Study of Geopolymer Composites Based on Volcanic Ash, Fly Ash, Pozzolan, Metakaolin and Mining Tailing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Geopolymer Synthesis
3. Results and Discussion
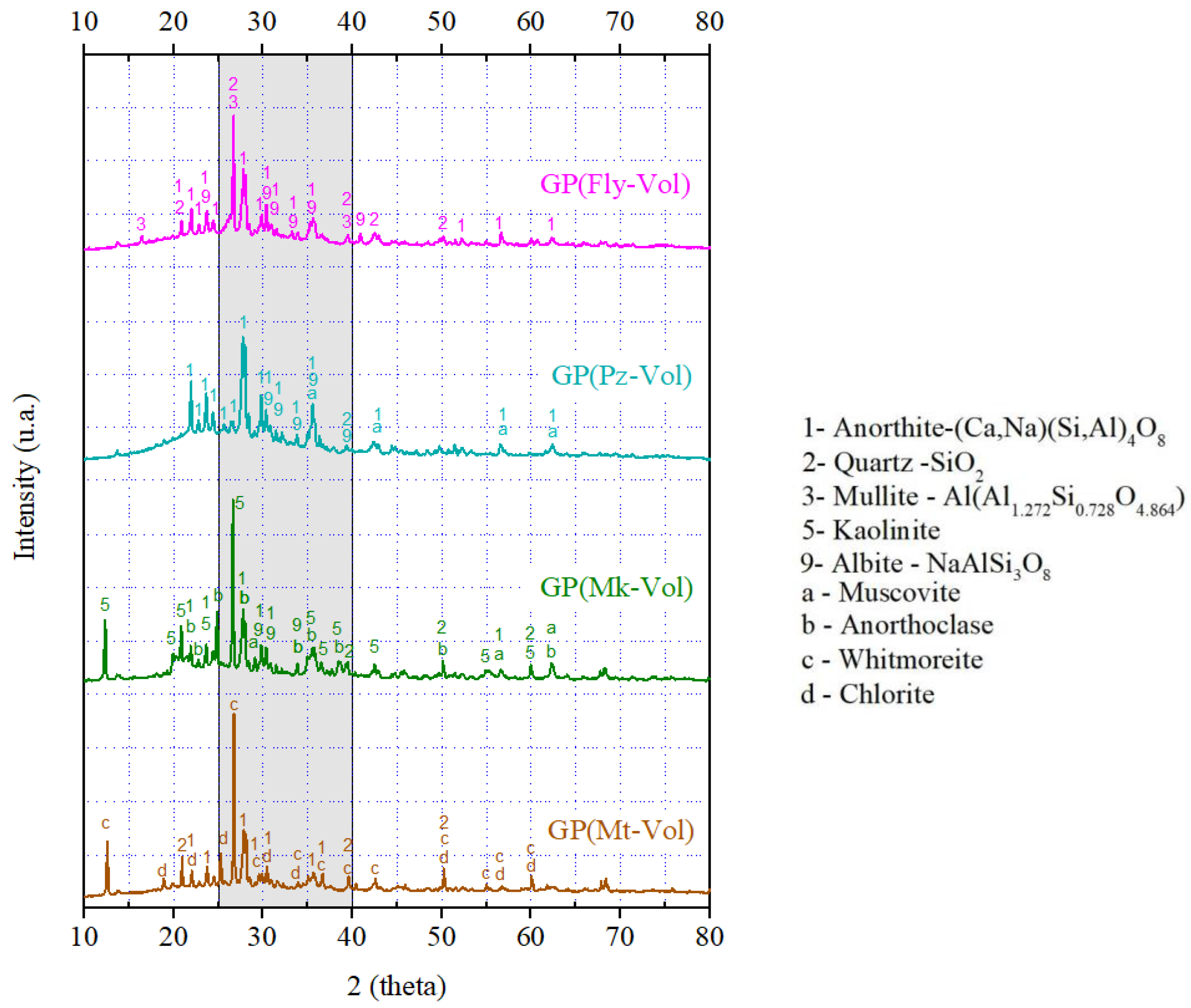
3.1. X-ray Diffraction Patterns of Geopolymers
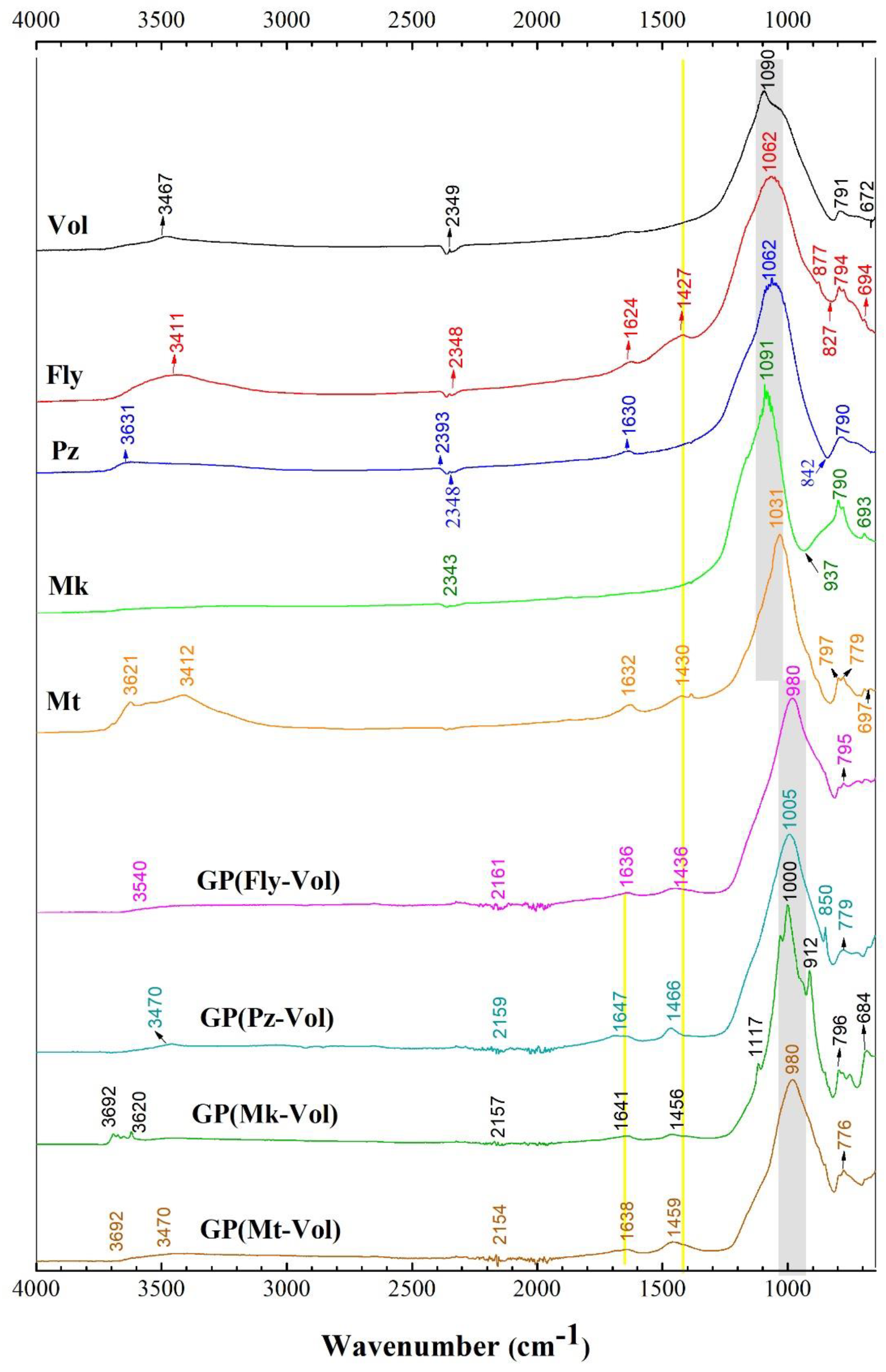
3.2. FTIR Spectra
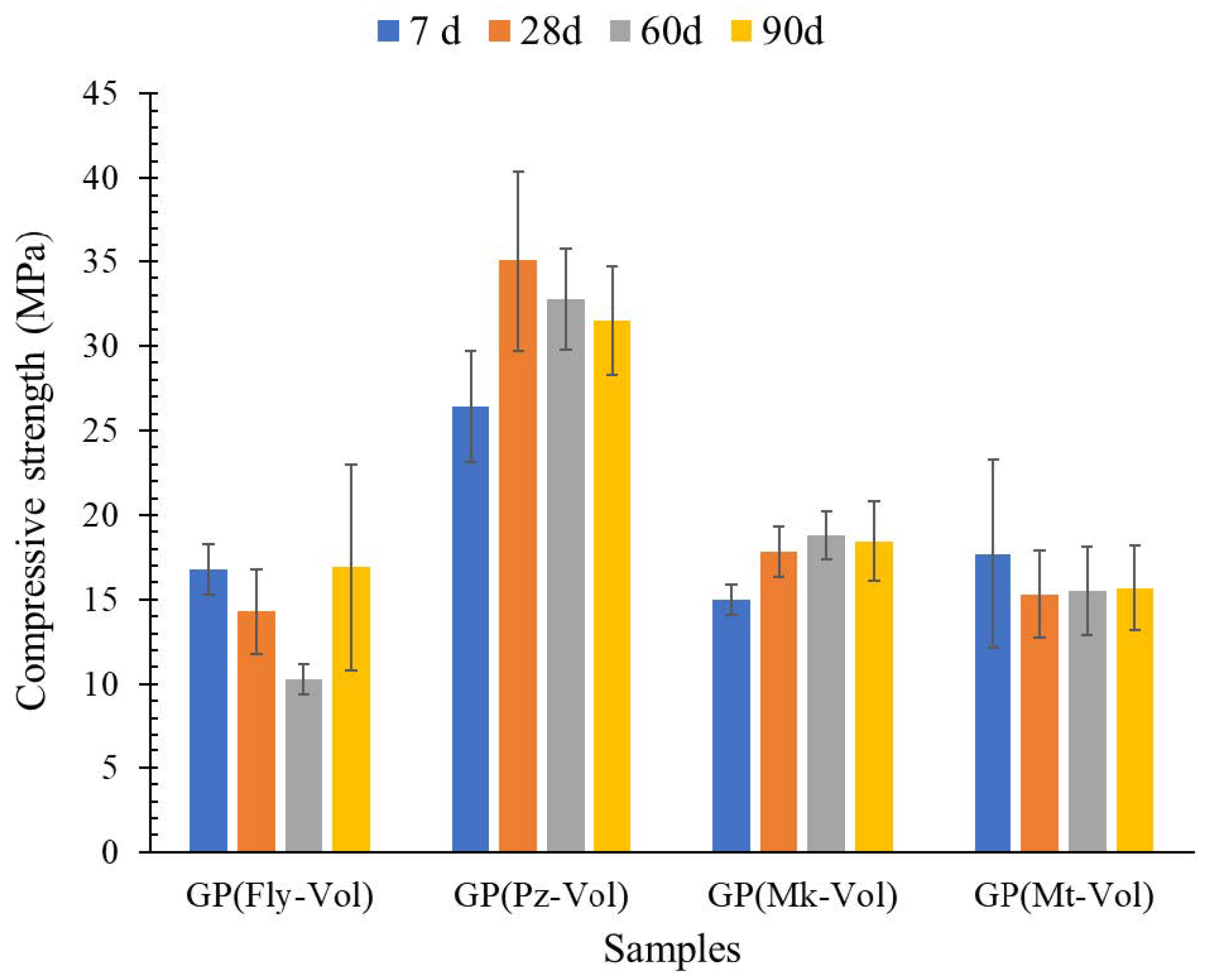
3.3. Compression Strength
4. Conclusions
- It has been shown that it is possible to use mixtures of natural waste (volcanic ash), hazardous solid waste (fly ash and mining tailings), natural material (pozzolan), and metakaolin as raw materials for the production of geopolymers. According to XRD and FTIR analysis, the final products are identified as geopolymer compounds. Additionally, it has been seen that the combination of precursor materials has an influence on the geopolymerization, and the variation trend of the amorphous phase contained in the geopolymers was detected by qualitative XRD analysis.
- The FTIR analysis revealed that the main band located in the range of 1014–1042 cm−1 proves geopolymerization related to Si-O-Si (Al) bonds and the increase in the amount of SiO2. The main band (related to N-A-S-H gel) shifts to a lower wavenumber. Additionally, it was observed that a change took place in the intensity and location in the range of 1417–1440 cm−1 of the groups related to the carbonate vibration which is probably detrimental to the mechanical properties that tend to cause cracks or micro-cracks on the geopolymer.
- The XRD analysis showed that the geopolymer composites have amorphous and semi-crystalline phases even in the raw materials that had crystalline phases. Among the four geopolymers, the GP(Pz-Vol) geopolymer had the highest amount of amorphous glassy phase, it has been seen that this promotes the development of the degree of geopolymerization and, therefore, the highest achieved mechanical strength. This is probably due to the unreacted phases that could act as microaggregates or fillers by reinforcing the geopolymer matrix, resulting in a compact and high-strength structure
- Additionally, a strong correlation was found between the strength values of the mortars and the SiO2/Al2O3 ratios obtained from the precursor mixtures. Thus, the compressive strength of the GP(Pz-Vol) reached values of 35.1 MPa, with this mixture having a value of 7.09 SiO2/Al2O3 ratio. This value obtained for GP(Pz-Vol) geopolymer is even higher than the reported for Portland cement mortar (29.36 ± 2.57 MPa), so this mortar could even show better values and applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Ceramic-like Inorganic Polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar] [CrossRef]
- Tchakoute Kouamo, H.; Elimbi, A.; Mbey, J.A.; Ngally Sabouang, C.J.; Njopwouo, D. The Effect of Adding Alumina-Oxide to Metakaolin and Volcanic Ash on Geopolymer Products: A Comparative Study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Zongjin, L. Composition Design and Microstructural Characterization of Calcined Kaolin-Based Geopolymer Cement. Appl. Clay Sci. 2010, 47, 271–275. [Google Scholar] [CrossRef]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH Solution on the Synthesis of Fly Ash Geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Bondar, D.; Lynsdale, C.J.; Milestone, N.B.; Hassani, N.; Ramezanianpour, A.A. Effect of Adding Mineral Additives to Alkali-Activated Natural Pozzolan Paste. Constr. Build. Mater. 2011, 25, 2906–2910. [Google Scholar] [CrossRef]
- Tekin, I. Properties of NaOH Activated Geopolymer with Marble, Travertine and Volcanic Tuff Wastes. Constr. Build. Mater. 2016, 127, 607–617. [Google Scholar] [CrossRef]
- Churata, R.; Almirón, J.; Roudet, F.; Bautista, A.; Torres-Carrasco, M.; Tupayachy-Quispe, D. Influence of the Activating Solution on the Mechanical Properties of Compacted Volcanic Ash Based Geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1150, 012011. [Google Scholar] [CrossRef]
- Tupayachy-Quispe, D.; Almirón, J.; Apaza, F.; Churata, R.; Paredes, E.; Torres-Carrasco, M.; Bautista, A. Peruvian Volcanic Ashes as New Alternative Material in Geopolymer Preparation: Influence of Dissolution Concentration and Wear Resistance. Proc. LACCEI Int. Multi-Conf. Eng. Educ. Technol. 2020, 27–31. [Google Scholar] [CrossRef]
- Calderón, N.; Vargas, M.; Almirón, J.; Bautista, A.; Velasco, F.; Tupayachy-Quispe, D. Influence of the Activating Solution and Aggregates in the Physical and Mechanical Properties of Volcanic Ash Based Geopolymer Mortars. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1054, 012003. [Google Scholar] [CrossRef]
- Ma, C.K.; Awang, A.Z.; Omar, W. Structural and Material Performance of Geopolymer Concrete: A Review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Alnahhal, M.F.; Alengaram, U.J.; Jumaat, M.Z.; Abutaha, F.; Alqedra, M.A.; Nayaka, R.R. Assessment on Engineering Properties and CO2 Emissions of Recycled Aggregate Concrete Incorporating Waste Products as Supplements to Portland Cement. J. Clean. Prod. 2018, 203, 822–835. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean Production and Properties of Geopolymer Concrete; A Review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential Applications of Geopolymer Concrete in Construction: A Review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Song, D.; Huang, T.; Fang, Q.; Liu, A.; Gu, Y.F.; Liu, Y.Q.; Liu, L.F.; Zhang, S.W. Feasibility Exploration on the Geopolymerization Activation of Volcanic Tuff, Parametrical Optimization, and Reaction Mechanisms. J. Mater. Res. Technol. 2021, 11, 618–632. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Tarallo, O.; Ferone, C.; Colangelo, F.; Roviello, V.; Cioffi, R. Innovative Fly Ash Geopolymer-Epoxy Composites: Preparation, Microstructure and Mechanical Properties. Materials 2016, 9, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhimova, N.R.; Rakhimov, R.Z. Toward Clean Cement Technologies: A Review on Alkali-Activated Fly-Ash Cements Incorporated with Supplementary Materials. J. Non-Cryst. Solids 2019, 509, 31–41. [Google Scholar] [CrossRef]
- Haddaji, Y.; Majdoubi, H.; Mansouri, S.; Tamraoui, Y.; el bouchti, M.; Manoun, B.; Oumam, M.; Hannache, H. Effect of Synthetic Fibers on the Properties of Geopolymers Based on Non-Heat Treated Phosphate Mine Tailing. Mater. Chem. Phys. 2021, 260, 124147. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A. A Critical Review on Application of Alkali Activated Slag as a Sustainable Composite Binder. Case Stud. Constr. Mater. 2019, 11, e00268. [Google Scholar] [CrossRef]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of Metakaolin-Based Geopolymer Concrete for Different Mix Design Parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Pradhan, P.; Panda, S.; Kumar Parhi, S.; Kumar Panigrahi, S. Factors Affecting Production and Properties of Self-Compacting Geopolymer Concrete—A Review. Constr. Build. Mater. 2022, 344, 128174. [Google Scholar] [CrossRef]
- Atabey, İ.İ.; Karahan, O.; Bilim, C.; Atiş, C.D. The Influence of Activator Type and Quantity on the Transport Properties of Class F Fly Ash Geopolymer. Constr. Build. Mater. 2020, 264, 120268. [Google Scholar] [CrossRef]
- Aygörmez, Y. Assessment of Performance of Metabentonite and Metazeolite-Based Geopolymers with Fly Ash Sand Replacement. Constr. Build. Mater. 2021, 302, 124423. [Google Scholar] [CrossRef]
- Jamil, N.H.; al Bakri Abdullah, M.M.; Pa, F.C.; Mohamad, H.; Ibrahim, W.M.A.W.; Chaiprapa, J. Influences of SiO2, Al2O3, CaO and MgO in Phase Transformation of Sintered Kaolin-Ground Granulated Blast Furnace Slag Geopolymer. J. Mater. Res. Technol. 2020, 9, 14922–14932. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, W.; Shi, Y. The Effects of Alkaline Dosage and Si/Al Ratio on the Immobilization of Heavy Metals in Municipal Solid Waste Incineration Fly Ash-Based Geopolymer. Chemosphere 2010, 79, 665–671. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of Geopolymer Concrete for a Cleaner and Sustainable Environment – A Review of Mechanical Properties and Microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Colangelo, F.; Cioffi, R. Low Temperature Alkaline Activation of Weathered Fly Ash: Influence of Mineral Admixtures on Early Age Performance. Constr. Build. Mater. 2015, 86, 169–177. [Google Scholar] [CrossRef]
- Çelikten, S.; Erdoğan, G. Effects of Perlite/Fly Ash Ratio and the Curing Conditions on the Mechanical and Microstructural Properties of Geopolymers Subjected to Elevated Temperatures. Ceram. Int. 2022, in press. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly Ash-Based Eco-Friendly Geopolymer Concrete: A Critical Review of the Long-Term Durability Properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Moussallam, Y.; Peters, N.; Masias, P.; Apaza, F.; Barnie, T.; Ian Schipper, C.; Curtis, A.; Tamburello, G.; Aiuppa, A.; Bani, P.; et al. Magmatic Gas Percolation through the Old Lava Dome of El Misti Volcano. Bull. Volcanol. 2017, 79, 1–11. [Google Scholar] [CrossRef]
- Rivera, M.; Thouret, J.C.; Samaniego, P.; le Pennec, J.L. The 2006-2009 Activity of the Ubinas Volcano (Peru): Petrology of the 2006 Eruptive Products and Insights into Genesis of Andesite Magmas, Magma Recharge and Plumbing System. J. Volcanol. Geotherm. Res. 2014, 270, 122–141. [Google Scholar] [CrossRef]
- Rivera, M.; Thouret, J.C.; Mariño, J.; Berolatti, R.; Fuentes, J. Characteristics and Management of the 2006-2008 Volcanic Crisis at the Ubinas Volcano (Peru). J. Volcanol. Geotherm. Res. 2010, 198, 19–34. [Google Scholar] [CrossRef]
- Yadav, A.L.; Sairam, V.; Srinivasan, K.; Muruganandam, L. Synthesis and Characterization of Geopolymer from Metakaolin and Sugarcane Bagasse Ash. Constr. Build. Mater. 2020, 258, 119231. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Zhang, H.; Li, M.; Wu, Y.; Guo, L.; Wang, W.; Duan, P.; Zhang, W.; Zhang, Z. Thermal Stability and Microstructure of Metakaolin-Based Geopolymer Blended with Rice Husk Ash. Appl. Clay Sci. 2020, 196, 105769. [Google Scholar] [CrossRef]
- Zain, M.F.M.; Islam, M.N.; Mahmud, F.; Jamil, M. Production of Rice Husk Ash for Use in Concrete as a Supplementary Cementitious Material. Constr. Build. Mater. 2011, 25, 798–805. [Google Scholar] [CrossRef]
- Sontia Metekong, J.V.; Kaze, C.R.; Deutou, J.G.; Venyite, P.; Nana, A.; Kamseu, E.; Melo, U.C.; Tatietse, T.T. Evaluation of Performances of Volcanic-Ash-Laterite Based Blended Geopolymer Concretes: Mechanical Properties and Durability. J. Build. Eng. 2021, 34, 101935. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Utilization of Cement Kiln Dust (CKD) to Enhance Mine Tailings-Based Geopolymer Bricks. Constr. Build. Mater. 2013, 40, 1002–1011. [Google Scholar] [CrossRef]
- Yaseri, S.; Masoomi Verki, V.; Mahdikhani, M. Utilization of High Volume Cement Kiln Dust and Rice Husk Ash in the Production of Sustainable Geopolymer. J. Clean. Prod. 2019, 230, 592–602. [Google Scholar] [CrossRef]
- Sore, S.O.; Messan, A.; Prud’homme, E.; Escadeillas, G.; Tsobnang, F. Synthesis and Characterization of Geopolymer Binders Based on Local Materials from Burkina Faso – Metakaolin and Rice Husk Ash. Constr. Build. Mater. 2016, 124, 301–311. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, H.; Zhang, Z.; Wu, Q. Effect of Rice Husk Ash Addition on the Compressive Strength and Thermal Stability of Metakaolin Based Geopolymer. Constr. Build. Mater. 2019, 222, 872–881. [Google Scholar] [CrossRef]
- Kusbiantoro, A.; Nuruddin, M.F.; Shafiq, N.; Qazi, S.A. The Effect of Microwave Incinerated Rice Husk Ash on the Compressive and Bond Strength of Fly Ash Based Geopolymer Concrete. Constr. Build. Mater. 2012, 36, 695–703. [Google Scholar] [CrossRef]

| Oxide | Vol | Fly | Pz | Mk | Mt |
|---|---|---|---|---|---|
| SiO2 | 44.91 | 44.44 | 53.11 | 42.78 | 45.54 |
| Al2O3 | 13.30 | 25.75 | 10.16 | 30.91 | 12.99 |
| Fe2O3 | 6.67 | 4.54 | 1.49 | 0.06 | 9.25 |
| CaO | 5.14 | 3.14 | 1.14 | 0.09 | 3.13 |
| MgO | 3.55 | 1.27 | 0.25 | 0.00 | 2.96 |
| K2O | 1.47 | 1.80 | 2.68 | 0.58 | 1.12 |
| Na2O | 0.26 | 0.01 | 0.01 | 0.01 | 0.01 |
| P2O5 | 0.37 | 0.14 | 0.09 | 0.24 | 0.12 |
| SO3 | 0.36 | 0.54 | 0.09 | 0.24 | 0.89 |
| TiO2 | 1.07 | 1.13 | 0.22 | 0.35 | 0.41 |
| CuO | 0.00 | 0.01 | 0.00 | 0.00 | 0.44 |
| LOI * | 22.39 | 16.75 | 30.37 | 22.80 | 21.93 |
| Formulation Label | SiO2 | Al2O3 | SiO2/Al2O3 | Solid/Liquid Ratio |
|---|---|---|---|---|
| Fly-Vol | 44.68 | 19.53 | 3.88 | 0.16 |
| Pz-Vol | 49.01 | 11.73 | 7.09 | 0.16 |
| Mk-Vol | 43.85 | 22.11 | 3.37 | 0.16 |
| Mt-Vol | 44.91 | 13.15 | 5.73 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Churata, R.; Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Torres-Almirón, J.; Ortiz-Valdivia, Y.; Velasco, F. Study of Geopolymer Composites Based on Volcanic Ash, Fly Ash, Pozzolan, Metakaolin and Mining Tailing. Buildings 2022, 12, 1118. https://doi.org/10.3390/buildings12081118
Churata R, Almirón J, Vargas M, Tupayachy-Quispe D, Torres-Almirón J, Ortiz-Valdivia Y, Velasco F. Study of Geopolymer Composites Based on Volcanic Ash, Fly Ash, Pozzolan, Metakaolin and Mining Tailing. Buildings. 2022; 12(8):1118. https://doi.org/10.3390/buildings12081118
Chicago/Turabian StyleChurata, Rossibel, Jonathan Almirón, María Vargas, Danny Tupayachy-Quispe, Jeniffer Torres-Almirón, Yosheff Ortiz-Valdivia, and Francisco Velasco. 2022. "Study of Geopolymer Composites Based on Volcanic Ash, Fly Ash, Pozzolan, Metakaolin and Mining Tailing" Buildings 12, no. 8: 1118. https://doi.org/10.3390/buildings12081118







