Selective Extraction of Zirconium from Sulfuric Acid Solutions at High Concentration with Trioctylamine (TOA)
Abstract
1. Introduction
2. Experimental
2.1. Experimental Materials and Instruments
2.2. Experimental Methods
2.2.1. Solvent Extraction Procedure
2.2.2. Mechanism Study
- Acidification
- 2.
- Saturation capacity method
- 3.
- Slope method
2.3. Calculation
3. Results
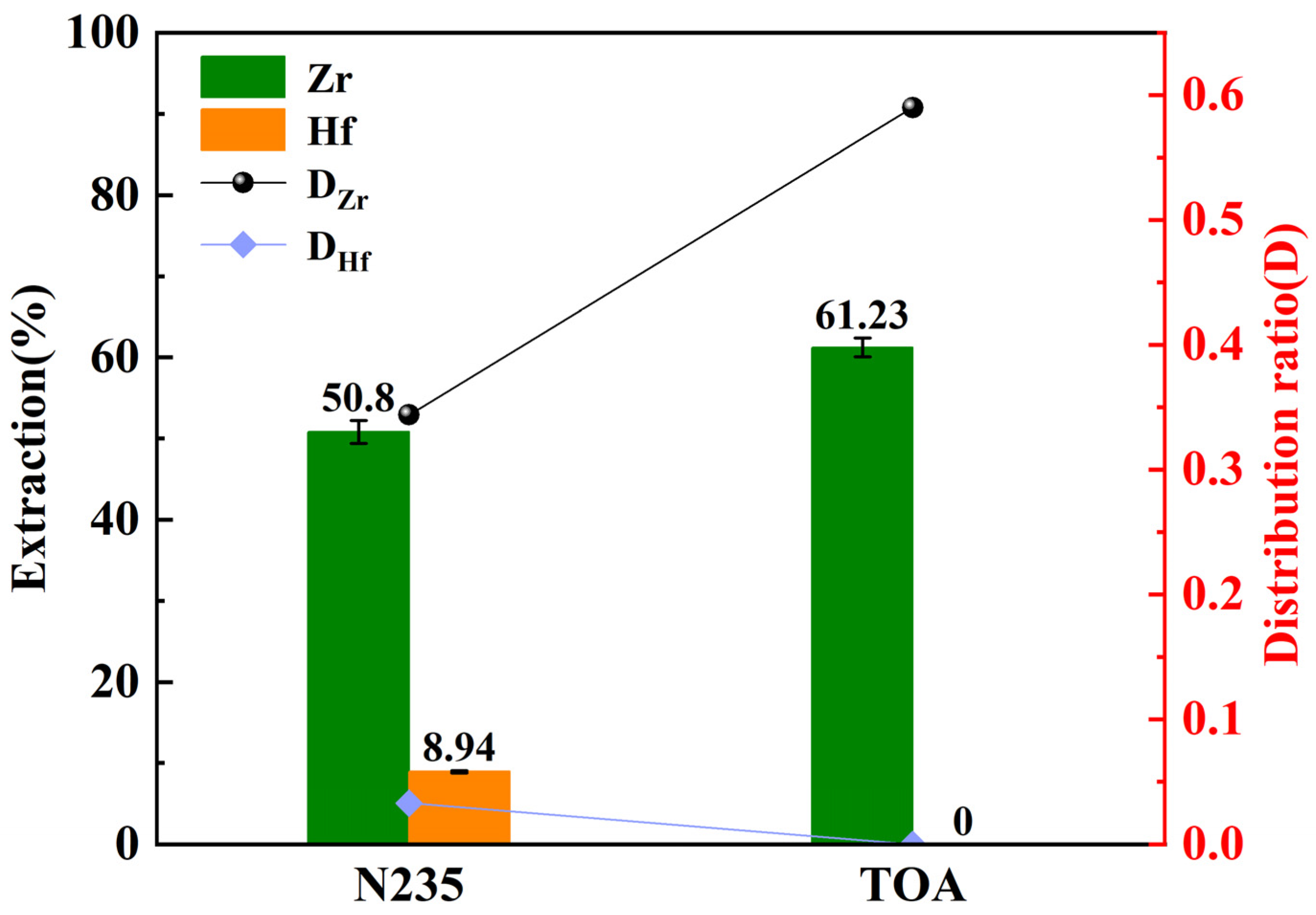
3.1. Extractant Type Influence on Zr/Hf Separation Efficiency
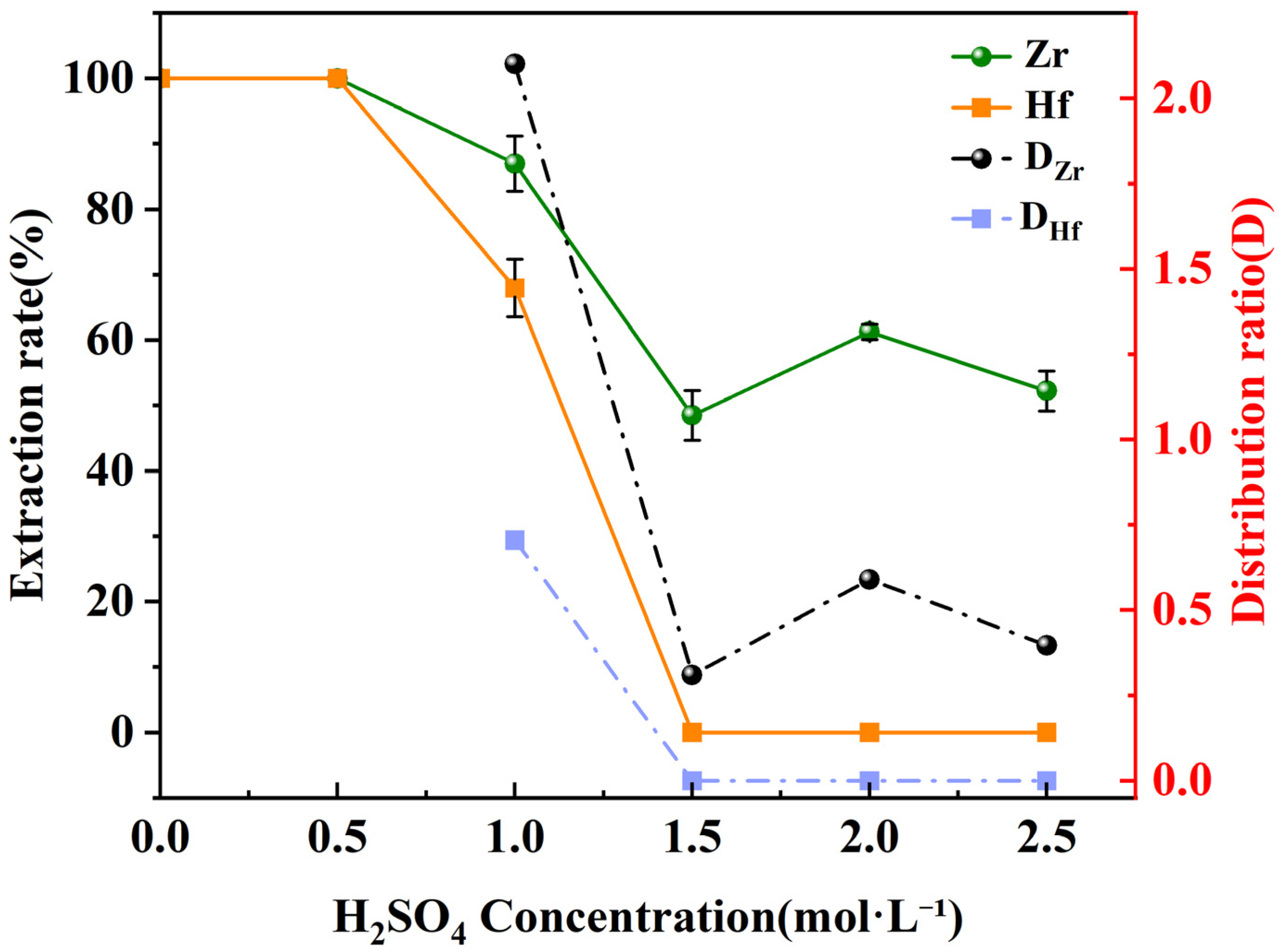
3.2. Impact of Sulfuric Acid Concentration
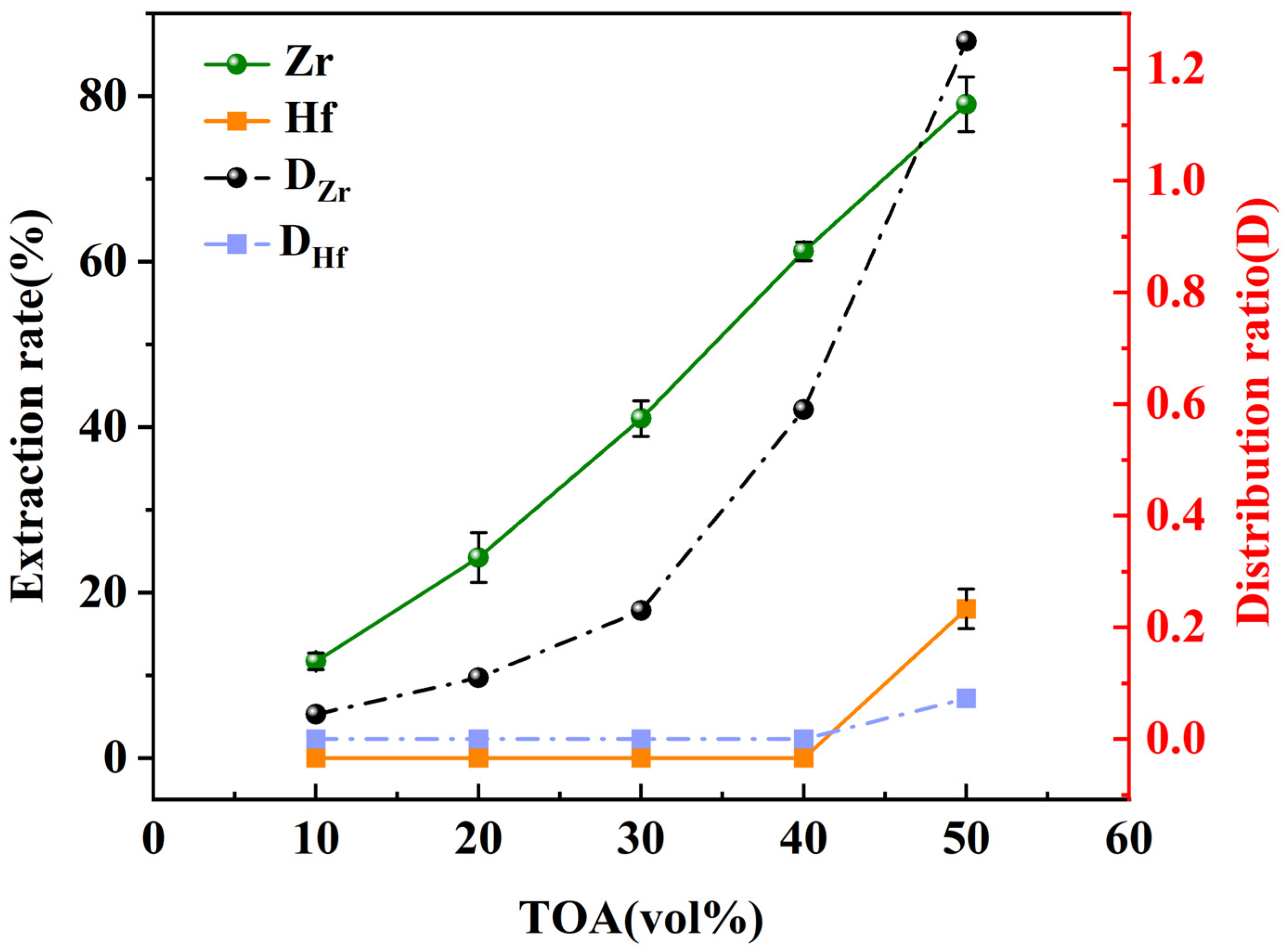
3.3. Effect of the Concentration of TOA
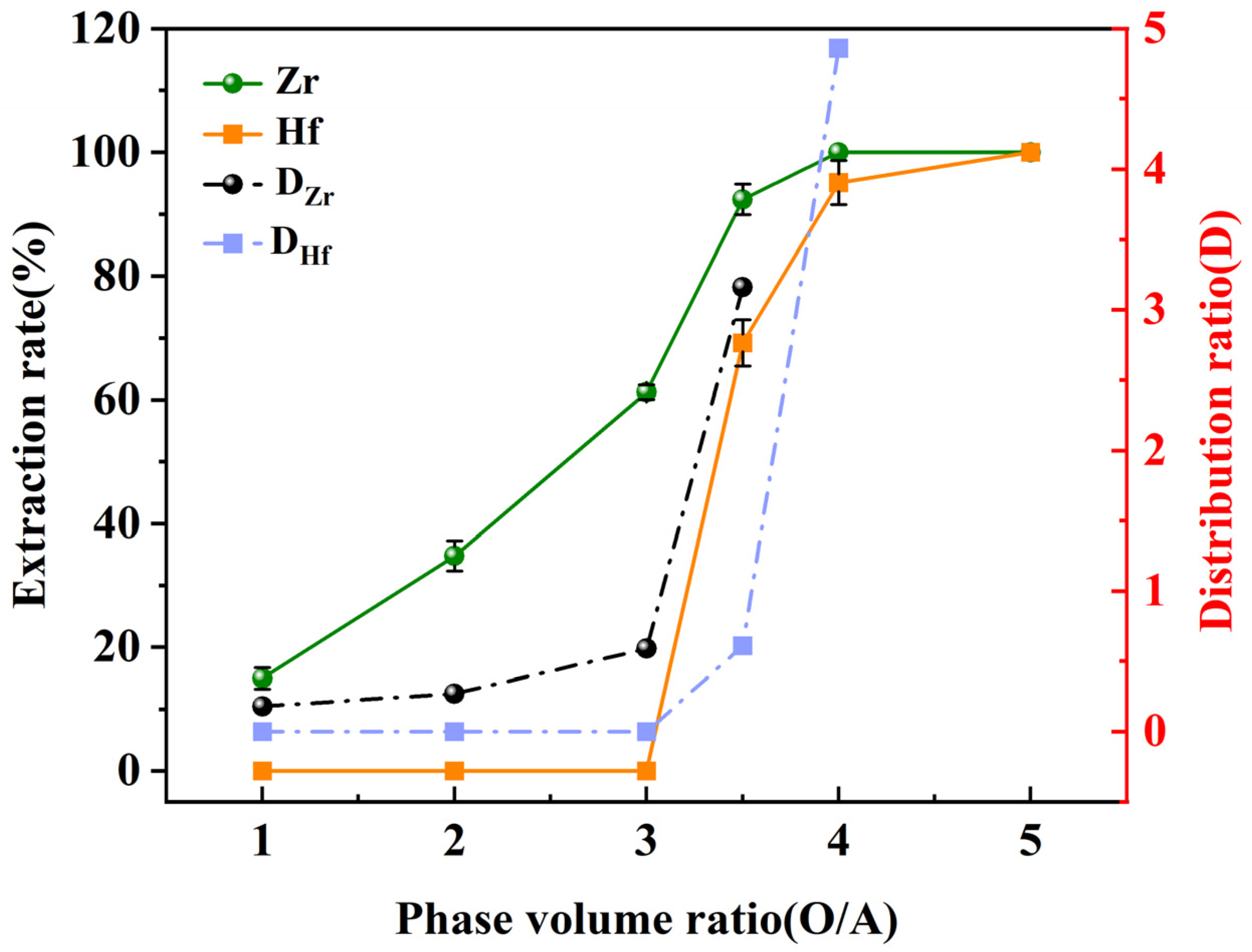
3.4. O/A Ratio Effects on Extraction
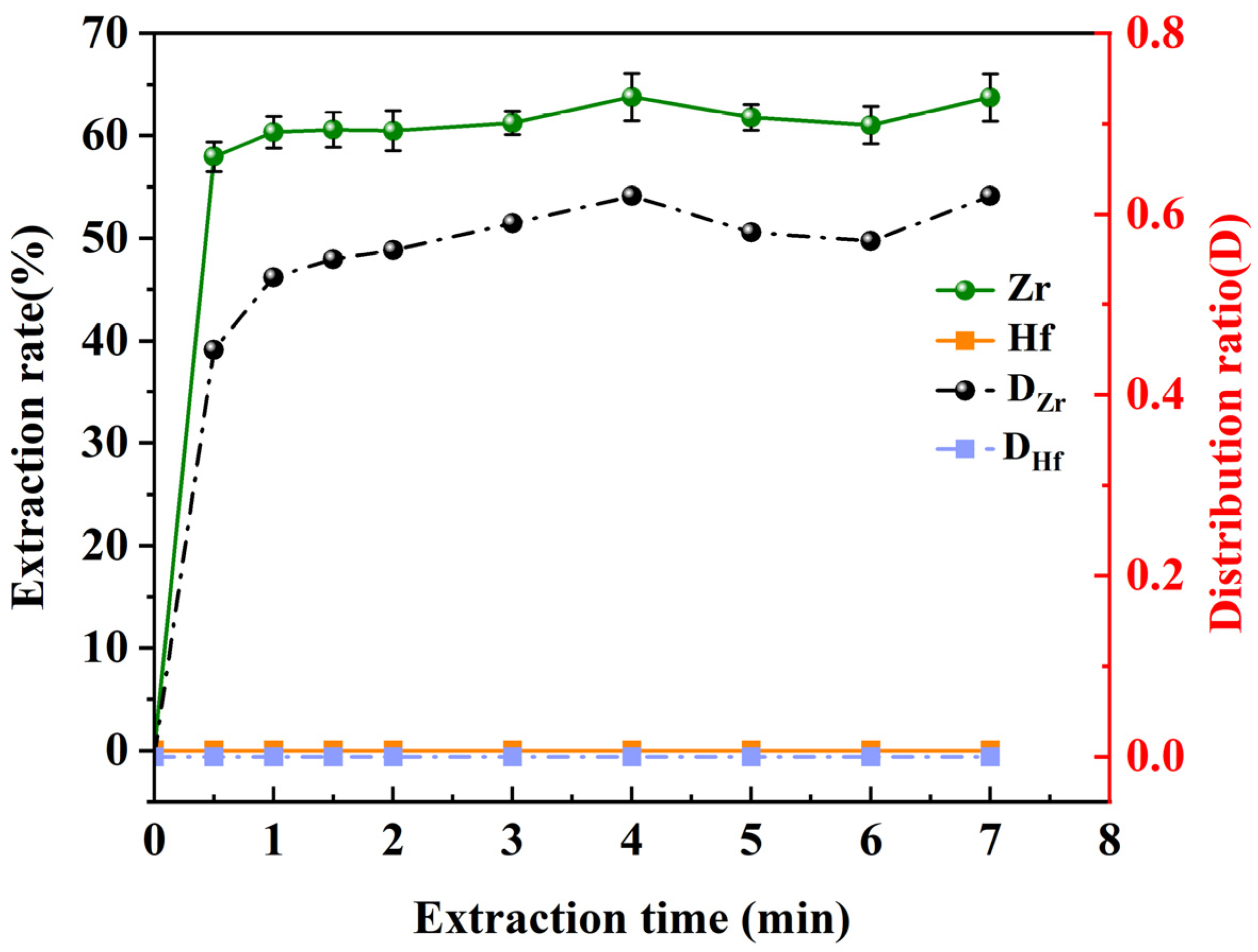
3.5. Effect of Extraction Time on Metal Extraction
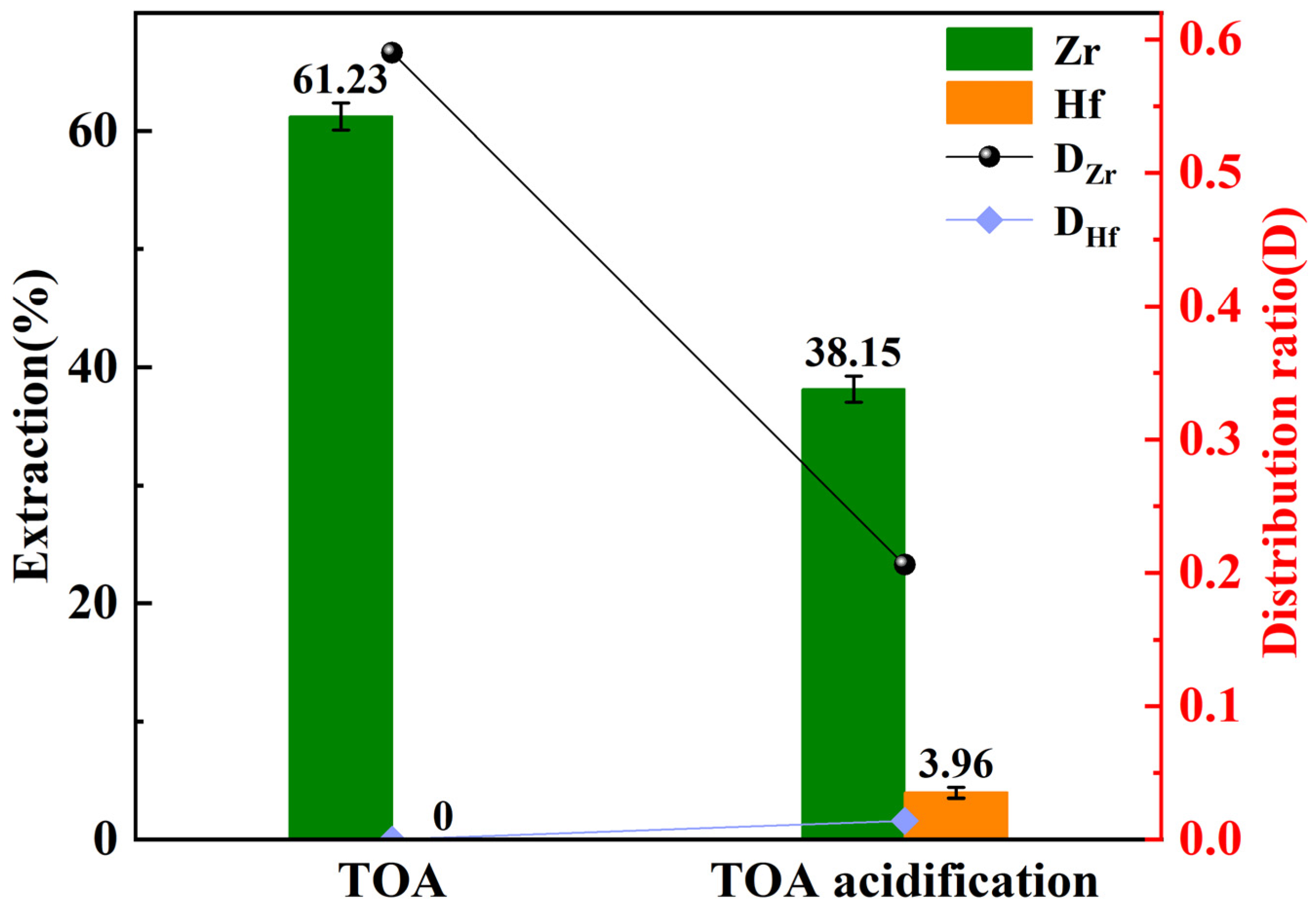
3.6. Effect of TOA Acidification
3.7. Extraction Mechanism of Zr by TOA
3.7.1. Saturation Capacity Method Analysis
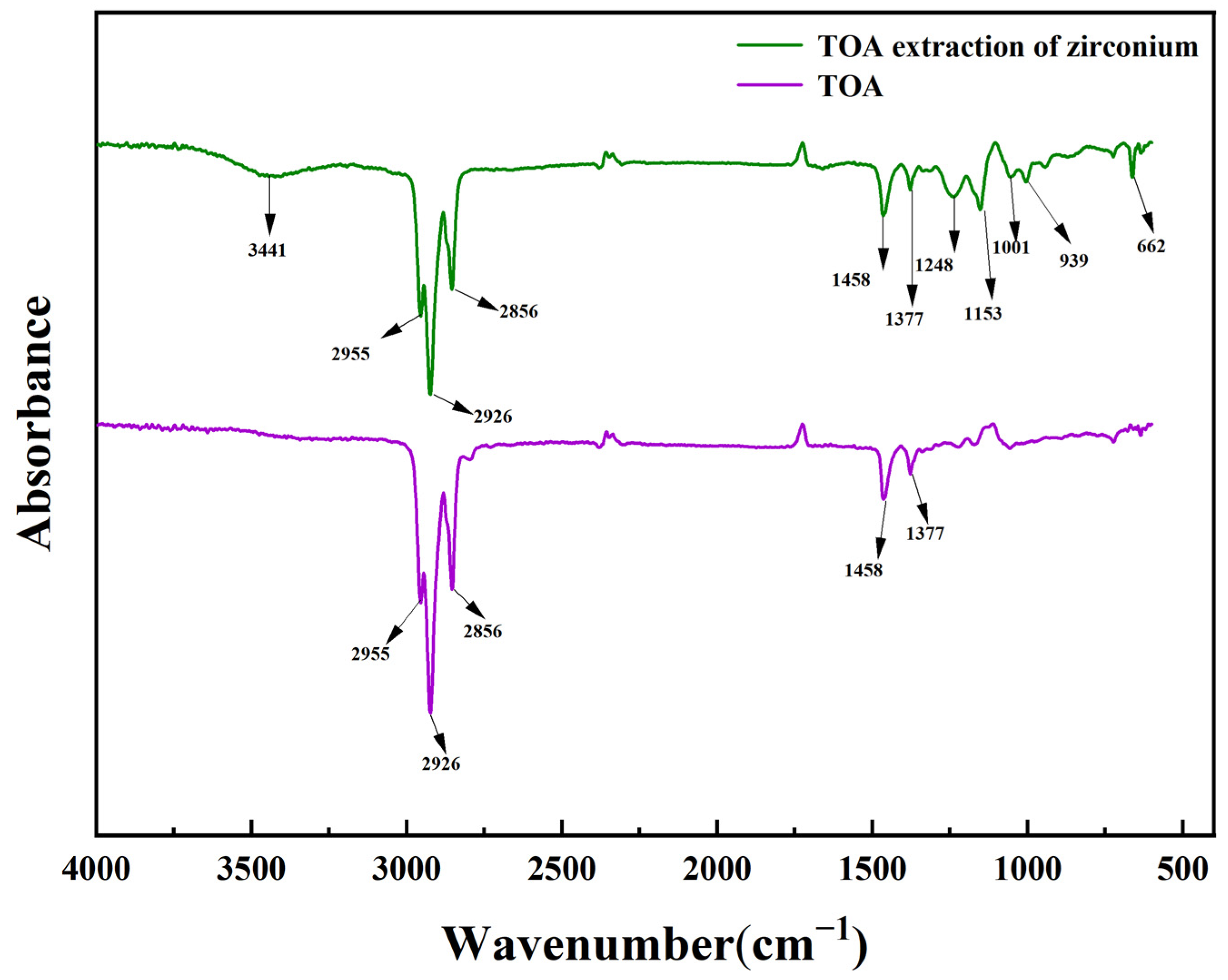
3.7.2. Infrared Spectroscopy Analysis of the Extraction Process
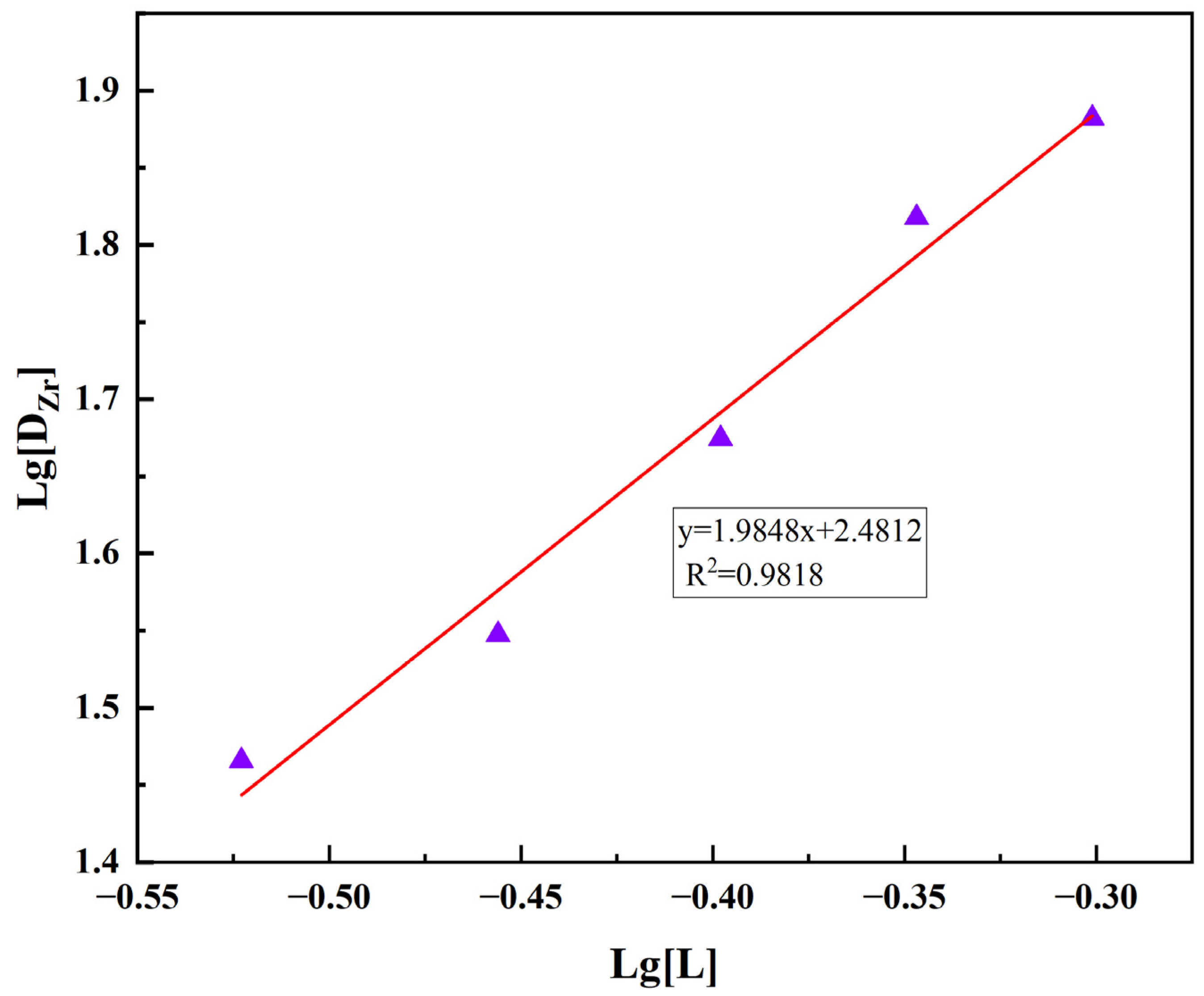
3.7.3. Slope Analysis for Determining TOA/Zr Molar Ratio in Extraction Complexes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, S.; Yang, F.; Tan, F.; Xie, M.; Yu, S.; Xue, L.; Li, Z.; Hu, T. Highly efficient and selective solvent extraction of zirconium and hafnium from chloride acid solution including amic acid extractant. Sep. Purif. Technol. 2021, 279, 119779. [Google Scholar] [CrossRef]
- Tu, H.-L.; Zhao, H.-B.; Fan, Y.-Y.; Zhang, Q.-Z. Recent developments in nonferrous metals and related materials for biomedical applications in China: A review. Rare Met. 2022, 41, 1410–1433. [Google Scholar] [CrossRef]
- Ismail, Z.H.; Rizk, S.E.; Elgoud, E.A.; Aly, H.F. Separation of hafnium from zircon leach solution using anion-exchange resin and production of high-purity zirconia for nuclear applications. Hydrometallurgy 2025, 231, 106411. [Google Scholar] [CrossRef]
- Sun, X.-D.; Yu, H.-W.; Zhao, C.-Y.; Zhang, J.; Shi, Y.; Pan, L.-J. Tunable structural coloration of ultrathin zirconia nanotubes film. Rare Met. 2023, 42, 3304–3310. [Google Scholar] [CrossRef]
- Bock, R. Über die Trennung anorganischer Stoffgemische durch Verteilung zwischen zwei Lösungsmitteln. 6. Fre. Für Anal. Chem. 1951, 133, 110–135. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, C.; Sui, N.; Liu, X.; Wang, J. Evaluation of novel solvent extraction systems for Zr/Hf separation with MIBK homologues. Arab. J. Chem. 2024, 17, 105425. [Google Scholar] [CrossRef]
- Xu, Z.-G.; Wu, Y.-K.; Zhang, J.-D.; Zhang, L.; Wang, L.-J. Equilibrium and kinetic data of adsorption and separation for zirconium and hafnium onto MIBK extraction resin. Trans. Nonferrous Met. Soc. China 2010, 20, 1527–1533. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. Solvent Extraction of Zr(IV) and Hf(IV) from Sulfuric Acid Solutions by Acidic Extractants and Their Mixtures with TBP. KCI Accredit. J. 2016, 25, 3–9. [Google Scholar]
- Xu, Z.; Wang, L.; Wu, M.; Xu, Y.; Chi, R.; Li, P.; Zhao, J. Separation of zirconium and hafnium by solvent extraction using mixture of DIBK and P204. Hydrometallurgy 2016, 165, 275–281. [Google Scholar] [CrossRef]
- Li, T.; Dong, S.; Chen, Z.; Wei, Q.; Ren, X. Breaking through the Separation Barrier of Zr(IV) and Hf(IV): Magical Effect of the Bisamide Ligand. Inorg. Chem. 2022, 61, 13915–13923. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, Y.; van Sandwijk, A.; Xu, Q.; Yang, Y. Production of nuclear grade zirconium: A review. J. Nucl. Mater. 2015, 466, 21–28. [Google Scholar] [CrossRef]
- Nayak, A.K.; Devi, N.; Sarangi, K. Recovery of Mo and V from the Spent Catalyst Using Organophosphorus Extractants. Trans. Indian Inst. Met. 2021, 74, 3155–3162. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Z.; Zhang, W.; Li, P.; Xu, Y. Extraction kinetics of zirconium and hafnium in DIBK-TOPO system. Rare Met. 2017, 41, 108–112. [Google Scholar]
- Lee, M.S.; Banda, R.; Min, S.H. Separation of Hf(IV)–Zr(IV) in H2SO4 solutions using solvent extraction with D2EHPA or Cyanex 272 at different reagent and metal ion concentrations. Hydrometallurgy 2015, 152, 84–90. [Google Scholar] [CrossRef]
- Banerjee, S.; Basu, S. Synergistic effect of neutral donors on the extraction of zirconium(IV) by salicylaldoxime in dichloromethane. J. Radioanal. Nucl. Chem. 2005, 262, 733–737. [Google Scholar] [CrossRef]
- Ekatova, T.Y.; Kazakov, A.G. Extraction-chromatographic behavior of Zr(IV) and Hf(IV) on TRU and LN resins in mixtures of HNO3 and HF. J. Radioanal. Nucl. Chem. 2019, 321, 557–563. [Google Scholar] [CrossRef]
- Banda, R.; Lee, H.Y.; Lee, M.S. Separation of Zr from Hf in Hydrochloric Acid Solution Using Amine-Based Extractants. Ind. Eng. Chem. Res. 2012, 51, 9652–9660. [Google Scholar] [CrossRef]
- Sato, T.; Watanabe, H. The extraction of zirconium(IV) from sulphuric acid solutions by long-chain aliphatic amines. J. Inorg. Nucl. Chem. 1974, 36, 2585–2589. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. A review on the aqueous chemistry of Zr(IV) and Hf(IV) and their separation by solvent extraction. J. Ind. Eng. Chem. 2016, 39, 1–9. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. Separation of Zr and Hf from sulfuric acid solutions with amine-based extractants by solvent extraction. Sep. Purif. Technol. 2015, 142, 83–89. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. Development of a separation process for the selective extraction of hafnium(IV) over zirconium(IV) from sulfuric acid solutions by using D2EHPA. Hydrometallurgy 2016, 160, 12–17. [Google Scholar] [CrossRef]
- Wu, M.; Dong, P.; Wu, C.; Zhang, Z.; Chi, R.; Xu, Z. Separation of Hf(IV) from Zr(IV) in thiocyanate medium with ionic liquid Aliquat 336. Hydrometallurgy 2022, 213, 105947. [Google Scholar] [CrossRef]
- Song, X.; Sun, S.; Yin, Z.; Zhang, W.; Yang, Y. The study on formation of the reversed micelle in extraction system of primary amine N1923 sulfate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 209, 57–63. [Google Scholar] [CrossRef]
- Qiu, X.; Chang, Z.; Li, W.; Zhou, H.; Dong, B.; Qiao, L. Structural analysis of NH⋯O in viscoelastic scum formation during solvent extraction of sulfuric acid with trioctylamine. Sep. Purif. Technol. 2012, 95, 196–201. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. CHAPTER 10—Compounds Containing –NH2, –NHR, and –NR2 Groups. In The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Lin-Vien, D., Colthup, N.B., Fateley, W.G., Grasselli, J.G., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 155–178. [Google Scholar] [CrossRef]
- Qiu, X.; Chang, Z.; Zhou, H.; Li, W.; Dong, B. Effect of hydroxyl on formation of viscoelastic scum during solvent extraction of sulfuric acid with trioctylamine. Sep. Purif. Technol. 2012, 86, 137–142. [Google Scholar] [CrossRef]
- Ershad, M.; Almeida, M.I.G.S.; Spassov, T.G.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) containing purified dinonylnaphthalene sulfonic acid (DNNS): Performance and selectivity. Sep. Purif. Technol. 2018, 195, 446–452. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, J.; Zhao, Y.; Dong, Z.; Wang, L.; Liu, Y. The Recovery of Sulfuric Acid in the Presence of Zr(IV) and Hf(IV) by Solvent Extraction with TEHA and Its Mixtures. Processes 2024, 12, 940. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Sridhar, R.; Jankovic, Z.; Halim, A. Separation and Purification of Zirconium with Amines from Sulphuric Acid System: A Review. In Proceedings of the ISEC2014—International Solvent Extraction Conference, Würzburg, Germany, 7–11 September 2014. [Google Scholar]
- He, H.; Xu, F.; Li, Q.; Dong, P.; Zheng, J.; Wu, C.; He, Z.; Qu, J.; Xu, Z.; Chi, R.; et al. Separation of hafnium from zirconium in HNO3 solution by solvent extraction with Cyanex572. Hydrometallurgy 2021, 202, 105600. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Kuang, S.; Li, Y.; Huang, X.; Liao, W. Separation of zirconium from hafnium in sulfate medium using solvent extraction with a new reagent BEAP. Hydrometallurgy 2017, 169, 607–611. [Google Scholar] [CrossRef]

| TOA Concentration | Distribution Ratio of Zr and Hf D | |
|---|---|---|
| DZr | DHf | |
| 10 vol% | 0.044 | 0 |
| 20 vol% | 0.11 | 0 |
| 30 vol% | 0.23 | 0 |
| 40 vol% | 0.59 | 0 |
| 50 vol% | 1.25 | 0.073 |
| Extractant | Extraction Type | Concentration Range (Zr/Hf) | Selectivity | Extraction Rate (Zr/Hf) | Separation Factor (β) | References |
|---|---|---|---|---|---|---|
| Trioctylamine (TOA) | H2SO4 | 1.096 mol·L−1 Zr/0.0112 mol·L−1 Hf | Zr | Zr: 61.23%; Hf: 0% | βZr/Hf > 100 | This work |
| MIPK | HSCN | 0.0221 mol·L−1 Zr/0.000477 mol·L−1 Hf | Hf | - | βHf/Zr = 12.61 | [6,7] |
| TBP + Cyanex 923 | HNO3 | 0.285 mol·L−1 Zr/0.0028 mol·L−1 Hf | Zr | Zr: 53%; Hf: <1% | βZr/Hf = 186 | [8,9] |
| Alamine 308 (TOA) | H2SO4 | 0.00219 mol·L−1 Zr/0.00112 mol·L−1 Hf | Zr | Zr:65%; Hf: 15% | βZr/Hf = 12.4 | [20] |
| Alamine 300 | H2SO4 | 0.00219 mol·L−1 Zr/0.00112 mol·L−1 Hf | Zr | Zr: 75%; Hf: 28% | βZr/Hf = 10.4 | [20] |
| D2EHPA | H2SO4 | 0.00219 mol·L−1 Zr/0.00112 mol·L−1 Hf | Hf | Hf: 90% | βHf/Zr = 66 | [14] |
| Cyanex 272 | H2SO4 | Hf | Hf: 50–80% | βHf/Zr up to 23.7 | [14,15] | |
| Aliquat 336 | HSCN | 1.052 mol·L−1 Zr/0.0126 mol·L−1 Hf | Hf | Hf: 94.5% | βZr/Hf = 19.4 | [22] |
| Organic Phase Concentration (mol·L−1) | Saturated Zr Concentration (mol·L−¹) | [Zr]/[TOA] Ratio |
|---|---|---|
| 0.1 | 0.0522 | 0.522 |
| 0.2 | 0.097 | 0.488 |
| 0.3 | 0.166 | 0.555 |
| Summary | - | All ratios are approximately 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Song, J.; Sun, H.; Zhao, C.; Zhang, Z.; Han, M.; Qi, T. Selective Extraction of Zirconium from Sulfuric Acid Solutions at High Concentration with Trioctylamine (TOA). Metals 2025, 15, 468. https://doi.org/10.3390/met15050468
Tian S, Song J, Sun H, Zhao C, Zhang Z, Han M, Qi T. Selective Extraction of Zirconium from Sulfuric Acid Solutions at High Concentration with Trioctylamine (TOA). Metals. 2025; 15(5):468. https://doi.org/10.3390/met15050468
Chicago/Turabian StyleTian, Shuo, Jing Song, Hongqian Sun, Congcong Zhao, Zhiyu Zhang, Mingming Han, and Tao Qi. 2025. "Selective Extraction of Zirconium from Sulfuric Acid Solutions at High Concentration with Trioctylamine (TOA)" Metals 15, no. 5: 468. https://doi.org/10.3390/met15050468
APA StyleTian, S., Song, J., Sun, H., Zhao, C., Zhang, Z., Han, M., & Qi, T. (2025). Selective Extraction of Zirconium from Sulfuric Acid Solutions at High Concentration with Trioctylamine (TOA). Metals, 15(5), 468. https://doi.org/10.3390/met15050468





