Abstract
Copper-containing sludge and spent petrochemical catalyst (SPC) were investigated for recovering palladium (Pd) and silver (Ag). Increasing the mixing ratio of alumina-based SPC leads to reduced recovery rates at 1500 °C. Specifically, as the SPC mixing ratio increases from 10% to 30%, the recovery rate of Pd and Ag sharply decreases to 62.1% and 91.0%, respectively. This is attributed to an increase in the slag viscosity as well as to the higher sulfur content in the metal phase by decreasing the CaO/Al2O3 ratio of the slag. An increase in the slag viscosity causes a decrease in the metal recovery, as it lowers the settling velocity of metal droplets, resulting in imperfect metal separation, i.e., an increase in physical loss. Additionally, the presence of sulfur at the slag–metal interface was found to reduce interfacial tension, facilitating the entrapment of copper droplets within the slag. This further hindered phase separation and contributed to an increase in physical loss. This study highlights that physical loss is more serious in metal recovery rather than chemical loss, which is dependent on the thermochemical solubility of the target metals in the slag. The results emphasize the need for the precise control of slag properties to maximize the metal recovery processes in conjunction with a mitigation of CO2 emissions.
1. Introduction
Rapid industrialization has resulted in the production of millions of tonnes of waste worldwide. The global production of electronic waste in 2022 reached approximately 62 million tonnes (Mt), with only 22.3% being documented as properly managed through collection and recycling processes [1]. The management of solid and household waste around industrial sites is often poorly designed, resulting in disposal in landfills. Such poor management methods threaten human health and can lead to severe ecological harm. The improper management of waste can result in contamination from metals, which, upon leaching into the environment, can cause significant ecological problems. However, these wastes also serve as potential sources of valuable metals, functioning as anthropogenic ores for the recovery of heavy metals. Spent catalysts, discarded batteries, red mud, municipal solid waste incineration ash, electroplating residue (sludge), and electronic scrap (e-scrap) contain a variety of elements, including Au, Ag, and Pd, which are ideal for recycling [2].
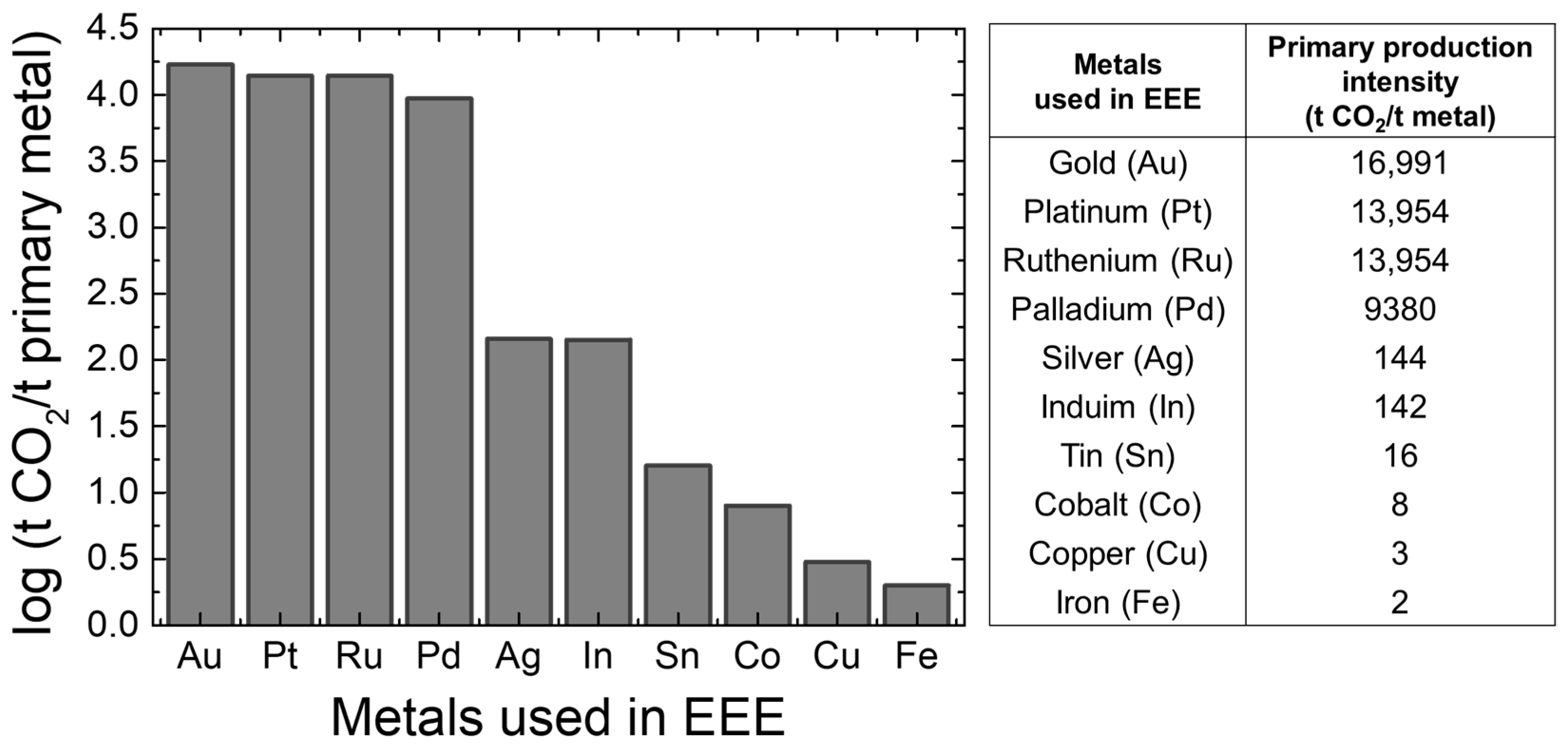
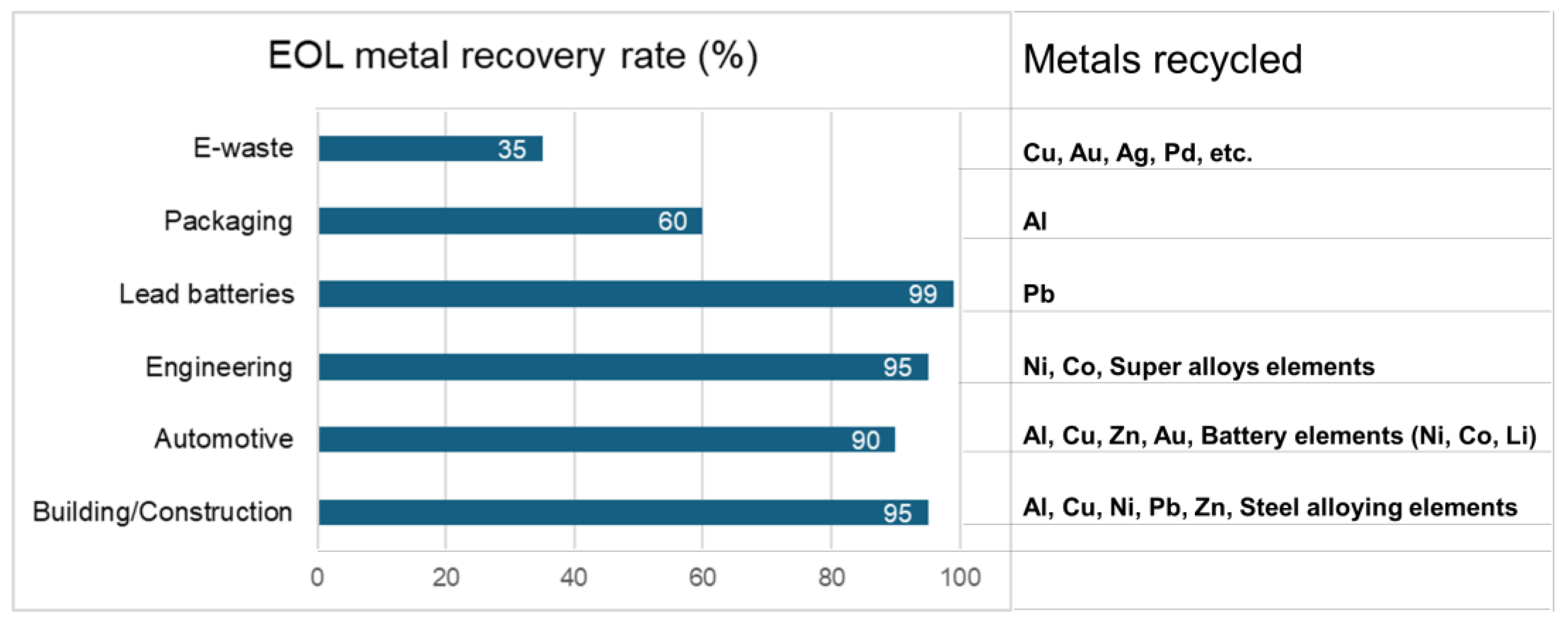
Recycling valuable metals is more energy-efficient and environmentally friendly than extracting metals from natural ore. As shown in Figure 1, producing valuable metals like Pd and Ag releases 9380 tons and 144 tons of CO2 per ton of metal, respectively [3,4]. Consequently, the primary production of metals needed for electrical and electronic equipment (EEE) from natural resources results in the emission of 23.4 million tonnes of CO2, which is nearly 1/2000 of the global CO2 emissions. Consequently, approaches to managing industrial waste, including reuse, remanufacturing, and materials recovery, have received significant interest. These strategies enhance long-term economic efficiency and enable metal recovery enterprises to achieve sustained profitability [5]. Specifically, as shown in Figure 2, the recovery rate of valuable metals from the end-of-life (EOL) material sources are relatively high (90–99%) in building and construction, and in the automotive industries, engineering alloys, and lead batteries. However, the recovery of Al from packaging and that of Cu, Au, Ag, and Pd from e-scrap is not more than about 60% and 35%, respectively.
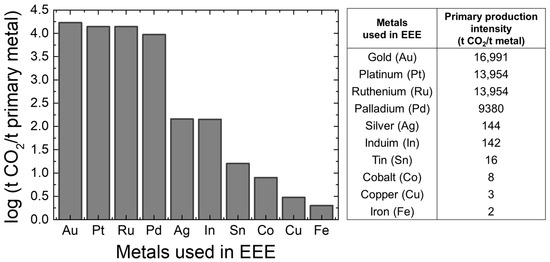
Figure 1.
CO2 emissions per tonne of primary metal produced.
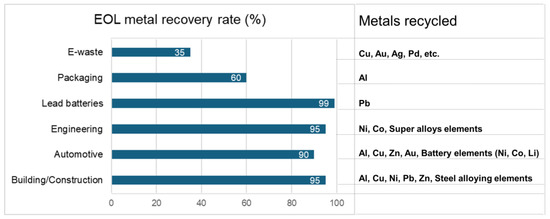
Figure 2.
Metal recovery rate of end-of-life (EOL) sources.
The annual production of municipal solid waste worldwide is expected to reach around 3.5 billion tonnes by 2050 [6], which highlights the necessity for improved recovery technologies. Various methods, such as pyrometallurgical, hydrometallurgical, electrochemical, and bio-hydrometallurgical processes, are used to extract valuable metals, which are crucial for reducing the environmental impact of waste and for utilizing valuable resources from these waste streams. Pyrometallurgical recovery entails the thermal treatment of ore and waste materials containing metals, which results in physical and chemical transformations that enable the extraction of valuable metals.
From the perspective of pyrometallurgical processing, extensive studies have been conducted on the behavior and recovery of valuable metals. Avarmaa et al. [7] conducted a study to determine the distribution coefficients for the valuable metals Rh, Ag, Pt, Au, and Pd between slag and copper matte within FeOx/SiO2 slag at 1250–1350 °C. In their findings, the solubility of silver in the slag ranged from 40 to 80 ppm, and for other valuable metals, solubility in iron silicate slag ranged from 1 to 20 ppm, decreasing with increasing matte grade. The study found that the distribution coefficients of these metals increased with a matte grade and were minimally affected by temperature. The highest distribution coefficients were observed for rhodium and the lowest for silver. Wan et al. [8] investigated the behavior of copper concentrate, iron oxide–silica slag, and PMs (Au, Ag, Pt, and Pd) by pre-mixing and reacting them at a typical copper smelting temperature of 1300 °C in both air and argon atmospheres. They reported that, as the molten matte and slag began to form, the studied valuable metals almost instantly migrated to the matte phase. The order of the distribution coefficients in both air and argon atmospheres was observed to be Pd, followed by Pt, Au, and Ag.
Jiang et al. [9] carried out smelting by mixing the leaching residue with NiSO4, a reducing agent such as carbon, a slagging agent (e.g., CaO, SiO2, and Al2O3), and flux (e.g., Na2CO3, CaF2 and Na2B4O7) in various ratios at 1400 °C for 2 h. They then performed acid leaching on the obtained alloy to determine the recovery rates of PGMs, which were 90.13% for Pt, 91.02% for Pd, and 90.54% for Rh under optimal smelting conditions. After acid leaching, the enrichment ratio of PGMs increased by 8.66 times compared to the raw materials. Ghodrat et al. [10] investigated the thermodynamic analysis of secondary copper smelting using PCBs, and predicted the recovery rates for two defined scenarios, SCE1 (copper recycling with addition of PCB waste) and SCE2 (general copper recycling) at temperatures of 1100–1600 °C and oxygen partial pressures of p(O2) = 10−7–10−10 atm. The study found that the copper recovery rate in the SCE1 scenario was 83.3%, higher than the 78.8% in the SCE2 scenario. Additionally, the recovery rates of valuable metals (Ag, Au) were predicted to be 88.5% and 96.5%, respectively. The analysis highlighted that optimal reduction conditions () and temperatures above 1300 °C are desirable for the process.
Park [11] conducted a fundamental study on the recovery behavior of Au, Ag, and Ni using CaO–Al2O3–SiO2 and CaO–SiO2–CaF2 slag from waste PCB at high temperatures. This study aimed to extract valuable metals like silver and gold, along with essential rare metals such as nickel. The calcium fluorosilicate slag containing CaF2 was controlled to have a lower melting point and viscosity than calcium aluminosilicate slag, resulting in higher recovery of Au, Ag, and Ni. Kim et al. [12] tested mixtures of incinerated PCB and automobile catalysts in ratios of 70:30, 78:22, and 84:16, melting at 1250 °C for 60 min. The most effective slag composition was 28CaO–45SiO2–17Al2O3–10MgO (wt%), with recoveries of 99.0% for Au, 98.0% for Pd, and 98.5% for Pt. They demonstrated that valuable metals are recoverable from spent PCB and automobile catalysts by utilizing metals from PCB as a collecting bath and automobile catalysts as a slag former. Park et al. [13] conducted a smelting experiment to recover valuable metals, such as gold, silver, nickel, and tin, from the insoluble residue of waste mobile phones. They used different synthetic flux compositions with varying CaO/SiO2 ratios to control the slag composition. A high-silica flux (34CaO/61SiO2/5Al2O3, wt%) showed poor recovery rates for Au, Ni, and Sn because of its high viscosity. Martinez et al. [14] investigated the recovery of platinum group metals (PGM) from waste automobile catalyst monolithic honeycomb using a method combining pyrometallurgical and electrolysis steps. The pyrometallurgical step enriched the PGM and prepared conductive material for the electrolysis, which was performed in a molten chloride electrolyte, achieving a recovery rate of nearly 100% PGM.
The present study focused on extracting valuable metals from industrial waste using a pyrometallurgical process. Two industrial waste materials, i.e., copper-containing sludge and spent petrochemical catalyst (SPC) were used to recover silver and palladium, respectively. We focused on the physicochemical properties of the slag that affect the recovery rates of target elements.
2. Experimental Procedure
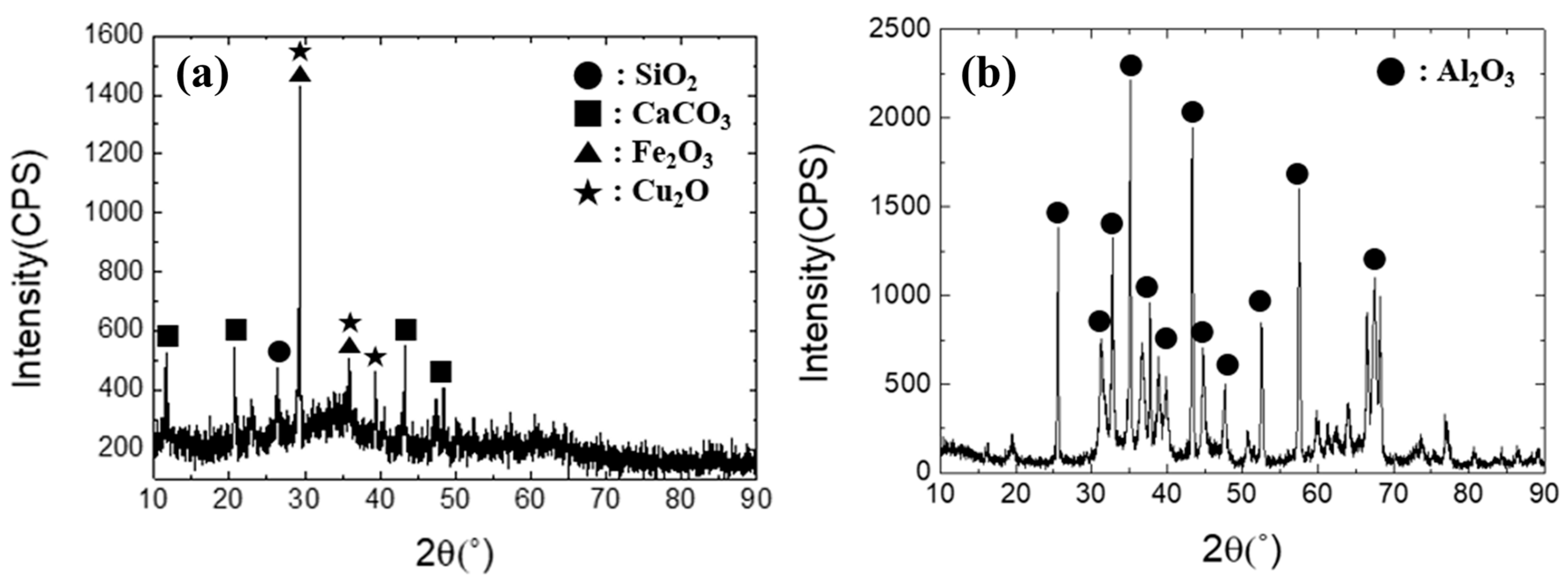
The industrial waste used in the present study was copper sludge and spent petrochemical catalyst (SPC), with an image of each shown in Figure 3. The X-ray diffraction patterns of each material are shown in Figure 4. The chemical compositions of the industrial waste were examined using an inductively coupled plasma atomic emission spectroscopy (ICP-AES, SPECTRO ARCOS, Kleve, Germany) and a combustion analyzer (CS800, ELTRA, Haan, Germany). Details of the findings are presented in Table 1. The Al2O3-based SPC was mixed with copper-containing sludge at a proportion varied from 10% to 30% SPC. The objective of this mixing condition is to ensure that the Al2O3 content in the slag does not exceed 30 wt% in order to avoid a significant rise in the melting point of the slag.
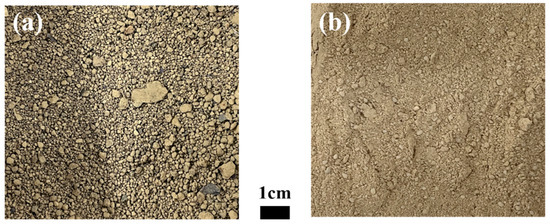
Figure 3.
Image of the materials used in the experiments: (a) copper sludge; (b) SPC.
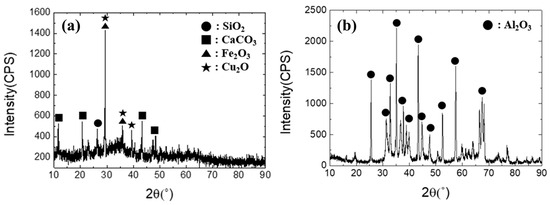
Figure 4.
X-ray diffraction patterns of (a) copper sludge and (b) SPC.

Table 1.
Composition of copper sludge and spent petrochemical catalysts (wt%).
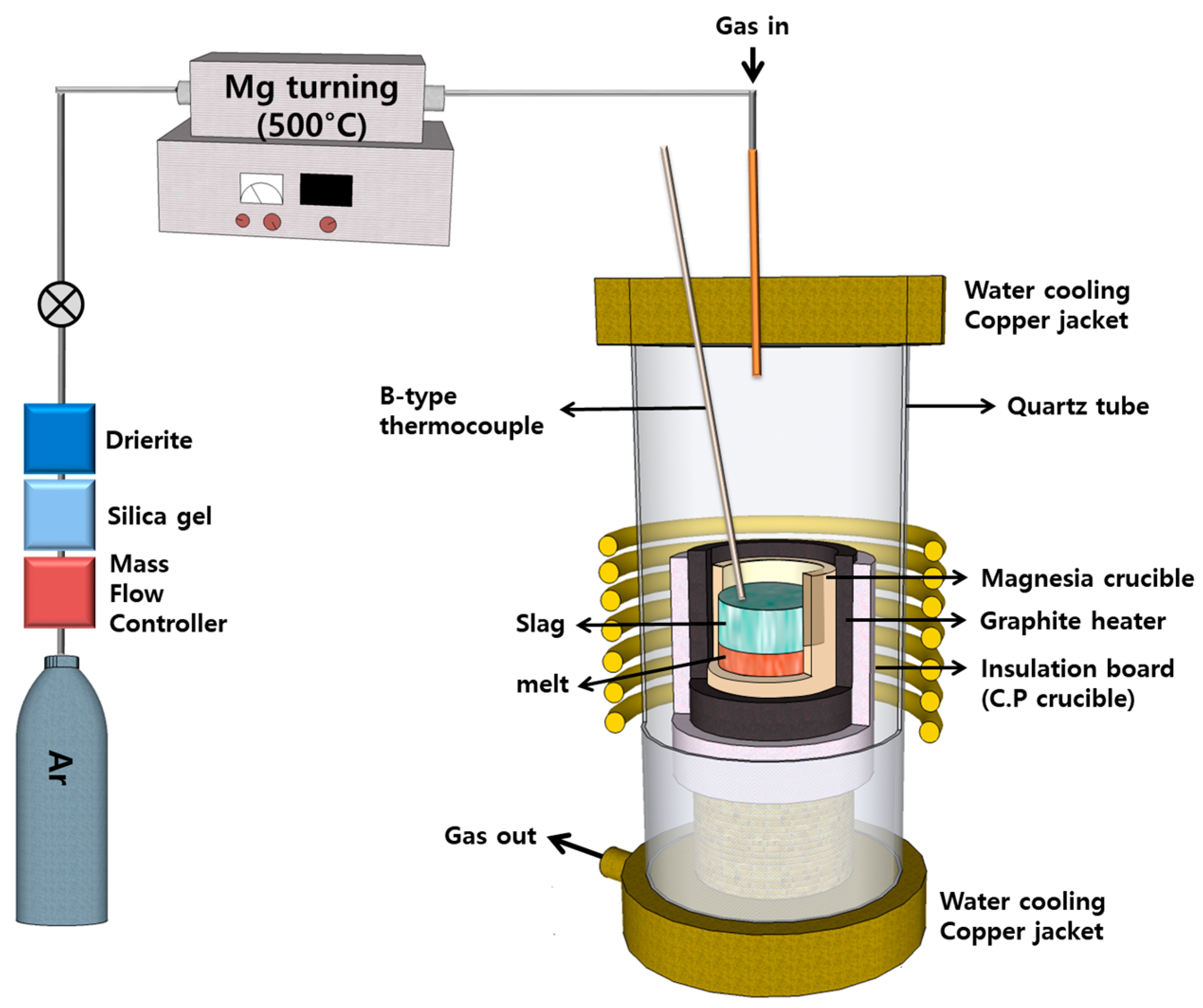
Three experiments were conducted utilizing a high-frequency induction furnace equipped with a graphite heating component. A schematic diagram of the experimental apparatus is presented in Figure 5. To ensure efficient heating, 100 g of a blend consisting of copper sludge and SPC, 60 g of copper powder to create a 5 mm melting pool, and about 3 g of solid carbon to facilitate the reduction reaction were placed in a fused magnesia crucible (60 × 50 × 120, mm) with a graphite heating element. The furnace was supplied with purified Ar gas at a flow rate of 500 mL/min, regulated by a mass flow controller. After maintaining the reduction reaction for 60 min at 1500 °C, the samples were cooled in an Ar gas atmosphere.
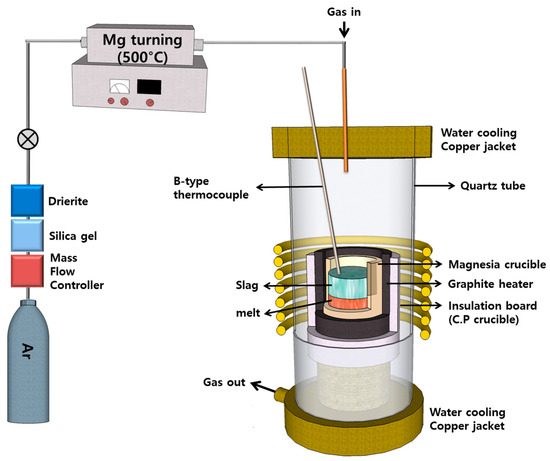
Figure 5.
Schematic diagram of the experimental apparatus.
To perform the chemical analysis, the slag was pulverized to a fine powder with particle sizes lower than 100 μm. Valuable metal concentration and the final composition of the slag were analyzed using ICP-AES and CS800.
For SEM imaging of the slag, samples were cold mounted in molds and the surfaces were ground using sandpaper. Metal droplets dispersed in the slag were examined with field emission scanning electron microscopy and energy-dispersive X-ray spectroscopy (FE-SEM and EDS, Nova Nano SEM 450, FEI, Waltham, MA, USA).
3. Results and Discussion
All analytical data for the metal ingots and slag obtained from the smelting experiments are listed in Table 2 and Table 3, respectively.

Table 2.
Composition of metal ingot after the experiments.

Table 3.
Composition of slag phase after the experiments.
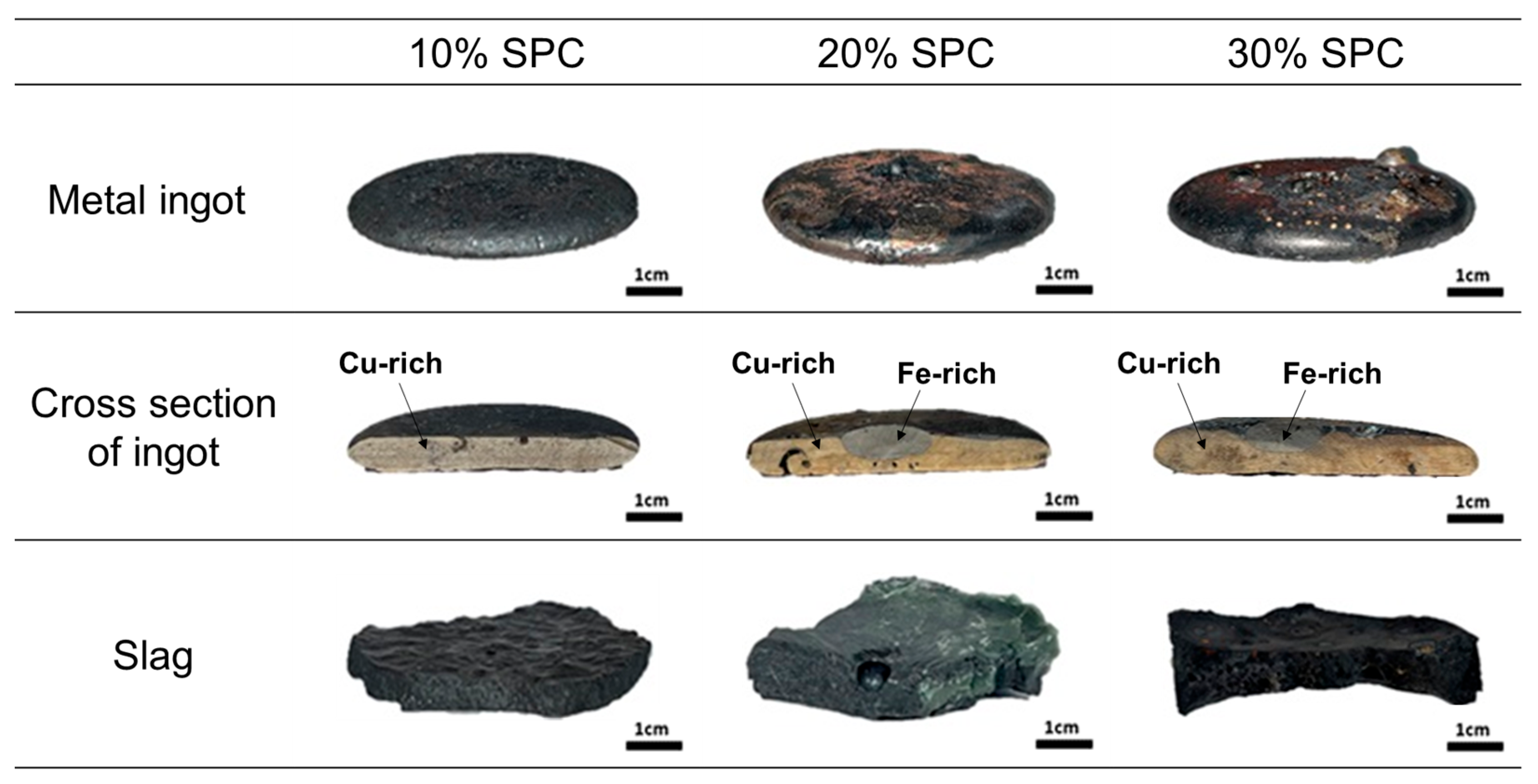
After the experiment, quenched samples were obtained. As shown in Figure 6, the metal ingot, primarily composed of a copper-rich phase and an iron-rich phase, and the slag were successfully separated. The amount of copper present in the slag is crucial because copper metal droplets significantly influence the overall metal recovery rate.
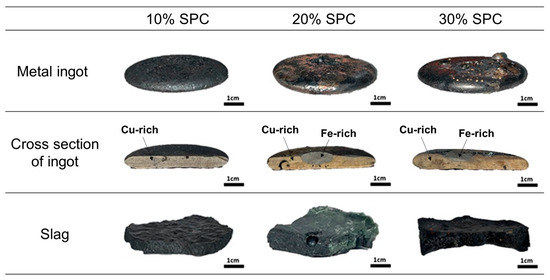
Figure 6.
Morphology of metal ingot and slag separated after experiments.
In the current study, metal recovery was evaluated by employing Equation (1) [11,12,13,14,15,16].
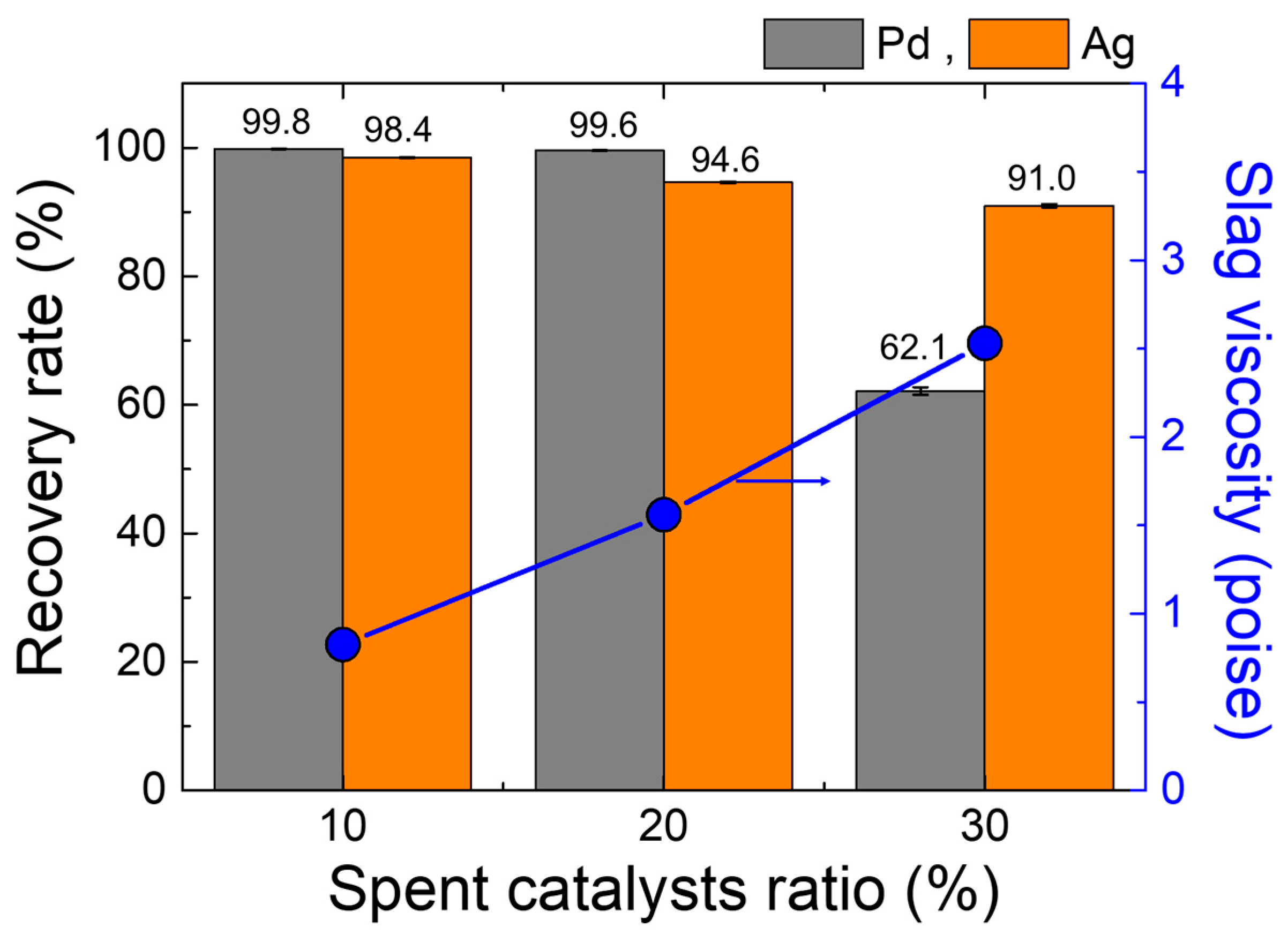
The recovery rate of Pd and Ag calculated from Equation (1) and the content of each element in metal ingot and slag given in Table 2 and Table 3 is presented in Figure 7. It is demonstrated that, as the SPC ratio increases, the recovery rate of Pd and Ag decreases. A decreasing tendency in the recovery rate is significant in the case of the Pd, i.e., it drops sharply when the SPC ratio increases to 30%. This trend aligns with the changes in copper content in the slag, i.e., the total copper content in the slag increases from about 0.6 wt% to 1.8 wt% with increasing the ratio of SPC from 10% to 30% (Table 3). Hence, it is suggested that the copper content in the slag is a critical factor influencing the recovery rates of valuable metals.
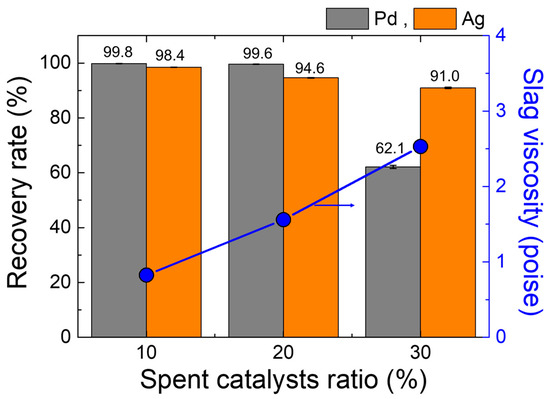
Figure 7.
Recovery rate of Pd and Ag, and slag viscosity as a function of the SPC mixing ratio.
The copper content in the slag is closely related to the slag viscosity, which directly impacts the efficiency of physical separation between the slag and the metal. In particular, an increase in the SPC ratio results in a decrease in the CaO/Al2O3 (=C/A) ratio of the slag from C/A = 2.6 to 0.8 (Table 3), and this change likely influenced the viscosity and physical properties of the slag. Several researchers have investigated how the structure affects the viscosity of calcium aluminosilicate melts with low silica content (SiO2 < 10 wt%), which is similar to the present slag system [17,18,19]. It was found that increasing the C/A ratio causes a sharp decrease in the proportion of polymerized aluminate structure unit (non-bridging oxygen per aluminum, NBO/Al = 0), while the amounts of simpler units (NBO/Al = 2) and (NBO/Al = 1) rise, which in turn reduces the viscosity of the slag. Consequently, the relatively low C/A ratio of the slag in the 30% SPC led to a substantial rise in viscosity, which trapped the copper droplets within the slag.
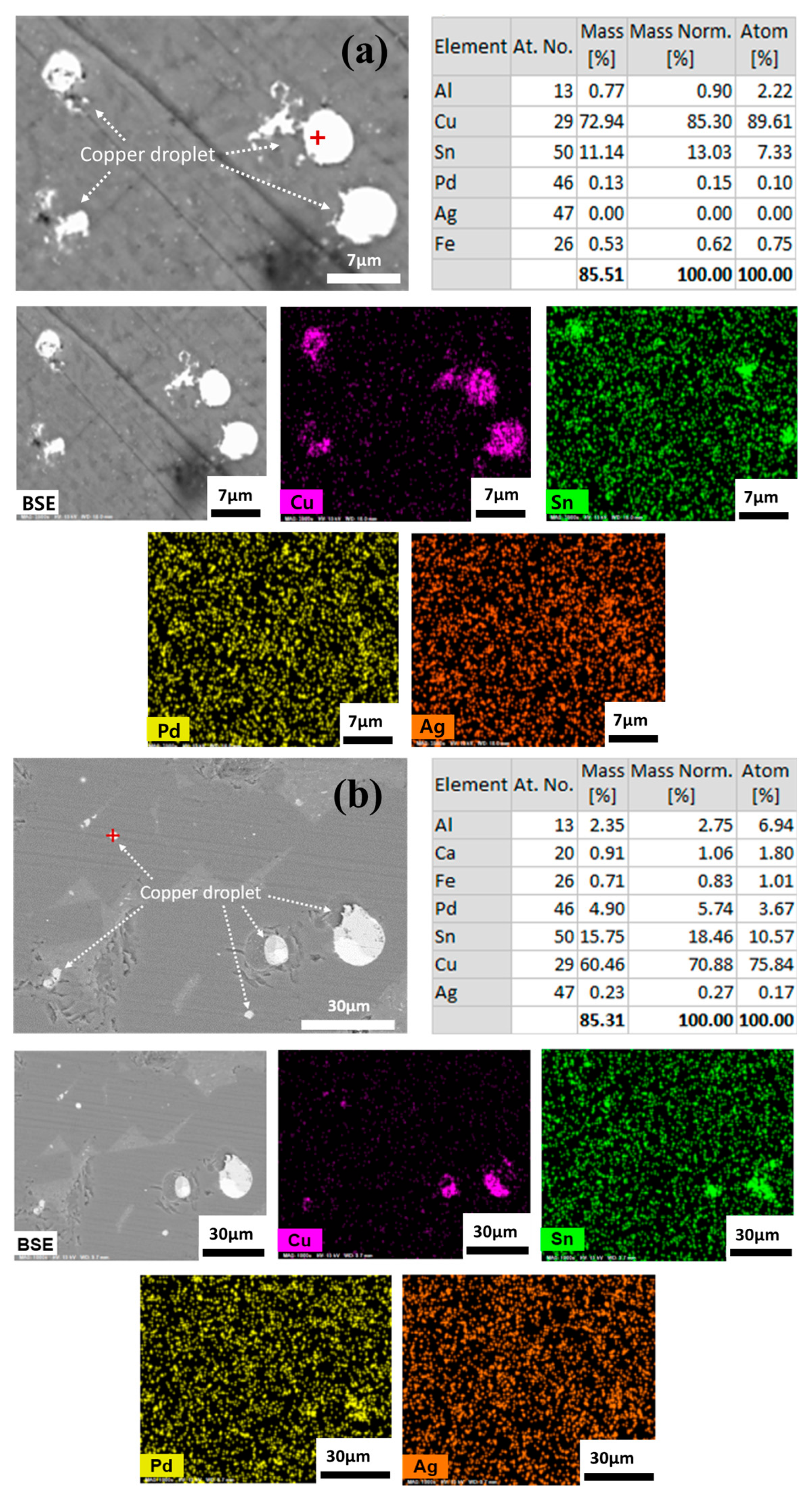
The smelting process uses gravitational forces to separate liquid metal from slag. The efficiency of metal recovery is determined by physical separation along with chemical reactions. A lower C/A ratio for the slag in the 30% SPC mixing condition resulted in the higher viscosity, which in turn led to a decreased recovery rate for valuable metals. The copper droplets entrapped and dispersed in the slag phase indicates an incomplete metal separation form the slag, as shown in Figure 8. A comparison between the 20% SPC mixing condition (Figure 8a), which had a higher recovery rate (99.6% for Pd, 94.6% for Ag, Figure 7), and the 30% SPC mixing condition (Figure 8b), which had a lower recovery rate (62.1% for Pd, 91.0% for Ag, Figure 7), shows that the 30% SPC mixing condition resulted in the larger (several tens of micrometers with spherical morphology) copper droplets trapped in the slag compared to that of 20% SPC mixing condition with the relatively smaller (several micrometers with spherical morphology) copper droplets.
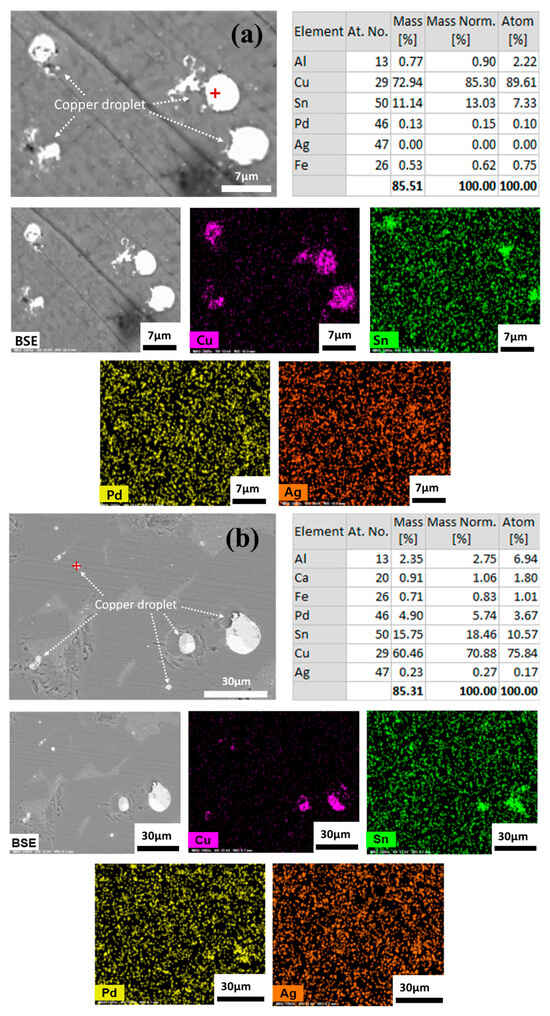
Figure 8.
SEM image, EDX, and element mapping results for the copper droplets confined to the slag: (a) for 20% SPC; (b) for 30% SPC mixing experiment. (For 10% SPC experiments, no copper droplets were dispersed).
As shown in Figure 8, EDX analysis qualitatively confirmed that Pd and Ag have relatively high solubility in the liquid copper phase. Alternatively, the chemical solubility of Pd (=3 to 320 ppm) in the FetO-SiO2-CaO-Al2O3-MgO slag at 1600 °C was measured as a function of slag composition as well as oxygen partial pressure [20]. From the equilibrium solubility data, the following dissolution reaction was proposed.
This reaction indicates that the chemical solubility of Pd in the slag increases with increasing oxygen partial pressure () and the activity of free oxygen ion (), i.e., which is qualitatively proportional to that of CaO (Equation (3)) at a fixed temperature, assuming that the activity of the ion would not be seriously changed by slag composition [20,21,22].
Therefore, from Equation (2), the chemical dissolution of Pd (as a form of ionic complex, ) in the slag decreases with decreasing C/A ratio, i.e., with increasing SCP mixing ratio in the present study. However, as shown in Figure 6, the recovery rate of Pd sharply decreased by increasing the mixing ratio of SPC to 30%. This means that the physical loss of Pd as a form of Cu(-Pd) metal droplets trapped and dispersed in the slag is much higher than the chemical loss of Pd due to an ionic dissolution based on Equation (2). A similar understanding is available for the changes in the recovery rate of Ag against the SPC mixing ratio.
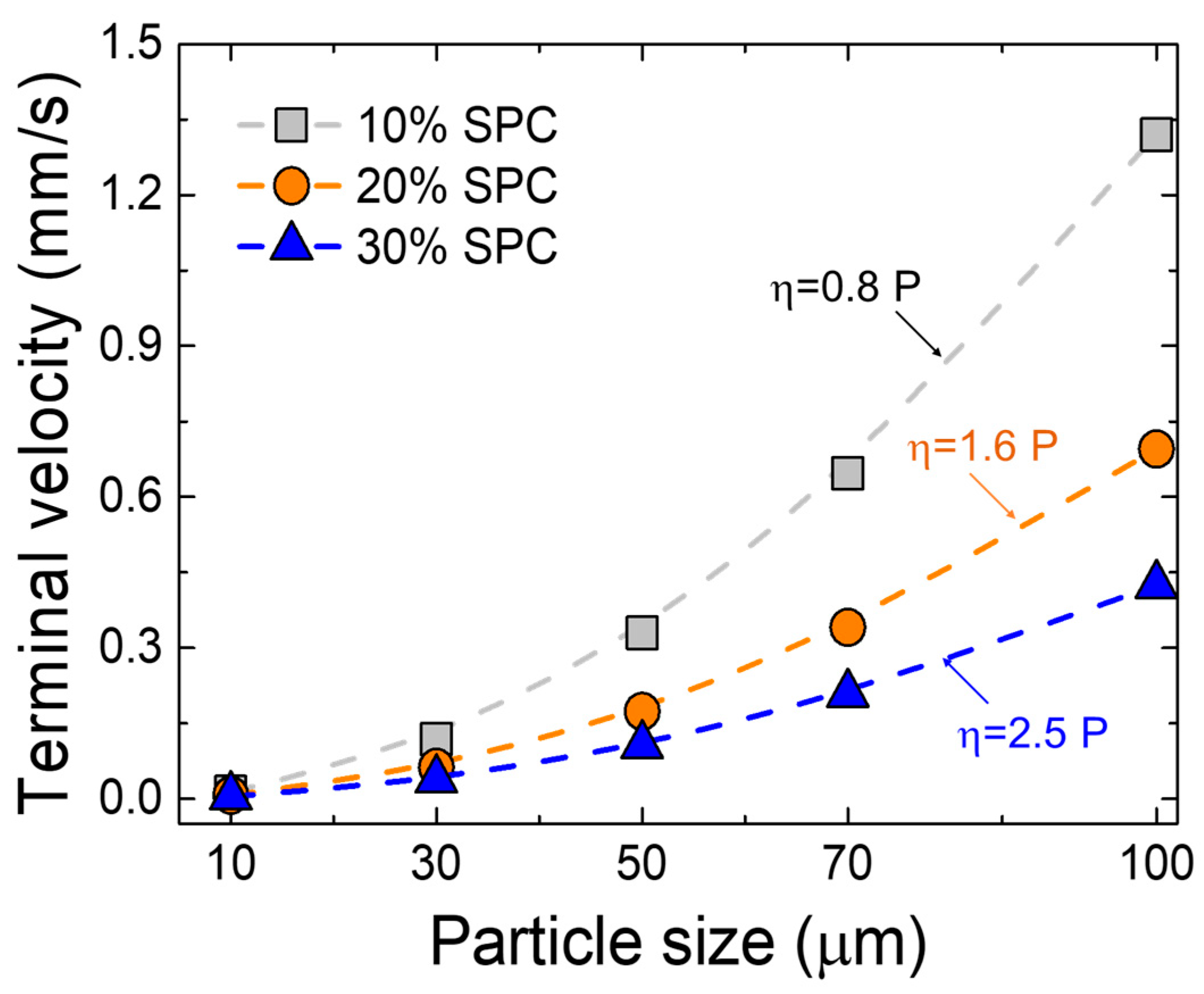
The main reason for metal loss in commercial smelters is the physical entrapment of copper droplets in the slag [13,23]. Therefore, we calculated the terminal settling velocity of metal droplets in the slag. The relationship among gravitational, buoyant, and frictional forces acting on the particles was considered. To estimate the terminal velocity of metallic phases settling through molten slag, assuming spherical particles and a low Reynolds number (Re < 1) under laminar flow regime, Stokes’ law was applied, as shown in Equation (4) [11,12,13,24,25,26].
where, denotes the terminal velocity (m/s), is the density (kg/m3), is the radius of the metal droplets (m), is the gravitational constant (m/s2), and is the slag viscosity (dPa·s = poise). In the present study, FactSageTM (version 8.2, ESM Software, Hamilton, OH), a commercial thermochemical computing software, was used to calculate the slag viscosity. The software has been widely used to predict the physicochemical properties of the slag in ferrous and non-ferrous metallurgical systems [20,21,22,23,24,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. The viscosities of the slag from 10%, 20%, and 30% SPC additions were 0.8 poise, 1.6 poise, and 2.5 poise at 1500 °C, respectively. Since copper was the primary element in the metal droplets, the density of liquid copper (7680 kg/m3) was used [44]. After the experiment, the slag composition was based on the CaO/Al2O3 based system (Table 3), the density values of the CaO/Al2O3 melt at 1500 °C were obtained from the literature, i.e., 2680 kg/m3, 2700 kg/m3, and 2750 kg/m3 for SPC ratios of 10%, 20%, and 30%, respectively [45]. Using Equation (4), the terminal velocities for metal droplets of various sizes (from 10 μm to 100 μm) in the CaO/Al2O3 based slag system were calculated. The results are presented in Figure 9. The most pivotal property in the settling process is slag viscosity. The settling velocity increases with increasing metallic particle size at a given slag composition and decreases by adding the SPC from 10% to 30%, which contributes to an increase in the viscosity of molten slag from about 0.8 to 2.5 poise.
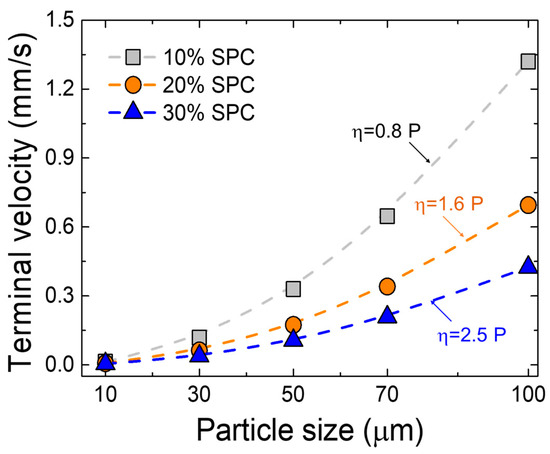
Figure 9.
Calculated terminal velocity of copper droplet in the slag as a function of particle size and viscosity at 1500 °C.
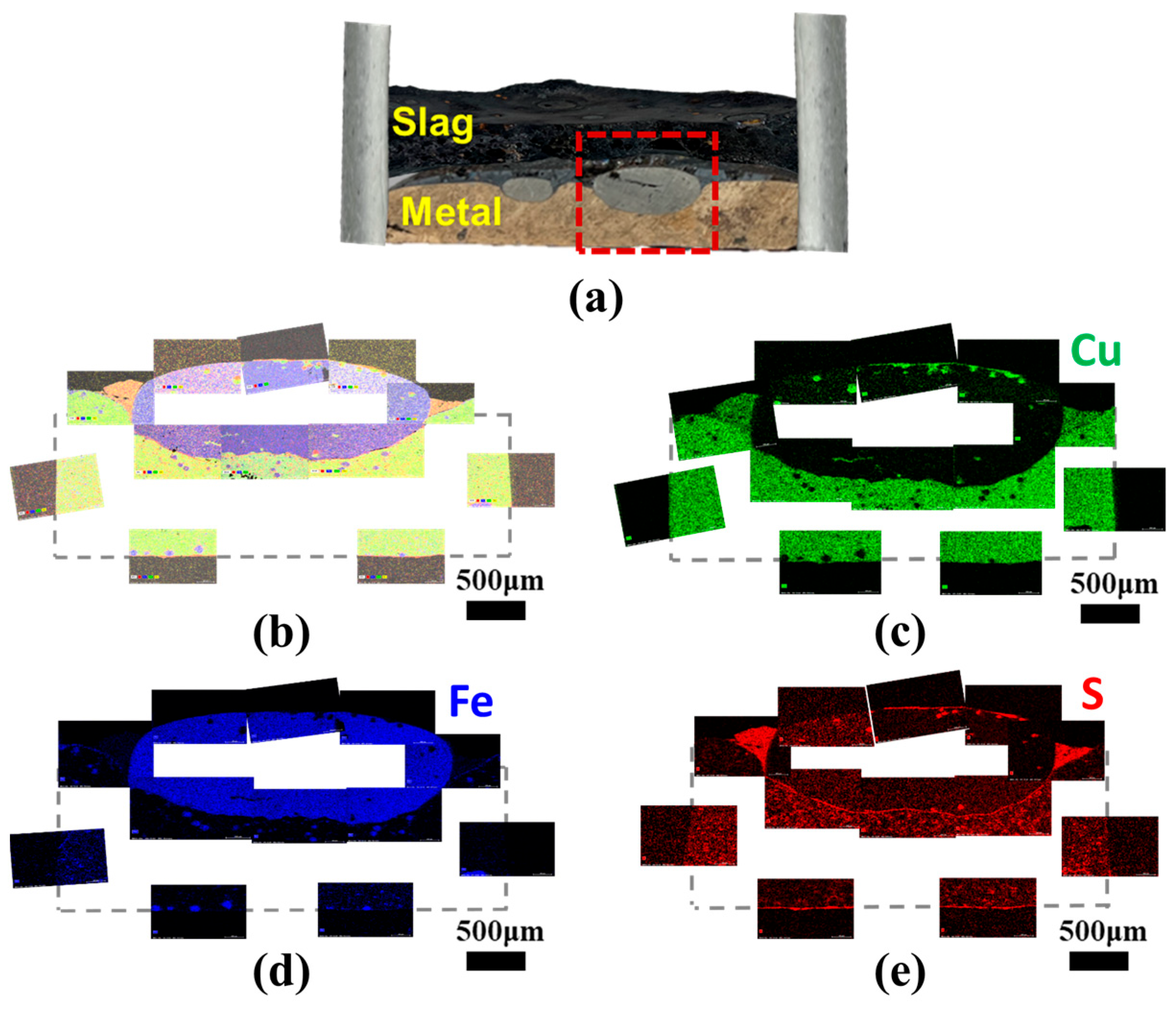
Finally, as illustrated in Figure 10, the presence of sulfur at the interface between slag and metal was confirmed, substantiating its function as a surface-active element in liquid metals. The surface-active elements exert an influence on the interatomic bond at the metal surface, leading to a decrease in surface tension [46]. Then, the presence of sulfur at the slag–metal interface contributes to a reduction in the slag–metal interfacial tension. As the interfacial tension decreases, copper droplets become more readily entrapped within the slag due to an instability of the interface, impeding their efficient separation [47,48,49,50,51,52,53,54]. Consequently, this interfacial phenomenon is potentially another factor affecting the recovery rate of valuable metals in the smelting process. Hence, more specific investigations for the slag–metal interfacial phenomena are necessary in further works.
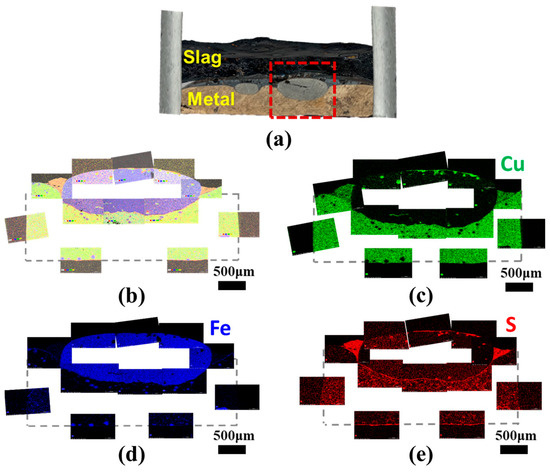
Figure 10.
(a) Morphological image of slag–metal interface for 30% SPC experiment. (b) Element mapping results for the box area in (a), and single mapping of (c) copper, (d) iron, and (e) sulfur.
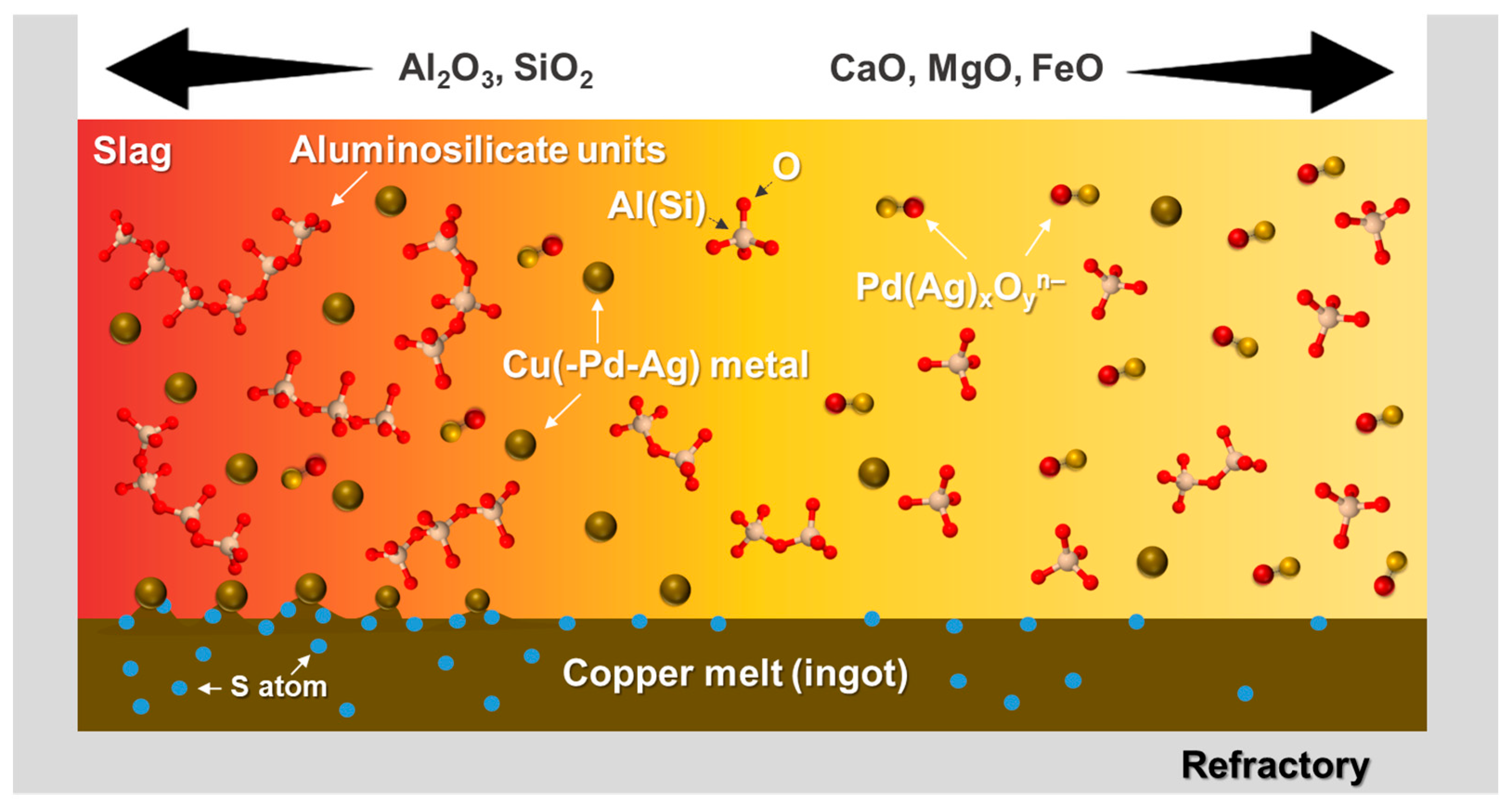
In summary, the thermophysical properties, such as slag viscosity, surface or interfacial tensions, in conjunction with thermochemical solubility of target elements in the slag, contribute to the recovery rate of valuable metals in terms of physical loss and chemical loss, respectively. To maximize the recovery of target metals, the lower slag viscosity, resulting in the higher terminal velocity of metal droplets, as well as the removal of sulfur, resulting in a higher surface or interfacial tension, are necessary for minimizing a physical loss. Alternatively, the lower solubility of target metals in the slag is required to minimize a chemical loss [20,24]. A graphical summary of the metal loss mechanism according to the slag properties is schematically shown in Figure 11.
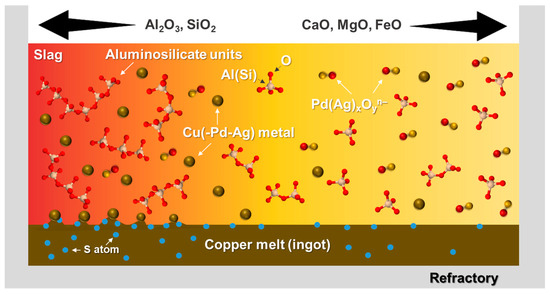
Figure 11.
Graphical summary of the metal loss mechanism according to slag chemistry: chemical loss as Pd(Ag)xOyn– ionic form is dominant in higher CaO, MgO, and FeO slag, while physical loss as Cu(-Pd-Ag) metallic particles dispersed in the slag phase is dominant in higher SiO2 and Al2O3 slag.
4. Conclusions
The current study explored the optimal slag conditions for the pyrometallurgical processing to recover palladium (Pd) and silver (Ag) from hazardous industrial wastes such as copper containing sludge and spent petrochemical catalyst (SPC) at 1500 °C. The major findings can be summarized as follows.
- Effective separation of slag and metal is essential for maximizing metal recovery rate, which requires a high settling velocity of metal droplets. This study demonstrates that slag viscosity significantly influences settling velocity, with lower viscosity facilitating higher settling velocity, and thus more efficient phase separation and metal recovery.
- As the mixing ratio of SPC increases from 10% to 30%, the recovery rate of Pd and Ag sharply decreases to 62.1% and 91.0%, respectively. This is attributed to the increase in slag viscosity as well as to the higher sulfur content in the metal phase, resulting in a decrease in the slag–metal interfacial tension. The lower interfacial tension enhances the instability of the interface, resulting in an emulsification of copper droplets in the slag phase, and hence hindering the metal–slag separation, ultimately decreasing the recovery of Pd and Ag.
- Even though the chemical solubility of target precious metals (i.e., chemical loss) decreases with the decreasing value of the CaO/Al2O3 ratio of the slag (i.e., with increasing SPC mixing ratio), an increase in the viscosity of the slag, and thus lower settling velocity of metal particles in slag phase (e.g., 0.1 mm/s for 50 μm diameter droplets for 30% SPC mixing with slag viscosity of 2.5 Poise). results in the higher physical loss. Hence, from the present experimental findings, physical loss is a much more dominant factor affecting the metal recovery rate rather than chemical loss.
Author Contributions
Conceptualization, J.P.; methodology, H.K. and J.P.; software, H.K.; validation, H.K., H.P. and J.P.; formal analysis, H.K. and J.P.; investigation, H.K. and H.P.; data curation, H.K., H.P. and J.P.; writing—original draft preparation, H.K.; writing—review and editing, H.K., H.P. and J.P.; visualization, H.K.; supervision, J.P.; project administration, J.P.; funding acquisition, J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP, Grant No. 20217510100080) as well as by the Korea Institute for Advancement of Technology with the Competency Development Program for Industry Specialists (KIAT, Grant No. P0023676), funded by the Ministry of Trade, Industry and Energy (MOTIE), Korea.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Hyunju Kim was employed by the company Hyundai Steel Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Balde, C.P.; Kuehr, R.; Yamamoto, T.; McDonald, R.; D’Angelo, E.; Althaf, S. The Global E-Waste Monitor 2024; International Telecommunication Union, United Nations Institute for Training and Resources: Bonn, Germany; Geneva, Switzerland, 2024. [Google Scholar]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.B.M.; Abd Majid, Z.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current technologies for recovery of metals from industrial wastes: An overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Meskers, C.E.; Hagelüken, C. Closed loop WEEE recycling? Challenges and opportunities for a global recycling society. In Proceedings of the 2009 EPD Congress, San Francisco, CA, USA, 15–19 February 2009; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Reck, B.K.; Graedel, T.E. Challenges in metal recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef]
- Schluep, M.; Hagelüken, C.; Kuehr, R.; Magalini, F.; Maurer, C.; Meskers, C.; Thiébaud, E.; Wang, F. Recycling—From E-Waste to Resources; United Nations Environment Programme & United Nations University: Berlin, Germany, 2009; pp. 41–50. [Google Scholar]
- Yatoo, A.M.; Hamid, B.; Sheikh, T.A.; Ali, S.; Bhat, S.A.; Ramola, S.; Ali, M.N.; Baba, Z.A.; Kumar, S. Global perspective of municipal solid waste and landfill leachate: Generation, composition, eco-toxicity, and sustainable management strategies. Environ. Sci. Pollut. Res. Int. 2024, 31, 23363–23392. [Google Scholar] [CrossRef]
- Avarmaa, K.; O’Brien, H.; Johto, H.; Taskinen, P. Equilibrium distribution of precious metals between slag and copper matte at 1250–1350 °C. J. Sustain. Metall. 2015, 1, 216–228. [Google Scholar] [CrossRef]
- Wan, X.; Kleemola, L.; Klemettinen, L.; O’Brien, H.; Taskinen, P.; Jokilaakso, A. On the kinetic behavior of recycling precious metals (Au, Ag, Pt, and Pd) through copper smelting process. J. Sustain. Metall. 2021, 7, 920–931. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, X.; He, Y.; Sun, L.; Wu, N. Enrichment of Platinum Group Metals from Spent Automotive Catalyst Leaching Residue by a Combined Smelting and Wet Extraction Method. J. Sustain. Metall. 2023, 9, 1180–1189. [Google Scholar] [CrossRef]
- Ghodrat, M.; Rhamdhani, M.A.; Khaliq, A.; Brooks, G.; Samali, B. Thermodynamic analysis of metals recycling out of waste printed circuit board through secondary copper smelting. J. Mater. Cycles Waste Manag. 2018, 20, 386–401. [Google Scholar] [CrossRef]
- Park, J.H. Recovery of Au, Ag and Ni from PCB Wastes by CaF2-containing Slag. Resour. Recycl. 2011, 20, 58–64. [Google Scholar]
- Kim, B.S.; Lee, J.C.; Seo, S.P.; Park, Y.K.; Sohn, H.Y. A process for extracting precious metals from spent printed circuit boards and automobile catalysts. JOM 2004, 56, 55–58. [Google Scholar] [CrossRef]
- Park, H.S.; Han, Y.S.; Park, J.H. Massive recycling of waste mobile phones: Pyrolysis, physical treatment, and pyrometallurgical processing of insoluble residue. ACS Sustain. Chem. Eng. 2019, 7, 14119–14125. [Google Scholar] [CrossRef]
- Martinez, A.M.; Tang, K.; Sommerseth, C.; Osen, K.S. Extraction of platinum group metals from spent catalyst material by a novel pyro-metallurgical process. In Rare Metal Technology 2021; Springer: Berlin/Heidelberg, Germany, 2021; pp. 101–113. [Google Scholar]
- Terakado, O.; Saeki, T.; Irizato, R.; Hirasawa, M. Pyrometallurgical recovery of indium from dental metal recycling sludge by chlorination treatment with ammonium chloride. Mater. Trans. 2010, 51, 1136–1140. [Google Scholar] [CrossRef]
- Lee, B.D.; Jung, H.J.; Baek, U.H.; Hong, S.H.; Han, J.W.; Yoo, B.D.; Lee, J.C. Recovery of Valuable Metals Using Pyrometallurgical Treatment. J. Korean Soc. Geosystem Eng. 2010, 47, 628–646. [Google Scholar]
- Kim, T.S.; Park, J.H. Structure-viscosity relationship of low-silica calcium aluminosilicate melts. ISIJ Int. 2014, 54, 2031–2038. [Google Scholar] [CrossRef]
- Poe, B.T.; McMillan, P.F.; Cote, B.; Massiot, D.; Coutures, J.P. Structure and dynamics in calcium aluminate liquids: High-temperature 27Al NMR and Raman Spectroscopy. J. Am. Ceram. Soc. 1994, 77, 1832–1838. [Google Scholar] [CrossRef]
- McMillan, P.F.; Petuskey, W.T.; Cote, B.; Massiot, D.; Landron, C.; Coutures, J.P. A structural investigation of CaO-Al2O3 glasses via 27Al MAS-NMR. J. Non-Cryst. Solids 1996, 195, 261–271. [Google Scholar] [CrossRef]
- Kim, R.R.; Kim, H.J.; Park, H.S.; Park, J.H. Thermodynamics of Palladium Dissolution Behavior in FetO–SiO2–CaO–Al2O3–MgO Slag at 1873 K. Metall. Mater. Trans. B 2024, 55, 2664–2672. [Google Scholar] [CrossRef]
- Kang, Y.B.; Park, J.H. On the dissolution behavior of sulfur in ternary silicate slags. Metall. Mater. Trans. B 2011, 42, 1211–1217. [Google Scholar] [CrossRef]
- Park, J.H.; Park, G.H.; Lee, Y.E. Carbide capacity of CaO–SiO2–MnO slag for the production of manganese alloys. ISIJ Int. 2010, 50, 1078–1083. [Google Scholar] [CrossRef]
- Jang, D.Y.; Min, D.J. The thermodynamic behavior of copper in the CaO-B2O3 and BaO-B2O3 slag system. Metall. Mater. Trans. B 2011, 42, 1144–1149. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, R.R.; Park, H.S.; Park, J.H. Solubility of Gold in FeO–SiO2–CaO–Al2O3–MgO Slag for Smelting of Gold-Containing Secondary Resources. Metall. Mater. Trans. B 2025, 56, 333–341. [Google Scholar] [CrossRef]
- Poirier, D.R.; Geiger, G.H. Laminar flow and momentum equation. In Solutions Manual To Accompany Transport Phenomena in Materials Processing; Springer: Berlin/Heidelberg, Germany, 1994; pp. 39–75. [Google Scholar]
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour. Conserv. Recycl. 2000, 31, 1–7. [Google Scholar] [CrossRef]
- Duan, S.; Kim, T.S.; Cho, J.; Park, J.H. Thermodynamic and Kinetic Analysis of Non-Metallic Inclusions Evolution in Si-Killed 316L Stainless Steel with Various Refining Slags. Met. Mater. Int. 2024, 30, 3483–3496. [Google Scholar] [CrossRef]
- Jing, J.; Guo, Y.; Wang, S.; Chen, F.; Yang, L.; Yang, J.; Xu, F.; Yu, L. Melting Properties and Phase Composition Transformation of Ti-Bearing Electric Furnace Slags in CaO–SiO2–MgO–Al2O3–50%TiO2 System. Met. Mater. Int. 2024, 30, 2045–2056. [Google Scholar] [CrossRef]
- Khartcyzov, G.; Shevchenko, M.; Nekhoroshev, E.; Jak, E. Integrated Experimental and Thermodynamic Modelling Study of Phase Equilibria in the PbO-AlO1.5-SiO2 System in Air. Met. Mater. Int. 2025. [Google Scholar] [CrossRef]
- Seo, J.G.; Song, J.M.; Lee, D.Y.; Park, T.J.; Kwon, H.J. Effect of Al2O3 Content and Particle Size of Iron Ore on the Assimilation Characteristics of Sintered Ore. Korean J. Met. Mater. 2024, 62, 696–704. [Google Scholar] [CrossRef]
- Fan, X.; Huang, Y.; Han, J.; Gao, S.; Zhang, J.; Jiao, K.; Chen, Z. Viscosity and Structure Studies of Iron-Based Quaternary Melts: The Effect of S. Met. Mater. Int. 2024, 30, 1783–1793. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, G.; Lu, J.; Long, H. Characteristics and Formation Mechanism of Ca–Mg–Al–Si–O+(Ca,Mn)S Duplex Inclusions in Ca–S Free-Cutting Steel. Met. Mater. Int. 2025, 31, 134–152. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.; Cheon, H.S.; Kim, B.J.; Lim, C.Y.; Kim, S.H.; Choi, Y.S. Effect of Solution Temperature and Quench Delay on the Microstructure and Mechanical Properties of an Extruded Al-Mg-Si-Cu-Mn alloy. Korean J. Met. Mater. 2024, 62, 132–141. [Google Scholar] [CrossRef]
- Choi, K.; Jung, H.Y. The Effects of Post-Weld Heat Treatment on the Microstructure and Mechanical Properties of 9% Ni Steel Weld Joint with Fe-Based Filler Material. Korean J. Met. Mater. 2023, 61, 669–678. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Wang, L. Modification of Desulfurization Slag for Hot Metal Bearing V-Ti and Industry Application. Metals 2025, 15, 245. [Google Scholar] [CrossRef]
- Lu, L.; Wang, F.; Wang, H.; Qiu, J.; Ping, X. Kinetic Analysis of Molten Oxide Reduction Using Bottom-Blown Hydrogen Injection. Metals 2024, 14, 1255. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Zeng, H.; Liao, J.; Wang, J.; Lai, C. Distribution Behavior of Impurities during the Hydrogen Reduction Ironmaking Process. Metals 2024, 14, 718. [Google Scholar] [CrossRef]
- Yan, Z.; Htet, T.T.; Hage, J.; Meijer, K.; Li, Z. HIsarna Process Simulation Model: Using FactSage with Macro Facility. Metall. Mater. Trans. B 2023, 54, 868–879. [Google Scholar] [CrossRef]
- Singha, P. Refining Contribution at Hotspot and Emulsion Zones of Argon Oxygen Decarburization: Fundamental Analysis Based upon the FactSage-Macro Program Approach. Metall. Mater. Trans. B 2024, 55, 2181–2193. [Google Scholar] [CrossRef]
- Yadav, S.; Srishilan, C.; Shukla, A.K. Thermodynamic Model of MIDREX Ironmaking Process Using FactSageTM and Macro Facility. Metall. Mater. Trans. B 2023, 54, 3508–3525. [Google Scholar] [CrossRef]
- Liu, P.; Gao, Y.; Liu, X.; Wang, Q.; Li, G. Effect of Applied Voltage on Melting and Wetting Behaviors of CaO-SiO2-MgO-Al2O3 slag on Alumina Substrate. Met. Mater. Int. 2024. [Google Scholar] [CrossRef]
- Fan, X.; Gao, S.; Zhang, J.; Jiao, K. High Temperature Melt Viscosity Prediction Model Based on BP Neural Network. Met. Mater. Int. 2024, 30, 2067–2076. [Google Scholar] [CrossRef]
- Chen, G.; Yang, J.; Li, Y.; He, S. Mathematical Simulation of Decarburization with CO2 Injection During RH Refining of Ultra-Low-Carbon Steel. Met. Mater. Int. 2025, 31, 167–181. [Google Scholar] [CrossRef]
- Assael, M.J.; Kalyva, A.E.; Antoniadis, K.D.; Banish, R.M.; Egry, I.; Wu, J.; Wakeham, W.A. Reference data for the density and viscosity of liquid copper and liquid tin. J. Phys. Chem. Ref. Data 2010, 39, 033105. [Google Scholar] [CrossRef]
- Keene, B.J.; Mills, K.C. Densities of molten slags (Chapter 8). In Slag Atlas, 2nd ed.; VDEh, Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995; pp. 313–347. [Google Scholar]
- Richardson, F.D. Interfacial phenomena and metallurgical processes. Can. Metall. Q. 1982, 21, 111–119. [Google Scholar] [CrossRef]
- Sun, H.; Nakashima, K.; Mori, K. Influence of Slag Composition on Slag–Iron Interfacial Tension. ISIJ Int. 2006, 46, 407–412. [Google Scholar] [CrossRef]
- Tanaka, T.; Goto, H.; Nakamoto, M.; Suzuki, M.; Hanao, M.; Zeze, M.; Yamamura, H.; Yoshikawa, T. Dynamic Changes in Interfacial Tension Between Liquid Fe Alloy and Molten Slag Induced by Chemical Reactions. ISIJ Int. 2016, 56, 944–952. [Google Scholar] [CrossRef]
- Muhmood, L. A New Insight to Interfacial Phenomena Occurring at Slag-Metal Interfaces. Steel Res. Int. 2011, 82, 1375–1384. [Google Scholar] [CrossRef]
- Yang, S.; Liu, W.; Li, J. Motion of Solid Particles at Molten Metal–Liquid Slag Interface. JOM 2015, 67, 2993–3001. [Google Scholar] [CrossRef]
- Ni, P.; Tanaka, T.; Suzuki, M.; Nakamoto, M.; Jönsson, P.G. A Kinetic Model of Mass Transfer and Chemical Reactions at a Steel/Slag Interface under Effect of Interfacial Tensions. ISIJ Int. 2019, 59, 737–748. [Google Scholar] [CrossRef]
- Rhamdhani, M.A.; Coley, K.S.; Brooks, G.A. Analysis of the source of dynamic interfacial phenomena during reaction between metal droplets and slag. Metall. Mater. Trans. B 2005, 36, 591–604. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakamoto, M.; Tanaka, T.; Tsukaguchi, Y.; Mishima, K.; Hanao, M. Effect of Sulfur in Slag on Dynamic Change Behavior of Liquid Iron/Molten Slag Interfacial Tension. ISIJ Int. 2020, 60, 2332–2338. [Google Scholar] [CrossRef]
- Muhmood, L.; Viswanathan, N.N.; Seetharaman, S. Some Investigations into the Dynamic Mass Transfer at the Slag–Metal Interface Using Sulfur: Concept of Interfacial Velocity. Metall. Mater. Trans. B 2011, 42, 460–470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).