Research Progress on Preparation, Microstructure, Properties, and Optimization of Ta and Its Compounds’ Coatings
Abstract
1. Introduction
2. Preparation Technology of Ta and Its Compounds Coating
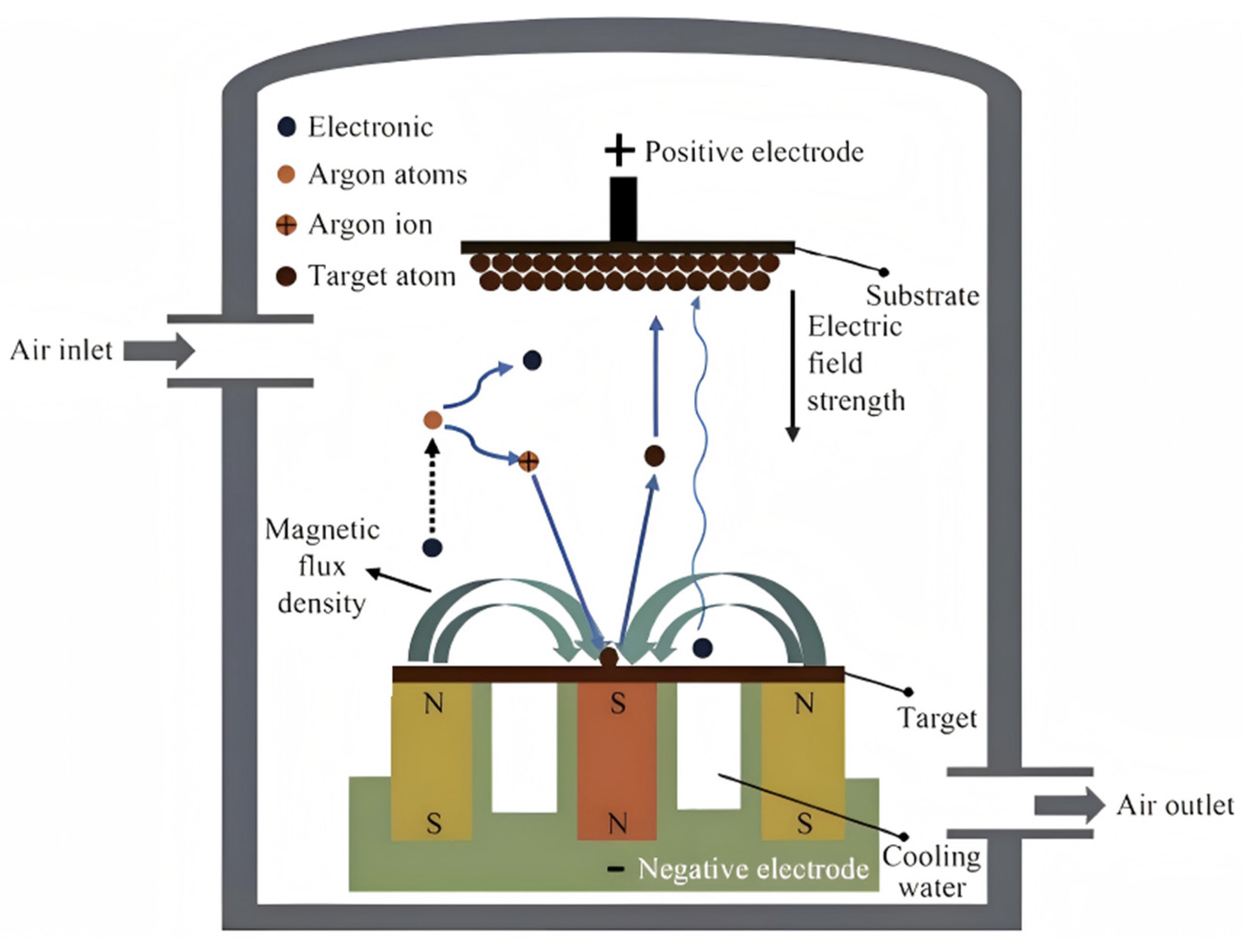
2.1. Physical Vapor Deposition
2.2. Chemical Vapor Deposition
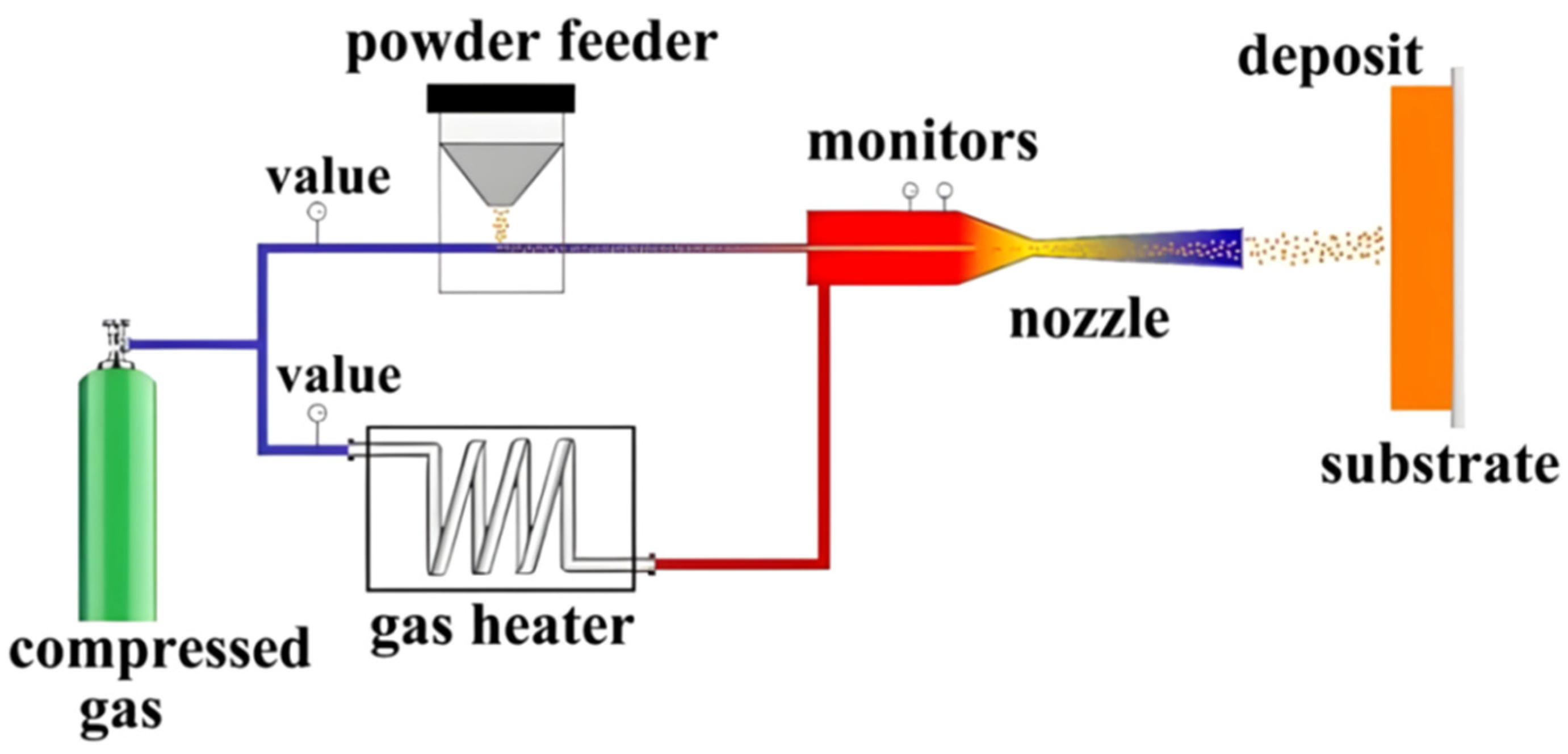
2.3. Cold Spraying
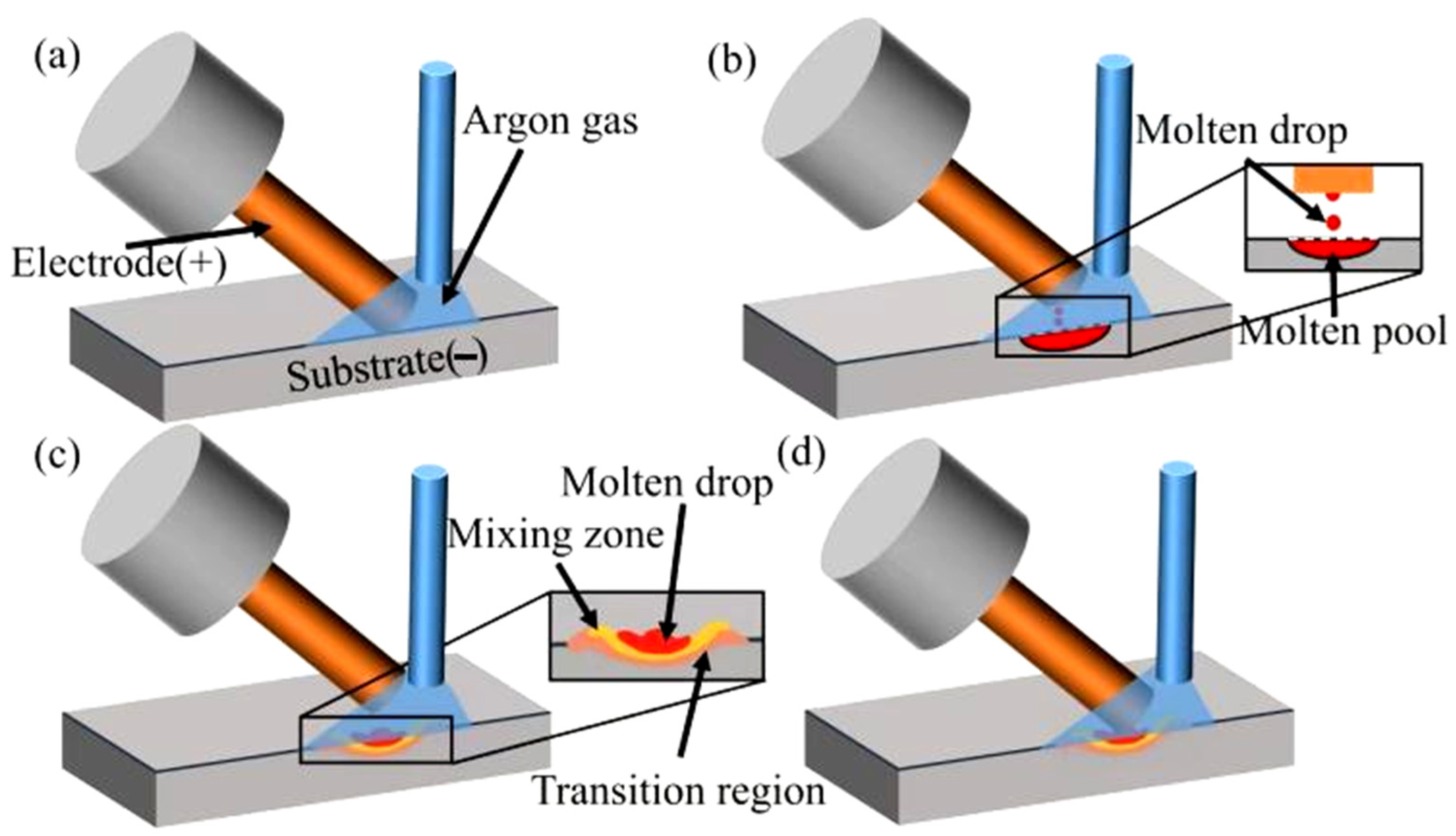
2.4. Electrospark Deposition
2.5. Other Coating Deposition Techniques
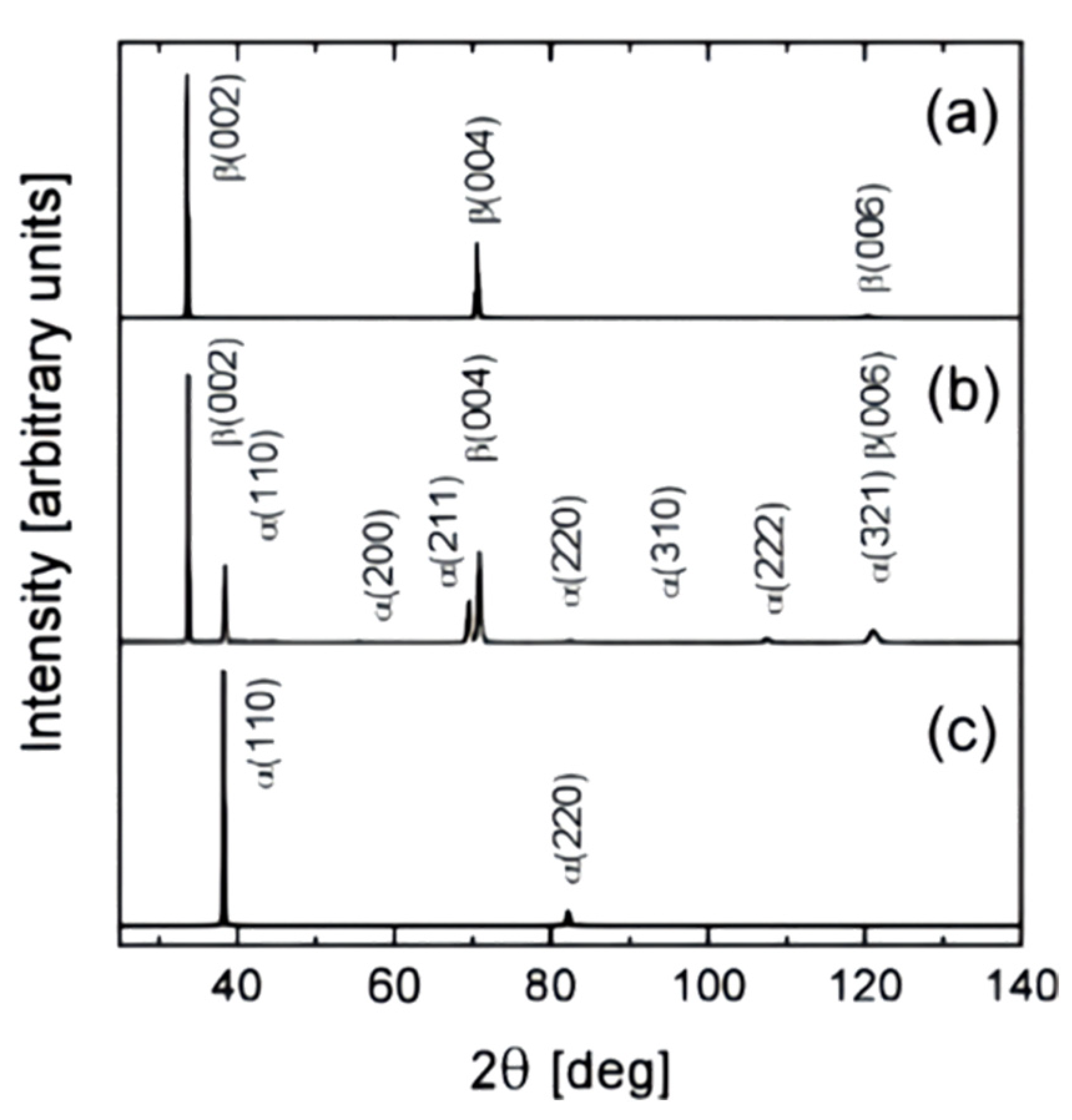
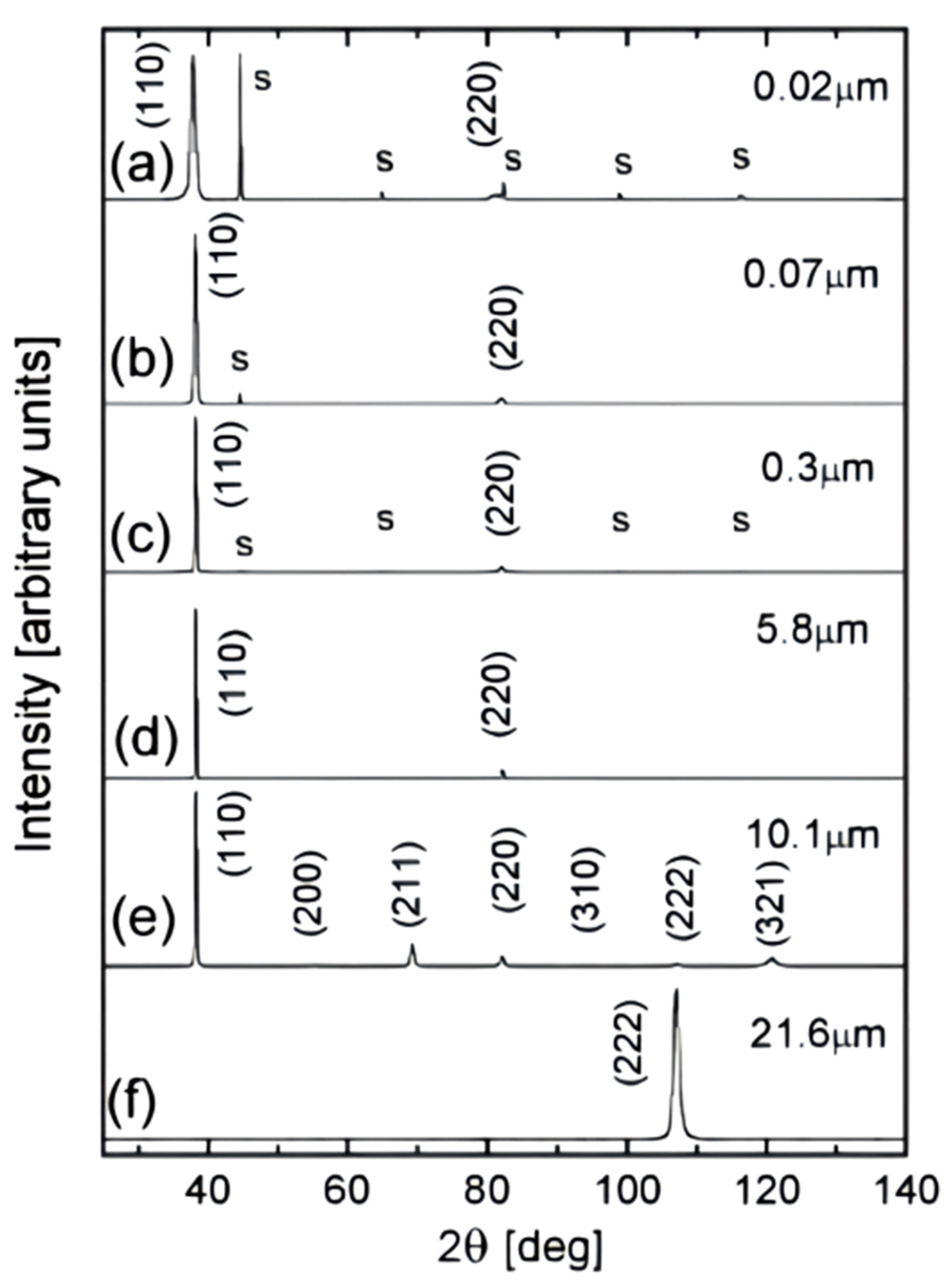
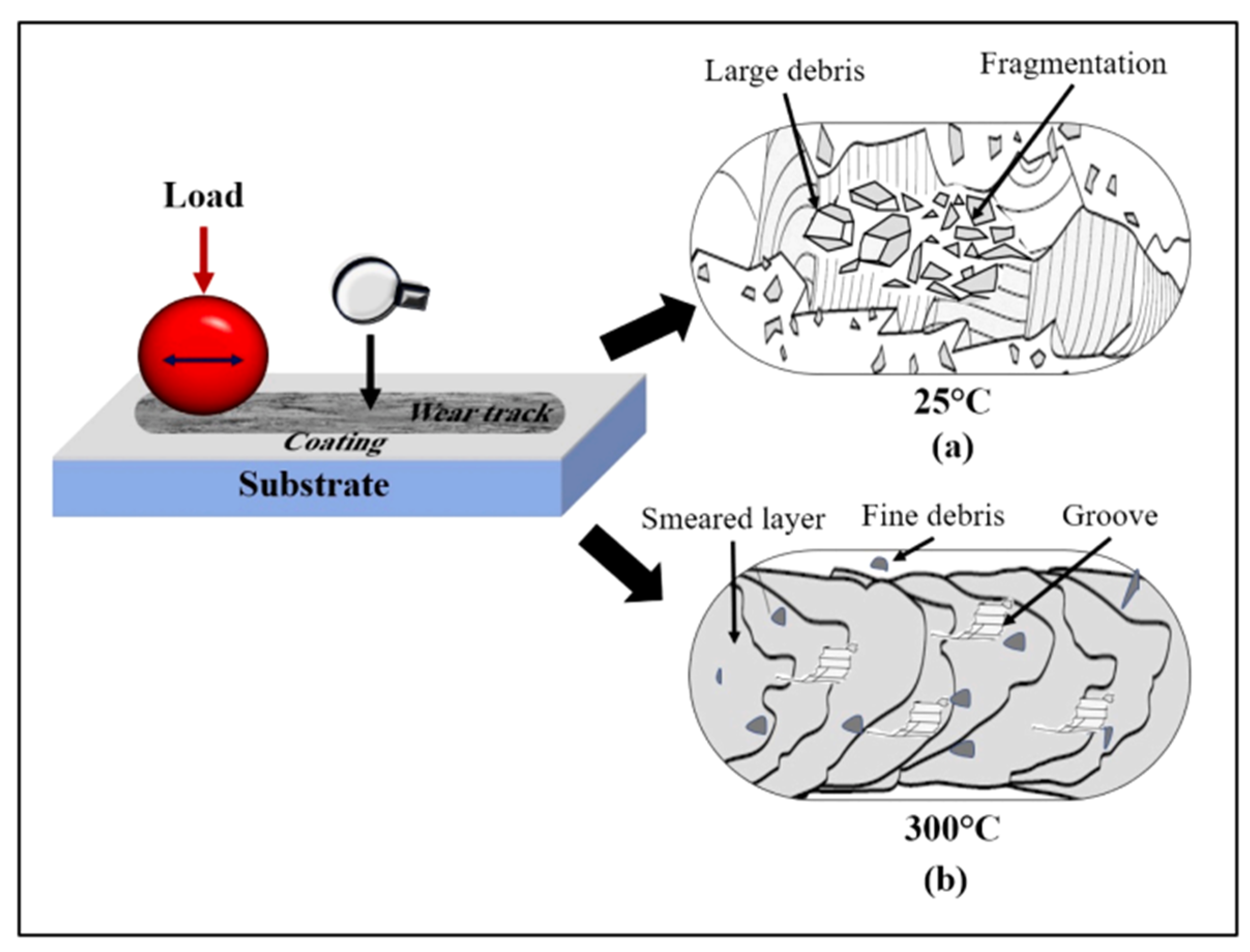
3. Microstructure and Properties of Ta Coatings
4. Microstructure and Properties of Ta Compound Coating
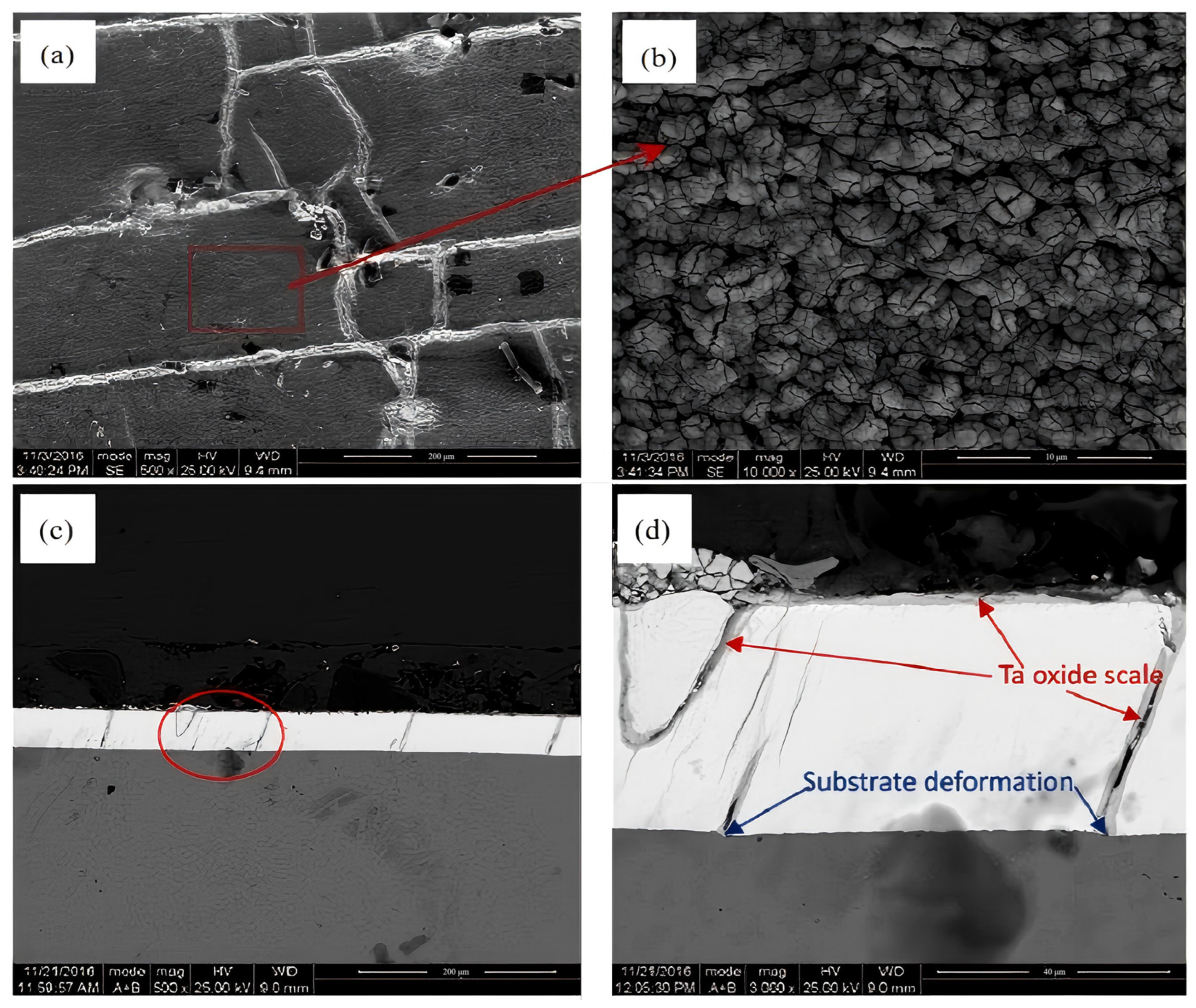
4.1. Ta Oxide Coating
4.2. Ta Carbide Coating
4.3. Ta Nitride Coating
5. Microstructure and Properties of Ta Alloy Coating
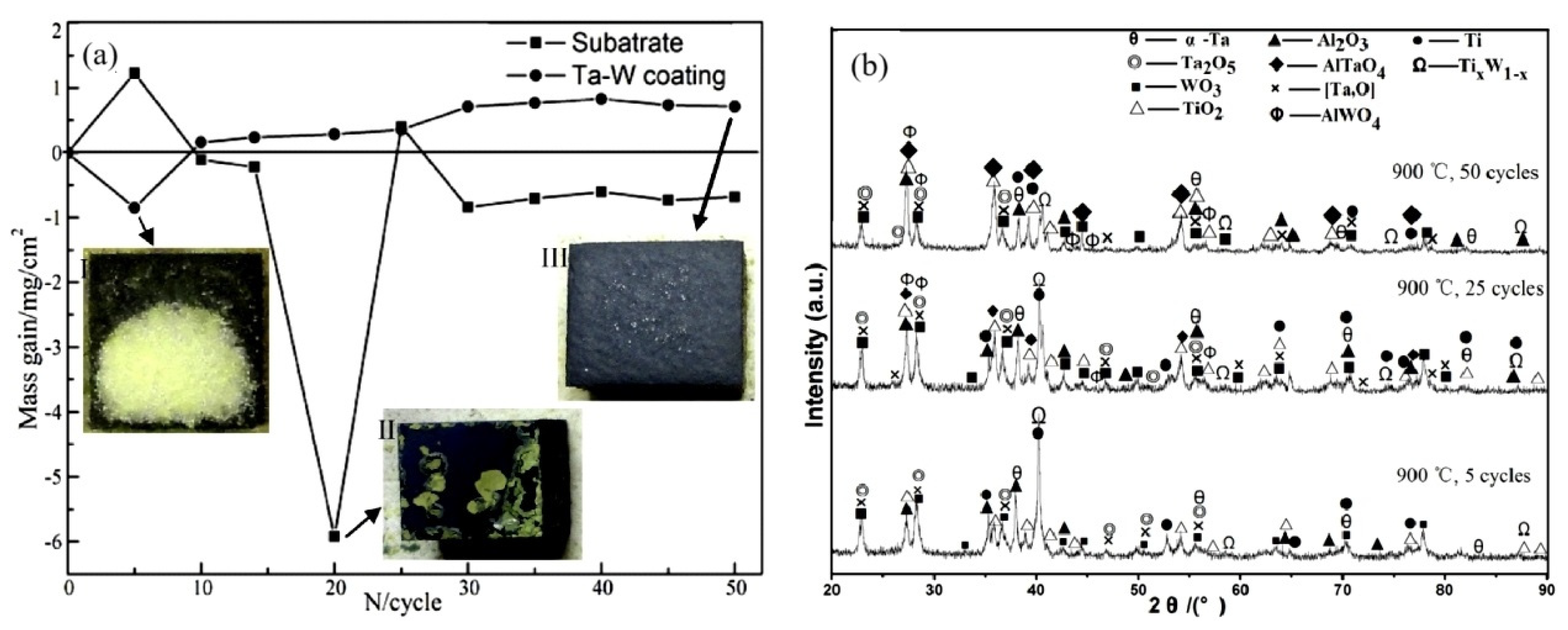
5.1. Ta-W Alloy Coating
5.2. Ta-Cr Alloy Coating
6. Ta and Its Compounds Coating Improvement Technique
6.1. Pre-Treatment
6.2. Buffer Layer
6.3. Annealing Treatment
7. Summary and Prospect
- Optimization of coating preparation process
- 2.
- Exploration of coating formation mechanism
- 3.
- Development of new coating types
- 4.
- Interdisciplinary application research of Ta and its compounds’ coatings
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhang, L.; Niu, H.; Bai, X.; Yu, S.; Ma, X.; Yu, Z. Deformation twinning and localized amorphization in nanocrystalline tantalum induced by sliding friction. Mater. Lett. 2014, 127, 4–7. [Google Scholar]
- Navid, A.A.; Hodge, A.M. Nanostructured alpha and beta tantalum formation-relationship between plasma parameters and microstructure. Mater. Sci. Eng. A 2012, 536, 49–56. [Google Scholar] [CrossRef]
- Byun, T.S.; Maloy, S.A. Dose dependence of mechanical properties in tantalum and tantalum alloys after low temperature irradiation. J. Nucl. Mater. 2008, 377, 72–79. [Google Scholar] [CrossRef]
- Kommel, L.; Shahreza, B.O.; Mikli, V. Microstructure and physical-mechanical properties evolution of pure tantalum processed with hard cyclic viscoplastic deformation. Int. J. Refract. Met. Hard Mater. 2019, 83, 104983. [Google Scholar] [CrossRef]
- Balaji, T.; Govindaiah, R.; Sharma, M.; Purushotham, Y.; Kumar, A.; Prakash, T. Sintering and electrical properties of tantalum anodes for capacitor applications. Mater. Lett. 2002, 56, 560–563. [Google Scholar] [CrossRef]
- Pu, J.; Zhang, J.; Zhang, S.; Liu, C.; Zhao, J.; Kang, J.; Yu, X.; Hong, N.; Li, Z.; Song, Y.; et al. Surface enlargement of tantalum capacitor foils by pulsed direct current etching and laser cladding. Surf. Coat. Technol. 2023, 467, 129693. [Google Scholar]
- Pickering, E.; Christofidou, K.; Stone, H.; Jones, N. On the design and feasibility of tantalum-base superalloys. J. Alloys Compd. 2019, 804, 314–321. [Google Scholar] [CrossRef]
- Sing, S.L.; Yeong, W.Y.; Wiria, F.E. Selective laser melting of titanium alloy with 50 wt% tantalum: Microstructure and mechanical properties. J. Alloys Compd. 2016, 660, 461–470. [Google Scholar]
- Koivuluoto, H.; Näkki, J.; Vuoristo, P. Corrosion properties of cold-sprayed tantalum coatings. J. Therm. Spray Technol. 2009, 18, 75–82. [Google Scholar]
- Wang, C.C.; Wang, F.; Han, Y. The structure, bond strength and apatite-inducing ability of micro-arc oxidized tantalum and their response to annealing. Appl. Surf. Sci. 2016, 361, 190–198. [Google Scholar] [CrossRef]
- Booth-Morrison, C.; Noebe, R.D.; Seidman, D.N. Effects of tantalum on the temporal evolution of a model Ni-Al-Cr superalloy during phase decomposition. Acta Mater. 2009, 57, 909–920. [Google Scholar]
- Niu, Y.; Chen, M.; Wang, J.; Yang, L.; Guo, C.; Zhu, S.; Wang, F. Preparation and thermal shock performance of thick α-Ta coatings by direct current magnetron sputtering (DCMS). Surf. Coat. Technol. 2017, 321, 19–25. [Google Scholar]
- Suh, Y.; Chen, W.; Maeng, S.; Gu, S.; Levy, R.; Thridandam, H. Synthesis and characterization of plasma assisted chemically vapor deposited tantalum. Thin Solid Films 2010, 518, 5452–5456. [Google Scholar]
- Arshad, A.; Yajid, M.A.M.; Daroonparvar, M.; Idris, M.H.; Zulkifli, I.S.M.; Hassan, A.G.; Halim, Z.A.A. Effect of Laser-Glazed Treatment on Thermal Cyclic Behavior of Plasma-Sprayed Lanthanum Zirconate/Yttria-Stabilized Zirconia Double Ceramic Layered on NiCoCrAlYTa-coated Inconel. J. Therm. Spray Technol. 2023, 32, 2603–2619. [Google Scholar]
- Zulkifli, I.S.M.; Yajid, M.A.M.; Idris, M.H.; Uday, M.B.; Daroonparvar, M.; Emadzadeh, A.; Arshad, A. Microstructural evaluation and thermal oxidation behaviors of YSZ/NiCoCrAlYTa coatings deposited by different thermal techniques. Ceram. Int. 2020, 46, 22438–22451. [Google Scholar]
- Maeng, S.M.; Axe, L.; Tyson, T.A.; Gladczuk, L.; Sosnowski, M. Corrosion behavior of magnetron sputtered α-Ta coatings on smooth and rough steel substrates. Surf. Coat. Technol. 2006, 200, 5717–5724. [Google Scholar]
- Maeng, S.; Axe, L.; Tyson, T.; Gladczuk, L.; Sosnowski, M. Corrosion behaviour of magnetron sputtered α- and β-Ta coatings on AISI 4340 steel as a function of coating thickness. Corros. Sci. 2006, 48, 2154–2171. [Google Scholar]
- Myers, S.; Lin, J.; Souza, R.M.; Sproul, W.D.; Moore, J.J. The β to α phase transition of tantalum coatings deposited by modulated pulsed power magnetron sputtering. Surf. Coat. Technol. 2013, 214, 38–45. [Google Scholar]
- Li, X.; Wang, L.; Yu, X.; Feng, Y.; Wang, C.; Yang, K.; Su, D. Tantalum coating on porous Ti6Al4V scaffold using chemical vapor deposition and preliminary biological evaluation. Mat. Sci. Eng. C-Mater. 2013, 33, 2987–2994. [Google Scholar]
- Monette, Z.; Kasar, A.K.; Daroonparvar, M.; Menezes, P.L. Supersonic particle deposition as an additive technology:methods, challenges, and applications. Int. J. Adv. Manuf. Technol. 2020, 106, 2079–2099. [Google Scholar]
- Daroonparvar, M.; Khan, M.F.; Saadeh, Y.; Kay, C.; Gupta, R.; Kasar, A.; Kumar, P.; Misra, M.; Menezes, P.L.; Bakhsheshi-Rad, H. Enhanced corrosion resistance and surface bioactivity of AZ31B Mg alloy by high pressure cold sprayed monolayer Ti and bilayer Ta/Ti coatings in simulated body fluid. Mater. Chem. Phys. 2020, 256, 123627. [Google Scholar]
- Su, Q.N. Research on Simulation of Droplet Transfer and Coating Formation Process in Electro-Spark Deposition. Master’s Thesis, Dalian Jiaotong University, Dalian, China, 2022. [Google Scholar]
- Trexler, M.D.; Carter, R.; de Rosset, W.S.; Gray, D.; Helfritch, D.J.; Champagne, V.K. Cold Spray Fabrication of Refractory Materials for Gun Barrel Liner Applications. Mate. Manuf. Process. 2012, 27, 820–824. [Google Scholar]
- Yang, M.L.; Xu, J.L.; Huang, J.; Zhang, L.W.; Luo, J.M. Wear Resistance of N-Doped CoCrFeNiMn High Entropy Alloy Coating on the Ti-6Al-4V Alloy. J. Therm. Spray Technol. 2024, 33, 2408–2418. [Google Scholar]
- Bai, M.; Liu, T.; Liu, B.; Li, Y.; Yu, H.; Zhao, Y.; Yang, C.; Song, L.; Liu, W. Preparation and properties of polyurethane cold galvanizing coatings withphosphoric acid modified zinc powder. Surf. Coat. Technol. 2024, 489, 131128. [Google Scholar]
- Tian, J.; Zhu, J.; Zheng, J.; Li, Y.; Yang, K.; Li, M.; Wang, H.; He, J. Thermal protection mechanism of novel high-entropy rare-earth niobate coating deposited by atmospheric plasma spraying. Appl. Surf. Sci. 2025, 688, 162315. [Google Scholar]
- Wang, C.; Wang, F.; Han, Y. Structural characteristics and outward-inward growth behavior of tantalum oxide coatings on tantalum by micro-arc oxidation. Surf. Coating. Technol. 2013, 214, 110–116. [Google Scholar]
- Carette, L.; Jacquet, P.; Cotton, D.; Vignal, V.; Faure, S. (TaC/Ta2C) bilayer formed on carburized and annealed tantalum: Development of a numerical growth model. Appl. Surf. Sci. 2019, 467, 84–88. [Google Scholar]
- Cotton, D.; Jacquet, P.; Faure, S.; Vignal, V. Epitaxial growth of tantalum carbides by low carbon flow carburizing. Mater. Chem. Phys. 2017, 192, 170–180. [Google Scholar]
- Xing, Y.-Z.; Jiang, C.-P.; Hao, J.-M. Time dependence of microstructure and hardness in plasma carbonized Tie6Ale4V alloys. Vacuum 2013, 95, 12–17. [Google Scholar]
- Zhao, Z.; Hui, P.; Liu, F.; Wang, X.; Li, B.; Xu, Y.; Zhong, L.; Zhao, M. Fabrication of TaC coating on tantalum by interstitial carburization. J. Alloys Compd. 2019, 790, 189–196. [Google Scholar]
- Balani, K.; Gonzalez, G.; Agarwal, A.; Hickman, R.; O’Dell, J.S.; Seal, S. Synthesis, microstructural characterization, and mechanical property evaluation of vacuum plasma sprayed tantalum carbide. J. Am. Ceram. Soc. 2010, 89, 1419–1425. [Google Scholar] [CrossRef]
- Firouzabadi, S.; Dehghani, K.; Naderi, M.; Mahboubi, F. Numerical investigation of sputtering power effect on nano-tribological properties of tantalum-nitride film using molecular dynamics simulation. Appl. Surf. Sci. 2016, 367, 197–204. [Google Scholar] [CrossRef]
- Firouzabadi, S.; Naderi, M.; Dehghani, K.; Mahboubi, F. Effect of nitrogen flow ratio on nano-mechanical properties of tantalum nitride thin film. J. Alloys Compd. 2017, 719, 63–70. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y. Valence Bond Structure of Ta-W Alloys. Acta Metall. Sin. 2009, 22, 275–283. [Google Scholar] [CrossRef]
- Pérez-Prado, M.; Hines, J.; Vecchio, K. Microstructural Evolution in Adiabatic Shear Bands in Ta and Ta-W Alloys. Acta Mater. 2001, 49, 2905–2917. [Google Scholar] [CrossRef]
- Zhai, Y.; Yi, G.U.; Huang, H. Study on Interface Reactions of Ta-10W Coatings and Titanium Substrate at High Temperature. Mater. Rev. 2016, 30, 103–107. [Google Scholar]
- Lee, Y.-J.; Lee, T.-H.; Nersisyan, H.H.; Lee, K.-H.; Jeong, S.-U.; Kang, K.-S.; Bae, K.-K.; Park, K.-T.; Lee, J.-H. Characterization of ta–W alloy films deposited by molten salt multi-anode reactive alloy coating (MARC) method. Int. J. Refract. Met. Hard Mater. 2015, 53, 23–31. [Google Scholar] [CrossRef]
- Peng, X.; Xia, C.; Zhou, L.; Huang, L.; Li, D.; Wu, A. Study of arc ion plated Ta-W coating on a titanium alloy. Surf. Coating. Technol. 2018, 349, 622–635. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, L.; Yuan, J.; Chen, X.; Zhang, D. Microstructure, wear behaviour and laser ablation behaviour of double glow plasma alloyed Ta–W coating. Int. J. Refract. Met. Hard Mater. 2024, 123, 106764. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Daneshvar, H.; Safavi, M.S.; Khalili, V. A survey on crystallization kinetic behavior of direct current magnetron sputter deposited NiTi thin films. Phys. B 2021, 615, 413086. [Google Scholar] [CrossRef]
- Safavi, M.S.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. RF-magnetron sputter deposited hydroxyapatite-based composite & multilayer coatings: A systematic review from mechanical, corrosion, and biological points of view. Ceram. Int. 2021, 47, 3031–3053. [Google Scholar]
- Sarraf, M.; Dabbagh, A.; Razak, B.A.; Nasiri-Tabrizi, B.; Hosseini, H.R.M.; Saber-Samandari, S.; Abu Kasim, N.H.; Yean, L.K.; Sukiman, N.L. Silver oxide nanoparticles-decorated tantala nanotubes for enhanced antibacterial activity and osseointegration of Ti6Al4V. Mater. Des. 2018, 154, 28–40. [Google Scholar] [CrossRef]
- Colin, J.J.; Abadias, G.; Michel, A.; Jaouen, C. On the origin of the metastable β-ta phase stabilization in tantalum sputtered thin films. Acta Mater. 2017, 126, 481–493. [Google Scholar] [CrossRef]
- Knepper, R.; Stevens, B.; Baker, S.P. Effect of oxygen on the thermomechanical behavior of tantalum thin films during the β–α phase transformation. J. Appl. Phys. 2006, 100, 123508–123511. [Google Scholar] [CrossRef]
- Gladczuk, L.; Patel, A.; Paur, C.S.; Sosnowski, M. Tantalum films for protective coatings of steel. Thin Solid Films 2004, 467, 150–157. [Google Scholar] [CrossRef]
- Alami, J.; Eklund, P.; Andersson, J.; Lattemann, M.; Wallin, E.; Bohlmark, J.; Persson, P.; Helmersson, U. Phase tailoring of Ta thin films by highly ionized pulsed magnetron sputtering. Thin Solid Films 2007, 515, 3434–3438. [Google Scholar] [CrossRef]
- Lin, J.; Moore, J.J.; Sproul, W.D.; Lee, S.L.; Wang, J. Effect of negative substrate bias on the structure and properties of Ta coatings deposited using modulated pulse power magnetron sputtering. IEEE Trans. Plasma Sci. 2010, 38, 3071–3078. [Google Scholar] [CrossRef]
- Hallmann, L.; Ulmer, P. Effect of sputtering parameters and substrate composition on the structure of tantalum thin films. Appl. Surf. Sci. 2013, 282, 1–6. [Google Scholar] [CrossRef]
- Matson, D.W.; McClanahan, E.D.; Lee, S.L.; Windover, D. Properties of thick sputtered Ta used for protective gun tube coatings. Surf. Coat. Technol. 2001, 146–147, 344–350. [Google Scholar] [CrossRef]
- Ren, H.; Sosnowski, M. Tantalum thin films deposited by ion assisted magnetron sputtering. Thin Solid Films 2008, 516, 1898–1905. [Google Scholar] [CrossRef]
- Yu, X.; Tan, L.; Yang, H.; Yang, K. Surface Characterization and Preparation of Ta Coating on Ti6Al4V Alloy. J. Alloys Compd. 2015, 644, 698–703. [Google Scholar] [CrossRef]
- Stoltenhoff, T.; Kreye, H.; Richter, H. An analysis of the cold spray process and its coatings. J. Therm. Spray. Technol. 2002, 11, 542–550. [Google Scholar]
- Kosarev, V.F.; Klinkov, S.V.; Alkhimov, A.P.; Papyrin, A.N. On some aspects of gas dynamics of the cold spray process. J. Therm. Spray. Technol. 2003, 12, 265–281. [Google Scholar]
- Grujicic, M.; Zhao, C.; Tong, C.; DeRosset, W.; Helfritch, D. Analysis of the impact velocity of powder particles in the cold-gas dynamic-spray process. Mat. Sci. Eng. A 2004, 368, 222–230. [Google Scholar] [CrossRef]
- Borchers, C.; Gärtner, F.; Stoltenhoff, T.; Assadi, H.; Kreye, H. Microstructural and Macroscopic Properties of Cold Sprayed Copper Coatings. J. Appl. Phys. 2003, 93, 10064–10070. [Google Scholar]
- Maev, R.G.; Leshchynsky, V. Air Gas Dynamic Spraying of Powder Mixtures: Theory and Application. J. Therm. Spray Technol. 2006, 15, 198–205. [Google Scholar] [CrossRef]
- Chang, Y.; Mohanty, P.; Karmarkar, N.; Khan, M.T.; Wang, Y.; Wang, J. Microstructure and properties of Cu-Cr coatings deposited by cold spraying. Vacuum 2020, 171, 109032. [Google Scholar] [CrossRef]
- Balani, K.; Laha, T.; Agarwal, A.; Karthikeyan, J.; Munroe, N. Effect of Carrier Gases on Microstructural and Electrochemical Behavior of Cold-Sprayed 1100 Aluminum Coating. Surf. Coat. Technol. 2005, 195, 272–279. [Google Scholar]
- Liu, J.; Wang, R.; Qian, Y. The formation of a single-pulse electrospark deposition spot. Surf. Coat. Technol. 2005, 200, 2433–2437. [Google Scholar]
- Wang, Z.; Zhu, G.; Lu, F.; Zhang, L.; Wang, Y.; Zhao, S.; Guo, C.; Zhang, J. Friction and wear performance of an electrospark-deposited Ta coating on CrNi3MoVA steel. Mater. Technol. 2024, 58, 17–23. [Google Scholar]
- Ziewiec, K.; Wojciechowska, M.; Ferenc, J.; Lis, M.; Mucha, D.; Morgiel, J.; Ziewiec, A. Thermal characteristics and amorphization in plasma spray deposition of Ni-Si-B-Ag alloy. J. Alloys Comp. 2017, 710, 685–691. [Google Scholar]
- Ghasemi, R.; Vakilifard, H. Plasma-sprayed nanostructured YSZ thermal barrier coatings: Thermal insulation capability and adhesion strength. Ceram. Int. 2017, 43, 8556–8563. [Google Scholar]
- Lashmi, P.G.; Ananthapadmanabhan, P.V.; Chakravarthy, Y.; Unnikrishnan, G.; Balaji, N.; Aruna, S.T. Hot corrosion studies on plasma sprayed bi-layered YSZ/La2Ce2O7 thermal barrier coating fabricated from synthesized powders. J. Alloys Comp. 2017, 711, 355–364. [Google Scholar]
- Yang, Y.; Li, K.Z.; Zhao, Z.G.; Li, H.J. Deposition and ablation resistance of HfC-based coatings prepared on SiC-coated C/C composites by supersonic atmospheric plasma spraying. Adv. Appl. Ceram. 2016, 115, 473–482. [Google Scholar]
- Han, Y.; Yan, Y.; Lu, C.; Zhang, Y.; Xu, K. Bioactivity and osteoblast response of the micro-arc oxidized zirconia films. J. Biomed. Mater. Res. B 2009, 88A, 117–127. [Google Scholar]
- Yan, Y.; Sun, J.; Han, Y.; Li, D.; Cui, K. Microstructure and bioactivity of Ca, Pand Sr doped TiO2 coating formed on porous titanium by micro-arc oxidation. Surf. Coat. Technol. 2010, 205, 1702–1713. [Google Scholar]
- Men, X.; Tao, F.; Gan, L.; Zhao, F.; Xu, Z. Erosion behavior and surface cracking mechanism of co-based coating deposited via PTA under high-speed propellant airflow. Surf. Coat. Technol. 2019, 372, 369–375. [Google Scholar]
- Chen, J.; Jia, Z.; Wang, J.; Jin, P.; Cao, Y.; Zhao, C.; Dou, C.; Li, R.; Zhang, C.; Huang, J. Newly developed high-strength martensitic stainless steel for the sniper rifle barrel with high shooting accuracy. J. Mater. Res. Technol. 2023, 24, 3956–3968. [Google Scholar]
- Lawton, B. Thermo-chemical erosion in gun barrels. Wear 2001, 251, 827–838. [Google Scholar]
- Sopok, S.; Rickard, C.; Dunn, S. Thermal–chemical–mechanical gun bore erosion of an advanced artillery system part one: Theories and mechanisms. Wear 2005, 258, 659–670. [Google Scholar]
- Wang, Y.; Ding, S.; Zhang, X. A novel structure to inhibit barrel erosion induced by thermal effects in a propulsion system. Int. Commun. Heat Mass. 2023, 147, 106991. [Google Scholar] [CrossRef]
- Hou, Z.; Peng, S.; Yu, Q.; Shao, X. Interface crack behavior of thermal protection coating of gun bores under transient convective cooling. Eng. Fail. Anal. 2022, 137, 106411. [Google Scholar] [CrossRef]
- Hu, M.; Pan, M.; Shen, M.; Guo, C.; Tang, Y.; Yu, H. Thermal shock behaviour and failure mechanism of two-kind Cr coatings on non-planar structure. Eng. Fail. Anal. 2022, 141, 106697. [Google Scholar] [CrossRef]
- Underwood, J.H.; Vigilante, G.N.; Mulligan, C.P.; Todaro, M.E. Thermomechanically controlled Erosion in Army cannons: A review. J. Press. Vessel. Technol. 2006, 128, 168–172. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Q.; Ma, Q. Influence of the laser pre-quenched substrate on an electroplated chromium coating/steel substrate. Appl. Surf. Sci. 2017, 405, 273–279. [Google Scholar] [CrossRef]
- Li, H.; Chen, G.; Zhang, K.; Luo, G.; Ye, Z. Degradation failure features of chromiumplated gun barrels with a laser-discrete-quenched substrate. Surf. Coat. Technol. 2007, 201, 9558–9564. [Google Scholar] [CrossRef]
- Underwood, J.H.; Parker, A.P.; Vigilante, G.N.; Cote, P.J. Thermal damage, cracking and rapid Erosion of Cannon bore coatings. J. Press. Vessel. Technol. 2003, 125, 299–304. [Google Scholar] [CrossRef]
- Wang, L.; Kim, D.; Nam, K.; Kim, M.; Kwon, S. Microstructure of electroplated hard chromium coatings after plasma nitrocarburizing. Surf. Coat. Technol. 2005, 190, 151–154. [Google Scholar] [CrossRef]
- Shukla, P.; Awasthi, S.; Ramkumar, J.; Balani, K. Protective trivalent Cr-based electrochemical coatings for gun barrels. J. Alloys Compd. 2018, 768, 1039–1048. [Google Scholar] [CrossRef]
- Ellis, E.A.I.; Chmielus, M.; Baker, S.P. Effect of sputter pressure on ta thin films: Beta phase formation, texture, and stresses. Acta Mater. 2018, 150, 317–326. [Google Scholar] [CrossRef]
- Bernoulli, D.; Müller, U.; Schwarzenberger, M.; Hauert, R.; Spolenak, R. Magnetron sputter deposited tantalum and tantalum nitride thin films: An analysis of phase, hardness and composition. Thin Solid Films 2013, 548, 157–161. [Google Scholar] [CrossRef]
- Traving, M.; Zienert, I.; Zschech, E.; Schindler, G.; Steinhögl, W.; Engelhardt, M. Phase analysis of TaN/Ta barrier layers in sub-micrometer trench structures for Cu interconnects. Appl. Surf. Sci. 2005, 252, 11–17. [Google Scholar] [CrossRef]
- Lee, S.; Doxbeck, M.; Mueller, J.; Cipollo, M.; Cote, P. Texture, structure and phase transformation in sputter beta tantalum coating. Surf. Coat. Technol. 2004, 177–178, 44–51. [Google Scholar]
- Niu, Y.; Xing, L.; Yang, F.; Li, H.; Chen, M.; Zhu, S.; Wang, F. Phase structure of sputtered ta coating and its ablation behavior by laser pulse heating (LPH). J. Mater. Sci. Technol. 2021, 65, 7–17. [Google Scholar] [CrossRef]
- Schmidt, T.; Gaertner, F.; Kreye, H. New Developments inCold Spray Based on Higher Gas- and Particle Temperatures. J. Therm. Spray Technol. 2006, 15, 488–494. [Google Scholar] [CrossRef]
- Corbella, C.; Vives, M.; Pinyol, A.; Porqueras, I.; Person, C.; Bertran, E. Influence of the porosity of RF sputtered Ta2O5 thin films ontheir optical properties for electrochromic applications. Solid State Ion. 2003, 165, 15–22. [Google Scholar] [CrossRef]
- Hála, M.; Vernhes, R.; Zabeida, O.; Bousser, E.; Klemberg-Sapieha, J.; Sargent, R.; Martinu, L. Growth and properties of high index Ta2O5 optical coatings prepared by HiPIMS and other methods. Surf. Coat. Technol. 2014, 241, 33–37. [Google Scholar]
- Chen, X.; Bai, R.; Huang, M. Optical properties of amorphous Ta2O5 thin films deposited by RF magnetron sputtering. Opt. Mater. 2019, 97, 109404. [Google Scholar] [CrossRef]
- Chiba, H.; Tada, K.-I.; Furukawa, T.; Yamamoto, T.; Yotsuya, T.; Oshima, N.; Funakubo, H. Low temperature MOCVD of Ta2O5 dielectric thin films from Ta[NC(CH3)3][OC(CH3)3]3 and O2. J. Ceram. Soc. Jpn. 2016, 124, 510–514. [Google Scholar] [CrossRef][Green Version]
- Muduli, R.; Pattanayak, R.; Kumar, S.; Kar, S.K.; Kumar, P.; Panigrahi, S.; Panda, R.K. Dielectric, ferroelectric and impedance spectroscopic study of Ta2O5, Sb2O5, and V2O5-doped AgNbO3 ceramic. J. Alloys Comp. 2016, 656, 33–44. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Hasegawa, T.; Terabe, K.; Aono, M. Conductance quantization and synaptic behavior in a Ta2O5-based atomic switch. Nanotechnology 2012, 23, 435705. [Google Scholar] [PubMed]
- Ndiege, N.; Wilhoite, T.; Subramanian, V.; Shannon, M.A.; Masel, R.I. Sol−Gel Synthesis of Thick Ta2O5 Films. Chem. Mater. 2007, 19, 3155–3161. [Google Scholar]
- Gritsenko, V.A.; Volodin, V.A.; Perevalov, T.V.; Kruchinin, V.N.; Gerasimova, A.K.; Aliev, V.S.; Prosvirin, I.P. Nanoscale potential fluctuations in nonstoichiometrics tantalum oxide. Nanotechnology 2018, 29, 425202. [Google Scholar]
- Zibrov, I.P.; Filonenko, V.P.; Sundberg, M.; Werner, P.E. Structures and phase transitions of B-Ta2O5 and Z-Ta2O5: Two high-pressure forms of Ta2O5. Acta Crystallogr. B 2000, 56, 659–665. [Google Scholar] [PubMed]
- Grey, I.; Mumme, W.; Roth, R. The crystal chemistry of L-Ta2O5 and related structures. J. Solid State Chem. 2005, 178, 3308–3314. [Google Scholar]
- Myers, D.L.; Kulis, M.J.; Horwath, J.P.; Jacobson, N.S.; Fox, D.S. Interactions of Ta2O5 with water vapor at elevated temperatures. J. Am. Ceram. Soc. 2017, 100, 2353–2357. [Google Scholar]
- Moldovan, M.; Weyant, C.M.; Johnson, D.L.; Faber, K.T. Tantalum oxide coatings as candidate environmental barriers. J. Therm. Spray Technol. 2004, 13, 51–56. [Google Scholar]
- Zheng, J.; Wen, K.; Liu, Y.; Gao, L.; Ma, Z.; Diao, X. Laser irradiation behavior of plasma-sprayed tantalum oxide coatings. Ceram. Int. 2020, 46, 3875–3881. [Google Scholar]
- Roy, A.; Munagala, V.N.V.; Patel, P.; Sharifi, N.; Alidokht, S.A.; Makowiec, M.; Chromik, R.R.; Moreau, C.; Stoyanov, P. Friction and wear behavior of suspension plasma sprayed tantalum oxide coatings at elevated temperatures. Surf. Coat. Technol. 2023, 452, 129097. [Google Scholar]
- Alidokht, S.; Manimunda, P.; Vo, P.; Yue, S.; Chromik, R. Cold spray deposition of a Ni-WC composite coating and its dry sliding wear behavior. Surf. Coat. Technol. 2016, 308, 424–434. [Google Scholar]
- Mahade, S.; Mulone, A.; Björklund, S.; Klement, U.; Joshi, S. Incorporation of graphene nano platelets in suspension plasma sprayed alumina coatings for improved tribological properties. Appl. Surf. Sci. 2021, 570, 151227. [Google Scholar]
- Viat, A.; Guillonneau, G.; Fouvry, S.; Kermouche, G.; Joao, S.S.; Wehrs, J.; Michler, J.; Henne, J.-F. Brittle to ductile transition of tribomaterial in relation to wear response at high temperatures. Wear 2017, 392, 60–68. [Google Scholar]
- Dreano, A.; Fouvry, S.; Guillonneau, G. Understanding and formalization of the fretting-wear behavior of a cobalt-based alloy at high temperature. Wear 2020, 452, 203297. [Google Scholar]
- Patel, P.; Alidokht, S.A.; Sharifi, N.; Roy, A.; Harrington, K.; Stoyanov, P.; Chromik, R.R.; Moreau, C. Microstructural and tribological behavior of thermal spray CrMnFeCoNi high entropy alloy coatings. J. Therm. Spray Technol. 2022, 31, 1285–1301. [Google Scholar]
- Ali, M.; Ürgen, M. Tantalum carbide-graphite composite film synthesized by hot-filament chemical vapor deposition. Pure Appl. Chem. 2012, 84, 2499–2506. [Google Scholar]
- Alangi, N.; Mukherjee, J. Liquid gadolinium corrosion study of TaC coating ontantalum. Ceram. Int. 2018, 44, 19619–19623. [Google Scholar]
- Zhao, Z.; Hui, P.; Wang, T.; Wang, X.; Xu, Y.; Zhong, L.; Zhao, M. New strategy tomgrow TiC coatings on titanium alloy: Contact solid carburization by cast iron. J. Alloys Compd. 2018, 745, 637–643. [Google Scholar]
- Zhao, Z.; Hui, P.; Wang, T.; Xu, Y.; Zhong, L.; Zhao, M.; Yang, D.; Wei, R. Fabrication of Mo2C coating on molybdenum by contact solid carburization. Appl. Surf. Sci. 2018, 462, 48–54. [Google Scholar]
- Grosser, M.; Münch, M.; Seidel, H.; Bienert, C.; Roosen, A.; Schmid, U. The impactof substrate properties and thermal annealing on tantalum nitride thin films. Appl. Surf. Sci. 2012, 258, 2894–2900. [Google Scholar]
- Liu, E.; Jin, G.; Cui, X.; Xiao, Q.; Shao, T. Effect of gas pressure on the mechanical properties of sputtered TaN films. Phys. Proced. 2013, 50, 438–441. [Google Scholar]
- Liu, X.; Ma, G.; Sun, G.; Duan, Y.; Liu, S. Effect of deposition and annealing temperature on mechanical properties of TaN film. Appl. Surf. Sci. 2011, 258, 1033–1037. [Google Scholar] [CrossRef]
- Nikravesh, M.; Akbari, G.; Poladi, A. A comprehensive study on the surface tribology of Ta thin film using molecular dynamics simulation: The effect of TaN interlayer, power and temperature. Tribol. Int. 2017, 105, 185–192. [Google Scholar] [CrossRef]
- Chang, J.-C.; Birch, J.; Gueorguiev, G.K.; Bakhit, B.; Greczynski, G.; Eriksson, F.; Sandström, P.; Hultman, L.; Hsiao, C.-L. Domain epitaxial growth of Ta3N5 film on c-plane sapphire substrate. Surf. Coat. Technol. 2022, 443, 128581. [Google Scholar] [CrossRef]
- Kim, D.K.; Lee, H.; Kim, D.; Kim, Y.K. Electrical and mechanical properties of tantalum nitride thin films deposited by reactive sputtering. J. Cryst. Growth 2005, 283, 404–408. [Google Scholar]
- Venkataraj, S.; Kittur, H.; Drese, R.; Wuttig, M. Multi-technique characterization of tantalum oxynitride films prepared by reactive direct current magnetron sputtering. Thin Solid Films 2005, 514, 1–9. [Google Scholar]
- Alishahi, M.; Mahboubi, F.; Khoie, S.M.; Aparicio, M.; Hübner, R.; Soldera, F.; Gago, R. Electrochemical behavior of nanocrystalline Ta/TaN multilayer on 316L stainless steel: Novel bipolar plates for proton exchange membrane fuel-cells. J. Power Sources 2016, 322, 1–9. [Google Scholar]
- Shah, H.N.; Jayaganthan, R.; Kaur, D.; Chandra, R. Influence of sputtering parameters and nitrogen on the microstructure of chromium nitride thin films deposited on steel substrate by direct-current reactive magnetron sputtering. Thin Solid Films 2010, 518, 5762–5768. [Google Scholar]
- Kumar, D.D.; Kumar, N.; Kalaiselvam, S.; Dash, S.; Jayavel, R. Micro-tribomechanical properties of nanocrystalline TiN thin films for small scale device applications. Tribol. Int. 2015, 88, 25–30. [Google Scholar]
- Liao, S.; Yang, L.; Song, Z.; Zhou, Z.; Zhang, Q.; Yang, C.; Zheng, J. Effect of nitrogen content on the mechanical and biological properties of tantalum nitride coatings. Surf. Coat. Technol. 2023, 464, 129544. [Google Scholar]
- Chen, C.; Wang, M.P.; Wang, S.; Jia, Y.L.; Lei, R.S.; Xia, F.Z.; Zuo, B.; Yu, H.C. The evolution of cold-rolled deformation microstructure of {001} <110> grains in Ta-7.5 wt% W alloy foils. J. Alloys Compd. 2012, 513, 208–212. [Google Scholar]
- Wang, C.; Zhang, M.; Chu, J.; Nieh, T. Structures and nanoindentation properties of nanocrystalline and amorphous Ta-W thin films. Scr. Mater. 2008, 58, 195–198. [Google Scholar] [CrossRef]
- Knezevic, M.; Beyerlein, I.J.; Lovato, M.L.; Tomé, C.N.; Richards, A.W.; McCabe, R.J. A strain-rate and temperature dependent constitutive model for BCC metals incorporating non-Schmid effects: Application to tantalum–tungsten alloys. Int. J. Plast. 2014, 62, 93–104. [Google Scholar] [CrossRef]
- Fritze, S.; Hans, M.; Riekehr, L.; Osinger, B.; Lewin, E.; Schneider, J.; Jansson, U. Influence of carbon on microstructure and mechanical properties of magnetron sputtered TaW coatings. Mater. Des. 2020, 196, 109070. [Google Scholar] [CrossRef]
- Li, R.; Gu, Y.; Zeng, F.; Li, Y.; Wu, A. High temperature diffusion behavior between Ta-10W coating and CP-Ti and TC4 alloy. Surf. Coat. Technol. 2021, 406, 126669. [Google Scholar] [CrossRef]
- Sun, P.; Gu, Y.; Li, Y.; Liu, B. Corrosion Behavior of Ta-10W Coatings on CP-Ti and TC4 Substrates. J. Mater. Eng. Perform. 2019, 28, 4152–4162. [Google Scholar] [CrossRef]
- Hsieh, J.; Li, C.; Wang, C.; Tang, Z. Boundary-free multi-element barrier films by reactive co-sputtering. Surf. Coat. Technol. 2005, 198, 335–339. [Google Scholar] [CrossRef]
- Chang, L.; Chen, Y.; Kao, H. Annealing of sputter-deposited nanocrystalline Cr–Ta coatings in a low-oxygen-containing atmosphere. Thin Solid Films. 2012, 520, 6929–6934. [Google Scholar] [CrossRef]
- Kumar, P.K.; Manikandan, V.; Raj, P.D.; Sridharan, M. Characterization of Magnetron Sputtered Si3N4 Thin Films Deposited on Aluminum Alloy Substrates. Mater. Today 2016, 3, 1536–1540. [Google Scholar]
- Du, X.Q.; Liu, Y.W.; Chen, Y. Enhancing the Corrosion Resistance of Aluminum by Superhydrophobic Silane/Graphene Oxide Coating. Appl. Phys. A 2021, 127, 580. [Google Scholar] [CrossRef]
- Moffitt, C.; Wieliczka, D.; Yasuda, H. An XPS Study of the Elemental Enrichment on Aluminum Alloy Surfaces From Chemical Cleaning. Surf. Coat. Technol. 2001, 137, 188–196. [Google Scholar] [CrossRef]
- Trinidad, C.; Światowska, J.; Zanna, S.; Seyeux, A.; Mercier, D.; Viroulaud, R.; Marcus, P. Effect of Surface Preparation Treatments on Copper Enrichment on 2024 Aluminium Alloy Surface. Appl. Surf. Sci. 2021, 560, 149991. [Google Scholar]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Corrosion of AA2024-T3 Part I: Localised Corrosion of Isolated IM Particles. Corr. Sci. 2011, 53, 17–26. [Google Scholar] [CrossRef]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Conversion Coating Growth on 2024-T3 Al Alloy. The Effect of Pre-Treatments. Surf. Coat. Technol. 2008, 202, 3396–3402. [Google Scholar]
- Zlamal, T.; Mrkvica, I.; Szotkowski, T.; Malotova, S. The Influence of Surface Treatment of PVD Coating on Its Quality and Wear Resistant. Coatings 2019, 9, 439. [Google Scholar] [CrossRef]
- Fernández-Abia, A.I.; Barreiro, J.; de Lacalle, L.N.L.; González-Madruga, D. Effect of Mechanical Pre-Treatments in the Behaviour of Nanostructured PVD-Coated Tools in Turning. J. Adv. Manuf. Technol. 2014, 73, 1119–1132. [Google Scholar]
- Bahcepinar, A.I.; Aydin, I. Investigation of the Effect of Shot Blasting on the Surface Properties of the HA Coatings Processed by the EPD Method. Kovove. Mater. 2022, 60, 293–302. [Google Scholar]
- Türkmen, I.; Kanbur, K.; Sargin, F. Characteristics of Boronized Ti6Al4V Alloy Using Boric Acid Based Boronizing Mixture. Mater. Charact. 2022, 192, 112180. [Google Scholar]
- Gül, C.; Albayrak, S.; Çinici, H.; Aytaç, A. Improvement in Corrosion Resistance of Tantalum Oxide and Tantalum Oxide With Diethanolamine Sol–Gel Coated Magnesium Alloys. Prot. Met. Phys. Chem. Surf. 2022, 58, 603–614. [Google Scholar]
- Gül, C.; Albayrak, S.; Çinici, H.; Algan, İ.B. The Effects of Alkali, Alkali-Acid and Sandblasting Surface Treatments Applied Before Tantalum-Oxide Coating With Magnetron Sputtering on the Wear Behavior of 7075 Aluminum Alloys. J. Fac. Eng. Archit. 2023, 38, 795–806. [Google Scholar]
- Albayrak, S.; Gül, C.; Çinici, H.; Şahin, Ö. Tribological Characteristics of Tantalum-Oxide Coating Fabricated on Pre-Treated 2024 Aluminum Alloys. J. Tribol. Oct. 2023, 145, 101706. [Google Scholar] [CrossRef]
- Hee, A.C.; Jamali, S.S.; Bendavid, A.; Martin, P.J.; Kong, C.; Zhao, Y. Corrosion Behaviour and Adhesion Properties of Sputtered Tantalum Coating on Ti6Al4V Substrate. Surf. Coat. Technol. 2016, 307, 666–675. [Google Scholar] [CrossRef]
- Chen, F.; Lin, J.; Chen, Y.; Dong, B.; Yin, C.; Tian, S.; Sun, D.; Xie, J.; Zhang, Z.; Li, H.; et al. Enhancement of electrochemical performance in lithium-ion battery via tantalum oxide coated nickel-rich cathode materials. Chin. Phys. B 2022, 31, 058101. [Google Scholar] [CrossRef]
- Corona-Gomez, J.; Jack, T.; Feng, R.; Yang, Q. Wear and corrosion characteristics of nano-crystalline tantalum nitride coatings deposited on CoCrMo alloy for hip joint applications. Mater. Charact. 2021, 182, 111516. [Google Scholar]
- Balagna, C.; Spriano, S.; Faga, M.G. Characterization of Co–Cr–Mo alloys after a thermal treatment for high wear resistance. Mater. Sci. Eng. C 2012, 32, 1868–1877. [Google Scholar]
- Chang, Y.Y.; Huang, H.L.; Chen, H.J.; Lai, C.H.; Wen, C.Y. Antibacterial properties and cytocompatibility of tantalum oxide coatings. Surf. Coat. Technol. 2012, 259, 193–198. [Google Scholar]
- Sarraf, M.; Razak, B.A.; Nasiri-Tabrizi, B.; Dabbagh, A.; Kasim NH, A.; Basirun, W.J.; Sulaiman, E.B. Nanomechanical properties, wear resistance and in-vitro characterization of Ta2O5 nanotubes coating on biomedical grade Ti–6Al–4V. J. Mech. Behav. Biomed. Mater. 2017, 66, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Oh, J.S.; Kim, H.J.; Oh, I.H.; Lee, K.A. The Effect of Annealing Heat Treatment on the Microstructure and Macroscopic Properties of Kinetic-Sprayed Ta Coating Layer. Adv. Mater. Res. 2014, 893, 64–68. [Google Scholar] [CrossRef]
- Clevenger, L.A.; Mutscheller, A.; Harper JM, E.; Cabral, C., Jr.; Barmak, K. The relationship between deposition conditions, the beta to alpha phase transformation, and stress relaxation in tantalum thin films. J. Appl. Phys. 1992, 72, 4918–4924. [Google Scholar] [CrossRef]
- Liao, S.; Yang, L.; Zhang, Q.; Zheng, B.; Yang, C.; Zheng, J.; Zou, D.; Song, Z. Effect of annealing on the mechanical properties and cytocompatibility of CoCrMo alloy with tantalum coating. Surf. Coat. Technol. 2023, 469, 129789. [Google Scholar] [CrossRef]



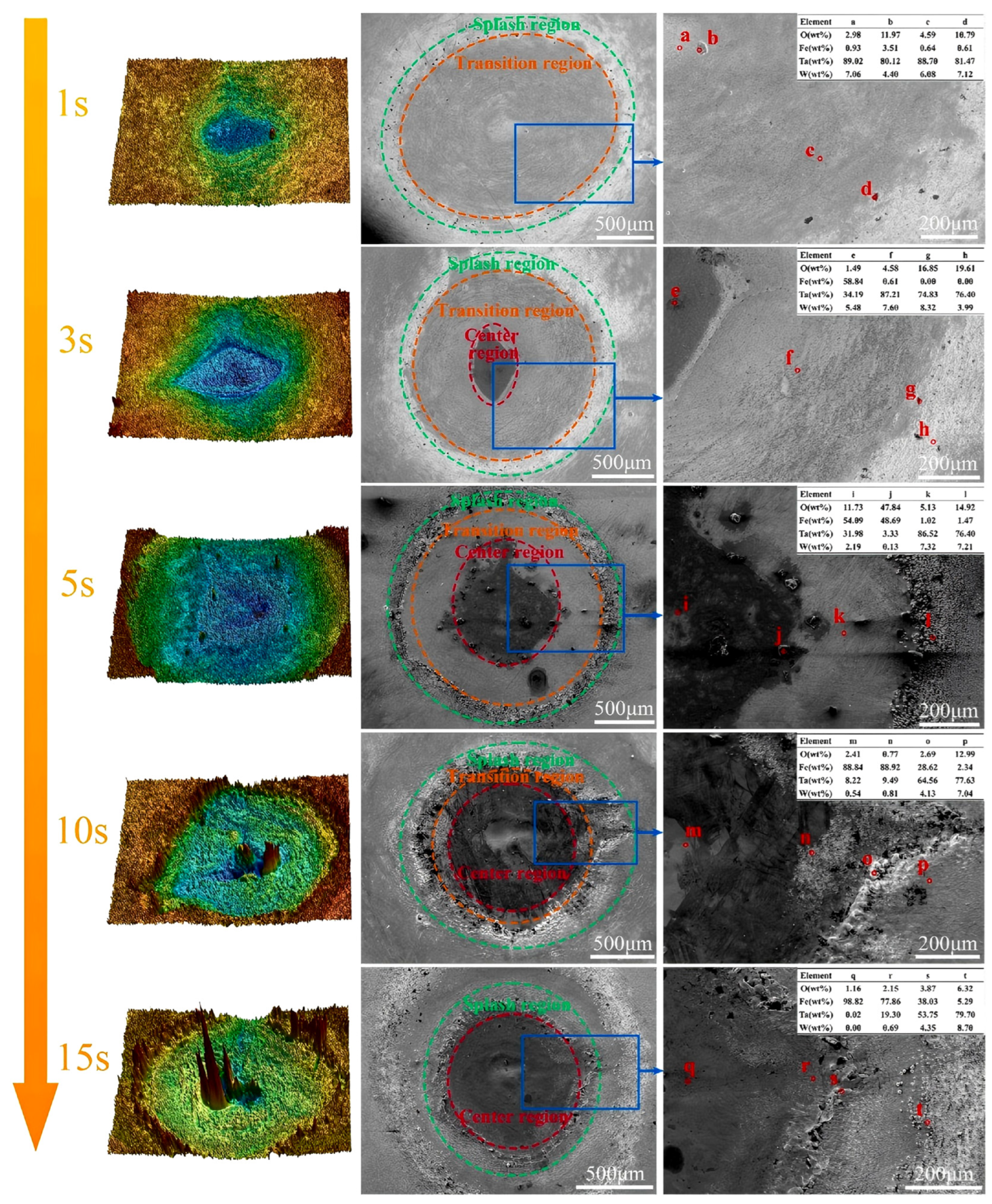
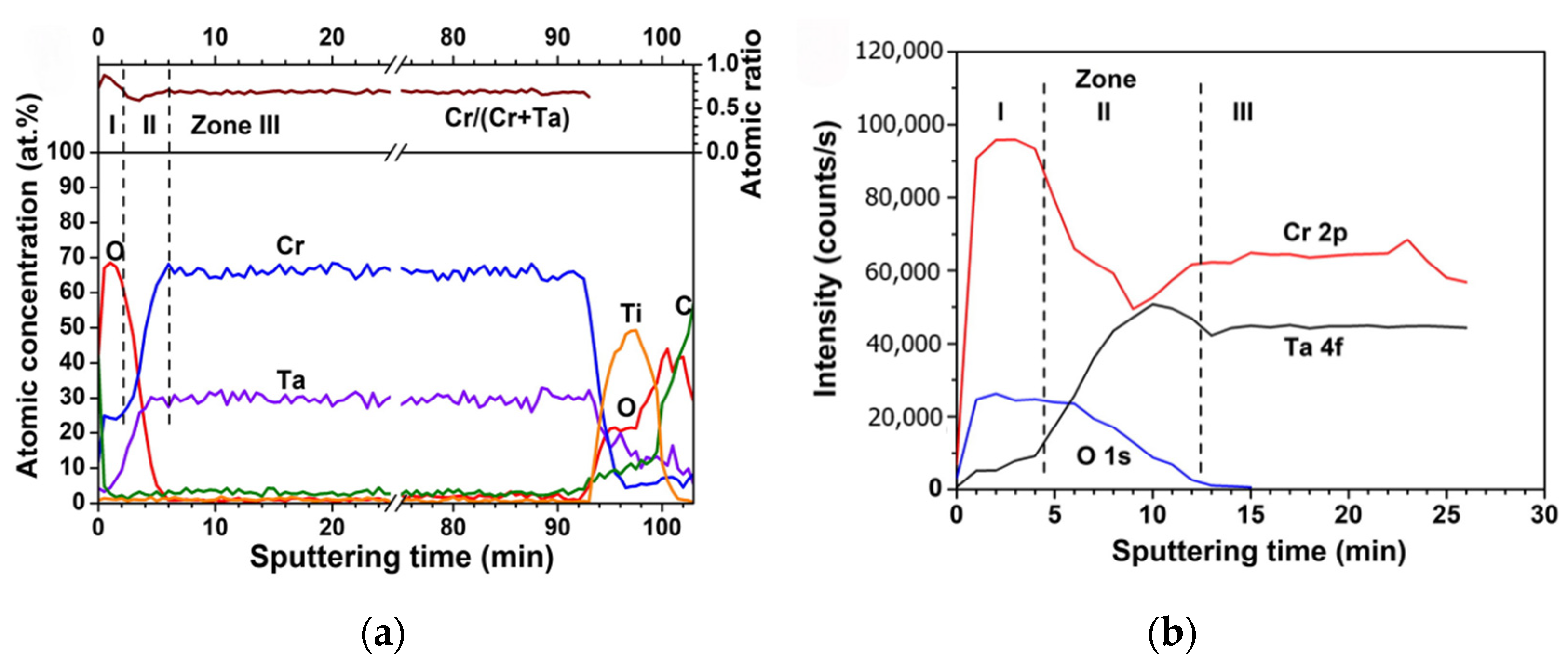
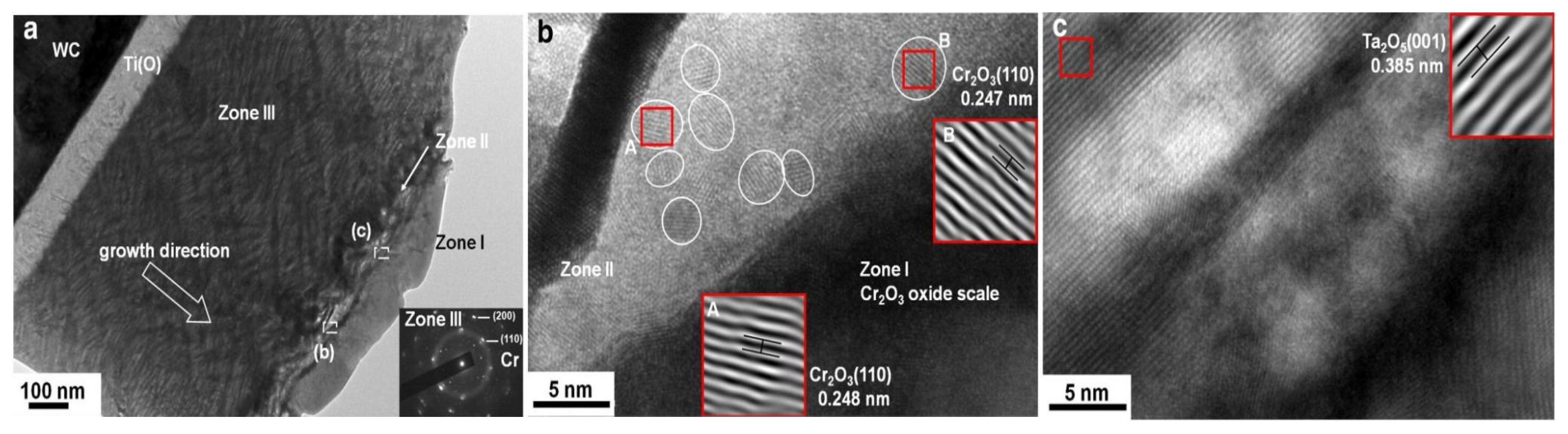

| Coatings | Synthesis Method | Properties |
|---|---|---|
| α-Ta [12,84,85] | Magnetron sputtering | Excellent ablative resistance |
| β-Ta [13,46] | Magnetron sputtering | Brittleness |
| Ta oxide [99,100] | Plasma spraying | Laser ablation resistance and low wear rate |
| Ta carbide [31] | Interstitial carburizing | High hardness |
| Ta nitride [33,34,120] | Sputtering | Increased hardness and wear resistance |
| Ta-W alloy [37,40,126] | Multi-arc ion plating | Excellent corrosion resistance and cyclic oxidation resistance |
| Ta-Cr alloy [128] | Magnetron sputtering | High hardness |
| Ta oxide [141] | Surface pretreatment | Improve wear resistance |
| α-Ta [142] | Buffer Layer | Improve wear resistance |
| Ta [148,149,150] | Annealing Treatment | Increase hardness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhu, G.; Lv, K.; Li, J.; Yu, X.; Yu, Y.; Guo, C.; Zhang, J. Research Progress on Preparation, Microstructure, Properties, and Optimization of Ta and Its Compounds’ Coatings. Metals 2025, 15, 416. https://doi.org/10.3390/met15040416
Wang Z, Zhu G, Lv K, Li J, Yu X, Yu Y, Guo C, Zhang J. Research Progress on Preparation, Microstructure, Properties, and Optimization of Ta and Its Compounds’ Coatings. Metals. 2025; 15(4):416. https://doi.org/10.3390/met15040416
Chicago/Turabian StyleWang, Zijun, Guanglin Zhu, Ke Lv, Jie Li, Xinfeng Yu, Yonghao Yu, Cean Guo, and Jian Zhang. 2025. "Research Progress on Preparation, Microstructure, Properties, and Optimization of Ta and Its Compounds’ Coatings" Metals 15, no. 4: 416. https://doi.org/10.3390/met15040416
APA StyleWang, Z., Zhu, G., Lv, K., Li, J., Yu, X., Yu, Y., Guo, C., & Zhang, J. (2025). Research Progress on Preparation, Microstructure, Properties, and Optimization of Ta and Its Compounds’ Coatings. Metals, 15(4), 416. https://doi.org/10.3390/met15040416





