Effects of Annealing on Carbide Size Distribution and Mechanical Properties of 1.0C-1.5Cr Bearing Steel Prepared by Continuous Casting with 510 mm × 390 mm × 250 mm Dimensions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Microstructural Characterization
2.3. Mechanical Properties Test
3. Results and Discussion
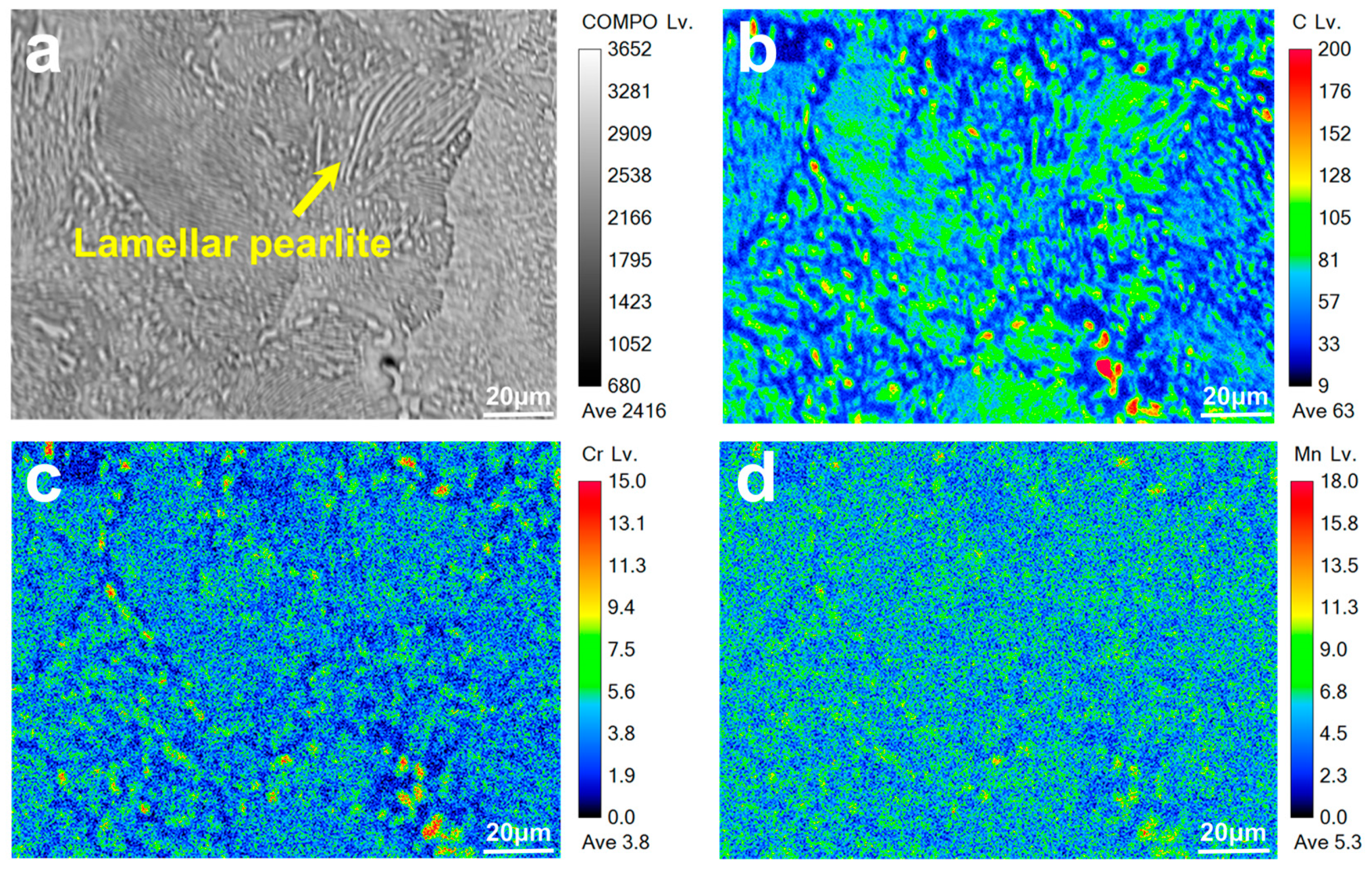
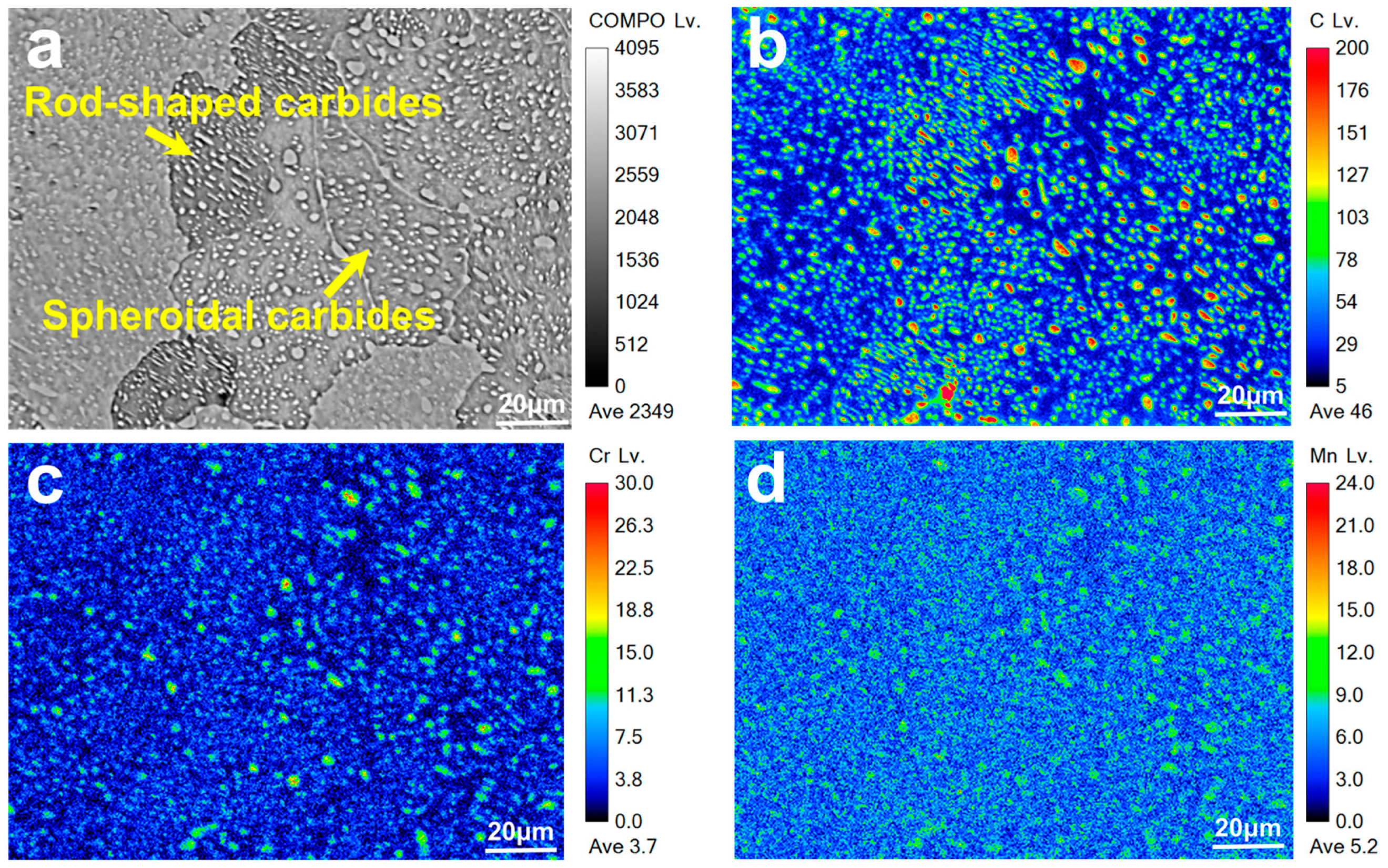
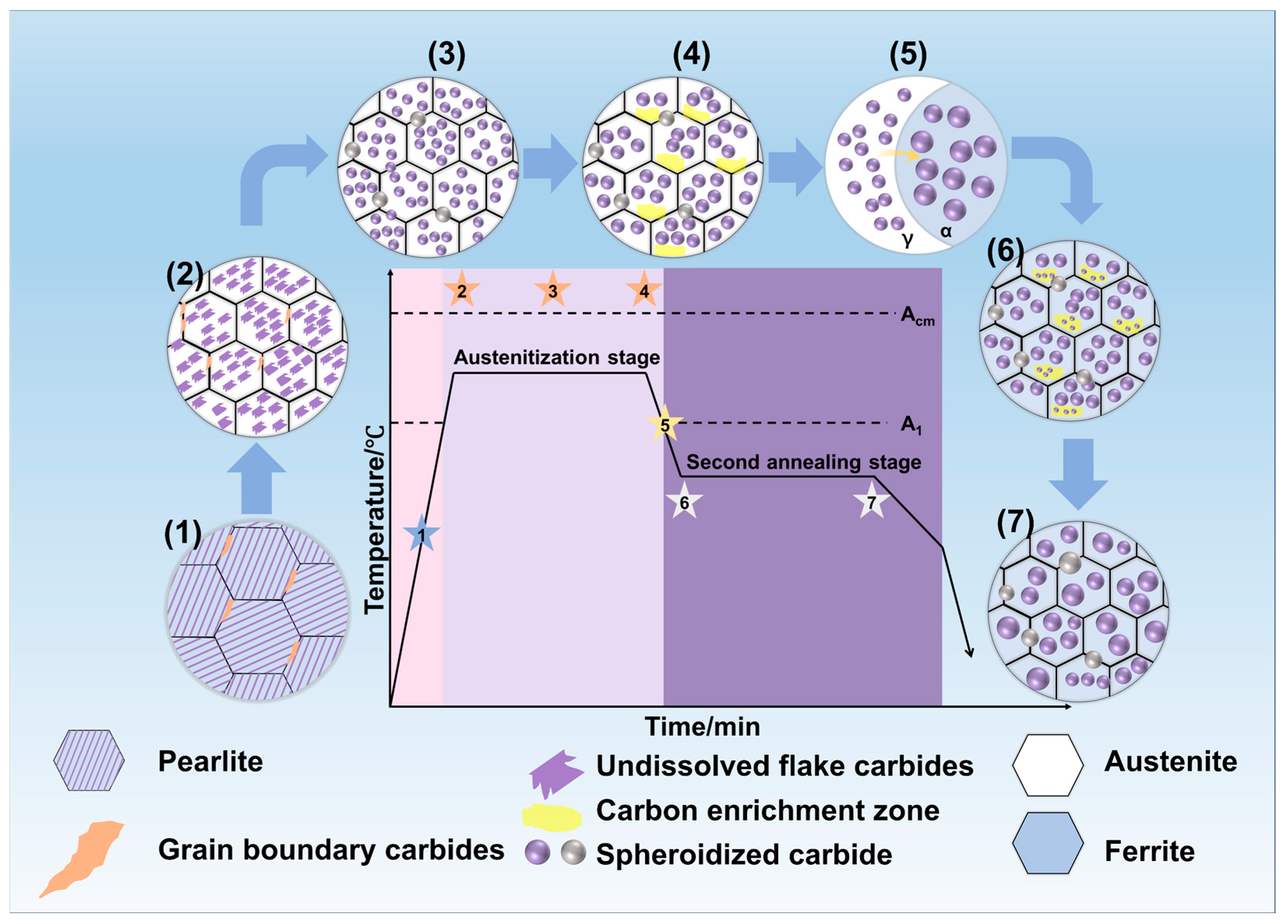
3.1. Effect of Annealing on Carbide Distribution
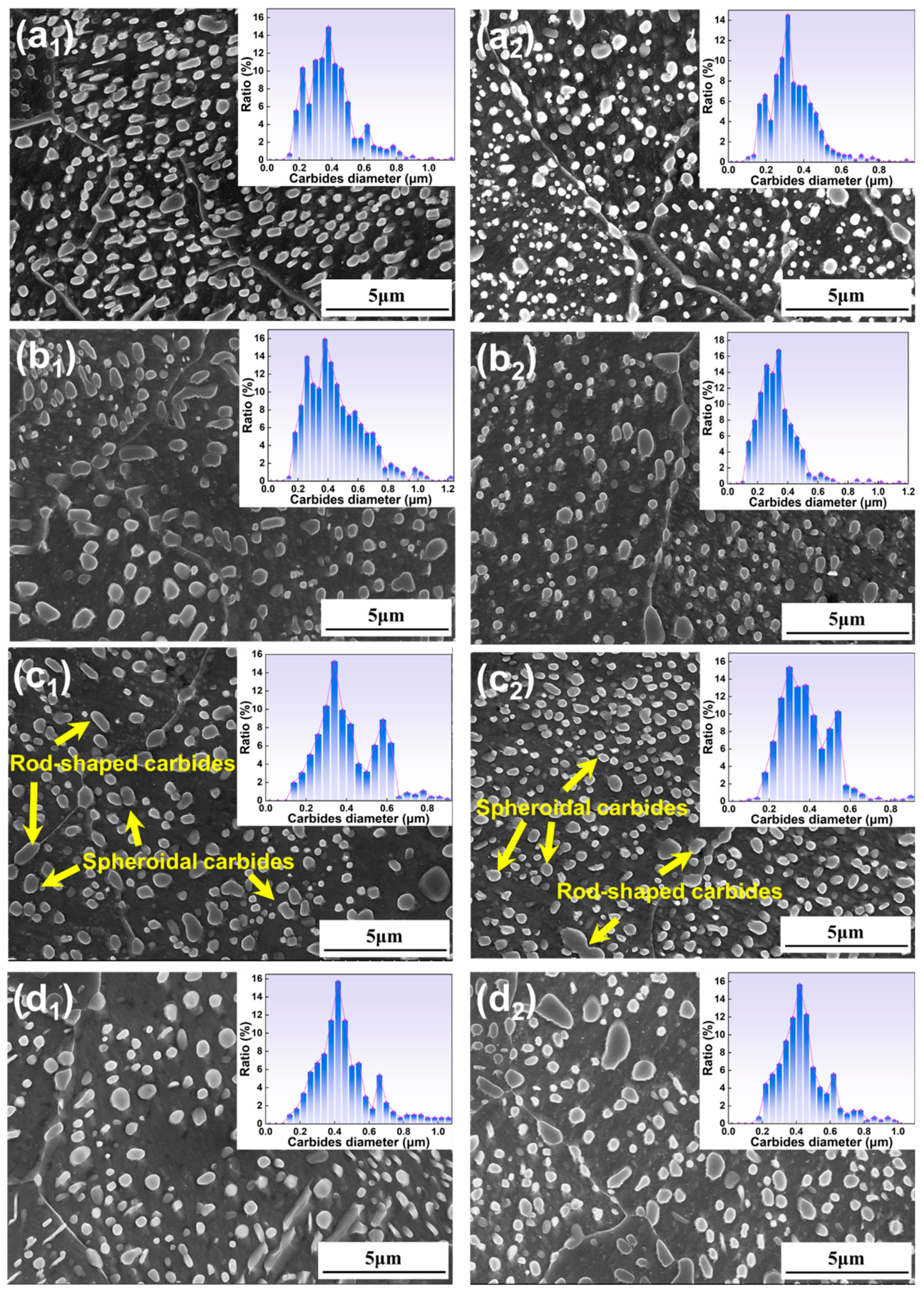
3.1.1. Microstructure Under Different Austenitizing Temperatures
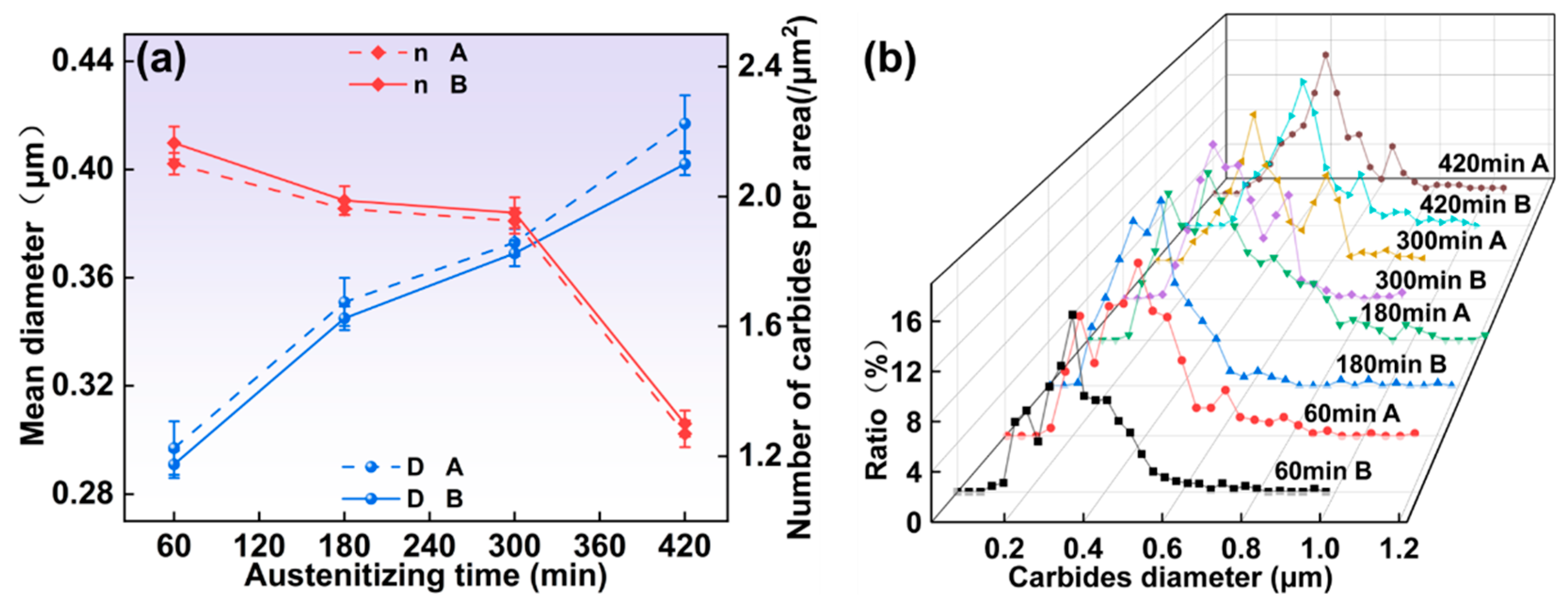
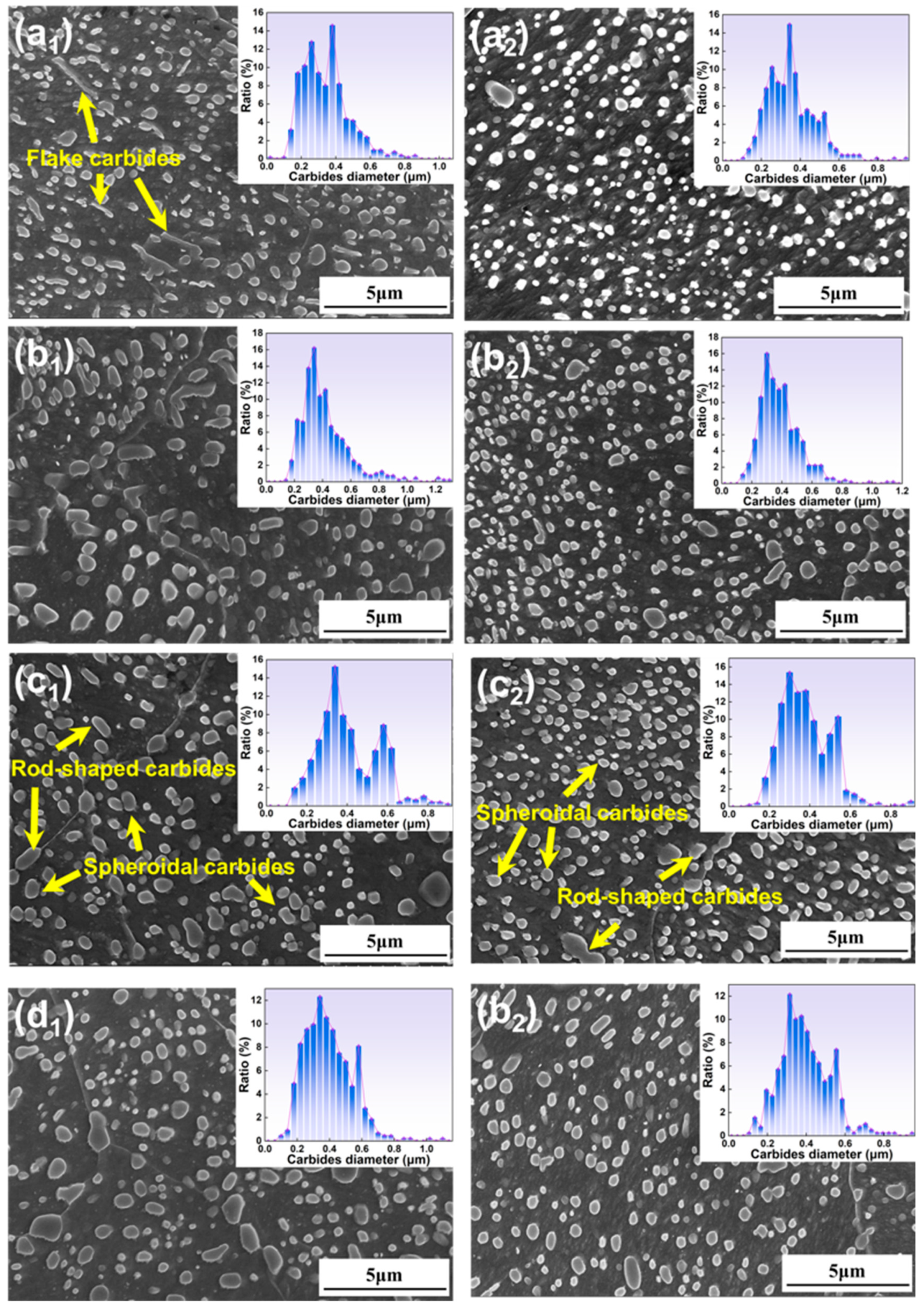
3.1.2. Microstructure Under Different Austenitizing Times
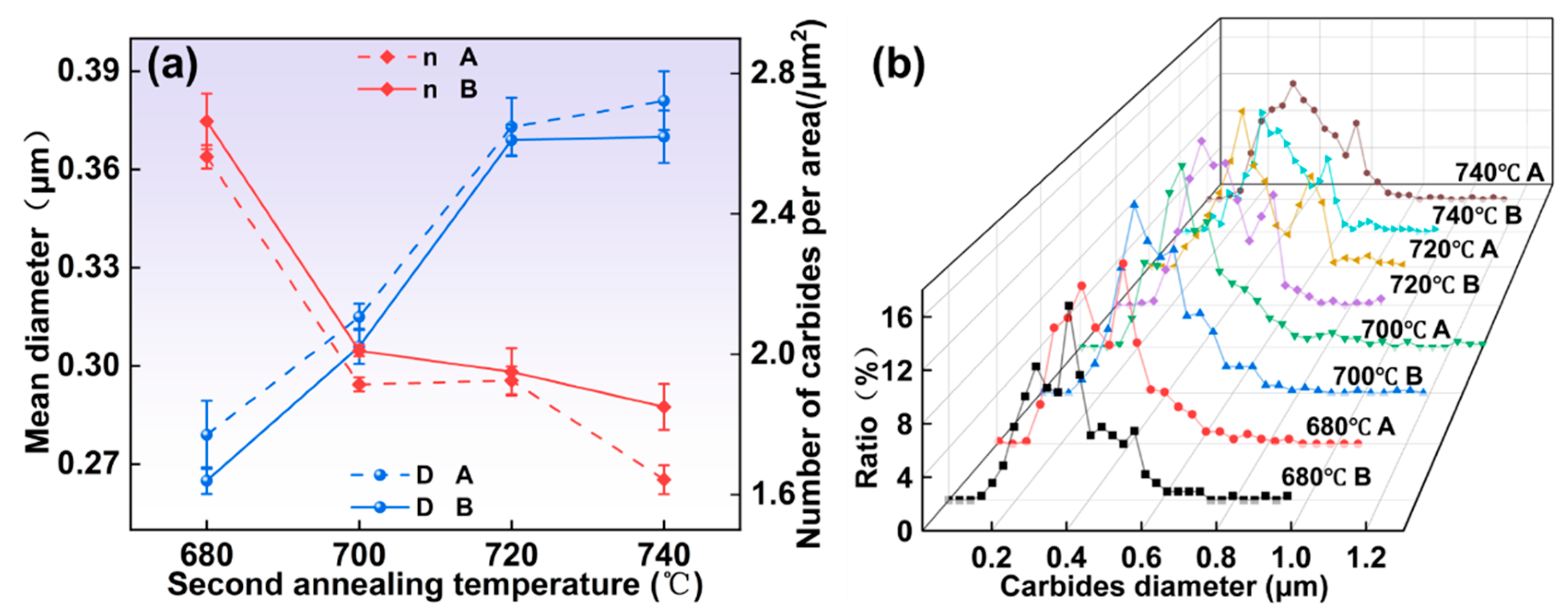
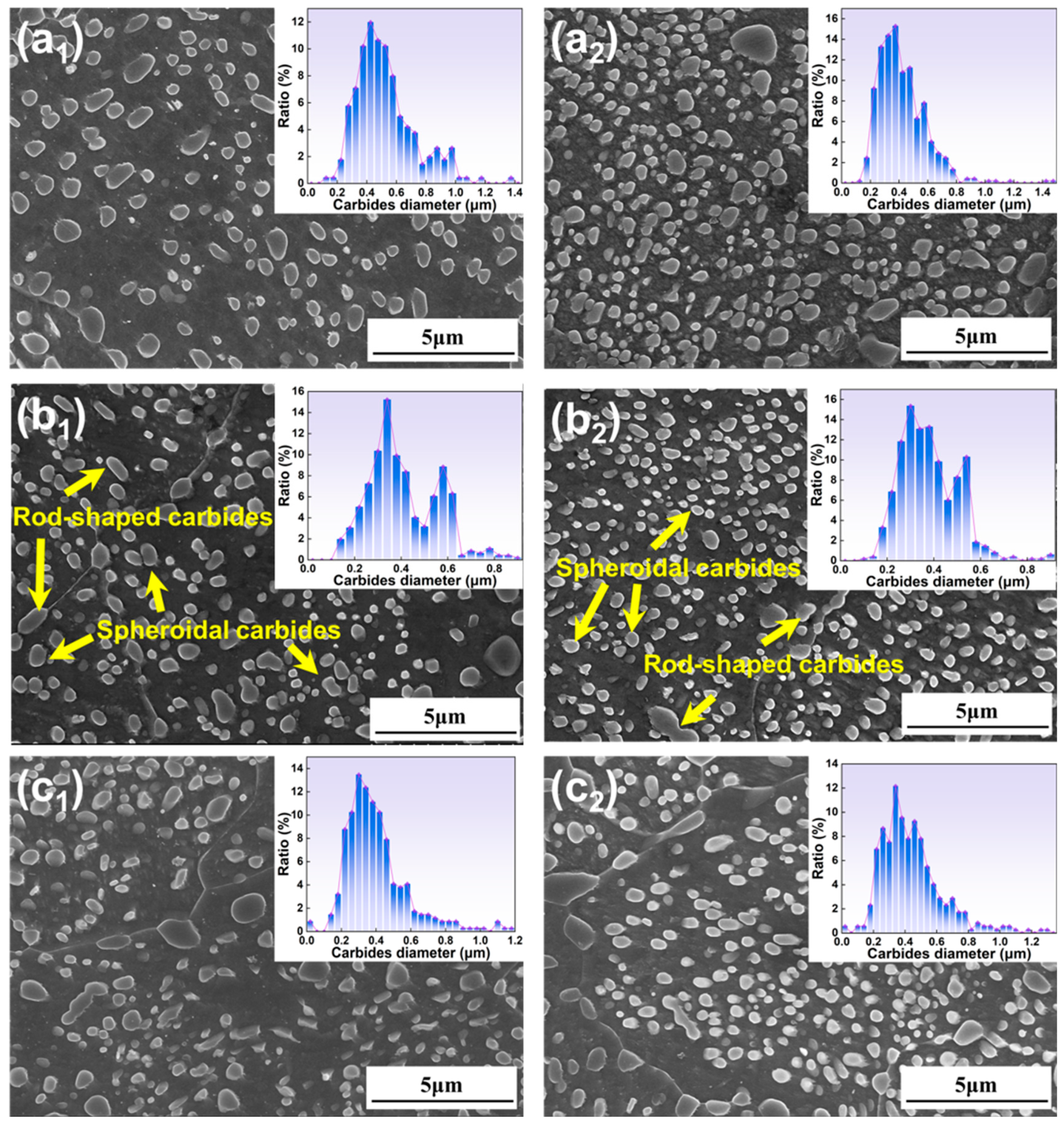
3.1.3. Microstructure Under Different Second Annealing Temperatures
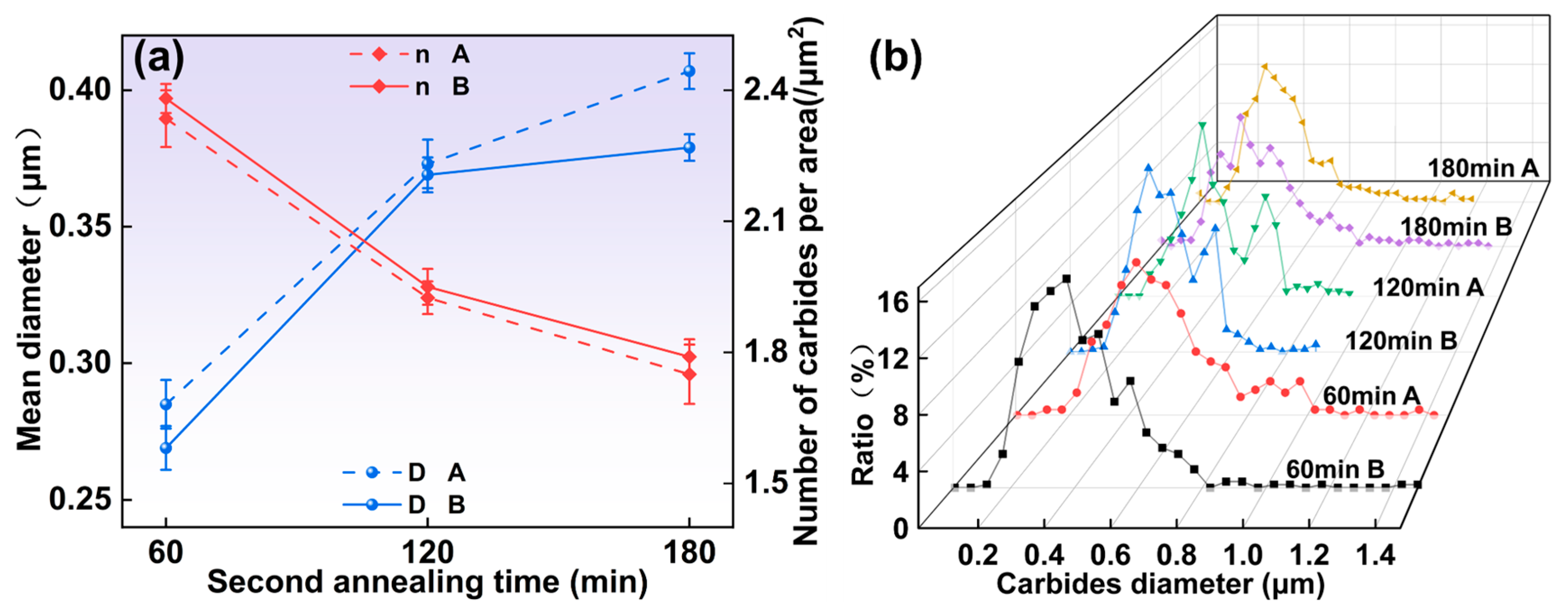
3.1.4. Microstructure Under Different Second Annealing Times
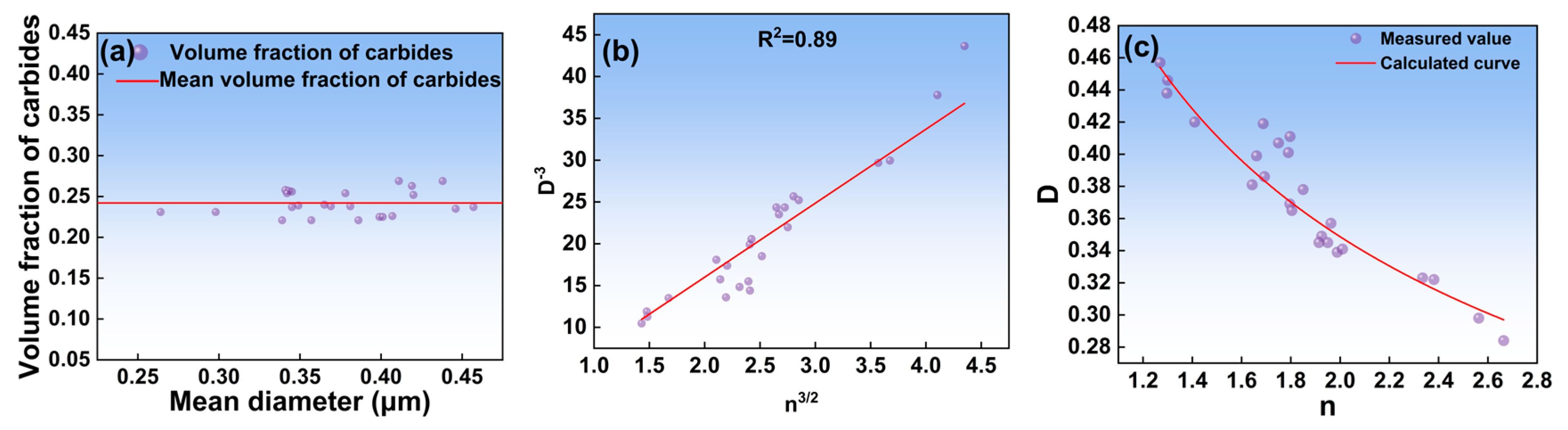
3.2. Model Building
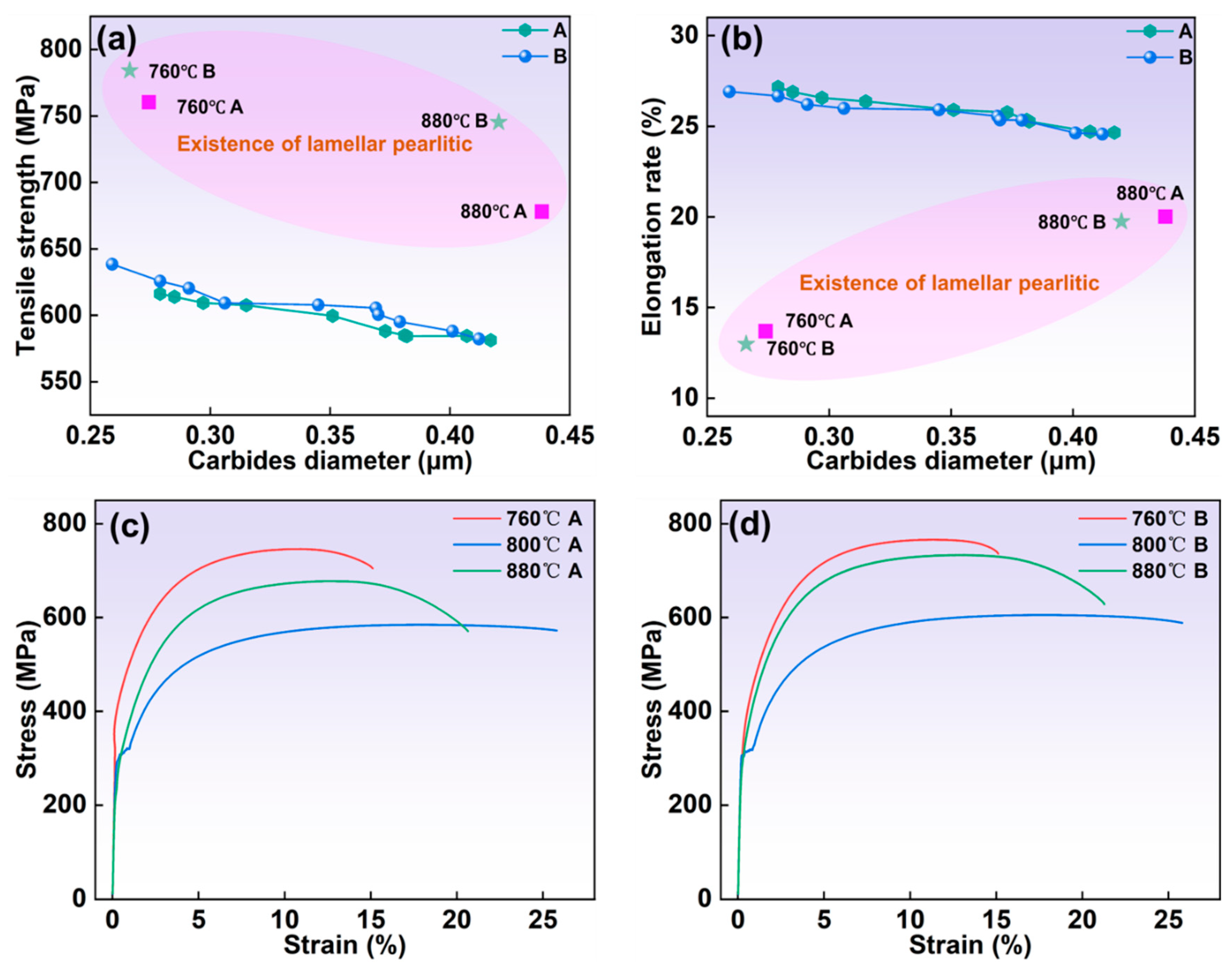
3.3. Effect of Carbide Size and Primary Cast Billet Position on Mechanical Properties
3.4. Fracture Behavior
4. Conclusions
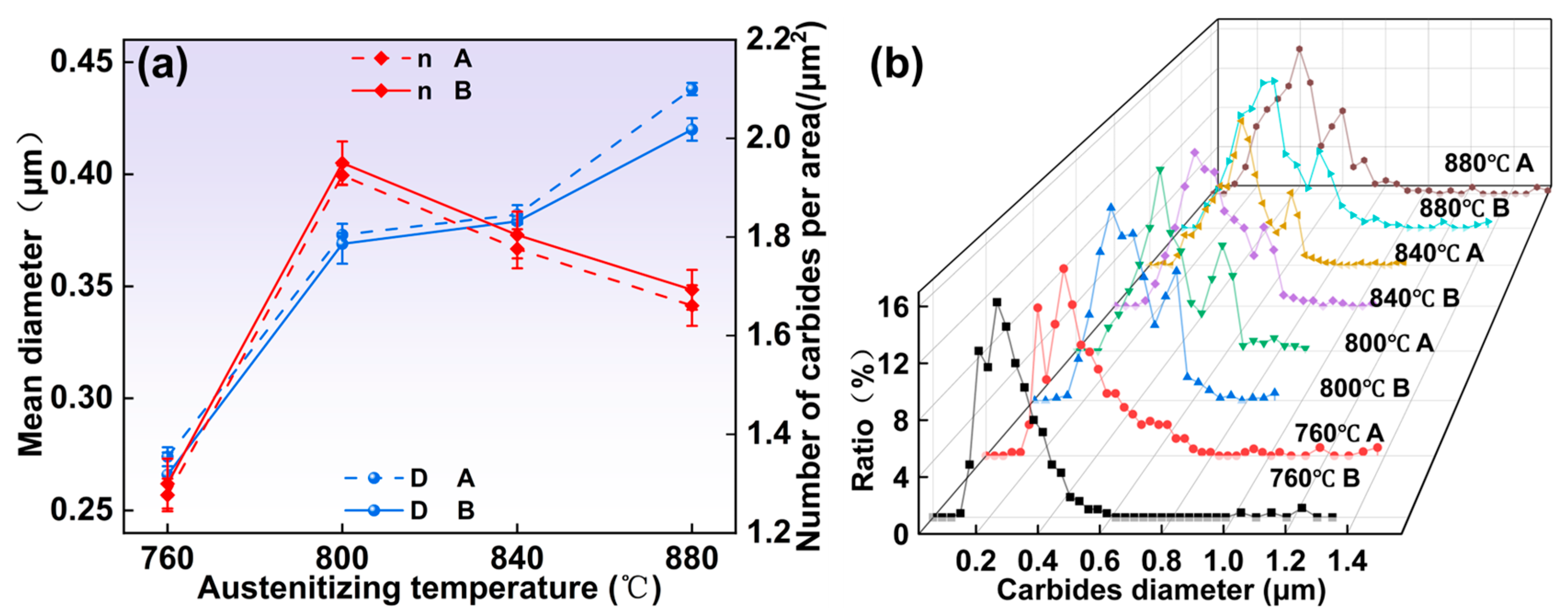
- As the austenitizing and second annealing stages progress, with increased temperatures and extended holding times, the average carbide size in both the 1/2 and center positions gradually increase, while the number of carbides per area decreases. Carbides in the center position are smaller in size but denser compared to those in the 1/2 region. At austenitizing temperatures of 760 °C and 880 °C, the persistence of lamellar pearlite structures can impede further mechanical processing. Hence, maintaining the austenitizing temperature within an optimal range, ideally around 800 °C, is critical. The austenitizing time should be between 120 and 300 min, while the second annealing temperature should be approximately 720 °C with a duration of around 120 min for optimal results.
- All specimens exhibit a bimodal distribution of carbides, which shifts toward larger sizes as temperature and time increase. This bimodal pattern is primarily due to the cooling process, wherein the new carbides nucleate and grow in localized carbon-rich regions, combined with varying carbide growth rates.
- The mean diameter of carbides and the number of carbides per area were modeled. The relationship between them can be expressed as .
- As the mean diameter of carbides increases, both the tensile strength and elongation decrease. Specimens austenitized at 760 °C and 880 °C, whether from the 1/2 or center positions, exhibit continuous yielding behavior, which results in higher strength that can be detrimental to machining. In contrast, other specimens show discontinuous yielding, indicating a more favorable distribution of carbon along the ferrite surface and better carbide formation, ultimately improving machinability.
- Fracture analysis of the tensile specimens showed that the fracture of most specimens showed uniform small toughness dimples, reflecting the typical toughness fracture characteristics. However, some specimens showed large carbides of about 0.76 μm in the fracture, which would lead to early nucleation of micropores due to interfacial mismatch, resulting in low-stress fracture and strength reduction. In addition, the fracture morphology of some specimens is characterized by a mixture of fracture surface and tough dimples, which indicates that both brittle and ductile fractures exist in the fracture process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Azevedo, H.D.M.; Araújo, A.M.; Bouchonneau, N. A review of wind turbine bearing condition monitoring: State of the art and challenges. Renew. Sust. Energ. Rev. 2016, 56, 368–379. [Google Scholar] [CrossRef]
- Ding, H.H.; Mermertas, U.; Hagemann, T.; Schwarze, H. Calculation and validation of planet gear sliding bearings for a three-stage wind turbine gearbox. Lubricants 2024, 12, 95. [Google Scholar] [CrossRef]
- Yang, B.; Liu, B.X.; Fan, K.Y.; Zhang, B.Y.; Ji, P.G.; Feng, J.H.; Tong, H.C.; Yin, F.X. Obvious different formability and mechanical properties of warm caliber rolling Q345 steel with normalized and tempered states. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2023, 887, 12. [Google Scholar] [CrossRef]
- Yan-kun, S.; Di, W. Effect of Ultra-Fast Cooling on Microstructure of Large Section Bars of Bearing Steel %J Journal of Iron and Steel Research (International). J. Iron Steel Res. (Int.) 2009, 16, 61–65,80. [Google Scholar]
- Härtel, S.; Awiszus, B.; Graf, M.; Nitsche, A.; Böhme, M.; Wagner, M.F.X.; Jirkova, H.; Masek, B. Influence of austenite grain size on mechanical properties after quench and partitioning treatment of a 42SiCr steel. Metals 2019, 9, 577. [Google Scholar] [CrossRef]
- Yuan, Q.Q.; Yin, H.Q.; Qiao, Z.Q.; Zhang, C.; Wang, Y.W.; Zhang, R.J.; Jiang, X.; Khan, D.F.; Li, D.; Liang, J.B.; et al. Homogenization Treatment and Plastic Deformation Improve the Carbide Homogeneity of M42 High-Speed Steel. Steel Res. Int. 2024, 95, 12. [Google Scholar] [CrossRef]
- Teng, H.; Qian, S.S.; Xie, J.H.; Zhao, H.S.; Wei, X.C.; Dong, H. Effect of Spheroidizing Annealing Process on Microstructure and Properties of Quenching and Tempering 60Cr16MoMA Martensitic Stainless Steel. Steel Res. Int. 2024, 95, 2300781. [Google Scholar] [CrossRef]
- Huang, H.; De Cooman, B.C. Influence of the Initial Microstructure on the Spheroidization of SAE 52100 Bearing Steel. Steel Res. Int. 2016, 87, 112–125. [Google Scholar] [CrossRef]
- Zhang, C.C.; Yi, X.L.; Yuan, Q.Q.; Wang, L. Microstructure and mechanism of three spheroidizing treatment for the carbide of GCr15 steel. In Proceedings of the 3rd International Conference on Manufacturing Science and Engineering (ICMSE 2012), Xiamen, China, 27–29 March 2012; p. 351. [Google Scholar]
- Huang, Z.Y.; Du, L.X.; Li, M.Z.; Fu, S.Y.; Lu, J.N. Study on rolling and hot delivery spheroidizing annealing of GCr15 bearing steel. J. Mater. Eng. Perform. 2023, 32, 7779–7784. [Google Scholar] [CrossRef]
- Ankit, K.; Mukherjee, R.; Nestler, B. Deviations from cooperative growth mode during eutectoid transformation: Mechanisms of polycrystalline eutectoid evolution in Fe–C steels. Acta Mater. 2015, 97, 316–324. [Google Scholar] [CrossRef]
- Verhoeven, J.D. The role of the divorced eutectoid transformation in the spheroidization of 52100 steel. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2000, 31, 2431–2438. [Google Scholar] [CrossRef]
- Liu, K.Q.; Zhang, X.J.; Song, X.Y.; Zhao, L.; Zhu, L. Evaluation and improvement of pearlite microstructure and mechanical properties in 45Cr4NiMoV and 50Cr5NiMoV steels. J. Iron Steel Res. Int. 2022, 29, 1995–2005. [Google Scholar] [CrossRef]
- Li, Z.X.; Li, C.S.; Kim, S.H.; Suh, D.W. Influence of initial pearlite morphology on the microstructure evolution during heat treatment of 1.0C-1.5Cr steel. Met. Mater. Int. 2019, 25, 9–17. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chae, J.Y.; Kim, K.H.; Suh, D.W. Effects of Mn, Si and Cr addition on the dissolution and coarsening of pearlitic cementite during intercritical austenitization in Fe-1mass%C alloy. Mater. Charact. 2013, 81, 56–67. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, J.S.; Lee, D.L. Effect of silicon on the spheroidization of cementite in hypereutectoid high carbon chromium bearing steels. Met. Mater. Int. 2010, 16, 871–876. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Hwang, B.; Lee, B.S.; Lee, C.G. Effect of carbide distribution on the fracture toughness in the transition temperature region of an SA 508 steel. Acta Mater. 2002, 50, 4755–4762. [Google Scholar] [CrossRef]
- Caballero, F.; de Andrés, C.G.; Capdevila, C. Characterization and morphological analysis of pearlite in a eutectoid steel. Mater. Charact. 2000, 45, 111–116. [Google Scholar] [CrossRef]
- Pang, J.; Wang, G.; Yi, H. Effect of isothermal annealing on microstructure and hardness of low-density bearing steel. J. Iron Steel Res. 2020, 32, 1077–1083. [Google Scholar]
- Chen, M.; Zhang, C.; Zhang, G.H.; Zhou, J.; Hu, F.; Wu, K.M. Acceleration of Spheroidization in Low-Density Ultrafine Pearlitic Steel by κ-Carbide. Steel Res. Int. 2024, 95, 2300564. [Google Scholar] [CrossRef]
- Hong, L.X.; Jin, Q.Y.; Li, X.S.; Huang, Y.Z. Image and medical annotations using non-homogeneous 2D ruler learning models. Comput. Electr. Eng. 2016, 50, 102–110. [Google Scholar] [CrossRef]
- Wu, D.; Li, Z. A New Pb-Free Machinable Austenitic Stainless Steel. J. Iron Steel Res. Int. 2010, 17, 59–63. [Google Scholar] [CrossRef]
- Ning, A.G.; Liu, Y.; Gao, R.; Yue, S.; Wang, M.B.; Guo, H.J. Effect of austenitizing condition on mechanical properties, microstructure and precipitation behavior of AISI H13 steel. J. Iron Steel Res. Int. 2024, 31, 143–156. [Google Scholar] [CrossRef]
- Qian, M. In-situ observations of the dissolution of carbides in an Fe-Cr-C alloy. Scr. Mater. 1999, 41, 1301–1303. [Google Scholar] [CrossRef]
- Hou, Z.Y.; Babu, R.P.; Hedström, P.; Odqvist, J. Early stages of cementite precipitation during tempering of 1C-1Cr martensitic steel. J. Mater. Sci. 2019, 54, 9222–9234. [Google Scholar] [CrossRef]
- Li, Z.X.; Li, C.S.; Zhang, J.; Qiao, B.; Li, Z.Z. Effects of annealing on carbides size and distribution and cold formability of 1.0C-1.5Cr bearing steel. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2015, 46A, 3220–3231. [Google Scholar] [CrossRef]
- Michal, G.M.; Novak, M.D. The divorced eutectoid transformation and spheroidization of steels. Symp. Thermodyn. Kinet. Charact. Model. Austenite Form. Decompos. 2003, 397–413. [Google Scholar]
- Zhang, Z.Q.; Jing, H.Y.; Xu, L.Y.; Han, Y.D.; Zhao, L.; Lv, X.Q.; Zhang, J.Y. The impact of annealing temperature on improving microstructure and toughness of electron beam welded duplex stainless steel. J. Manuf. Process. 2018, 31, 568–582. [Google Scholar] [CrossRef]
- Kamyabi-Gol, A.; Sheikh-Amiri, M. Spheroidizing kinetics and optimization of heat treatment parameters in ck60 steel using taguchi robust design. J. Iron Steel Res. Int. 2010, 17, 45–52. [Google Scholar] [CrossRef]
- Yang, H.B.; Wang, Y.M.; Luo, L.; Zhang, P.P. Ostwald Growth of Carbides in Cyclic Annealing Process of GCr15 Bearing Steel. In Proceedings of the 4th International Conference on Technology of Architecture and Structure (ICTAS 2011), Xi’an University of Architecture and Technology, Xi’an, China, 22–24 September 2021; pp. 1805–1808. [Google Scholar]
- Lu, X.; Fang, F.; Zhang, Y.X.; Wang, Y.; Yuan, G.; Xu, Y.B.; Misra, R.D.K.; Zhang, W.N.; Wang, G.D. Microstructure and magnetic properties of strip-cast grain-oriented 4.5%Si steel under isochronal and isothermal secondary annealing. J. Mater. Sci. 2018, 53, 2928–2941. [Google Scholar] [CrossRef]
- Erdogan, M.; Kilicli, V.; Demir, B. The influence of the austenite dispersion on phase transformation during the austempering of ductile cast iron having a dual matrix structure. Int. J. Mater. Res. 2008, 99, 751–760. [Google Scholar] [CrossRef]
- Di, H.; Zhang, X.; Wang, G.; Liu, X. Spheroidizing kinetics of eutectic carbide in the twin roll-casting of M2 high-speed steel. J. Mater. Process. Technol. 2005, 166, 359–363. [Google Scholar] [CrossRef]
- Oyama, T.; Sherby, O.D.; Wadsworth, J.; Walser, B. Application of the divorced eutectoid transformation to the development of fine-grained, spheroidized structures in ultrahigh carbon steels. Scr. Metall. 1984, 18, 799–804. [Google Scholar] [CrossRef]
- Doppler, T.; Pfeiler, W. Isothermal annealing at various temperatures—Effect of temperature-change on the process kinetics observed. J. Mater. Sci. 1991, 26, 2533–2542. [Google Scholar] [CrossRef]
- Caruso, M.; Verboomen, H.; Godet, S. Direct spheroidization of high carbon steels: Effect of thermomechanical processing. In Proceedings of the International Conference on Solid-Solid Phase Transformations in Inorganic Materials (PTM 2010), Avignon, France, 6–11 June 2010; pp. 922–927. [Google Scholar]
- Qian, D.S.; Chen, B.; Wang, F.; Wu, L.Y. Rapid Spheroidizing Annealing via Combining Warm Deformation with Divorced Eutectoid Transformation in M50 Steel. Metals 2022, 12, 359. [Google Scholar] [CrossRef]
- Taleff, E.M.; Lewandowski, J.J.; Pourladian, B. Microstructure-property relationships in pearlitic eutectoid and hypereutectoid carbon steels. JOM-J. Miner. Met. Mater. Soc. 2002, 54, 25–30. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; He, S.; Huang, C.; Huang, M. Enhancing yield stress and uniform elongation in an ultrathin packaging steel via controlling dislocation density. Int. J. Plast. 2022, 155, 18. [Google Scholar] [CrossRef]



| Position | C | Cr | Si | Mn | Ni | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| A (1/2 position) | 0.99 | 1.40 | 0.25 | 0.29 | 0.031 | 0.008 | 0.004 | 96.8 |
| B (center position) | 1.02 | 1.37 | 0.25 | 0.30 | 0.031 | 0.019 | 0.007 | 96.8 |
| Austenitizing Temperature (°C) | Austenitizing Time (min) | Second Annealing Temperature (°C) | Second Annealing Time (min) |
|---|---|---|---|
| 760 | 300 | 720 | 120 |
| 800 | 300 | 720 | 120 |
| 840 | 300 | 720 | 120 |
| 880 | 300 | 720 | 120 |
| 800 | 60 | 720 | 120 |
| 800 | 180 | 720 | 120 |
| 800 | 420 | 720 | 120 |
| 800 | 300 | 680 | 120 |
| 800 | 300 | 700 | 120 |
| 800 | 300 | 740 | 120 |
| 800 | 300 | 720 | 60 |
| 800 | 300 | 720 | 180 |
| Position | 760 (°C) | 800 (°C) | 840 (°C) | 880 (°C) |
|---|---|---|---|---|
| A (1/2 position) | 84.7 | 88.4 | 93.5 | 95.3 |
| B (center position) | 85.8 | 86.9 | 92.2 | 93.1 |
| Position | 60 (min) | 180 (min) | 300 (min) | 420 (min) |
|---|---|---|---|---|
| A (1/2 position) | 80.7 | 84.5 | 88.4 | 96.5 |
| B (center position) | 81.9 | 83.3 | 86.9 | 94.7 |
| Position | 680 (°C) | 700 (°C) | 720 (°C) | 740 (°C) |
|---|---|---|---|---|
| A (1/2 position) | 85.2 | 87.1 | 88.4 | 90.5 |
| B (center position) | 83.1 | 84.9 | 86.9 | 89.1 |
| Position | 60 (min) | 120 (min) | 180 (min) |
|---|---|---|---|
| A (1/2 position) | 85.7 | 88.4 | 92.5 |
| B (center position) | 84.3 | 86.9 | 88.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, P.; Zhang, J.; Shu, C.; Yu, S.; Yao, Z. Effects of Annealing on Carbide Size Distribution and Mechanical Properties of 1.0C-1.5Cr Bearing Steel Prepared by Continuous Casting with 510 mm × 390 mm × 250 mm Dimensions. Metals 2025, 15, 467. https://doi.org/10.3390/met15040467
Ding P, Zhang J, Shu C, Yu S, Yao Z. Effects of Annealing on Carbide Size Distribution and Mechanical Properties of 1.0C-1.5Cr Bearing Steel Prepared by Continuous Casting with 510 mm × 390 mm × 250 mm Dimensions. Metals. 2025; 15(4):467. https://doi.org/10.3390/met15040467
Chicago/Turabian StyleDing, Peiheng, Jicong Zhang, Changqing Shu, Shuaipeng Yu, and Zhengjun Yao. 2025. "Effects of Annealing on Carbide Size Distribution and Mechanical Properties of 1.0C-1.5Cr Bearing Steel Prepared by Continuous Casting with 510 mm × 390 mm × 250 mm Dimensions" Metals 15, no. 4: 467. https://doi.org/10.3390/met15040467
APA StyleDing, P., Zhang, J., Shu, C., Yu, S., & Yao, Z. (2025). Effects of Annealing on Carbide Size Distribution and Mechanical Properties of 1.0C-1.5Cr Bearing Steel Prepared by Continuous Casting with 510 mm × 390 mm × 250 mm Dimensions. Metals, 15(4), 467. https://doi.org/10.3390/met15040467





