Effects of Trace Alloying Elements Fe and Cr on the Microstructure and Aging Properties of Cu-3Ti Alloy Foils
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
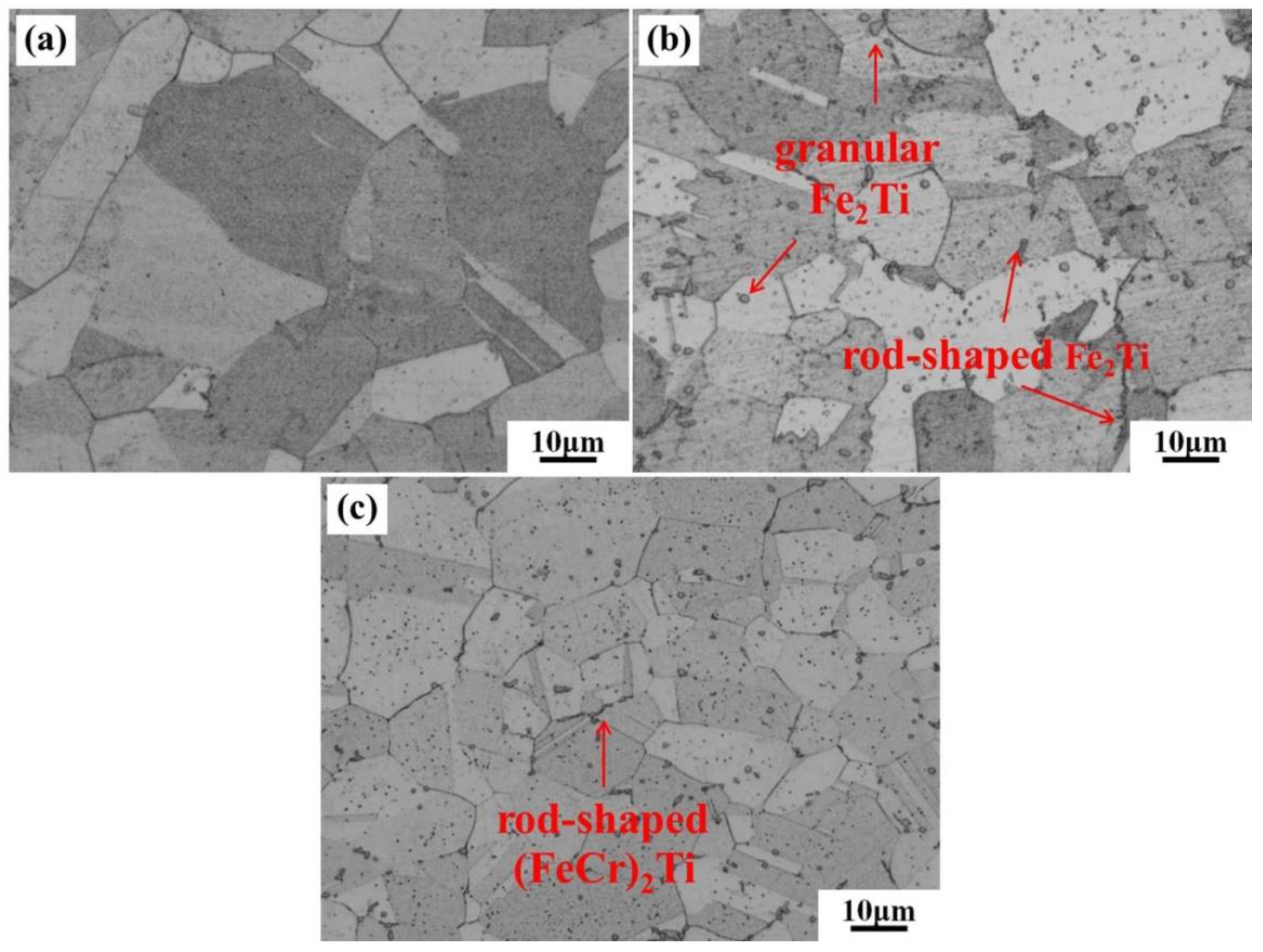
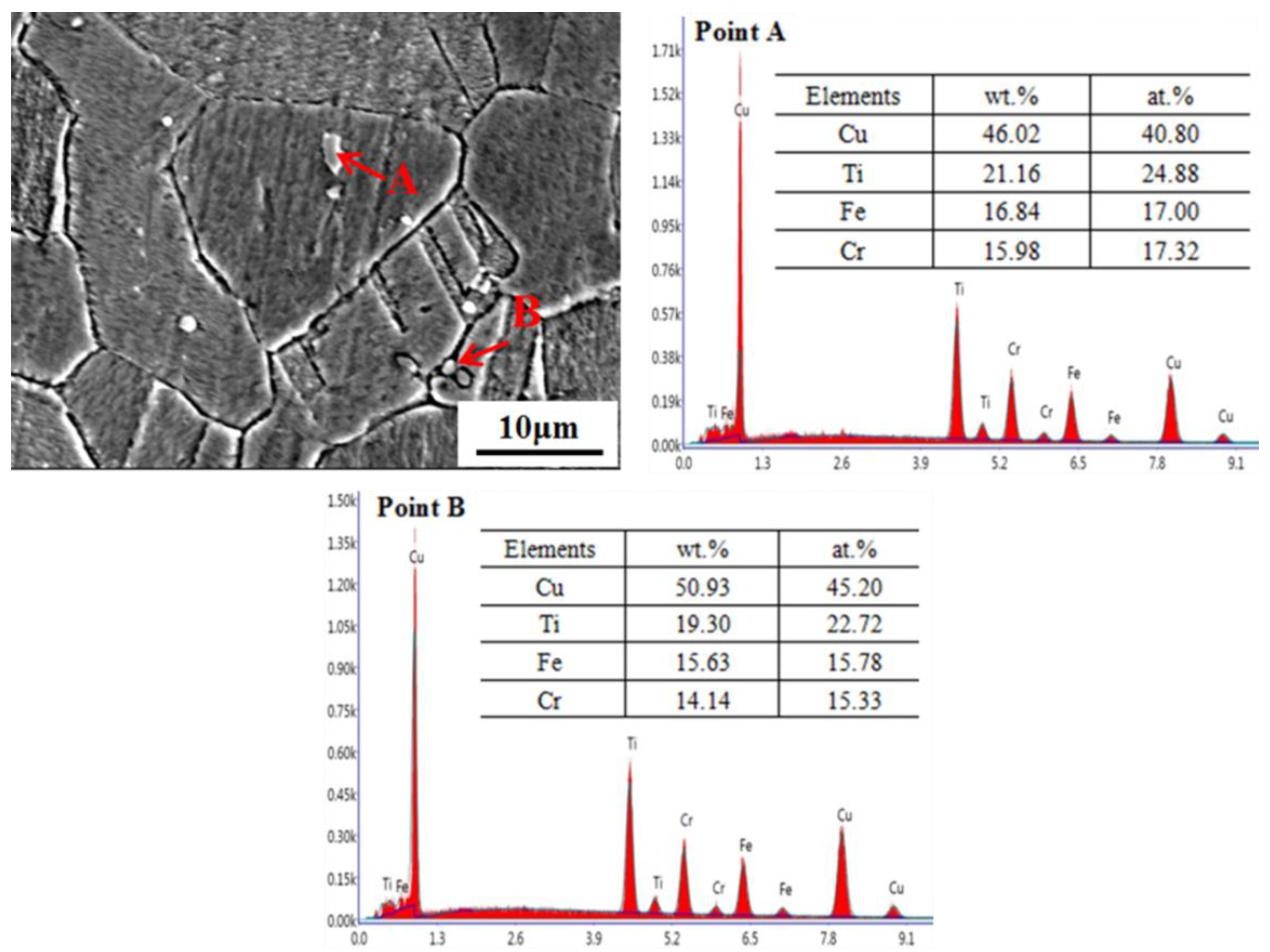
3.1. Microstructure of the Specimens in the Solution-Treated Condition
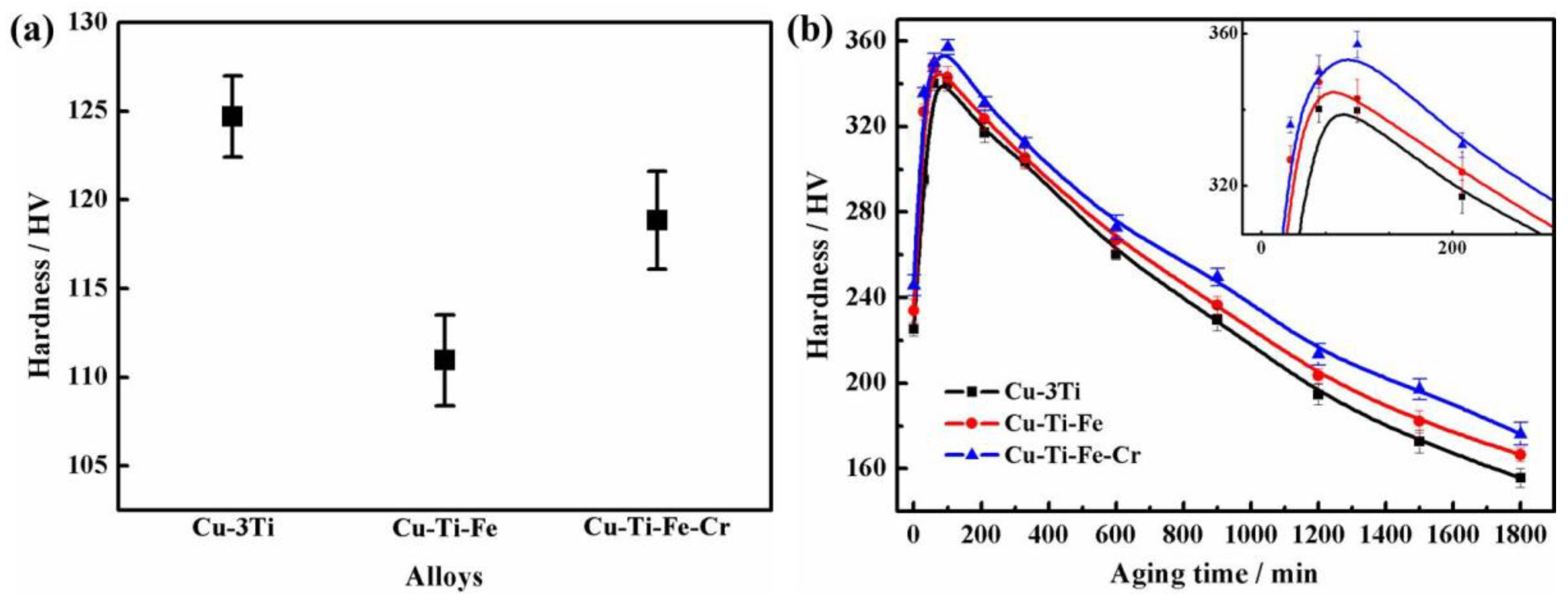
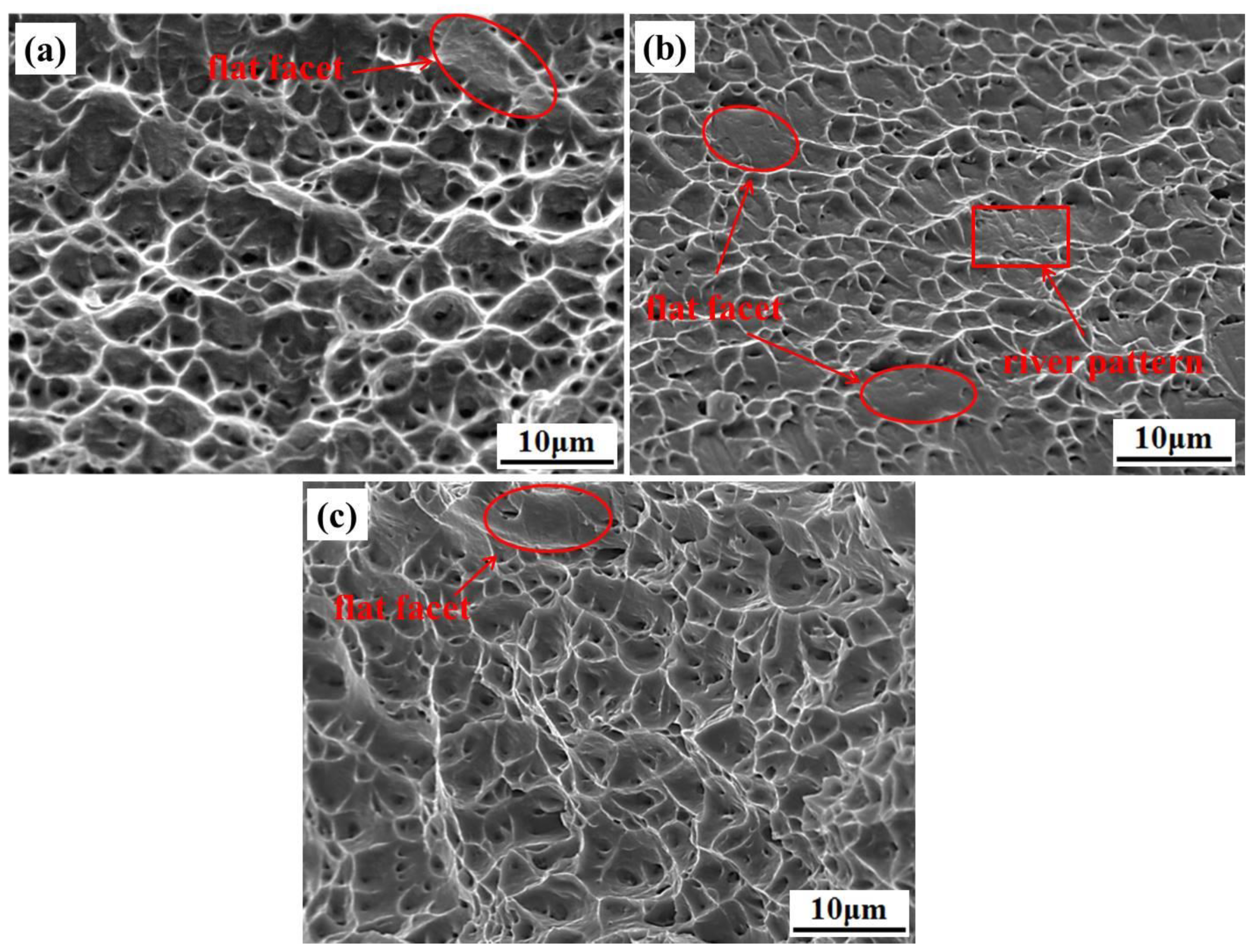
3.2. Mechanical Properties
3.3. Electrical Properties
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nagarjuna, S.; Sharma, K.K.; Sudhakar, I.; Sarma, D.S. Age hardening studies in a Cu-4.5Ti-0.5Co alloy. Mater. Sci. Eng. A 2001, 313, 251–260. [Google Scholar] [CrossRef]
- Xiao, X.P.; Yi, Z.Y.; Chen, T.T.; Liu, R.Q.; Wang, H. Suppressing spinodal decomposition by adding Co into Cu-Ni-Si alloy. J. Alloys Compd. 2016, 660, 178–183. [Google Scholar] [CrossRef]
- Wei, H.; Cui, Y.C.; Cui, H.Q.; Wei, Y.H.; Hou, L.F. Effects of multiple trace alloying elements on the microstructure and properties of Cu-4wt% Ti alloys. Mater. Sci. Eng. A 2017, 707, 392–398. [Google Scholar] [CrossRef]
- Nagarjuna, S.; Srinivas, M.; Balasubramanian, K.; Sarma, D.S. The alloy content and grain size dependence of flow stress in Cu-Ti alloys. Acta Mater. 1996, 44, 2285–2293. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Xiao, Z.; Li, S.H.; Shen, L.N.; Dong, Q.Y. Microstructure and property of Cu-2.7Ti-0.15Mg-0.1Ce-0.1Zr alloy treated with a combined aging process. Mater. Sci. Eng. A 2016, 650, 345–353. [Google Scholar]
- Nagarjuna, S.; Balasubramanian, K.; Sarma, D.S. Effect of prior cold work on mechanical properties, electrical conductivity and microstructure of aged Cu-Ti alloys. J. Mater. Sci. 1999, 34, 2929–2942. [Google Scholar] [CrossRef]
- Betra, I.S.; Laik, A.; Kale, G.B.; Dey, G.K.; Kulkarni, U.D. Microstructure and properties of a Cu-Ti-Co alloy. Mater. Sci. Eng. A 2005, 402, 118–125. [Google Scholar] [CrossRef]
- Suzuki, S.; Hirabayashi, K.; Shibata, H.; Mimura, K.; Isshiki, M.; Waseda, Y. Electrical and thermal conductivities in quenched and aged high–purity Cu-Ti alloys. Scripta Mater. 2003, 48, 431–435. [Google Scholar] [CrossRef]
- Soffa, W.A.; Laughlin, D.E. High-strength age hardening copper-titanium alloys: redivivus. Prog. Mater. Sci. 2004, 49, 347–366. [Google Scholar] [CrossRef]
- Nagarjuna, S.; Balasubramanian, K.; Sarma, D.S. Effect of Ti additions on the electrical resistivity of copper. Mater. Sci. Eng. A 1997, 225, 118–124. [Google Scholar] [CrossRef]
- Markandeya, R.; Nagarjuna, S.; Sarma, D.S. Effect of prior cold work on age hardening of Cu-3Ti-1Cr alloy. Mater. Charact. 2006, 57, 348–357. [Google Scholar] [CrossRef]
- Markandeya, R.; Nagarjuna, S.; Sarma, D.S. Influence of prior cold work on age hardening of Cu-Ti-Zr alloys. Mater. Sci. Tech-lond 2005, 21, 1171–1180. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.H.; Ran, Q.N.; Zhao, G.; Zhu, X.X. Microstructure and properties of Cu-3Ti-1Ni alloy with aging process. T. Nonferr. Metal. Soc. 2016, 26, 3183–3188. [Google Scholar] [CrossRef]
- Toyohiko, J.K.; Rimi, N.; Satoshi, S.; Tetsu, O.; Eiji, O. Aging behavior of Cu-Ti-Al alloy observed by transmission electron microscopy. J. Mater. Sci. 2008, 43, 3761–3768. [Google Scholar]
- Wang, X.H.; Chen, C.Y.; Guo, T.T.; Zou, J.T.; Yang, X.H. Microstructure and properties of ternary Cu-Ti-Sn alloy. J. Mater. Eng. Perform. 2015, 24, 2738–2743. [Google Scholar] [CrossRef]
- Fernee, H.; Nairn, J.; Atrens, A. Precipitation hardening of Cu-Fe-Cr alloys. J. Mater. Sci. 2001, 36, 2711–2719. [Google Scholar] [CrossRef]
- Wei, H.; Wei, Y.H.; Hou, L.F.; Dang, N. Correlation of aging precipitates with the corrosion behavior of Cu-4wt.% Ti alloys in 3.5wt.% NaCl solution. Corros. Sci. 2016, 111, 382–390. [Google Scholar] [CrossRef]
- Mineau, L.; Hamar-Thibault, S.J.; Allibert, C.H. Precipitation in Cu-rich Cu-Fe-Ti ternary alloys. Phys. Status. Solidi. A 1993, 137, 87–100. [Google Scholar] [CrossRef]
- Bo, H.; Wang, J.; Duarte, L.; Leinenbach, C.; Liu, L.B.; Liu, H.S.; Jin, Z.P. Thermodynamic re-assessment of Fe-Ti binary system. T. Nonferr. Metal. Soc. 2012, 22, 2204–2211. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhao, J.Z.; He, J. Investigation on the microstructure and mechanical properties of the spray-formed Cu-Cr alloys. Mater. Sci. Eng. A 2007, 460, 69–76. [Google Scholar] [CrossRef]
- Atsunori, K.; Takahiro, K.; Mauo, O. Effects of dehydrogenation heat-treatment on electrical-mechanical properties for hydrogenated Cu-3 mass% Ti alloys. J. Alloys Compd. 2013, 566, 1–4. [Google Scholar]
- Satoshi, S.; Mikio, I.; Shigeo, S.; Kazuaki, W.; Takayuki, T. Extraction of precipitates from age-hardenable Cu-Ti alloys. Mater. Charact. 2013, 82, 23–31. [Google Scholar]
- Satoshi, S.; Shintaro, A.; Jie, F.; Akihiro, I.; Takayuki, T. Kinetics and equilibrium of age-induced precipitation in Cu-4at.% Ti binary alloy. Metall. Mater. Trans. A 2017, 48, 1501–1511. [Google Scholar]
- Lei, Q.; Li, Z.; Pan, Z.Y.; Wang, M.P.; Xiao, Z.; Chen, C. Dynamics of phase transformation of Cu-Ni-Si alloy with super-high strength and high conductivity during aging. T. Nonferr. Metal. Soc. 2010, 20, 1006–1011. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change I general theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Cahn, J.W. Thermodynamic aspects of Cottrell atmospheres. Philos. Mag. 2013, 93, 3741–3746. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization I. A Theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]


| Mechanical properties | Cu-3Ti | Cu-Ti-Fe | Cu-Ti-Fe-Cr |
|---|---|---|---|
| Peak hardness (HV) | 340.2 ± 4.0 | 347.3 ± 5.4 | 357.1 ± 4.6 |
| Ultimate tensile strength (MPa) | 945 ± 16 | 996 ± 17 | 1068 ± 21 |
| Yield strength (MPa) | 923 ± 8 | 926 ± 6 | 976 ± 11 |
| Elongation (%) | 2.2 ± 0.1 | 1.9 ± 0.1 | 2.2 ± 0.1 |
| Alloys | Peak aging EC (%IACS) |
|---|---|
| Cu-3Ti | 13.1 ± 0.1 |
| Cu-Ti-Fe | 12.1 ± 0.2 |
| Cu-Ti-Fe-Cr | 12.5 ± 0.1 |
| t/min | Cu-3Ti | Cu-Ti-Fe | Cu-Ti-Fe-Cr | |||
|---|---|---|---|---|---|---|
| σ/%IACS | f | σ/%IACS | f | σ/%IACS | f | |
| 0 | 4.5 ± 0.1 | 0 | 4.8 ± 0.1 | 0 | 4.5 ± 0.1 | 0 |
| 60 | 13.1 ± 0.1 | 0.3613 | 12.1 ± 0.1 | 0.3303 | 10.6 ± 0.1 | 0.2919 |
| 100 | 15.0 ± 0.1 | 0.4412 | 14.0 ± 0.1 | 0.4163 | 12.5 ± 0.1 | 0.3828 |
| 330 | 21.0 ± 0.1 | 0.6933 | 20.0 ± 0.1 | 0.6878 | 18.2 ± 0.2 | 0.6555 |
| 600 | 22.4 ± 0.2 | 0.7521 | 21.7 ± 0.1 | 0.7647 | 20.8 ± 0.1 | 0.7799 |
| 900 | 24.3 ± 0.1 | 0.8319 | 23.5 ± 0.2 | 0.8462 | 22.4 ± 0.1 | 0.8565 |
| 1200 | 25.3 ± 0.1 | 0.8740 | 24.9 ± 0.1 | 0.9095 | 23.7 ± 0.1 | 0.9187 |
| 1500 | 27.4 ± 0.1 | 0.9622 | 26.0 ± 0.2 | 0.9593 | 24.6 ± 0.2 | 0.9617 |
| 1800 | 28.3 ± 0.1 | 1 | 26.9 ± 0.2 | 1 | 25.4 ± 0.1 | 1 |
| Alloys | icorr (μA⋅cm-2) | Ecorr (mV/SCE) | Epit (mV/SCE) | ΔE (V/SCE) | bc (mV/dec) | ba (mV/dec) | Rp (kΩ⋅cm2) |
|---|---|---|---|---|---|---|---|
| Cu-3Ti | 2.887 ± 0.055 | -171.3 ± 4.2 | -121.2 ± 2.5 | 50.1 | 111.1 ± 3.3 | 10.1 ± 1.8 | 1.392 |
| Cu-Ti-Fe | 10.244 ± 0.036 | -185.1 ± 3.4 | -122.4 ± 2.9 | 62.7 | 170.2 ± 5.1 | 21.5 ± 2.7 | 0.809 |
| Cu-Ti-Fe-Cr | 2.689 ± 0.034 | -165.8 ± 3.7 | -115.2 ± 3.1 | 50.6 | 116.7 ± 3.9 | 62.0 ± 2.4 | 6.538 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Dong, Y.; Dong, X.; Wu, M.; Li, R. Effects of Trace Alloying Elements Fe and Cr on the Microstructure and Aging Properties of Cu-3Ti Alloy Foils. Metals 2018, 8, 881. https://doi.org/10.3390/met8110881
Zhao H, Dong Y, Dong X, Wu M, Li R. Effects of Trace Alloying Elements Fe and Cr on the Microstructure and Aging Properties of Cu-3Ti Alloy Foils. Metals. 2018; 8(11):881. https://doi.org/10.3390/met8110881
Chicago/Turabian StyleZhao, Hongliang, Yaguang Dong, Xianglei Dong, Mingwei Wu, and Rongping Li. 2018. "Effects of Trace Alloying Elements Fe and Cr on the Microstructure and Aging Properties of Cu-3Ti Alloy Foils" Metals 8, no. 11: 881. https://doi.org/10.3390/met8110881
APA StyleZhao, H., Dong, Y., Dong, X., Wu, M., & Li, R. (2018). Effects of Trace Alloying Elements Fe and Cr on the Microstructure and Aging Properties of Cu-3Ti Alloy Foils. Metals, 8(11), 881. https://doi.org/10.3390/met8110881




