Casting Process and Quality Control Analysis of Zr705C Zirconium Alloy
Abstract
1. Introduction
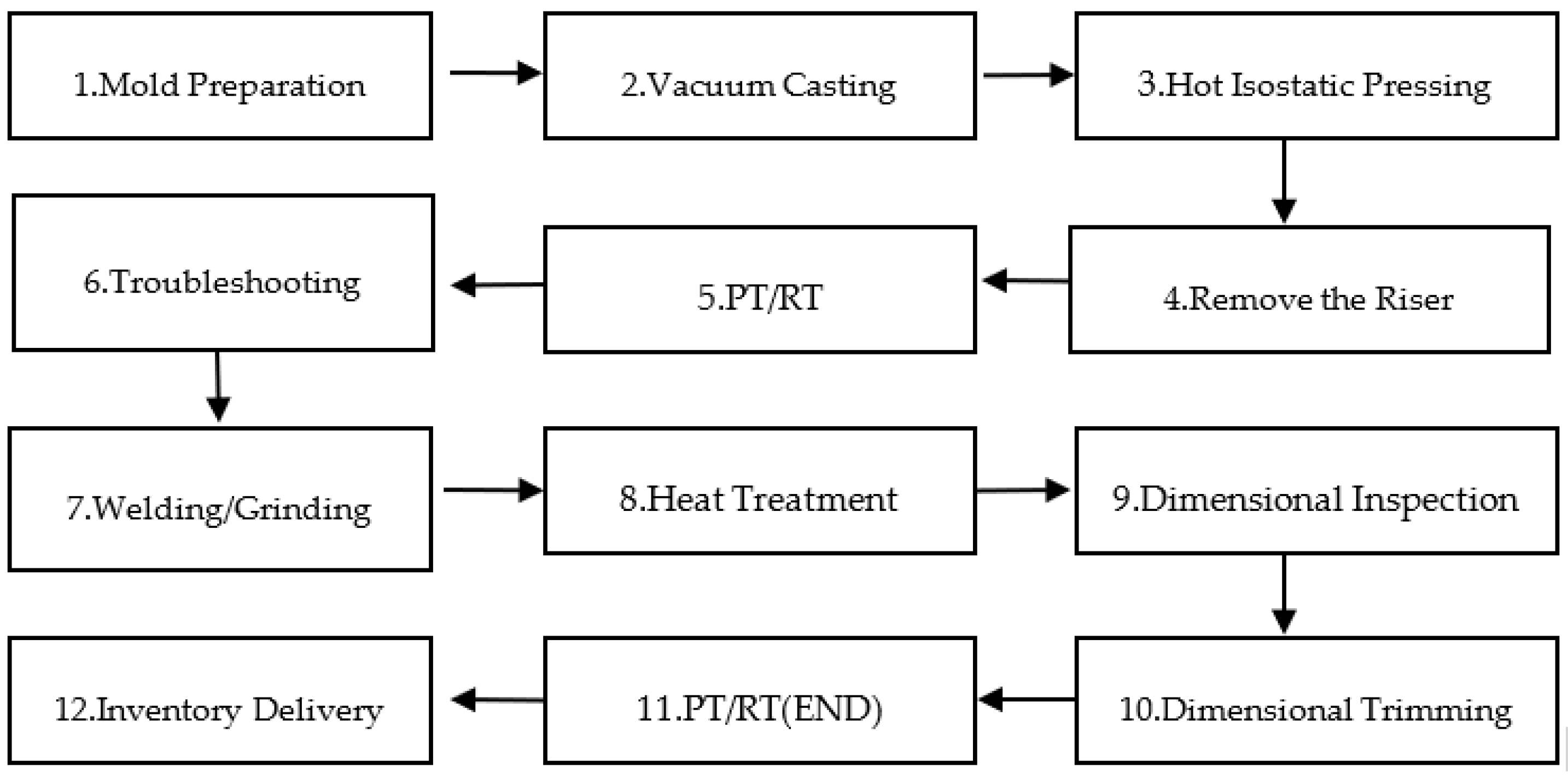
2. Zirconium Alloy Processing Technology
2.1. Melting Method
2.2. Molding Process
2.3. Casting Process
2.4. Welding Process
3. Process Design and Simulation Optimization
3.1. Process Design
- (1)
- Casting method;
- (2)
- Difficulty in shell preparation;
- (3)
- Casting shrinkage compensation effect;
- (4)
- Craft output yield.
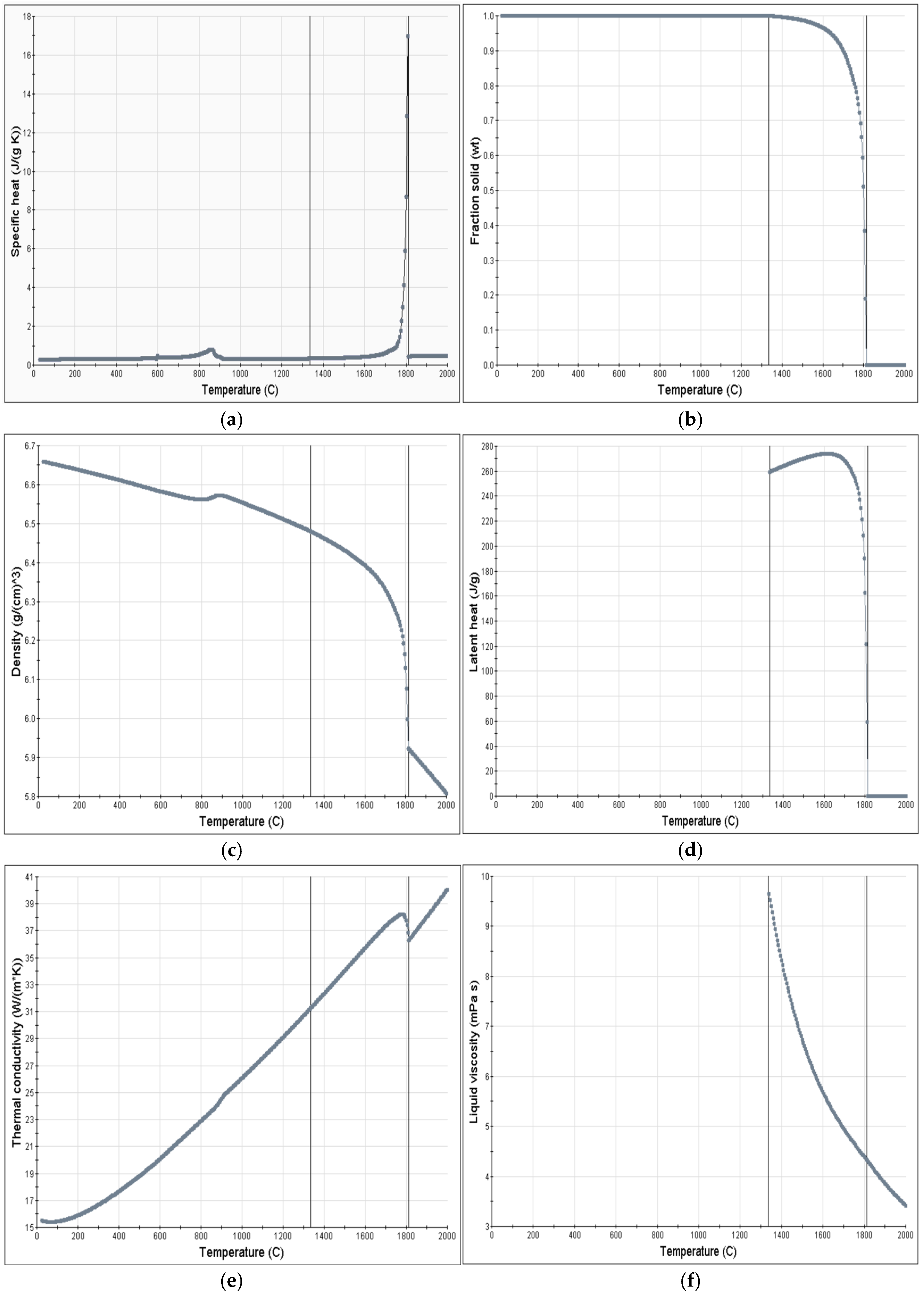
3.2. Preparation Before Simulation
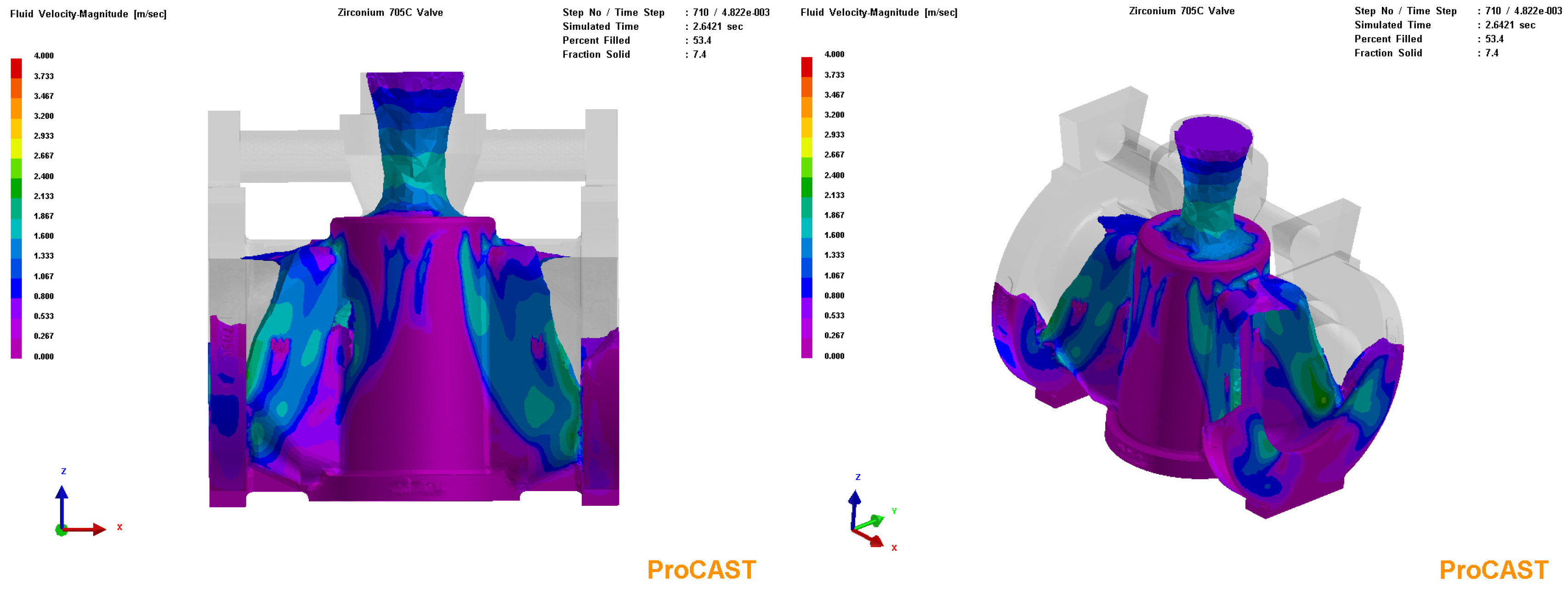
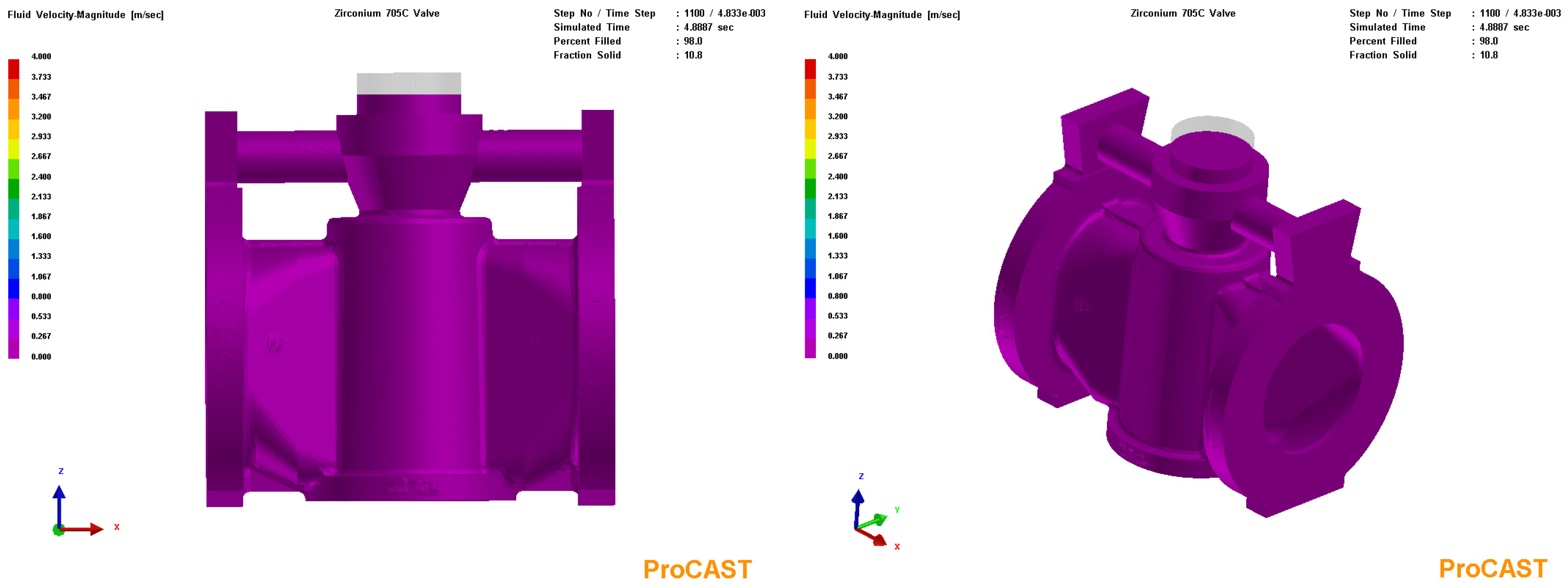
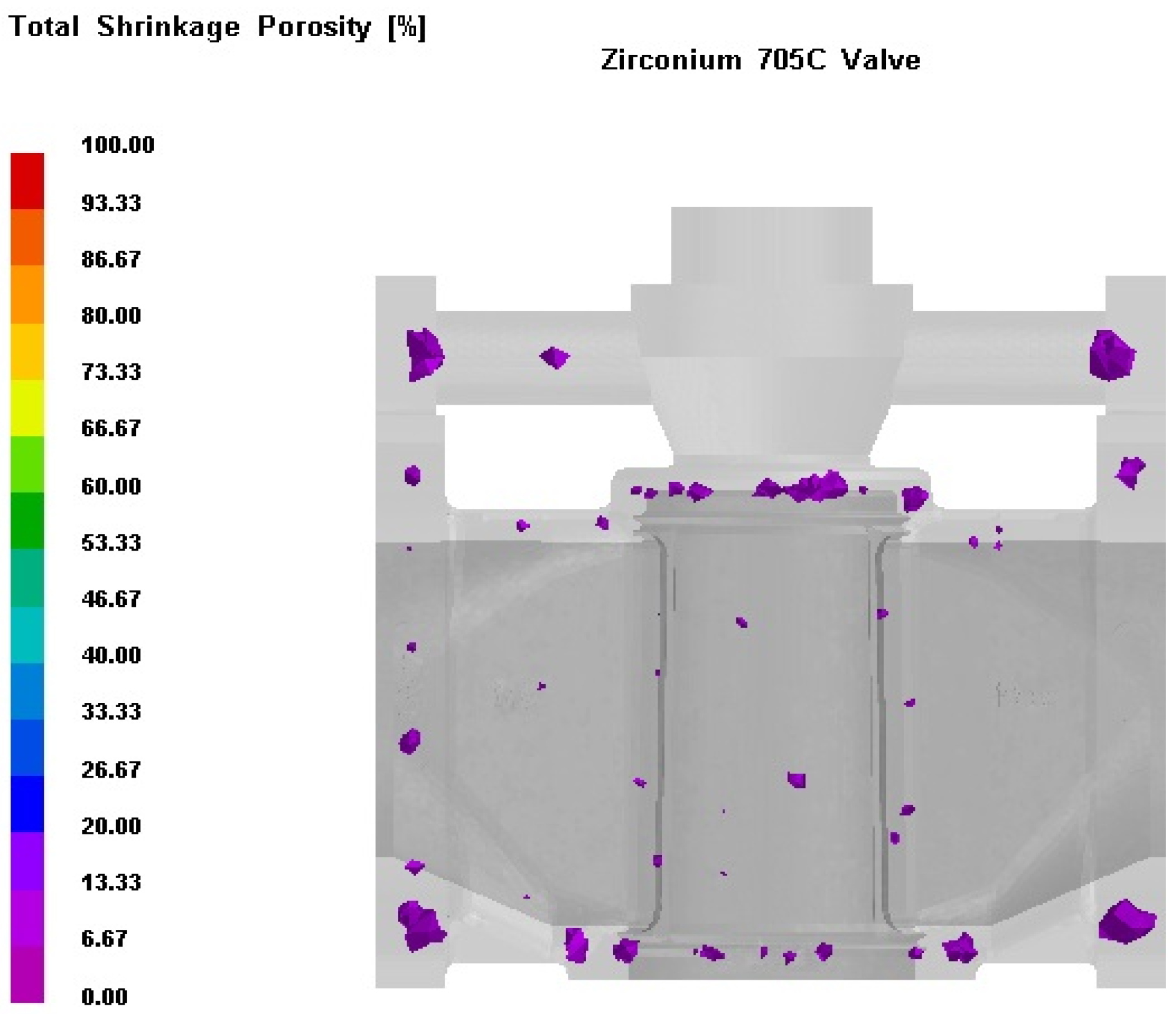
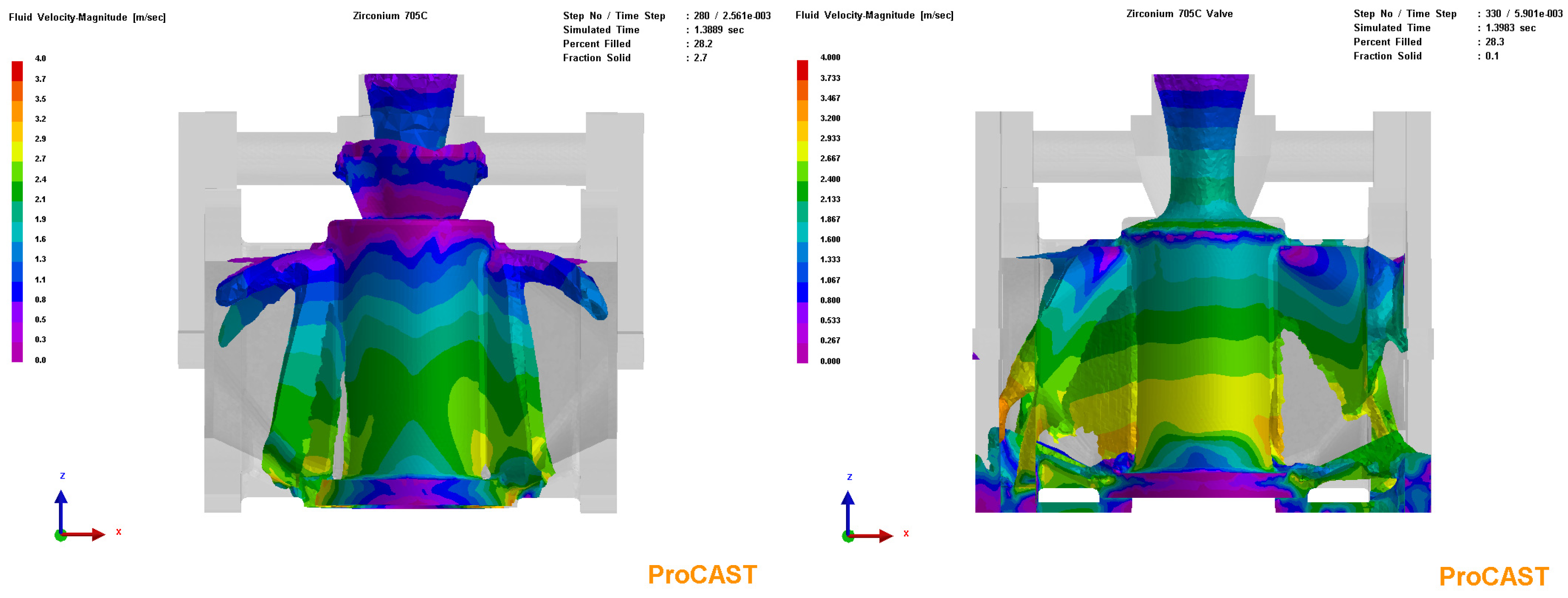
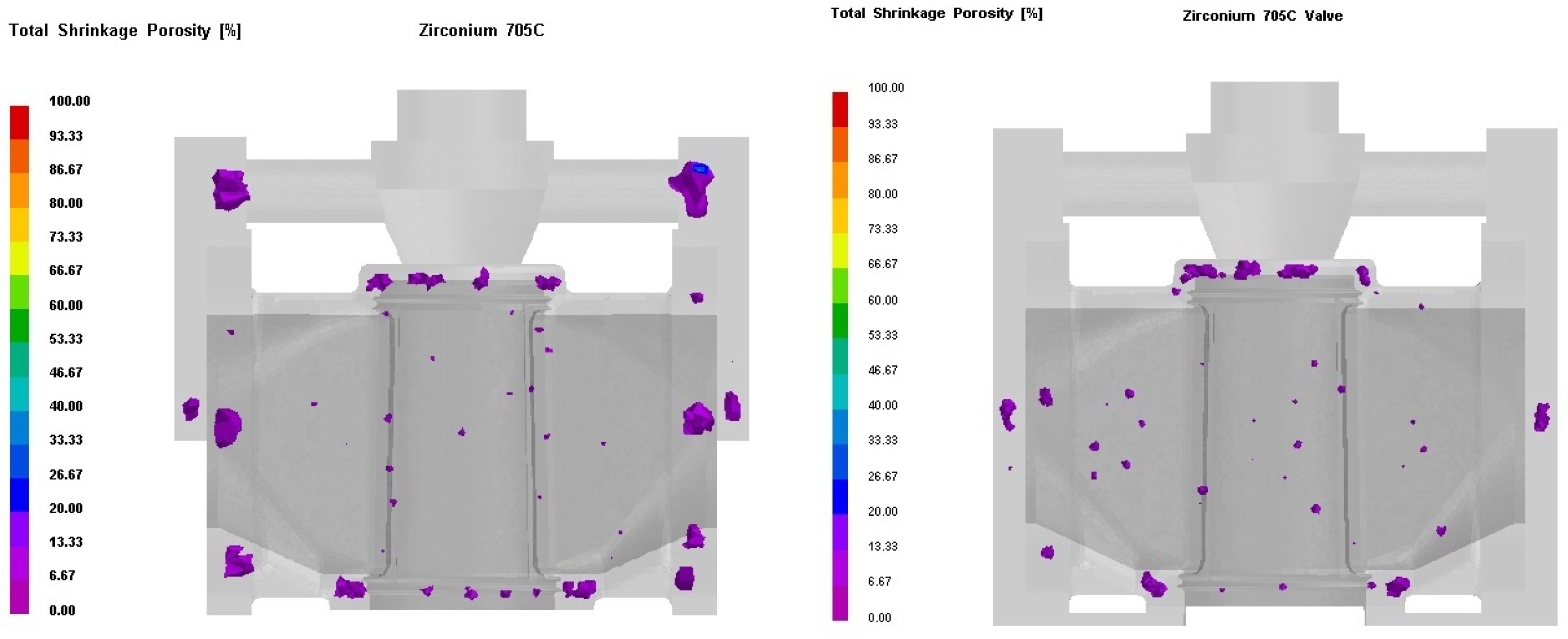
3.3. Process Simulation Analysis
3.4. Process Optimization
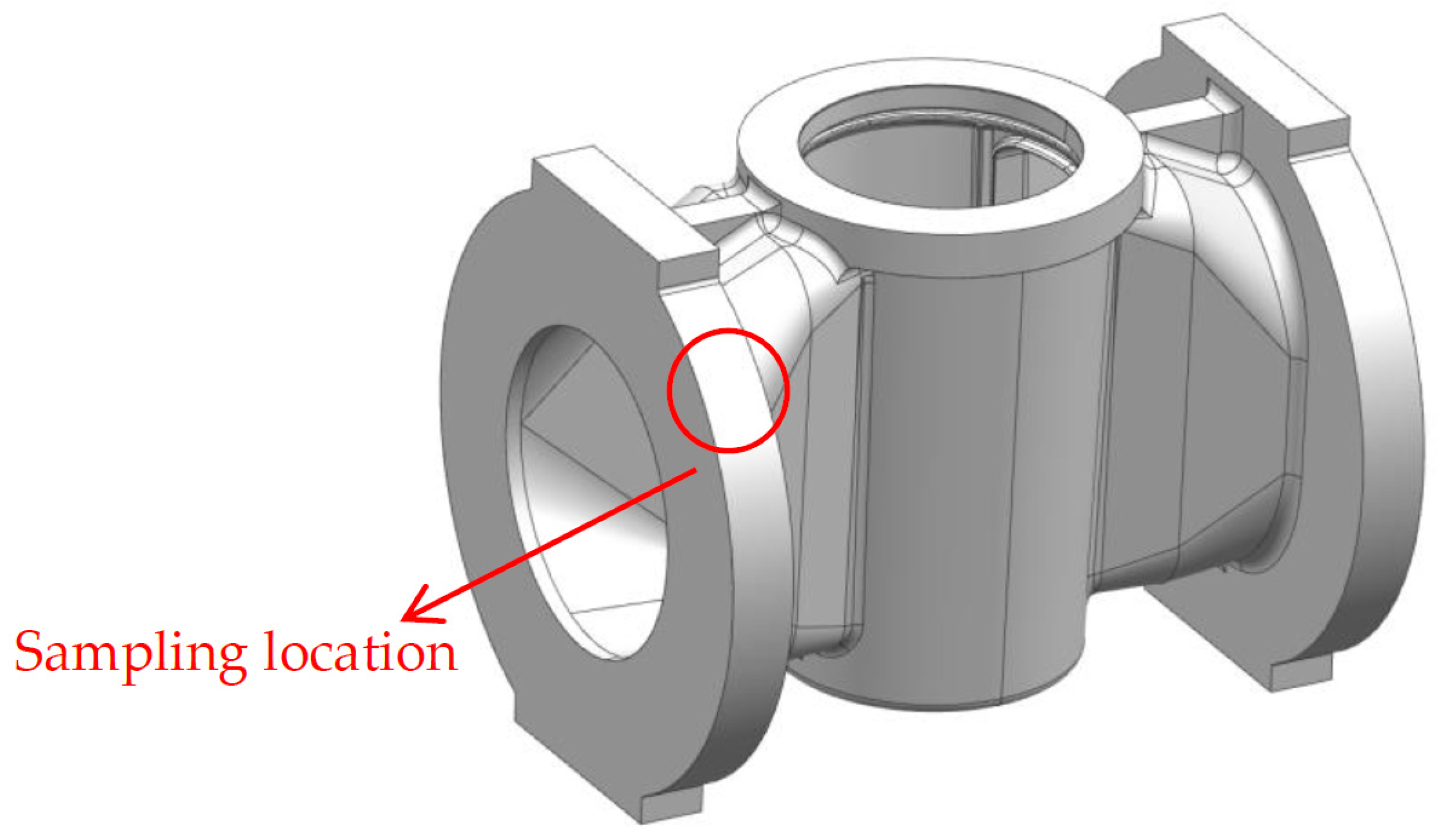
4. Experiment
5. Results and Analysis
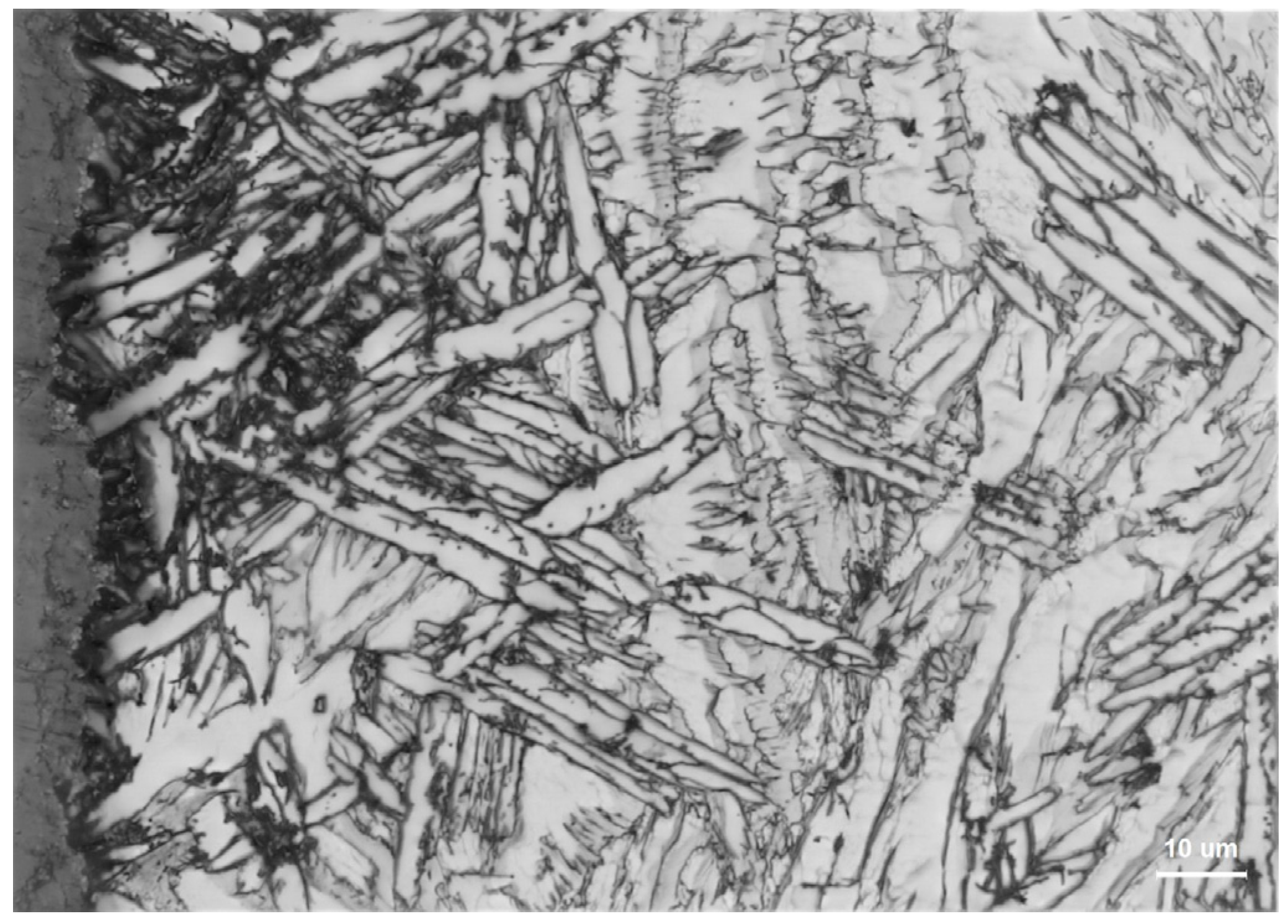
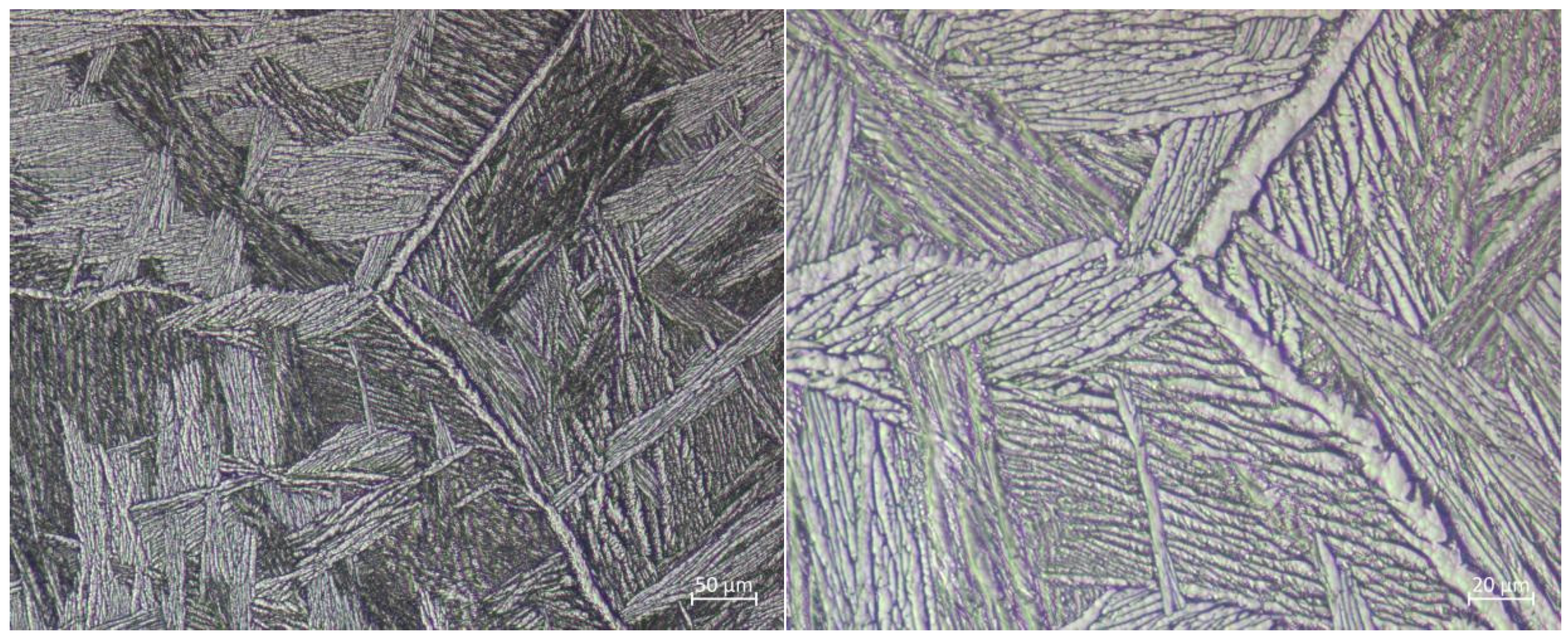
5.1. Metallographic Analysis
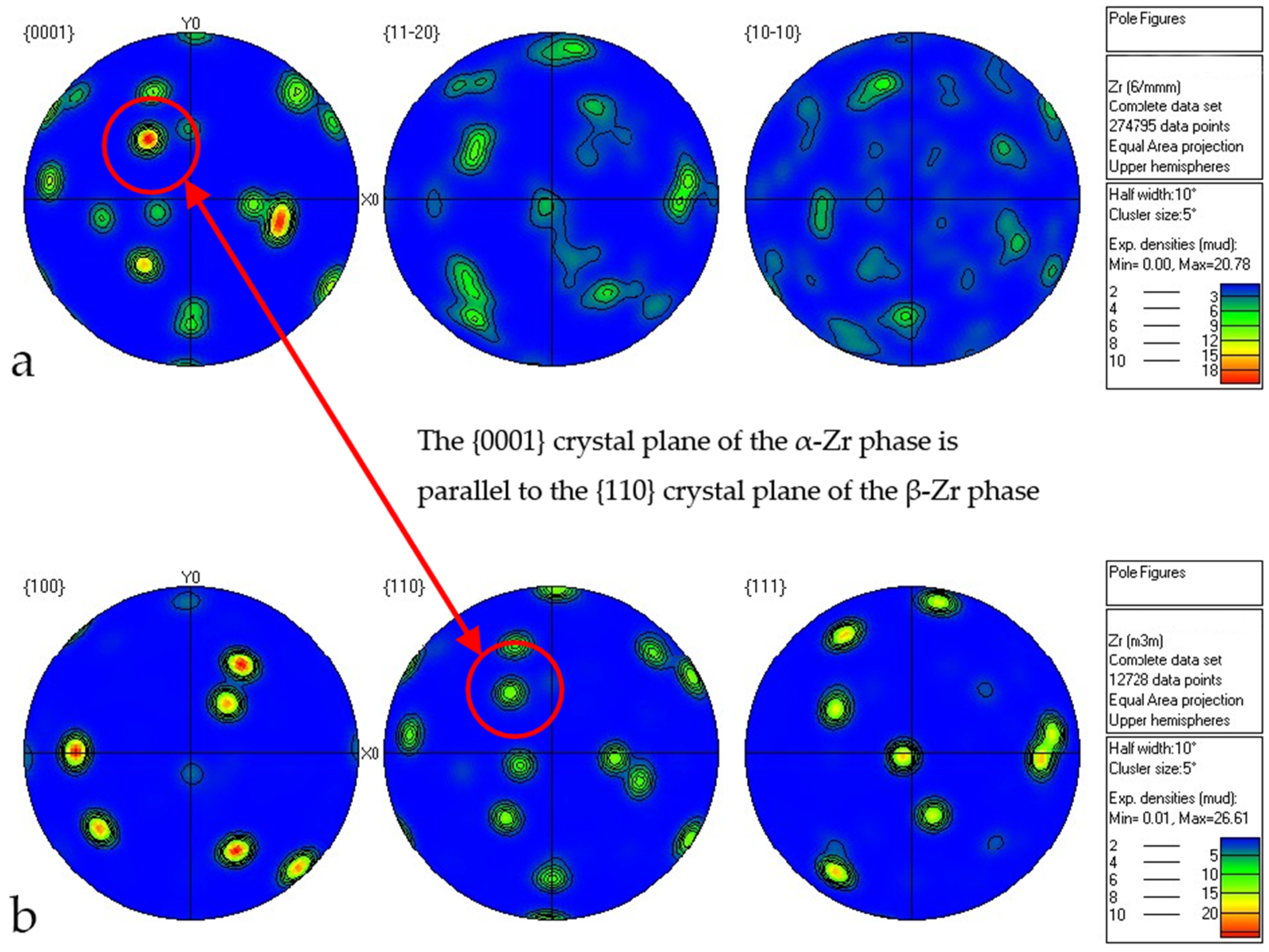
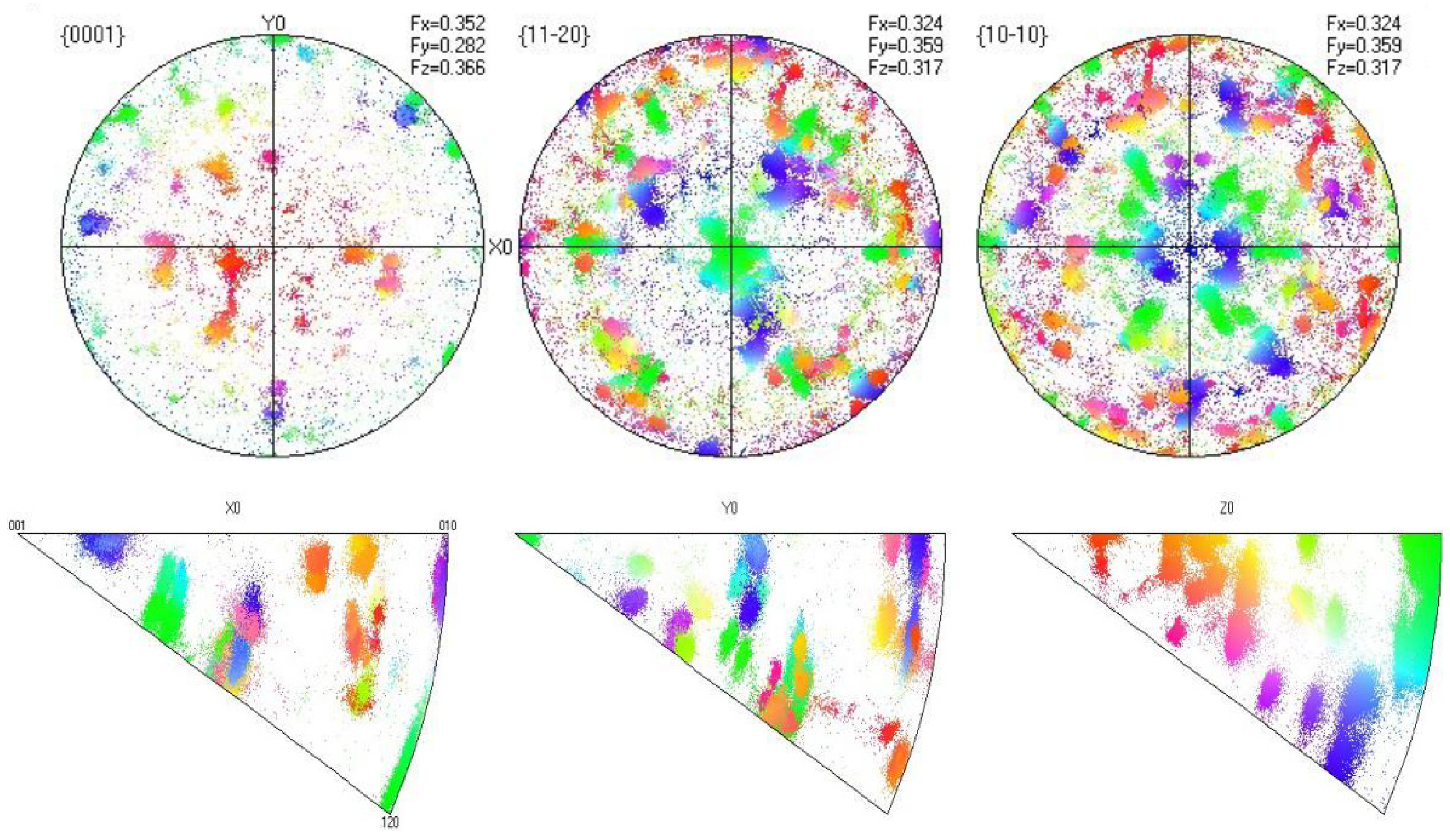
5.2. EBSD Analysis
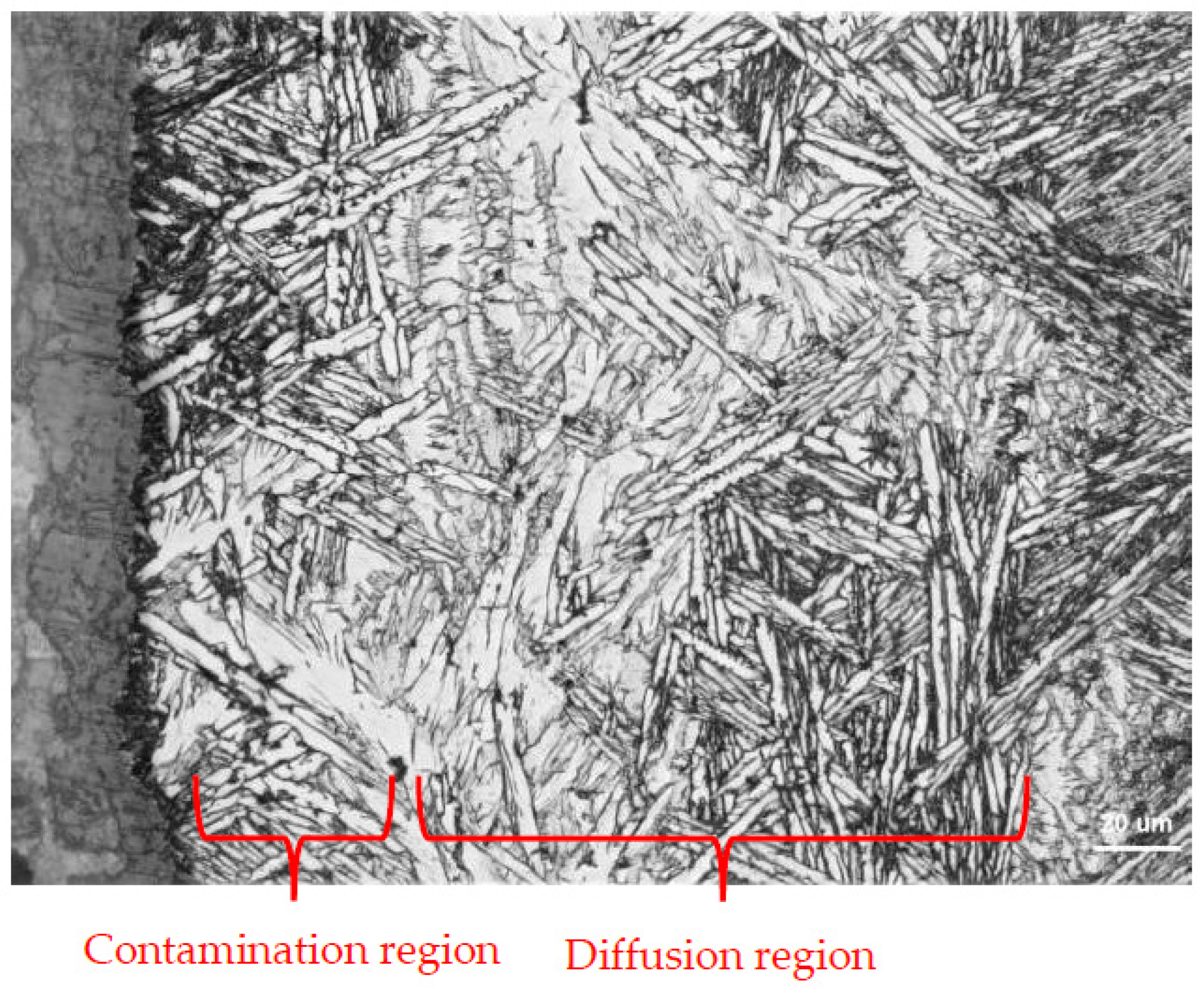
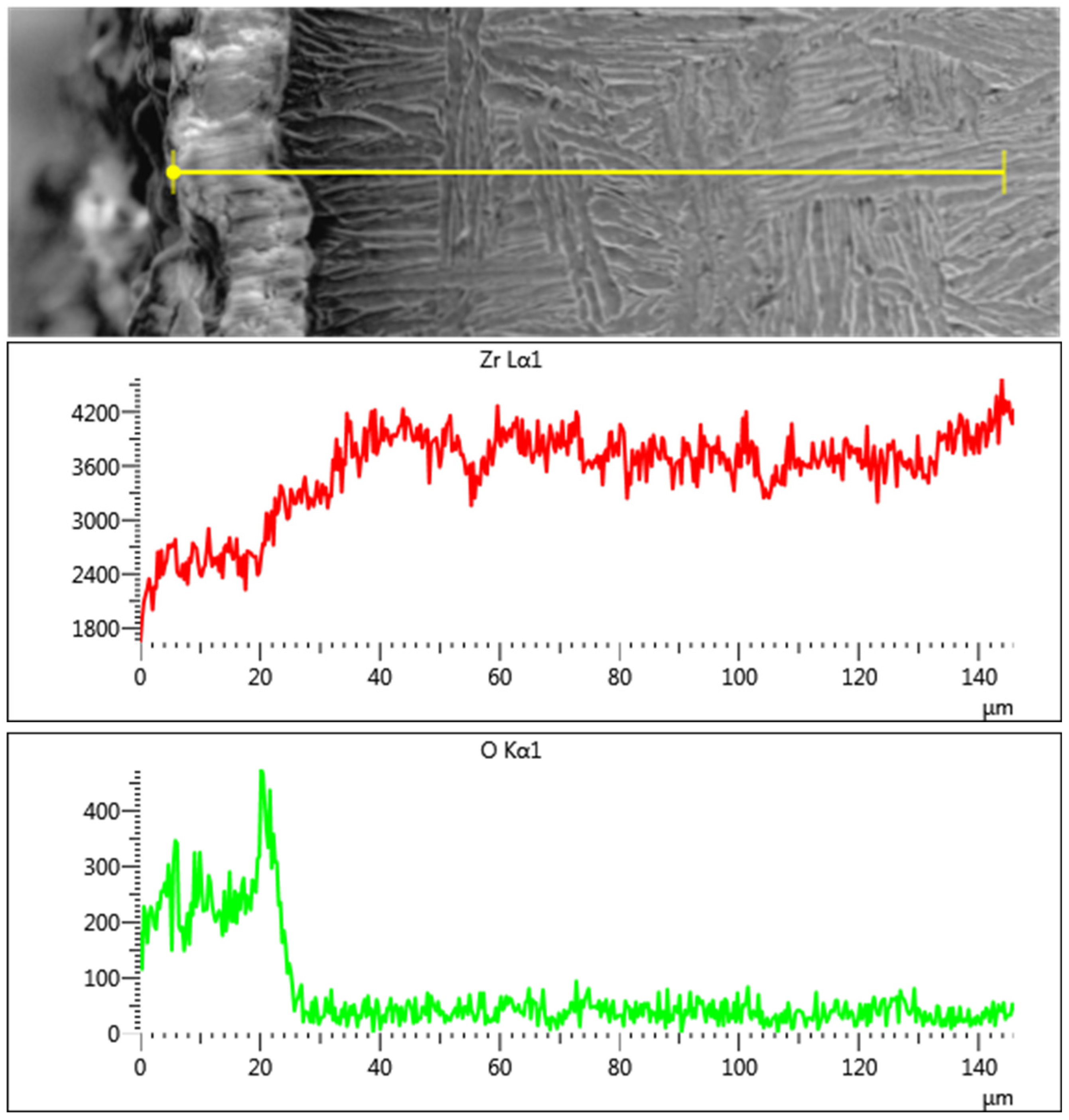
5.3. Analysis of Surface Diffusion Contamination Layer of Castings
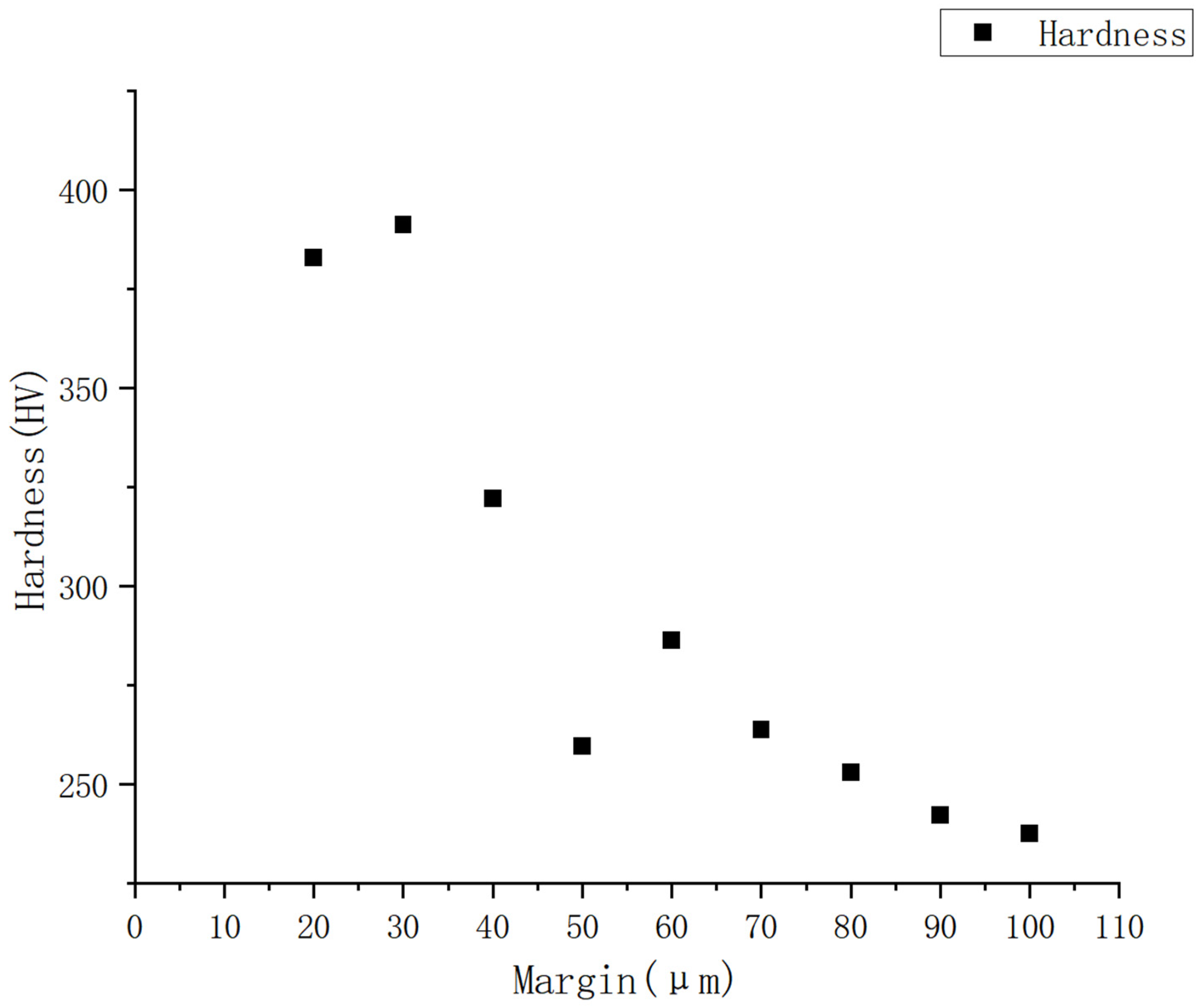
5.4. Mechanical Property Testing
6. Key Points of Welding Quality Control
- (1)
- The actual castings should be slightly thicker than the required castings to allow for later surface finishing and defect removal.
- (2)
- The cutting and gouging of zirconium alloy castings are carried out using oxygen, plasma, or water jet or laser equipment, and it is not recommended to use carbon electrodes for arc cutting, which can easily cause more pollution.
- (3)
- During the thermal cutting process, an oxidation and contamination layer will form near the cutting surface, which should be removed before welding, with a thickness of at least 0.8 mm, 1.6 mm after oxygen, 1.6 mm after plasma, and 0.8 mm after laser.
- (4)
- Sandblasting, shot blasting, and steam sandblasting are used to remove high-temperature oxide layers and lubricants from surfaces. Mechanical descaling media can include alumina, silicon carbide, silica sand, zircon sand, and steel shot, and methods such as ultrasonic cleaning, steam degreasing, or the use of electrolytic alkaline cleaning systems are used to remove grease and lubricants.
- (5)
- Pickling, as the final cleaning step for castings, requires attention: maintaining a ratio of nitric acid to hydrofluoric acid of 10:1 can minimize the casting’s absorption of hydrogen. All oxides and scale should be removed to prevent the finished product from being preferentially etched. After pickling, the product should be immediately rinsed with cold water to avoid the contamination of the surface by residual fluorides or chlorides.
- (1)
- Protective gas: High-purity argon gas (purity > 99.999%) should be used.
- (2)
- Welding wire material and cleanliness: The welding wire should be made of wire with lower levels of N, H, and O, and it must be ensured to be clean before use.
- (3)
- Base material cleaning: It must be ensured that the base material is clean and tidy.
7. Conclusions
- (1)
- Process Optimization: The numerical simulation-guided redesign of the casting system and riser configuration (YH-2 process) effectively addressed shrinkage porosity in thick-walled regions, achieving stable mold filling and meeting stringent quality requirements for high-pressure Zr705C valve bodies. This approach offers a replicable methodology for large-scale zirconium alloy casting production.
- (2)
- Microstructural Insights: The microstructure of Zr705C castings exhibits three distinct zones: a surface fine-grained layer, a transition columnar grain region, and an internal equiaxed grain structure. The metallurgical composition comprises a lamellar α-phase and granular β-phase, with crystallographic orientation relationships between phases confirmed via EBSD analysis.
- (3)
- Surface Contamination Control: A diffusion reaction layer (20–30 μm thick) formed due to melt–shell interactions during casting. This layer, rich in oxygen, necessitates rigorous surface machining and pre-welding treatments to eliminate contamination-induced defects.
- (4)
- Welding Quality Assurance: Comprehensive surface preparation, including mechanical descaling, pickling, and contamination layer removal, is critical to prevent welding cracks. Process parameters such as argon purity (>99.999%) and welding wire cleanliness were identified as key factors for defect-free welds.
- (5)
- Practical Implications: The integration of simulation, optimized casting parameters, and post-processing protocols provides a robust foundation for the domestic production of high-integrity zirconium alloy components, which can effectively reduce reliance on imported castings. Future work should explore advanced mold materials to further minimize contamination and enhance casting surface quality.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.J. Overview of zirconium and zirconium alloys. Titan. Ind. Prog. 2011, 28, 38. [Google Scholar]
- Zinkle, S.J.; Was, G.S. Materials Challenges in Nuclear Energy. Acta Mater. 2013, 61, 735–758. [Google Scholar]
- Odette, G.R.; Zinkle, S.J. Structural Alloys for Nuclear Energy Applications; Elsevier: Amsterdam, The Netherlands; Munich, Germany; Oxford, UK; Cambridge, MA, USA, 2019; ISBN 978-0-12-397046-6. [Google Scholar]
- Hu, J.; Li, G.; Zhang, T.G.; Wang, J.B. Research status of industrial grade zirconium and zirconium alloy properties. Met. World 2020, 3, 23–26. [Google Scholar]
- Zhang, X.Q. Major applications of zirconium in the chemical industry. Rare Met. Mater. Eng. 1983, 2, 84–85. [Google Scholar]
- Luo, X.W.; Luo, F.C. Properties, application, production technology and development prospect of zirconium and hafnium material. Jiangxi Metall. 2009, 29, 17–21. [Google Scholar]
- YS/T 853-2012; Zirconium and Zirconium Alloy Casting Parts. China Standards Press: Beijing, China, 2012.
- ASME 2633-2011; 2633 Cast ASTM B 752-06 Zirconium Alloy Grades 702C and 705C Section VIII, Division 1. American Society for Testing and Materials: West Conshohocken, PA, USA, 2011.
- Kautz, E.; Gwalani, B.; Yu, Z.; Geelhood, T.V.K.; Devaraj, A.; Senor, D. Investigating zirconium alloy corrosion with advanced experimental techniques: A review. J. Nucl. Mater. 2023, 585, 154586. [Google Scholar]
- Jia, Y.J.; Lin, X.H.; Zou, X.W.; Han, W.Z. History, current situation and development trend of zirconium alloy. Prog. Chin. Mater. 2022, 41, 354–370. [Google Scholar]
- Qin, W. Improvement and Application of Zirconium Alloys. Metals 2018, 8, 794. [Google Scholar] [CrossRef]
- Chen, J.H.; Xie, Y.Q.; Wang, Y.T.; Zhou, Z.Q.; Yuan, H.Z.; Wang, L.N. Application of the elastic modulus with zirconium. Sci. Technol. Innov. Appl. 2021, 11, 32–34. [Google Scholar]
- Zhou, Y. Application of zirconium and zirconium alloys in chemical equipment. Chem. Eng. Des. 2003, 13, 19–22. [Google Scholar]
- Chen, X.; Li, Z.K.; Zhou, J.; Tian, F. Overview of the effects of alloy elements on the corrosion resistance of zirconium alloys. Therm. Work. Process 2015, 44, 14–19. [Google Scholar]
- Ma, M.L.; Zhu, Z.B.; Wang, S.J.; Yang, X.D.; Liu, Y.Q. Research progress of domestic zirconium alloy and its casting process. Foundry Eng. 2020, 41, 986–989. [Google Scholar]
- Coleman, C.E. The Metallurgy of Zirconium; International Atomic Energy Agency. IAEA: Vienna, Austria, 2022; ISBN 978-92-0-109221-2. [Google Scholar]
- Wang, S.; Fan, H.Y.; Zhou, C.C.; Zhang, L.J.; Ying, S.H. Research on the melting technology of niobium-containing zirconium alloy. Therm. Process. Process 2008, 9, 15–18. [Google Scholar]
- Yao, Q.; Zhang, Y.W.; Bao, C.L.; You, T. Research on the casting process of zirconium alloy valve body casting. Foundry 2016, 65, 632–638. [Google Scholar]
- Li, B.T.; Chen, G.Y.; Kang, J.Y.; Ali, W.; Qin, Z.W.; Lu, X.G.; Li, C.H. Research status of refractory materials for precision casting and smelting of zirconium alloy. Titan. Ind. Prog. 2017, 34, 6–11. [Google Scholar]
- Klotz, U.E.; Legner, C.; Bulling, F.; Freitag, L.; Faßauer, C.; Schafföner, S.; Aneziris, C.G. Investment Casting of Titanium Alloys with Calcium Zirconate Moulds and Crucibles. Int. J. Adv. Manuf. Technol. 2019, 103, 343–353. [Google Scholar] [CrossRef]
- Tian, Y.W.; Zheng, S.Q.; Li, B.B.; Li, W.D.; Yang, X.D.; Liu, Y.Q. Study on the welding technology of 705C zirconium alloy thick plate GTAW. Therm. Process. Process 2023, 52, 99–101+117. [Google Scholar]
- Zhang, T.G.; Wang, J.B.; Li, G. Research status of welding method of industrial zirconium alloy. Chin. Foreign Entrep. 2020, 2, 154. [Google Scholar]
- Tong, L.; He, X.M.; Diao, L.F.; Sun, W.C.; Cui, S.P. Study on welding and forming of zirconium alloy square pipe. Weld. Tech. 2019, 48, 55–57. [Google Scholar]
- Zhou, Y. Recent research progress of welding technology of industrial grade zirconium and zirconium alloy. Chin. Foreign Entrep. 2019, 34, 87. [Google Scholar]
- Jiang, F. Study on the welding properties of the new zirconium alloy pipe and rod. Weld. Tech. 2019, 48, 40–43. [Google Scholar]
- Wu, J.C. Key points for welding quality control for zirconium pipes. Weld. Tech. 2020, 49, 100–104. [Google Scholar]
- Chen, X.; Chen, W.H.; Jolanta, Ś.; He, W.; Liu, Q. Quasi-in-situ EBSD quantification investigation of the {10-12} deformation twinning in a zirconium alloy. Mater. Charact. 2024, 217, 114443. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, A.; Liu, X.; Ye, X.; Wang, J.; Zhang, Y.; Zhang, Z. An investigation of slip and twinning behavior of a zirconium alloy during plastic deformation based on in-situ SEM-EBSD. J. Alloys Compd. 2025, 1010, 177918. [Google Scholar] [CrossRef]
- Hai, M.N.; Wang, W.; Huang, F.; Xianjun, L.I.; Xiaogang, Y.U.N.; Yongqiang, Z.H.N.; Jixiong, L.I.; Fuchang, X.I.N.; Kuaishe, W.A.G. Research progress of industrial grade zirconium and zirconium alloy welding. Electr. Weld. Mach. 2018, 48, 87–91. [Google Scholar]
- Bi, Y.B.; Chen, B.B.; Sun, Z.Q.; Xu, Z.; Lu, L.; Zhang, X.; Luo, Z. Solid-state welding for dissimilar zirconium alloy under joule heating effect: Material flowing behavior, characteristics, evolution and formation of interface. J. Nucl. Mater. 2024, 600, 155296. [Google Scholar] [CrossRef]
- Wang, H.Z.; Fan, Q.R.; Li, Q.W.; Zhu, Q.M. Welding of zirconium tube. China Chem. Equip. 2011, 13, 44–48. [Google Scholar]
- Li, X.M. Welding of the zirconium pipes. Electr. Weld. Mach. 2006, 36, 57–59. [Google Scholar]
- Deng, J.; Luo, Y.; Yin, J.C. Study on welding technology and properties of zirconium alloy for spent fuel. Guangdong Chem. Ind. 2023, 50, 28–31. [Google Scholar]
- Zhao, Z.M.; Zhang, L.X. On the welding of the zirconium materials. Weld. Tech. 2003, 32, 20–21. [Google Scholar]
- Mikhail, S. Dissimilar welding and brazing of zirconium and its alloys: Methods, parameters, metallurgy and properties of joints. J. Manuf. Process. 2022, 75, 928–1002. [Google Scholar]















| Material | Zr + Hf | Nb | Hf | N | C | H | Fe + Cr | P | O |
|---|---|---|---|---|---|---|---|---|---|
| Zr705C | ≥95.1 | 2.0~3.0 | ≤4.5 | ≤0.03 | ≤0.1 | ≤0.005 | ≤0.3 | ≤0.01 | ≤0.3 |
| 95.465 | 2.22 | 2.05 | 0.0013 | 0.016 | 0.0037 | 0.16 | 0.002 | 0.082 |
| Project | Tensile Strength Rm/MPa | Yield Strength Rp0.2/MPa | Elongation at Break A/% | Brinell Hardness/HBW |
|---|---|---|---|---|
| Standard | ≥483 | ≥345 | ≥12 | ≤235 |
| Sample1 | 509 | 392 | 15 | 169 |
| Sample2 | 499 | 380 | 18.5 | 171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shan, Z.; Zang, Y.; Jin, D.; Bao, C.; Liang, X.; Yao, Q. Casting Process and Quality Control Analysis of Zr705C Zirconium Alloy. Metals 2025, 15, 417. https://doi.org/10.3390/met15040417
Zhang Y, Shan Z, Zang Y, Jin D, Bao C, Liang X, Yao Q. Casting Process and Quality Control Analysis of Zr705C Zirconium Alloy. Metals. 2025; 15(4):417. https://doi.org/10.3390/met15040417
Chicago/Turabian StyleZhang, Youwei, Zhongde Shan, Yong Zang, Dehua Jin, Chunling Bao, Xiao Liang, and Qian Yao. 2025. "Casting Process and Quality Control Analysis of Zr705C Zirconium Alloy" Metals 15, no. 4: 417. https://doi.org/10.3390/met15040417
APA StyleZhang, Y., Shan, Z., Zang, Y., Jin, D., Bao, C., Liang, X., & Yao, Q. (2025). Casting Process and Quality Control Analysis of Zr705C Zirconium Alloy. Metals, 15(4), 417. https://doi.org/10.3390/met15040417








