The Influence of Chemical Activators on the Hydration Behavior and Technical Properties of Calcium Sulfoaluminate Cements Blended with Ground Granulated Blast Furnace Slags
Abstract
:1. Introduction
2. Experimental Set-Up
2.1. Materials
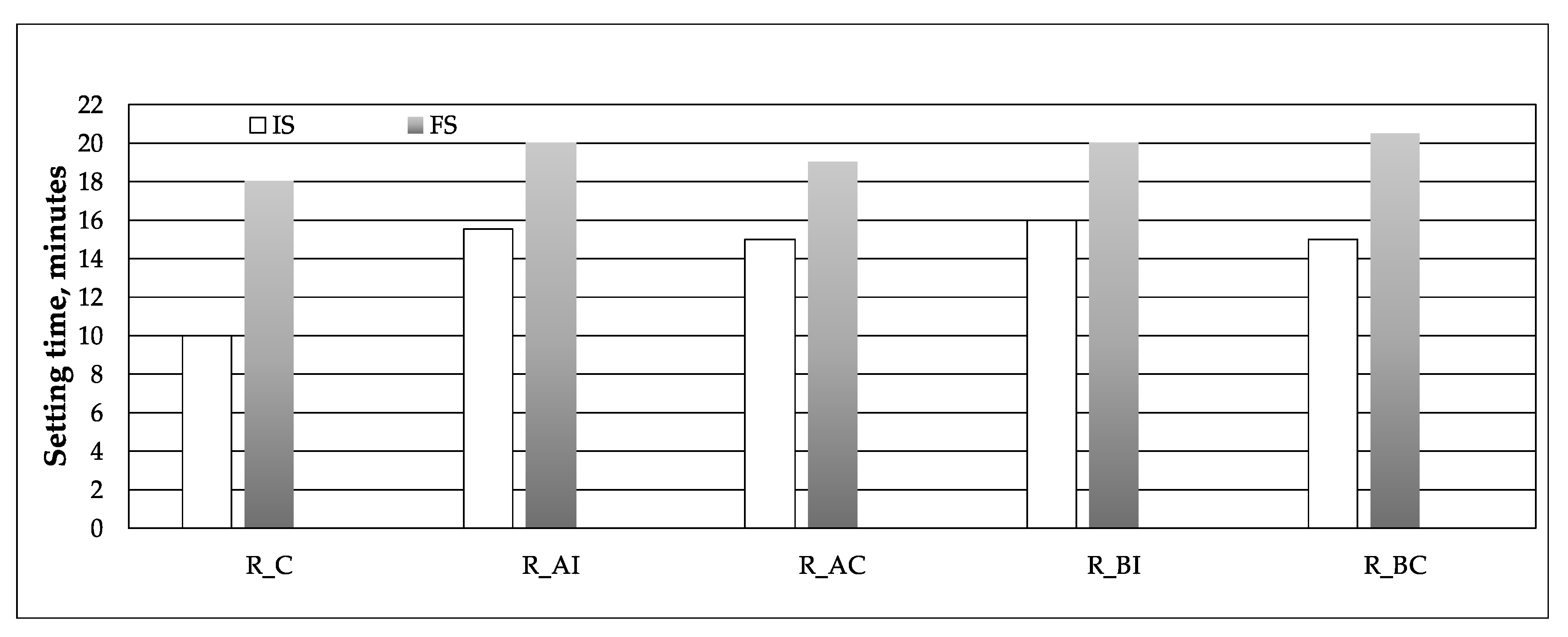
2.2. Hydration Procedures, Setting Times and Expansion/Shrinkage Measurements
2.3. Mechanical Tests
2.4. Characterization Techniques
2.4.1. X-ray Fluorescence (XRF) Analysis
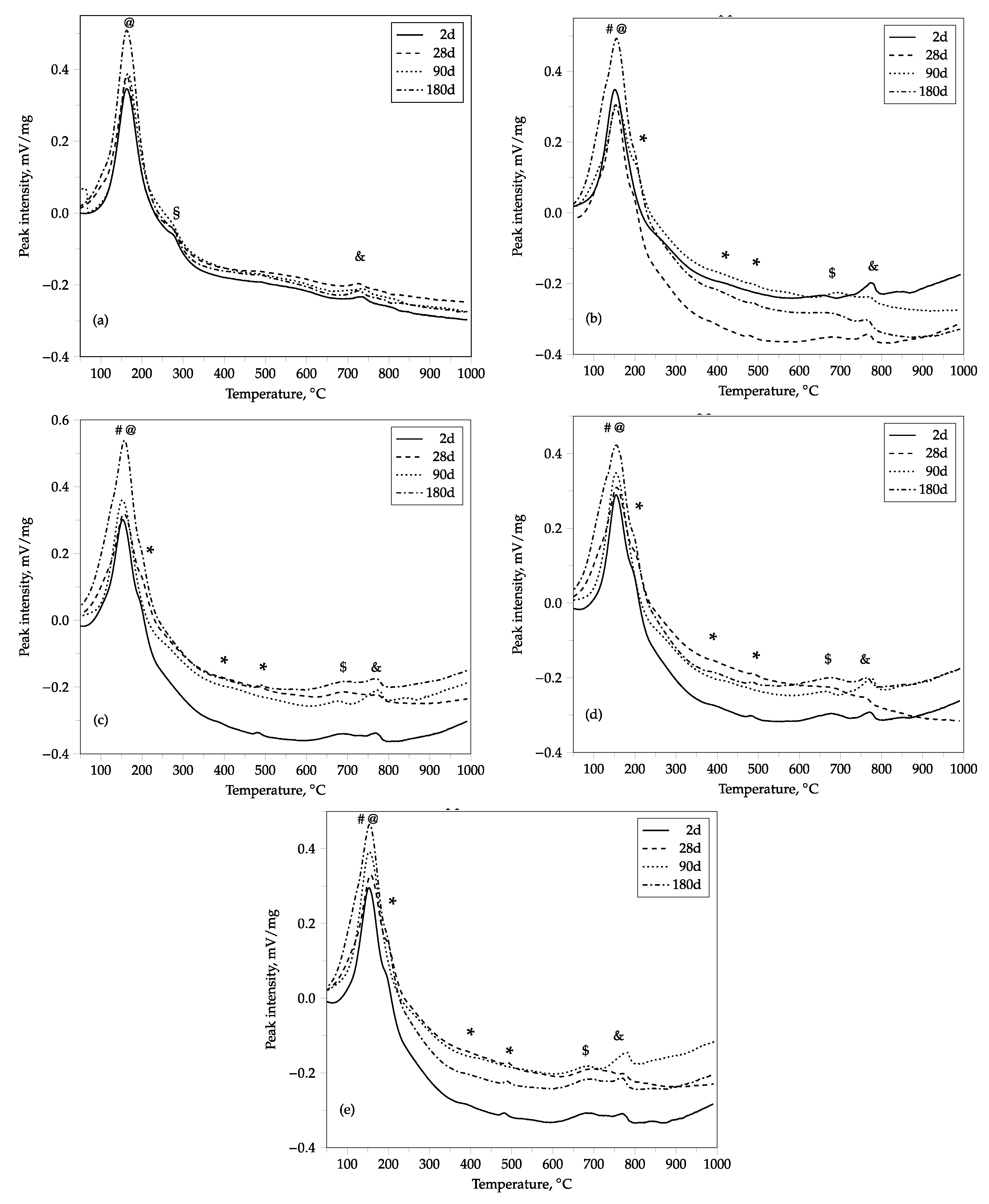
2.4.2. Simultaneous DT-TG Analysis
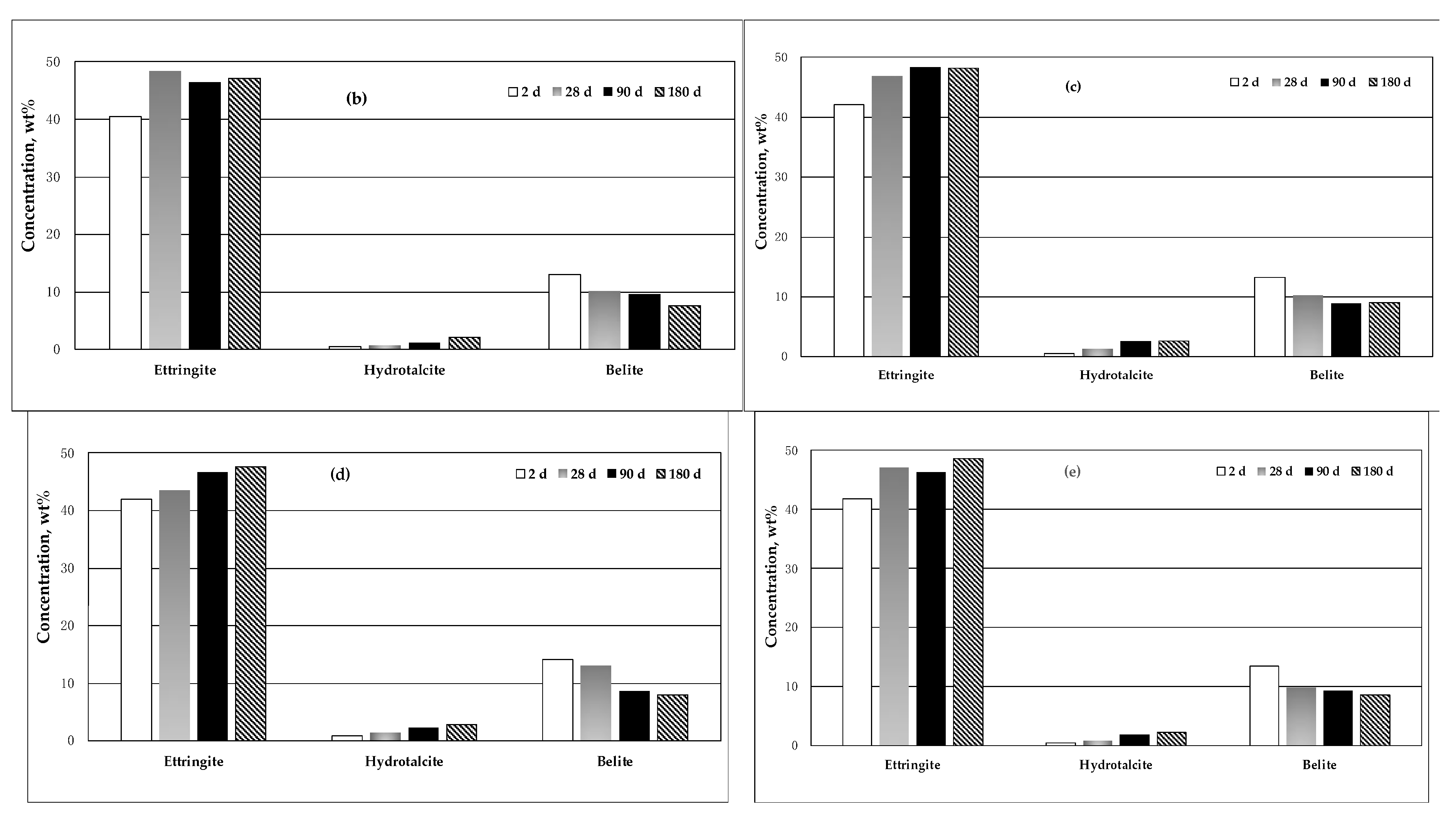
2.4.3. XRD Analysis
2.4.4. MIP Analysis
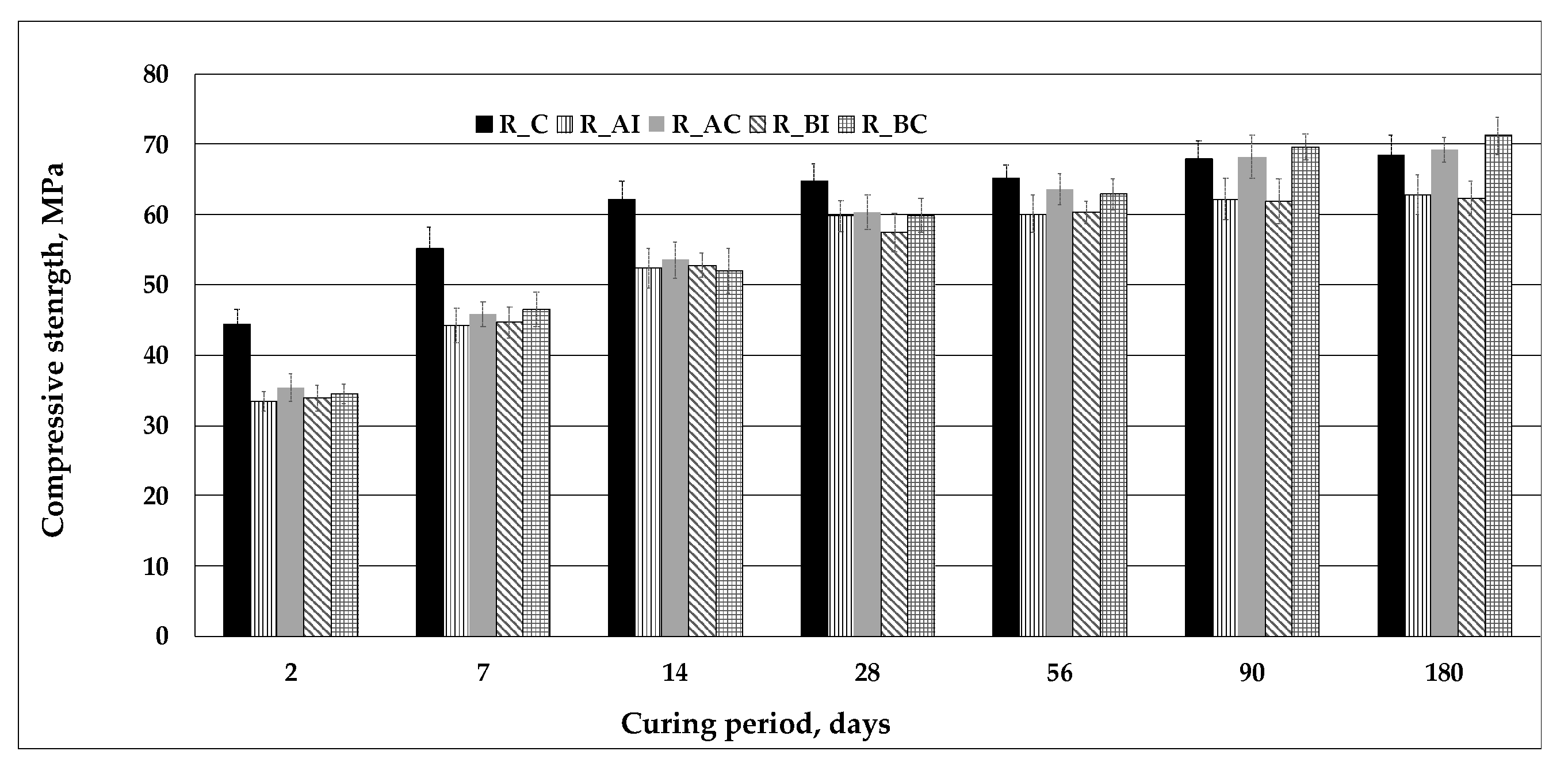
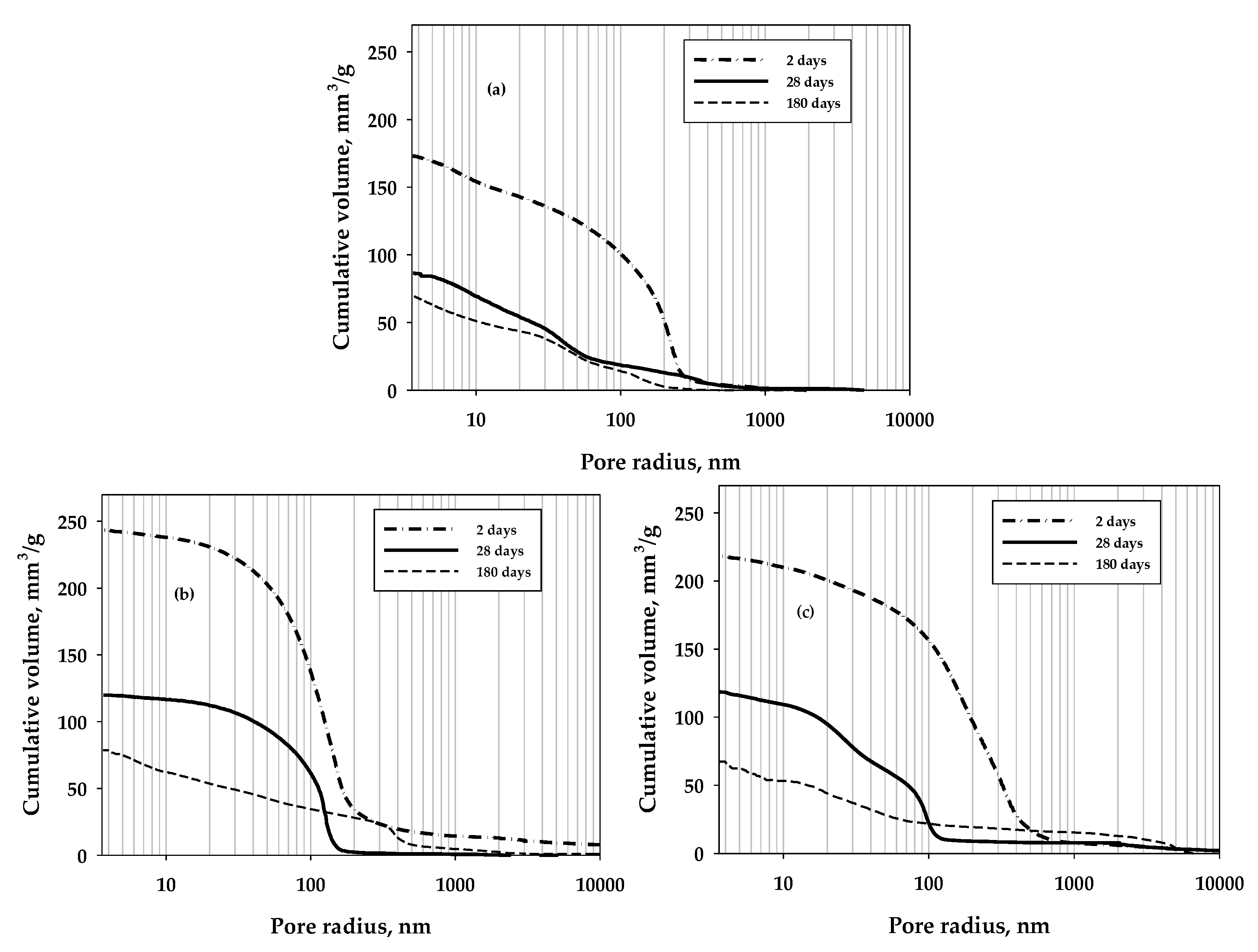
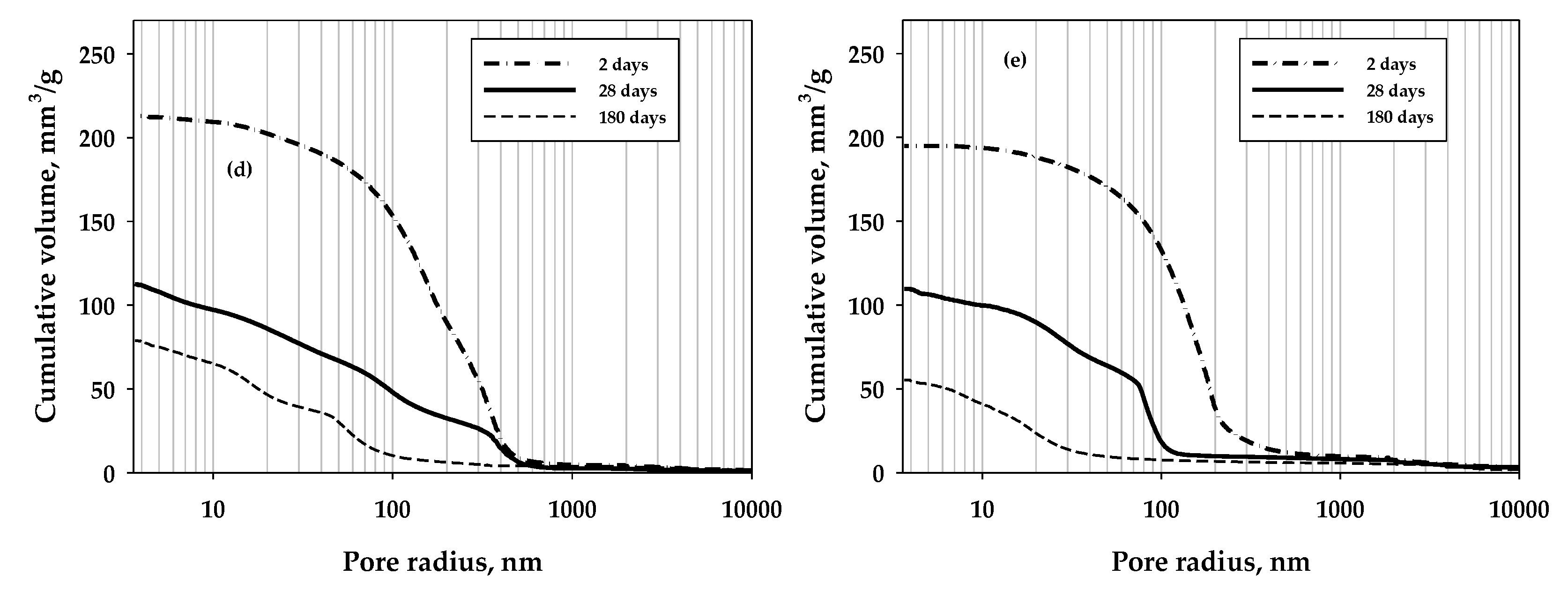
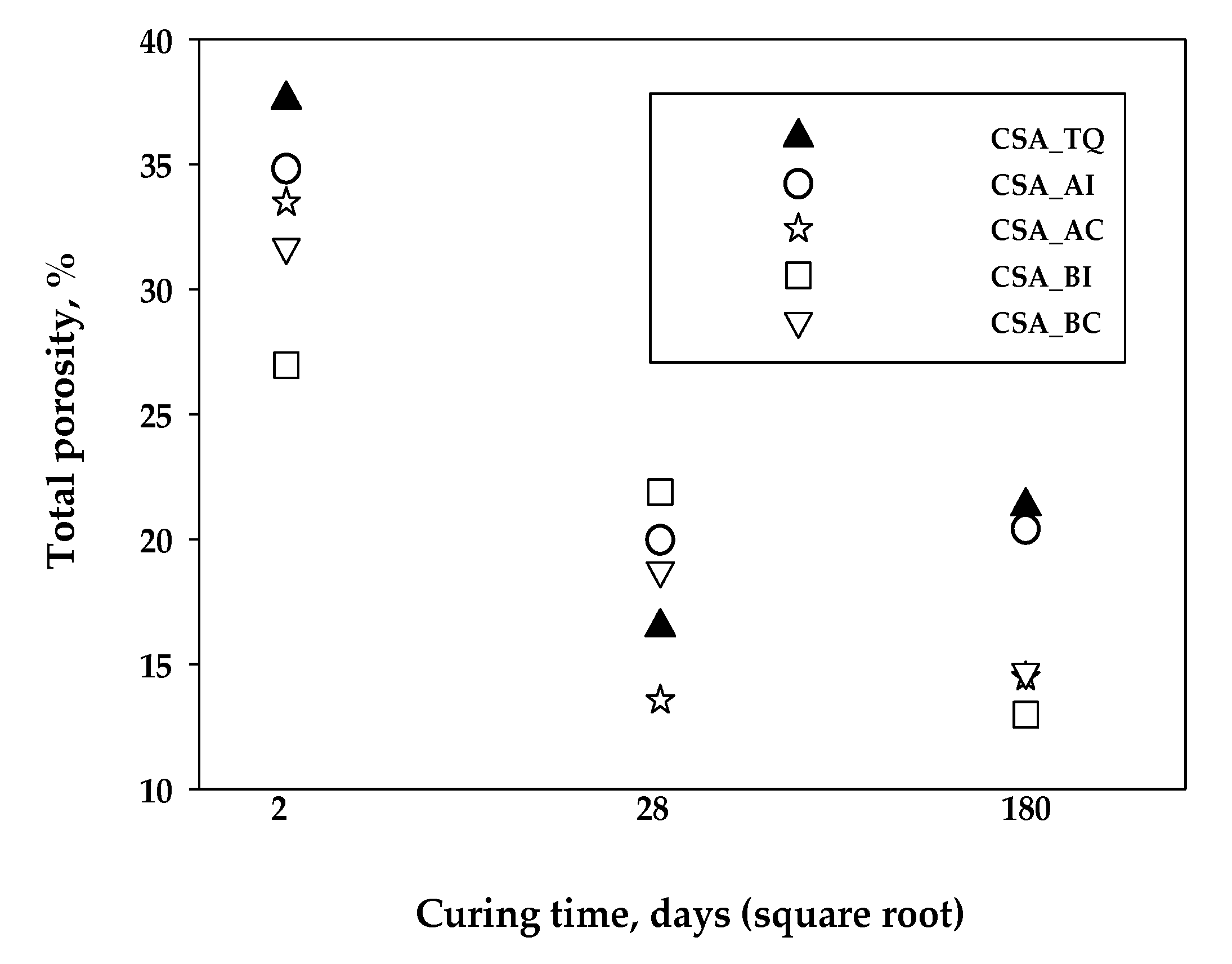
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Telesca, A.; Matschei, T.; Marroccoli, M. Study of eco-friendly belite-calcium sulfoaluminate cements obtained from special wastes. Appl. Sci. 2020, 10, 8650. [Google Scholar] [CrossRef]
- IEA. Global Energy and CO2 Emissions in 2020. Available online: https://www.iea.org/reports/global-energy-review-2020/global-energy-and-co2-emissions-in-2020 (accessed on 28 May 2021).
- Tregambi, C.; Solimene, R.; Montagnaro, F.; Salatino, P.; Marroccoli, M.; Ibris, N.; Telesca, A. Solar-driven production of lime for ordinary Portland cement formulation. Sol. Energy 2018, 173, 759–768. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Miller, S.A.; Monteiro, P.J.M.; Ostertag, C.P.; Horvath, A. Concrete mixture proportioning for desired strength and reduced global warming potential. Constr. Build. Mater. 2016, 128, 410–421. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Myers, R.J.; Lothenbach, B.; Bernal, S.A.; Provis, J.L. Thermodynamic modelling of alkali-activated slag cements. Appl. Geochem. 2015, 61, 233–247. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Alkali-Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Springer: Berlin, Germany; RILEM: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Dung, N.T.; Unluer, C. Development of MgO concrete with enhanced hydration and carbonation mechanisms. Cem. Concr. Res. 2018, 103, 160–169. [Google Scholar] [CrossRef]
- Gomez, C.M.; de Oliveira, A.D.S. Chemical phases and microstructural analysis of pastes based on magnesia cement. Con. Build. Mater. 2018, 188, 615–620. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Mobili, A.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate and alkali-activated fly ash cements as alternative to Portland cement: Study on chemical, physical-mechanical, and durability properties of mortars with the same strength class. Constr. Build. Mater. 2020, 246, 118436. [Google Scholar] [CrossRef]
- Ben Haha, M.; Winnefeld, F.; Pisch, A. Advances in understanding ye’elimite-rich cements. Cem. Concr. Res 2019, 123, 105778. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Tomasulo, M.; Valenti, G.L.; Dieter, H.; Montagnaro, F. Low-CO2 cements from fluidized bed process wastes and other industrial byproducts. Comb. Sci. Tech. 2016, 188, 492–503. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Comp. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Irico, S.; Gastaldi, D.; Canonico, F.; Magnacca, G. Investigation of the microstructural evolution of calcium sulfoaluminate cements by thermoporometry. Cem. Concr. Res. 2013, 53, 239–247. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Selçuk, N.; Soner, I.; Selçuk, E. Synthesis of special cement with fluidized bed combustion ashes. Adv. Cem. Res. 2010, 22, 107–113. [Google Scholar] [CrossRef]
- Marroccoli, M.; Pace, M.L.; Telesca, A.; Valenti, G.L. Synthesis of Calcium sulfoaluminate cements from Al2O3-rich by-products from Aluminium Manufacture. In Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; Zachar, J., Claisse, P., Naik, T.R., Ganjiam, E., Eds.; UWM Center for By-Products Utilization: Milwaukee, WI, USA, 2010; p. 161. [Google Scholar]
- Marroccoli, M.; Montagnaro, F.; Pace, M.L.; Valenti, G.L. Use of fluidized bed combustion ash and other industrial wastes as raw materials for the manufacture of calcium sulphoaluminate cements. In Proceedings of the 20th International Conference on Fluidized Bed Combustion, Xi’an, China, 18–21 May 2009; Yue, G., Zhang, H., Zhao, C., Luo, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 389–395. [Google Scholar]
- Chaunsali, P.; Vaishnav, K. Calcium-sulfoaluminate-belite cements: Opportunities and challenges. Indian Concr. J. 2020, 94, 18–25. [Google Scholar]
- Telesca, A.; Marroccoli, M.; Winnefeld, F. Synthesis and characterization of calcium sulfoaluminate cements produced by different chemical gypsums. Adv. Cem. Res. 2019, 31, 113–123. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.J.; Müller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Const. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]
- Gallardo-Heredia, M.; Almanza-Robles, J.M.; Magallanes-Rivera, R.X.; Cortes-Hernández, D.A.; Escobedo-Bocardo, J.C.; Avila-López, U. Calcium sulfoaluminate cement pastes from industrial wastes: Effect of hemihydrate content. Mater. Struct. 2017, 50, 93. [Google Scholar] [CrossRef]
- Garcia-Mate, M.; De la Torre, A.G.; Leon-Reina, L.; Losilla, E.R.; Aranda, M.A.G.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Alvarez-Pinazo, G.; Santacruz, I.; Aranda, M.A.G.; De la Torre, A.G. Hydration of belite-ye’elimite-ferrite cements with different calcium sulfate sources. Adv. Cem. Res. 2016, 28, 529–543. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Telesca, A.; Marchon, D.; Xu, K.; Marroccoli, M.; Jiang, Z.; Monteiro, P.J.M. Effect of polycarboxylate ether on the expansion of ye’elimite hydration in the presence of anhydrite. Cem. Concr. Res. 2021, 140, 106321. [Google Scholar] [CrossRef]
- Zhang, L. Microstructure and Performance of Calcium Sulfoaluminate Cements. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2000. [Google Scholar]
- Zhang, L.; Su, M.; Wang, Y. The third cement series in China. World Cem. 1994, 25, 6–10. [Google Scholar]
- Zhang, L.; Su, M.Z.; Wang, Y.M. Development of the use of sulfo-and ferroaluminate cements in China. Adv. Cem. Res. 1999, 11, 15–21. [Google Scholar] [CrossRef]
- Mobili, A.; Belli, A.; Giosué, C.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate, geopolimeric, and cementitious mortars for structural applications. Environment 2017, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Oh, J.E.; Jun, Y.; Park, J.; Ha, J. Influence of four additional activators on hydrated-lime [Ca(OH)2] activated ground granulated blast-furnace slag. Cem. Concr. Comp. 2016, 65, 1–10. [Google Scholar] [CrossRef]
- Kolani, B.; Buffo-Lacarrière, L.; Sellier, A.; Escadeillas, G.; Boutillon, L.; Linger, L. Hydration of slag-blended cement. Cem. Concr. Comp. 2012, 34, 1009–1018. [Google Scholar] [CrossRef]
- Sajedi, F.; Abdul Razak, H. Comparison of different methods for activation of ordinary Portland cement-slag mortars. Constr. Build. Mater. 2011, 25, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Matthes, W.; Vollpracht, A.; Villagrán, Y.; Kamali-Bernard, S.; Hooton, D.; Gruyaert, E.; Soutsos, M.; De Belie, N. Ground granulated blast-furnace slag. RILEM State Art Rep. 2018, 25, 1–53. [Google Scholar] [CrossRef]
- Yuksel, I. Blast-furnace slag. Waste Suppl. Cem. Mater. Concr. 2018, 361–415. [Google Scholar] [CrossRef]
- Cardinale, T.; D’Amato, M.; Sulla, R.; Cardinale, N. Mechanical and Physical Characterization of Papercrete as New Eco-Friendly Construction Material. Appl. Sci. 2021, 11, 1011. [Google Scholar] [CrossRef]
- Diaz-Loya, I.; Juenger, M.; Seraj, S.; Minkara, R. Extending supplementary cementitious material resources: Reclaimed and remediated fly ash and natural pozzolans. Cem. Concr. Compos. 2019, 101, 44–51. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, understanding and unlocking supplementary cementitious materials. RILEM Tech. Lett. 2016, 1, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Z.; Meng, Y.; Tang, J.; Yang, L. Effect of Flue Gas Desulfurization Gypsum on the Properties of Calcium Sulfoaluminate Cement Blended with Ground Granulated Blast Furnace Slag. Materials 2021, 14, 382. [Google Scholar] [CrossRef]
- Kim, T.; Ki_Young, Y.S.; Kang, C.; Lee, T.-K. Development of Eco-Friendly Cement Using a Calcium Sulfoaluminate Expansive Agent Blended with Slag and Silica Fume. Appl. Sci. 2021, 11, 394. [Google Scholar] [CrossRef]
- Yoon, H.N.; Seo, J.; Kim, S.; Lee, H.K.; Park, S. Hydration of calcium sulfoaluminate cement blended with blast-furnace slag. Const. Build. Mater. 2021, 268, 121214. [Google Scholar] [CrossRef]
- Bertola, F.; Gastaldi, D.; Canonico, F.; Paul, G. CSA and slag: Towards CSA composite binders. Adv. Cem. Res. 2019, 31, 147–158. [Google Scholar] [CrossRef]
- Gao, D.; Meng, Y.; Yang, L.; Tang, J.; Lv, M. Effect of ground granulated blast furnace slag on the properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 227, 116665. [Google Scholar] [CrossRef]
- Jun, Y.; Hong Kim, J.; Kim, T. Hydration of calcium sulfoaluminate-based binder incorporating red mud and silica fume. Appl. Sci. 2019, 9, 2270. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.H.J.; Winnefeld, F.; Tschopp, E.; Müller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Contribution of limestone to the hydration of calcium sulfoaluminate cement. Cem. Concr. Comp. 2015, 62, 204–211. [Google Scholar] [CrossRef]
- Hargis, G.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (ye’elimite) hydration in the presence of gypsum, calcite and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Aranda, M.A.; Santacruz, I. Hydration studies of calcium sulfoaluminate cements blended with fly ash. Cem. Concr. Compos. 2013, 54, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Telesca, A.; Ibris, N.; Marroccoli, M. Use of potabilized water sludge in the production of low-energy blended calcium sulfoaluminate cements. Appl. Sci. 2021, 11, 1679. [Google Scholar] [CrossRef]
- Samui, P.; Kim, D.; Iyer, N.R.; Chaudhary, S. New Materials in Civil Engineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Academic Press: London, UK, 1997; p. 480. [Google Scholar] [CrossRef]

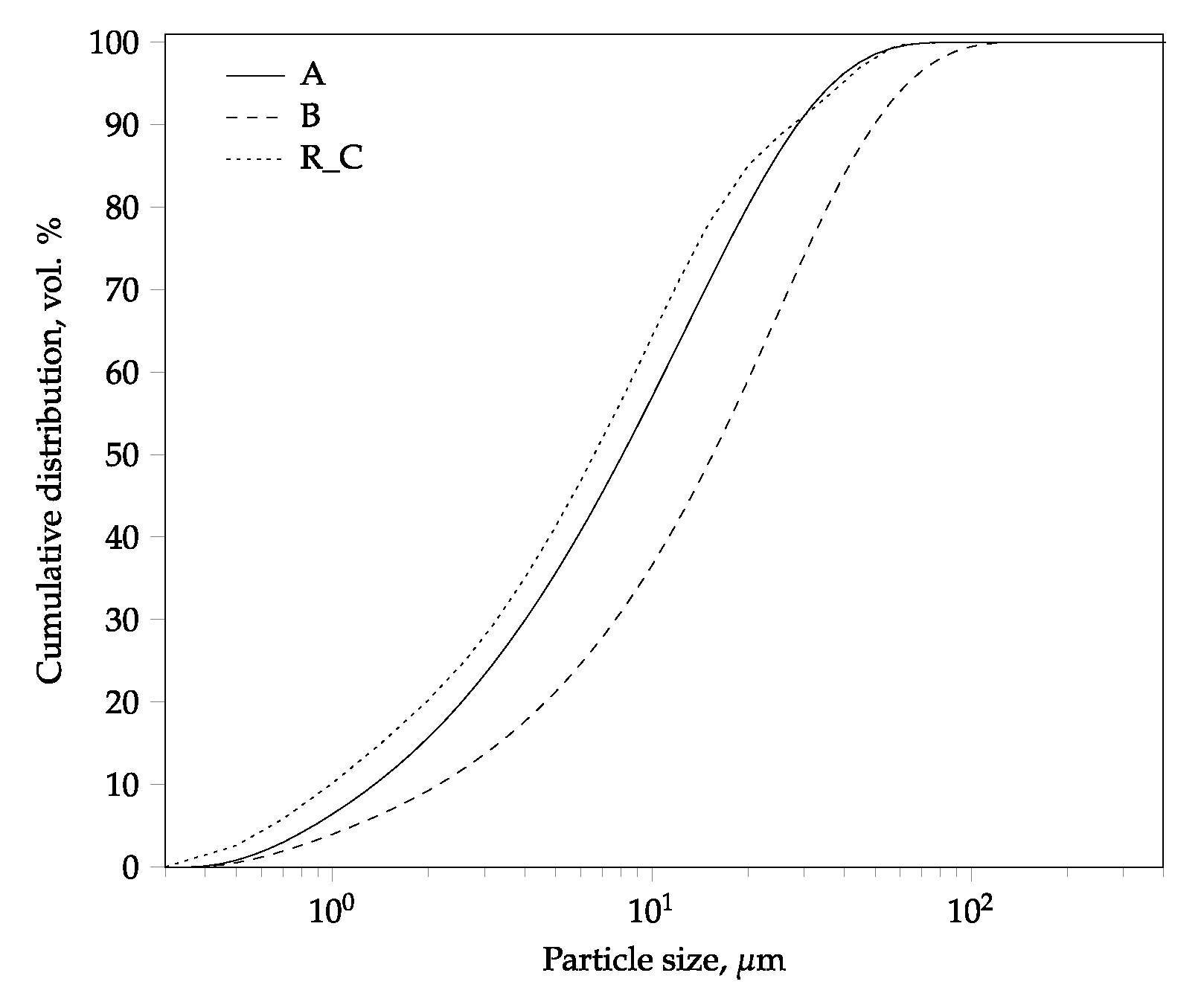




| Chemical Composition | Mineralogical Phase Composition | |||||
|---|---|---|---|---|---|---|
| R_C | A | B | R_C | ICDD Ref. Number | ||
| CaO | 44.58 | 41.02 | 37.32 | Ye’elimite | 30-0256 | 43.0 |
| SiO2 | 8.95 | 35.27 | 35.28 | β-belite | 33-0302 | 21.7 |
| Al2O3 | 22.42 | 11.85 | 11.17 | Celite | 38-1429 | 3.8 |
| Fe2O3 | 1.86 | 0.88 | 1.03 | Anhydrite | 37-1496 | 19.1 |
| TiO2 | 1.10 | 0.52 | 0.53 | Calcite | 05-0586 | 1.1 |
| K2O | 0.30 | 0.58 | 0.34 | Brownmillerite | 30-0256 | 4.5 |
| MnO | 0.08 | 0.33 | 0.75 | Gehlenite | 73-2041 | 1.6 |
| Na2O | 0.08 | 0.31 | 0.42 | Others | - | 5.2 |
| MgO | 0.94 | 7.15 | 10.55 | |||
| Cl− | 0.07 | - | - | |||
| SO3 | 16.85 | 0.33 | 1.80 | |||
| P2O5 | 0.05 | - | - | |||
| l.o.i * | 2.16 | 1.10 | 0.70 | |||
| Total | 9.44 | 99.59 | 99.89 | Total | 100.0 | |
| AI | AC | BI | BC | R_AI | R_AC | R_BI | R_BC | |
|---|---|---|---|---|---|---|---|---|
| R_C | - | 80.0 | 80.0 | 80.0 | 80.0 | |||
| A | 92.0 | 92.0 | - | - | 18.4 | 18.4 | - | - |
| B | - | - | 92.0 | 92.0 | - | - | 18.4 | 18.4 |
| I | 8.0 | - | 8.0 | - | 1.6 | - | 1.6 | - |
| C | - | 8.0 | - | 8.0 | - | 1.6 | - | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marroccoli, M.; Telesca, A. The Influence of Chemical Activators on the Hydration Behavior and Technical Properties of Calcium Sulfoaluminate Cements Blended with Ground Granulated Blast Furnace Slags. Buildings 2021, 11, 268. https://doi.org/10.3390/buildings11070268
Marroccoli M, Telesca A. The Influence of Chemical Activators on the Hydration Behavior and Technical Properties of Calcium Sulfoaluminate Cements Blended with Ground Granulated Blast Furnace Slags. Buildings. 2021; 11(7):268. https://doi.org/10.3390/buildings11070268
Chicago/Turabian StyleMarroccoli, Milena, and Antonio Telesca. 2021. "The Influence of Chemical Activators on the Hydration Behavior and Technical Properties of Calcium Sulfoaluminate Cements Blended with Ground Granulated Blast Furnace Slags" Buildings 11, no. 7: 268. https://doi.org/10.3390/buildings11070268






