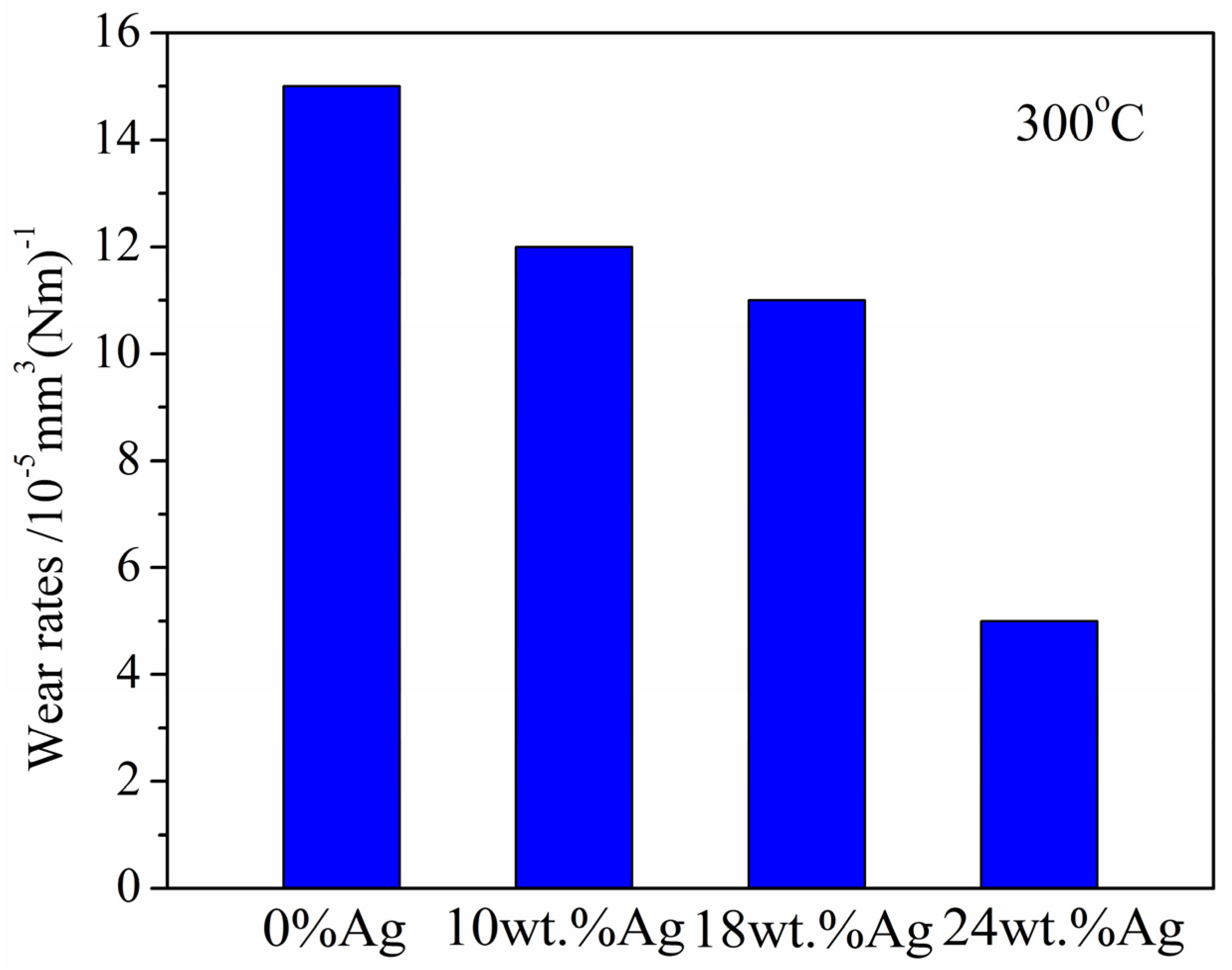
3.2.3. At 300 °C
- CoF
- 2.
- Wear rate
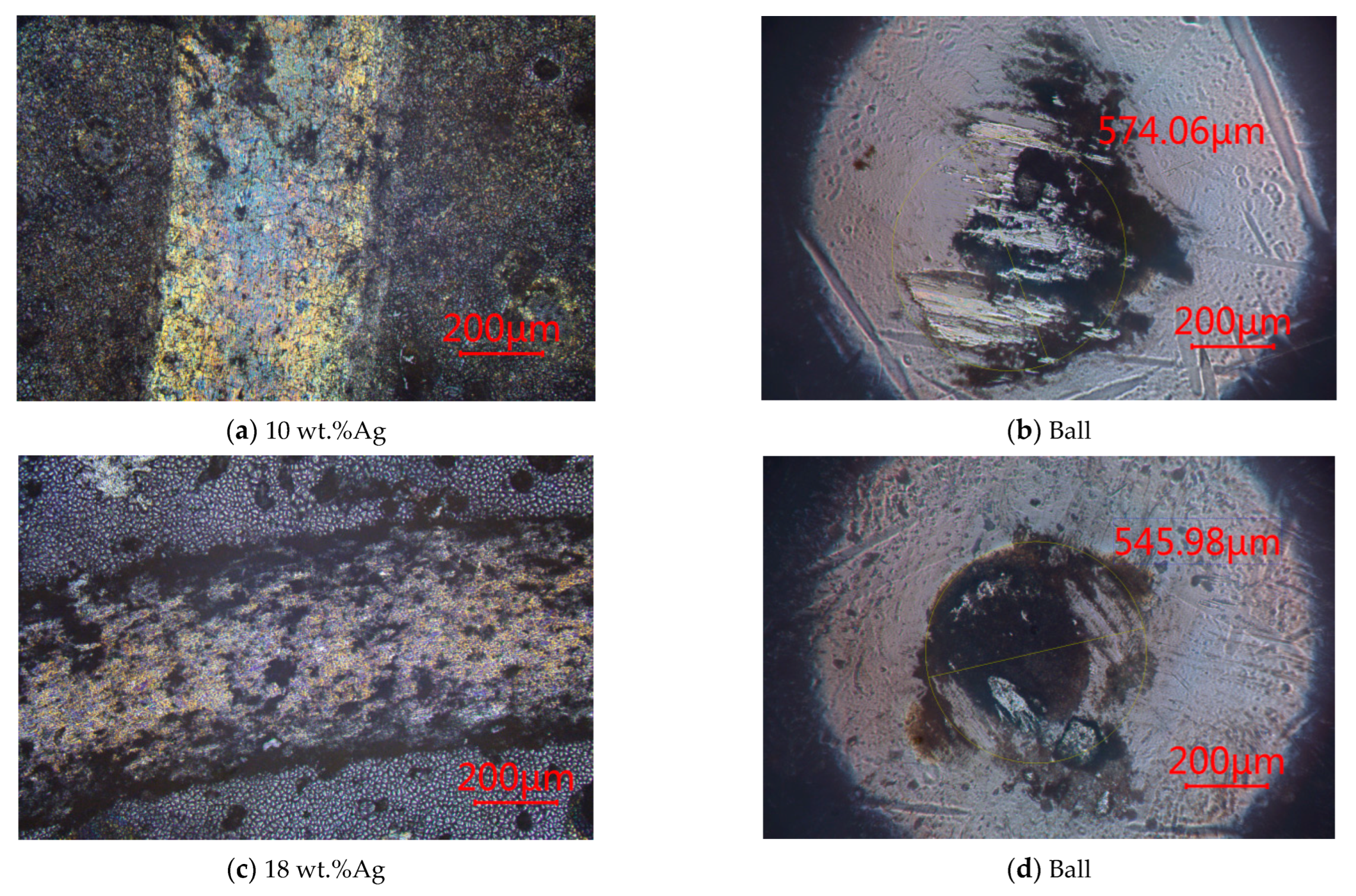
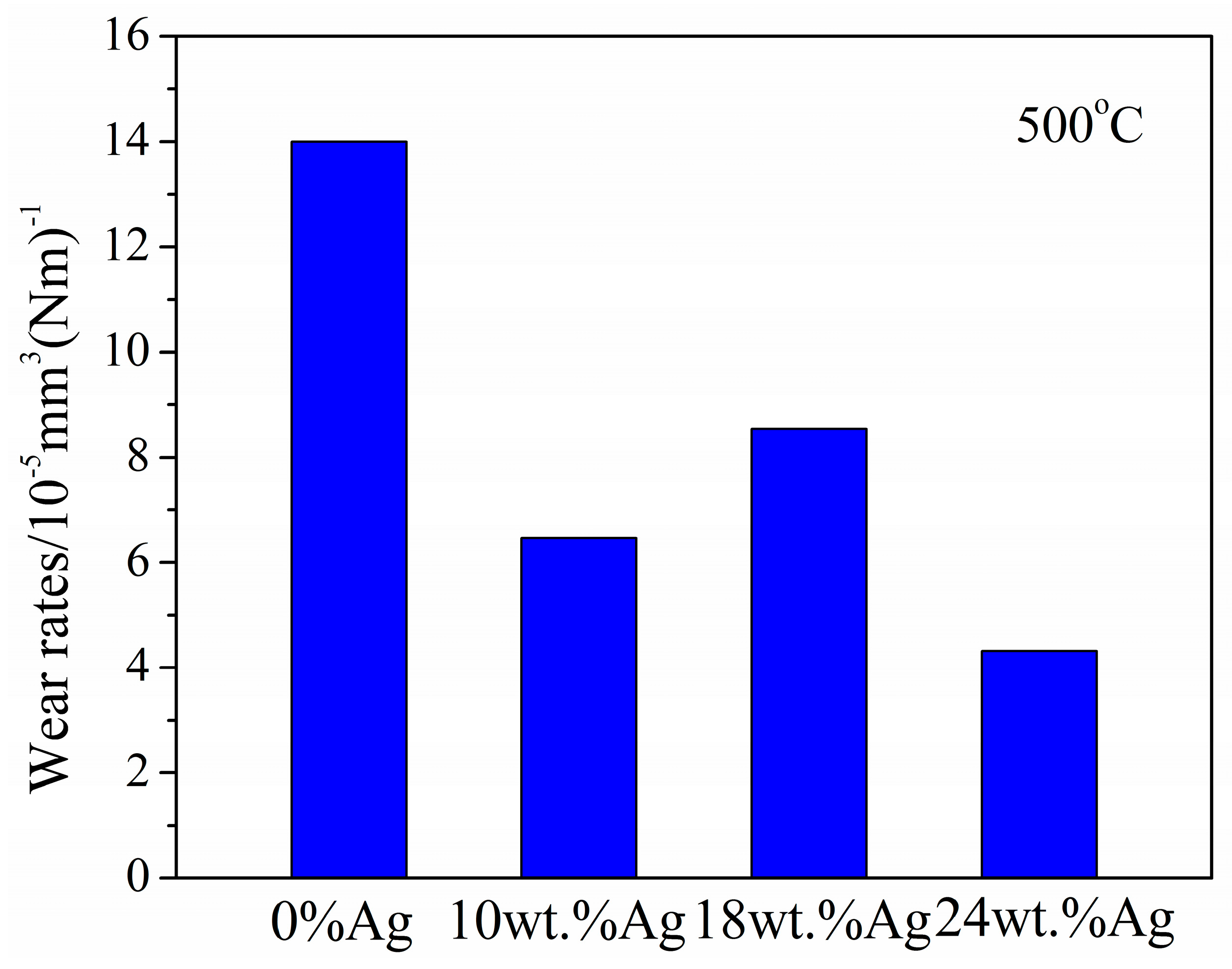
3.2.4. At 500 °C
- CoF
- 2.
- Wear rate
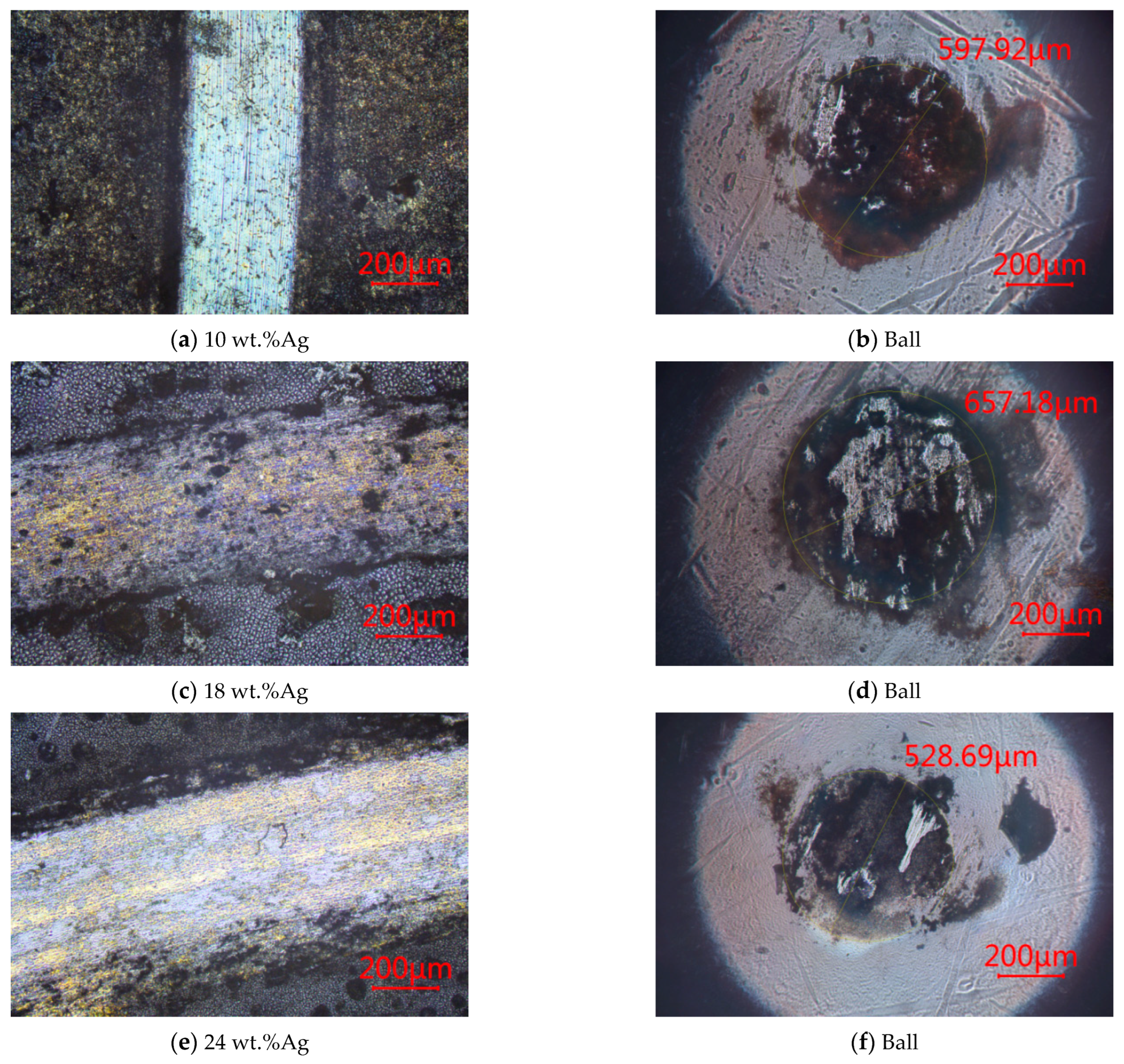
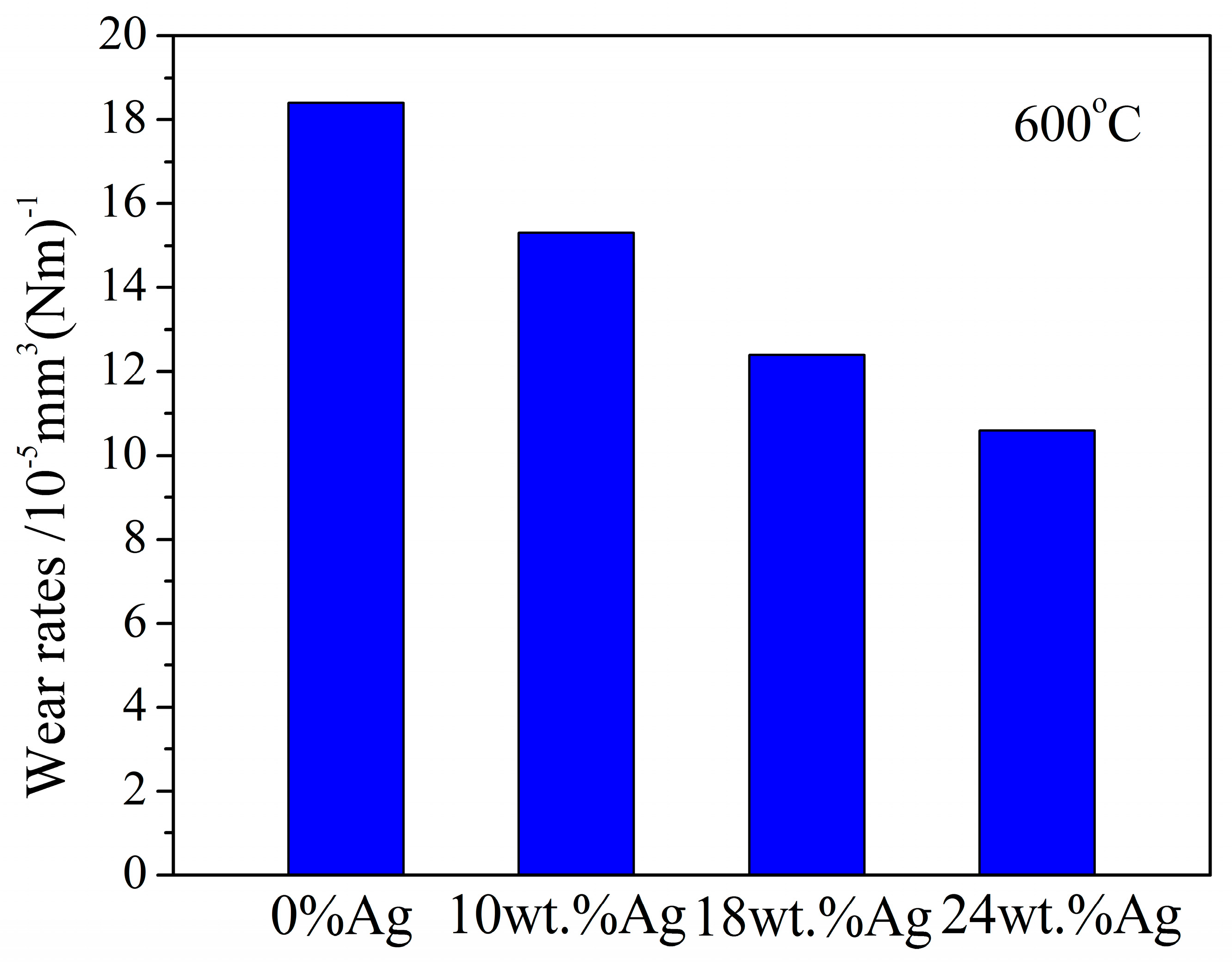
3.2.5. At 600 °C
- CoF
- 2.
- Wear rate
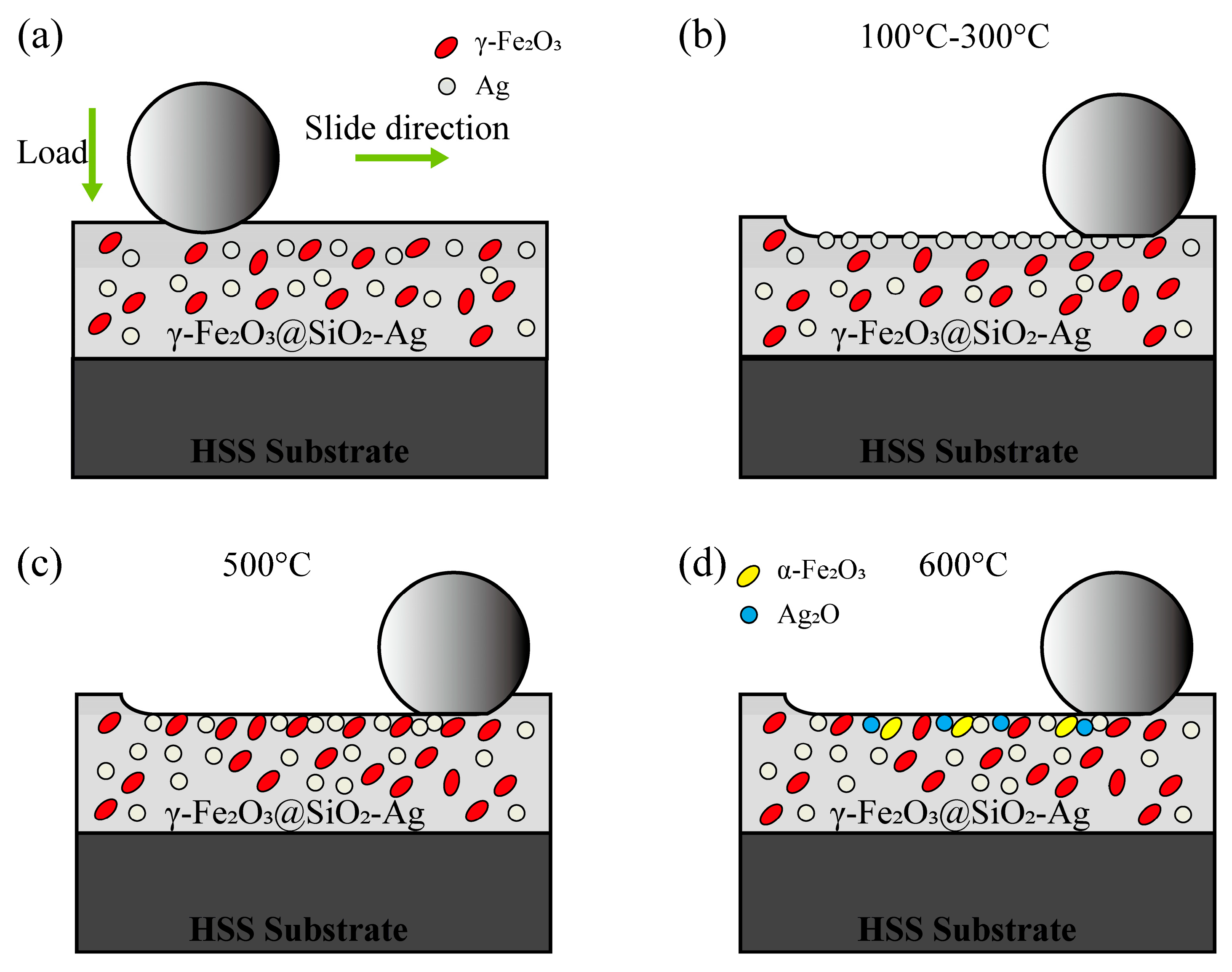
3.3. High-Temperature Lubrication Mechanism of the Nanocomposite Coatings
4. Conclusions
- (1)
- The microstructure and mechanical properties of γ-Fe2O3@SiO2-Ag nanocomposite coatings are influenced by the Ag content. There are holes in the nanocomposite coatings at the Ag content of 10 wt.% and 18 wt.%. The microstructure of the nanocomposite coatings is dense at the Ag content of 24 wt.%.
- (2)
- The CoF and wear rate of γ-Fe2O3@SiO2-Ag nanocomposite coatings decrease gradually with the increase in Ag content from RT to 300 °C.
- (3)
- At 500 °C, the lowest CoFs of 10 wt.%Ag, 18 wt.%Ag, and 24 wt.%Ag in the γ-Fe2O3@SiO2 nanocomposite coatings are 0.08, 0.05, and 0.06, respectively. At 600 °C, the CoFs of 10 wt.%Ag, 18 wt.%Ag, and 24 wt.%Ag in the γ-Fe2O3@SiO2 nanocomposite coatings decrease first and then increase, and the wear rate of the Ag-doped nanocomposite coatings is low compared with that of the nanocomposite coatings without Ag.
- (4)
- Ag plays a role in lubricating and reducing wear from RT to 300 °C. At 500 °C, γ-Fe2O3 in the γ-Fe2O3@SiO2 nanocomposite coatings becomes soft due to the friction heat and the environment temperature and is easy to shear and lubricate with Ag. The CoF of the nanocomposite coatings is as low as 0.1. At 600 °C, the tribological properties of the nanocomposite coatings become worse due to the oxidation of Ag and the phase transition of γ-Fe2O3 in the nanocomposite coatings.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouyang, J.-H.; Li, Y.-F.; Zhang, Y.-Z.; Wang, Y.-M.; Wang, Y.-J. High-temperature solid lubricants and self-lubricating composites: A critical review. Lubricants 2022, 10, 177. [Google Scholar] [CrossRef]
- Voevodin, A.; Zabinski, J. Nanocomposite and nanostructured tribological materials for space applications. Compos. Sci. Technol. 2005, 65, 741–748. [Google Scholar] [CrossRef]
- Erdemir, A.; Voevodin, A.A. Nanocomposite coatings for severe application. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 679–715. [Google Scholar] [CrossRef]
- Wang, J.Y.; Shan, Y.; Guo, H.; Li, B.; Wang, W.; Jia, J. Friction and wear characteristics of hot-pressed NiCr–Mo/MoO3/Ag self-lubrication composites at elevated temperatures up to 900 °C. Tribol. Lett. 2015, 59, 48. [Google Scholar] [CrossRef]
- Findik, F. Latest progress on tribological properties of industrial materials. Mater. Des. 2014, 57, 218–244. [Google Scholar] [CrossRef]
- Semenov, A. Tribology at high temperatures. Tribol. Inter. 1995, 28, 45–50. [Google Scholar] [CrossRef]
- Kasar, A.K.; Scalaro, K.; Menezes, P.L. Tribological properties of high-entropy alloys under dry conditions for a wide temperature range—A review. Materials 2021, 14, 5814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Cheng, J.; Qiao, Z.; Yang, J. High temperature solid-lubricating materials: A review. Tribol. Int. 2019, 133, 206–223. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, W. High temperature superlubricity behaviors achieved by AlSiN coatings against WS2 coatings at 600 °C. Ceram. Int. 2024, 50, 3787–3796. [Google Scholar] [CrossRef]
- Jin, B.; Zhao, J.; Chen, G.; He, Y.; Huang, Y.; Luo, J. In situ synthesis of Mn3O4/graphene nanocomposite and its application as a lubrication additive at high temperatures. Appl. Surf. Sci. 2021, 546, 149019. [Google Scholar] [CrossRef]
- Zeng, Q.; Cai, S. Low-friction behaviors of Ag-doped γ-Fe2O3 @SiO2 nanocomposite coatings under a wide range of temperature conditions. J. Sol-Gel Sci. Technol. 2019, 90, 271–280. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.; Su, F.; Li, Z.; Chen, Y.; Xu, Z. Microstructure and tribological performance of adaptive MoN–Ag nanocomposite coatings with various Ag contents. Wear 2022, 488, 204170. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Liu, Y.; Li, Q.; Xiao, S.; Su, F. Microstructure, mechanical and high-temperature tribological properties of MoS2 -Cr-Ag composite films. Surf. Coat. Technol. 2023, 452, 129135. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, J.; Shi, X.; Wang, C.; Lu, X.; Hao, J. Tribological properties and transfer behaviors of WS2-Ag nanocomposite films with structure evolution for aerospace application. Appl. Surf. Sci. 2024, 674, 160928. [Google Scholar] [CrossRef]
- Huang, K.; Cao, X.; Kong, L.; Lu, Z.; Zhang, G.; Ding, Q.; Hu, H. Effect of Ag content on friction and wear properties of Ag and V co-doped CrN coatings at 25–700 °C. Ceram. Int. 2021, 47, 35021–35028. [Google Scholar] [CrossRef]
- Patnaik, L.; Maity, S.R.; Kumar, S. Comprehensive structural, nanomechanical and tribological evaluation of silver doped DLC thin film coating with chromium interlayer (Ag-DLC/Cr) for biomedical application. Ceram. Int. 2020, 46, 22805–22818. [Google Scholar] [CrossRef]
- Zeng, Q. Thermal stability and high-temperature super low friction of γ-Fe2O3 @SiO2 nanocomposite coatings on steel. Lubricants 2024, 12, 223. [Google Scholar] [CrossRef]
- Zeng, Q.; Cai, S.; Li, S. High-temperature low-friction behaviors of γ-Fe2O3 @SiO2 nanocomposite coatings obtained through sol–gel method. J. Sol-Gel Sci. Technol. 2018, 85, 558–566. [Google Scholar] [CrossRef]
- Ju, H.; He, S.; Yu, L.; Asempah, I.; Xu, J. The improvement of oxidation resistance, mechanical and tribological properties of W2 N films by doping silicon. Surf. Coat. Technol. 2017, 317, 158–165. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Li, J.; Luo, J. Enhancement of friction performance enabled by a synergetic effect between graphene oxide and molybdenum disulfide. Carbon 2019, 154, 266–276. [Google Scholar] [CrossRef]
- Velasco, S.; Cavaleiro, A.; Carvalho, S. Functional properties of ceramic-Ag nanocomposite coatings produced by magnetron sputtering. Prog. Mater. Sci. 2016, 84, 158–191. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.; Ji, L.; Liu, X.; He, N.; Zhou, H.; Chen, J. Preparation and high-temperature tribological properties of CrAlVYN-Ag nanocomposite coatings. Mater. Manuf. Process. 2017, 32, 409–415. [Google Scholar] [CrossRef]
- Feng, X.; Lu, C.; Jia, J.; Xue, J.; Wang, Q.; Sun, Y.; Wang, W.; Yi, G. High temperature tribological behaviors and wear mechanisms of NiAl–NbC–Ag composites formed by in-situ decomposition of AgNbO3. Tribol. Int. 2020, 141, 105898. [Google Scholar] [CrossRef]
- Bondarev, A.; Golizadeh, M.; Shvyndina, N.; Shchetinin, I.; Shtansky, D. Microstructure, mechanical, and tribological properties of Ag-free and Ag-doped VCN coatings. Surf. Coat. Technol. 2017, 331, 77–84. [Google Scholar] [CrossRef]
- Vidiš, M.; Truchlý, M.; Izai, V.; Fiantok, T.; Rajninec, M.; Roch, T.; Satrapinskyy, L.; Haršáni, M.; Nagy, Š.; Turiničová, V.; et al. Mechanical and tribological properties of Ag/TiBx nanocomposite thin films with strong antibacterial effect prepared by magnetron co-sputtering. Coatings 2023, 13, 989. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Yuan, J.; Wang, Z.; Zhang, Y.; Ma, G.; Wang, A. Temperature-adaptive lubrication of Ag doped Cr2 AlC nanocomposite coatings. Wear 2024, 540, 205221. [Google Scholar] [CrossRef]
- Lozano, D.; Mercado-Solis, R.; Perez ATalamantes, J.; Morales, F.; Hernandez-Rodriguez, M. Tribological behaviour of cast hypereutectic Al–Si–Cu alloy subjected to sliding wear. Wear 2009, 267, 545–549. [Google Scholar] [CrossRef]
- Weibel, A.; Bouchet, R.; Boulc, F.; Knauth, P. The big problem of small particles: A comparison of methods for determination of particle size in nanocrystalline anatase powders. Chem. Mater. 2005, 17, 2378–2385. [Google Scholar] [CrossRef]








| Number | Reagent | Parameter | The Mass Content of Ag/% |
|---|---|---|---|
| TEOS | 5 mL | ||
| Fe(NO3)3·9H2O | 4.5283 g | ||
| 1 | 0.3847 g | 10 | |
| 2 | AgNO3 | 0.7692 g | 18 |
| 3 | 1.1539 g | 24 | |
| EtoH | 10 mL |
| Ag Content | Hardness (HV) |
|---|---|
| 10 wt.%Ag | 577 |
| 18 wt.%Ag | 493 |
| 24 wt.%Ag | 547 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Sun, S.; Jia, Q. Influence of Ag Doping on Wide-Emperature Tribological Properties of γ-Fe2O3@SiO2 Nanocomposite Coatings on Steel. Metals 2024, 14, 996. https://doi.org/10.3390/met14090996
Zeng Q, Sun S, Jia Q. Influence of Ag Doping on Wide-Emperature Tribological Properties of γ-Fe2O3@SiO2 Nanocomposite Coatings on Steel. Metals. 2024; 14(9):996. https://doi.org/10.3390/met14090996
Chicago/Turabian StyleZeng, Qunfeng, Shichuan Sun, and Qian Jia. 2024. "Influence of Ag Doping on Wide-Emperature Tribological Properties of γ-Fe2O3@SiO2 Nanocomposite Coatings on Steel" Metals 14, no. 9: 996. https://doi.org/10.3390/met14090996
APA StyleZeng, Q., Sun, S., & Jia, Q. (2024). Influence of Ag Doping on Wide-Emperature Tribological Properties of γ-Fe2O3@SiO2 Nanocomposite Coatings on Steel. Metals, 14(9), 996. https://doi.org/10.3390/met14090996







