Transient Evolution of Inclusions during Al and Ti Additions in Fe-20 Mass pct Cr Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Chemical Analysis
2.3. Inclusion Characterization
3. Results and Discussion
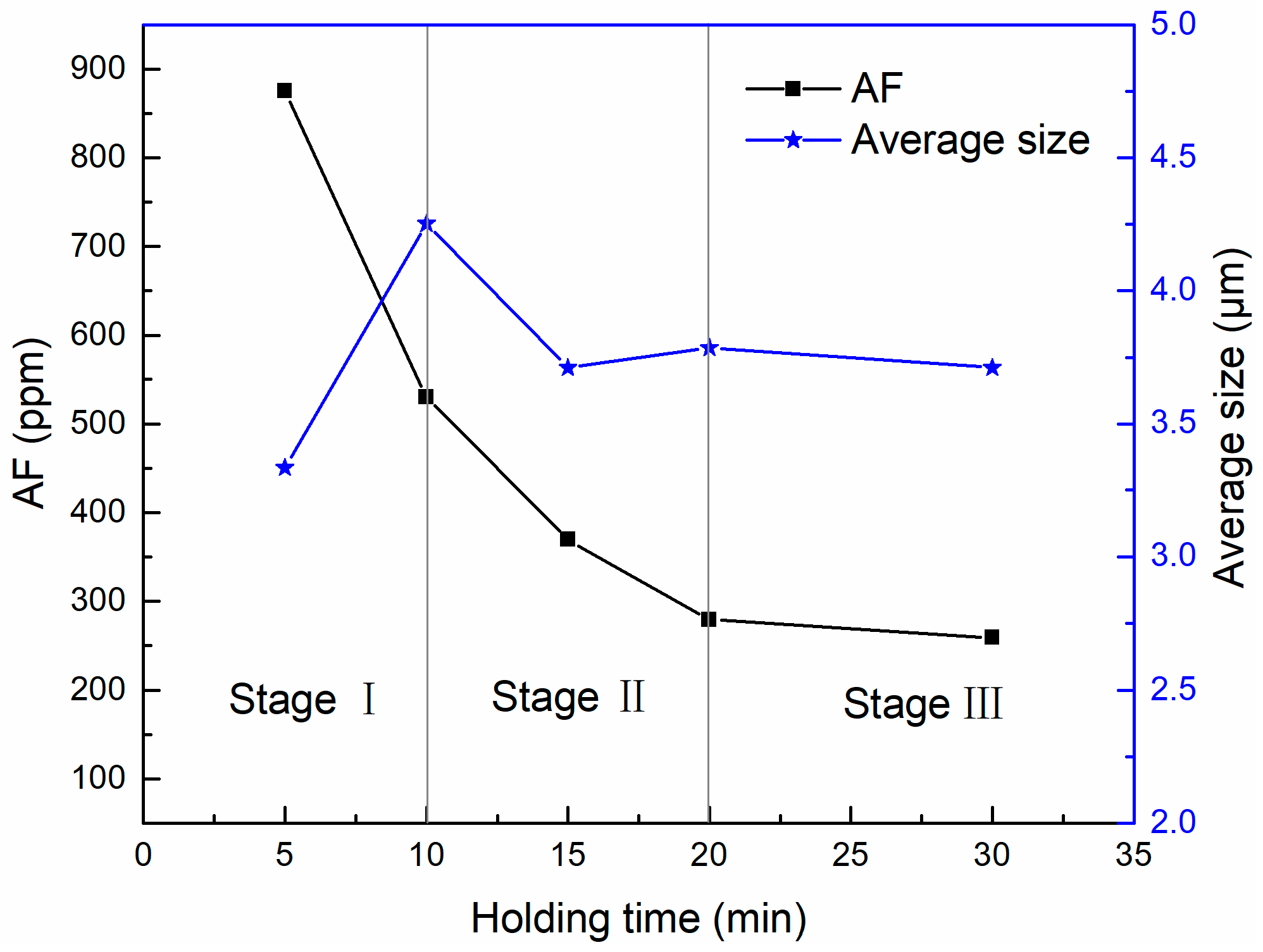
3.1. Two-Dimensional Characteristics of Inclusions after Al Addition
3.2. Three-Dimensional Characteristics of Inclusions after Al Addition
3.3. Two-Dimensional Inclusion Characteristics after Ti Addition
3.4. Three-Dimensional Characteristics of Inclusions after Ti Addition
3.5. Thermodynamic Conditions in the Fe-20 Mass pct Cr–Al–Ti–O–N System
3.6. Formation Mechanism of Transient Inclusions after Ti Addition
3.7. Agglomeration Mechanism of Inclusions after Ti Addition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Thomas, B.G. State of the art in the control of inclusions during steel ingot casting. Metall. Mater. Trans. B 2006, 37, 733–761. [Google Scholar] [CrossRef]
- Ito, Y.I.; Nara, S.; Kato, Y.; Suda, M. Shape control of alumina inclusions by double calcium addition treatment. Tetsu-to-Hagane 2007, 93, 355–361. [Google Scholar] [CrossRef]
- Nakajima, K.; Hasegawa, H.; Khumkoa, S.; Hayashi, M. Effect of catalyst on heterogeneous nucleation in Fe-Ni-Cr alloys. ISIJ Int. 2006, 46, 801–806. [Google Scholar] [CrossRef]
- Ito, A.; Suito, H.; Inoue, R. Size distribution of multi-phase deoxidation particles for heterogeneous crystallization of TiN and solidification structure in Ti-added ferritic stainless steel. ISIJ Int. 2012, 52, 1196–1205. [Google Scholar] [CrossRef]
- Kim, S.K.; Suito, H.; Inoue, R. Effect of multi-phase oxide particles on TiN crystallization and solidification ftructure in Ti-added ferritic stainless steel. ISIJ Int. 2012, 52, 1935–1944. [Google Scholar] [CrossRef]
- Fujimura, H.; Tsuge, S.; Komizo, Y.; Nishizawa, T. Effect of oxide composition on solidification structure of Tiadded ferritic stainless steel. Tetsu-to-Hagane 2001, 87, 29–34. [Google Scholar] [CrossRef]
- Basu, S.; Choudhary, S.K.; Girase, N.U. Nozzle clogging behaviour of Ti-bearing Al-killed ultra low carbon steel. ISIJ Int. 2004, 44, 1653–1660. [Google Scholar] [CrossRef]
- Gao, Y.; Sorimachi, K. Formation of clogging materials in an immersed nozzle during continuous casting of titanium stabilized stainless steel. ISIJ Int. 2007, 33, 291–297. [Google Scholar] [CrossRef]
- Hasegawa, M.; Maruhashi, S.; Kamidate, Y.; Muranaka, Y.; Hoshi, F. Tundish nozzle on constriction in continuous casting of titanium bearing stainless steel. Tetsu-to-Hagane 2009, 70, 1704–1711. [Google Scholar] [CrossRef]
- Sun, Y.H.; Bai, X.F.; Yin, X.; Duan, D.B.; Jin, J.N.; Fu, S.P.; Zhu, X.Z. Research on submerged entry nozzles clogging during AISI 321 stainless steel billet casting. Chin. J. Eng. 2016, 38, S109–S118. [Google Scholar]
- Lee, J.-H.; Kang, M.-H.; Kim, S.-K.; Kim, J.; Kim, M.-S.; Kang, Y.-B. Influence of Al/Ti Ratio in Ti-ULC Steel and Refractory Components of Submerged Entry Nozzle on Formation of Clogging Deposits. ISIJ Int. 2019, 59, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Dorrer, P.; Michelic, S.K.; Bernhard, C.; Penz, A.; Rössler, R. Study on the Influence of FeTi-Addition on the Inclusion Population in Ti-Stabilized ULC Steels and Its Consequences for SEN-Clogging. Steel Res. Int. 2019, 0, 1800635. [Google Scholar] [CrossRef]
- Kang, Y.B.; Lee, J.H. Consideration of Phase Equilibria in Fe-Al-Ti-O System and Its Importance in Steel Cleanliness during Casting Process. Metall. Ital. 2019, 1, 5–11. [Google Scholar]
- Ruby-Meyer, F.; Lehmann, J.; Gaye, H. Thermodynamic analysis of inclusions in Ti-deoxidised steels. Scand. J. Metall. 2000, 29, 206–212. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jo, J.O.; Lee, C.O.; Kim, D.S.; Pak, J.J. Thermodynamic relation between aluminum and titanium in liquid iron. ISIJ Int. 2008, 48, 17–22. [Google Scholar] [CrossRef]
- Jung, I.H.; Eriksson, G.; Wu, P.; Pelton, A. Thermodynamic modeling of the Al2O3-Ti2O3-TiO2 system and its applications to the Fe-Al-Ti-O inclusion diagram. ISIJ Int. 2009, 49, 1290–1297. [Google Scholar] [CrossRef]
- Kang, Y.B.; Lee, J.H. Reassessment of Oxide Stability Diagram in the Fe-Al-Ti-O System. ISIJ Int. 2017, 57, 1665–1667. [Google Scholar] [CrossRef]
- Wang, C.; Verma, N.; Kwon, Y.; Tiekink, W.; Kikuchi, N.; Sridhar, S. A study on the transient inclusion evolution during reoxidation of a Fe-Al-Ti-O melt. ISIJ Int. 2011, 51, 375–381. [Google Scholar] [CrossRef]
- Marie-Aline, V.E.; Guo, M.; Dekkers, R.; Burty, M.; Joris, V.D.; Tom, J.P.; Blanpain, B.; Wollants, P. Formation and evolution of Al–Ti oxide inclusions during secondary steel refining. ISIJ Int. 2009, 49, 1133–1140. [Google Scholar]
- Matsuura, H.; Wang, C.; Wen, G.; Sridhar, S. The transient stages of inclusion evolution during Al and/or Ti additions to molten iron. ISIJ Int. 2007, 47, 1265–1274. [Google Scholar] [CrossRef]
- Lee, S.B.; Choi, J.H.; Lee, H.G.; Rhee, P.C.H.; Jung, S.M. Aluminum deoxidation equilibrium in liquid Fe-16 pct Cr alloy. Metall. Materi. Trans. B 2005, 36, 414–416. [Google Scholar] [CrossRef]
- Wada, H.; Pehlke, R.D. Nitrogen solution and titanium nitride precipitation in liquid Fe-Cr-Ni alloys. Metall. Trans. B 1977, 8, 443–450. [Google Scholar] [CrossRef]
- Choi, H.Y.; Slye, W.E.; Fruehan, R.J.; Nunnington, R.C. Activity of titanium in Fe-Cr melts. Metall. Mater. Trans. B 2005, 36, 537–541. [Google Scholar] [CrossRef]
- Jo, J.-O.; Kim, W.-Y.; Lee, C.-O.; Pak, J.-J. Thermodynamic interaction between chromium and titanium in liquid Fe-Cr alloys containing 30 mass% Cr. ISIJ Int. 2010, 50, 1373–1379. [Google Scholar] [CrossRef]
- Kishi, M.; Inoue, R.; Suito, H. Thermodynamics of oxygen and nitrogen in liquid Fe-20 mass%Cr alloy equilibrated with titania-based slags. ISIJ Int. 1994, 34, 859–867. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Thermodynamics of aluminum and manganese deoxidation equilibria in Fe-Ni and Fe-Cr alloys. ISIJ Int. 2003, 43, 1301–1308. [Google Scholar] [CrossRef]
- Jo, J.-O.; Jung, M.-S.; Park, J.-H.; Lee, C.-O.; Pak, J.-J. Thermodynamic interaction between chromium and aluminum in liquid Fe-Cr alloys containing 26 mass% Cr. ISIJ Int. 2011, 51, 208–213. [Google Scholar] [CrossRef]
- Ozturk, B.; Matway, R.; Fruehan, R. Thermodynamics of inclusion formation in Fe-Cr-Ti-N alloys. Metall. Mater. Trans. B 1995, 26, 563–567. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, C. Interaction coefficients in Fe-C-Ti-i (i= Si, Cr, AI, Ni) systems. Metall. Trans. B 1990, 21, 543–547. [Google Scholar]
- Itoh, T.; Nagasaka, T.; Hino, M. Equilibrium between Dissolved Chromium and Oxygen in Liquid High Chromium Alloyed Steel Saturated with pure Cr2O3. ISIJ Int. 2000, 40, 1051–1058. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Lee, C.-O.; Yun, C.-W.; Pak, J.-J. Effect of chromium on nitrogen solubility in liquid Fe–Cr alloys containing 30 mass% Cr. ISIJ Int. 2009, 49, 1668–1672. [Google Scholar] [CrossRef]
- Sun, M.-K.; Jung, I.-H.; Lee, H.-G. Morphology and chemistry of oxide inclusions after Al and Ti complex deoxidation. Met. Mater. Int. 2008, 14, 791. [Google Scholar] [CrossRef]
- Xuan, C.J.; Karasev, A.V.; Jonsson, P.G. Evaluation of agglomeration mechanisms of non-metallic inclusions and cluster characteristics produced by Ti/Al complex deoxidation in Fe-10mass% Ni alloy. ISIJ Int. 2016, 56, 1204–1209. [Google Scholar] [CrossRef]
- Wang, C.; Nuhfer, N.T.; Sridhar, S. Transient behavior of inclusion chemistry, shape, and structure in Fe-Al-Ti-O melts: Effect of titanium source and laboratory deoxidation simulation. Metall. Mater. Trans. B 2009, 40, 1005–1021. [Google Scholar] [CrossRef]
- Wang, C.; Nuhfer, N.T.; Sridhar, S. Transient behavior of inclusion chemistry, shape, and structure in Fe-Al-Ti-O melts: Effect of titanium/aluminum ratio. Metall. Mater. Trans. B 2009, 40, 1022. [Google Scholar] [CrossRef]
- Wang, C.; Nuhfer, N.T.; Sridhar, S. Transient behavior of inclusion chemistry, shape, and structure in Fe-Al-Ti-O melts: Effect of gradual increase in Ti. Metall. Mater. Trans. B 2010, 41, 1084–1094. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, M.; Matsuura, H.; Tsukihashi, F. Dynamic evolution of inclusions in Ti-bearing Al-deoxidized molten Irons at 1873 K. Steel Res. Int. 2014, 85, 16–25. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, C.; Jiang, M. Effect of Mg on behavior and particle size of inclusions in Al-Ti deoxidized molten steels. Metall. Mater. Trans. B 2016, 47, 2253–2262. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L. Modelling inclusion evolution in Al–Ti-killed steels during ladle mixing process. Ironmak. Steelmak. 2018, 45, 585–591. [Google Scholar] [CrossRef]
- Nagata, Y.; Kawashima, Y.; Shinme, K.; Nishio, K. Formation mechanism of inclusion on Al-Ti deoxidation (Behavior of inclusion on Al-Ti deoxidation—I). CAMP-ISIJ 1991, 4, 1236. [Google Scholar]
- Kunisada, K.; Iwai, H. Morphology of oxides formed by deoxidation in molten steel. CAMP-ISIJ 1993, 6, 1173. [Google Scholar]
- Kunisada, K.; Iwai, H. Al-Ti deoxidation and morphology of the deoxidation products. CAMP-ISIJ 1994, 7, 1130. [Google Scholar]
- Doo, W.C.; Kim, D.Y.; Kang, S.C. The morphology of Al-Ti-O complex oxide inclusions formed in an ultra low-carbon steel melt during the RH process. Met. Mater. Int. 2007, 13, 249. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.; Yang, Y.; Bai, X.; Barati, M.; Mclean, A. Formation of inclusions in Ti-stabilized 17Cr austenitic stainless steel. Metall. Mater. Trans. B 2016, 47, 3274–3284. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Zhang, L.; Wang, W. Characteristics of alumina-based inclusions in low carbon Al-killed steel under no-stirring condition. Steel Res. Int. 2013, 84, 878–891. [Google Scholar] [CrossRef]
- Braun, T.B.; Elliott, J.F.; Flemings, M.C. The clustering of alumina inclusions. Metall. Trans. B 1979, 10, 171–184. [Google Scholar] [CrossRef]
- Dekkers, R.; Blanpain, B.; Wollants, P. Crystal growth in liquid steel during secondary metallurgy. Metall. Mater. Trans. B 2003, 34, 161–171. [Google Scholar] [CrossRef]
- Sunagawa, I. Morphology of Crystals; Terra Science Publishing: Tokyo, Japan, 1987; pp. 509–587. [Google Scholar]
- Flemings, M.C. Solidification processing. Metall. Trans. 1974, 5, 2121–2134. [Google Scholar] [CrossRef]
- Van Ende, M.-A.; Guo, M.; Zinngrebe, E.; Dekkers, R.; Proost, J.; Blanpain, B.; Wollants, P. Morphology and growth of alumina inclusions in Fe–Al alloys at low oxygen partial pressure. Ironmak. Steelmak. 2009, 36, 201–208. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, J.; Liu, D.Z.; Li, H.B.; Zhu, K.R.; Chen, B. Formation mechanism and control technology of Al-Ti inclusions in ultra low carbon steel. Iron Steel 2018, 53, 24–30. [Google Scholar]
- Nabeel, M.; Alb, M.; Sun, S.; Karasev, A.; Jönsson, P.; Dogan, N. Characterization of inclusions in high-Mn steel using two-dimensional and three-dimensional methods. In AISTech 2018; AIST: Warrendale, PA, USA, 2018; pp. 1483–1491. [Google Scholar]
- Itoh, H.; Hino, M.; Ban-Ya, S. Thermodynamics on the formation of non-metallic inclusion of spinel (MgO·Al2O3) in liquid steel. Tetsu-to-Hagane 1998, 84, 85–90. [Google Scholar] [CrossRef]
- Fujii, K.; Nagasaka, T.; Hino, M. Activities of the constituents in spinel solid solution and free energies of formation of MgO, MgO·Al2O3. ISIJ Int. 2000, 40, 1059–1066. [Google Scholar] [CrossRef]
- Maddalena, R.; Rastogi, R.; Bassem, S.; Cramb, A.W. Nozzle deposits in titanium treated stainless steels. Iron Steelmaker 2000, 27, 71–79. [Google Scholar]
- Robinson, S.; Martin, I.; Pickering, F. Formation of alumina in steel and its dissemination during mechanical working. Met. Technol. 1979, 6, 157–169. [Google Scholar] [CrossRef]
- Shi, X.F.; Guo, G.C.; Zhao, P. Effect of Al on inclusions in 430 stainless steel containing titanium. Iron Steel Vanadium Titanium 2011, 32, 57–61. [Google Scholar]
- Taniguchi, S.; Kikuchi, A. Mechanisms of collision and coagulation between fine particles in fluid. Tetsu-to-Hagane 1992, 78, 527–535. [Google Scholar] [CrossRef]
- Lindborg, U. A collision model for the growth and separation of deoxidation products. Trans. Metall. Soc. AIME 1968, 242, 94. [Google Scholar]
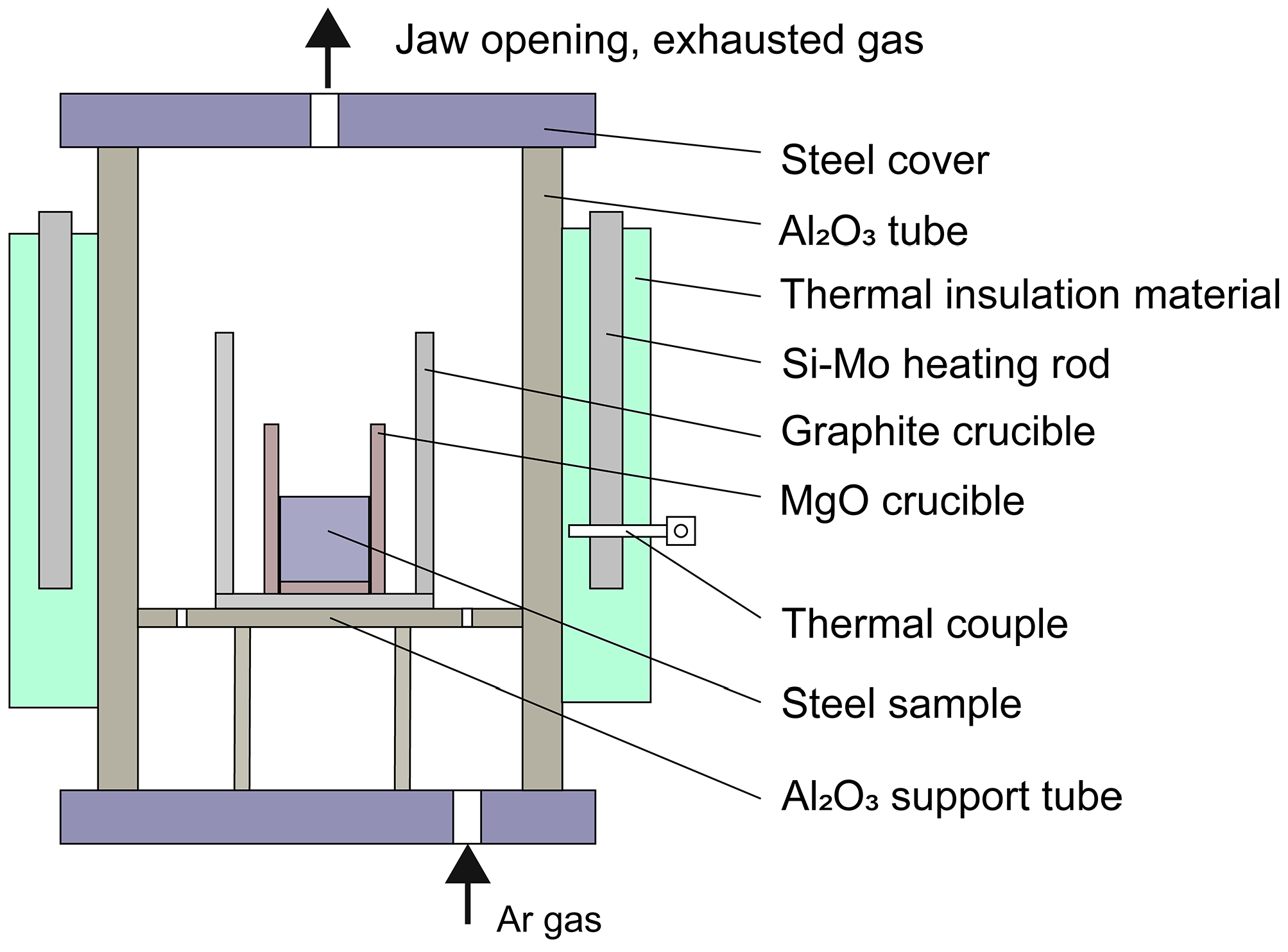


















| Experiment No. | Time | Al | Ti | O | N |
|---|---|---|---|---|---|
| 1 | Before Al addition | - | - | 0.0312 | 0.0018 |
| 5 min after Al addition | 0.082 | - | 0.0252 | 0.0020 | |
| 10 min after Al addition | 0.068 | - | 0.0107 | 0.0021 | |
| 15 min after Al addition | 0.056 | - | 0.0064 | 0.0022 | |
| 20 min after Al addition | 0.039 | - | 0.0054 | 0.0023 | |
| 30 min after Al addition | 0.053 | 0.0047 | 0.0023 | ||
| 2 | 20 min after Al addition | 0.048 | - | 0.0067 | 0.0016 |
| 10 min after Ti addition | 0.055 | 0.15 | 0.0056 | 0.0024 |
| Inclusion | Al2O3 | MgO·Al2O3 | Ti2O3 |
|---|---|---|---|
| Disregistry | 17.48% | 4.88% | 16.17% |
| K/(J/K) | T/K | µ/(kg/m·s) | α/(m2/s3) | ε/(m2/s3) |
|---|---|---|---|---|
| 1.38 × 10−23 | 1873 | 0.005 | 0.3 | 0.0018 |
| /(kg/m3) | /(kg/m3) | /(kg/m3) | /(kg/m3) | g/(m/s2) |
| 8000 | 5220 | 3900 | 4486 | 9.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Sun, Y.; Zhang, Y. Transient Evolution of Inclusions during Al and Ti Additions in Fe-20 Mass pct Cr Alloy. Metals 2019, 9, 702. https://doi.org/10.3390/met9060702
Bai X, Sun Y, Zhang Y. Transient Evolution of Inclusions during Al and Ti Additions in Fe-20 Mass pct Cr Alloy. Metals. 2019; 9(6):702. https://doi.org/10.3390/met9060702
Chicago/Turabian StyleBai, Xuefeng, Yanhui Sun, and Yimin Zhang. 2019. "Transient Evolution of Inclusions during Al and Ti Additions in Fe-20 Mass pct Cr Alloy" Metals 9, no. 6: 702. https://doi.org/10.3390/met9060702
APA StyleBai, X., Sun, Y., & Zhang, Y. (2019). Transient Evolution of Inclusions during Al and Ti Additions in Fe-20 Mass pct Cr Alloy. Metals, 9(6), 702. https://doi.org/10.3390/met9060702




