Performance Analysis of Industrial-Waste-Based Artificial Aggregates: CO2 Uptake and Applications in Bituminous Pavement
Abstract
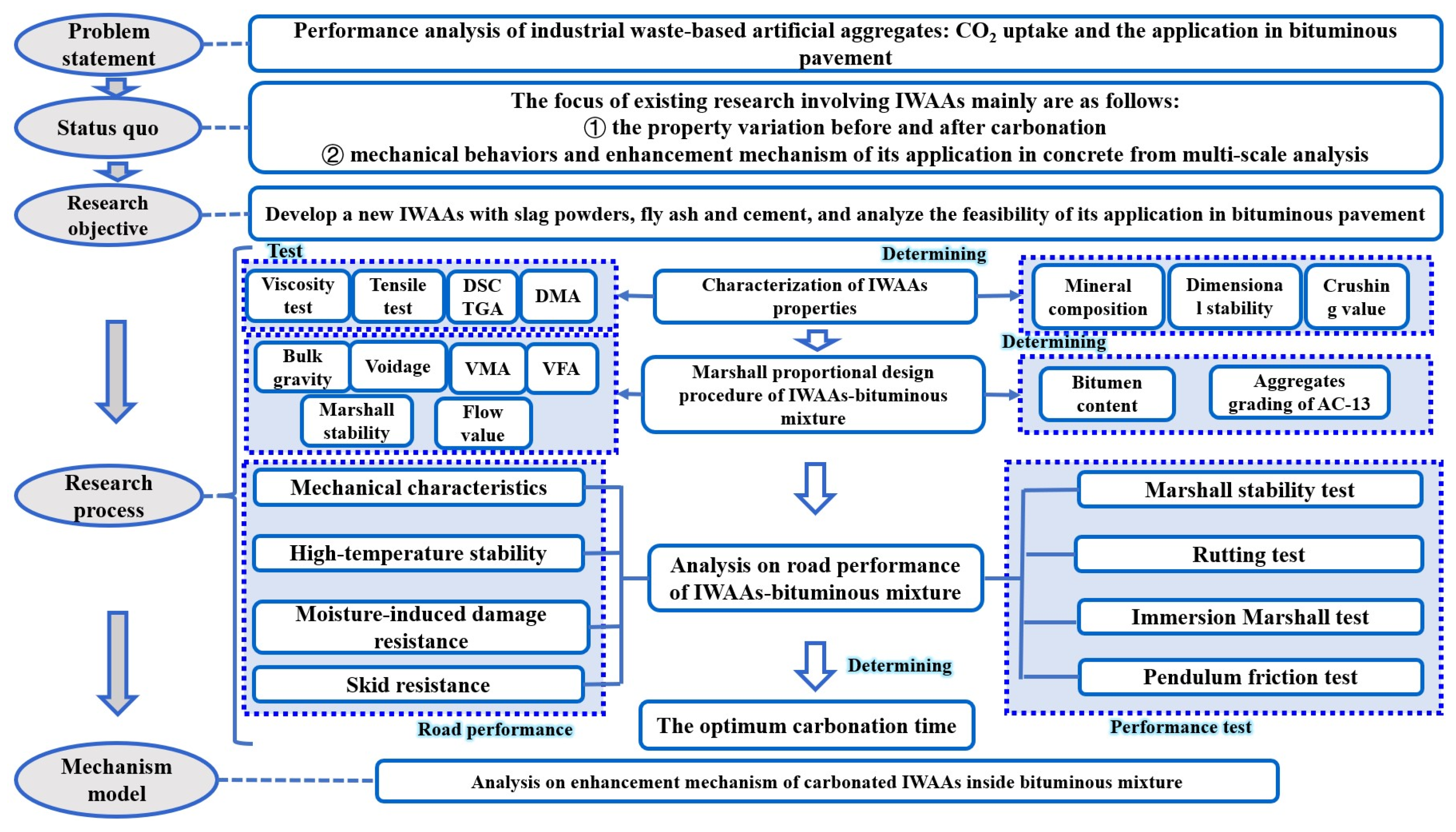
:1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Preparation and Carbonation Process of IWAAs Specimens
2.3. Characterization of IWAAs Properties
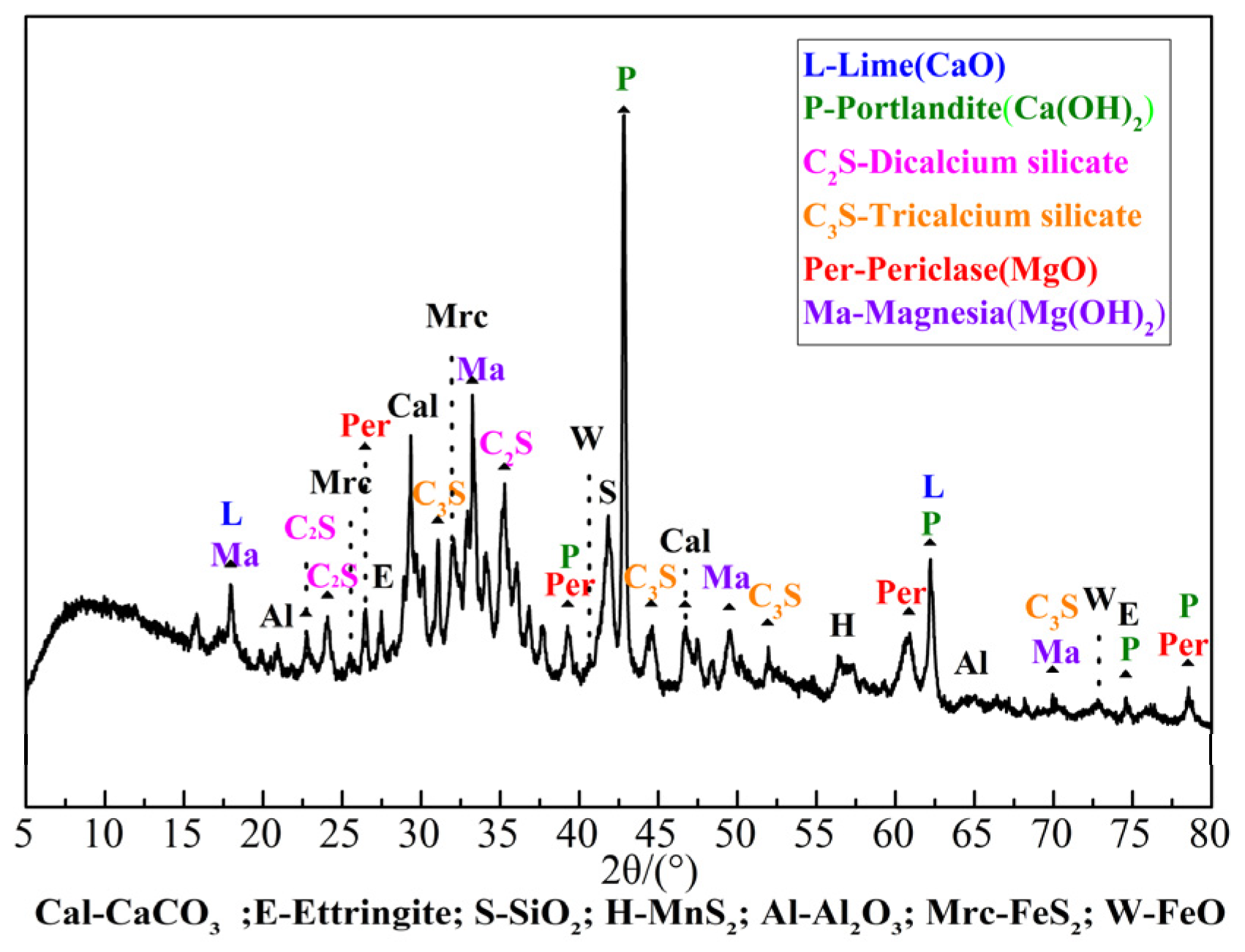
2.3.1. Mineral Composition Detection
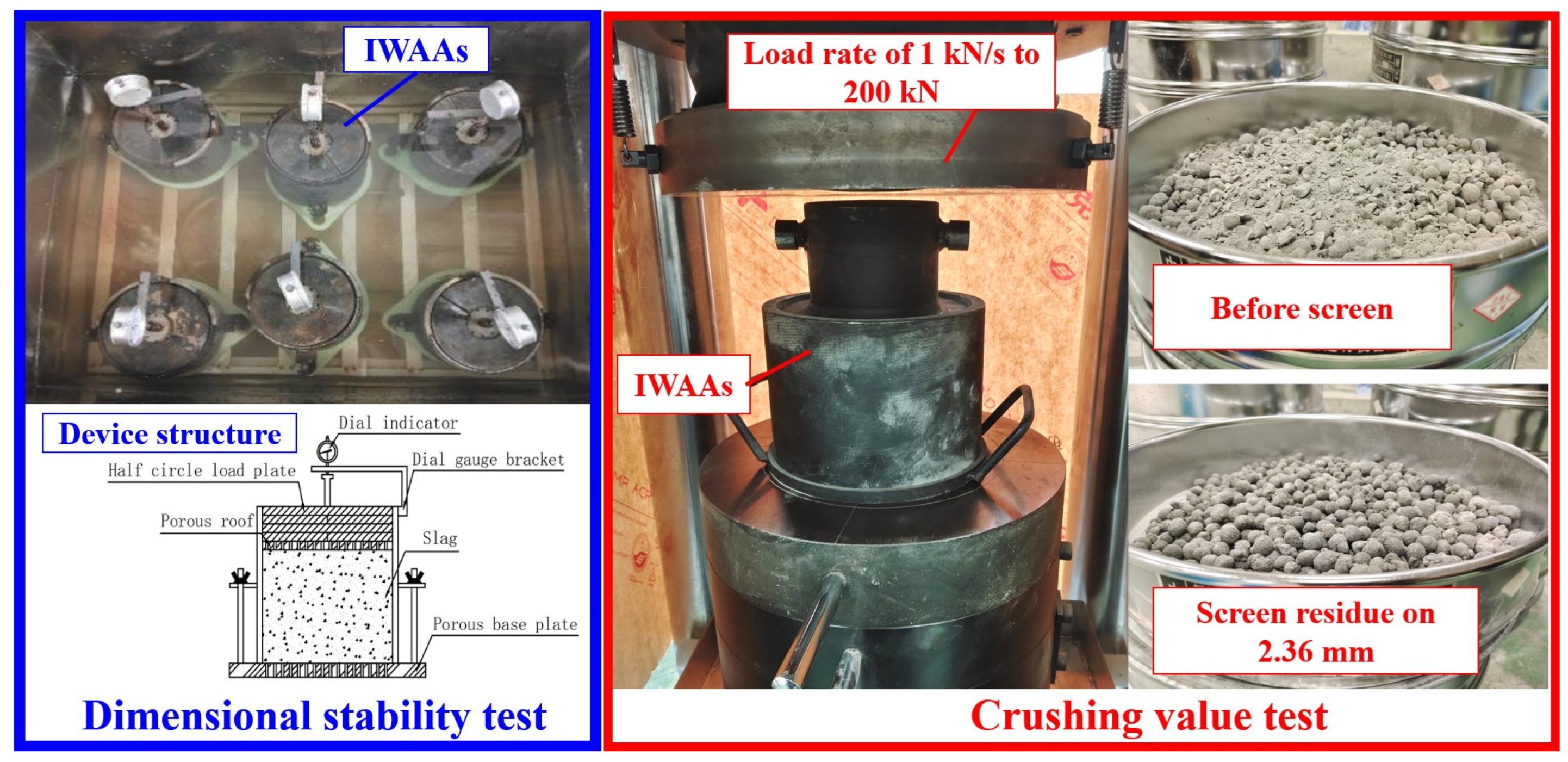
2.3.2. Dimensional Stability and Crushing Value of IWAAs
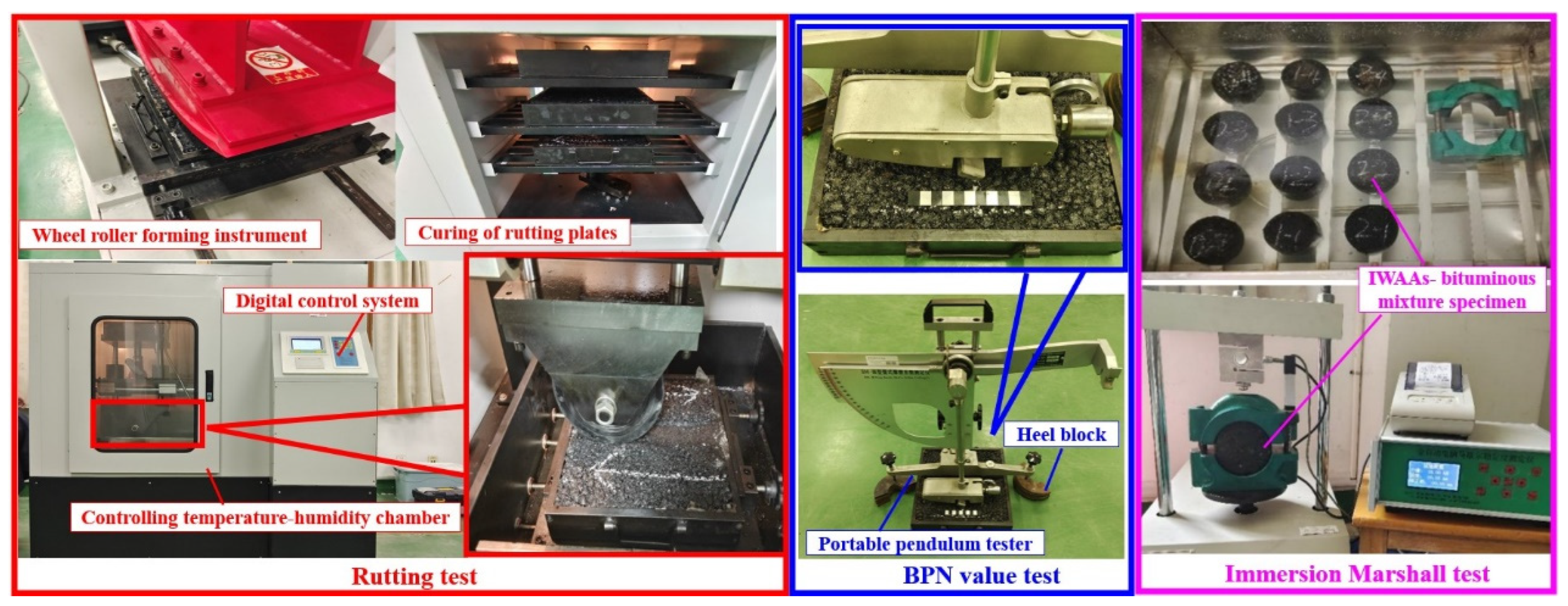
2.4. Performance of the IWAAs–Bituminous Mixture
3. Results and Discussion
3.1. Properties and Characteristics of the IWAAs
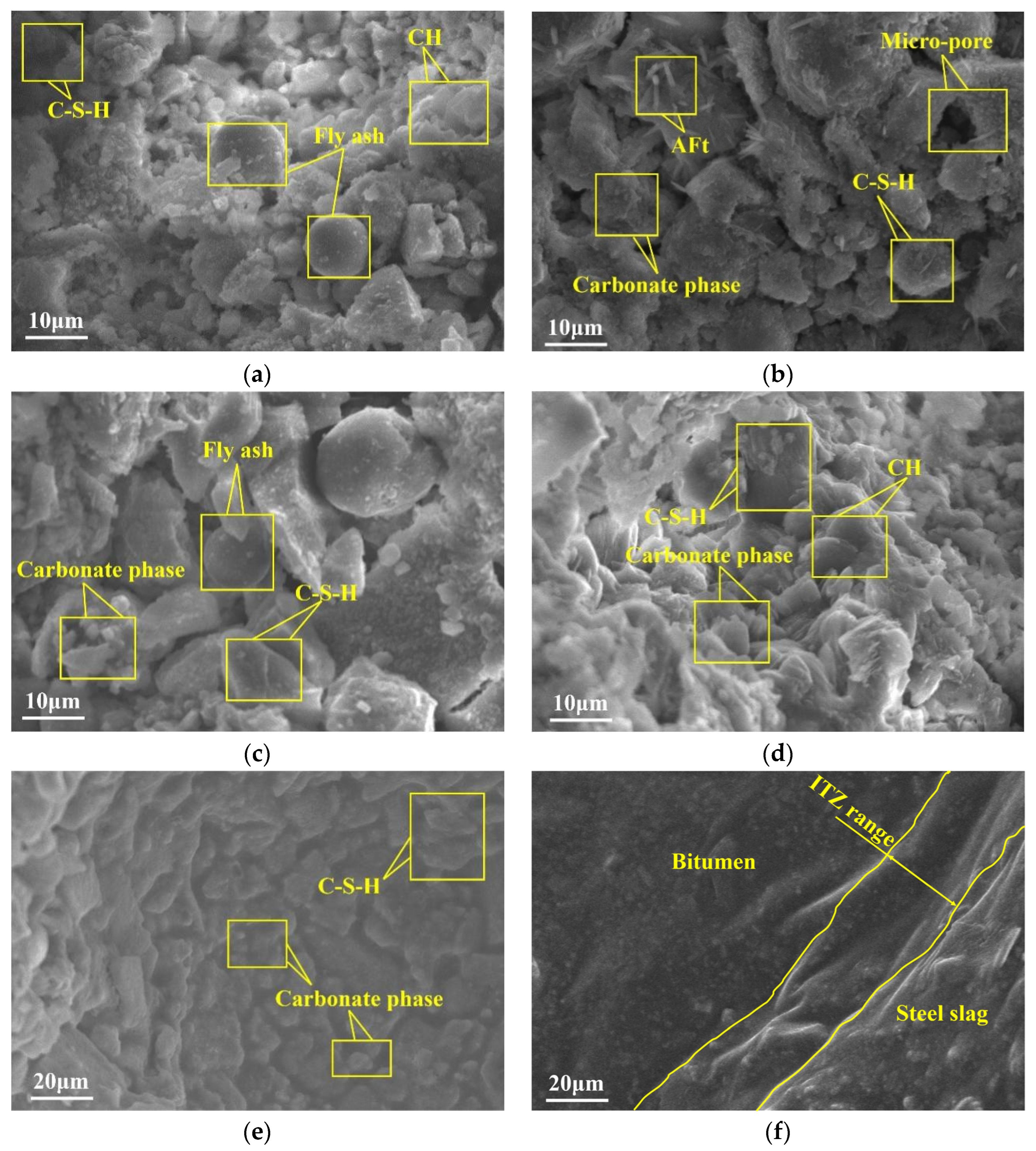
3.1.1. Microscopic Morphology
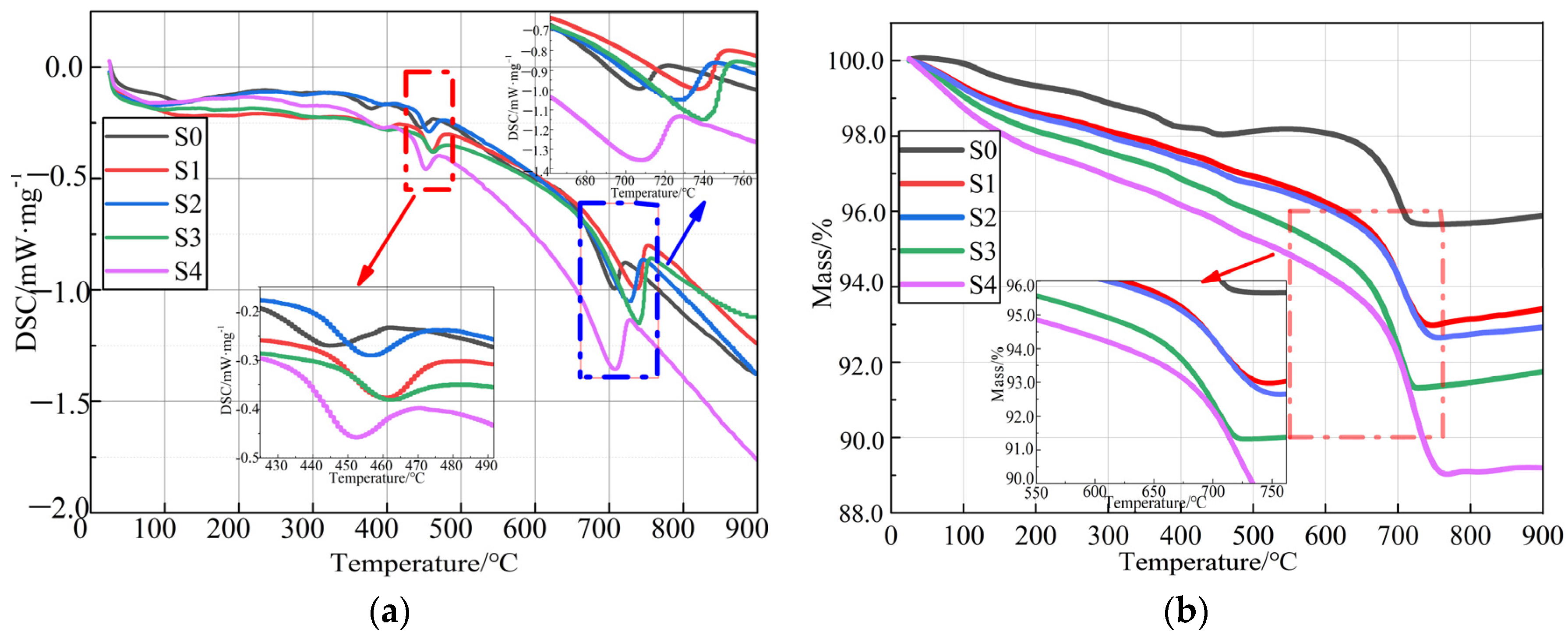
3.1.2. TG/DSC Thermal Analysis
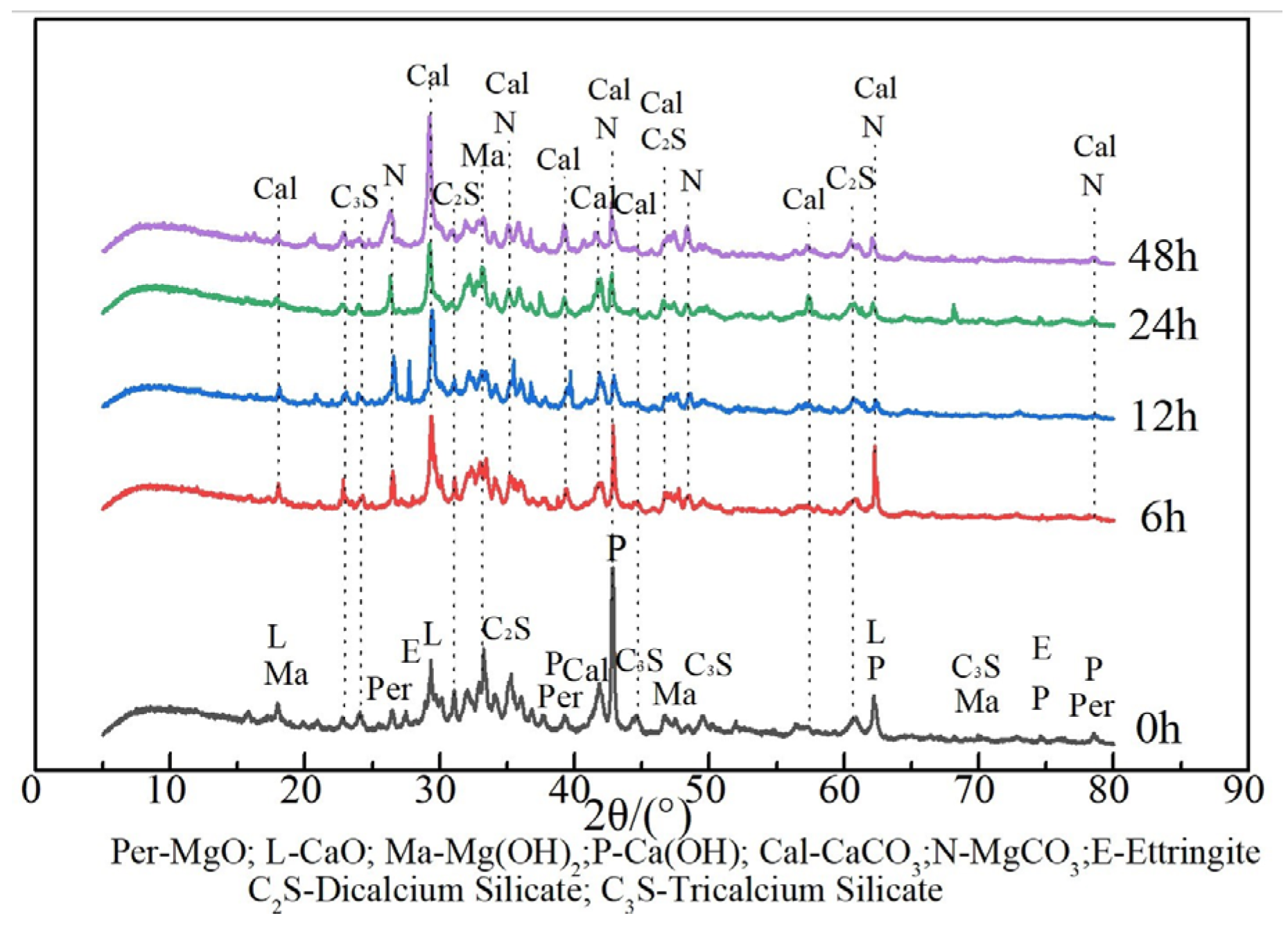
3.1.3. Mineral Composition Analysis
3.2. Performance Analysis of IWAAs
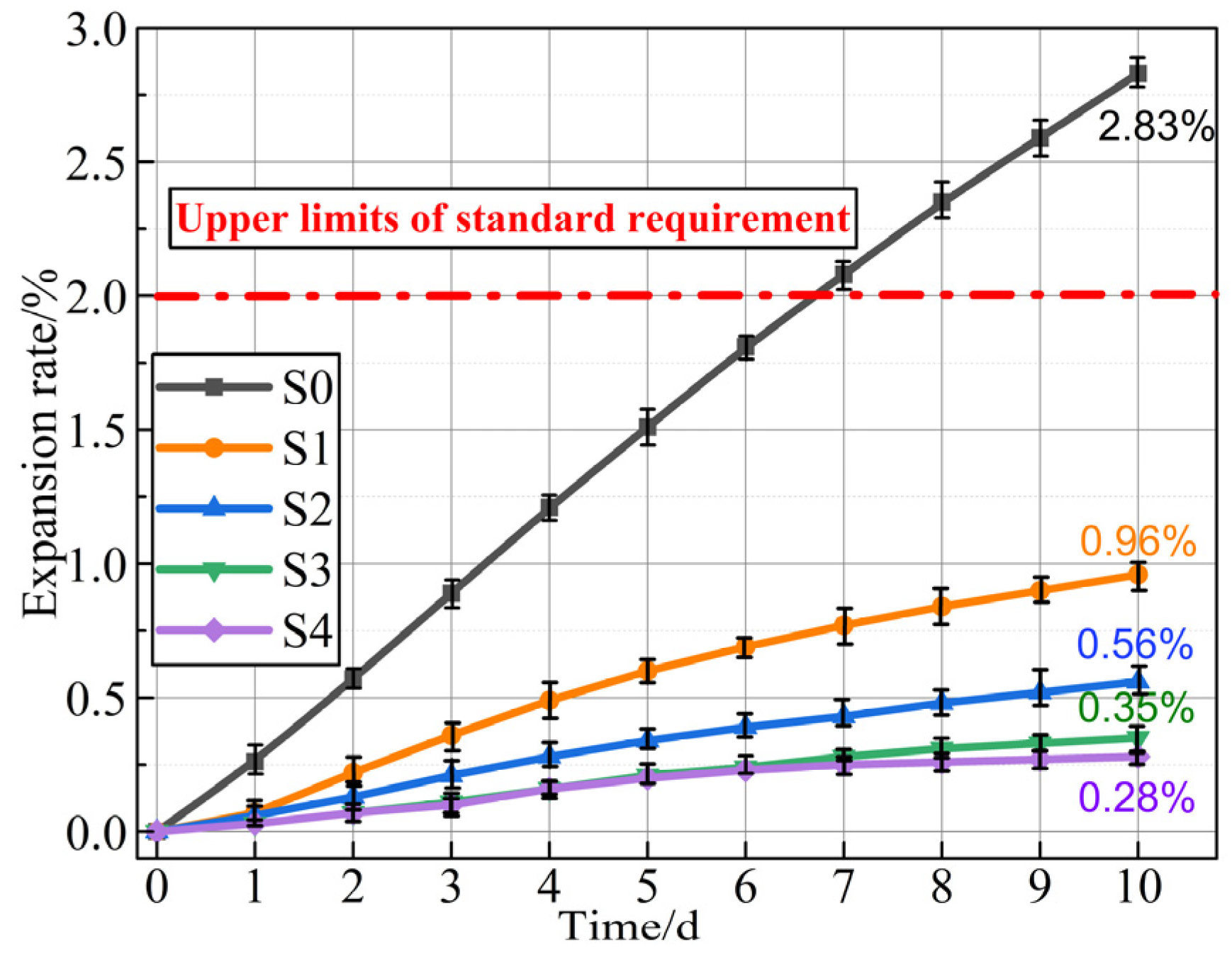
3.2.1. Dimensional Stability
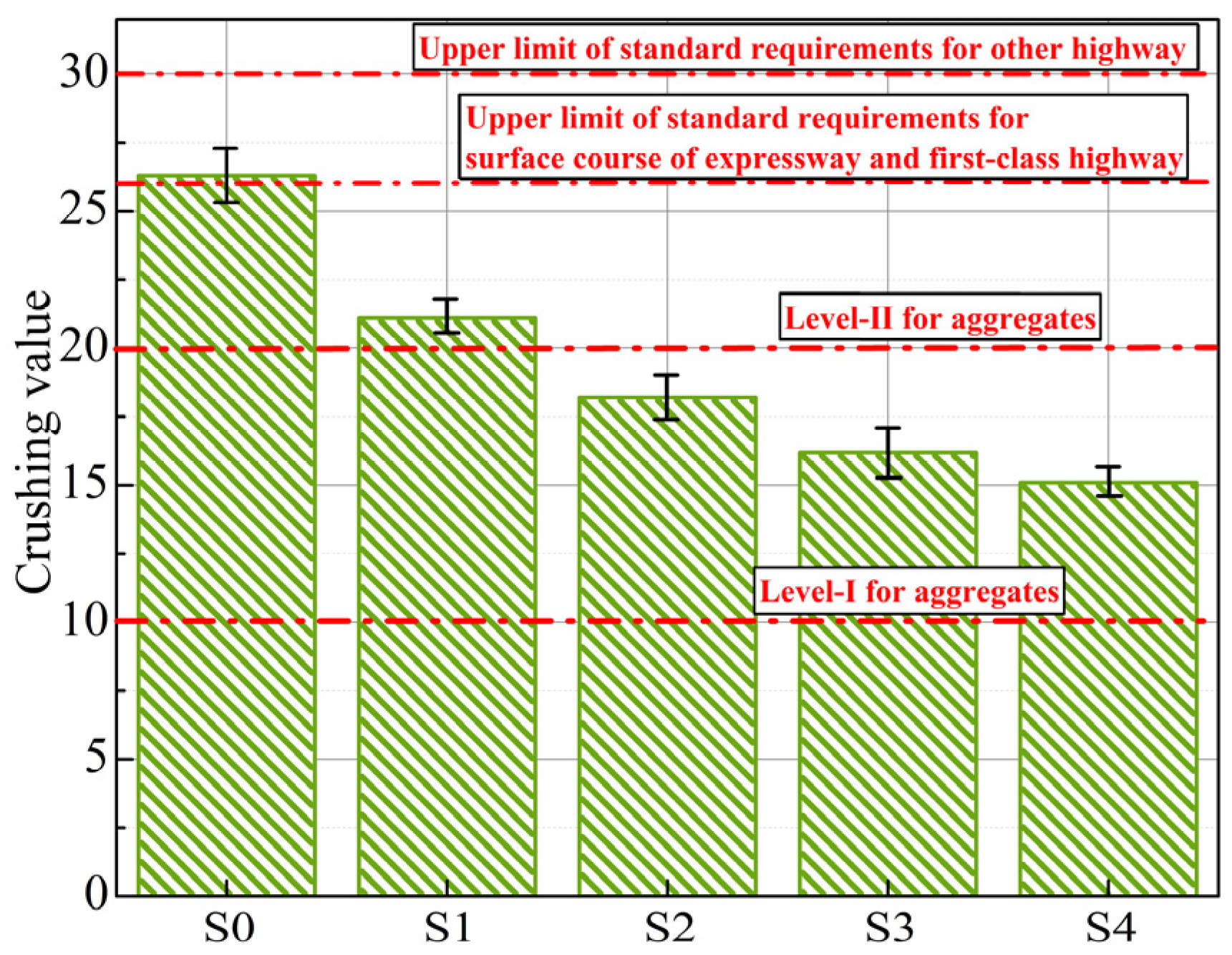
3.2.2. Crushing Value
3.3. Performance Characterization of IWAAs–Bituminous Mixture
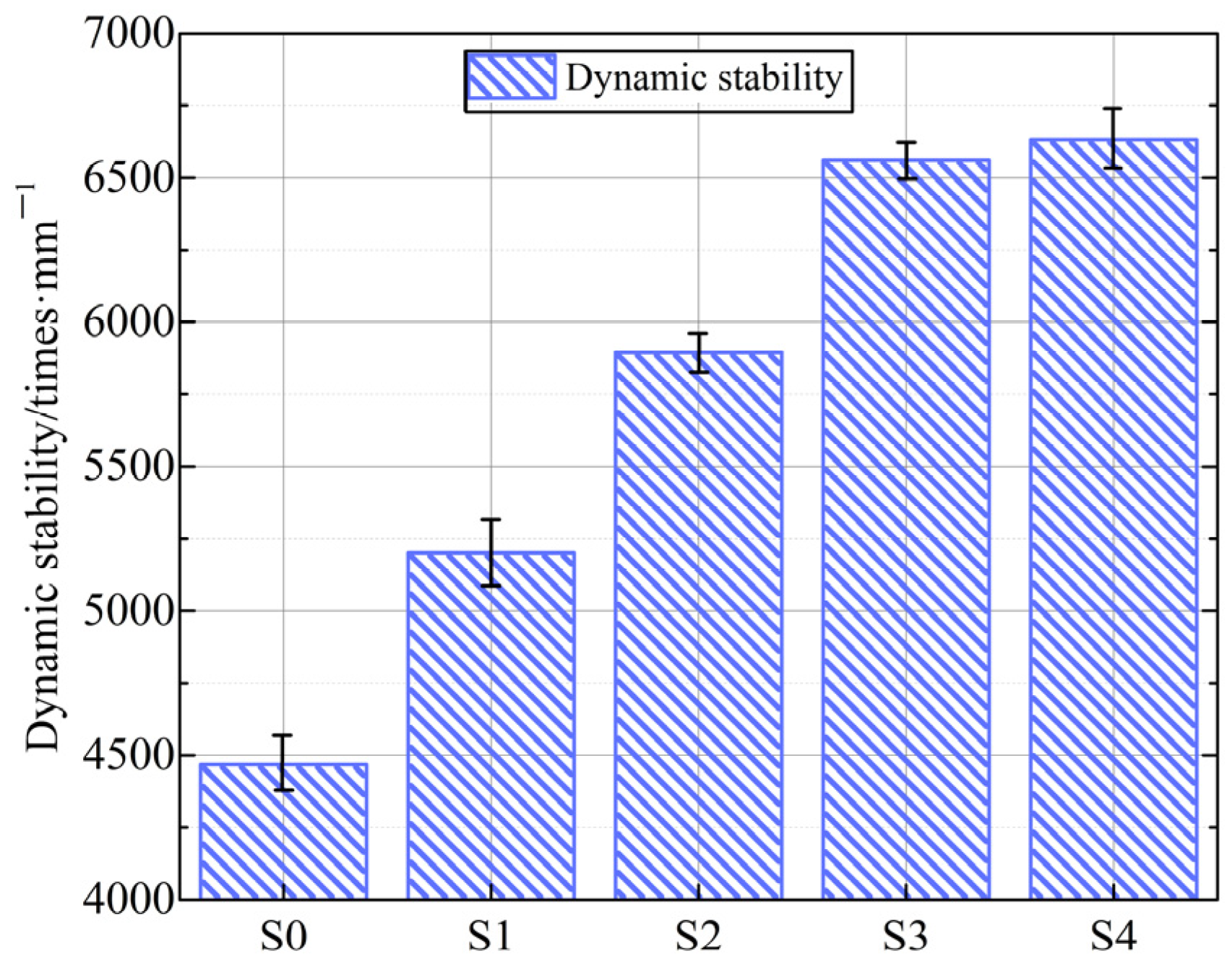
3.3.1. High-Temperature Rutting Resistance
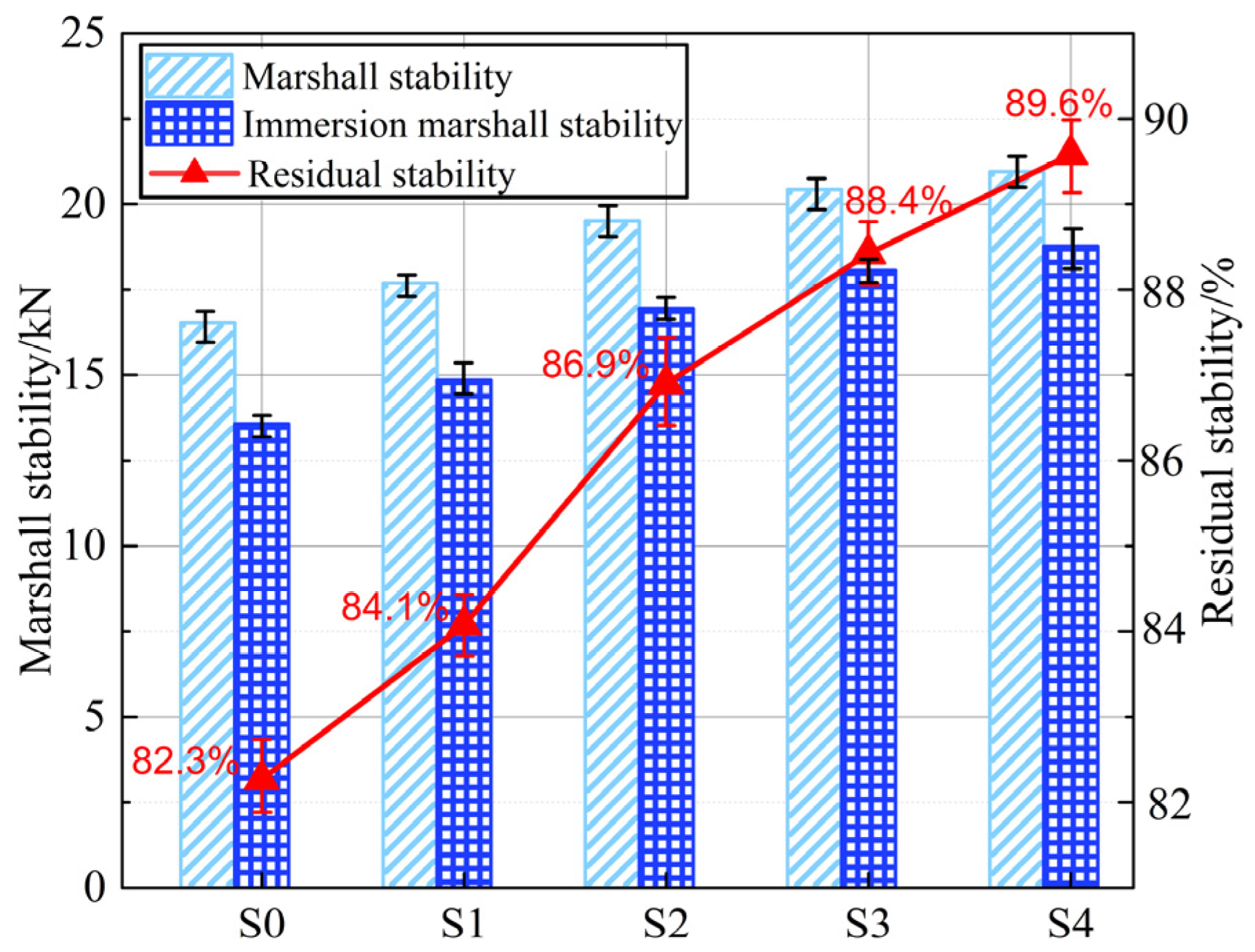
3.3.2. Moisture Damage Resistance
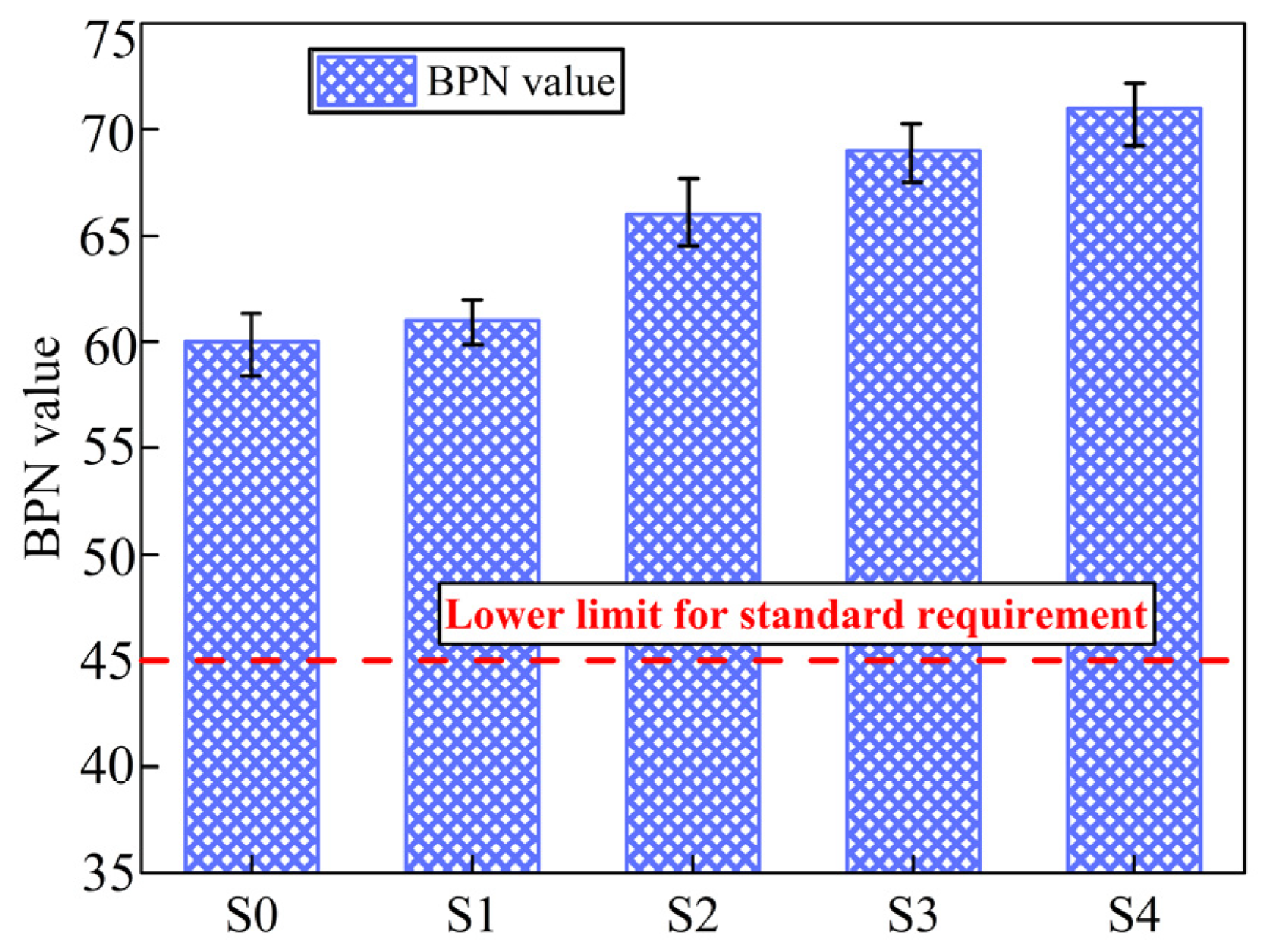
3.3.3. Skid-Resistant Performance
4. Conclusions
- (1)
- With the progress in the CO2 uptake, the Ca-based (e.g., f-CaO and Portlandite) and Mg-based (e.g., periclase and brucite) compounds, as well as the hydration products inside IWAAs, might have a chemical reaction with CO2 to generate stabilized carbonation crystals, effectively boosting the enhancement of the microstructural densification and homogenization. Herein, the 12 h carbonation time could improve both the anti-crushing properties and the dimensional stability by 82.21% and 41.48%, respectively.
- (2)
- Based on the mineral composition analysis, the carbonation efficiency of the IWAAs progressively decreased with the extension of the carbonation time, since the carbonated phase generated from the mineralization reaction would exert a replenishing and densification effect on the surface and hinder the CO2 uptake and diffusion into IWAAs during the further carbonation.
- (3)
- The high-temperature rut resistance, moisture damage resistance, and skid resistance gradually increased with prolonging the carbonation time from 6 h to 48 h for the IWAAs–bituminous mixture, where the optimal carbonation time was 12 h, and the corresponding increases were 31.92%, 5.59%, and 10.00%, respectively. The generated carbonate crystalline phase with abrasion resistance and irregular morphology could boost the enhancement of the microstructural densification and homogenization, together with excellent interfacial adhesion to bitumen due to its alkalinity.
- (4)
- After comprehensive consideration of the carbonation efficiency, the properties’ growth rates, and the limits values of the specification for IWAAs, as well as the IWAAs–bituminous mixture, the 12 h carbonation time is recommended during the preparation and carbonation process of IWAAs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhang, Z.; Lv, J.; Zhang, G. Effect of Lithium Silicate–Impregnated Limestone Aggregate on Skid Resistance Properties of Bituminous Mixture. J. Mater. Civ. Eng. 2022, 34, 04022251. [Google Scholar] [CrossRef]
- Khoshsepehr, Z.; Alinejad, S.; Alimohammadlou, M. Exploring industrial waste management challenges and smart solutions: An integrated hesitant fuzzy multi-criteria decision-making approach. J. Clean. Prod. 2023, 420, 138327. [Google Scholar] [CrossRef]
- Shi, M.; Ling, T.C.; Gan, B.; Guo, M.Z. Turning concrete waste powder into carbonated artificial aggregates. Constr. Build. Mater. 2019, 199, 178–184. [Google Scholar] [CrossRef]
- Ren, P.; Ling, T.; Mo, K. Recent advances in artificial aggregate production. J. Clean. Prod. 2021, 291, 125215. [Google Scholar] [CrossRef]
- Bekkeri, G.; Shetty, K.; Nayak, G. Synthesis of artificial aggregates and their impact on performance of concrete: A review. J. Mater. Cycles Waste Manag. 2023, 25, 1988–2011. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q. Preparation of artificial aggregates from concrete slurry waste and waste brick masonry powder: CO2 uptake and performance evaluation. Constr. Build. Mater. 2023, 382, 131356. [Google Scholar] [CrossRef]
- Fan, X.; Li, Z.; Zhang, W.; Jin, W.; Liu, J.; Xing, F.; Tang, L. New applications of municipal solid waste incineration bottom ash (MSWIBA) and calcined clay in construction: Preparation and use of an eco-friendly artificial aggregate. Constr. Build. Mater. 2023, 387, 131629. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T. Production of artificial aggregates from steel-making slag: Influences of accelerated carbonation during granulation and/or post-curing. J. CO2 Util. 2020, 36, 135–144. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, M.; Wu, P.; Wen, S.; Huang, L.; Ning, P. A Review of the Application of Steel Slag in CO2 Fixation. ChemBioEng Rev. 2021, 8, 189–199. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, R.; Yan, F.; Dong, R.; Kong, C.; Li, J. Analysis of Asphalt Mixtures Modified with Steel Slag Surface Texture Using 3D Scanning Technology. Materials 2023, 16, 3256. [Google Scholar] [CrossRef]
- Wan, J.; Wu, S.; Hu, X.; Li, Y.; Pan, P.; Gan, W. Assessment on Steel Slag-Based SMA-5 and AC-5 Asphalt Mixtures for Maintenance and Induction Heating. J. Mater. Civ. Eng. 2022, 34, 04021471. [Google Scholar] [CrossRef]
- Shen, A.; Liu, B.; Guo, Y.; Yu, P.; Yu, M. Skid Resistance Attenuation of Steel Slag Asphalt Mixture on Tunnel Pavement. J. Build. Mater. 2019, 22, 284–291. [Google Scholar]
- Pasetto, M.; Baliello, A.; Giacomello, G.; Pasquini, E. The Use of Steel Slags in Asphalt Pavements: A State-of-the-Art Review. Sustainability 2023, 15, 8817. [Google Scholar] [CrossRef]
- José, N.; João, C. Performance Evaluation of Steel Slag Asphalt Mixtures for Sustainable Road Pavement Rehabilitation. Appl. Sci. 2023, 13, 5716. [Google Scholar]
- Lyu, Z.; Shen, A.; Li, D.; Guo, Y.; Zhai, C.; Yang, X. Effect of Dry–Wet and Freeze–Thaw Repeated Cycles on Water Resistance of Steel Slag Asphalt Mixture. Iran. J. Sci. Technol. Trans. Civ. Eng. 2021, 45, 291–301. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Shen, A.; Yang, X.; Cui, T. Study of the long-term water stability of asphalt mixtures containing steel slag aggregate. J. Adhes. Sci. Technol. 2019, 34, 877–902. [Google Scholar] [CrossRef]
- Cao, R.; Jia, Z.; Zhang, Z.; Zhang, Y.; Banthia, N. Leaching kinetics and reactivity evaluation of ferronickel slag in alkaline conditions. Cem. Concr. Res. 2020, 137, 106202. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Yang, S.; Chu, J.; Liu, Y. Study on the Early Effect of Excitation Method on the Alkaline Steel Slag. Sustainability 2023, 15, 4714. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Adhikari, R.; Chen, Y.-H.; Li, P.; Chiang, P.-C. Integrated and innovative steel slag utilization for iron reclamation, green material production and CO2 fixation via accelerated carbonation. J. Clean. Prod. 2016, 137, 617–631. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Huang, D. Polycarboxylate superplasticizer as strengthening additive in carbonated artificial steel slag aggregate. Constr. Build. Mater. 2023, 403, 133136. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.; Yang, S. Effects of internal CO2 curing provided by biochar on the carbonation and properties of steel slag-based artificial lightweight aggregates (SALAs). Cem. Concr. Compos. 2023, 142, 105197. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, C.; Li, Z.; Liu, G.; Zhang, W.; Xie, G.; Xing, F. Carbonation of steel slag at low CO2 concentrations: Novel biochar cold-bonded steel slag artificial aggregates. Sci. Total Environ. 2023, 902, 166065. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Yang, S.; Huang, B.; Xu, L.; Feng, S.; Deng, M. Preparation, microstructure and property of carbonated artificial steel slag aggregate used in concrete. Cem. Concr. Compos. 2020, 113, 103715. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.; Mu, Y.; Wang, F.; He, Y. Effect of chitosan on the carbonation behavior of γ-C2S. Cem. Concr. Compos. 2020, 111, 103637. [Google Scholar] [CrossRef]
- Khan, R.; Ashraf, W.; Olek, J. Amino acids as performance-controlling additives in carbonation-activated cementitious materials. Cem. Concr. Res. 2021, 147, 106501. [Google Scholar] [CrossRef]
- Khan, R.; Intesarul Haque, M.; Siddique, S.; Landis, E.N.; Ashraf, W. Effects of amino acids on the multiscale properties of carbonated wollastonite composites. Constr. Build. Mater. 2023, 374, 130816. [Google Scholar] [CrossRef]
- Baffoe, E.; Ghahremaninezhad, A. Effect of proteins on the mineralization, microstructure and mechanical properties of carbonation cured calcium silicate. Cem. Concr. Compos. 2023, 141, 105121. [Google Scholar] [CrossRef]
- MOT (Ministry of Transport of the People’s Republic of China). Technical Specification for Construction of Highway Asphalt Pavement; JTG F40-2004; MOT: Beijing, China, 2004. (In Chinese)
- Li, L.; Ling, T.C.; Pan, S.Y. Environmental benefit assessment of steel slag utilization and carbonation: A systematic review. Sci. Total Environ. 2022, 806, 150280. [Google Scholar] [CrossRef]
- Ma, J.; Dai, G.; Jiang, F. Effect of Carbonation Treatment on the Properties of Steel Slag Aggregate. Materials 2023, 16, 5768. [Google Scholar] [CrossRef]
- Yang, S.; Mo, L.; Deng, M. Effects of ethylenediamine tetra-acetic acid (EDTA) on the accelerated carbonation and properties of artificial steel slag aggregates. Cem. Concr. Compos. 2021, 118, 103948. [Google Scholar] [CrossRef]
- MOT (Ministry of Transport of the People’s Republic of China). Test Methods of Aggregate for Highway Engineering; JTG E42-2005; MOT: Beijing, China, 2005. (In Chinese)
- MOT (Ministry of Transport of the People’s Republic of China). Standard Test Methods of Bitumen and Bituminous Mixture for Highway Engineering; JTG E20-2011; MOT: Beijing, China, 2011. (In Chinese)
- Jiang, Y.; Ling, T.C.; Shi, C.; Pan, S.Y. Characteristics of steel slags and their use in cement and concrete-A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Liu, G.; Tang, Y.; Wang, J. Recycling and valorization of hydrated cement blends in mortars via semi-dry carbonation—The role of waste glass, granulated blast furnace slag and fly ash. Constr. Build. Mater. 2023, 401, 132987. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Y.; Xu, X.; Pan, H.; Tang, R. Transformation of amorphous calcium carbonate into aragonite. J. Cryst. Growth 2012, 343, 62–67. [Google Scholar] [CrossRef]
- Kravchenko, E.; Qin, C.; Lin, Z.; Ng, C.W.W. Effect of polyvinyl alcohol on the CO2 uptake of carbonated steel slag. Constr. Build. Mater. 2023, 375, 130761. [Google Scholar] [CrossRef]
- Ko, M.S.; Chang, T.B.; Lee, C.Y.; Huang, J.W.; Lim, C.F. Optimization of Cyclone-Type Rotary Kiln Reactor for Carbonation of BOF Slag. Sustainability 2021, 13, 11556. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, W.; Lyu, X.; Liu, X.; Su, H.; Li, C.; Wang, H. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Liu, G.; Schollbach, K.; van der Laan, S.; Tang, P.; Florea, M.V.; Brouwers, H.J.H. Recycling and utilization of high volume converter steel slag into CO2 activated mortars—The role of slag particle size. Resour. Conserv. Recycl. 2020, 160, 104883. [Google Scholar] [CrossRef]
- Song, Q.; Guo, M.Z.; Wang, L.; Ling, T.C. Use of steel slag as sustainable construction materials: A review of accelerated carbonation treatment. Resources. Conserv. Recycl. 2021, 173, 105740. [Google Scholar] [CrossRef]
- MOT (Ministry of Transport of the People’s Republic of China). Technical Specification for Construction of Steel Slag Mixture Used as Base Course; YB/T 4184-2009; MOT: Beijing, China, 2009. (In Chinese)
- SAC (Standardization Administration of China). Pebble and Crushed Stone for Construction; GB/T 14685-2011; The Industry Standards of the People’s Republic of China: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Shen, A.; Zhai, C.; Guo, Y.; Yang, X. Mechanism of adhesion property between steel slag aggregate and rubber asphalt. J. Adhes. Sci. Technol. 2018, 32, 2727–2740. [Google Scholar] [CrossRef]
- Rashid, A.S.A.; Shirazi, M.G.; Mohamad, H.; Sahdi, F. Bearing capacity of sandy soil treated by Kenaf fibre geotextile. Environ. Earth Sci. 2017, 76, 1–6. [Google Scholar] [CrossRef]



| Types | Mass Fraction (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | MgO | SiO2 | Al2O3 | Fe2O3 | K2O | Na2O | SO3 | Loss | |
| P.O 42.5 cement | 64.02 | 2.38 | 19.39 | 4.81 | 2.89 | 0.70 | 0.13 | 2.62 | 2.71 |
| Steel slag | 41.83 | 10.67 | 9.80 | 2.59 | 29.31 | 0.05 | 0.10 | 0.09 | 1.40 |
| Class-I fly ash | 4.02 | 1.08 | 48.79 | 27.86 | 6.08 | 1.12 | 0.39 | 0.88 | 6.17 |
| Properties | Penetration (25 °C, 100 g, 5 s)/0.1 mm | Ductility (5 cm/min, 15 °C)/cm | Softening Point/°C | Relative Density (25 °C) | Dynamic Viscosity (60 °C)/Pa·s |
|---|---|---|---|---|---|
| Results | 68.2 | >100 | 48.5 | 1.012 | 185.1 |
| Standard requirement | 60–80 | ≥15 | ≥46 | - | ≥160 |
| Properties | Crushing Value (%) | Polished Value | Los Angeles Abrasion Loss (%) | Soft Stone Content (%) | Needle-like Content (%) |
|---|---|---|---|---|---|
| Results | 23.9 | 40.7 | 18.6 | 0.51 | 8.5 |
| Standard requirement | ≤26 | ≥40 | ≤28 | ≤3 | ≤15 |
| Properties | Apparent Relative Density (g/cm3) | Angularity | Sand Equivalent (%) | Sturdiness (<0.3 mm) | Mud Content (<0.075 mm) (%) |
|---|---|---|---|---|---|
| Results | 2.781 | 44.1 | 75 | 7.2 | 0.9 |
| Standard requirement | ≥2.50 | ≥30 | ≥60 | - | ≤3 |
| Properties | Apparent Relative Density (g/cm3) | Particle Size Range (%) | Hydrophilic Coefficient | Plasticity Index (%) | ||
|---|---|---|---|---|---|---|
| <0.6 mm | <0.15 mm | <0.075 mm | ||||
| Results | 2.674 | 100 | 94.9 | 89.5 | 0.57 | 2.5 |
| Standard requirement | ≥2.50 | - | <1 | <4 | ||
| Bitumen Content (%) | Bulk Gravity (g/cm3) | Voidage (%) | VMA (%) | VFA (%) | Marshall Stability (kN) | Flow Value (mm) |
|---|---|---|---|---|---|---|
| 6.4 | 2.725 | 3.7 | 18.49 | 78.56 | 16.19 | 2.94 |
| Grouping | S0 | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| Carbonation time (h) | - | 6 | 12 | 24 | 48 |
| Grouping | Weight Loss within 600~750 °C/wt.% | Estimated Absorption of CO2/wt.% | Estimated Production of CaCO3/wt.% |
|---|---|---|---|
| S0 | 0.46 | 2.53 | 1.05 |
| S1 | 6.76 | 9.93 | 15.36 |
| S2 | 7.21 | 10.06 | 16.38 |
| S3 | 7.49 | 10.13 | 17.01 |
| S4 | 8.36 | 10.75 | 19.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, X.; Zhang, Z.; Dai, G.; Huo, Y.; Zhao, Y. Performance Analysis of Industrial-Waste-Based Artificial Aggregates: CO2 Uptake and Applications in Bituminous Pavement. Buildings 2023, 13, 2823. https://doi.org/10.3390/buildings13112823
Ma J, Wang X, Zhang Z, Dai G, Huo Y, Zhao Y. Performance Analysis of Industrial-Waste-Based Artificial Aggregates: CO2 Uptake and Applications in Bituminous Pavement. Buildings. 2023; 13(11):2823. https://doi.org/10.3390/buildings13112823
Chicago/Turabian StyleMa, Jian, Xiaodong Wang, Zhen Zhang, Guangjian Dai, Yifei Huo, and Yunfeng Zhao. 2023. "Performance Analysis of Industrial-Waste-Based Artificial Aggregates: CO2 Uptake and Applications in Bituminous Pavement" Buildings 13, no. 11: 2823. https://doi.org/10.3390/buildings13112823
APA StyleMa, J., Wang, X., Zhang, Z., Dai, G., Huo, Y., & Zhao, Y. (2023). Performance Analysis of Industrial-Waste-Based Artificial Aggregates: CO2 Uptake and Applications in Bituminous Pavement. Buildings, 13(11), 2823. https://doi.org/10.3390/buildings13112823






