Generation Mechanism of MgO and Al2O3 Inclusions in 51CrV4 Spring Steel Based on the Ion–Molecule Coexistence Theory
Abstract
:1. Introduction
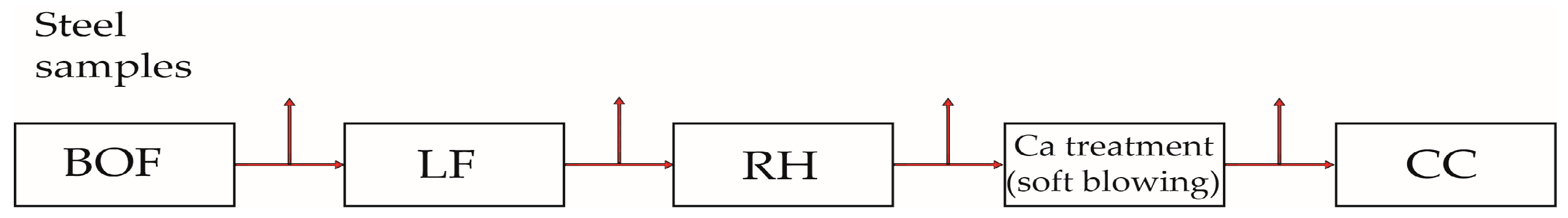
2. Experimental and Methods
2.1. Nozzle Clogging
2.2. Types of MgO·Al2O3 Spinel Inclusions in 51CrV4 Spring Steel
2.3. Generation Mechanism of MgO·Al2O3 Inclusions
2.4. MAC Model of the Constitutional Units in Slag Systems
3. Results and Discussions
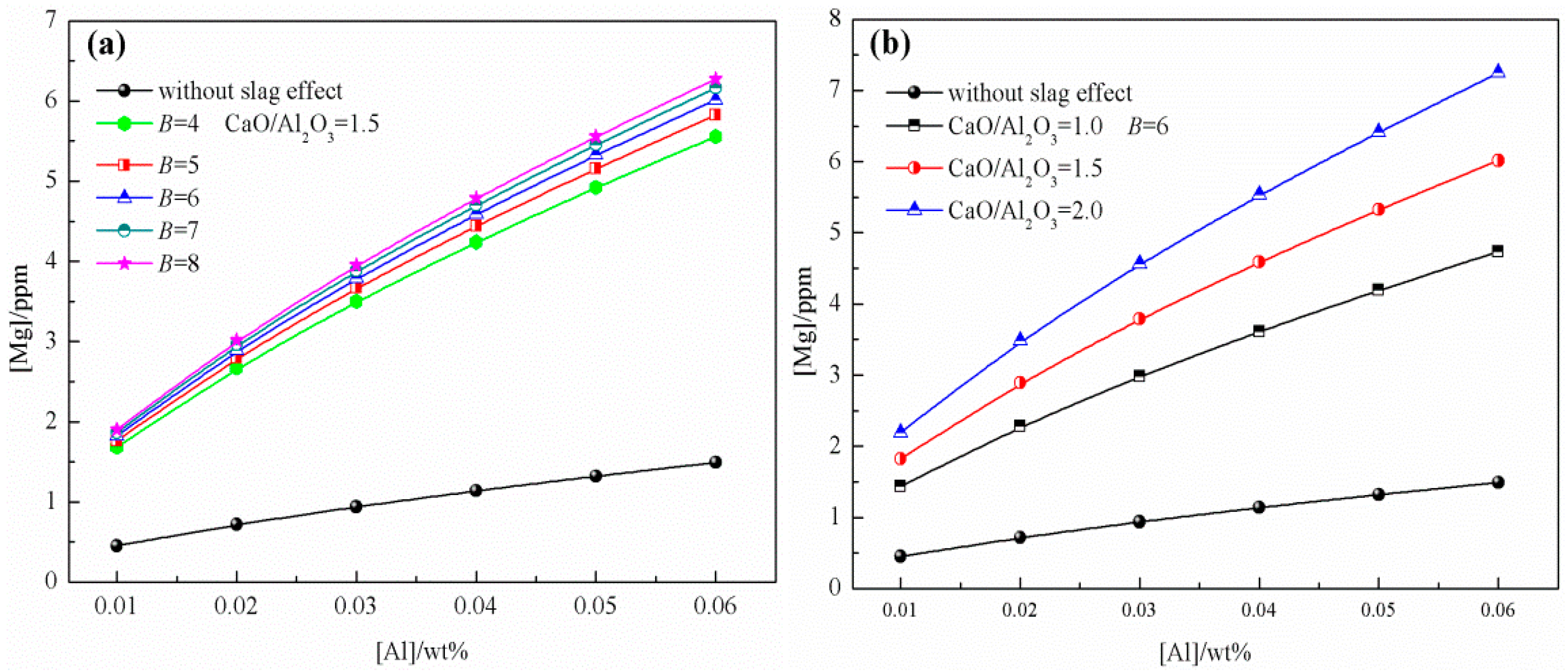
3.1. Effect of Slag Composition on Mg Content in Liquid Steel
3.2. Effect of Lining on the Mg Content in Liquid Steel
3.3. Effect of C on the Mg Content Under RH Conditions
4. Conclusions
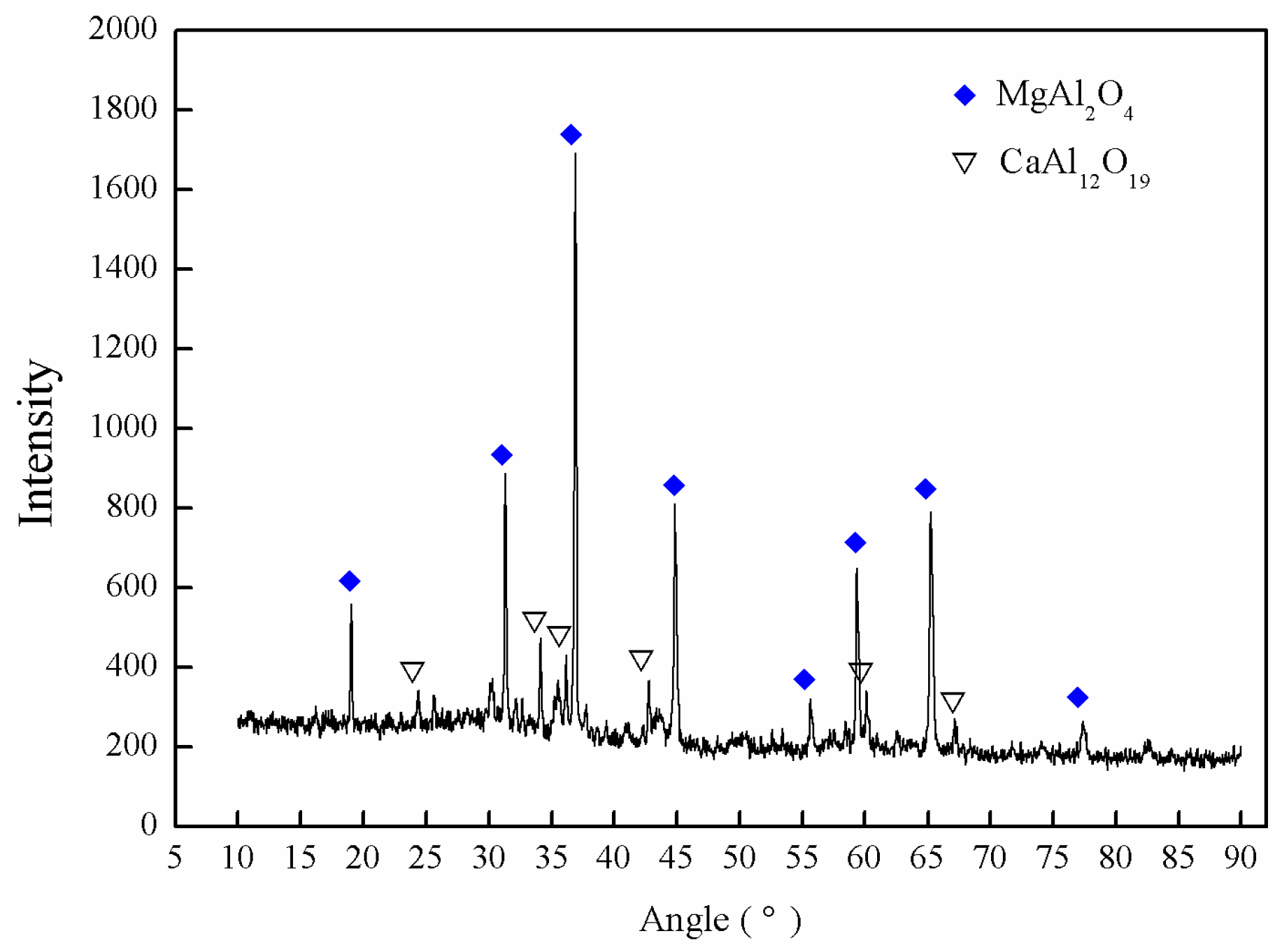
- The XRD and SEM/EDS analyses indicated that the nozzle clogging for 51CrV4 spring steel production was primarily due to the presence of MgAl2O4 spinel inclusions;
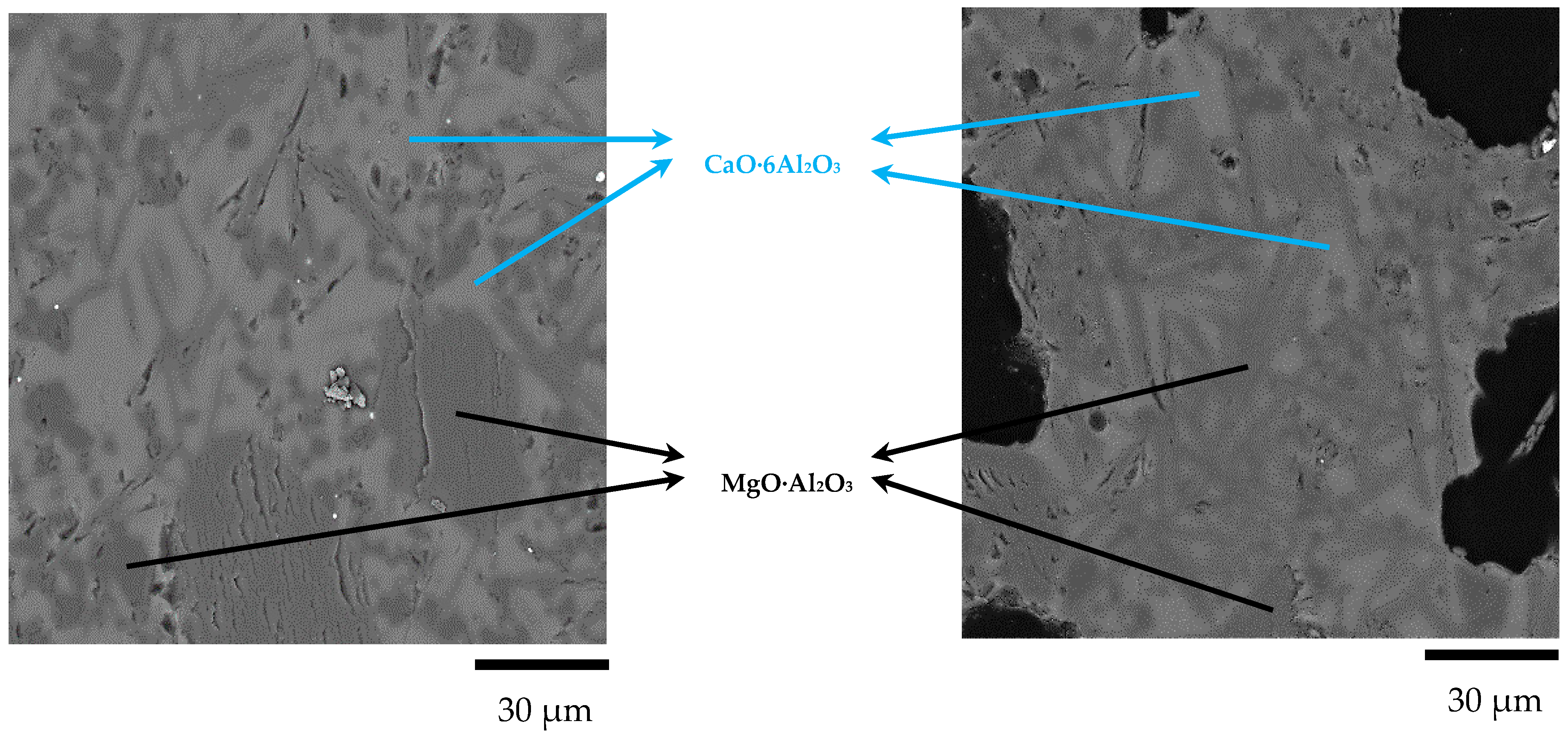
- Three types of MgO·Al2O3 spinel inclusions were observed in steel billets by non-aqueous electrolysis: Pure MgO·Al2O3 inclusions; modified MgO·Al2O3 spinel inclusions containing Mg, Al, Ca, and O, which was the dominant inclusion type; and modified spinel inclusions primarily containing Al, Ca, and O. The assessment of the inclusions in the specimens before and after LF and RH indicated that the inclusions transformed through Al2O3→MgO·Al2O3→MgO–Al2O3–CaO during the refining process;
- The generation mechanism of MgO·Al2O3 inclusions in 51CrV4 spring steel refined by CaO–SiO2–Al2O3–MgO–FeO–MnO slag was evaluated based on the IMCT combined with industrial results. The effects of slag composition, refractory, and RH conditions on the content of Mg in liquid steel were determined. Model calculation results indicated that the Mg content increased with an increasing basicity, CaO/Al2O3 ratio, and Al content during LF, with the CaO/Al2O3 ratio being the most critical factor. In contrast, under RH conditions, the effects of basicity and the CaO/Al2O3 ratio were insignificant, and the partial pressure of CO was the dominant factor.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yao, D.; Li, J.; Li, J.; Zhu, Q. Effect of cold rolling on morphology of carbides and properties of 7Cr17MoV stainless steel. Mater. Manuf. Process. 2015, 30, 111–115. [Google Scholar] [CrossRef]
- Park, J.H.; Todoroki, H. Control of MgO·Al2O3 spinel inclusions in stainless steels. ISIJ Int. 2010, 50, 1333–1346. [Google Scholar] [CrossRef]
- Fukaura, K.; Yokoyama, Y.; Yokoi, D.; Tsujii, N. Fatigue of cold-work tool steels: Effect of heat treatment and carbide morphology on fatigue crack formation, life, and fracture surface observations. Metall. Mater. Trans. A 2004, 35, 1289–1300. [Google Scholar] [CrossRef]
- Atkinson, H.V.; Shi, G. Characterization of inclusions in clean steels: A review including the statistics of extremes methods. Prog. Mater. Sci. 2003, 48, 457–520. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Xie, F.; Lai, C.; Li, H.; Zhang, Q. Dynamic recrystallization behavior and critical strain of 51CrV4 high-strength spring steel during hot deformation. JOM 2018, 70, 2385–2391. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, D.H.; Li, Y.C.; Wang, X.J.; Ren, X.X.; Wang, E.G. Effect of quenching conditions on the microstructure and mechanical properties of 51CrV4 spring steel. Metals 2018, 8, 1056. [Google Scholar] [CrossRef]
- Hasegawa, M.; Maruhashi, S. Synthetic slag refining of 18Cr steel in VOD. Tetsu-to-Hagane 1977, 63, 2087–2093. [Google Scholar] [CrossRef]
- Nishi, T.; Shinme, K. Formation of spinel inclusions in molten stainless steel under Al deoxidation with slags. Tetsu-to-Hagane 1998, 84, 837–843. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Chen, B.; Wang, W. Laboratory study on evolution mechanisms of non-metallic inclusions in high strength alloyed steel refined by high basicity slag. ISIJ Int. 2010, 50, 95–104. [Google Scholar] [CrossRef]
- Ganesh, I. A review on magnesium aluminate (MgAl2O4) spinel: Synthesis, processing and applications. Cheminform 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Sakata, K.; Co, A.S. Technology for production of austenite type clean stainless. ISIJ Int. 2006, 46, 1795–1799. [Google Scholar] [CrossRef]
- Beskow, K.; Tripathi, N.N.; Nzotta, M.; Sandberg, A.; Sichen, D. Impact of slag-refractory lining reactions on the formation of inclusions in steel. Ironmak. Steelmak. 2004, 31, 514–518. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Q.; Zhang, L.; Li, J.; Peaslee, K. Formation and modification of MgO·Al2O3-based inclusions in alloy steels. Metall. Mater. Trans. B 2012, 43, 731–750. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Wang, X.; Ren, Y.; Liu, X.; Shan, Q. Characteristics of inclusions in low carbon Al-killed steel during ladle furnace refining and calcium treatment. ISIJ Int. 2013, 53, 1401–1410. [Google Scholar] [CrossRef]
- Itoh, H.; Hino, M.; Ban-Ya, S. Thermodynamics on the formation of spinel nonmetallic inclusion in liquid steel. Metall. Mater. Trans. B 1997, 28, 953–956. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Chen, B.; Wang, W. Formation of MgO·Al2O3 inclusions in high strength alloyed structural steel refined by CaO–SiO2–Al2O3–MgO slag. ISIJ Int. 2008, 48, 885–890. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.H.; Wang, W.J. Control of non-metallic inclusions by slag-metal reactions for high strength alloying steels. Steel Res. Int. 2010, 81, 759–765. [Google Scholar] [CrossRef]
- Liu, C.; Huang, F.; Suo, J.; Wang, X. Effect of magnesia-carbon refractory on the kinetics of MgO·Al2O3 spinel inclusion generation in extra-low oxygen steels. Metall. Mater. Trans. B 2016, 47, 989–998. [Google Scholar] [CrossRef]
- Giordani, E.J.; Guimara, V.A.; Pinto, T.B.; Ferreira, I. Effect of precipitates on the corrosion-fatigue crack initiation of ISO 5832-9 stainless steel biomaterial. Int. J. Fatigue 2004, 26, 1129–1136. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Y.H.; Yang, Y.D.; Bai, X.F.; Deng, X.X.; Barati, M.; McLean, A. Inclusion evolution during refining and continuous casting of 316L stainless steel. Ironmak. Steelmak. 2016, 43, 533–540. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.S. Effect of CaO–Al2O3–MgO slags on the formation of MgO–Al2O3 inclusions in ferritic stainless steel. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2005, 36, 495–502. [Google Scholar] [CrossRef]
- Todoroki, H.; Mizuno, K.; Noda, M.; Tohge, T. Formation mechanism of spinel type inclusion in 304 stainless steel deoxidized with ferosilicon alloys. In Proceedings of the 84th Steelmaking Conference; ISS: Warrendale, PA, USA, 2001; Volume 331. [Google Scholar]
- Todoroki, H.; Mizuno, K. Effect of silica in slag on inclusion compositions in 304 stainless steel deoxidized with aluminum. ISIJ Int. 2004, 44, 1350–1357. [Google Scholar] [CrossRef]
- Kawakami, K.; Taniguchi, T.; Nakashima, K. Generation mechanisms of non-metallic inclusions in high-cleanliness steel. Tetsu-to-Hagane 2007, 93, 743–752. [Google Scholar] [CrossRef]
- Brabie, V. Mechanism of reaction between refractory materials and aluminum deoxidised molten steel. ISIJ Int. 1996, 36, 109–112. [Google Scholar] [CrossRef]
- Okuyama, G.; Yamaguchi, K.; Takeuchi, S.; Sorimachi, K. Effect of slag composition on the kinetics of formation of Al2O3-MgO inclusions in aluminum killed ferritic stainless steel. ISIJ Int. 2000, 40, 121–128. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H. Effect of CaO/Al2O3 ratio of ladle slag on formation behavior of inclusions in Mn and V alloyed steel. ISIJ Int. 2018, 58, 88–97. [Google Scholar] [CrossRef]
- Chen, G.; Guo, Y.; He, S. Effect of FeO content in slag on formation of MgO·Al2O3 inclusion for Al-killed steel. Metall. Res. Technol. 2016, 113, 204–213. [Google Scholar] [CrossRef]
- Suito, H.; Inoue, R. Thermodynamics on control of inclusions composition in ultra-clean steels. ISIJ Int. 1996, 36, 528–536. [Google Scholar] [CrossRef]
- Shin, J.H.; Chung, Y.; Park, J.H. Refractory–slag–metal–inclusion multiphase reactions modeling using computational thermodynamics: Kinetic model for prediction of inclusion evolution in molten steel. Metall. Mater. Trans. B 2017, 48, 46–59. [Google Scholar] [CrossRef]
- Liu, C.; Huang, F.; Wang, X. The effect of refining slag and refractory on inclusion transformation in extra low oxygen steels. Metall. Mater. Trans. B 2016, 47, 999–1009. [Google Scholar] [CrossRef]
- Brabie, V. A study on the mechanism of reaction between refractory materials and aluminium deoxidised molten steel. Steel Res. 1997, 68, 54–60. [Google Scholar] [CrossRef]
- Liu, C.; Yagi, M.; Gao, X.; Kim, S.J.; Huang, F.; Ueda, S.; Kitamura, S.Y. Dissolution behavior of Mg from magnesia-chromite refractory into Al-killed molten steel. Metall. Mater. Trans. B 2018, 49, 2298–2307. [Google Scholar] [CrossRef]
- Alhussein, A.; Yang, W.; Zhang, L.F. Effect of interactions between Fe–Al alloy and MgO-based refractory on the generation of MgO·Al2O3 spinel. Ironmak. Steelmak. 2019, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Glaser, B.; Sichen, D. Improvement of resistance of MgO-based refractory to slag penetration by in situ spinel formation. Metall. Mater. Trans. B 2015, 46, 749–757. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, L.; Zhang, Y.; Ren, Y. Kinetic modeling for the dissolution of MgO lining refractory in Al-killed steels. Metall. Mater. Trans. B 2017, 48, 2195–2206. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.F. Thermodynamic model for prediction of slag-steel-inclusion reactions of 304 stainless steels. ISIJ Int. 2017, 57, 68–75. [Google Scholar] [CrossRef]
- Park, J.H. Thermodynamic investigation on the formation of inclusions containing MgAl2O4 spinel during 16Cr–14Ni austenitic stainless steel manufacturing processes. Mater. Sci. Eng. A 2008, 472, 43–51. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.F.; Duan, H.; Ren, Y.; Wang, J.; Liu, X. Formation of non-metallic inclusions in the molten steel in MgO crucibles. In EPD Congress 2014; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 269–276. [Google Scholar]
- Jansson, S.; Brabie, V.; Jönsson, P. Magnesia–carbon refractory dissolution in Al killed low carbon steel. Ironmak. Steelmak. 2006, 33, 389–397. [Google Scholar] [CrossRef]
- Duan, S.C.; Guo, X.L.; Guo, H.J.; Guo, J. A manganese distribution prediction model for CaO–SiO2–FeO–MgO–MnO–Al2O3 slags based on IMCT. Ironmak. Steelmak. 2017, 44, 168–184. [Google Scholar] [CrossRef]
- Duan, S.C.; Li, C.; Guo, X.L.; Guo, H.J.; Guo, J.; Yang, W.S. A thermodynamic model for calculating manganese distribution ratio between CaO–SiO2–MgO–FeO–MnO–Al2O3–TiO2–CaF2 ironmaking slags and carbon saturated hot metal based on the IMCT. Ironmak. Steelmak. 2017, 45, 655–664. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Guo, H.; Guo, J.; Duan, S.; Sun, W. A phosphorus distribution prediction model for CaO–SiO2–MgO–FeO–Fe2O3–Al2O3–P2O5 slags based on the IMCT. Ironmak. Steelmak. 2019, 1–10. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Duan, D.P.; Zhang, J. A Thermodynamic model for predicting phosphorus partition between CaO-based slags and hot metal during hot metal dephosphorization pretreatment process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2016, 47, 2279–2301. [Google Scholar] [CrossRef]
- Yang, X.M.; Duan, J.P.; Shi, C.B.; Zhang, M.; Zhang, Y.L.; Wang, J.C. A thermodynamic model of phosphorus distribution ratio between CaO–SiO2–MgO–FeO–Fe2O3–MnO–Al2O3–P2O5 slags and molten steel during a top-bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2011, 42, 738–770. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Duan, J.P.; Zhang, J. A thermodynamic model of phosphate capacity for CaO–SiO2–MgO–FeO–Fe2O3–MnO–Al2O3–P2O5 slags equilibrated with molten steel during a top-bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2011, 42, 951–977. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, M.; Chai, G.M.; Li, J.Y.; Liang, Q.; Zhang, J. Thermodynamic models for predicting dephosphorisation ability and potential of CaO–FeO–Fe2O3–Al2O3–P2O5 slags during secondary refining process of molten steel based on ion and molecule coexistence theory. Ironmak. Steelmak. 2016, 43, 663–687. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, M.; Guo, M.; Yang, X.M. Enrichment mechanism of phosphate in CaO–SiO2–FeO–Fe2O3–P2O5 steelmaking slags. Metall. Mater. Trans. B 2014, 45, 1666–1682. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Yan, F.J.; Duan, D.P.; Zhang, J. A further evaluation of the coupling relationship between dephosphorization and desulfurization abilities or potentials for CaO-based slags: Influence of slag chemical composition. Metals 2018, 8, 1083. [Google Scholar] [CrossRef]
- Shi, C.B.; Yang, X.M.; Jiao, J.S.; Li, C.; Guo, H.J. A sulphide capacity prediction model of CaO–SiO2–MgO–Al2O3 ironmaking slags based on the ion and molecule coexistence theory. ISIJ Int. 2010, 50, 1362–1372. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Chai, G.M.; Wang, F. A thermodynamic model of sulfur distribution ratio between CaO–SiO2–MgO–FeO–MnO–Al2O3 slags and molten steel during LF refining process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2011, 42, 1150–1180. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, M.; Shi, C.B.; Chai, G.M.; Zhang, J. A sulfide capacity prediction model of CaO–SiO2–MgO–FeO–MnO–Al2O3 slags during the LF refining process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2012, 43, 241–266. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Chai, G.M.; Zhang, J. Prediction model of sulfide capacity for CaO–FeO–Fe2O3–Al2O3–P2O5 slags in a large variation range of oxygen potential based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2014, 45, 2118–2137. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Zhang, J. Prediction model of sulphur distribution ratio between CaO–FeO–Fe2O3–Al2O3–P2O5 slags and liquid iron over large variation range of oxygen potential during secondary refining process of molten steel based on ion and molecule coexistence theory. Ironmak. Steelmak. 2016, 43, 39–55. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, M.; Zhang, J.L.; Li, P.C.; Li, J.Y.; Zhang, J. Representation of oxidation ability for metallurgical slags based on the ion and molecule coexistence theory. Steel Res. Int. 2014, 85, 347–375. [Google Scholar] [CrossRef]
- Zhang, J. Computational Thermodynamics of Metallurgical Melts and Solutions; Metallurgical Industry Press: Beijing, China, 2007. [Google Scholar]
- Verein Deutscher Eisenhuttenleute. Slag Atlas, 2nd ed.; Woodhead Publishing Limited: Cambridge, UK, 1995. [Google Scholar]
- Chen, J.X. Common Charts and Databook for Steelmaking, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Rein, R.H.; Chipman, J. Activities in the liquid solution SiO2–CaO–MgO–Al2O3 at 1600 °C. Trans. Met. Soc. AIME 1965, 233, 415–425. [Google Scholar]
- Turkdogan, E.T. Physical Chemistry of High Temperature Technology; Academic Press: New York, NY, USA, 1980; pp. 8–12. [Google Scholar]
- Gaye, H.; Welfringer, J. Proceedings of the Second International Symposium on Metallurgical Slags and Fluxes; Fine, H.A., Gaskell, D.R., Eds.; TMS–AIME: Lake Tahoe, NV, USA, 1984; pp. 357–375. [Google Scholar]
- Ban-ya, S.; Chiba, A.; Hikosaka, A. Thermodynamics of FetO–MxOy(MxOy = CaO, SiO2, TiO2, and Al2O3) binary melts in equilibrium with solid iron. Tetsu-to-Hagane 1980, 66, 1484–1493. [Google Scholar] [CrossRef]
- Timucin, M.; Muan, A. Activity–composition relations in NiAl2O4–MnAl2O4 solid solutions and stabilities of NiAl2O4 and MnAl2O4 at 1300 °C and 1400 °C. J. Am. Ceram. Soc. 1992, 75, 1399–1406. [Google Scholar] [CrossRef]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances (Supplement); Springer: New York, NY, USA, 1977; pp. 392–445. [Google Scholar]
- Itoh, H.; Hino, M.; Ban-ya, S. Deoxidation equilibrium of magnesium in liquid iron. Tetsu-to-Hagane 1997, 83, 623–628. [Google Scholar] [CrossRef]
- Huang, X.H. Theory of Iron and Steel Metallurgy, 3rd ed.; Metallurgical Industry Press: Beijing, China, 2002. [Google Scholar]
- Sun, Y.H.; Zeng, Y.N.; Xu, R.; Cai, K.K. Formation mechanism and control of MgO·Al2O3 inclusions in non-oriented silicon steel. Int. J. Min. Met. Mater. 2014, 21, 1068–1076. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.F.; Fang, W. Effect of addition of Al-based slag deoxidizer on MgO·Al2O3 inclusions in 3Si-Fe steels. Metall. Res. Technol. 2016, 114, 108–116. [Google Scholar] [CrossRef]
- Tang, H.Y.; Wu, T.; Wang, J.L.; Liang, Y.C.; Li, J. Mass action concentration model of CaO–MgO–FeO–Al2O3–SiO2 slag systems and its application to the formation mechanism of MgO·Al2O3 spinel-type inclusion in casing steel. Metall. Res. Technol. 2015, 112, 409–423. [Google Scholar] [CrossRef]





| Steels | Slag/Refractory | Inclusions | Main Finding | Ref. |
|---|---|---|---|---|
| High strength alloyed structural steel | CaO–SiO2–Al2O3–MgO | MgO·Al2O3 | log(XMgO/XAl2O3) of inclusions and log(aMgO/aAl2O3) in slag exhibiting a good linear relation. | [16] |
| High strength alloying steel | CaO–SiO2–Al2O3 | MgO·Al2O3– CaO | MgO·Al2O3 inclusions can be modified to liquid ones by high-basicity slag. | [17] |
| Ferritic stainless steel | CaO–Al2O3–MgO | MgO·Al2O3 | log(XMgO/XAl2O3) of inclusions and log(aMgO/aAl2O3) in slag showing a good linear relation. | [21] |
| Al-killed ferritic stainless steel | CaO–SiO2–Al2O3–MgO | MgO·Al2O3 | MgO contents in inclusions decreased with the declining of CaO/SiO2 and CaO/Al2O3 ratio. | [26] |
| Mn and V alloyed steel | CaO–SiO2–Al2O3–MgO– CaF2 | Spinel | The generation behavior of inclusion was influenced by aMgO in the initial slag. | [27] |
| Al-killed steel | CaO–SiO2–Al2O3–MgO– FeO | MgO·Al2O3 | The content of FeO in slag plays an important role in the formation of MgO·Al2O3 inclusions. | [28] |
| Bearing steel | CaO–SiO2–Al2O3–MgO | CaO–SiO2–Al2O3–MgO | Thermodynamic for the formation of spinel inclusions was made. | [29] |
| Fe–Mn–S–C–Al steel | CaO–Al2O3–SiO2–CaF2– MgO/MgO–C refractory | MgAl2O4 | The calculated results at different composition of slag and steel were in good agreement with the experimental results. | [30] |
| Extra-low- oxygen steel | MgO-based refractory and MgO bearing slag | MgO·Al2O3 spinel | MgO-based refractory supplied more Mg into liquid steel than refining slag. | [31] |
| Al deoxidized molten steel | MgO–C refractory | MgO·Al2O3 | An internal oxidation-reduction occurs in the MgO–C refractory at elevated temperature. | [32] |
| Al-killed molten steel | Mg–Cr refractory | MgO·Al2O3 spinel | Mg and Cr dissolved from the refractory, and lead to the increasing contents of Mg and Cr in the liquid steel. | [33] |
| Fe–Al alloy | MgO-based refractory | MgO·Al2O3 spinel | The generation of a spinel layer at the interface was attributing to oxidation–reduction reactions and phase transformation. | [34] |
| Steels | MgO-based refractory | Spinel | Improving the resistance of MgO-based refractory to slag penetration is good to improve steel cleanness. | [35] |
| Al-killed steel | MgO refractory | Spinel | The decomposing of MgO refractory plays a key role in the dissolution of MgO refractory in Al-killed steels. | [36] |
| 304 stainless steel | CaO–Al2O3-based slag | Spinel | A thermodynamic model was developed to predict slag–steel–inclusion reactions. | [37] |
| C | Si | Mn | P | S | Al | Cr | V | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.51 | 0.23 | 0.94 | 0.01 | 0.008 | 0.024 | 1.02 | 0.16 | balance |
| CaO | SiO2 | MgO | FeO | MnO | Al2O3 | CaO/Al2O3 |
|---|---|---|---|---|---|---|
| 50.19 | 8.2 | 7.49 | 0.35 | 0.18 | 33.59 | 1.49 |
| Items | Constitutional Units | Balanced Mole Number | Mass Action–Concentrations (MACs) |
|---|---|---|---|
| Simple cations and anions | |||
| Simple molecules | |||
| Complex molecules | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, J.; Zhu, H.; Zhao, D.; Xue, Z. Generation Mechanism of MgO and Al2O3 Inclusions in 51CrV4 Spring Steel Based on the Ion–Molecule Coexistence Theory. Metals 2019, 9, 830. https://doi.org/10.3390/met9080830
Lei J, Zhu H, Zhao D, Xue Z. Generation Mechanism of MgO and Al2O3 Inclusions in 51CrV4 Spring Steel Based on the Ion–Molecule Coexistence Theory. Metals. 2019; 9(8):830. https://doi.org/10.3390/met9080830
Chicago/Turabian StyleLei, Jialiu, Hangyu Zhu, Dongnan Zhao, and Zhengliang Xue. 2019. "Generation Mechanism of MgO and Al2O3 Inclusions in 51CrV4 Spring Steel Based on the Ion–Molecule Coexistence Theory" Metals 9, no. 8: 830. https://doi.org/10.3390/met9080830





