Definition of a First Process Window for Purification of Aluminum via “Cooled Finger” Crystallization Technique
Abstract
:1. Introduction
2. Experimental and Assessment Methodology
3. Results and Discussions
3.1. Effect of Combination of Rotation and Cooling Gas Flow Rate on Crystallization Growth
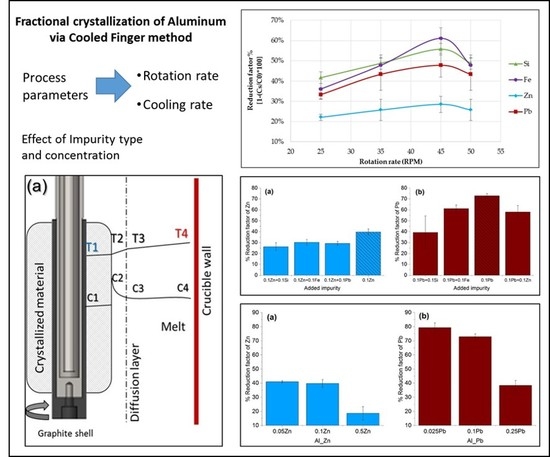
3.2. Effect of Rotation on Removal Efficiency (Reduction Factor)
3.3. Effect of Type and the Concentration of the Impuriies on the Removal Efficiency
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weiser, K. Theoretical calculation of distribution coefficients of impurities in germanium and silicon, heats of solid solution. J. Phys. Chem. Solids 1958, 7, 118–126. [Google Scholar] [CrossRef]
- Pfann, W.G. Zone melting: This technique offers unique advantages in purification and in control of composition in various substances. Science 1962, 135, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Bertazzoli, R.; Garcia, A. Experimental impurity segregation and numerical analysis based on variable solute distribution coefficients during multi-pass zone refining of aluminum. J. Cryst. Growth 2008, 310, 1274–1280. [Google Scholar] [CrossRef]
- Burton, J.A.; Prim, R.C.; Slichter, W.P. The Distribution of Solute in Crystals Grown from the Melt. Part II. Experimental. J. Chem. Phys. 1953, 21, 1991–1996. [Google Scholar] [CrossRef]
- Burton, J.A.; Prim, R.C.; Slichter, W.P. The Distribution of Solute in Crystals Grown from the Melt. Part I. Theoretical. J. Chem. Phys. 1953, 21, 1987–1991. [Google Scholar] [CrossRef]
- Tiller, W.; Jackson, K.; Rutter, J.; Chalmers, B. The redistribution of solute atoms during the solidification of metals. Acta Metall. 1953, 1, 428–437. [Google Scholar] [CrossRef]
- Ostrogorsky, A.G.; Glicksman, M.E. Segregation and Component Distribution. In Handbook of Crystal Growth: Bulk Crystal Growth, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume II-A, pp. 1008–1009. [Google Scholar]
- Porter, D.A.; Easterling, K.E. Solidification. In Phase Transformations in Metals and Alloys, 2nd ed.; Chapman & Hall: London, UK, 1992; pp. 209–215. [Google Scholar]
- Chatelain, M.; Albaric, M.; Pelletier, D.; Botton, V. Solute segregation in directional solidification: Scaling analysis of the solute boundary layer coupled with transient hydrodynamic simulations. J. Cryst. Growth 2015, 430, 138–147. [Google Scholar] [CrossRef]
- Wilson, L.O. On interpreting a quantity in the burton, prim and slichter equation as a diffusion boundary layer thickness. J. Cryst. Growth 1978, 44, 247–250. [Google Scholar] [CrossRef]
- Rudolph, P.; Nishinga, T. Handbook of Crystal Growth: Bulk Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2014; Volume II-B, pp. 389–397. [Google Scholar]
- Hannibal, W.D.; Ibe, G.; Kurre, K.; Peychal-Heiling, H. Entwicklung Eines Technischen Verfahrens zur Herstellung von Reinstaluminium für die Kyroelektrotechnik, Speziell für Kyromagnete; Zentralstelle für Luft-u. Raumfahrtdokumentation u.-information: Munich, Germany, 1974. [Google Scholar]
- Barthel, J.; Buhirg, E.; Al, E. Kristallisation Aus Schmelzen; Verlag für Grundstoffindustrie Leipzig: Leipzig, Germany, 1982. [Google Scholar]
- Widiatmo, J.V.; Harada, K.; Yamazawa, K.; Arai, M. Estimation of impurity effect in aluminium fixed-point cells based on thermal analysis. Metrologia 2006, 43, 561–572. [Google Scholar] [CrossRef]
- Hashimoto, E.; Ueda, Y.; Kino, T. Purification of Ultra-High Purity Aluminum. J. Phys. IV 1995, 5, C7-153–C7-157. [Google Scholar] [CrossRef]
- Shingu, H.; Arai, K.; Sakaguchi, M.; Nishide, T.; Watanabe, O.; Tashiro, Y.; Otsuka, R.; Tsukamoto, K. Process for Producing High-Purity Aluminum. U.S. Patent 4,469,512, 4 September 1984. [Google Scholar]
- Friedrich, S.; Curtolo, D.C.; Friedrich, B. Effect of process parameter variation on purity during rotary fractional crystallization of Aluminum. Open J. Met. 2017, 7, 25. [Google Scholar] [CrossRef]
- Altieri, A.L.; Davis, S.H. Instabilities in solidification of multi-component alloys. J. Cryst. Growth 2017, 467, 162–171. [Google Scholar] [CrossRef]
- Curtolo, D.C.; Friedrich, S.; Friedrich, B. High purity Germanium, a review on principle theories and technical production methodologies. J. Cryst. Process Technol. 2017, 7, 79–80. [Google Scholar] [CrossRef]
- Müller, A.; Wilhelm, M. Periodische Temperaturschwankungen in flüssigem InSb als Ursache schichtweisen Einbaus von Te in kristallisierendes InSb. Zeitschrift für Naturforschung A 1964, 19, 254–263. [Google Scholar] [CrossRef]
- Ueda, H. Resistivity Striations in Germanium Single Crystal. J. Phys. Soc. Jpn. 1960, 16, 61–66. [Google Scholar] [CrossRef]









| Element | Range of k | Element | Range of k |
|---|---|---|---|
| Fe [12,13,14] | 0.018–0.053 | Ti [12,14] * | 7–11 |
| Cu [12,13,14] | 0.15–0.153 | Si [12,13,14] * | 0.082–0.12 |
| Ag [12,13] | 0.2–0.3 | K [14] | 0.56 |
| Au [12,13] | 0.18 | Zr [14] * | 2.3–3 |
| Zn [12,14] * | 0.35–0.47 | Pb * | 0.0007 |
| Ni [12,13,14] * | 0.004–0.09 | P [12,13] | <0.01 |
| Mn [12,14] * | 0.55–0.9 | Sc [15] | 0.9 |
| Mg [12,14] * | 0.29–0.5 | Sb [12] | 0.09 |
| Ca [12,13,14] | 0.006–0.08 | V [12,14] * | 3.3–4.3 |
| Cr [14] | 1.8 | Na [14] | 0.013 |
| Rotation Rate (rpm) | 25 | 35 | 45 | 50 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cooling gas flow rate (L/min) | 45 | 50 | 55 | 45 | 50 | 55 | 45 | 50 | 55 | 45 | 50 | 55 |
| Average growth rate (μm/s) | 12.2 | 13.3 | 17 | 6.5 | 11.3 | 21.5 | 0.7 | 9.3 | 19.3 | - | 7.5 | 20.6 |
| Rotation (rpm) | Average Growth Rate (μm/s) |
|---|---|
| 25 | 22.09 |
| 35 | 17.48 |
| 45 | 10.83 |
| 50 | 10.86 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtolo, D.C.; Friedrich, S.; Bellin, D.; Nayak, G.S.; Friedrich, B. Definition of a First Process Window for Purification of Aluminum via “Cooled Finger” Crystallization Technique. Metals 2017, 7, 341. https://doi.org/10.3390/met7090341
Curtolo DC, Friedrich S, Bellin D, Nayak GS, Friedrich B. Definition of a First Process Window for Purification of Aluminum via “Cooled Finger” Crystallization Technique. Metals. 2017; 7(9):341. https://doi.org/10.3390/met7090341
Chicago/Turabian StyleCurtolo, Danilo C., Semiramis Friedrich, Dominic Bellin, Gargi S. Nayak, and Bernd Friedrich. 2017. "Definition of a First Process Window for Purification of Aluminum via “Cooled Finger” Crystallization Technique" Metals 7, no. 9: 341. https://doi.org/10.3390/met7090341
APA StyleCurtolo, D. C., Friedrich, S., Bellin, D., Nayak, G. S., & Friedrich, B. (2017). Definition of a First Process Window for Purification of Aluminum via “Cooled Finger” Crystallization Technique. Metals, 7(9), 341. https://doi.org/10.3390/met7090341