Effect of Co2O3 on Oxidation Induration and Reduction Swelling of Chromium-Bearing Vanadium Titanomagnetite Pellets with Simulated Coke Oven Gas
Abstract
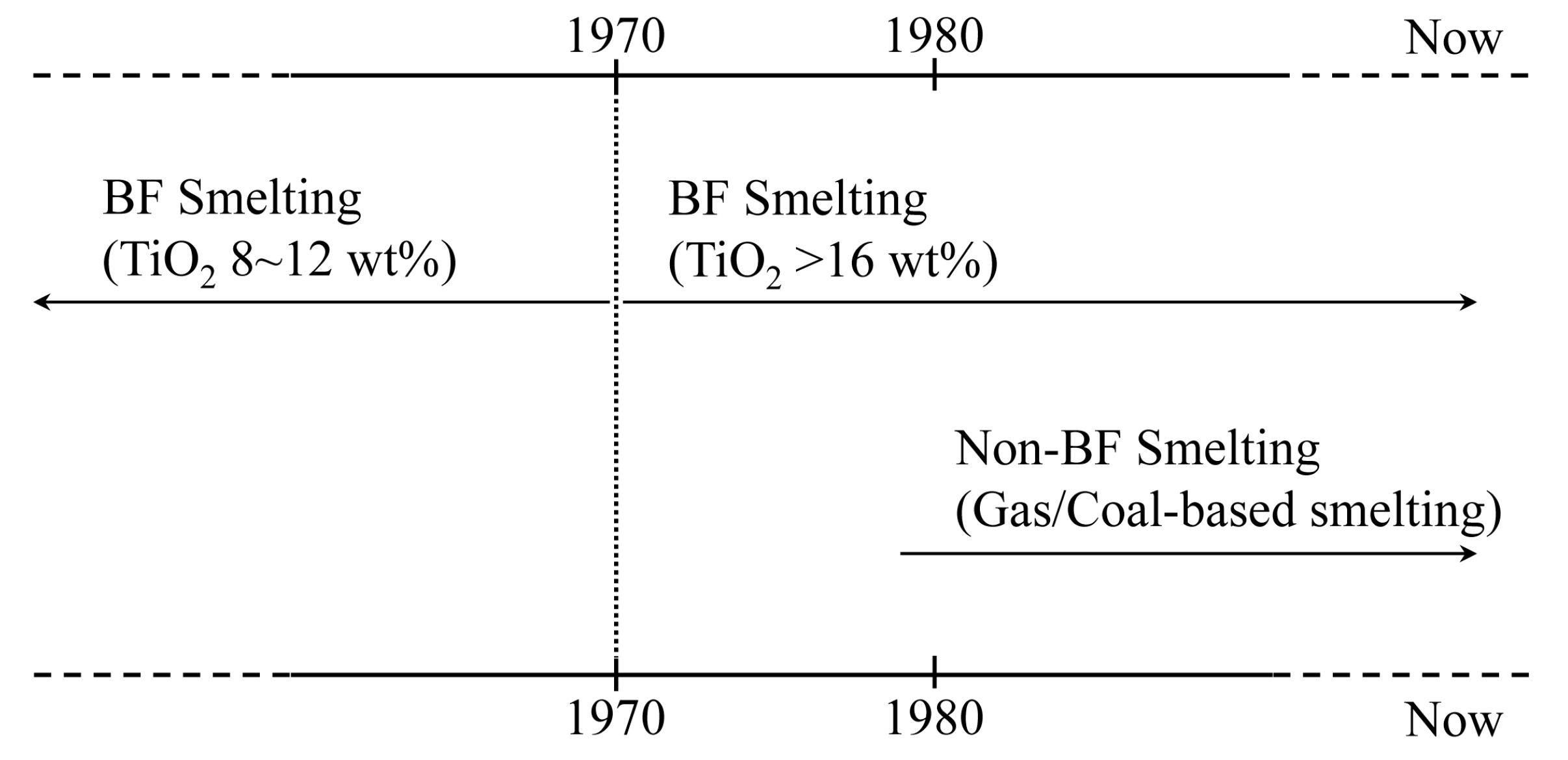
:1. Introduction
2. Materials and Methods
2.1. Materials
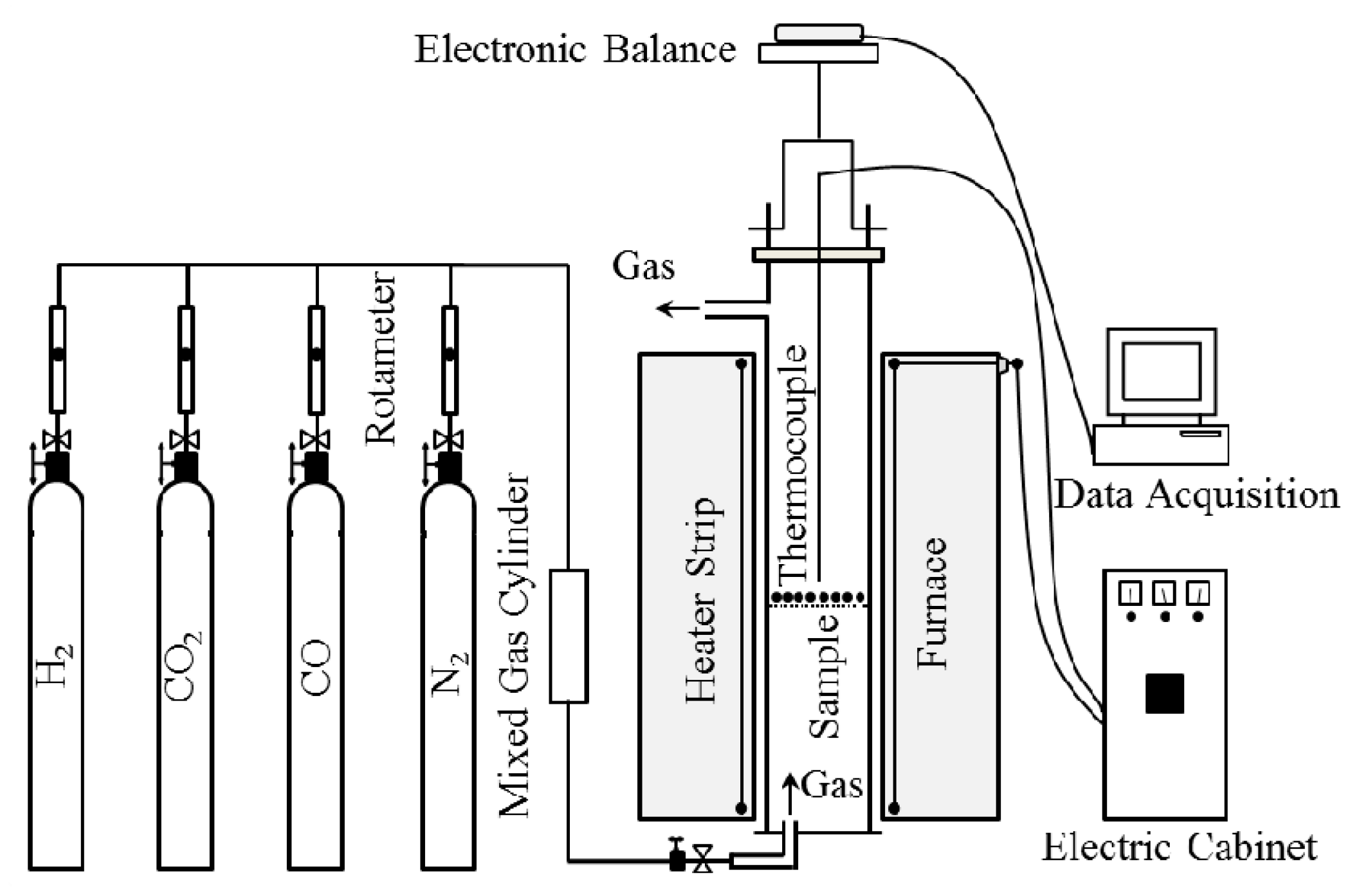
2.2. Apparatus and Procedure
2.3. Definition of Parameters
2.4. Analytical Methods
3. Results and Discussion
3.1. Oxidation Induration of CVTP
3.1.1. Phase Composition
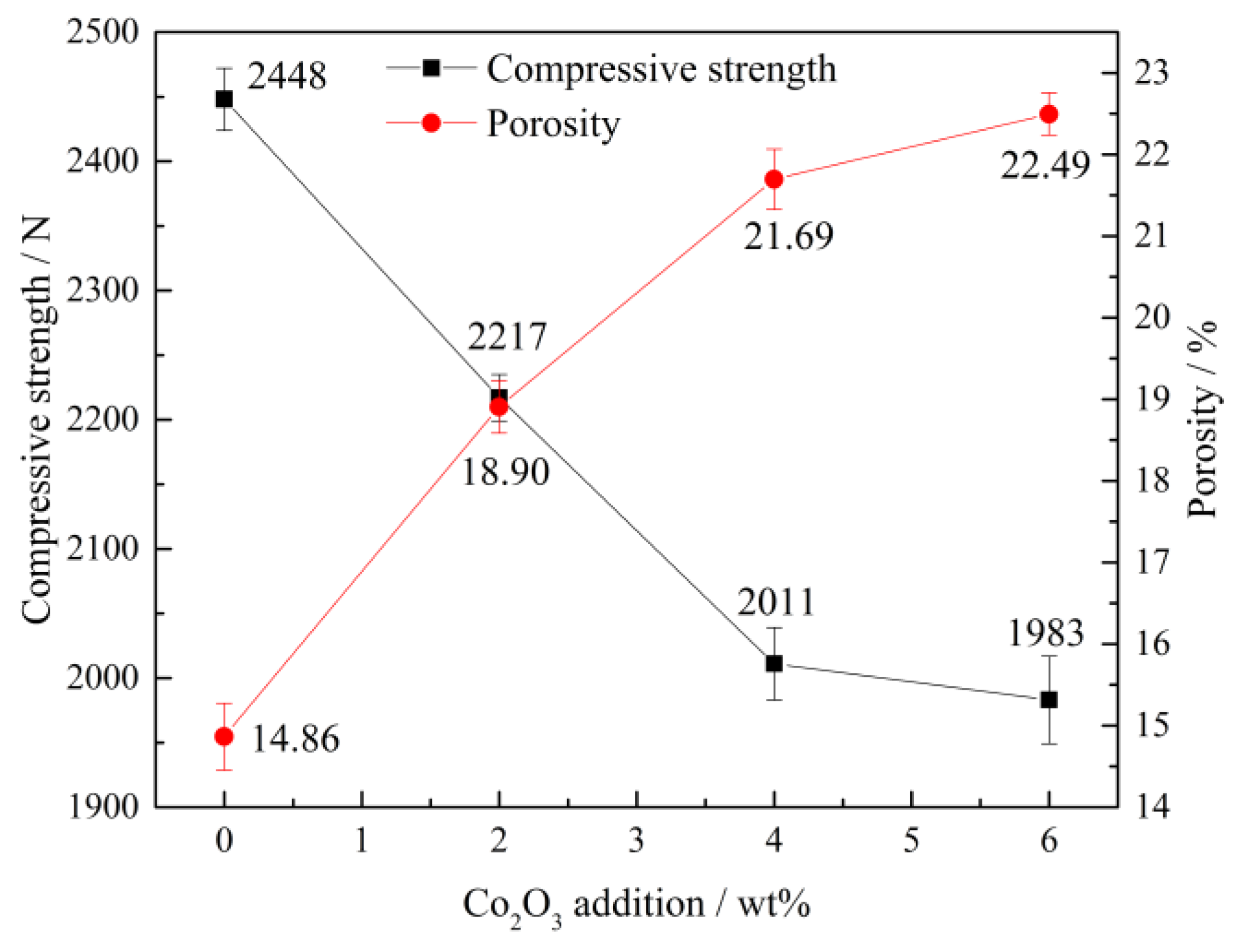
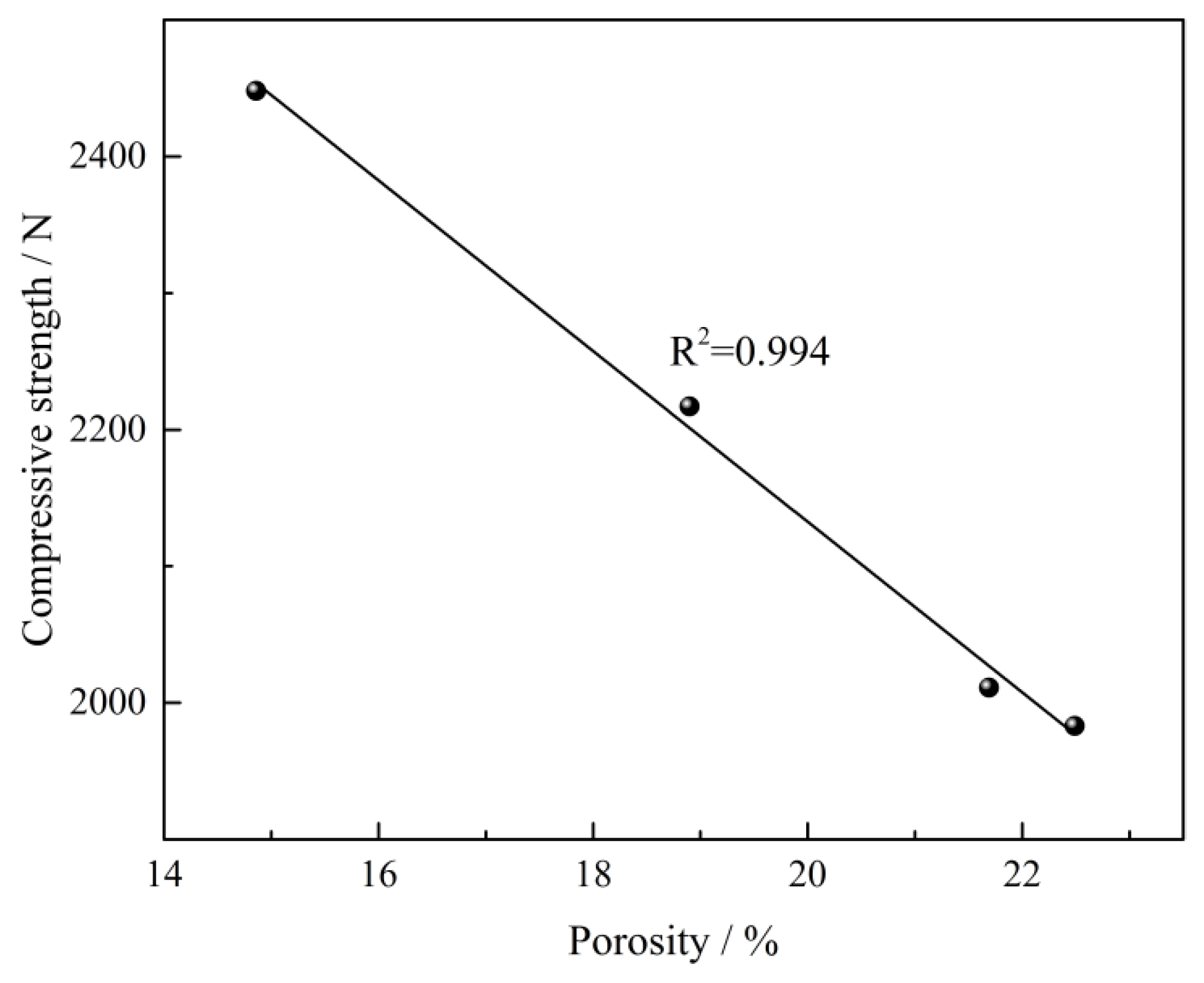
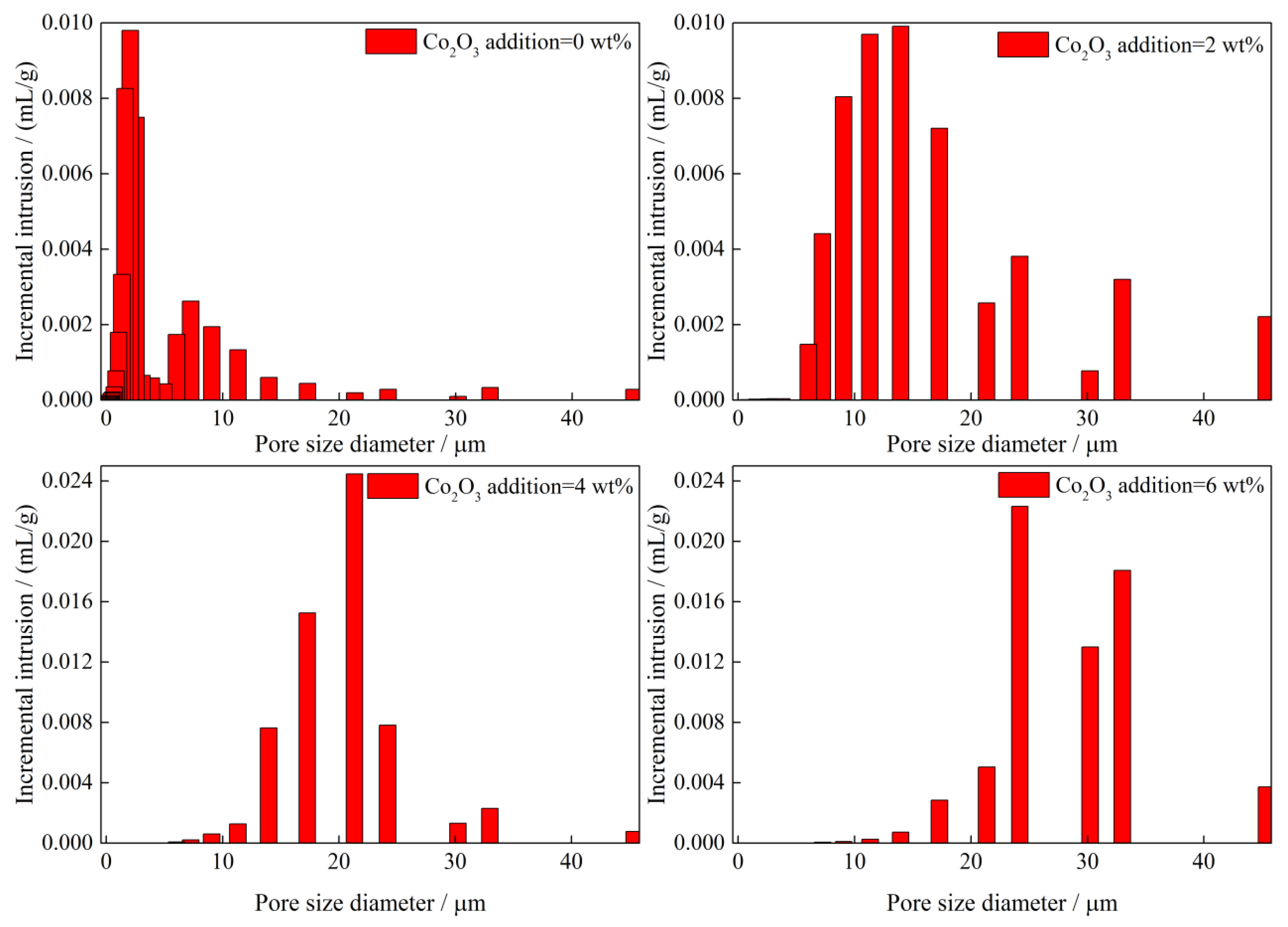
3.1.2. Compressive Strength and Porosity
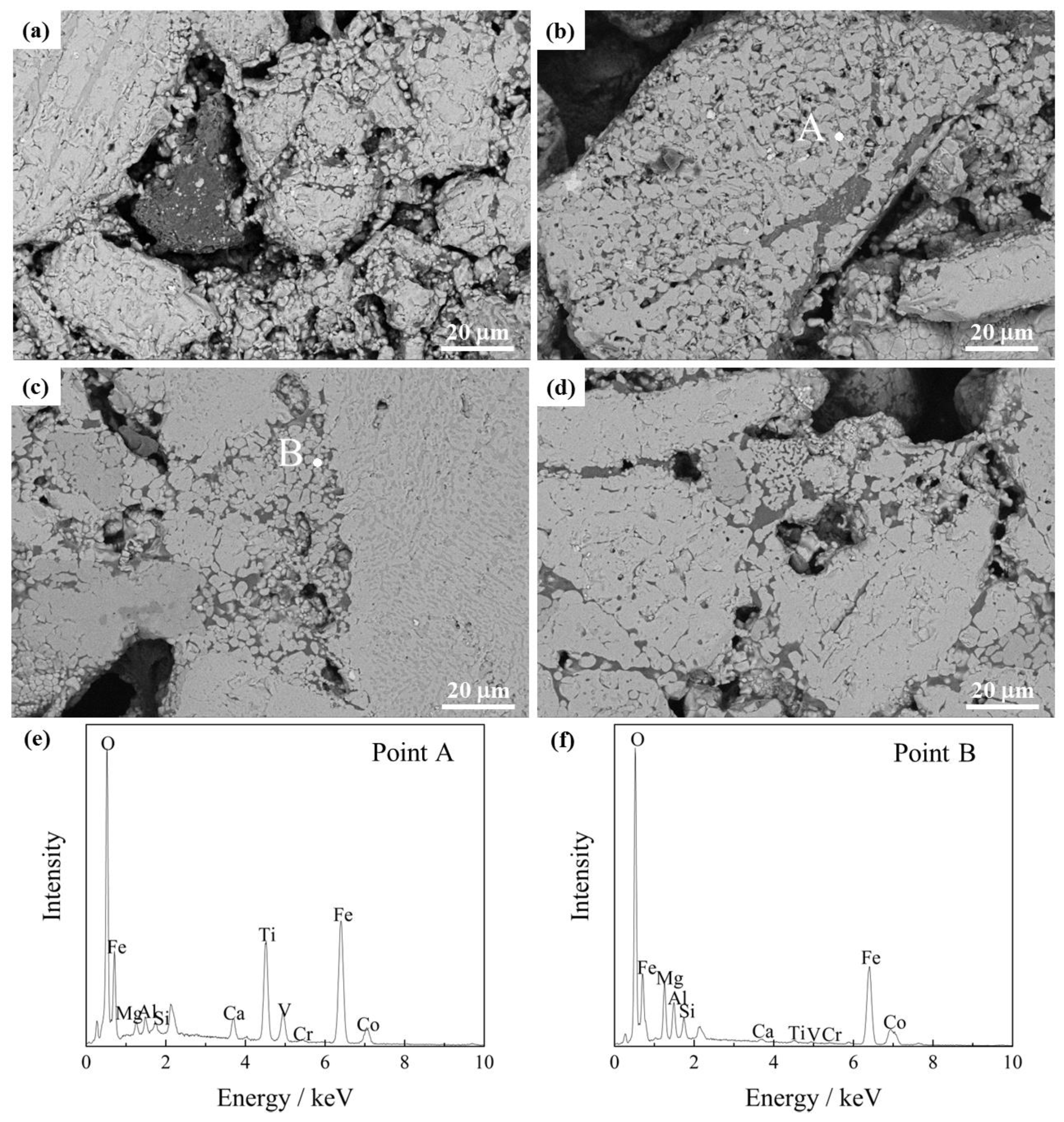
3.1.3. Microscopic Structure
3.2. Induration Mechanism
3.3. Swelling Behavior of CVTP
3.3.1. Phase Composition
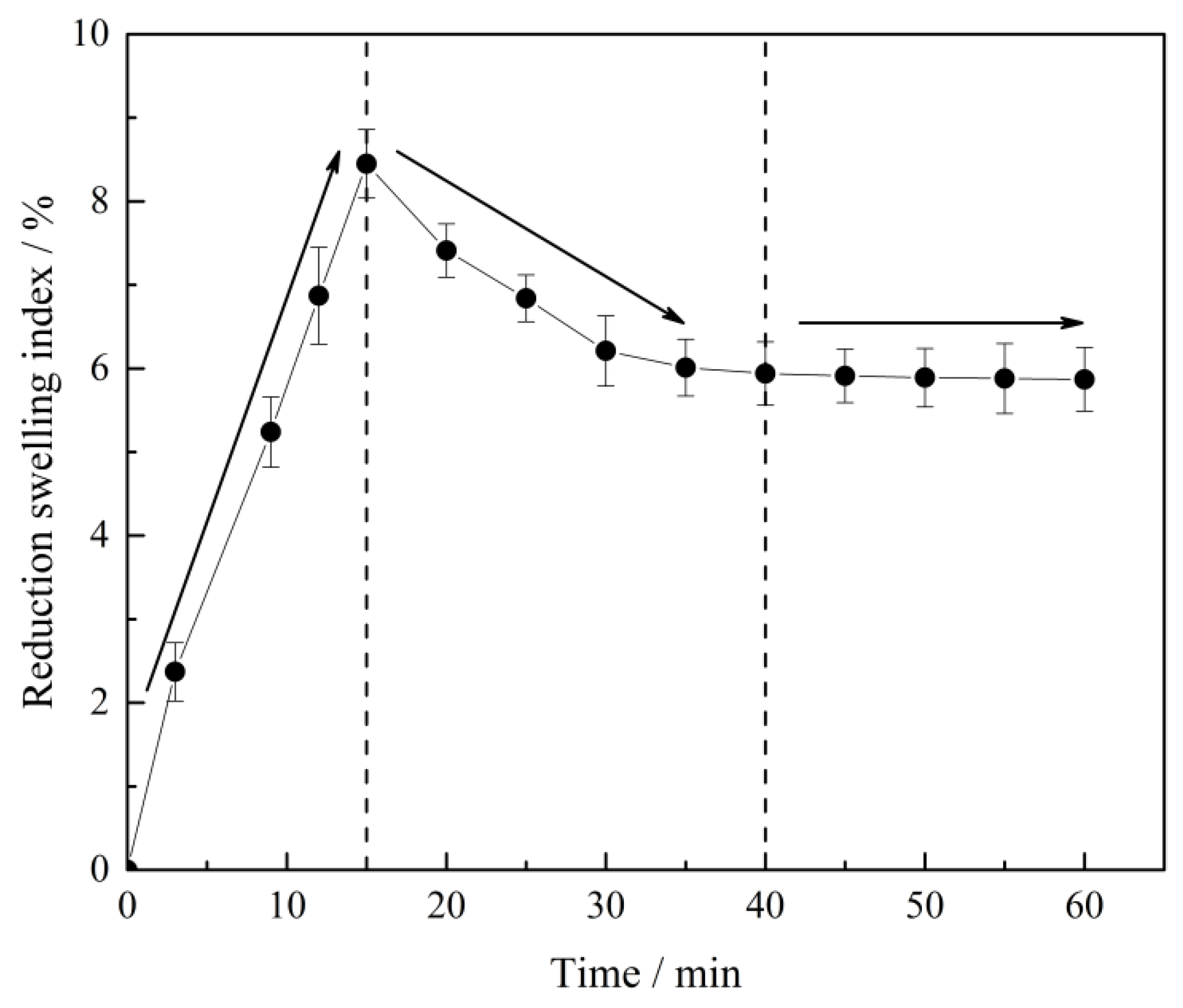
3.3.2. Effect of Time and Co2O3 Addition on RSI
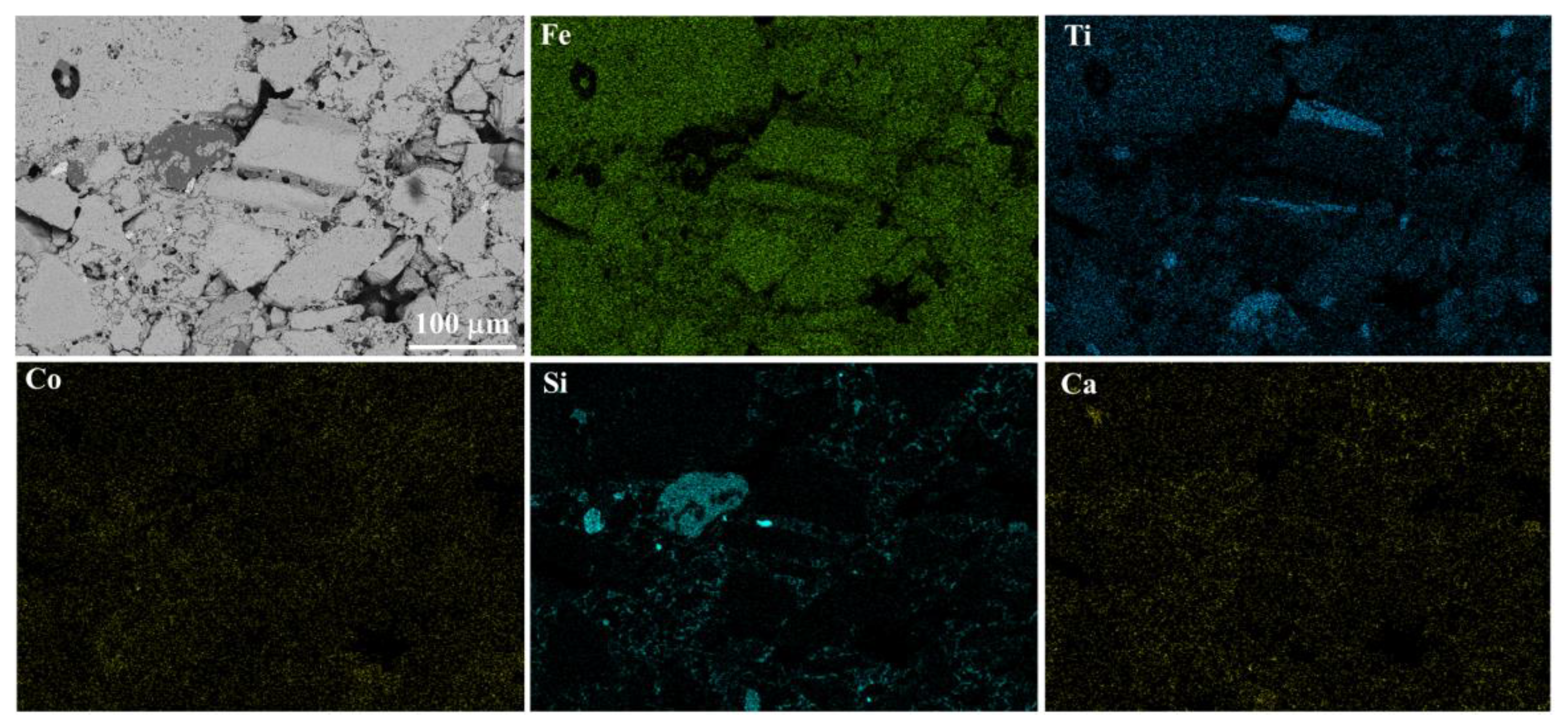
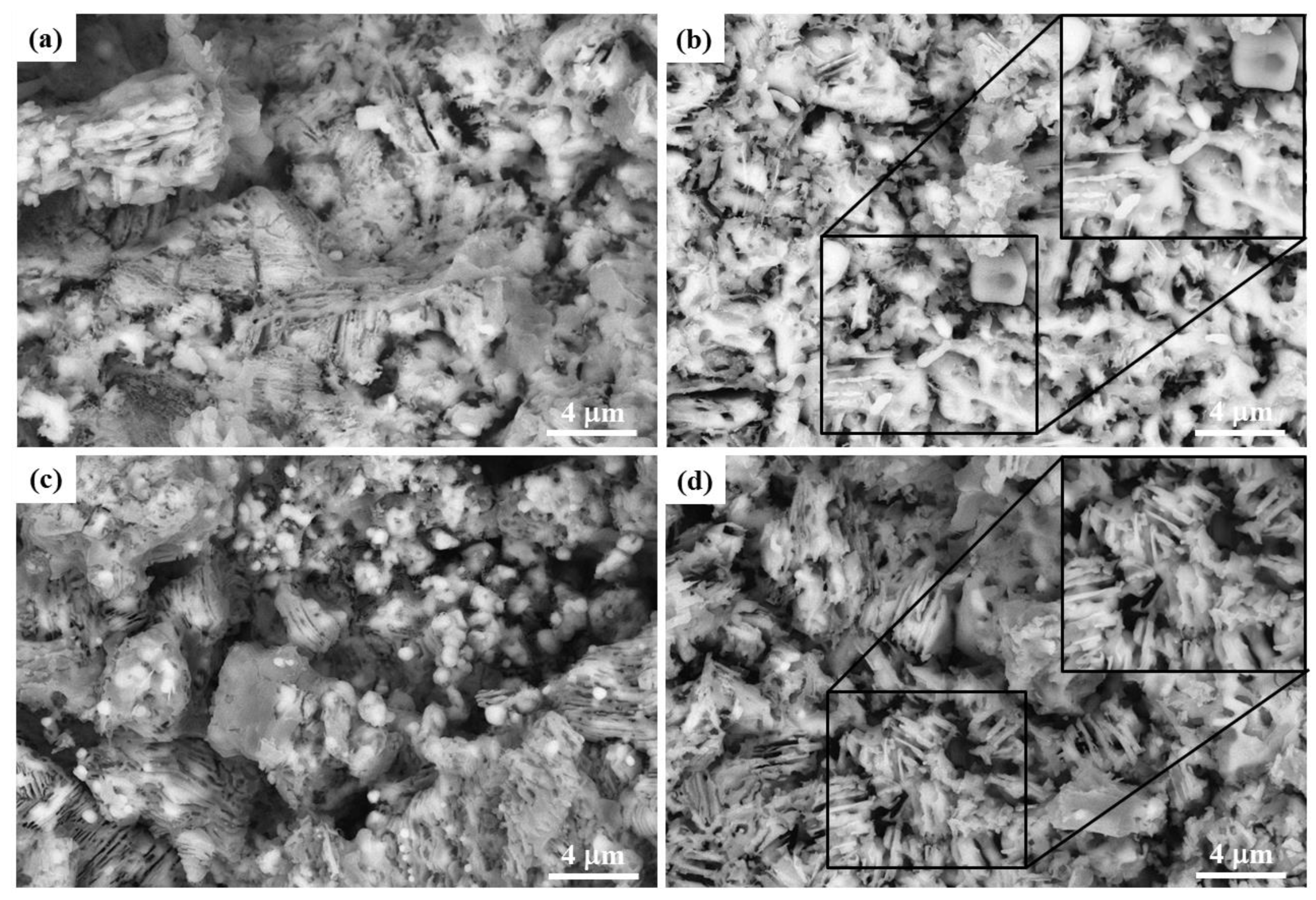
3.3.3. Microscopic Structure
4. Conclusions
- The primary phases of CVTP with Co2O3 addition are Fe2O3, Fe2TiO5, and CoFe2O4. The CS of CVTP decreases from 2448 to 1983 N and porosity increases from 14.86 to 22.49% with increasing Co2O3 additions.
- The Co-bearing phase mainly distributes on gap edges and among adjacent hematite grains. Many cracks and pores distribute along the grain boundaries and damage the connection of hematite grains.
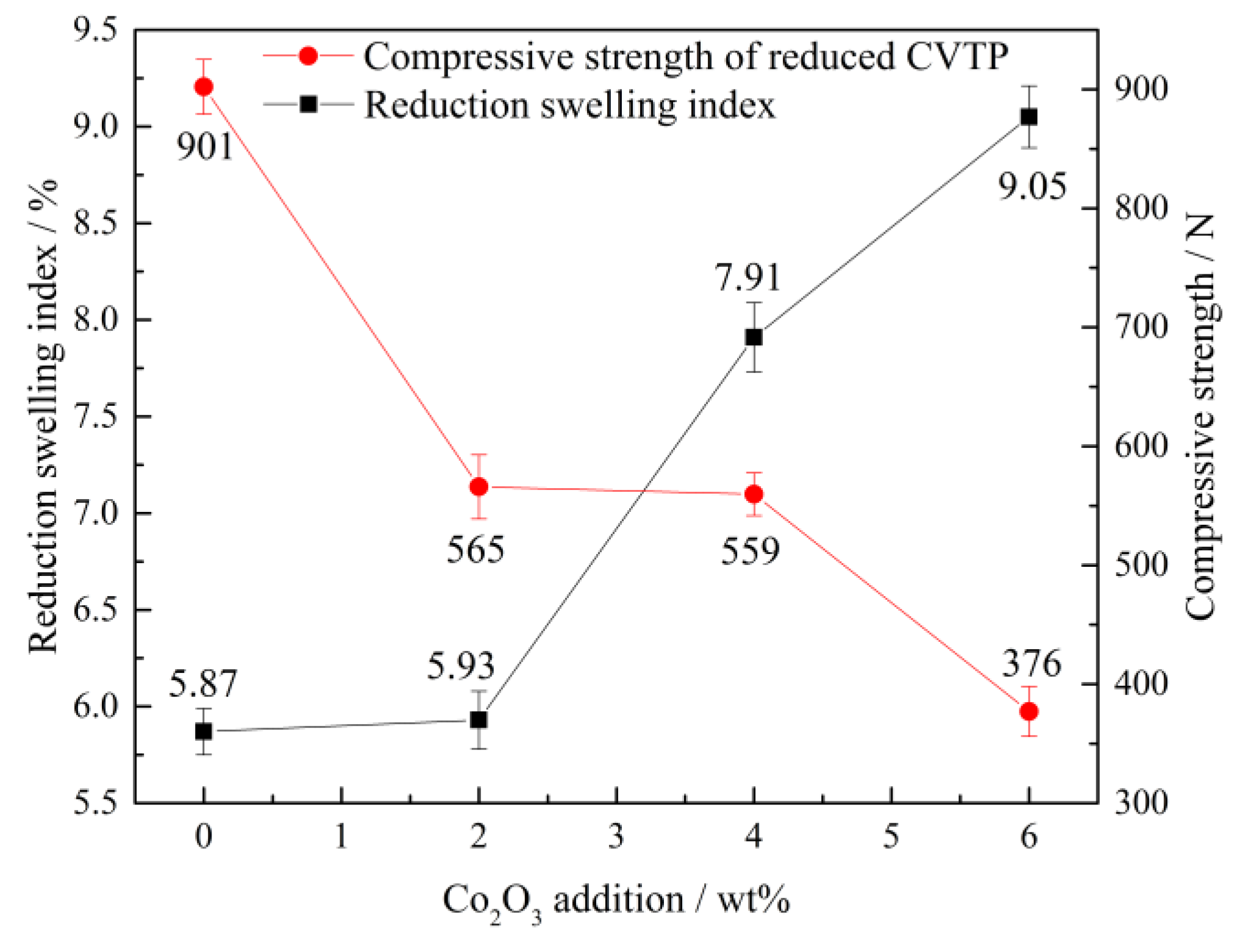
- The primary phases of reduced CVTP with simulated COG are Fe, FeO, CoFe2O4, and Fe2TiO4. The RSI of reduced CVTP increases rapidly with increasing time and reach the peak value of 8.45% at 15 min, then RSI decreases to 5.87% as a smooth curve with increasing time. The RSI of reduced CVTP increases from 5.87 to 9.05% while the CSRC decreases from 901 to 376 N with increasing Co2O3 additions.
- The Co2O3 addition facilitates the aggregation and diffusion of metallic iron particles, and the aggregations of metallic iron thicken the lamellar crystals in reduced CVTP. The pores and interval of grains enlarge with increasing Co2O3 additions.
Author Contributions
Funding
Conflicts of Interest
References
- Tang, J.; Chu, M.S.; Li, F.; Tang, Y.T.; Liu, Z.G.; Xue, X.X. Reduction mechanism of high-chromium vanadium-titanium magnetite pellets by H2-CO-CO2 gas mixtures. Int. J. Min. Met. Mater. 2015, 22, 562–572. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Feng, C.; Tang, Y.T.; Liu, Z.G. Coupled effect of valuable components in high-chromium vanadium-bearing titanomagnetite during oxidation roasting. ISIJ Int. 2016, 56, 1342–1351. [Google Scholar] [CrossRef]
- Takano, C.; Zambrano, A.P.; Nogueira, A.E.A.; Mourao, M.B.; Iguchi, Y. Chromites reduction reaction mechanisms in carbon-chromites composite agglomerates at 1773 k. ISIJ Int. 2007, 47, 1585–1589. [Google Scholar] [CrossRef]
- Lu, C.Y.; Zou, X.L.; Lu, X.G.; Xie, X.L.; Zheng, K.; Xiao, W.; Cheng, H.W.; Li, G.S. Reductive kinetics of panzhihua ilmenite with hydrogen. Trans. Nonferrous Met. Soc. China 2016, 26, 3266–3273. [Google Scholar] [CrossRef]
- Cheng, G.J.; Xue, X.X.; Gao, Z.X.; Jiang, T.; Yang, H.; Duan, P.N. Effect of Cr2O3 on the reduction and smelting mechanism of high-chromium vanadium-titanium magnetite pellets. ISIJ Int. 2016, 56, 1938–1947. [Google Scholar] [CrossRef]
- Jena, B.C.; Dresler, W.; Reilly, I.G. Extraction of titanium, vanadium and iron from titanomagnetite deposits at pipestone lake, manitoba, canada. Miner. Eng. 1995, 8, 159–168. [Google Scholar] [CrossRef]
- Mousa, E.A.; Babich, A.; Senk, D. Enhancement of iron ore sinter reducibility through coke oven gas injection into the modern blast furnace. ISIJ Int. 2013, 53, 1372–1380. [Google Scholar] [CrossRef]
- Liu, Z.; Chu, M.; Guo, T.; Wang, H.; Fu, X. Numerical simulation on novel blast furnace operation of combining coke oven gas injection with hot burden charging. Ironmak. Steelmak. 2016, 43, 64–73. [Google Scholar] [CrossRef]
- Wang, H.; Chu, M.; Guo, T.; Zhao, W.; Feng, C.; Liu, Z.; Tang, J. Mathematical simulation on blast furnace operation of coke oven gas injection in combination with top gas recycling. Steel Res. Int. 2016, 87, 539–549. [Google Scholar] [CrossRef]
- Nishioka, K.; Ujisawa, Y.; Tonomura, S.; Ishiwata, N.; Sikstrom, P. Sustainable aspects of co2 ultimate reduction in the steelmaking process (course50 project), part 1: Hydrogen reduction in the blast furnace. J. Sustain. Metall. 2016, 2, 200–208. [Google Scholar] [CrossRef]
- Mousa, E.A.; Babich, A.; Senk, D. Reduction behavior of iron ore pellets with simulated coke oven gas and natural gas. Steel Res. Int. 2013, 84, 1085–1097. [Google Scholar] [CrossRef]
- Mohan, H.; Shaikh, I.A.; Kulkarni, R.G. Magnetic properties of the mixed spinel CoFe2−xCrxO4. Phys. B Condens. Matter 1996, 217, 292–298. [Google Scholar] [CrossRef]
- Li, L.; Han, Y.; Zhang, P.; Li, J.; Cao, L.; Liao, Q. Effect of Co2O3 additive on the microstructures and dielectric properties of MgTiO3 ceramics. Ferroelectrics 2009, 388, 167–171. [Google Scholar] [CrossRef]
- Aleksandrov, A.A.; Dashevskii, V.Y.; Linchevskii, B.V. Thermodynamics of the oxygen solutions in chromium-containing melts of the Fe-Co system. Russ. Metall. 2014, 2014, 681–687. [Google Scholar] [CrossRef]
- Landolt, D. Corrosion and Surface Chemistry of Metals; EPFL Press: New York, NY, USA, 2007. [Google Scholar]
- Li, W.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Influence of V2O5 content on the gas-based direct reduction of hongge vanadium titanomagnetite pellets with simulated shaft furnace gases. JOM 2018, 70, 76–78. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Effect of V2O5 addition on the oxidation induration process of hongge vanadium titanomagnetite pellets. Ironmak. Steelmak. 2017, 45, 1–6. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Effect of TiO2 addition on the oxidation induration and reduction behavior of hongge vanadium titanomagnetite pellets with simulated shaft furnace gases. Powder Technol. 2018, 326, 137–145. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Influence of Cr2O3 addition on the gas-based direct reduction of hongge vanadium titanomagnetite pellets with simulated shaft furnace gases. ISIJ Int. 2018, 58, 604–611. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Effect of V2O5 addition on the oxidation induration process of hongge vanadium titanomagnetite pellets. Int. J. Min. Met. Mater. 2018, 25, 391–398. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloy. Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Berdzenishvili, I.G. Functional corrosion-resistant enamel coatings and their adherence strength. Acta Phys. Pol. A 2012, 121, 178–180. [Google Scholar] [CrossRef]
- Liu, Y.C.; Nachimuthu, S.; Chuang, Y.C.; Ku, Y.; Jiang, J.C. Reduction mechanism of iron titanium based oxygen carriers with H2 for chemical looping applications—A combined experimental and theoretical study. RSC Adv. 2016, 6, 106340–106346. [Google Scholar] [CrossRef]
- Sharma, T.; Gupta, R.C.; Prakash, B. Effect of firing condition and ingredients on the swelling behaviour of iron ore pellets. ISIJ Int. 1993, 33, 446–453. [Google Scholar] [CrossRef]
- Sharma, T.; Gupta, R.C.; Prakash, B. Swelling of iron ore pellets by statistical design of experiment. ISIJ Int. 1992, 32, 1268–1275. [Google Scholar] [CrossRef]
- Seaton, C.E.; Foster, J.S.; Velasco, J. Structural changes occurring during reduction of hematite and magnetite pellets containing coal char. Trans. Iron Steel Inst. Jpn. 1983, 23, 497–503. [Google Scholar] [CrossRef]
- Kang, T.; Gupta, S.; Sahajwalla, V. Characterizing swelling behaviour of iron oxides during solid state reduction for corex application and their implications on fines generation. ISIJ Int. 2007, 47, 1590–1598. [Google Scholar] [CrossRef]
- Wang, H.; Sohn, H.Y. Effects of firing and reduction conditions on swelling and iron whisker formation during the reduction of iron oxide compact. ISIJ Int. 2011, 51, 7. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Jiang, T.; Wang, L.; Qi, T. Swelling behavior of iron ore pellet reduced by H2–Co mixtures. Powder Technol. 2015, 269, 290–295. [Google Scholar] [CrossRef]







| Sample | FeTotal | FeO | TiO2 | Co2O3 | V2O5 | Cr2O3 | CaO | SiO2 | MgO | Al2O3 | P | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CVTM | 53.35 | 26.91 | 11.60 | 0.02 | 0.57 | 0.81 | 0.96 | 4.71 | 3.33 | 2.82 | 0.02 | 0.26 |
| Bentonite | - | - | - | - | - | - | 2.19 | 68.28 | 3.56 | 13.45 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Yang, S.; Yang, H.; Xue, X. Effect of Co2O3 on Oxidation Induration and Reduction Swelling of Chromium-Bearing Vanadium Titanomagnetite Pellets with Simulated Coke Oven Gas. Metals 2019, 9, 16. https://doi.org/10.3390/met9010016
Tang W, Yang S, Yang H, Xue X. Effect of Co2O3 on Oxidation Induration and Reduction Swelling of Chromium-Bearing Vanadium Titanomagnetite Pellets with Simulated Coke Oven Gas. Metals. 2019; 9(1):16. https://doi.org/10.3390/met9010016
Chicago/Turabian StyleTang, Weidong, Songtao Yang, He Yang, and Xiangxin Xue. 2019. "Effect of Co2O3 on Oxidation Induration and Reduction Swelling of Chromium-Bearing Vanadium Titanomagnetite Pellets with Simulated Coke Oven Gas" Metals 9, no. 1: 16. https://doi.org/10.3390/met9010016





