Numerical Predictions of Uniform CO2 Corrosion in Complex Fluid Domains Using Low Reynolds Number Models
Abstract
:1. Introduction
2. Models
2.1. Transport Model
2.2. Chemical Reactions
2.3. Electrochemical Reactions
2.4. Transport Equations
2.5. Source Terms
2.6. Boundary Conditions
3. Numerical Simulations of Mass Transfer and Corrosion
3.1. Mass Transfer Validation
3.2. Corrosion Predictions
4. Conclusions
- (1)
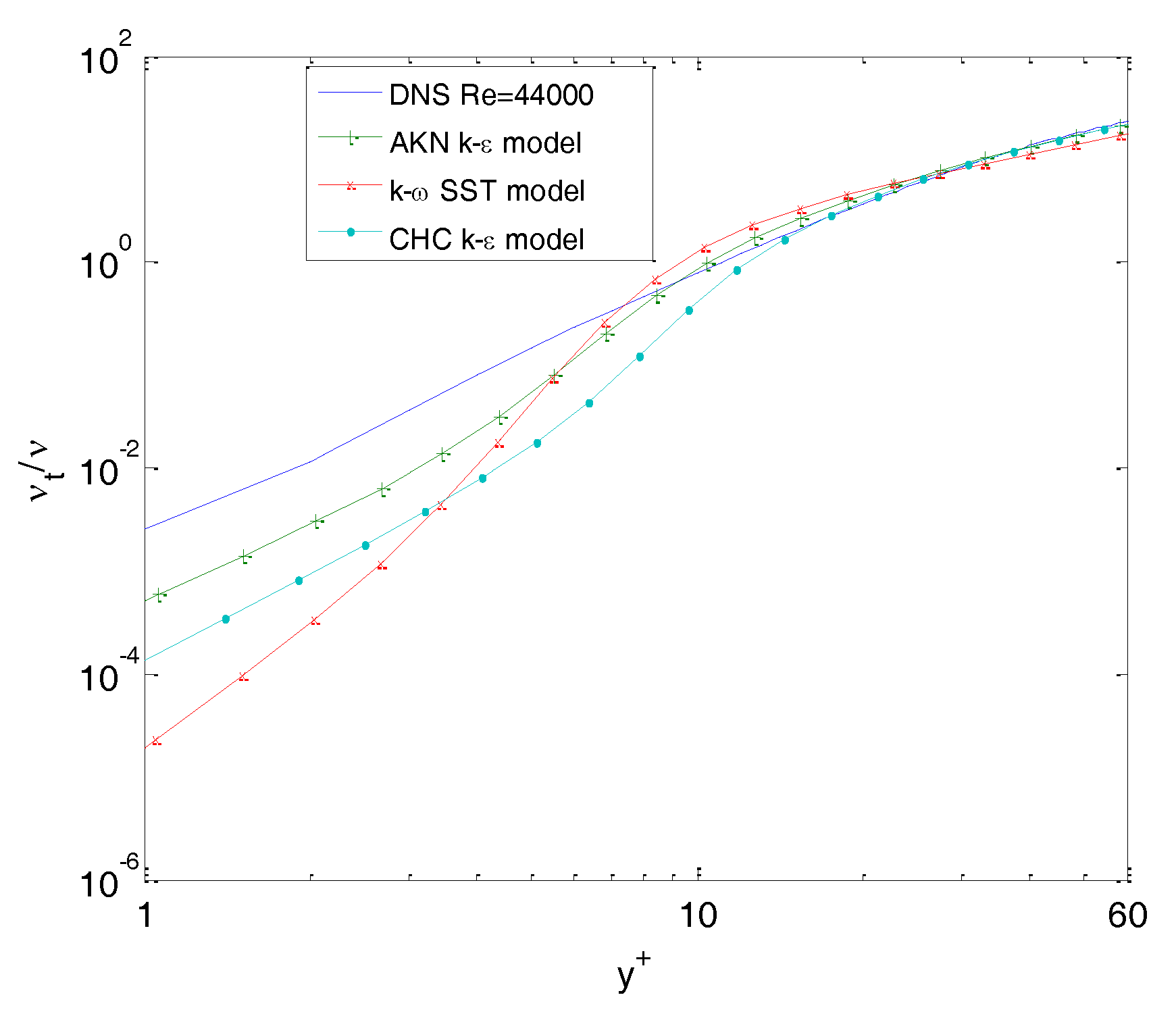
- Turbulent transport is appreciable and dominantly contributes to concentration verification in the viscous sublayer. All LRN models (AKN, CHC, SST) under predicted the turbulent viscosity in this sublayer, and thus also under predicted the local mass transfer coefficient. The AKN model gave the best estimation of turbulent viscosity in the viscous sublayer.
- (2)
- The AKN model accurately predicted the Stanton number with a relative error of 6.54% while the SST and CHC models’ estimate of the Stanton number with relative errors was even greater than 40% for straight pipe flow at Re = 44,000. Moreover, the Stanton number predicted by the AKN model was less than that derived from the experience model of Berger and Hau with a relative error of 4–10%, in the region of 10,000 < Re < 100,000.
- (3)
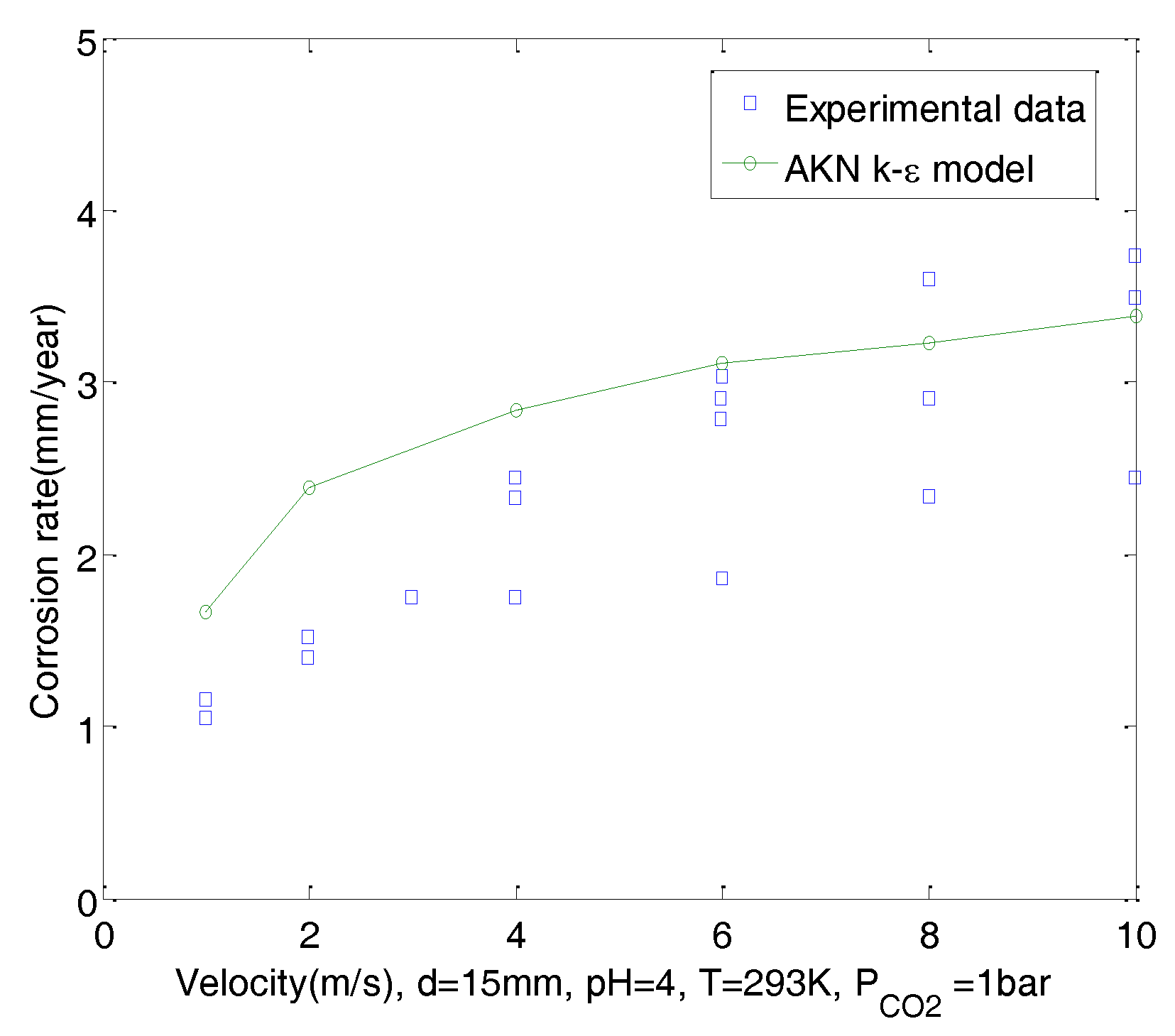
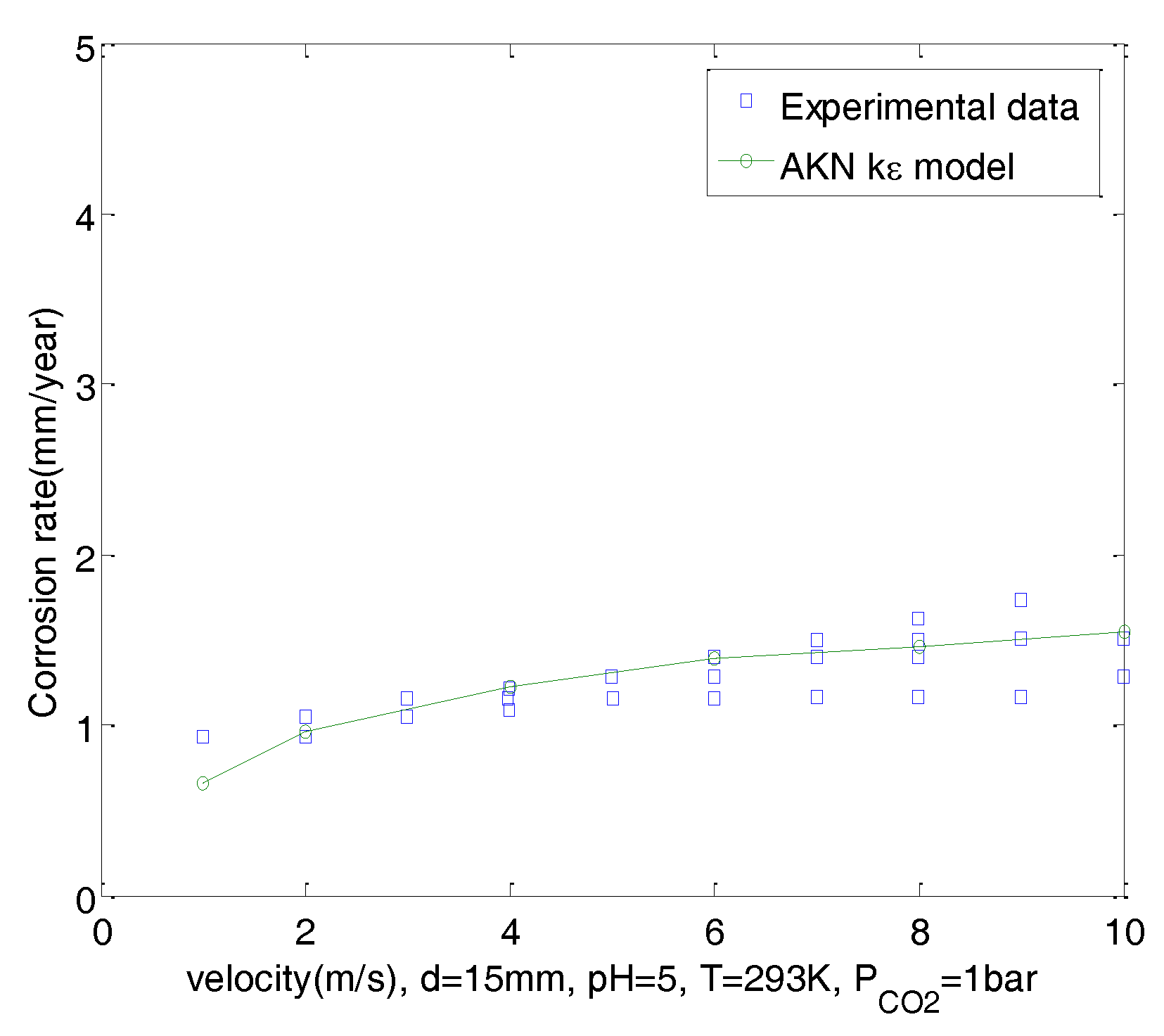
- Predictions of CO2 corrosion were made using UDFs and the AKN model for domains with 2D geometry under steady-state flow. Boundary conditions of corroded walls can be derived from the mixture potential, which was responsible for the charge balance of the corroded surface. Corrosion rates of CO2 solutions in a straight pipe with d = 15 mm under pH = 4, 5 and 6, PCO2 = 1 bar were predicted. Results show that predictions have good agreement with experimental data and correctly reflect the effect of velocity on the corrosion. Corrosion rates were not only more sensitive to change in flow at low pH than at high pH, but were also more sensitive to change in flow at low velocity than at high velocity.
- (4)
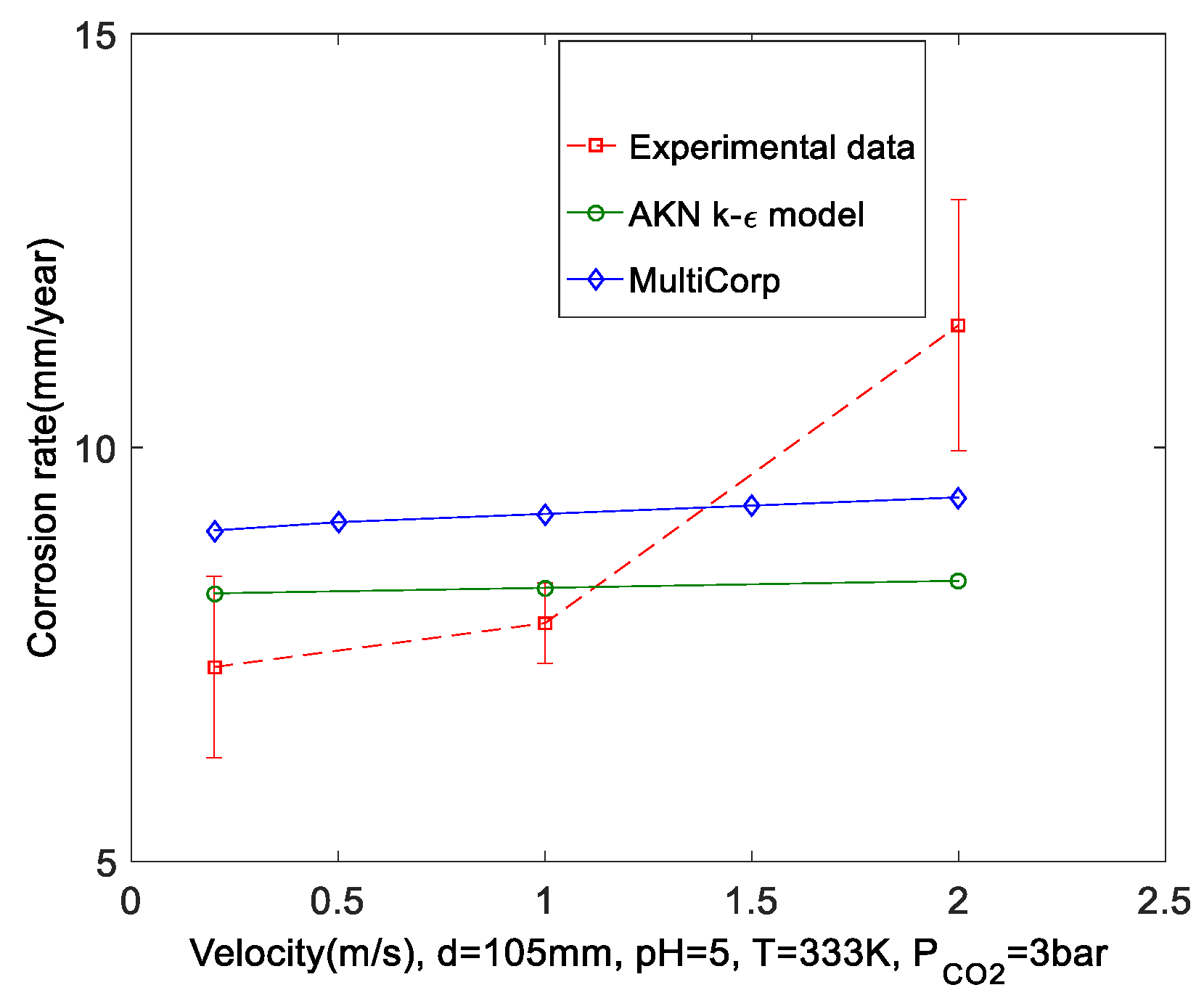
- The corrosion rates of CO2 solutions in a straight pipe with d = 25.4 mm under pH = 5, PCO2 = 1 bar, Um = 4 m/s, and TK = 20–80 °C were predicted using the AKN model and compared with those derived using the Multicorp V5.2.105. Both codes correctly show that corrosion rates increase as temperature rises. However, the UDFs gave lower predictions than the Multicorp. Predictions of CO2 corrosion in a straight pipe with d = 105 mm under pH = 5, PCO2 = 3 bar, TK = 333 K, Um = 0.2–2 m/s were made and compared with experimental data and Multicorp data. Results show that the predictions are reasonable and close to Multicorp data. The predictions correctly show that corrosion rates do not vary greatly with change of velocity since the corrosion process was mainly controlled by the CO2 hydration reaction, which is insensitive to velocity under the tested conditions.
- (5)
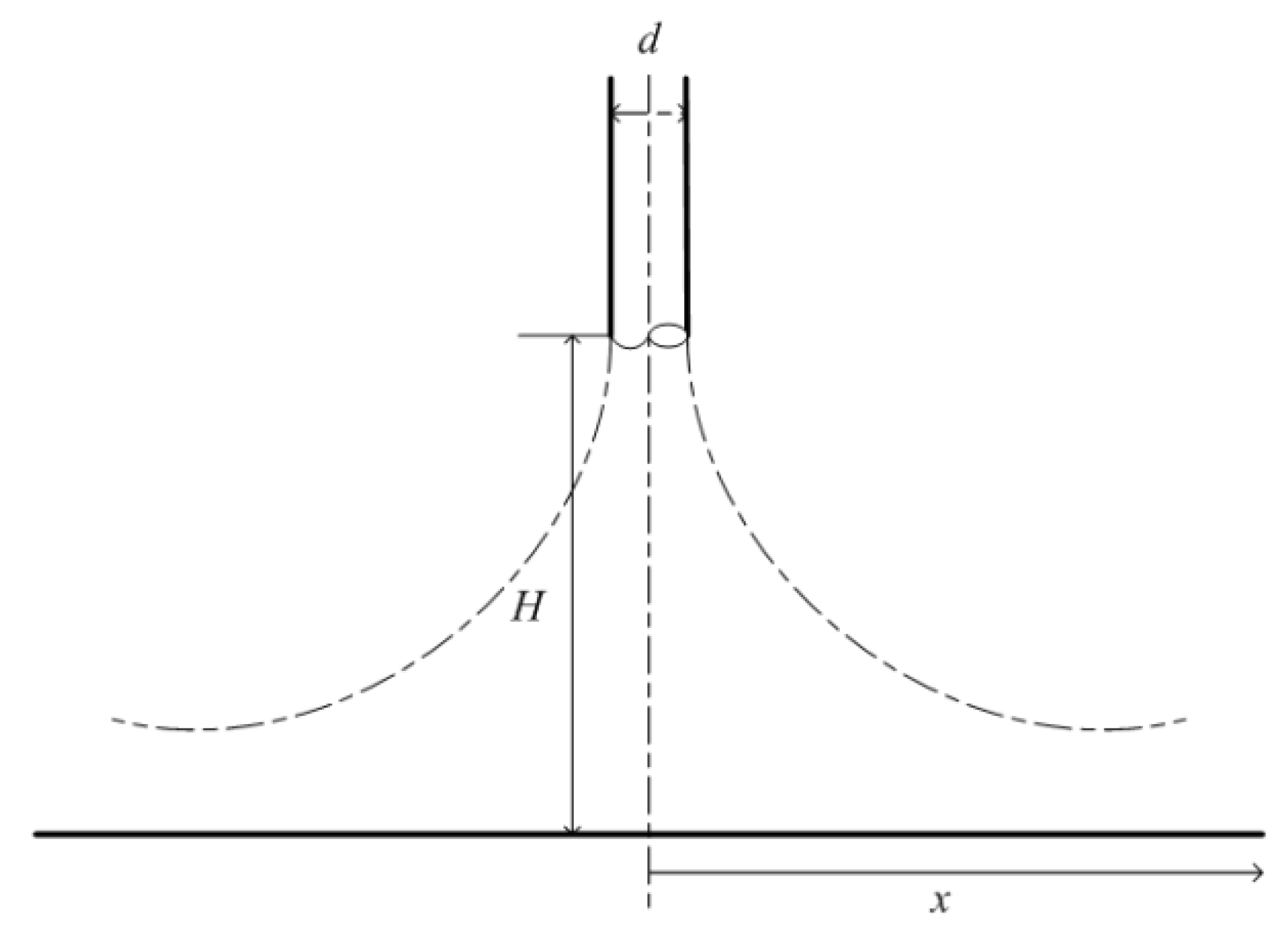
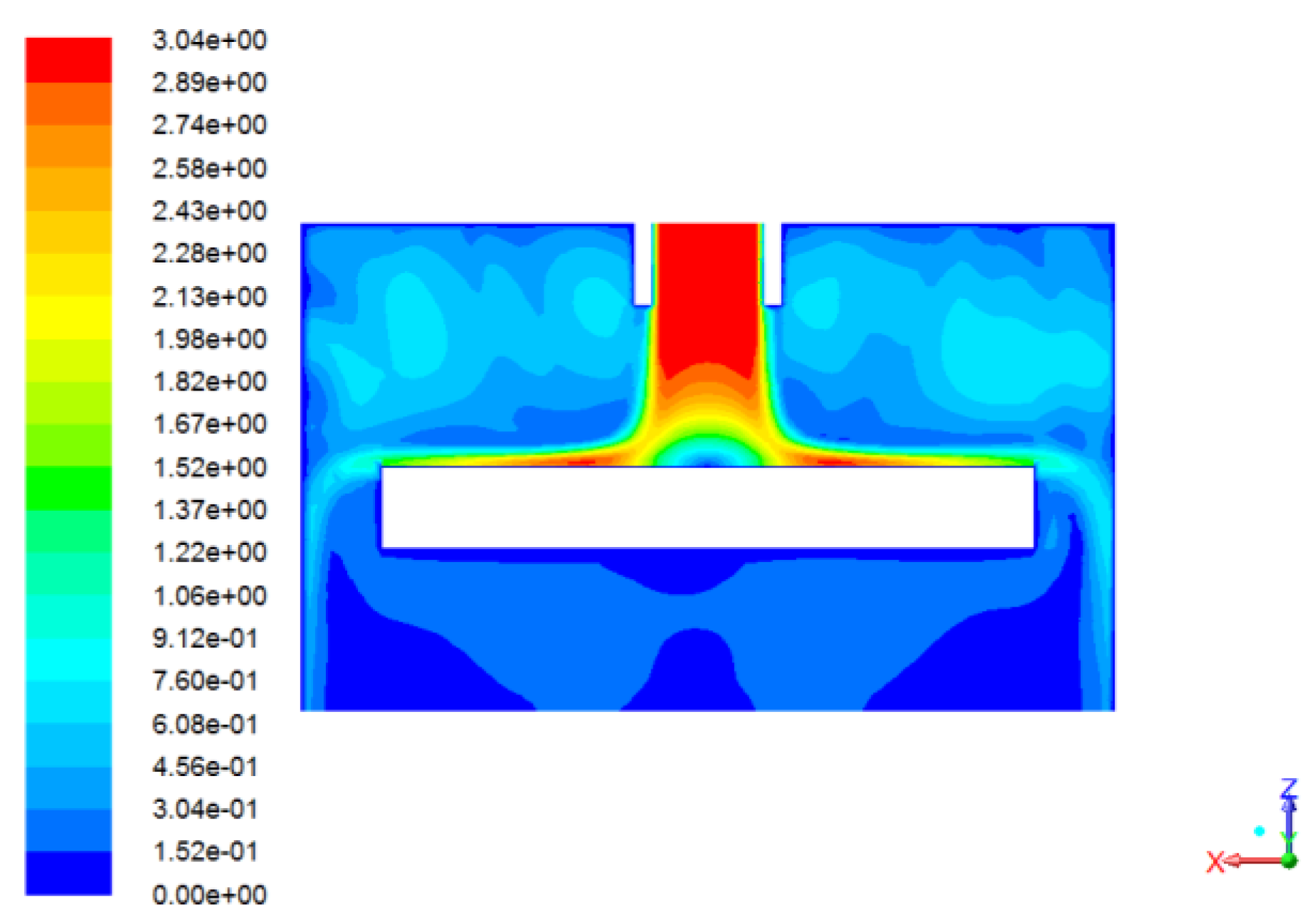
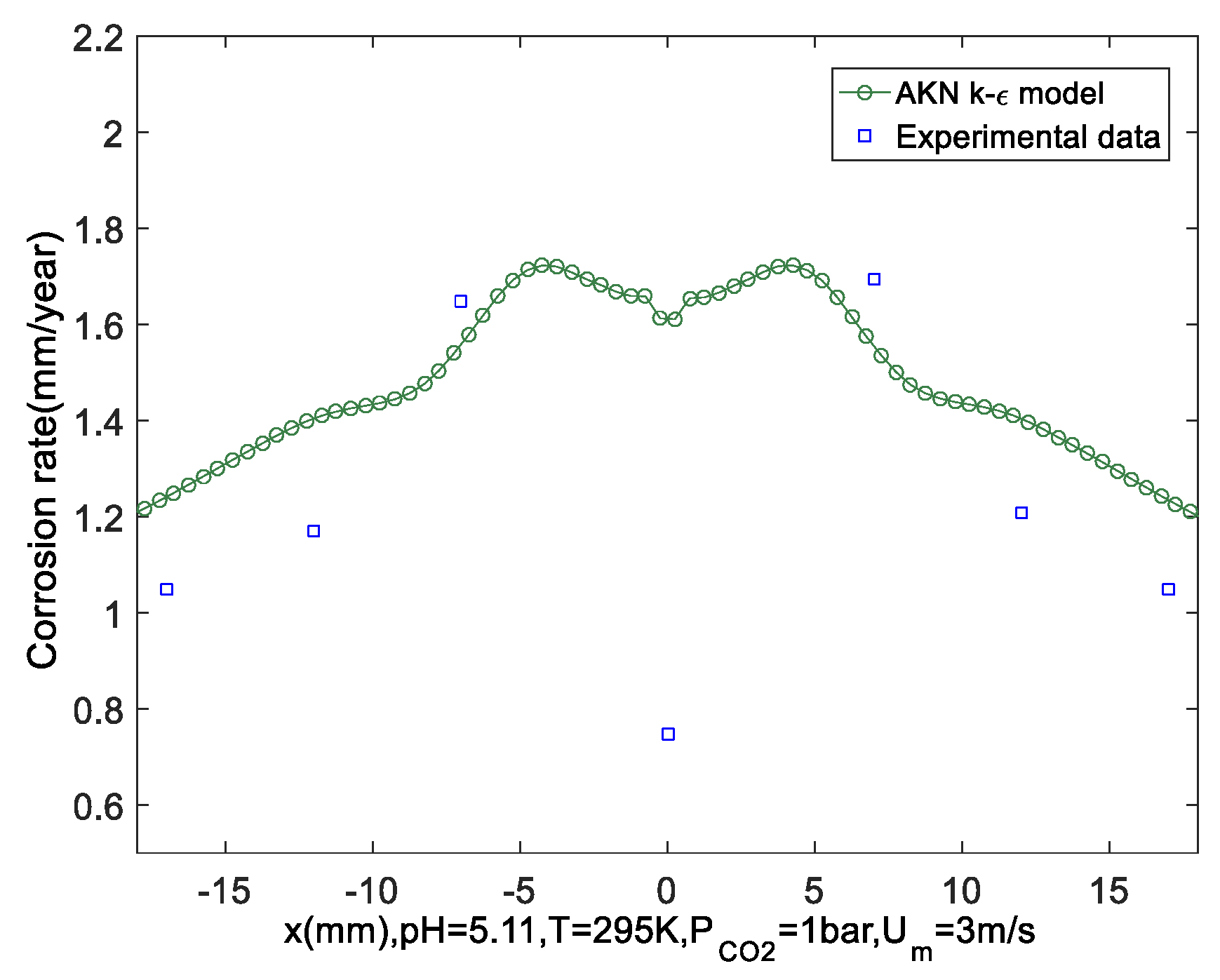
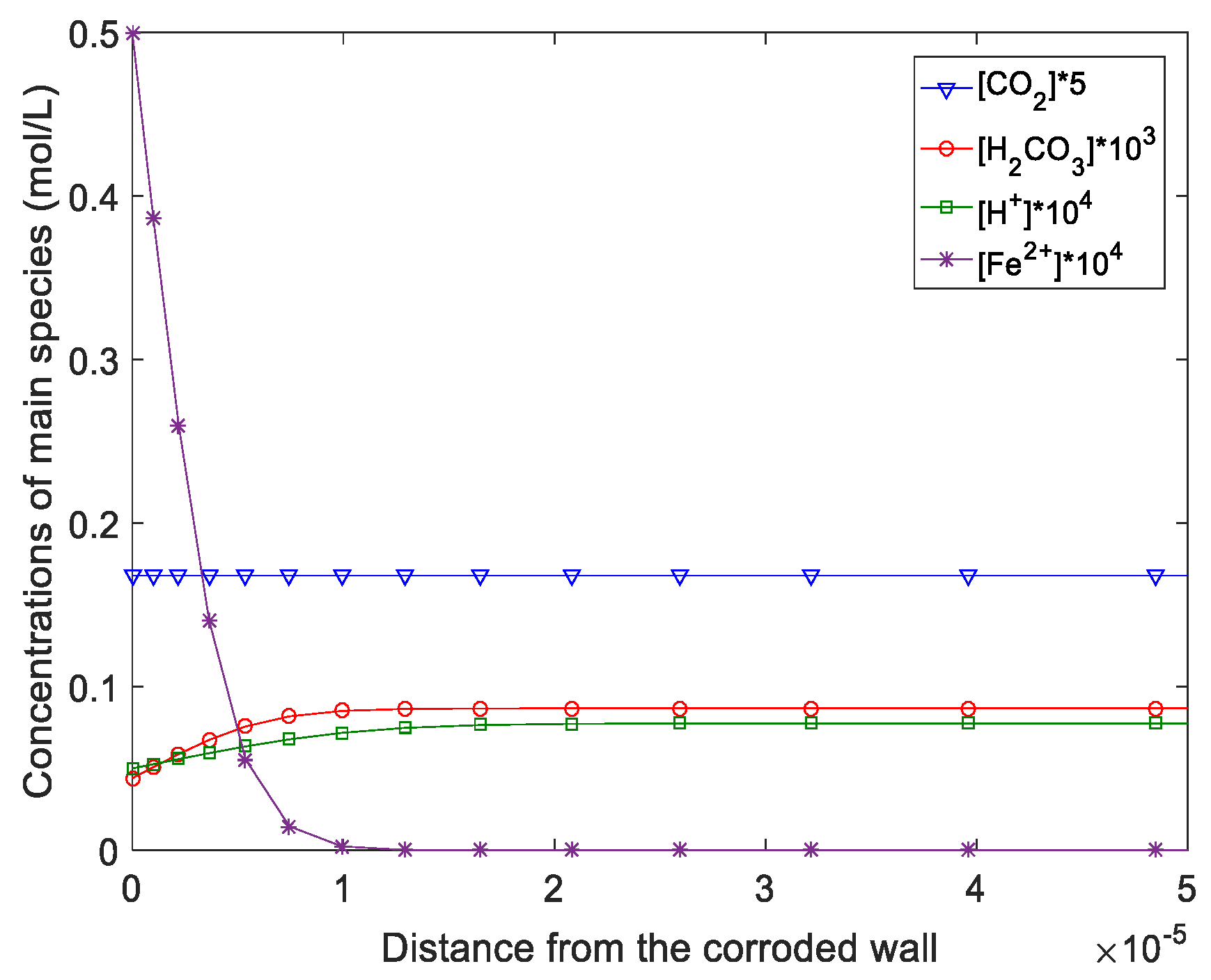
- The flow and corrosion in a jet impinging were predicted by the AKN model. The results show that numerical predictions of the corrosion rate have good agreement with experimental data. The position with the most serious local thinning was around 0.6*d from the wall center in the jet impinging system. The concentration profiles of ions in solutions denote that H2CO3 plays a greater role in corrosion.
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
Nomenclature
| area of face cell (m2) | |
| Tafel equation constants | |
| SST model constant | |
| SST model constants | |
| Tafel slope (V) | |
| concentrations (mol·m−3) | |
| model constants | |
| positive portion of the cross-diffusion term | |
| model additional term (m2·s−3) | |
| diameter of pipe (mm) or nozzle (inch, mm) | |
| SST model cross-diffusion term (kg·m−3·s−2) | |
| model additional term (m2·s−3) | |
| friction factor, | |
| Faraday constant (96485 s·A·mol−1) | |
| blending functions | |
| , | damping functions |
| production of (kg·m−3·s−2) | |
| production of (kg·m−1·s−3) | |
| nozzle height (inch, m) | |
| active energy (kJ·mol−1) | |
| current density (A·m−2) | |
| exchange current destiny (A·m−2) | |
| turbulent kinetic energy (m2·s−2) | |
| equilibrium constant | |
| , | forward and backward reactions rate coefficients |
| mass transfer coefficient (m·s−1) | |
| number of moles of electrons exchanged per mole of the -thspecies | |
| the -thspecies flux (mol·m−2·s−1) | |
| Reynolds averaged pressure (Pa) | |
| partial pressure (bar) | |
| production of (kg·m−1·s−3) | |
| universal gas constant (8.314 J·mol−1·K−1) | |
| source term of the -thspecies (mol·s−1·m−2) | |
| turbulent Reynolds number, , , | |
| invariant measure of the strain rate, (s−1) | |
| mean rate of deformation component, (s−1) | |
| laminar and turbulent Schmidt numbers | |
| Stanton number | |
| Kelvin temperature (K) | |
| Celsius temperature (°C) | |
| mean velocity (m·s−1) | |
| corrosion rate (m·s−1) | |
| axial velocity (m·s−1) | |
| dimensionless axial velocity, | |
| friction velocity (m·s−1) | |
| turbulent species flux (kg·mol·s−1·m−5) | |
| Reynolds stress (kg·m−1·s−2) | |
| electric potential (V vs. SHE) | |
| , | Cartesian coordinate (m) |
| normal distance from the inner wall (m), Cartesian coordinate (m) | |
| normal distance from the center of the first cell to the wall (m) | |
| y+ | normalized wall distance |
| dissipation of turbulence kinetic energy (kg·m−1·s−3) | |
| dissipation of (kg·m−3·s−2) | |
| electrical charge of species, Cartesian coordinate (m) |
Greek symbols
| , | SST model constants |
| , | laminar and turbulent diffusion coefficient (m2·s−1) |
| Kronecker delta | |
| dissipation rate of (m2·s−3) | |
| permittivity of the medium (F·m−1) | |
| ionic mobility (m2·V−1·s−1) | |
| θ | Impact angel (°) |
| , | laminar and turbulent dynamic viscosity (kg·m−1·s−1) |
| , | laminar and turbulent kinematic viscosity (m2·s−1) |
| density (kg·m−3) | |
| specific dissipation rate of turbulent kinetic energy (s−1) | |
| blend functions |
Subscripts
| ‘ | fluctuations |
| anode | |
| b | bulk fluid |
| bicarbonate dissociation | |
| cathode | |
| carbonic dissociation | |
| fc | first cell near the wall |
| hydration | |
| tensor components | |
| index of species | |
| mixture value | |
| ref | reference value |
| reversible value | |
| w | wall |
| water dissociation | |
| CO2, H2CO3, HCO3−, CO32−, H+, OH−, Fe2+, H2O | species |
Abbreviation
| AKN | Abe-Kondoh–Nagano |
| CHC | Change-Hsieh-Chen |
| CFD | Computational fluid dynamics |
| DNS | Direct Numerical Simulations |
| LB | Lam-Bremhorst |
| LPR | linear Polarization Resistance Probes |
| LRN | low Reynolds number |
| SST | shear stress transport |
| TCFC | thin-channel flow cell |
| UDFs | user defined functions |
References
- De Waard, C.; Lotz, U. Prediction of CO2 Corrosion of Carbon Steel; Corrosion/93; NACE International: Houston, TX, USA, 1993; p. 69. [Google Scholar]
- De Waard, C.; Lotz, U.; Dugstad, A. Influence of Liquid Flow Velocity on CO2 Corrosion: A Semi-Empirical Model; Corrosion/95; NACE International: Houston, TX, USA, 1995; p. 128. [Google Scholar]
- Jepson, W.P.; Stitzel, S.; Kang, C.; Gopal, M. Model for Sweet Corrosion in Horizontal Multiphase Slug Flow; Corrosion/97; NACE International: Houston, TX, USA, 1997; p. 11. [Google Scholar]
- De Waard, C.; Smith, L.; Bartlett, P.; Cunningham, H. Modelling Corrosion Rates in oil Production Tubing; Eurocorr: Riva del Garda, Italy, 2001; p. 254. [Google Scholar]
- De Waard, C.; Smith, L.; Craig, B.D. The Influence of Crude Oil on Well Tubing Corrosion Rates; Corrosion/03; NACE International: Houston, TX, USA, 2003; p. 3629. [Google Scholar]
- Nešić, S.; Postlethwaite, J.; Olsen, S. An electrochemical model for prediction of corrosion of mild steel in aqueous carbon dioxide solutions. Corrosion 1996, 52, 280. [Google Scholar] [CrossRef]
- Anderko, A.; McKenzie, P.; Young, R.D. Computation of rates of general corrosion using electrochemical and thermodynamic models. Corrosion 2001, 57, 202. [Google Scholar] [CrossRef]
- George, K.; Wang, S.; Nešić, S.; de Waard, C. Modeling of CO2 Corrosion of Mild Steel at High Pressures of CO2 and in Presence of Acetic Acid; Corrosion/04; NACE International: Houston, TX, USA, 2004; p. 4623. [Google Scholar]
- Sun, W.; Nešić, S. A mechanistic model of uniform hydrogen sulfide/carbon dioxide corrosion of mild steel. Corrosion 2009, 65, 291. [Google Scholar] [CrossRef]
- Zheng, Y.; Ning, J.; Brown, B.; Nešić, S. Electrochemical model of mild steel corrosion in a mixed H2S/CO2 aqueous environment in the absence of protective corrosion product layers. Corros. Sci. 2014, 71, 316. [Google Scholar] [CrossRef]
- Zheng, Y.; Ning, J.; Brown, B.; Nešić, S. Advancement in Predictive Modeling of Mild Steel Corrosion in CO2 and H2S Containing Environments; Corrosion/15; NACE International: Houston, TX, USA, 2005; p. 6146. [Google Scholar]
- Nešić, S.; Li, H.; Huang, J.; Sormaz, D. An open source mechanistic model for CO2/H2S corrosion of carbon steel. In Proceedings of the NACE International Corrosion Conference & Expo, Atlanta, GA, USA, 22–26 March 2009. [Google Scholar]
- Nordsveen, M.; Nešić, S.; Nyborg, R.; Stangeland, A. A mechanistic model for carbon dioxide corrosion of mild steel in the presence of protective iron carbonate films-Part 1: Theory and verification. Corrosion 2003, 59, 443. [Google Scholar] [CrossRef]
- Song, F.M. A comprehensive model for predicting CO2 corrosion rate in oil and gas production and transportation systems. Electrochimi. Acta 2010, 55, 689. [Google Scholar] [CrossRef]
- Berger, F.P.; Hau, K.F.F.L. Mass transfer in turbulent pipe flow measured by the electrochemical method. Int. J. Heat Mass Transf. 1977, 20, 1185. [Google Scholar] [CrossRef]
- Nešić, S. Key issues related to modelling of internal corrosion of oil and gas pipelines–A review. Corros. Sci. 2007, 49, 4308. [Google Scholar] [CrossRef]
- Kahyarian, A.; Singer, M.; Nesic, S. Modeling of uniform CO2 corrosion of mild steel in gas transportation systems: A review. J. Nat. Gas Sci. Eng. 2016, 29, 530. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Cheng, G.; Li, Y.; Xia, Y.; Wu, W. Numerical Predictions of Mild-Steel Corrosion in a H2S Aqueous Environment Without Protective Product Layers. Corrosion 2017, 73, 786. [Google Scholar] [CrossRef]
- Nešić, S.; Adamopoulos, G.; Postlethwaite, J.; Bergstrom, D.J. Modelling of turbulent flow and mass transfer with wall function and low Reynolds number closures. Can. J. Chem. Eng. 1993, 71, 28. [Google Scholar] [CrossRef]
- Nešić, S.; Postlethwaite, J.; Bergstrom, D.J. Calculation of wall-mass transfer rates in separated aqueous flow using a low Reynolds number κ-ε model. Int. J. Heat Mass Transfer. 1992, 35, 1977. [Google Scholar] [CrossRef]
- Xiong, J.; Koshizuka, S.; Sakai, M. Turbulence modeling for mass transfer enhancement by separation and reattachment with two-equation eddy-viscosity models. Nucl. Eng. Des. 2011, 241, 3190. [Google Scholar] [CrossRef]
- Wang, Y.; Postlethwaite, J. The application of low Reynolds number k-ε turbulence model to corrosion modelling in the mass transfer entrance region. Corros. Sci. 1997, 39, 1265. [Google Scholar] [CrossRef]
- Hsieh, W.D.; Chang, K.C. Calculation of wall heat transfer in pipe expansion turbulent flows. Int. J. Heat Mass Transf. 1996, 39, 3813. [Google Scholar] [CrossRef]
- Wang, S.J.; Mujumdar, A.S. A comparative study of five low Reynolds number k-ε models for impingement heat transfer. Appl. Therm. Eng. 2005, 25, 31. [Google Scholar] [CrossRef]
- Gorji, S.; Seddighi, M.; Ariyaratne, C.; Vardy, A.E. A comparative study of turbulence models in a transient channel flow. Comput. Fluids 2014, 89, 111. [Google Scholar] [CrossRef] [Green Version]
- Ansys. Fluent Theory Guide; Ansys: Canonsburg, PA, USA, 2015. [Google Scholar]
- Kharaka, Y.K.; Gunter, W.D.; Aggarwal, P.K.; DeBraal, J.D. SOLMINEQ 88: A Computer Program for Geochemical Modeling of Water-Rock Interactions; US Geological Survey: Menlo Park, CA, USA, 1989.
- Duan, Z.; Li, D. Coupled phase and aqueous species equilibrium of the H2O–CO2–NaCl–CaCO3 system from 0 to 250 °C, 1 to 1000 bar with NaCl concentrations up to saturation of halite. Geochim. Cosmochim. Acta 2008, 72, 5128. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Drazic, D.; Despic, A.R. The electrode kinetics of the deposition and dissolution of iron. Electrochim. Ascta 1961, 4, 325. [Google Scholar] [CrossRef]
- Wu, X.; Moin, P. A direct numerical simulation study on the mean velocity characteristics in turbulent pipe flow. J. Fluid Mech. 2008, 608, 81. [Google Scholar] [CrossRef]
- Rao, V.V.; Trass, O. Mass transfer from a flat surface to an impinging turbulent jet. Can. J. Chem. Eng. 1964, 42, 95. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Electrochemical characterization and computational fluid dynamics simulation of flow-accelerated corrosion of X65 steel in a CO2-saturated oilfield formation water. Corros. Sci. 2010, 52, 2716. [Google Scholar] [CrossRef]
- Nešić, S.; Solvi, G.T.; Enerhaug, J. Comparison of the rotating cylinder and pipe flow tests for flow-sensitive carbon dioxide corrosion. Corrosion 1995, 51, 773. [Google Scholar] [CrossRef]






| Model | |||||||
|---|---|---|---|---|---|---|---|
| AKN | 0.09 | 1/1.4 | 1/1.4 | 1.50 | 1.90 | 0 | |
| CHC | 0.09 | 1.0 | 1/1.3 | 1.44 | 1.92 | 0 |
| Reactions | Constant | Source |
|---|---|---|
| CO2 + H2O <----> H2CO3 | [13] | |
| s−1 | [27] | |
| H2CO3 <----> HCO3− + H+ | M | [28] |
| s−1 | [13] | |
| HCO3− <----> CO32− + H+ | M | [28] |
| s−1 | [13] | |
| H2O <----> HO− + H+ | M2 | [13] |
| M−1s−1 | [13] |
| Electrochemical Reaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 10−4 | 0.5 | N/A | 0 | 30 | 298.16 | pH | ||
| 0.06 | 10−5 | –0.5 | 10−4 | 1 | 57.5 | 298.16 | pH | ||
| 1 | 10−4 for and , or and | 0.5 | N/A | 0 | 50 | 298.16 | −0.488 | ||
| N/A for and | 0 | N/A | 0 | 50 | 298.16 | −0.488 | |||
| 1 | N/A for and | 0 | N/A | 0 | 50 | 298.16 | −0.488 |
| Species | Source Term |
|---|---|
| CO2 | |
| H2CO3 | |
| HCO3− | |
| CO32- | |
| H+ | |
| OH− |
| Models | Friction Factor | Stanton Number | ||
|---|---|---|---|---|
| Relative Error (%) | Relative Error (%) | |||
| Baseline | 0.0216 (Wu and Moin [30]) | --- | 2.887 (Berger and Hau [15]) | --- |
| AKN | 0.0285 | 5.81 | 2.70 | −6.54 |
| SST | 0.0264 | 4.81 | 1.52 | −47.4 |
| CHC | 0.0198 | −8.26 | 1.68 | −41.7 |
| Parameters | Value |
|---|---|
| Reference water viscosity () | 1.002 × 10−3 (kg·m−1s−1) |
| Reference diffusion coefficient of CO2 () | 1.96 × 10−9 (m2s−1) |
| Reference diffusion coefficient of H2CO3 () | 2.0 × 10−9 (m2s−1) |
| Reference diffusion coefficient of HCO3− () | 1.105 × 10−9 (m2s−1) |
| Reference diffusion coefficient of CO32− () | 0.92 × 10−9 (m2s−1) |
| Reference diffusion coefficient of OH− () | 5.26 × 10−9 (m2s−1) |
| Reference diffusion coefficient of H+ () | 9.312 × 10−9 (m2s−1) |
| Reference diffusion coefficient of Fe2+ () | 0.72 × 10−9 (m2s−1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Xu, H.; Huang, C.; Chen, X.; Li, X.; Li, Y. Numerical Predictions of Uniform CO2 Corrosion in Complex Fluid Domains Using Low Reynolds Number Models. Metals 2018, 8, 1001. https://doi.org/10.3390/met8121001
Hu H, Xu H, Huang C, Chen X, Li X, Li Y. Numerical Predictions of Uniform CO2 Corrosion in Complex Fluid Domains Using Low Reynolds Number Models. Metals. 2018; 8(12):1001. https://doi.org/10.3390/met8121001
Chicago/Turabian StyleHu, Haijun, Hao Xu, Changmeng Huang, Xing Chen, Xiufeng Li, and Yun Li. 2018. "Numerical Predictions of Uniform CO2 Corrosion in Complex Fluid Domains Using Low Reynolds Number Models" Metals 8, no. 12: 1001. https://doi.org/10.3390/met8121001





