Abstract
Crushed dolomite stone can be used as a part of concrete for hot weather. Fine dolomite as a filler is not commonly included in Portland cement. In this paper, the properties of a blended binder based on Portland cement and dolomite filler are presented. Dolomite filler was obtained from dust grains by mechanical activation in a laboratory ball mill to increase the specific surface area and its chemical activity. It is shown that the impact of mechanical activation allows to obtain dolomite filler with a median particle size of 1.4 μm and a specific surface area of 639.9 m2/kg. The content of dolomite filler in Portland cement was 10, 30 and 50%. The main properties of blended cements, i.e., the standard consistency, setting time, compressive strength, average density, and drying shrinkage, were determined on pastes. The mineralogical composition of the hydrated pastes was determined by XRD at 28 days. The presence of dolomite filler at levels higher than 10% decreases the compressive strength of blended cements. The dolomite filler decreases the water demand, shortens the setting time, and mitigates the development of drying shrinkage in the blended binder. To prevent concrete cracking, the application of dolomite filler in blended cement is relevant in hot weather due to its reduced drying shrinkage.
1. Introduction
A hot dry climate has a significant effect on the hardening of cement concretes at high ambient temperatures, low relative humidity, and intense solar radiation [1,2]. The strength of concrete in a hot dry climate at the age of 28 days is 10–20% lower than the strength of concrete hardening in normal temperature and humidity conditions [3]. If the wind speed increases, the influence of these factors is even greater. Temperature shrinkage deformations with a network of microcracks occur in the inner and outer layers of concrete construction exposed in hot dry climates. Temperature and relative humidity changes, as well as strong cyclic heating of open surfaces, negatively affect the durability of concrete [4,5]. Therefore, increasing the service life of concrete in hot weather is a relevant task [6].
The processing of dolomite rocks generates a huge amount of waste. The production of carbonate aggregate forms about 30% of dolomite quarry waste. It is noted that waste from dolomite quarries can be used as a filler and aggregate in concrete [7].
Supplementary cementitious materials based on industrial waste are used in Portland cement to reduce clinker consumption and environmental impact [8,9,10,11]. Various industrial wastes are used as fillers in concrete. Fillers play an important role in concrete mixtures [12,13]. The most commonly applied component in cement-based binders is ground calcium carbonate, whose market share is 34% [10]. According to the author of [14], it is recommended to consider filler materials with a particle size of less than 90 μm, but others refer to particles with a size of less than 125 μm.
Numerous studies have established that the limestone filler participates in hydration reactions and is not inert. The limestone filler accelerates the rate of hydration [15] and reacts with calcium aluminates to form calcium carboaluminates, especially in cements with a high content of C3A [16]. The reaction products are hemi- and monocarboaluminate phases. These components make the structure of cement paste denser, reduce the capillary porosity, and improve the surface structure of the interfacial transition zone between the aggregate and cement paste [17]. Finely ground limestone particles increase the hydration rate caused by the nucleation effect. Furthermore, with the presence of limestone particles, the hydration of cement (alite) is accelerated [18]. There is an interaction between calcium silicate (alite) and calcium carbonate; the latter accelerates the hydration of C3S and modifies the Ca/Si ratio of C-S-H [19]. During the reaction between the calcium carbonate filler and silicate phases, small amounts of filler are incorporated to the C–S–H [20] and the formation of a carbonated hydrated calcium silicate compound occurs [18]. As shown in [21], calcium carbonate does not dissociate under any conditions and does not dissolve in water, even in the pore solution alkalinity of cement paste, which is inherent in the pore electrolyte of cement paste. Reactions of dissolution of calcium carbonate in the presence of sulfate and nitrate ions are also thermodynamically impossible. Chemical interaction of CaCO3 is possible with aluminum ions AlO2− dissolved in pore solution alkalinity of cement paste, which is formed in a high pore solution alkalinity during the hydration of calcium aluminates of Portland cement. The authors have shown that, based on the values of the isobaric-isothermal potential, the presence of CaCO3 in the reaction enhances the hydration of both tricalcium silicate and dicalcium silicate, which is confirmed by an increase in the Gibbs energy of reactions in comparison with the hydration reactions of pure clinker minerals.
The addition of a limestone filler reduces the water demand and segregation of concrete mixtures, increases their water-retaining capacity and uniformity, reduces shrinkage, and improves the water and frost resistance of the concrete [22].
The use of a dolomite filler in concrete, as well as limestone, leads to a rapid growth of strength, which is associated with an increase in the number of the nucleation points of crystallization [23,24]. It not only accelerates the formation of hydration and carbonation products, but also improves the size of crystalline grains. The addition of dolomite filler in concrete with a content of 5–20% accelerates the hardening and can serve as a potential substitute for cement [25].
It has been established that finely ground dolomite can be used to produce dolomite Portland cement due to its sufficient durability, leaching resistance, carbonation, and chloride penetration [26]. The addition of a dolomite filler causes the formation of hemi- and monocarboaluminate phases and the stabilization of ettringite. Moreover, hydrotalcite is formed in dolomite cement, which occurs at elevated temperatures and long-term hardening. The formation of hydrotalcite in dolomite cement can improve the pore structure of the mortar and, thus, increase its compressive strength [27,28,29].
If limestone is added to the cement mill, the specific surface area of limestone fraction in Portland cement is in the range of 800 to 1100 m2/kg, and the clinker fraction is within 400 m2/kg. Limestone can be grinded separately and added to Portland cement [30]. The minimum fineness of the limestone filler in blended cement determined by the Blaine method should be equal to 600 m2/kg or more to ensure the necessary workability and reactivity. The median size of the limestone should be less than 5 μm [18]. Its effectiveness in cement depends not only on the degree of grinding, but also on the content of aluminate and SO3 [21,31].
The limestone filler reduces the water demand of cement-based Portland cement [32]. The presence of small clay particles in the limestone filler [32], as well as a denser packing of particles in the blended binder [33] affect the flowability of the mortar, which can either decrease or increase depending on the origin and amount of carbonate filler. This is due to a small roughness of surface, which reduces the friction between the particles. In this case, the natural carbonates, such as calcite, chalk, and dolomite, are round-shaped and close to spherical-shaped.
In hot weather, concrete is exposed to early cracking. To reduce shrinkage cracking, the water–cement ratio and the content of cement paste should be decreased. This problem can be solved by reducing the amount of cement paste with a partial replacement in the blended binder of a dolomite filler obtained by activating the dolomite quarry waste. The objective of this study is to investigate the influence of the dolomite filler on water demand, setting time, compressive strength, and shrinkage during drying in accordance with the standard requirements, and to compare its performance with pure Portland cement.
2. Materials and Methods
2.1. Materials
In the present work, the following materials were used:
- -
- A Portland cement CEM I 52.5N “Heidelbergcement”, in accordance with [34] with a specific surface area of 377.3 m2/kg; density of 2.9 g/cm3; standard consistency of 30.2%; initial setting time 150 min; end of setting time 175 min; compressive strength at the age of 2 days 29.9 MPa; compressive strength at the age of 28 days 62.6 MPa. Table 1 shows the chemical and mineralogical composition of the clinker.
 Table 1. Mineralogical and chemical composition of the clinker.
Table 1. Mineralogical and chemical composition of the clinker.
- -
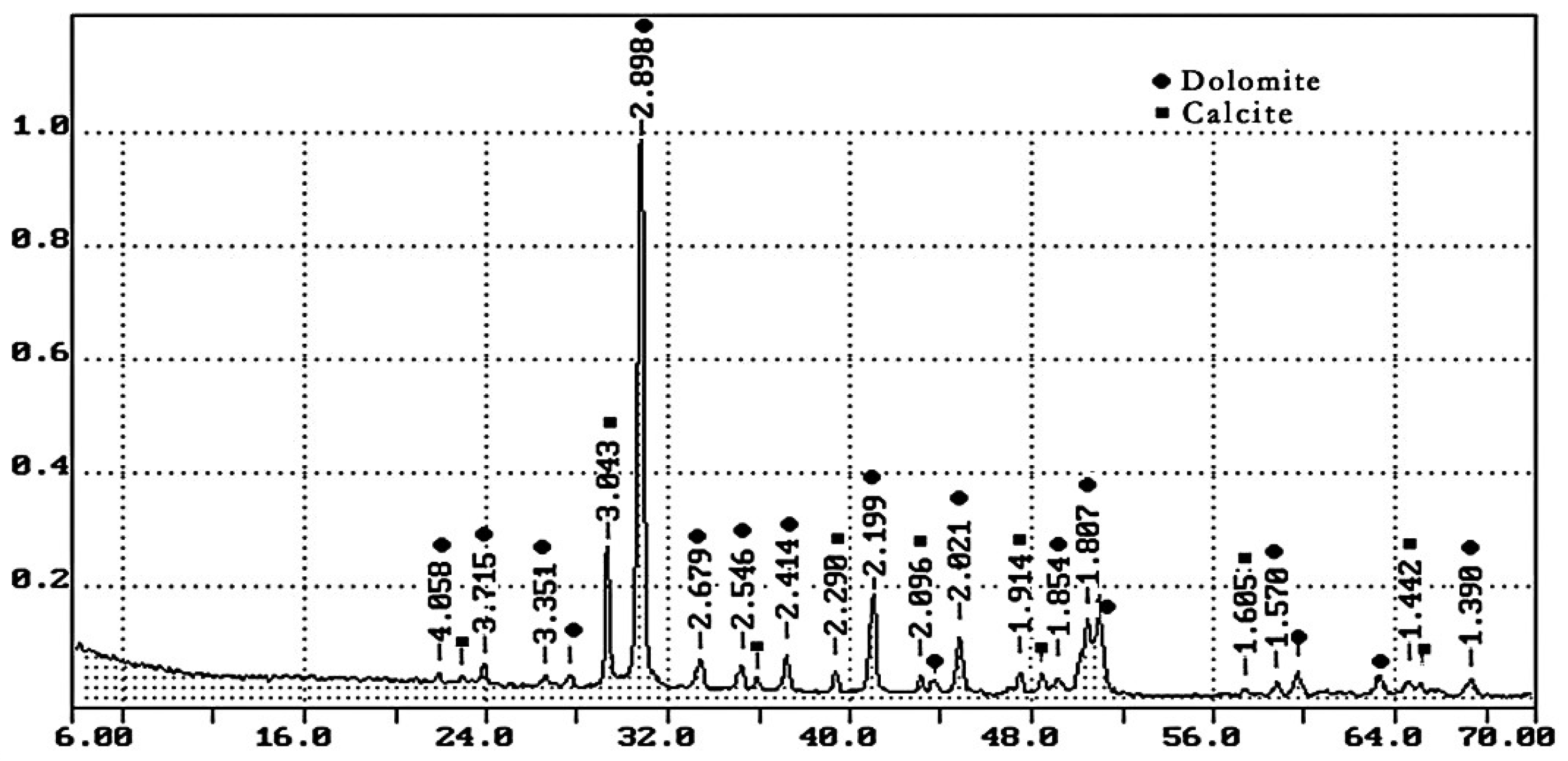
- Dolomite quarry waste was formed during the production of coarse aggregate in Kamensky Mining Quarry LLC with a density of 2.65 g/cm3. The particle size distribution of screenings is given in Table 2; the phase composition is shown in Figure 1. Crushed dolomite is a sedimentary carbonate rock; it consists of 90% mineral dolomite (CaCO3·MgCO3) and contains 10% calcite (calcium carbonate CaCO3). Table 3 shows the chemical composition of dolomite quarry waste.
 Table 2. Particle size distribution of dolomite quarry waste.
Table 2. Particle size distribution of dolomite quarry waste.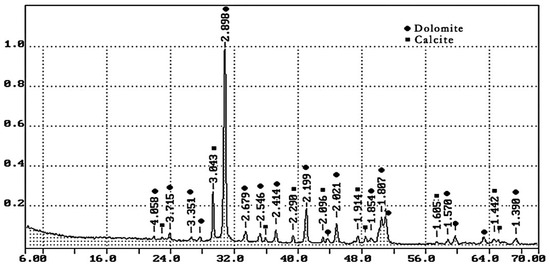 Figure 1. XRD pattern of dolomite quarry waste.
Figure 1. XRD pattern of dolomite quarry waste. Table 3. Chemical composition of quarry waste of dolomite.
Table 3. Chemical composition of quarry waste of dolomite.
2.2. Methods
The dust grains of dolomite quarry waste were crushed by grinding in a laboratory ball mill with dimensions of 680 × 580 × 550 mm for 420 min to obtain the filler. The specific surface area of dolomite filler was determined according to the Blaine method with the ToniPERM Standard equipment. Before measuring, the test filler was dried at the temperature of 60 °C for 1 h.
A particle size distribution analysis of the fillers was conducted using a laser diffraction analyzer HELOS Sympatec GmbH. The microstructure of the dust grains of dolomite crushed stone was analyzed using a FEI Quanta 200 scanning electron microscope. An X-ray phase analysis of the blended binder samples was performed in accordance with [35] The phase composition of the samples was carried out using the method of corundum numbers determination [36].
The chemical composition of Portland cement and dolomite filler was studied using the Bruker S4 Pioneer and Bruker S8 Tiger X-ray analyzers. The mineralogical composition of Portland cement was carried out on a Bruker D2 Phaser diffractometer.
The influence of the specific surface area and the content of the dolomite filler in the blended cement on standard consistency, setting time, and strength in accordance with [37] was researched. The hardening of the samples was carried out under standard curing conditions at the ages of 2, 7, 21, and 28 days.
The drying shrinkage of the blended binder was determined in accordance with [38]. The samples of the blended cement paste in sizes of 40 × 40 × 160 mm were prepared. After curing in chambers for 24 h, the drying shrinkage of the samples was measured at a relative humidity of 26% and a temperature of 27 °C to obtain maximum shrinkage value.
3. Results and Discussion
3.1. Dolomite Quarry Waste
It is necessary to reduce the environmental impact and the level of waste associated with dolomite rock extraction and aggregate processing. Dolomite quarry waste is often represented by a granulometric composition that does not meet the requirements of Russian standards for use in concrete. The increased content of large grains more than 5 mm can reach up to 15%, and the content of dust grains can reach 18%. Grains with large size and dust grains should be removed.
The granulometric composition of dolomite quarry waste is represented in Table 4. In this work, dolomite residues were subjected to additional screening for use in concrete as a dolomite fine aggregate to meet the requirements of the Russian standard [39]. The obtained fine dolomite aggregate was characterized as “very large” with a modulus of fineness Mk = 3.8 (Table 4) and can be used as a partial replacement of fine aggregate to improve the granulometric composition of fine quartz sand.

Table 4.
Granulometric composition of fine dolomite aggregate after additional screening.
The microstructure of the dust grains of dolomite quarry waste was analyzed in this work (Figure 2). The analysis of scanning microscopy data shows that the particle size of the dust grains is within wide limits; there are particles ranging in size from 32 to 365 μm. The use of dust grains as a filler in this form is impossible due to the presence of inhomogeneous particle sizes and the impossibility of their uniform distribution over the entire volume of the cement paste. The stabilization of the filler properties based on industrial waste includes mechanical activation in a ball mill. The result of the activation is an increase in the reactivity and homogeneity of the filler, and chemical reactions between both solid-phase and solid and liquid components accelerate. A change in composition and structure of the activated substance occurs. However, activation in a ball mill is inevitably accompanied by the opposite process of aggregation of small particles—flocculation. The production of finely dispersed and chemically active dolomite filler for use in concrete in hot weather can be achieved precisely by the use of activation in the form of grinding.

Figure 2.
Microstructure of dust grains of dolomite quarry waste.
To increase the specific surface area of dust grains and obtain chemically active dolomite filler, directed mechanical activation was carried out in a laboratory ball mill SLM-5. Metal balls with a diameter of 30 mm were used as grinding media. The volume of the grinding chamber was 5 L. The volume of balls filled into the mill was 40% of the volume of the drum. The volume of the material was 10% of the volume of the drum, that is, 1260 g of dolomite quarry waste per 1 L. The mill’s operating mode was shock-abrasion with a frequency of 40 Hz. A dry activation method was used. Dolomite quarry waste was pre-crushed to pieces less than 5 mm in size before being loaded into the mill. The specific surface area was determined every 30 min of activation for 420 min [40].
3.2. Particle Size
Carbonate rocks are crushed more easily in comparison with Portland cement clinker. It is possible to obtain particles with a size of less than 10 μm; they densify the cement paste and increase its durability [41]. The reactivity of limestone filler to the aluminate phase of Portland cement clinker appears precisely in a highly dispersed state.
The activation of the fillers is performed to enhance their adhesion to the binder and increase the structure-forming role. The selection of the fillers and the determination of the ways of their activation preferably lead to the formation of chemically non-equilibrium systems with high adhesive strength. In non-equilibrium systems, a chemical reaction occurs at the interface with a partial rupture of interatomic bonds in the volume of each phase. Intermediate compounds of various types are formed, and then diffusion of one phase into another and dissolution occur. In this case, the initial boundary between the phases is blurred, and a new one is often formed, for example, between one of the phases and a layer of an intermediate chemical compound. In adhesive systems, the substrate is a particle of powdered filler (chalk, soot, glass), glass and textile fibers, or polymer film.
The creation of a strongly pronounced adhesive interaction between cement and filler is possible only if the surface energy of the filler is significantly higher than that of the cement. This conclusion is based on the thermodynamic concept of adhesion [42]. In accordance with the thermodynamic theory of adhesion, the main role for building adhesive strength is assigned to the ratio of the values of the surface energy of the adhesive Wad and the substrate Ws. In this case, the mandatory condition is
Wad < Ws
Mechanical treatment is an effective way to activate fillers by increasing surface energy. The increase in the specific surface of the solid filler acquires a significant reserve of surface energy. It is known that the surface energy increases due to the rupture of the interatomic bonds of the solid structure. The work expended during the activation of a substance to break the bonds between molecules is converted into the potential energy of unsaturated bonds at the interface of phases. Due to the increase (many times) of the interface during the grinding of the substance, this excess surface energy reaches a significant value. Having more energy in the finely dispersed state, the crushed substance, therefore, has more activity than the non-crushed one. Mechanical activation increases the reactivity of the material due to the combined effect of an enlarged surface area and physico-chemical changes. These changes occur both inside the body in the entire volume and on the surface of the substance as a result of high-energy grinding in a mill [42,43]. For example, mechanical activation of fly ash is aimed to increase the amorphous content [44], and enlarge the specific surface area and fineness [45].
The surface energy is increased by breaking the interatomic bonds of the structure. This occurs during crushing, grinding, and abrasion of the solid state. Newly formed surfaces have significantly higher values of surface energy, which causes their higher adhesive activity. The special energetic state of the new surfaces of crushed mineral materials (quartz, limestone, magnesite, gypsum, etc.) can be explained by the formation of a large number of unsaturated valence bonds. Mechanical processes during the grinding of mineral and organic materials cause, along with an increase in their surface energy, an increase in the isobaric potential of fillers and, accordingly, their chemical activity. This ensures the creation of a high adhesive strength of the contact of the filler with the binder [32].
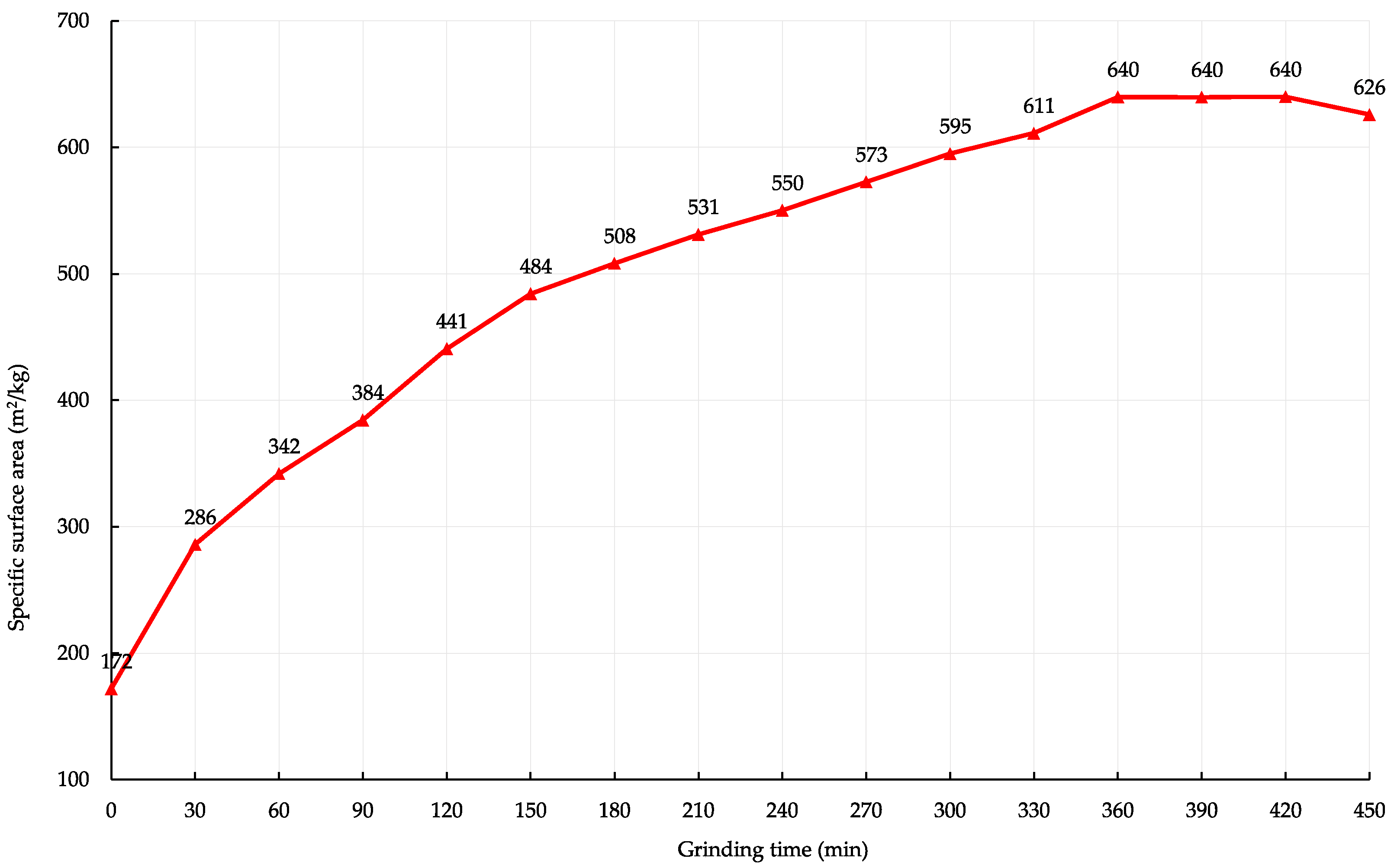
The influence of mechanical activation on particle size distribution was analysed. There is a change in the specific surface area and particle size of the dolomite filler. The specific surface area of the dolomite filler with 474.2, 557.4, and 639.9 m2/kg was obtained by grinding for 120, 180, and 360 min respectively. Figure 3 shows the influence of grinding time change on the specific surface area of the dolomite filler. The initial specific surface area of the dust grains was 206.3 m2/kg.
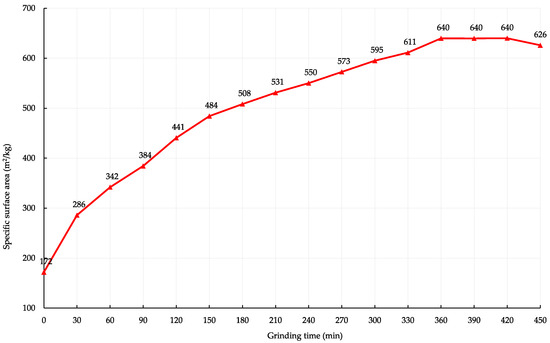
Figure 3.
The effect of activation time on the change in the specific surface area of the dolomite filler.
Grinding in a ball mill is necessarily accompanied by the flocculation of particles. The smaller the product becomes, the larger a floccule is obtained. It follows from Figure 3 that after the filler reaches 639.9 m2/kg, the specific surface area decreases with the grinding time. This indicates the appearance and development of agglomeration processes of fine particles. Further mechanical activation and energy costs are technically and economically inappropriate.
The particle size distribution analysis of Portland cement, the dust grains of dolomite quarry waste before activation, as well as the dolomite filler after activation in a ball mill were studied using the HELOS Sympatec GmbH laser diffraction granulometer. The granulometric analysis of dispersed components shows the main fractions of cement and dolomite filler. The ratio of the three main fractions and the presence of a plateau on the distribution curve affect the rate of hydration of Portland cement and its strength formation.
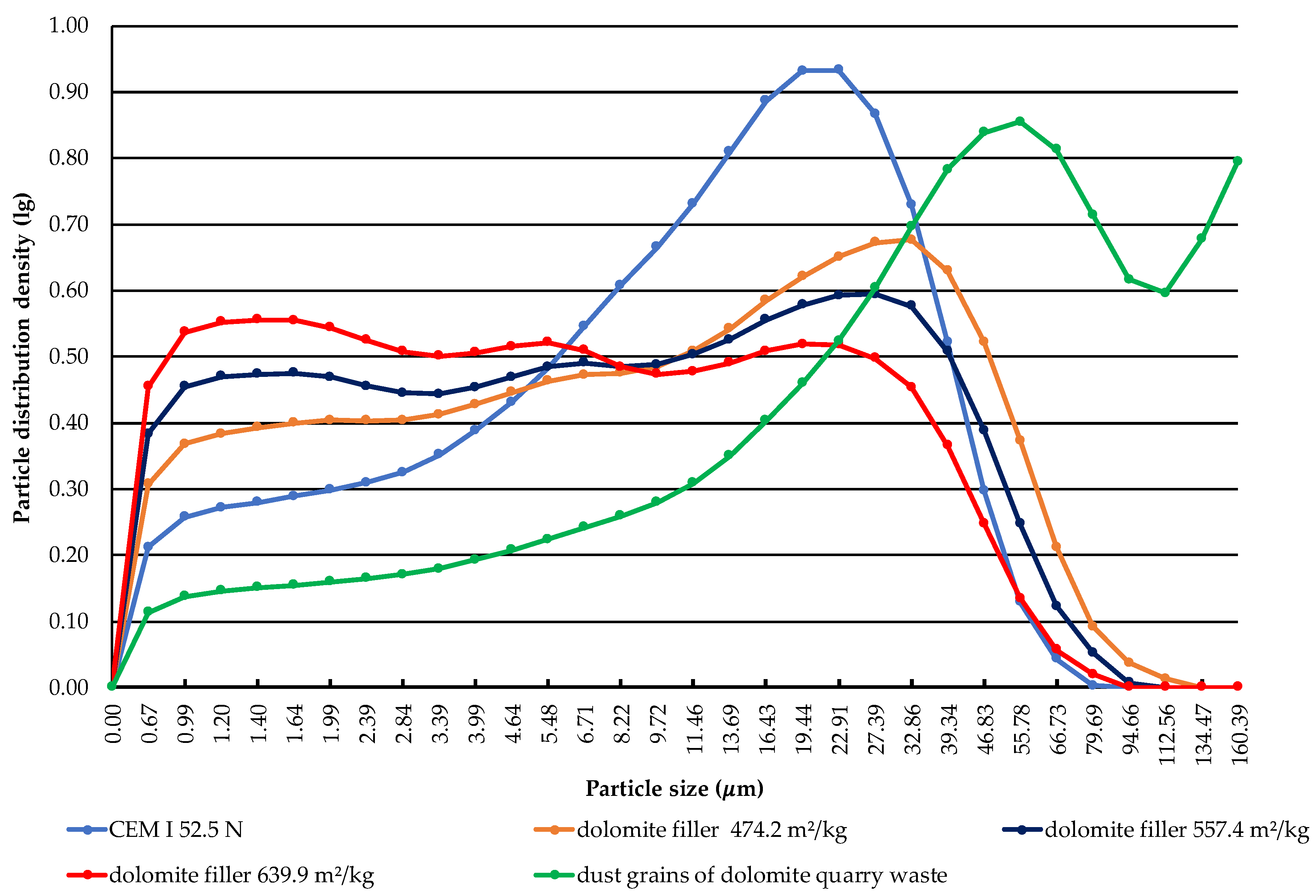
The results of the granulometric composition of Portland cement, the dust grains of dolomite quarry waste before activation, and the dolomite filler with specific surface areas of 474.2, 557.4, and 639.9 m2/kg are presented in Figure 4. Cumulative particle size distribution curves of dolomite filler with a specific surface area of 639.9 m2/kg show the presence of fine size particles of up to 5 μm. It was determined what size of particles dominates the sample of fine components.
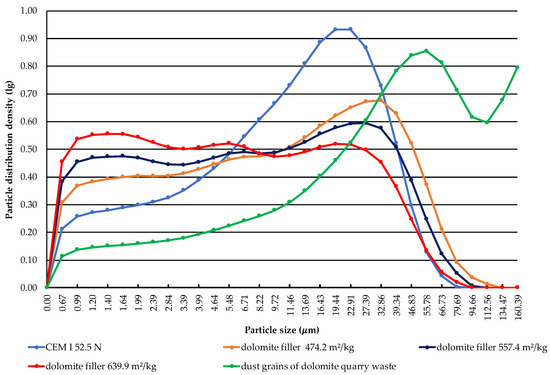
Figure 4.
Particle size distribution curve at different activation time of dolomite filler.
In Portland cement, 29.07% consists of small particles up to 5 μm in size. This fraction provides the strength in two days of hardening; the content of fraction 5–30 μm in cement is 57.56%, which gives the strength at the age of twenty-eight days of hardening. The content of fraction larger than 30 μm in cement is 13.37%, which provides strength at the long term of hardening. The obtained data show that Portland cement contains particles ranging in size from 0.67 to 79.69 μm. The highest content in Portland cement are particles with a size of 22.91 μm, which can be seen at the peak of the distribution curve. The estimated specific surface area of Portland cement is 377.3 m2/kg.
The granulometric composition of the dust grain fraction of dolomite quarry waste showed that the content of the particles up to 5 μm is 15.23%; that of medium particles from 5 to 30 μm is 28.17%; and that of particles larger than 30 μm is 56.6%. It was determined that the dust grain component has particles ranging in size from 0.67 to 160.39 μm. The highest content are particles with a size of 55.78 μm. The estimated specific surface area of the dust grain fraction of dolomite quarry waste is 206.5 m2/kg.
It was determined that the dolomite filler with a specific surface area of 474.2 m2/kg has particles up to 5 μm with a content of 37.84%. The highest content are particles with a size of 32.86 μm.
The granulometric composition of the dolomite filler with an estimated specific surface area of 557.4 m2/kg shows that the content of small particles up to 5 μm is 44.13%; that of medium particles from 5 to 30 μm is 41.15%; and that of particles larger than 30 μm is 14.72%. It was stated that the dolomite filler has particles ranging from 0.67 to 94.66 μm. The largest particle content in the filler is a fraction of 27.39 μm in size.
It was found that the dolomite filler with an estimated specific surface area of 639.9 m2/kg contains 90.09% of particles less than 30 μm in size. According to the data, it can be seen that there are particles ranging in size from 0.67 to 79.69 μm, similar to the size of Portland cement. However, the highest content in the filler are fine particles with a size of 1.4 μm.
3.3. Water Demand
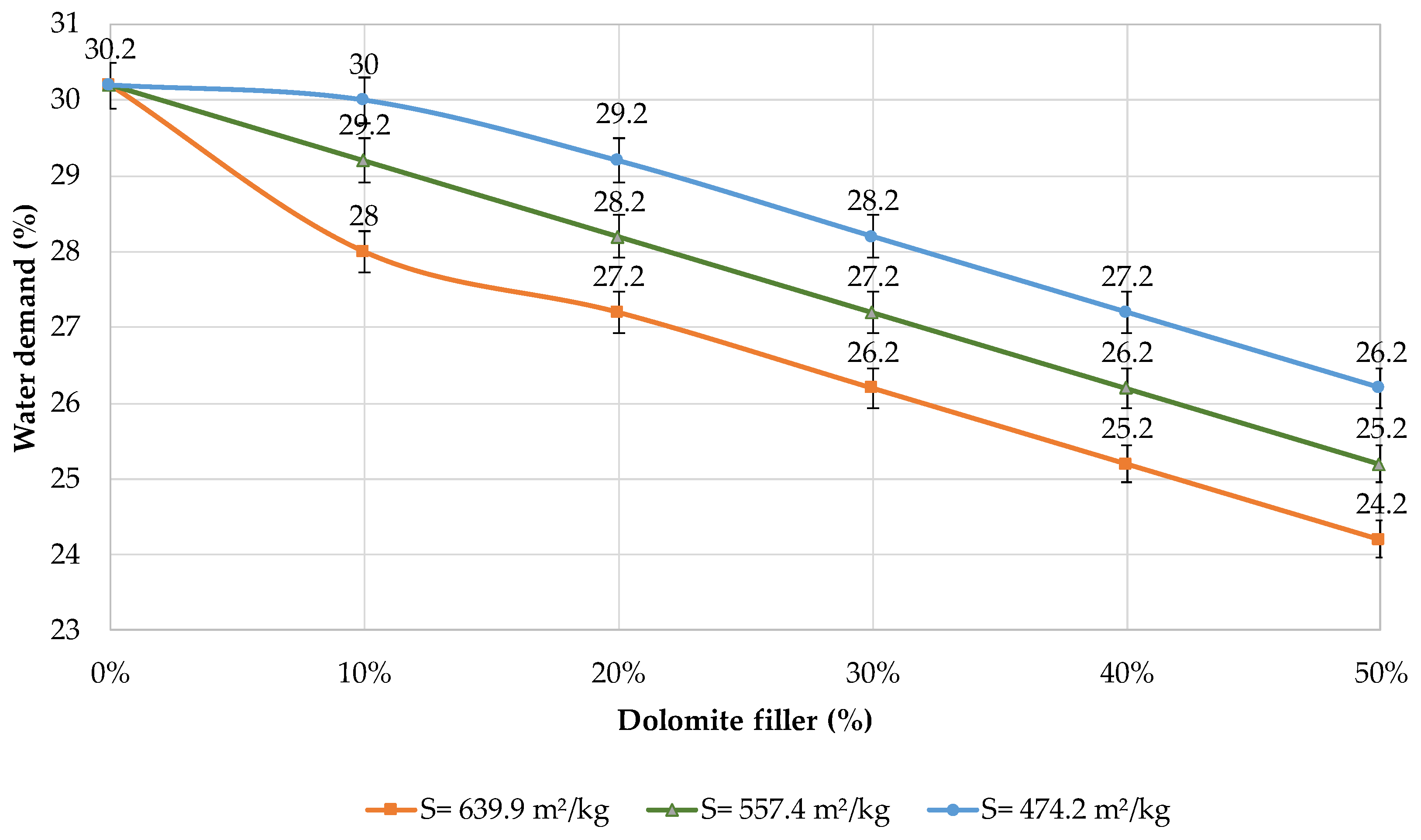
The influence of mechanical activation on the properties of blended cement was researched. Figure 5 shows the effect of the dolomite filler on the water demand of the blended cement. It was found that an increase in the filler content leads to a decrease in the water demand.
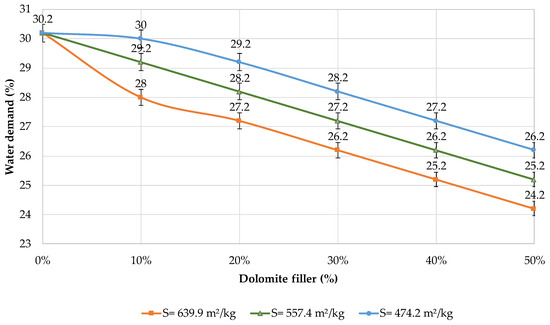
Figure 5.
Water demand of blended cements with different contents of dolomite filler.
The water demand of the fillers is an important property when the composition of concrete is designed. Portland cement and the fillers have the strongest influence on the water demand of the concrete mixture.
There are different approaches to assess the water requirements of the binders. The spreading test is a classic method for determining the water requirement for powder materials used in SCC [46]. The Puntke test is a method developed by Puntke for the rapid testing of fly ash [47].
The Vicat test is still the most commonly used method for the quantitative assessment of cement paste properties. The modified Marquardt test is suitable for powder materials, building mixes, and also for whole concrete mixtures. There are a number of alternative testing procedures, such as centrifugation and variants of the Proctor compaction test [14].
According to the Vicat test, the water demand of cement is equal to a certain amount of water to obtain the standard consistency of cement paste. The data show that despite the presence of the dolomite filler, less water is required to obtain the standard consistency of cement paste (Figure 5). The presence of fine particles less than 5 μm and the widening effect of the filler have a significant effect on the water demand of the binder, which corresponds with the data of other researchers [48].
The maximum reduction in water demand is observed when the binder contains 50% of the filler with a specific surface area of 639.9 m2/kg. The content of the water is 20% less (24.2%) in comparison with plain cement paste, which corresponds with the data of other researchers [49].
It was estimated that plain cement has a water demand of 30.2%. The addition of 10% of the filler with a specific surface area of 474.2 m2/kg does not significantly influence the water demand of the blended binder (1–3%). However, when 30–50% of the filler is added, the water demand decreases by 7–13%. If 30–50% of the filler with a specific surface area of 557.4 m2/kg is introduced, the water demand is reduced by 10–17%. With the addition of 30–50% of the filler with a specific surface area of 639.9 m2/kg, the water demand decreases by 13–20%, which can be confirmed by the data of other researchers [50]. This is due to the presence of particles smaller than 30 microns in the amount of 90.09%; it is the highest content of fine particles with a size of 1.4 microns. The decrease in the water demand can be explained by a better particle packing density, which corresponds with the data of other researchers [51].
3.4. Setting Time
The results of the setting time of cement paste with dolomite powder are shown in Table 5. Dolomite filler is less reactive in comparison with limestone filler [52]. It is determined that the addition of the filler to Portland cement affects the setting time. The acceleration of the initial setting time is noted with the presence of the filler in the blended binder, with a specific surface area of 557.4 and 639.9 m2/kg in comparison with pure Portland cement. However, the most rapid acceleration is observed with the presence of 10 and 50% dolomite filler and a specific surface area of 639.9 m2/kg. It was found that with the substitution at the range of 50% with a specific surface area of 639.9 m2/kg, the blended binder has the shortest setting time with an initial time of 125 min and a final time of 170 min. Such results correspond with the studies of other authors concerning limestone fillers [53]. The reduction in setting time can be explained by the acceleration of hydration of Portland cement, which is caused by the nucleation and growth sites offered by the fine dolomite particle surfaces, the formation of nuclei, and the short distance between the particles due to the inclusion of fine particles [54,55].

Table 5.
Setting time of blended cement with different contents of dolomite filler.
3.5. Compressive Strength
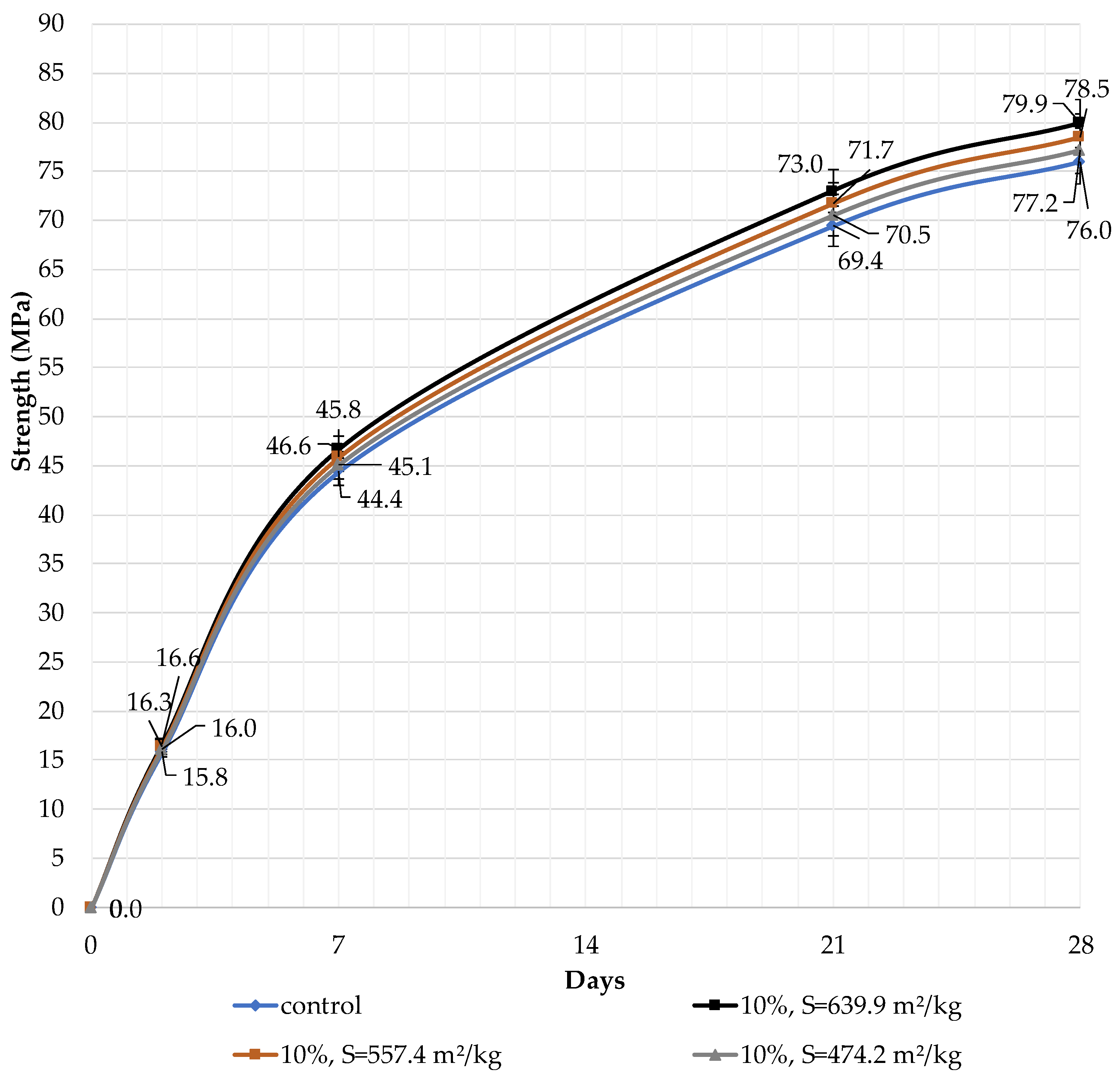
The effect of the specific surface area of the filler and its content in the blended cement on the compressive strength at the ages of 2, 7, 21, and 28 days of hardening is presented. The content of the filler in blended cement was 10, 30, and 50%. According to [33], blended cements with 20% dolomite and limestone filler have lower compressive strengths than the reference binder, at any age, as reported in previous studies. It was found that the highest strength at the age of 28 days is achieved with 10% dolomite filler with a specific surface area of 639.9 m2/kg (79.9 MPa) compared to pure Portland cement (76.0 MPa). The specific surface area has a strong influence on the properties of the blended binder.
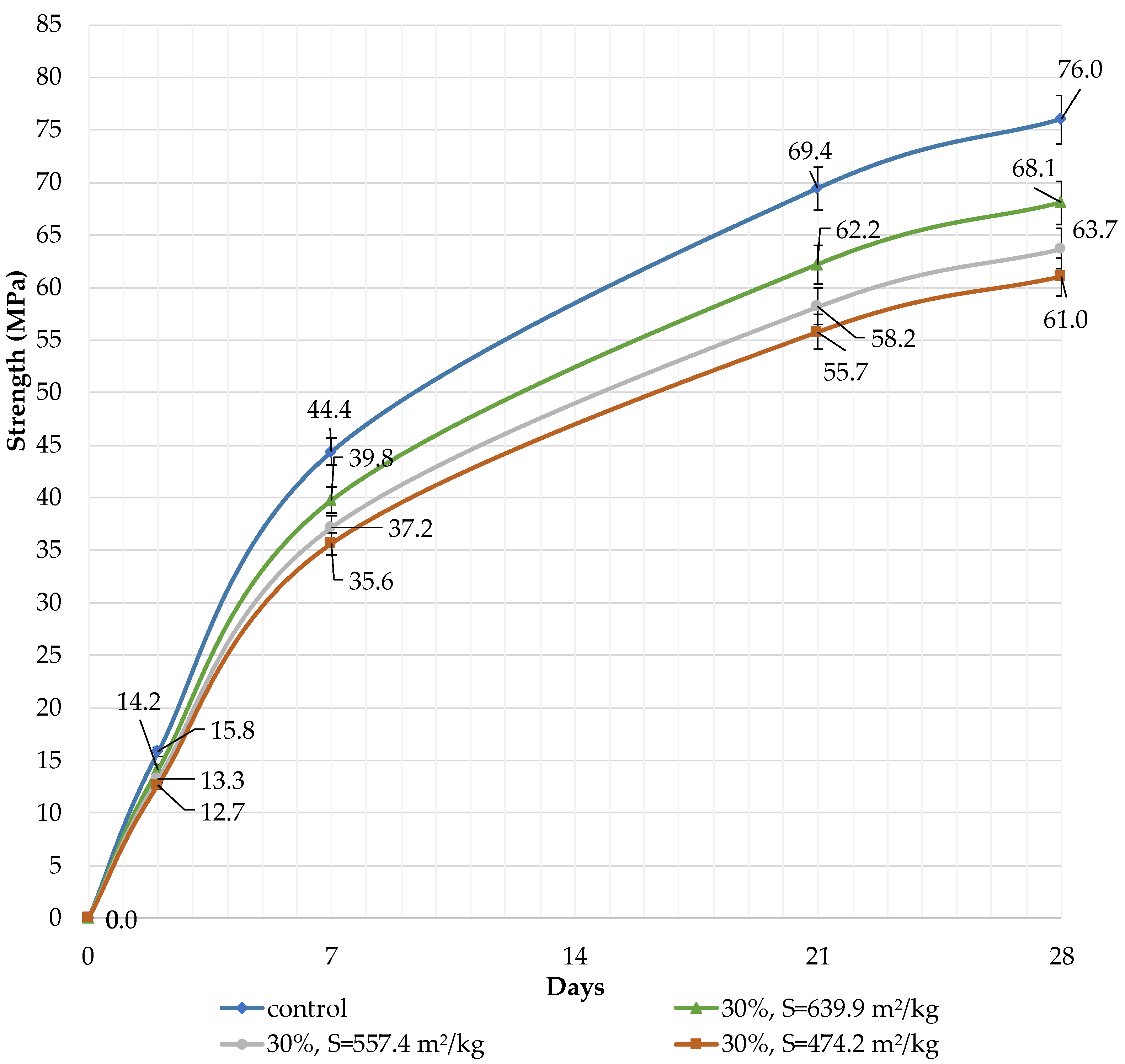
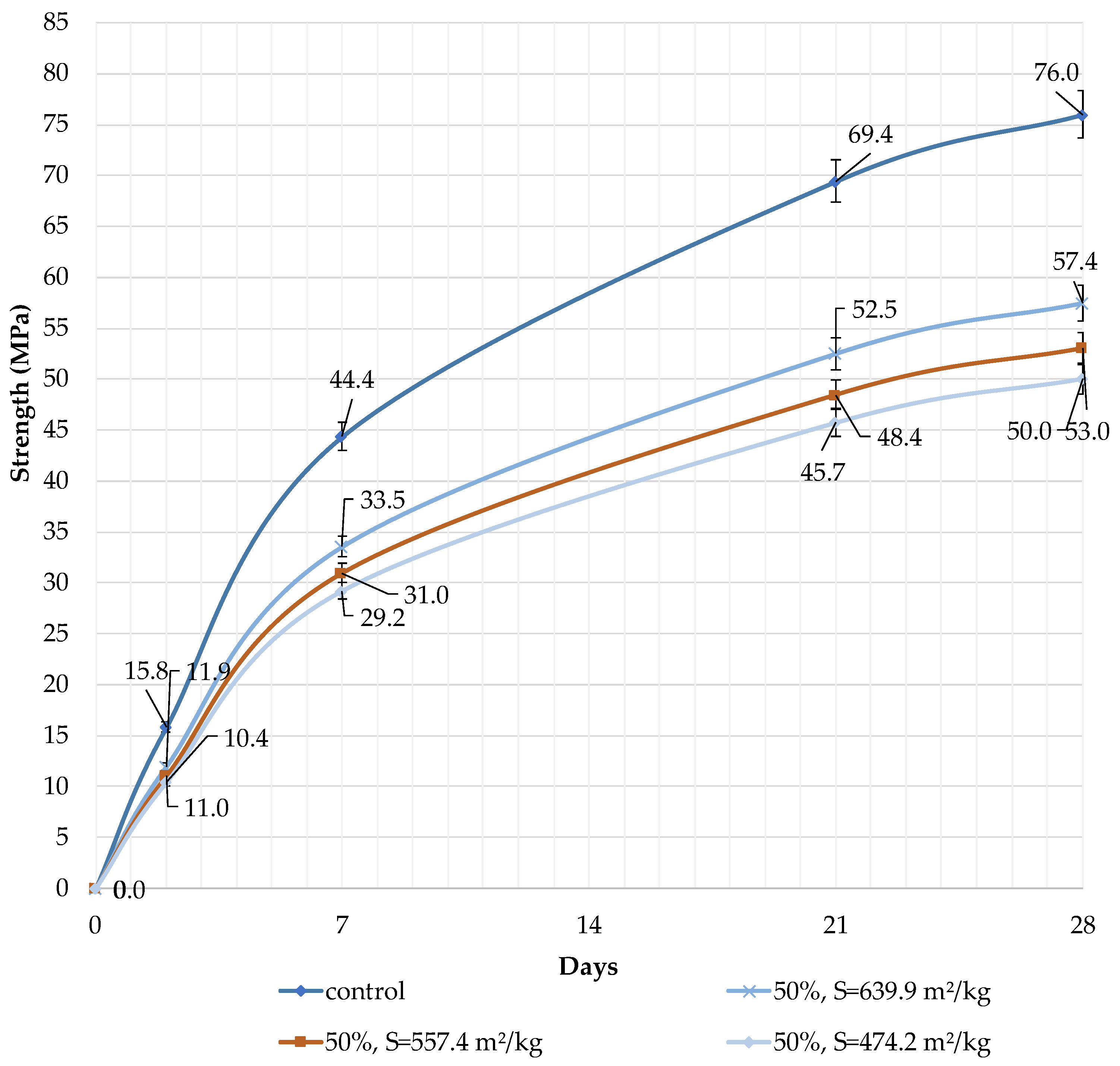
It can be concluded that the use of 10% dolomite filler with a specific surface area of 639.9 m2/kg increases the strength of the blended cement in 2, 7, and 28 days of hardening (Figure 6). Moreover, 10% of dolomite filler increases the strength after 2 days by 5%, after 7 days by 3%, and after 28 days by 1.5%. The replacement of cement with 10% dolomite filler does not significantly reduce the compressive strength, although the number of particles of chemically active clinker decreases. When the content of dolomite filler is 30 and 50%, as expected, the compressive strength decreases (Figure 7 and Figure 8). The same strength properties are observed for coarser ground dolomite filler with a specific surface area of 557.4 and 474.2 m2/kg. The increase in strength of blended cement is explained by the occurrence of chemical activity in the dolomite filler. This is due to the presence of calcium carbonate (CaCO3) in dolomite (Figure 1). Some studies show that calcium carbonate in small dosages can react with the aluminates of the clinker to form hydrocarbonates [54,55,56], which, in turn, increase the resistance of ettringite [57].
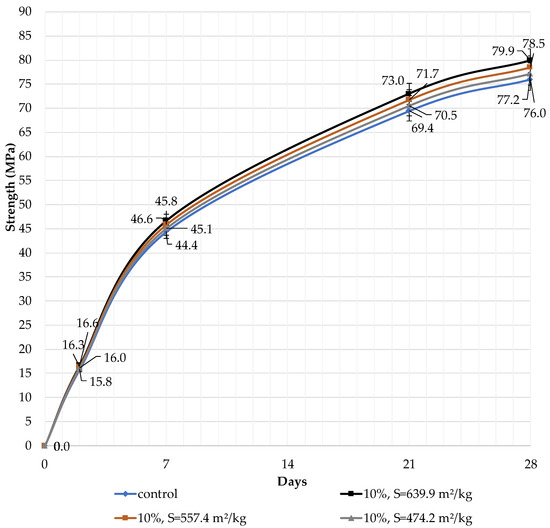
Figure 6.
Influence of specific surface area and dolomite filler with 10% content in blended cement on the compressive strength.
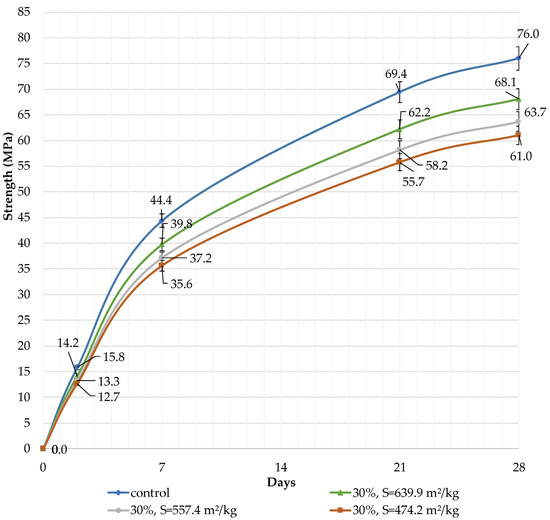
Figure 7.
Influence of specific surface area and dolomite filler with 30% content in blended cement on the compressive strength.
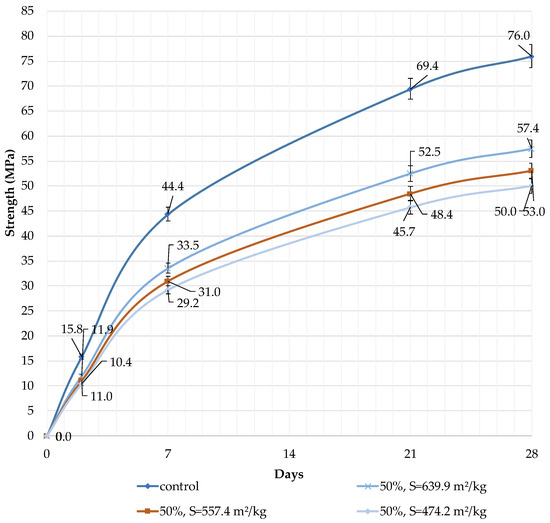
Figure 8.
Influence of specific surface area and dolomite filler with 50% content in blended cement on the compressive strength.
The interaction between the tricalcium aluminate of cement and finely ground calcium carbonate forms calcium hydrocarboaluminate 3CaO·Al2O3·CaCO3·12H2O [58], which assists to form the crystalline structure of the hardened cement matrix. The occurrence of additional crystalline crystallohydrates in the initially forming cement structure ensures the formation of a more dense, hardened cement paste structure, reduces the capillary porosity, and improves the surface structure of the interfacial transition zone between the filler and the cement paste [17]. This statement may explain the increase in strength.
The decrease in strength is due to the high content of the filler, and the “dilution” effect can be observed. If the content is high (30 and 50%), the dolomite filler plays the role of cement replacement at any specific surface area (639.9, 557.4, and 474.2 m2/kg), and the strength of the blended cement reduces after 2, 7, and 28 days by 10% and 24%, respectively (Figure 7 and Figure 8). The dolomite filler acts as an inert material, which loosens the initial cement structure and leads to a decrease in strength.
3.6. Drying Shrinkage
Drying shrinkage of concrete in hot weather is significant. It causes the early cracking of concrete. The combined use of a water-reducing admixture and a dolomite filler with low water demand and increased water retention capacity could save the water loss of concrete mixture, and maintain its desired properties under the influence of elevated temperatures and low relative humidity. This may ensure favorable conditions for the hydration of the binder, reduce shrinkage during hardening and heat generation, and affect the formation of the initial structure of hardening concrete.
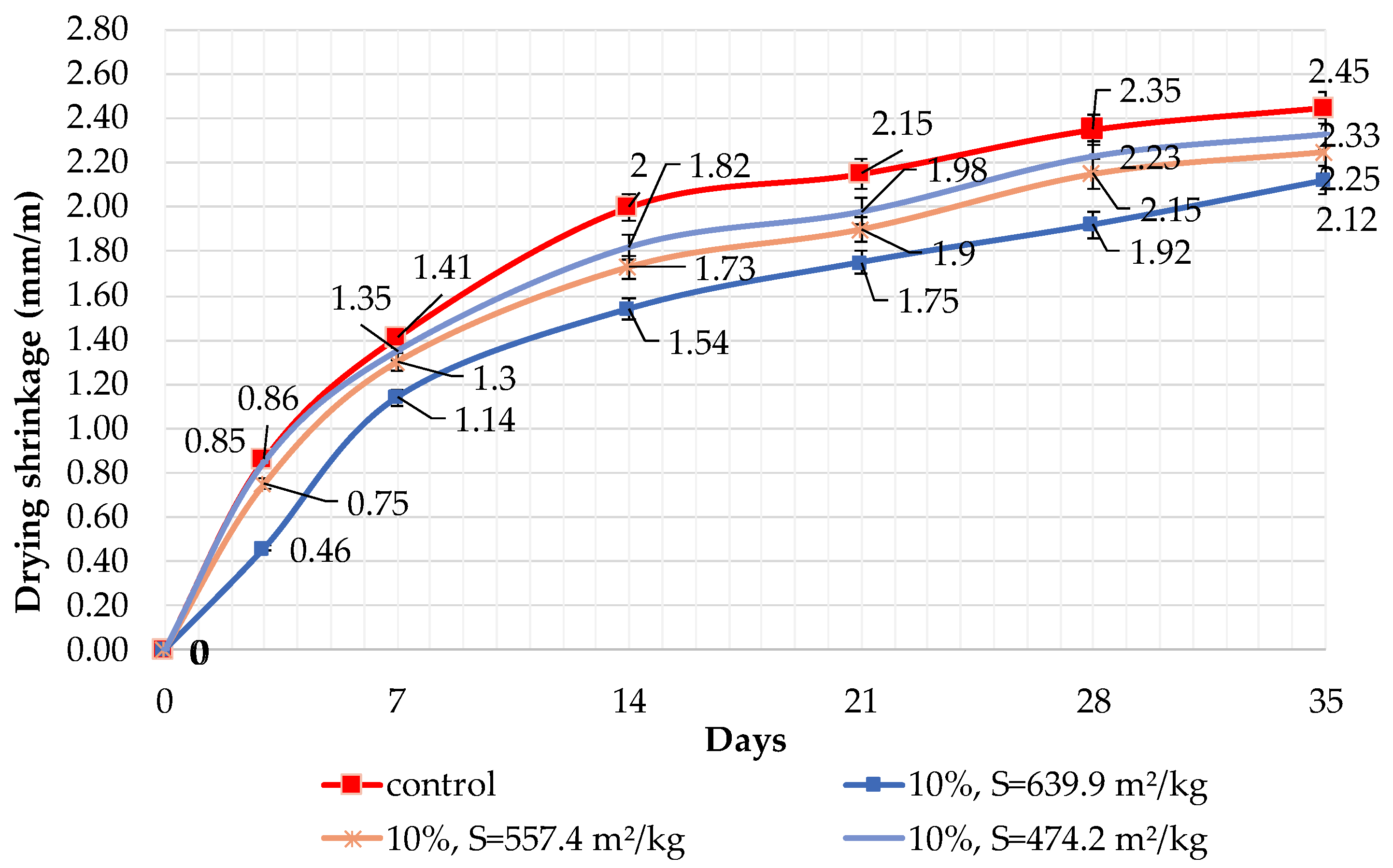
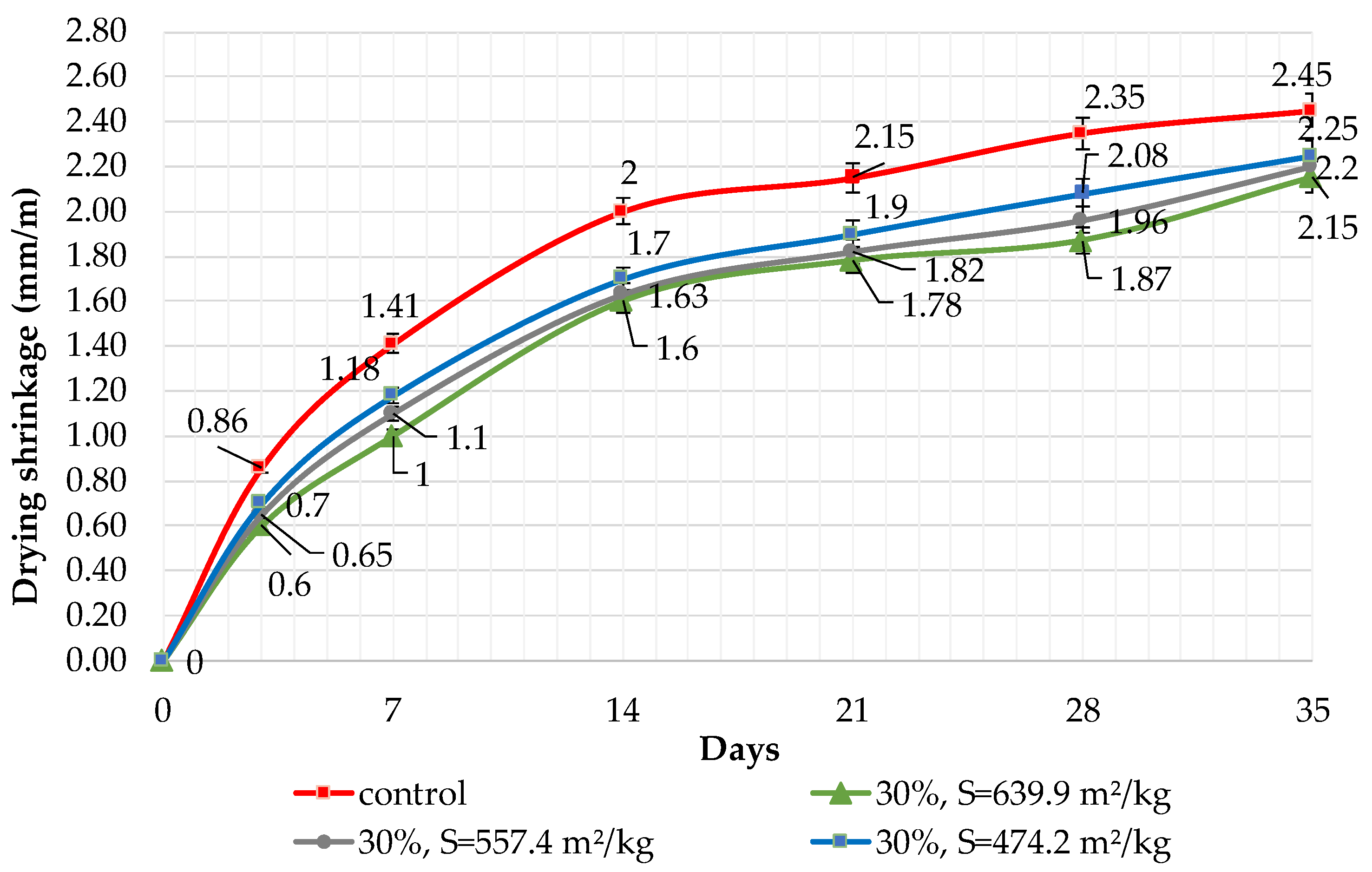
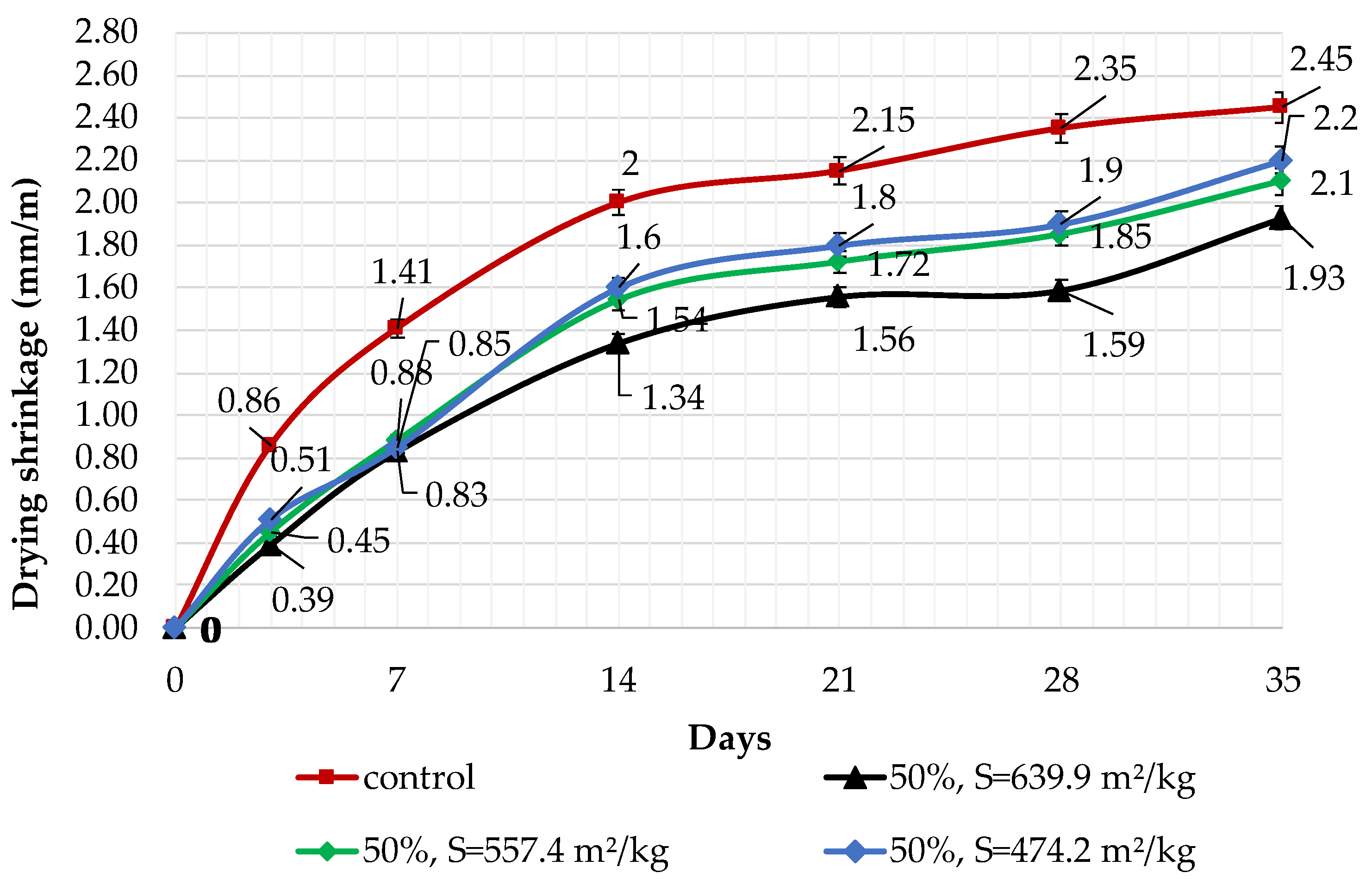
The results of shrinkage tests are shown in Figure 9, Figure 10 and Figure 11. It was determined that linear shrinkage after 35 days for cement containing dolomite filler is lower than for pure Portland cement. The composition with 50% filler with a specific surface area of 639.9 m2/kg has the minimum shrinkage. In this case, the shrinkage index is 21% less than the pure cement. The maximum linear shrinkage is demonstrated by Portland cement and a composition with 10% dolomite filler with a specific surface area of 474.2 m2/kg.
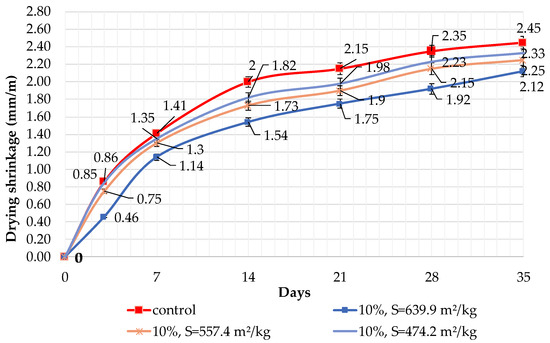
Figure 9.
Influence of specific surface area and 10% dolomite filler in blended cement on linear shrinkage.
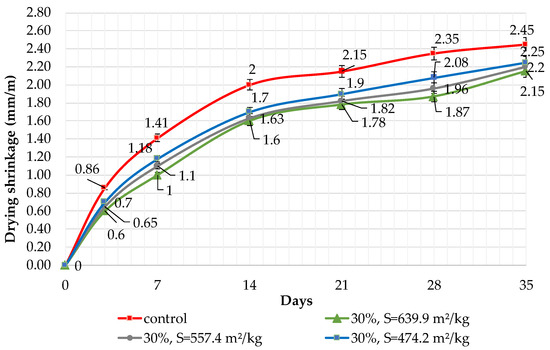
Figure 10.
Influence of specific surface area and 30% dolomite filler in blended cement on linear shrinkage.
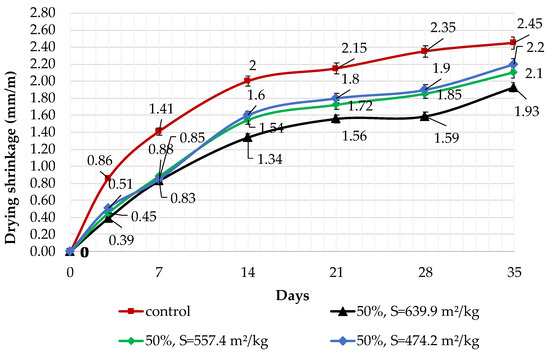
Figure 11.
Influence of specific surface area and 50% dolomite filler in blended cement on linear shrinkage.
3.7. Average Density
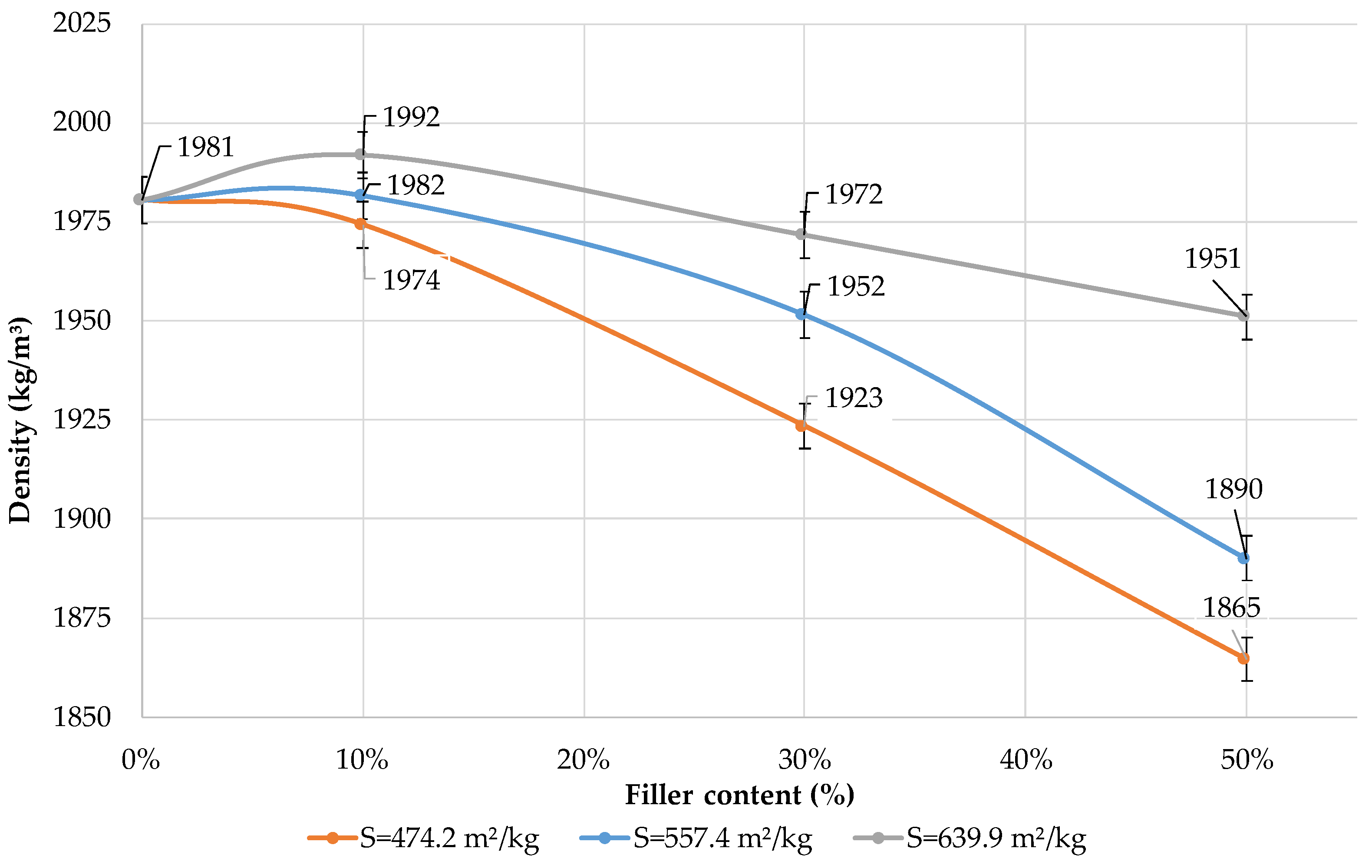
Figure 12 shows the effect of the specific surface area of the filler and its content in the blended binder on the average density of cement paste. The use of dolomite filler reduces the average density of blended cement if the substitution rate is 30 and 50%. The average density of blended binder with a specific surface area of 639.9 m2/kg increases if the content of the filler is 10%. This can be explained by the chemical activity of the dolomite filler and the formation of a more dense cement paste structure that corresponds with an increase in strength (Figure 6). If the content of the filler is 10% and its specific surface area is 557.4 and 474.2 m2/kg, the composition has a similar average density as pure Portland cement.
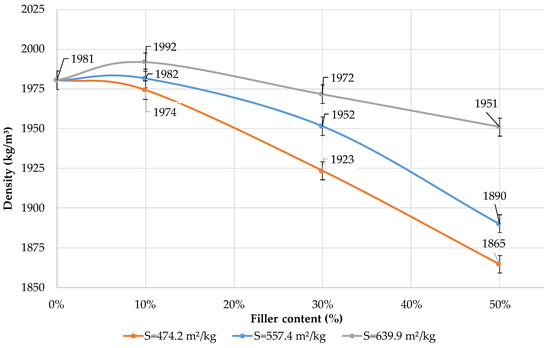
Figure 12.
Influence of specific surface area and content of dolomite filler in blended cement on the average density of the cement paste.
3.8. Hydration Products
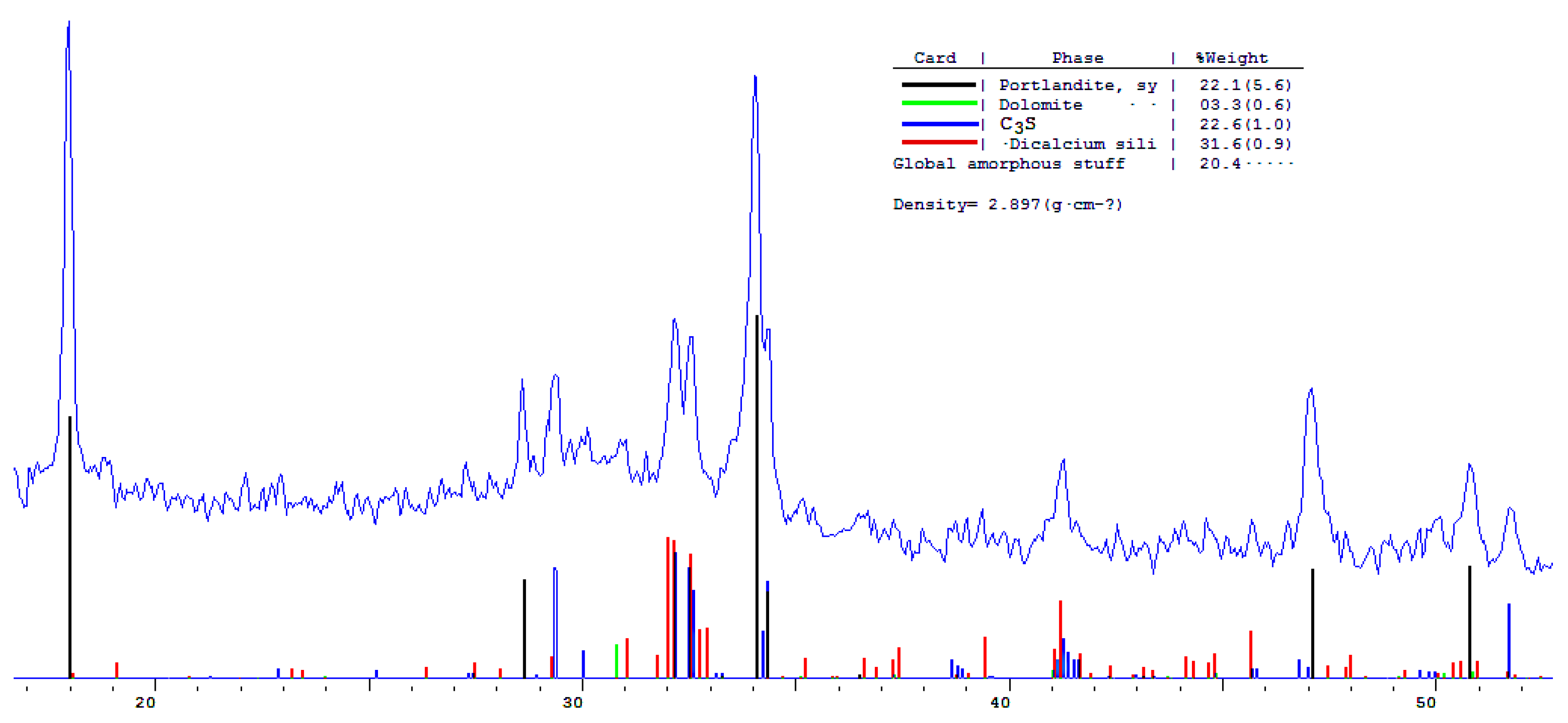
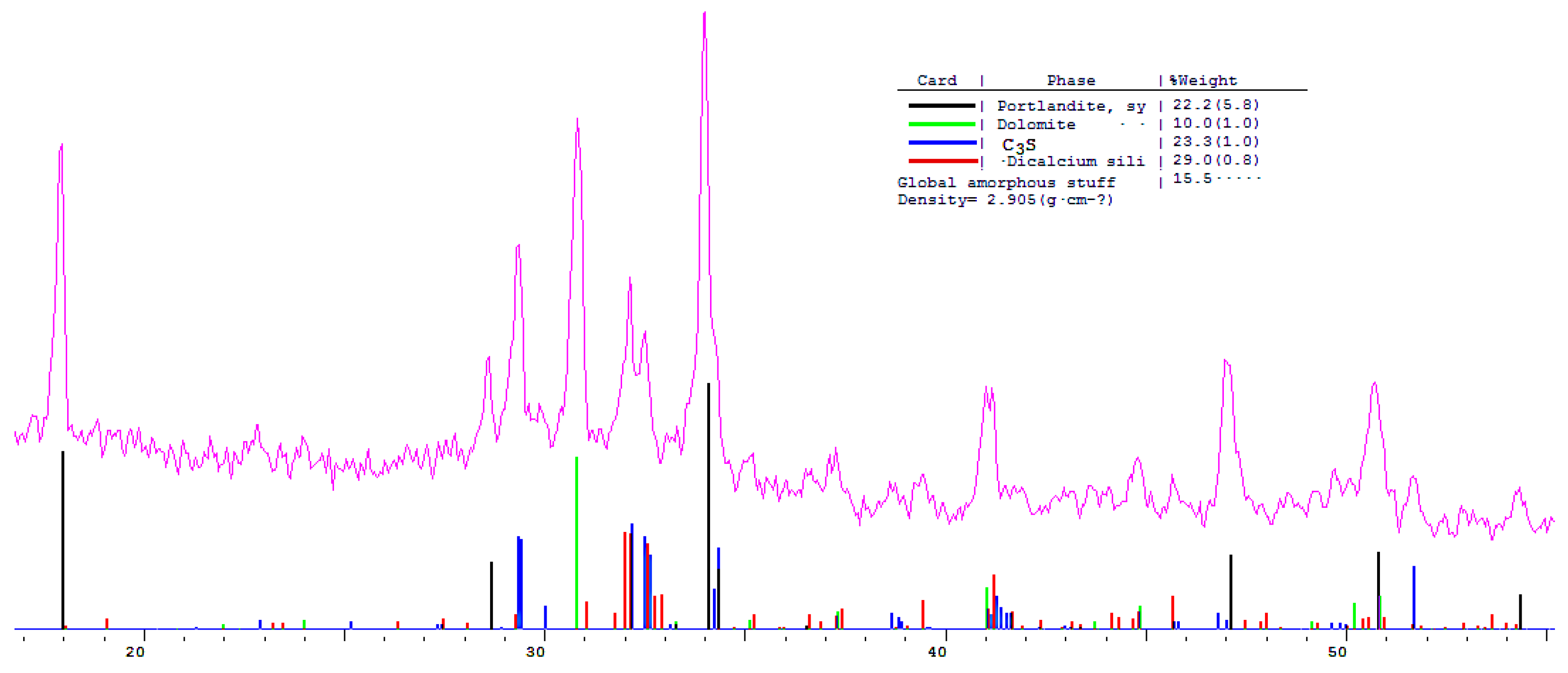
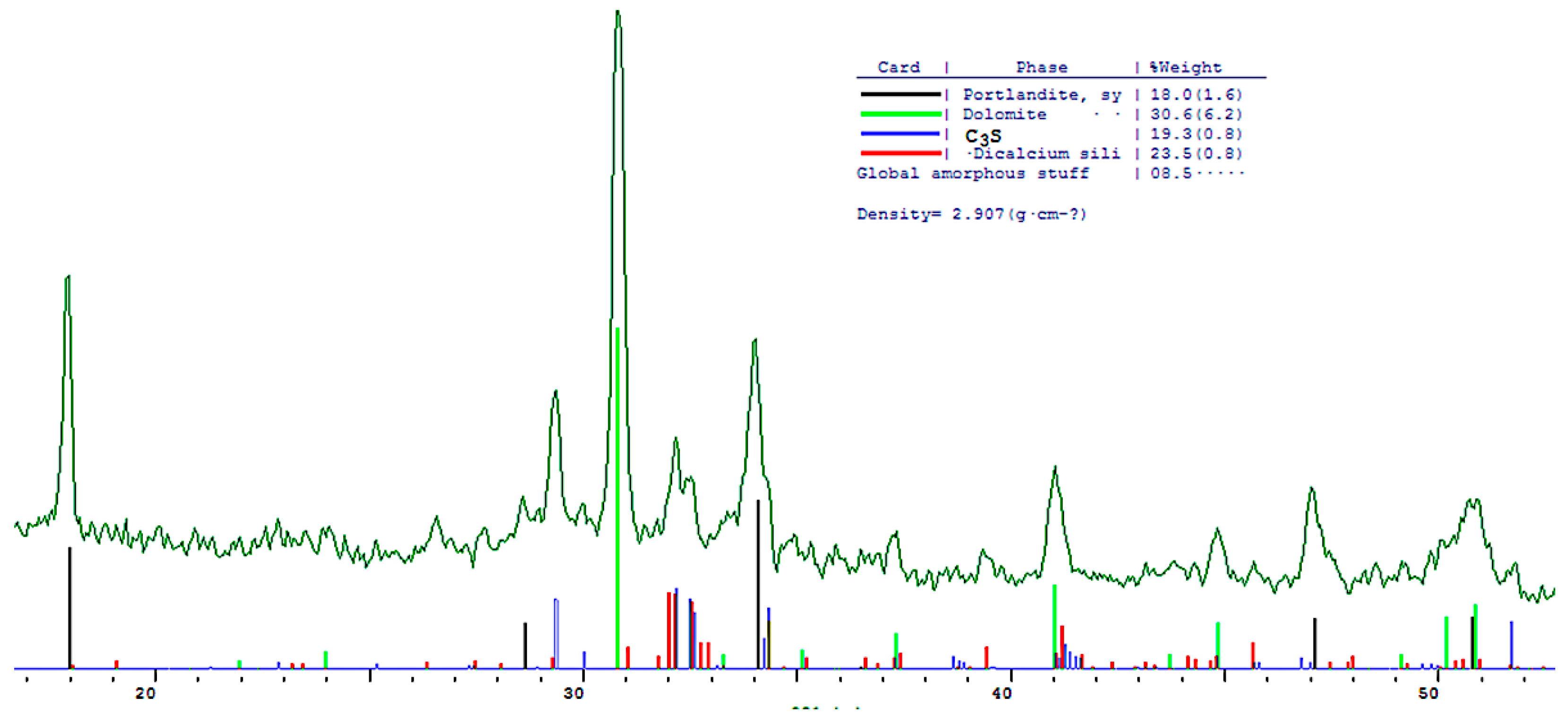
The phase composition of hydration products of Portland and blended cements with 10 and 30% dolomite filler with a specific surface area of 639.9 m2/kg was researched. The analysis of the XRF data showed that at the age of 28 days of standard curing condition, in addition to hydrate phases, such as Ca(OH)2 and X-ray amorphous calcium hydrosilicates, the main clinker minerals alite (C3S) and belite (C2S) are present in all samples (Figure 13, Figure 14 and Figure 15).
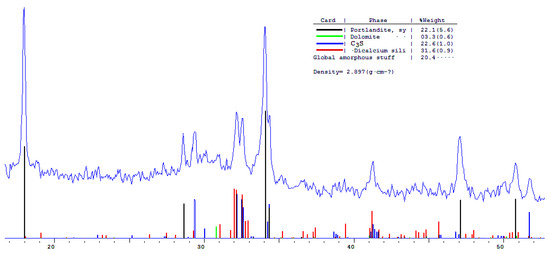
Figure 13.
Comparative phase analysis of pure Portland cement with a card file.
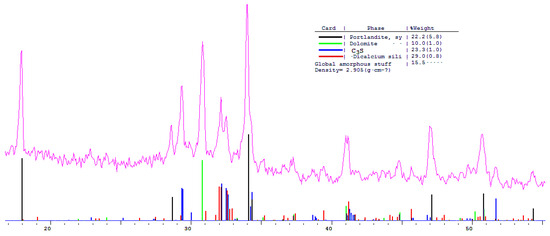
Figure 14.
Comparative phase analysis of blended cement with 10% dolomite filler with a card file.
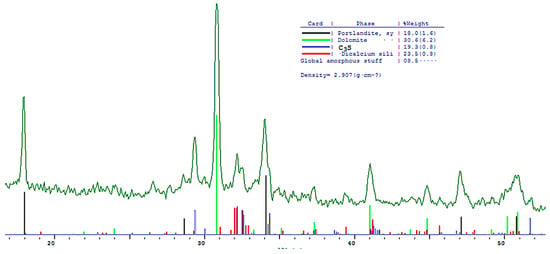
Figure 15.
Comparative phase analysis of blended cement with 30% dolomite filler with a card file.
A quantitative phase analysis showed that the sample with 30% dolomite filler contains 26% Ca(OH)2; 19% C3S (alite); 22% C2S (belite); and 8–10% amorphous phase. The sample with 10% dolomite filler contains 28% Ca(OH)2; 21% C3S (alite); 26% C2S (belite); and 15% amorphous phase. The pure Portland cement sample consisted of 33% Ca(OH)2; 21% C3S (alite); 28% C2S (belite); and 18–20% amorphous phase.
The content of Ca(OH)2 in pure Portland cement is increased, while its content in samples with 10 and 30% dolomite filler is reduced. Adding 10 and 30% of dolomite filler to Portland cement binder leads to a decrease in the content of the minerals alite (C3S) and belite (C2S), which indicates the influence of the dolomite filler on the acceleration of the hydration reaction of minerals with water.
4. Conclusions
The following conclusions can be drawn from this study:
Dolomite quarry waste activated in a laboratory ball mill can be used as a filler in blended cement. The necessary fineness of the dolomite filler is achieved by regulating the activation time. It was found that dolomite filler consists of ultrafine particles with a predominant size of less than 5 μm. The effect of the partial replacement of Portland cement with dolomite filler on the properties of blended binder was researched.
It was found that water demand decreases despite the presence of ultrafine particles of the filler. This can reduce water consumption and shrinkage deformation in hot weather.
The presence of dolomite filler in blended cement showed the influence on strength properties. If the content of the filler is low (10%), the strength will slightly increase, same as the average density of the cement paste. This enhancement can be explained by the particle size effect of the filler, which provides the additional nucleation sites. If the content of the filler is high, the strength of the binder decreases, and the “dilution” effect can be observed.
It was found that substitution of Portland cement with a filler up to 50% by weight leads to a decrease in drying shrinkage that can be explained by the low water demand of the blended binder.
It was found that the presence of the filler decreases the setting time of the blended binder, which can be explained by the acceleration of the hydration of Portland cement. The temperature and shrinkage deformations of the concrete can be reduced due to the rapid setting of the concrete mixture.
The results presented in this article clearly show that dolomite filler produced better properties in reference to pure Portland cement. The use of the filler must be limited in freeze–thaw environments. Future research should focus on the effect of the dolomite filler on concrete durability in hot weather with necessary properties. The influence of the filler on heat evolution during hardening, setting time, slump loss, and uniformity at elevated temperatures and low relative humidity should be researched.
Author Contributions
Conceptualization, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A.; methodology, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A.; software, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A.; validation, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A.; investigation, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A.; data curation, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A.; writing—original draft preparation, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A.; visualization, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A.; supervision, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A.; project administration, S.V.S.; funding acquisition, S.V.S., O.A.L., I.V.K., D.G.A. and D.A.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (grant # 075-15-2021-686). Tests were carried out using research equipment of The Head Regional Shared Research Facilities of the Moscow State University of Civil Engineering.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soroka, I.; Ravina, D. Hot weather concreting with admixtures. Cem. Concr. Compos. 1998, 20, 129–136. [Google Scholar] [CrossRef]
- Abbas, Y.M.; Fares, G.; Khan, M.I. Impact of Hot Weather Conditions on the Performance of Supplementary Cementitious Materials Concrete. Sustainability 2023, 15, 8393. [Google Scholar] [CrossRef]
- Ahmadi, B.H. Initial and final setting time of concrete in hot weather. Mater. Struct. 2000, 33, 511–514. [Google Scholar] [CrossRef]
- Samchenko, S.V.; Larsen, O.A.; Alobaidi, D.A.N.; Naruts, V.V.; Bakhrah, A.M.; Solodov, A.A. Concrete based on carbonate raw materials for dry hot climate. Ind. Civ. Constr. 2022, 9, 74–79. [Google Scholar]
- Fawzy, A.; Elshami, A.; Ahmad, S. Investigating the Effects of Recycled Aggregate and Mineral Admixtures on the Mechanical Properties and Performance of Concrete. Materials 2023, 16, 5134. [Google Scholar] [CrossRef]
- Mailar, G.; Sujay Raghavendra, N.; Sreedhara, B.M.; Manu, D.S.; Hiremath, P.; Jayakesh, K. Investigation of concrete produced using recycled aluminium dross for hot weather concreting conditions. Resour.-Effic. Technol. 2016, 2, 68–80. [Google Scholar]
- Fraser, J.; McBride, R.A. The utility of aggregate processing fines in the rehabilitation of dolomite quarries. Land Degrad. Dev. 2000, 1, 1–17. [Google Scholar] [CrossRef]
- Javali, S.; Chandrashekar, A.R.; Raghavendra Naganna, S.; Manu, D.S.; Hiremath, P.; Preethi, H.G.N.; Kumar, V. Eco-concrete for sustainability: Utilizing aluminium dross and iron slag as partial replacement materials. Clean Technol. Environ. Policy 2017, 19, 2291–2304. [Google Scholar] [CrossRef]
- Li, X.; Qin, D.; Hu, Y.; Ahmad, W.; Ahmad, A.; Aslam, F.; Joyklad, P. A systematic review of waste materials in cement-based composites for construction applications. J. Build. Eng. 2022, 45, 103447. [Google Scholar] [CrossRef]
- Market Study: Fillers. 2018. Available online: www.ceresana.com/en (accessed on 14 May 2020).
- Thorpe, D.; Zhuge, Y. Advantages and disadvantages in using permeable concrete pavement as a pavement construction material. In Proceedings of the 26th Annual ARCOM Conference, Leeds, UK, 6–8 September 2010; pp. 1341–1350. [Google Scholar]
- Moosberg-Bustnes, H.; Lagerblad, B.; Forssberg, E. The function of fillers in concrete. Mater. Struct. 2004, 37, 74–81. [Google Scholar] [CrossRef]
- Garg, A.; Aggarwal, P.; Aggarwal, Y.; Belarbi, M.O.; Chalak, H.D.; Tounsi, A.; Gulia, R. Machine learning models for predicting the compressive strength of concrete containing nano silica. Comput. Concr. 2022, 30, 33–42. [Google Scholar] [CrossRef]
- Hunger, M. An Integral Design Concept for Ecological Self-Compacting Concrete. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Lawrence, M.C.; Ringot, E. Mineral admixtures in mortars. Effect of inert materials on short-term hydration. Cem. Concr. Res. 2003, 33, 1939–1947. [Google Scholar] [CrossRef]
- Michel, F.; Piérard, J.; Courard, L.; Pollet, V. Influence of physico-chemical characteristics of limestone fillers on fresh and hardened mortar performances. In 5th International RILEM Symposium on Self-Compacting Concrete, Proceedings PRO 54; CONFpnb ERENCE: SCC 2007 GHENT; RILEM Publications SARL: Bagneux, France, 2007. [Google Scholar]
- Samchenko, S.V.; Alexandrova, O.V.; Gurkin, A.Y. Properties of cement composites based on limestone depending on its granulometric composition. Bull. MGSU 2020, 15, 999–1006. [Google Scholar]
- Pera, J.; Husson, S.; Guilhot, B. Influence of finely ground limestone on cement hydration. Cem. Concr. Compos. 1999, 21, 99–105. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Chun-Mei, Z. Dependence of the fineness of carbonate on the hydration behaviour of tricalcium silicate. Durab. Build. Mater. 1986, 4, 45–66. [Google Scholar]
- Ramachandran, V.S.; Zhang, C. Cement with calcium carbonate additions. In Proceedings of the 8th International Congress on the Chemistry of Cement, Rıo de Janeiro, Brazil, 22–27 September 1986; pp. 178–187. [Google Scholar]
- Samchenko, S.V.; Kudryashov, N.I.; Gurkin, A.Y. Thermodynamic assessment of the effect of calcium carbonate on cement hydration. Tech. Technol. Silic. 2020, 27, 6–12. [Google Scholar]
- Balakrishnan, S.D.; Paulose, K.C. Workability and strength characteristics of self-compacting concrete containing fly ash and dolomite powder. Am. J. Eng. Res. 2013, 24, 43–47. [Google Scholar]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Korjakins, A.; Gaidukovs, S.; Sahmenko, G.; Bajare, D.; Pizele, D. Investigation of alternative dolomite filler properties and their application in concrete production. Sci. Proceedia Riga Tech. Univ. Constr. Sci. 2008, 2, 64–71. [Google Scholar]
- Ranjith Kumar, L.; Kiran, J.; Rangarajan, P. Properties of Concrete Incorporating Dolomite Powder. IOSR J. Mech. Civ. Eng. 2017, 14, 78–80. [Google Scholar] [CrossRef]
- Schöne, S.; Dienemann, W.; Wagner, E. Portland dolomite cement as an alternative to Portland limestone cement. In Proceedings of the 13th International Congress on the Chemistry of Cement (13th ICCC), Madrid, Spain, 3–8 July 2011. [Google Scholar]
- Lu, J.; Xu, A.; Xu, J.; Lu, D.; Xu, Z. Sulfate resistance of Portland dolomite cement: Performance and mechanisms. Mater. Struct. 2020, 53, 125. [Google Scholar] [CrossRef]
- Chyad Liejy, M.; Al Zand, A.W.; Mutalib, A.A.; Alghaaeb, M.F.; Abdulhameed, A.A.; Al-Attar, A.A.; Tawfeeq, W.M.; Hilo, S.J. Flexural Performance of a Novel Steel Cold-Formed Beam–PSSDB Slab Composite System Filled with Concrete Material. Buildings 2023, 13, 432. [Google Scholar] [CrossRef]
- Abdulhameed, A.A.; Al-Zuhairi, A.H.; Al Zaidee, S.R.; Hanoon, A.N.; Al Zand, A.W.; Hason, M.M.; Abdulhameed, H.A. The Behavior of Hybrid Fiber-Reinforced Concrete Elements: A New Stress-Strain Model Using an Evolutionary Approach. Appl. Sci. 2022, 12, 2245. [Google Scholar] [CrossRef]
- Courard, L.; Herfort, D.; Villagran, Y. Limestone powder. In Properties of Fresh and Hardened Concrete Containing Supplementary Cementitious Materials. State-of-the-Art Report of the RILEM Technical Committee 238-SCM, Working Group 4; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Gallias, J.L.; Kara-Ali, R.; Bigas, J.P. The effect of fine mineral admixtures on water requirement of cement pastes. Cem. Concr. Res. 2000, 30, 1543–1549. [Google Scholar] [CrossRef]
- Boos, P.; Hardtl, R. Experience Report Portland Limestone Cement; Report HeidelbergCement Technology Center: Heidelberg, Germany, 2004; p. 34. [Google Scholar]
- Bentz, D.P.; Jones, S.Z.; Lootens, D. Minimizing Paste Content in Concrete Using Limestone Powders—Demonstration Mixtures. National Institute of Standards and Technology; Technical Note; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1906. [Google Scholar]
- GOST 31108-2020; Common Cements. Specifications Introduced. 1 March 2021. Standartinform: Moscow, Russia, 2016.
- GOST R 51795-2001; Cements. Methods for Determining the Content of Mineral Additives. Introduced 28 June 2001. Gosstroy of Russia: Moscow, Russia, 2001.
- Chung, F.H. Quantitative Interpretation of X-ray Diffraction Patterns of Mixtures. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar] [CrossRef]
- GOST 30744–2001; Cements. Test Methods Using Polyfraction Sand. Introduced. 1 March 2002. Gosstroy of Russia: Moscow, Russia, 2002.
- GOST 24544-2020; Concretes. Methods of Shrinkage and Creep Flow Determination Introduced. 1 June 2021. Standartinform: Moscow, Russia, 2021.
- GOST 31424-2010; Non-Metallic Construction Materials from Sifting of Crushing Solid Stone in Aggregate Manufacturing. Specifications Introduced 1 July 2011. Standartinform: Moscow, Russia, 2019.
- Mezhov, A.; Pott, U.; Stephan, D.; Kovler, K. Influence of mechanical activation of fly ash in presence of polynaphthalene sulfonate superplasticizer on rheology and hydration kinetics of cement—Fly ash pastes. Constr. Build. Mater. 2019, 210, 380–390. [Google Scholar] [CrossRef]
- Barbhuiya, S. Effects of fly ash and dolomite powder on the properties of self-compacting concrete. Constr. Build. Mater. 2011, 25, 3301–3305. [Google Scholar] [CrossRef]
- Dvorkin, L.I.; Solomatov, V.I.; Vyrovoy, V.N.; Chudnovsky, S.M. Cement Concretes with Mineral Fillers; Budivelnyk: Kyiv, Ukraine, 1991; p. 136. [Google Scholar]
- Basin, V.E. Adhesive Strength; Chemistry: Moscow, Russia, 1981; p. 208. [Google Scholar]
- Kumar, R.; Kumar, S.; Mehrotra, S.P. Towards sustainable solutions for fly ash through mechanical activation: Review. Resour. Conserv. Recycl. 2007, 52, 157–179. [Google Scholar] [CrossRef]
- Rajak, D.K.; Raj, A.; Guria, C.; Pathak, A.K. Grinding of Class-F fly ash using planetary ball mill: A simulation study to determine the breakage kinetics by direct- and back-calculation method. S. Afr. J. Chem. Eng. 2017, 24, 135–147. [Google Scholar] [CrossRef]
- Okamura, H.; Ouchi, M. Development of self-compacting concrete, present use and future. In 1st International Exhibition of Self–Compacting Concrete; Petersson, I.S., Ed.; RILEM Publications S.A.R.L.: Stockholm, Sweden, 1999; pp. 3–14. [Google Scholar]
- Puntke, W. Wasseranspruch von feinen Kornhaufwerken. Concrete 2002, 52, 242–248. [Google Scholar]
- Tsivilis, S.; Chaniotakis, E.; Badogiannis, E.; Pahoulasa, G.; Ilias, A. A study on the parameters affecting the properties of Portland limestone cements. Cem. Concr. Compos. 1999, 21, 107–116. [Google Scholar] [CrossRef]
- Schiller, B.; Ellerbrock, H.G. The grinding and properties of cements with several main constituents. Zement-Kalk-Gips 1992, 45, 223–231. [Google Scholar]
- Tobon, J.I.; Kazes Gomez, R. Behavior of Portland Cement blended with limestones of different purity degrees. Dyna 2008, 75, 177–184. [Google Scholar]
- Courard, L.; Degeimbre, R.; Darimont, A.; Michel, F.; Willem, X.; Flamant, S. Some effects of limestone aggregates as a partial replacement of cement in solutions of the composition. In Proceedings of the ConMat’05 Third International Conference on Building Materials: Performance, Innovation and Design Implications, Vancouver, BC, Canada, 22–24 August 2005. [Google Scholar]
- Voglis, N.; Kakali, G.; Chaniotakis, E.; Tsivilis, S. Portland-limestone cements. Their properties and hydration compared to those of other composite cements. Cem. Concr. Compos. 2005, 27, 191–196. [Google Scholar] [CrossRef]
- Topccu, I.B.; Baylavli, H. The use of concrete wastes as a limestone replacement in limestone-blended cement production. Kuwait J. Sci. 2019, 46, 67–73. [Google Scholar]
- Stark, J.; Moser, B.; Bellmann, F. Nucleation and growth of C-S-H phases on mineral admixtures. In Advances in Construction Materials; Springer: Berlin/Heidelberg, Germany, 2007; pp. 531–538. [Google Scholar]
- Brykov, A.S.; Voronkov, M.E.; Antonov, P.A. Influence of dolomite-containing fillers and fillers on the stability of Portland cement solutions in an alkaline medium. Cem. Its Appl. 2020, 3, 90–93. [Google Scholar]
- Zajac, M.; Rossberg, A.; Le Saout, G.; Lothenbach, B. Influence of limestone and anhydrite on thehydration of Portland cements. Cem. Concr. Compos. 2014, 46, 99–108. [Google Scholar] [CrossRef]
- Kouznetsova, T.V.; Samchenko, S.V. Resistance of the calcium sulphoaluminate phases to carbonation. Cem. Wapno Beton 2014, 5, 317–322. [Google Scholar]
- Briki, Y.; Zajac, M.; Haha, M.B.; Scrivener, K. Impact of limestone fineness on cement hydration at early age. Cem. Concr. Res. 2021, 147, 106515. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).