Effect of Stress on High-Temperature Molten Salt Corrosion of T91 Steel
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Material
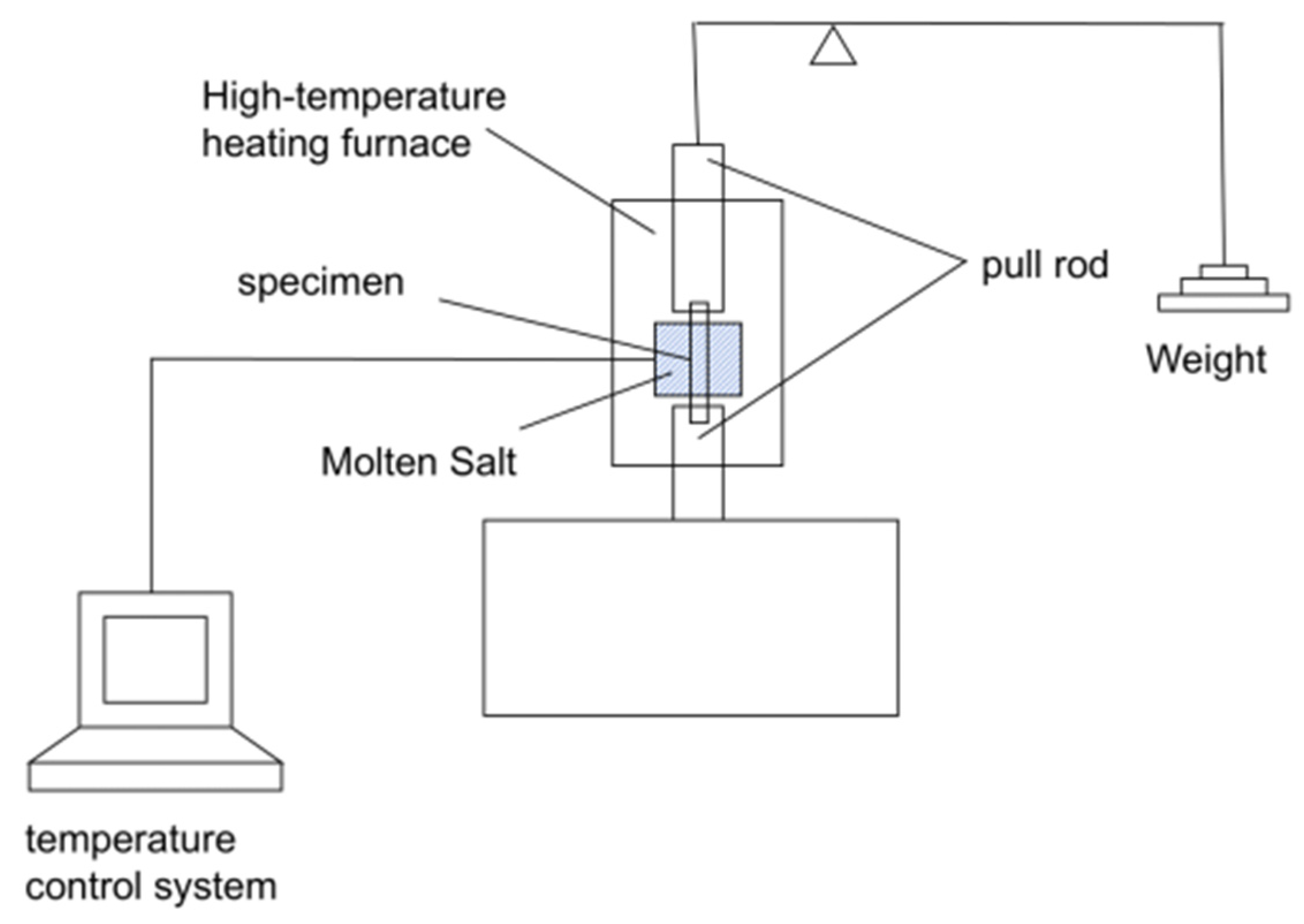
2.2. Experimental Method
3. Results and Discussion
3.1. Surface Morphology
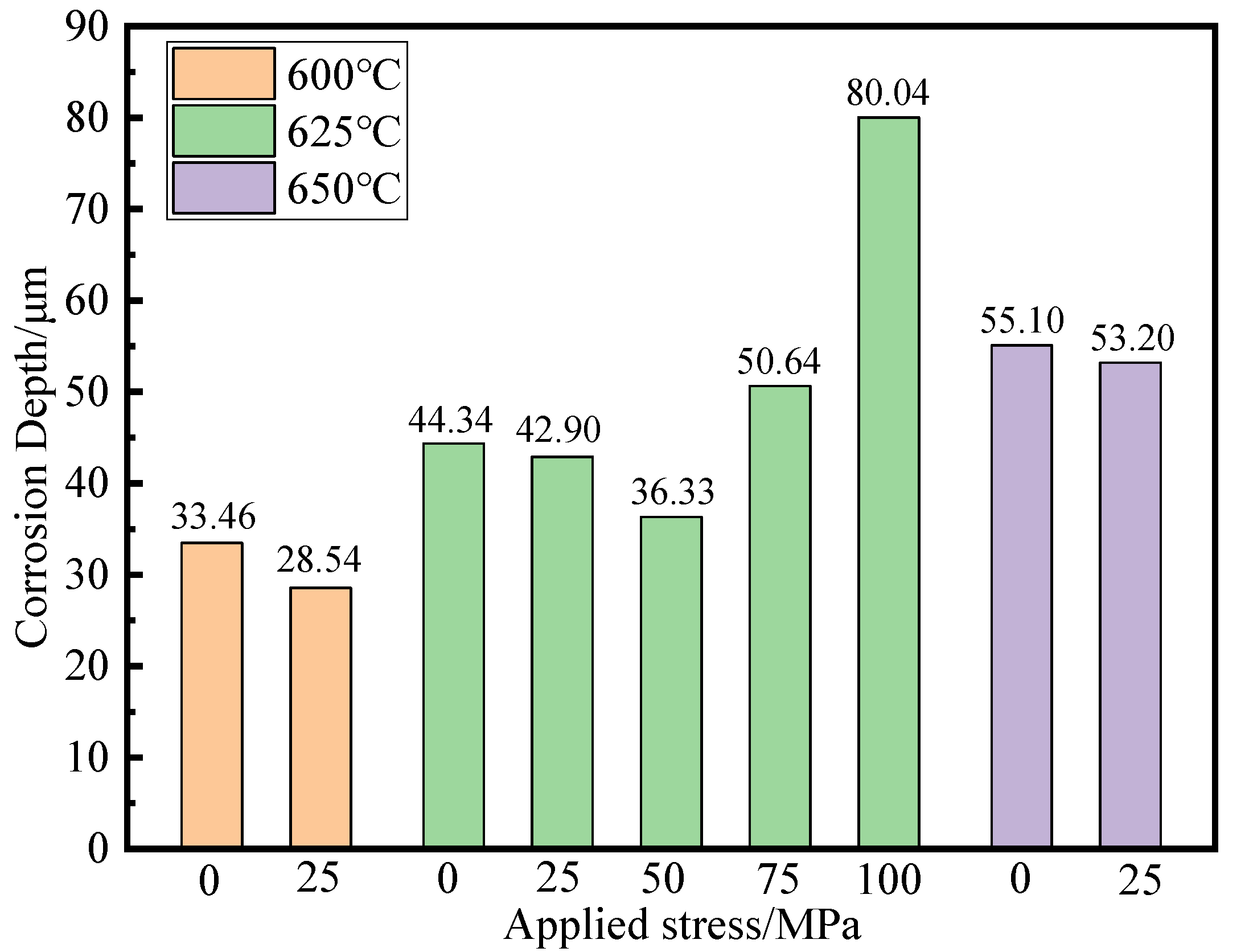
3.2. Thickness of Corrosion Layer
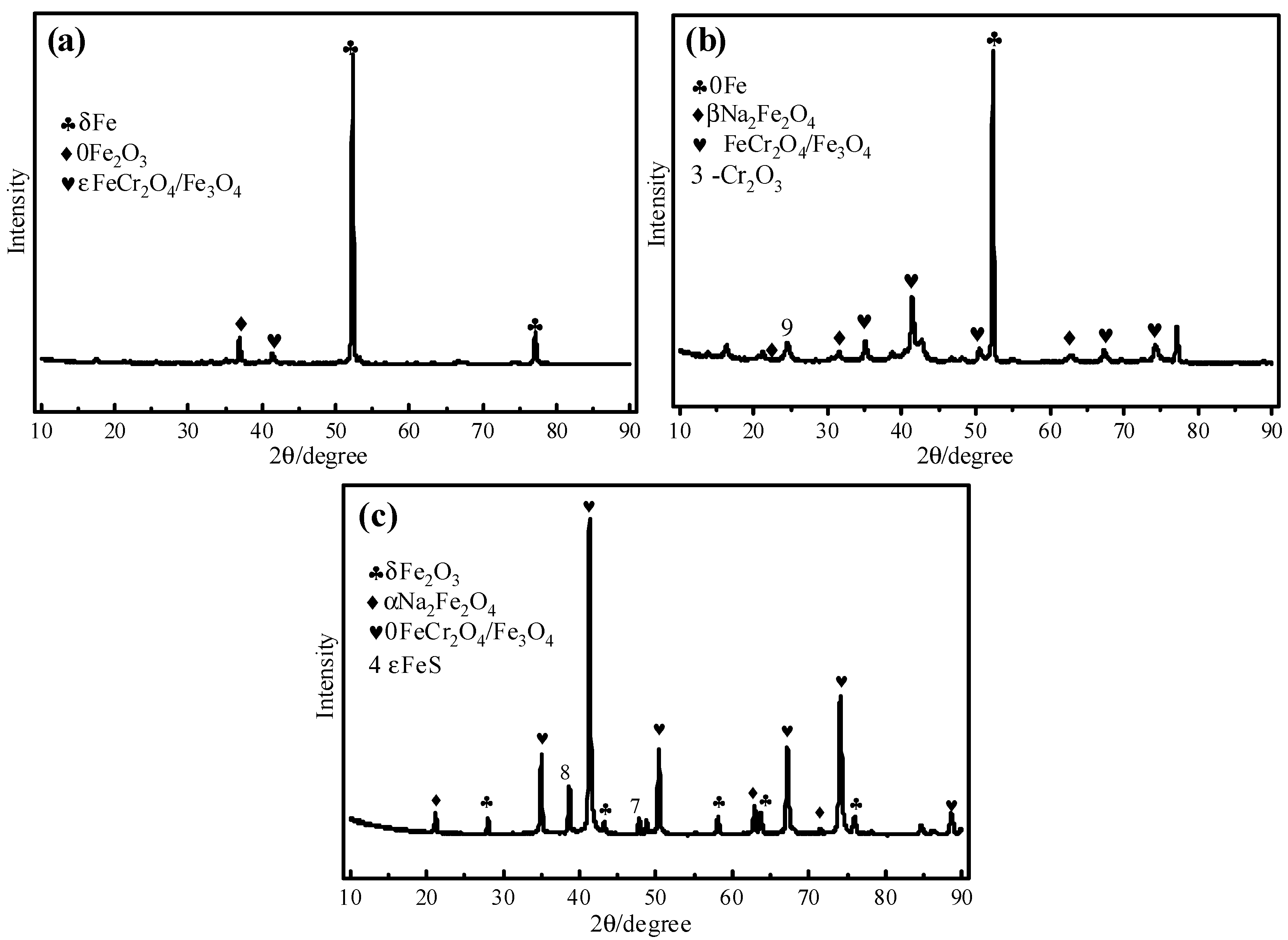
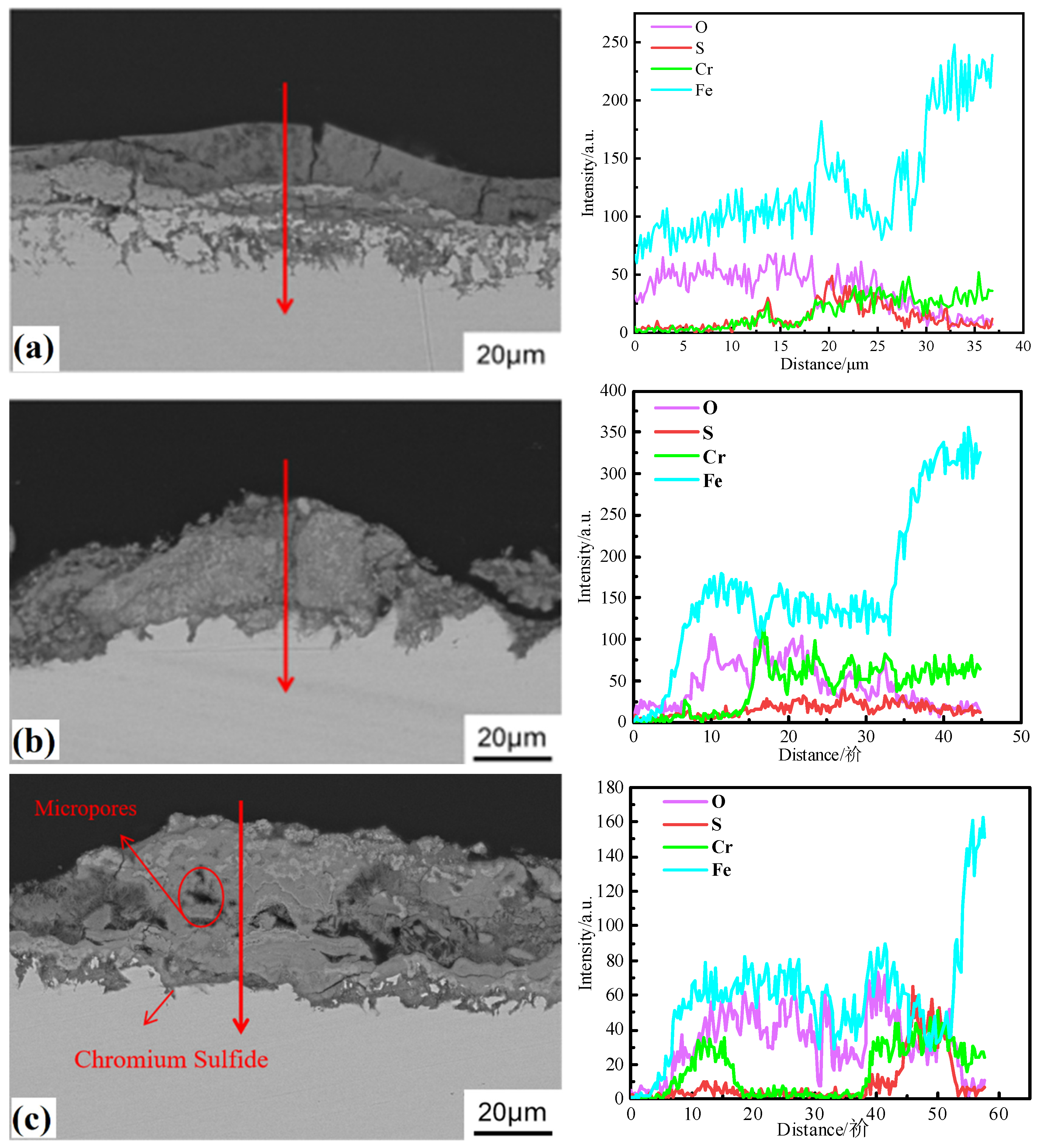
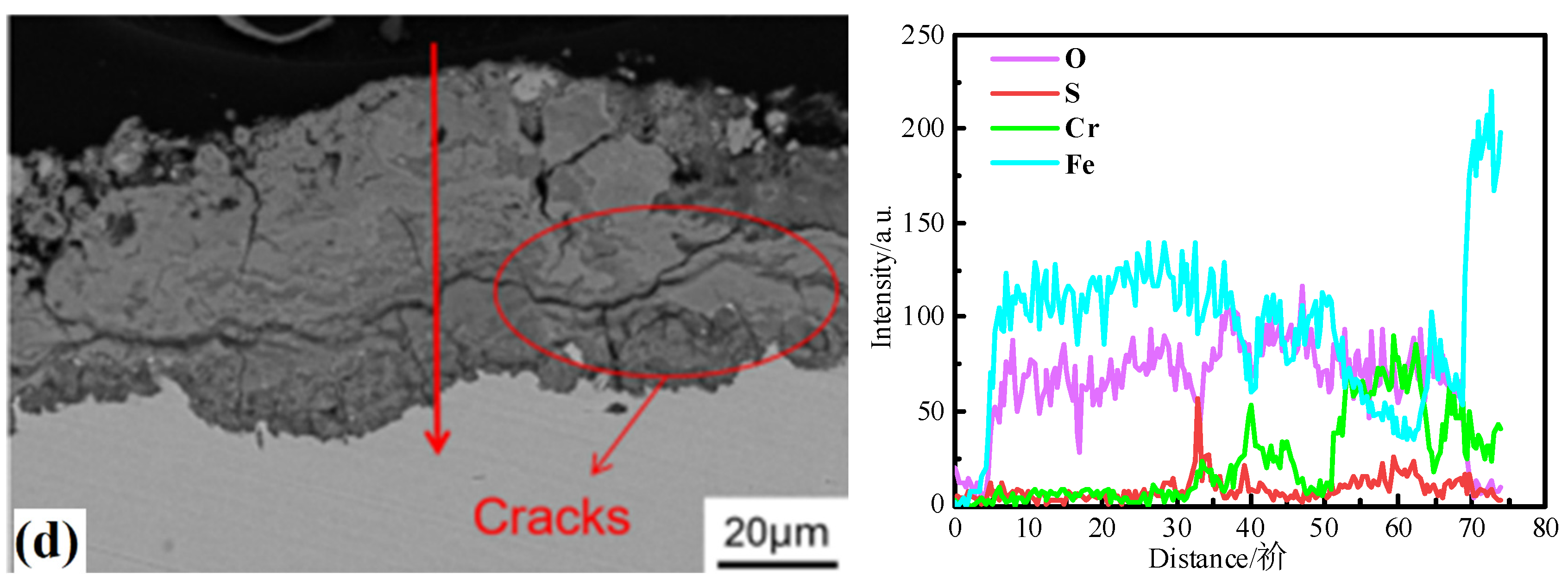
3.3. Corrosion Products
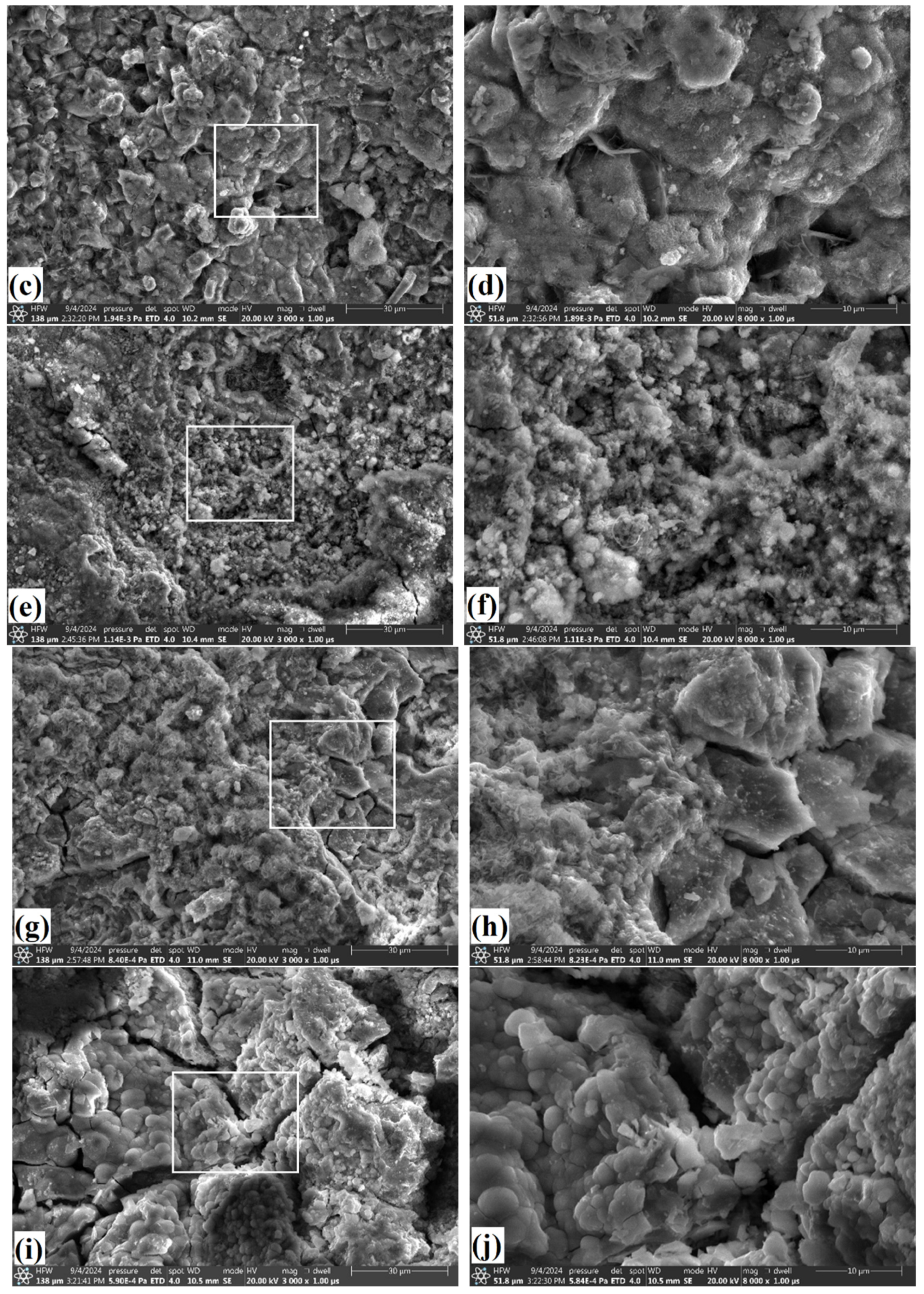
3.4. Oxide Morphology
3.5. Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Viswanathan, R.; Henry, J.F.; Tanzosh, J.; Stanko, G.; Shingledecker, J. US program on materials technology for ultra-supercritical coal power plants. J. Mater. Eng. Perform. 2005, 14, 281–292. [Google Scholar] [CrossRef]
- Natesan, K.; Park, J. Fireside and steamside corrosion of alloys for USC plants. Int. J. Hydrog. Energy 2007, 32, 3689–3697. [Google Scholar] [CrossRef]
- Viswanathan, R.; Bakker, W. Materials for ultrasupercritical coal power plants-Turbine materials: Part II. J. Mater. Eng. Perform. 2001, 10, 96–101. [Google Scholar] [CrossRef]
- Shim, H.S.; Valentine, J.R.; Davis, K.; Seo, S.-I.; Kim, T.-H. Development of fireside waterwall corrosion correlations using pilot-scale test furnace. Fuel 2008, 87, 3353–3361. [Google Scholar] [CrossRef]
- Lutz, B.S.; Holcomb, G.; Meier, G. Determination of the initiation and propagation mechanism of fireside corrosion. Oxid. Met. 2015, 84, 353–381. [Google Scholar] [CrossRef]
- Fu, J.; Li, N.; Zhou, Q.; Guo, P. Impacts of Applied Stresses on High Temperature Corrosion Behavior of HR3C in Molten Salt. Oxid. Met. 2015, 83, 317–333. [Google Scholar] [CrossRef]
- Douglass, D.L.; Kofstad, P.; Rahmel, A.; Wood, G.C. International Workshop on High-Temperature Corrosion. Oxid. Met. 1996, 45, 529–620. [Google Scholar] [CrossRef]
- Schmitt, J.F.; Pacia, N.; Pigeat, P.; Weber, B. Study of the initial oxidation of a Ni-20Cr alloy in the temperature range 550–830 ℃: Influence of mechanical deformation. Oxid. Met. 1995, 44, 429–452. [Google Scholar] [CrossRef]
- Khalid, F.A.; Benjamin, S.E. The Effect of Deformation Substructure on the High-Temperature Oxidation of Inconel 625. Oxid. Met. 2000, 54, 63–71. [Google Scholar] [CrossRef]
- Mathieu, C.; Toesca, S. Effects of mode-I stresses on the oxidation and failure mechanisms of Ni-20Cr and Ni-15Cr-8Fe alloys in sulfur dioxide. Oxid. Met. 1993, 39, 155–165. [Google Scholar] [CrossRef]
- Bell, S.; Stoker, D.; Will, G.; Steinberg, T. Effects of Mechanical Stress on High Temperature Corrosion in Molten Salt. In Proceedings of the International Conference on Concentrating Solar Power and Chemical Energy Systems, Daegu, Republic of Korea, 1–4 October 2019. [Google Scholar]
- ASTM A213/A213M-23; Standard Specification for Seamless Ferritic and Austenitic Alloy-Steel Boiler, Superheater, and Heat-Exchanger Tubes. ASTM: West Conshohocken, PA, USA, 2015.
- Bai, H.; Su, C.; Xie, Y.; Zhang, B.Y.; Wang, Z.T.; Peng, X. High temperature corrosion of laser additively manufactured CoNiCrAlY. Corros. Commun. 2022, 7, 35–42. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, M.H.; Yang, L.L.; Sun, W.Y.; Zhu, S.L.; Wang, F.H. Nanocrystalline coatings on superalloys against high temperature oxidation: A review. Corros. Commun. 2021, 1, 58–69. [Google Scholar] [CrossRef]
- Calvarin-Amiri, G.; Huntz, A.M.; Molins, R. Effect of an applied stress on the growth kinetics of oxide scales formed on Ni-20Cr alloys. Mater. High. Temp. 2001, 18, 91–99. [Google Scholar]
- Liu, Z.H.; Ning, Z.Y.; Zhou, Q.L.; Li, N.; Liu, T.S. Effects of External Stress on High-Temperature Corrosion Behavior of T92 Ferrite Steel with Na2SO4-K2SO4 Molten Salts. Oxid. Met. 2022, 97, 141–155. [Google Scholar] [CrossRef]
- Gonda, D.; Chawla, V.; Puri, D.; Prakash, S. High Temperature Corrosion Behaviour of T-91 and T-22 Bare Steel in 75wt.%Na2SO4+25wt.%NaCl Molten Salt Environment at 900 °C. J. Miner. Mater. Charact. Eng. 2010, 9, 593–606. [Google Scholar]
- Rizhang, Z.; Manjiou, G.; Yu, Z. A study of the mechanism of internal sulfidation-internal oxidation during hot corrosion of Ni-base alloys. Oxid. Met. 1987, 27, 253–265. [Google Scholar] [CrossRef]
- Shang, C.G.; Xin, L.; Xu, Q.L.; Lu, Y.H. Fireside Corrosion of P92 Steel with Mixed Sulfate Deposit at 650 °C. Oxid. Met. 2020, 94, 323–341. [Google Scholar] [CrossRef]
- Fu, J.P.; Zhou, Q.L.; Li, N.; Liu, Z.H.; Liu, T.S. Effects of external stresses on hot corrosion behavior of stainless steel TP347HFG. Corros. Sci. 2016, 104, 103–111. [Google Scholar] [CrossRef]
- Young, B.D.J. High Temperature Oxidation and Corrosion of Metals; Corrosion Series; Elsevier: Amsterdam, The Netherlands, 2008; Volume 1. [Google Scholar]
- Hussain, N.; Shahid, K.A.; Khan, I.H.; Rahman, S. Oxidation of high-temperature alloys (superalloys) at elevated temperatures in air. II. Oxid. Met. 1995, 43, 363–378. [Google Scholar] [CrossRef]
- Nazarov, A.; Vivier, V.; Thierry, D.; Vucko, F.; Tribollet, B. Effect of Mechanical Stress on the Properties of Steel Surfaces: Scanning Kelvin Probe and Local Electrochemical Impedance Study. J. Electrochem. Soc. 2017, 164, 66–74. [Google Scholar] [CrossRef]
- Töpfer, J.; Aggarwal, S.; Dieckmann, R. Point defects and cation tracer diffusion in (CrxFe1-x)3-δO4 spinels. Solid State Ionics 1995, 81, 25–266. [Google Scholar] [CrossRef]
- Chen, Y.; Sridharan, K.; Allen, T.R. Corrosion behavior of ferritic-martensitic steel T91 in supercritical water. Corros. Sci. 2006, 48, 2843–2854. [Google Scholar] [CrossRef]
- Spiegel, M.; Schroer, C.; Grabke, H.J. Corrosion of high alloy steels and Fe-Cr-alloys beneath deposits from waste incinerator plants. Mater. Sci. Forum 1997, 251–254, 527–534. [Google Scholar] [CrossRef]
- Ning, Z.Y.; Zhou, Q.L.; Liu, Z.H.; Li, N.; Luo, Q.L.; Wen, D. Effects of imposed stresses on high temperature corrosion behaviour of T91. Corros. Sci. 2021, 189, 109595. [Google Scholar] [CrossRef]
- Calvarin-Amiri, G.; Molins, R.; Huntz, A. Effect of the application of a mechanical load on the oxide-layer microstructure and on the oxidation mechanism of Ni-20Cr foils. Oxid. Met. 2000, 53, 399–426. [Google Scholar] [CrossRef]
- Rahmel, A.; Wood, G.; Kofstad, P.; Douglass, D. International Workshop on “Critical Issues Concerning the Mechanisms of High-Temperature Corrosion”. Oxid. Met. 1985, 23, 253–337. [Google Scholar] [CrossRef]
- Barnard, B.; Liaw, P.; Buchanan, R.; Klarstrom, D. Affects of applied stresses on the isothermal and cyclic high-temperature oxidation behavior of superalloys. Mater. Sci. Eng. A 2010, 527, 3813–3821. [Google Scholar] [CrossRef]
- Sánchez, L.; Bolívar, F.J.; Hierro, M.P.; Pérez, F.J. Temperature dependence of the oxide growth on aluminized 9–12%Cr ferritic-martensitic steels exposed to water vapour oxidation. Thin Solid Films 2009, 517, 3292–3298. [Google Scholar] [CrossRef]




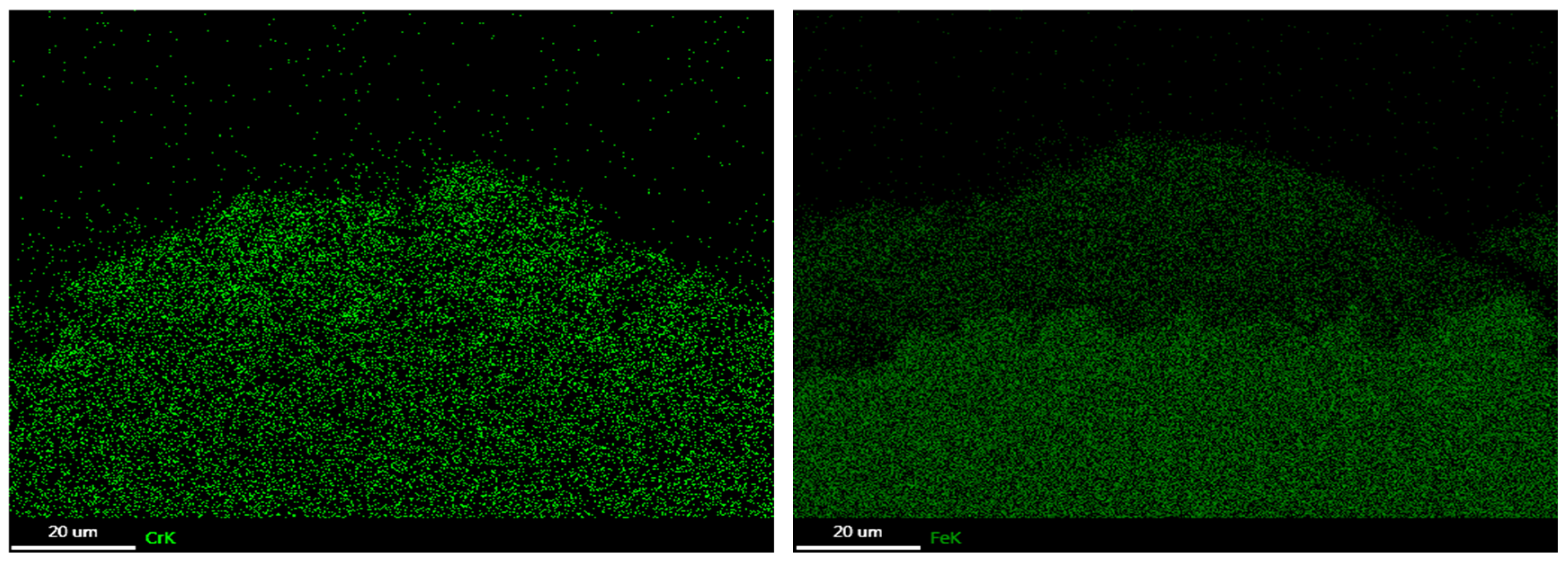
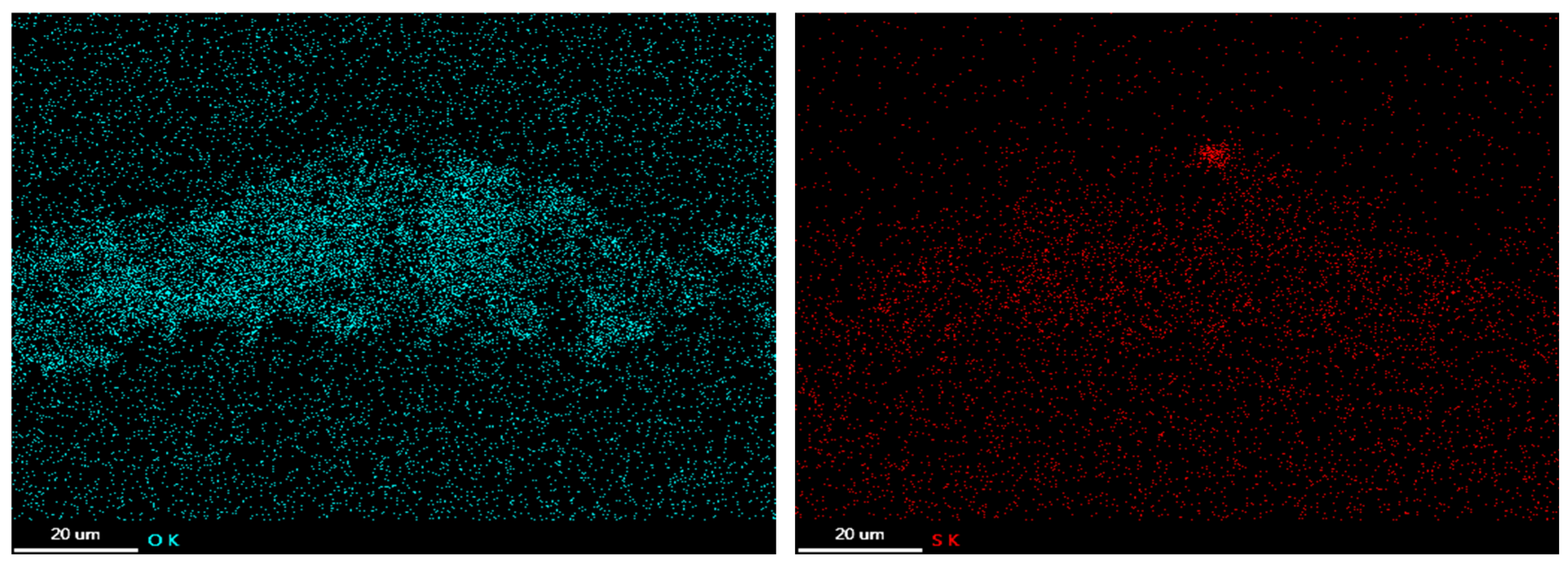
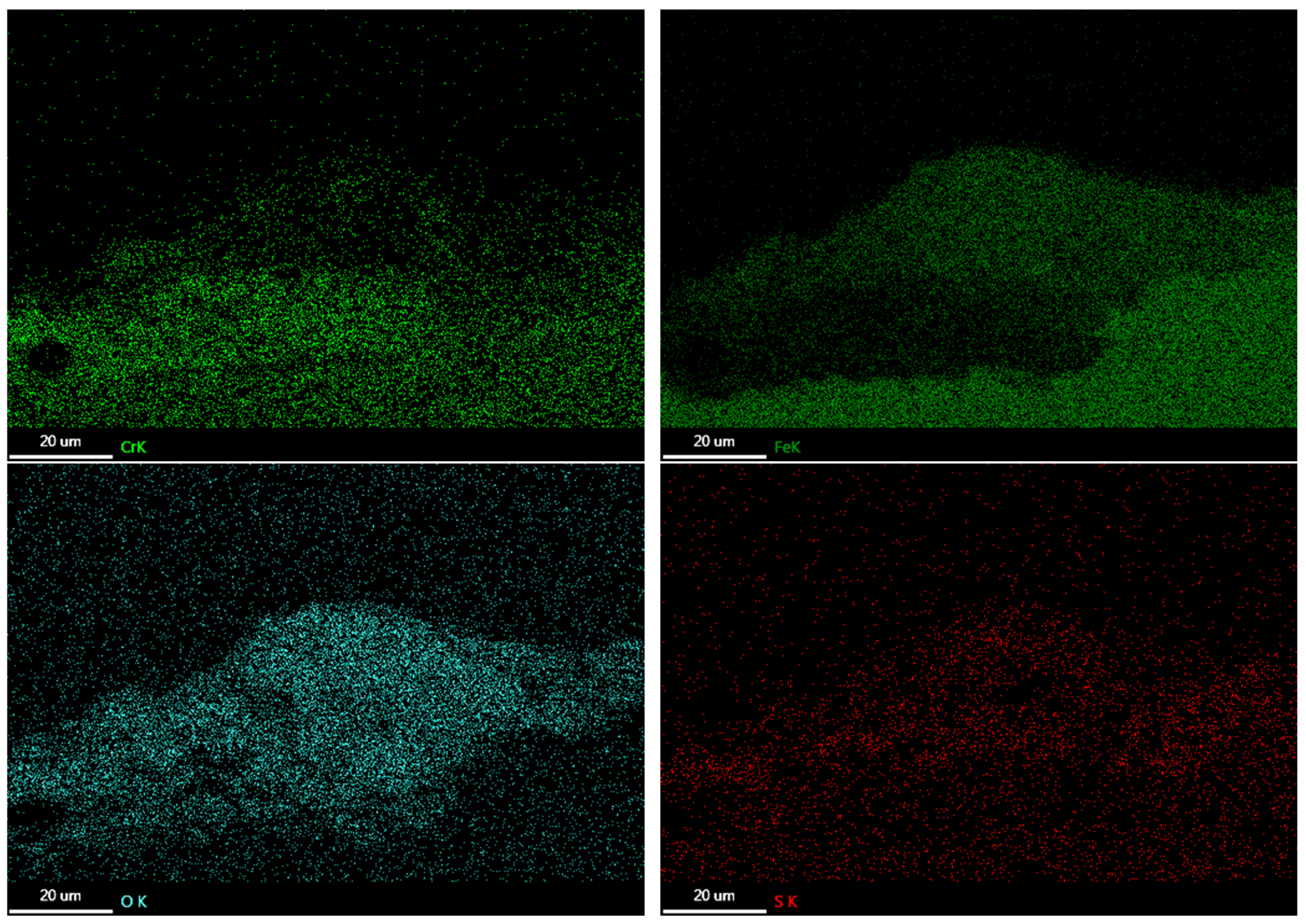
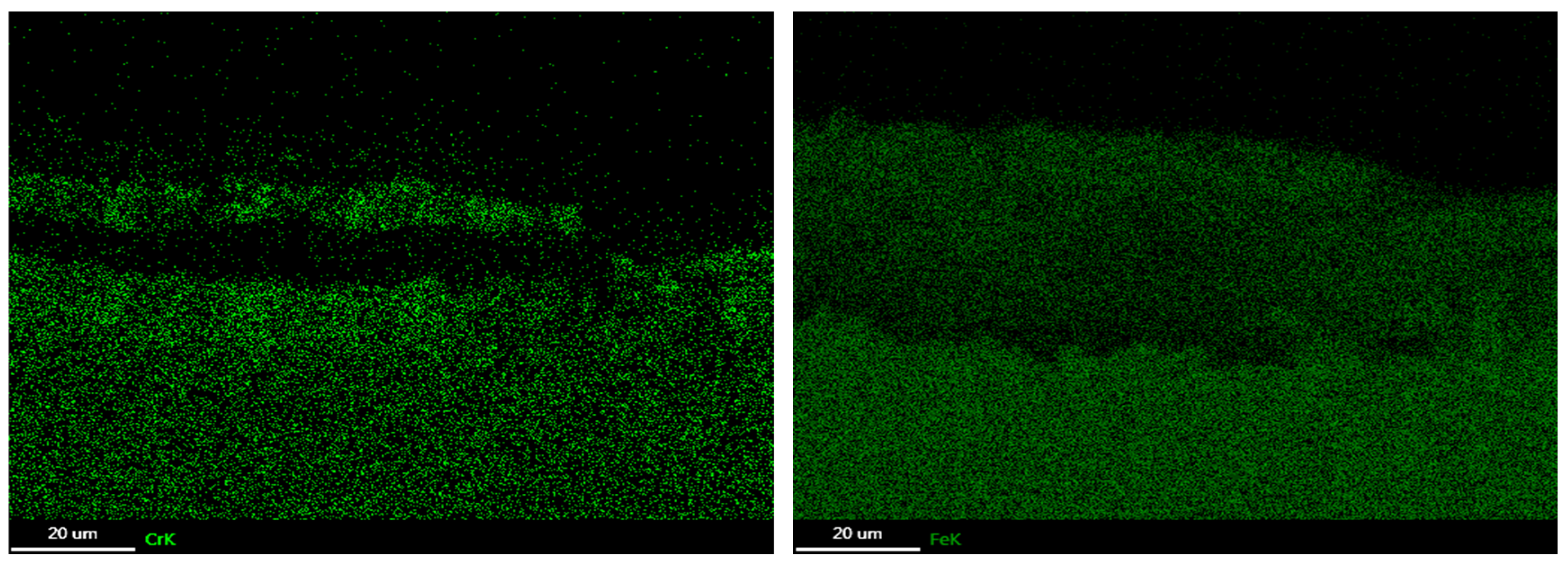

| C | Si | Mn | P | S | Ni | Cr | Mo | V | Nb | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.41 | 0.11 | ≤0.01 | 0.07 | 8.51 | 0.92 | 0.19 | 0.07 | Bal. |
| Element | 600 °C 0 MPa | 600 °C 25 MPa | 650 °C 0 MPa | 650 °C 25 MPa | 625 °C 0 MPa | 625 °C 25 MPa | 625 °C 50 MPa | 625 °C 75 MPa | 625 °C 100 MPa |
|---|---|---|---|---|---|---|---|---|---|
| O | 29.6 | 33.3 | 21.6 | 56.5 | 23.3 | 33 | 30.5 | 35.3 | 9.4 |
| S | 7.9 | 1.6 | 3.3 | 1.1 | 0.9 | 7.1 | 10.8 | 3.1 | 1.6 |
| Cr | 1.4 | 1.1 | 3.1 | 0.8 | 2.1 | 0.8 | 10 | 32.3 | 44.3 |
| Fe | 61.1 | 64 | 72 | 41.6 | 73.7 | 59 | 48.7 | 29.3 | 44.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, K.; Shi, B.; Ma, S.; Li, P.; Zhu, Z. Effect of Stress on High-Temperature Molten Salt Corrosion of T91 Steel. Metals 2025, 15, 446. https://doi.org/10.3390/met15040446
Yan K, Shi B, Ma S, Li P, Zhu Z. Effect of Stress on High-Temperature Molten Salt Corrosion of T91 Steel. Metals. 2025; 15(4):446. https://doi.org/10.3390/met15040446
Chicago/Turabian StyleYan, Kai, Bingjie Shi, Shaohai Ma, Peihan Li, and Zhongliang Zhu. 2025. "Effect of Stress on High-Temperature Molten Salt Corrosion of T91 Steel" Metals 15, no. 4: 446. https://doi.org/10.3390/met15040446
APA StyleYan, K., Shi, B., Ma, S., Li, P., & Zhu, Z. (2025). Effect of Stress on High-Temperature Molten Salt Corrosion of T91 Steel. Metals, 15(4), 446. https://doi.org/10.3390/met15040446





