Manufacturing of Fired Clay Bricks for Internal Walls with Dolomite Residue as a Secondary Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
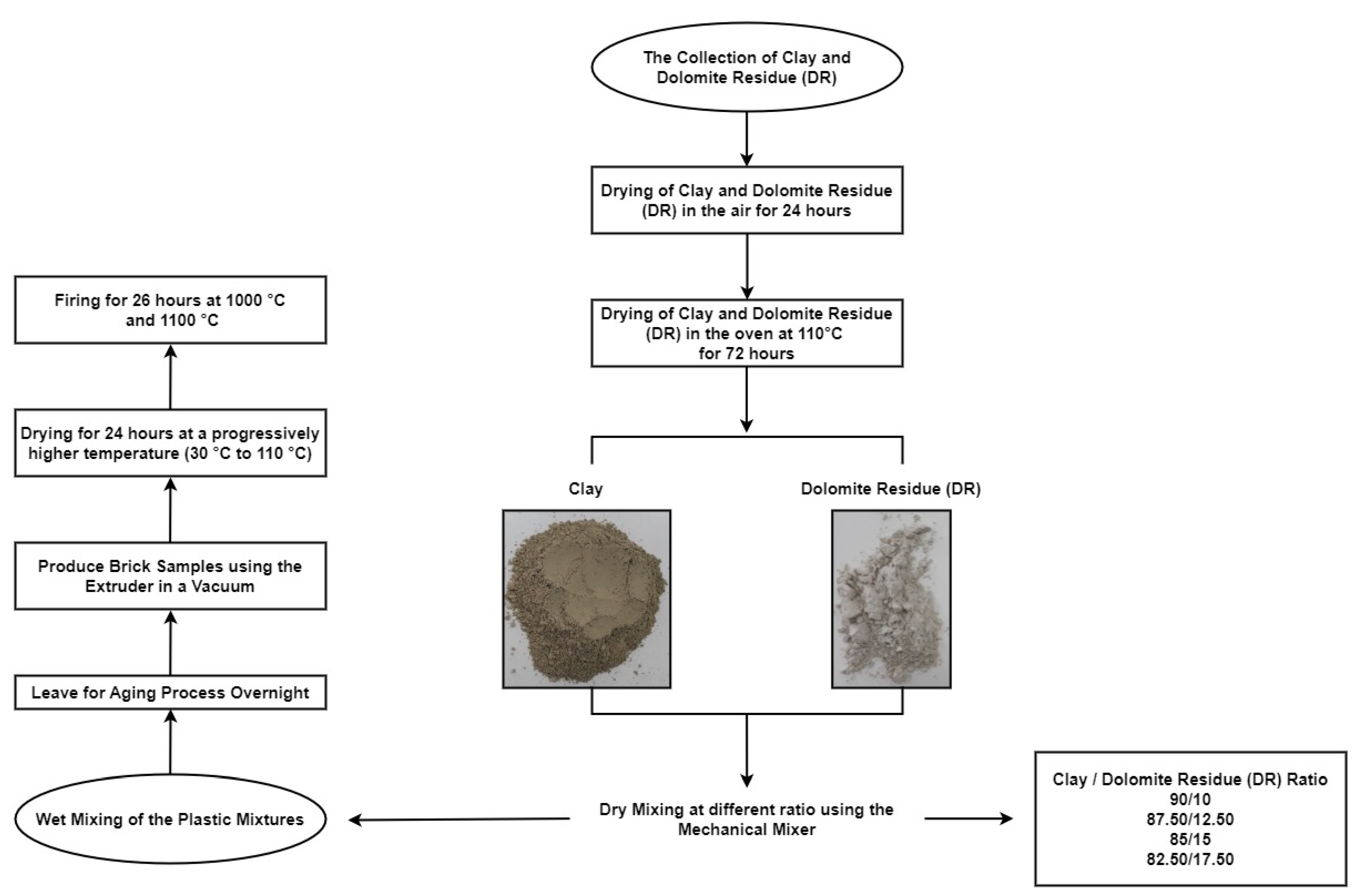
2.2. Preparation of Fired Brick Samples
2.3. Testing Methods
3. Results and Discussion
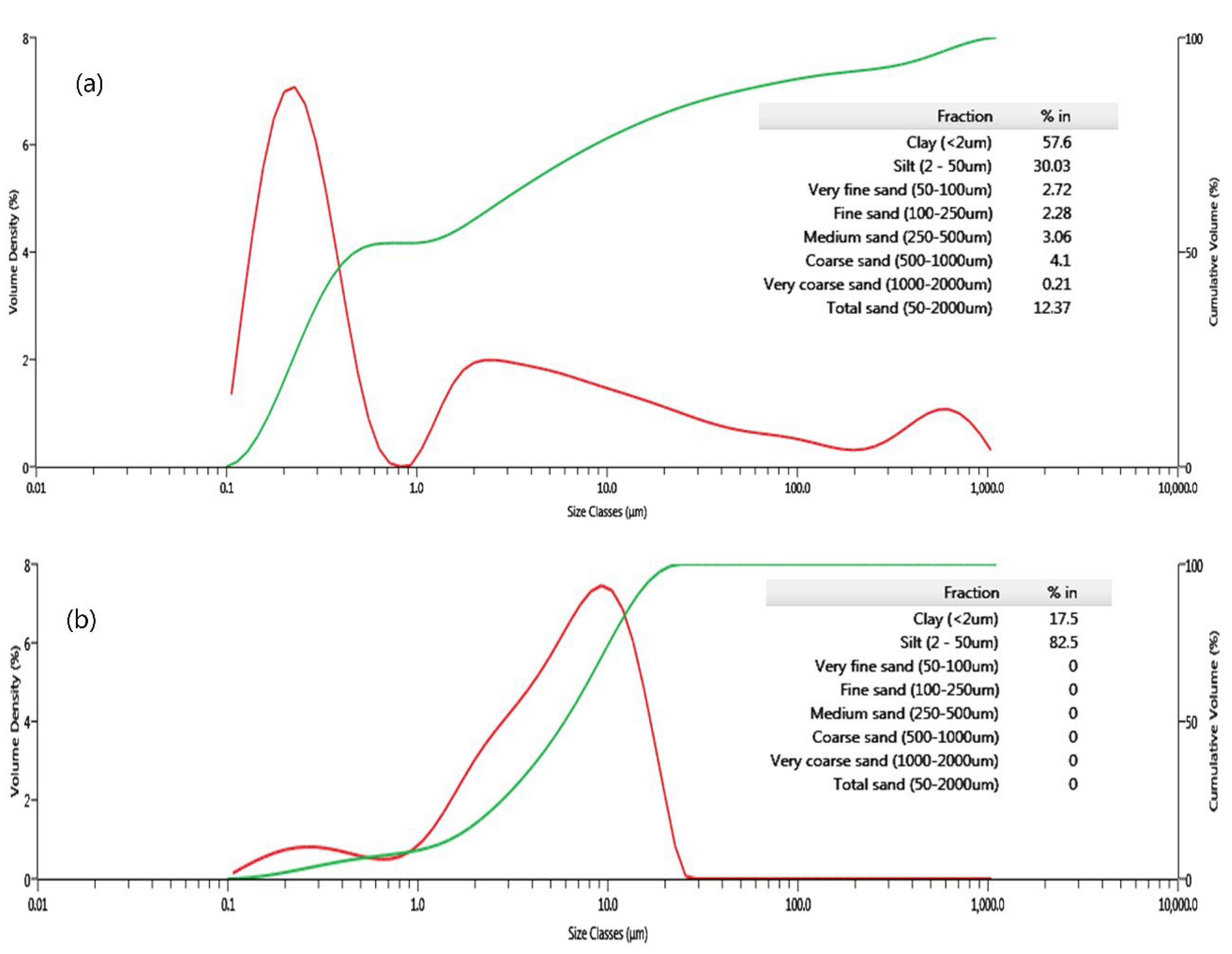
3.1. Properties of Raw Materials
3.2. The Physical and Mechanical Characteristics of Dried and Burnt Brick Sample
3.2.1. Physical Properties
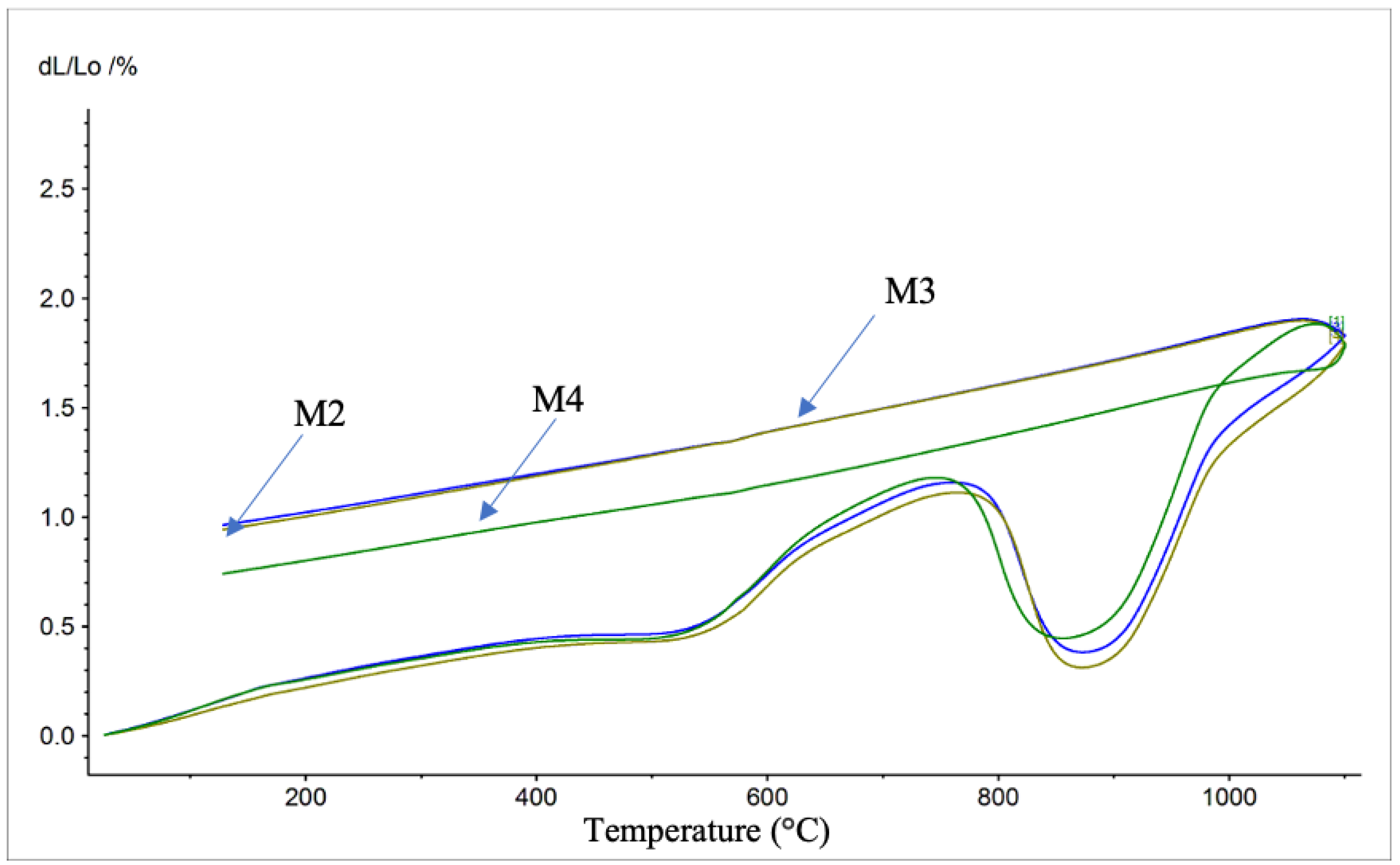
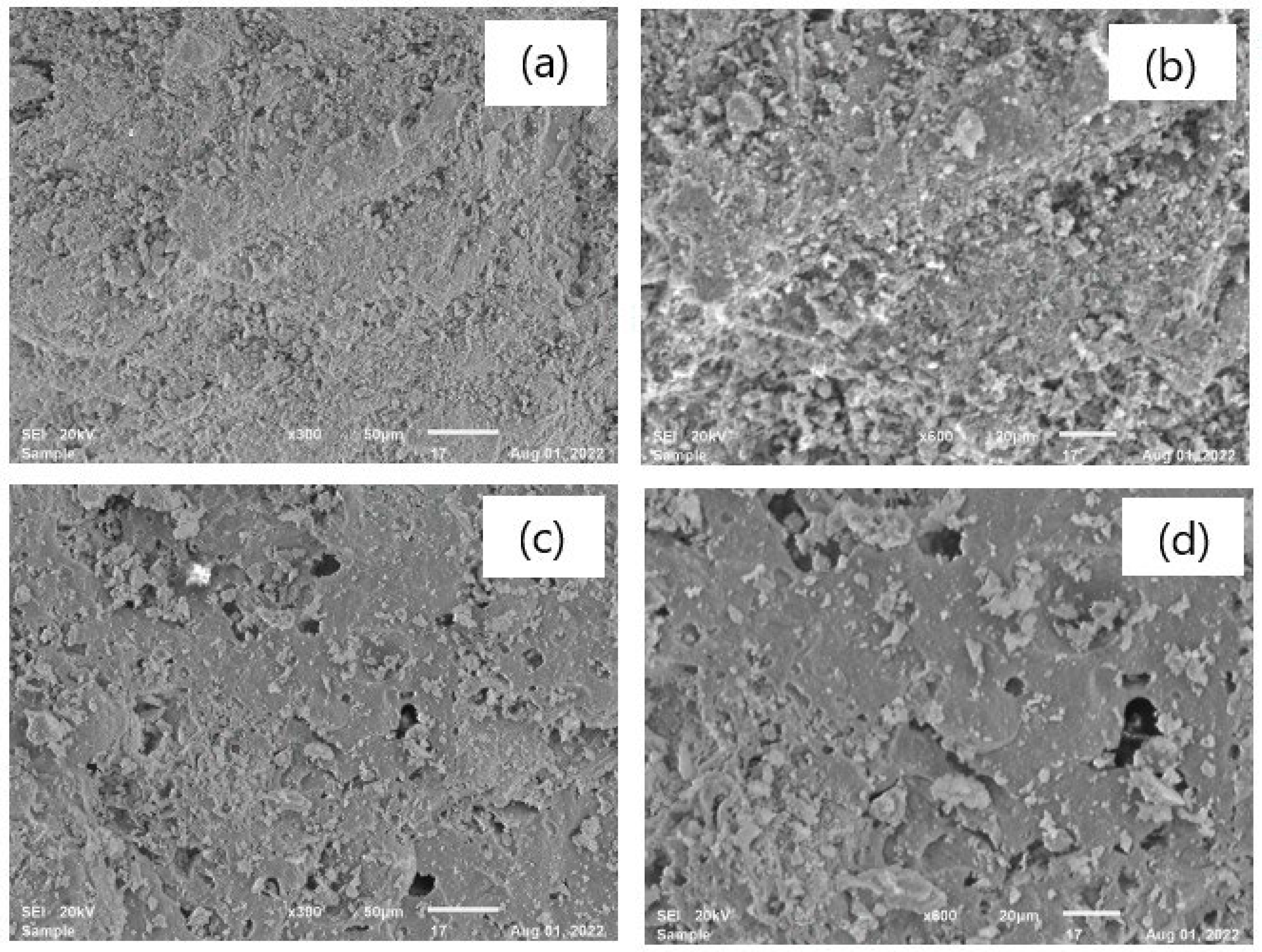
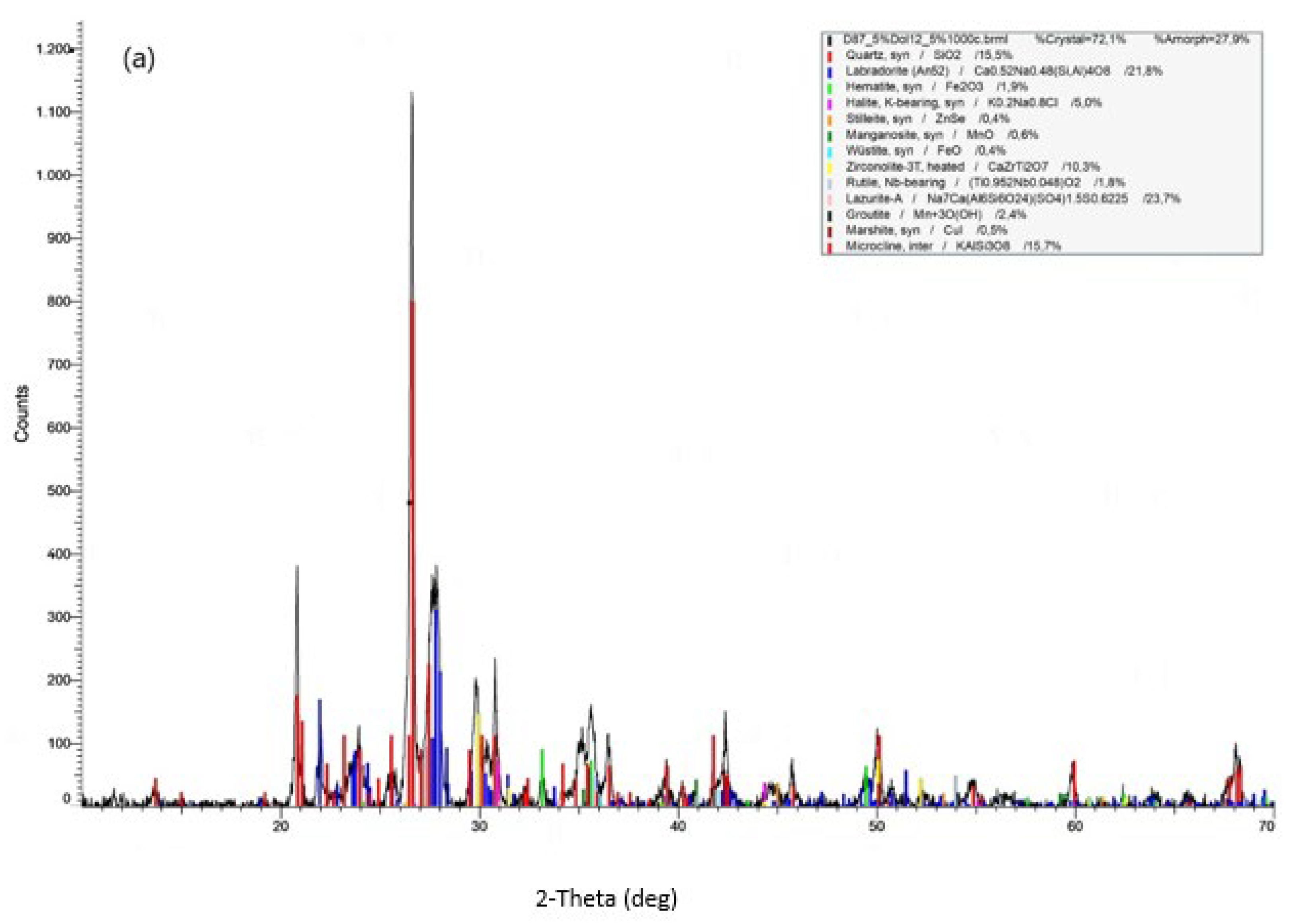
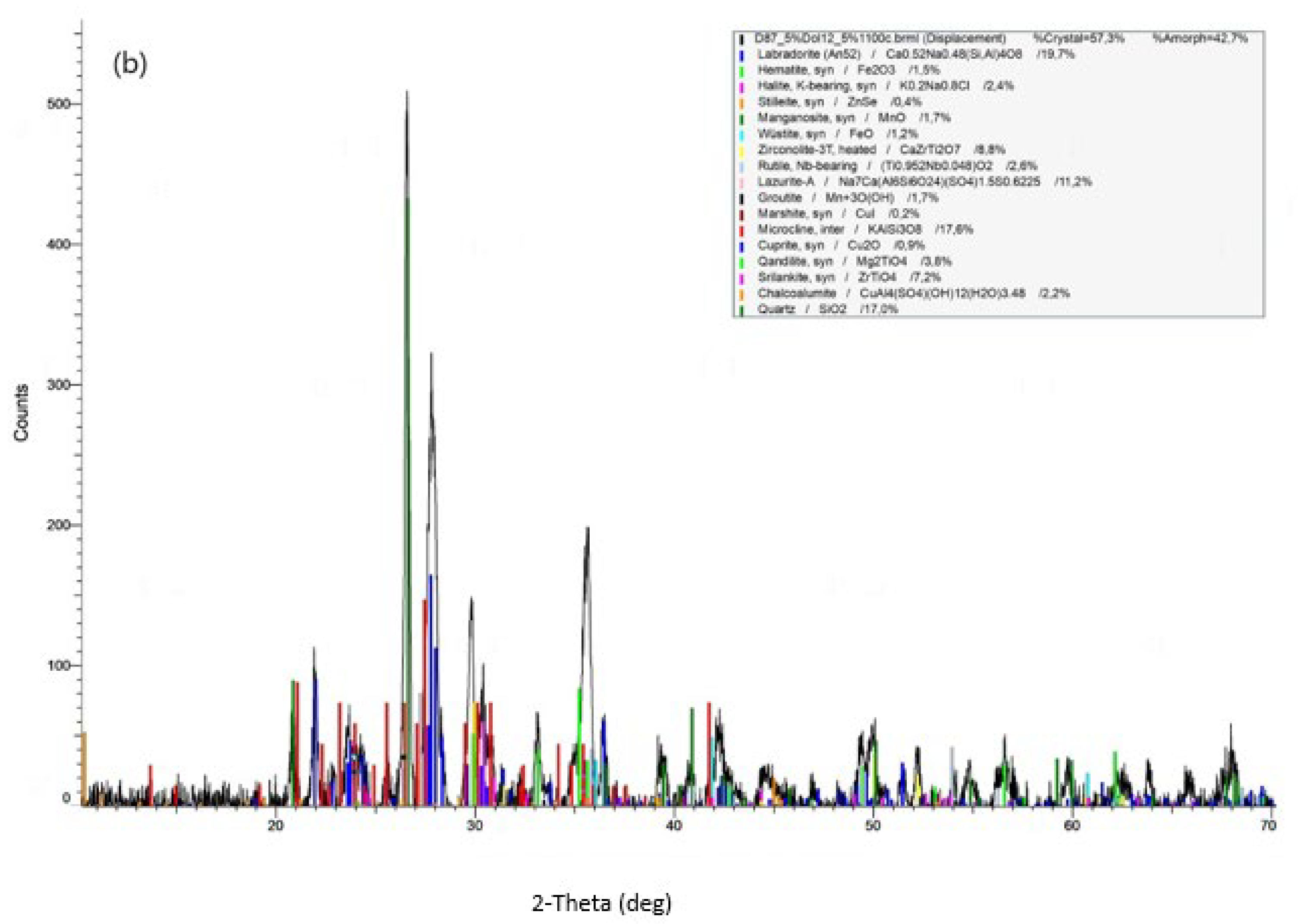
3.2.2. Microstructure and Phase Analysis
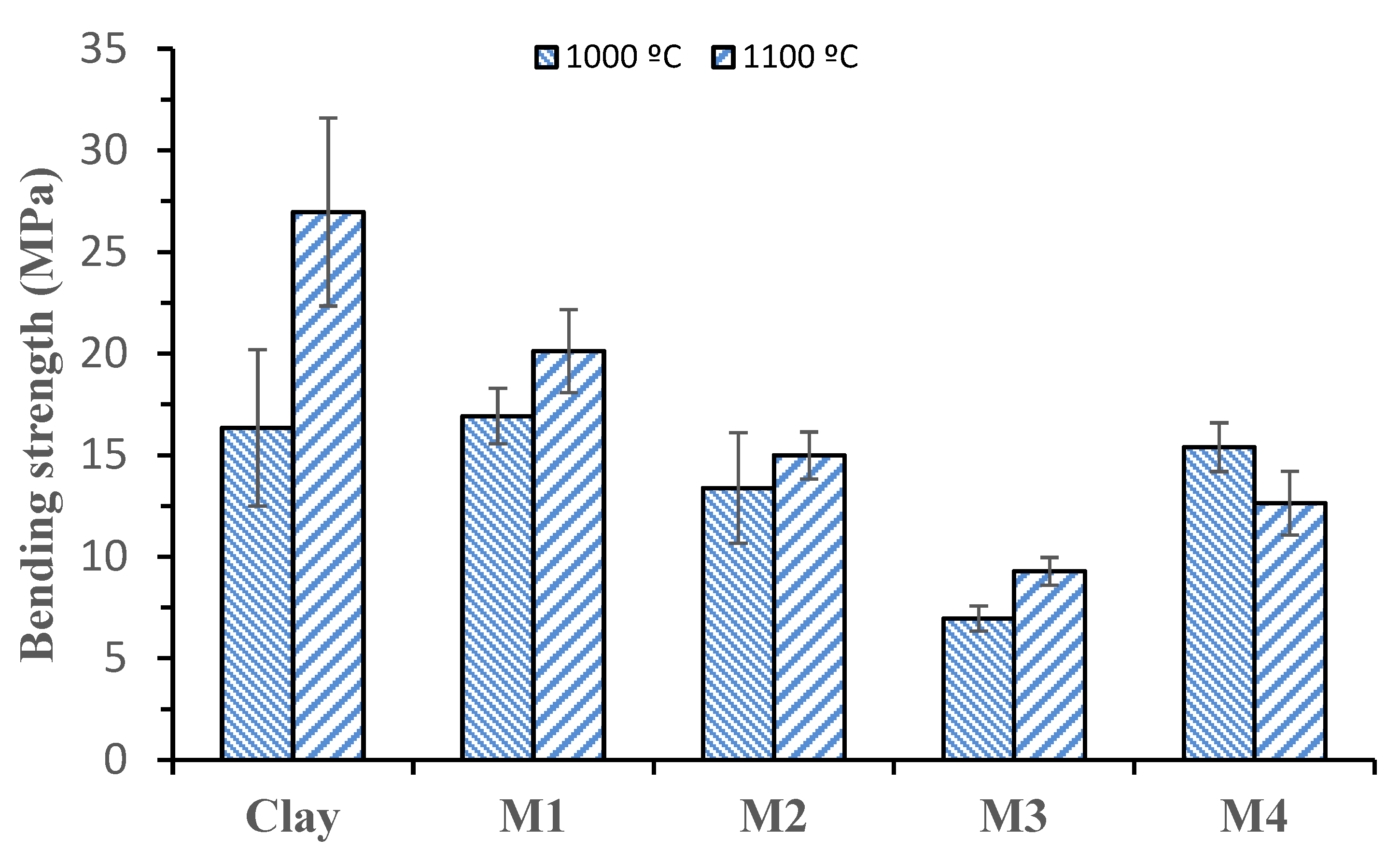
3.2.3. Bending Strength
3.2.4. Efflorescence Results
4. Conclusions
- A total of 2500 tons of dolomite residue is disposed annually from the mirror factory. Considering DOL as a secondary material in clay production can be considered as sustainable manufacturing. Its advantage is not only the reduction in clay consumption, but also in assisting the disposal of DOL.
- Firing temperature always plays a vital role in clay brick, and higher sintering means better brick properties. However, an increase in the firing temperature to 1100 °C with the clay of this specific basin was not possible as it melts down after 1050 °C. The addition of dolomite residue solved this problem, and bricks were produced in firing temperatures up to 1100 °C. Setting the sintering temperature to 1100 °C improved porosity, bending strength, and water absorption characteristics of the bricks. On the other hand, DOL addition mainly positively affected thermal conductivity, and thermal conductivity performed well at 1000 °C firing temperature rather than 1100 °C. Therefore, 1000 °C sintering temperature could be selected for general application by considering energy efficiency and gas releases.
- The decline in bending strength following dolomite addition to the clay may be attributed to factors such as phase decomposition, the introduction of porosity during dolomite thermal breakdown, and the potential incompatibility of phases. These effects collectively contribute to weakened bonds and altered microstructures, compromising the material’s overall bending integrity.
- DOL can be potentially used in the production of lighter bricks. It is recorded that substituting 17.5% of clay with DOL led to a decrease of approximately 15% in density compared to clay brick. This reduction in the weight of bricks incorporating DOL can decrease the overall dead load of the structures and, as a result, economical structures can be built.
- Although the use of DOL decreased some of the brick properties, they are still within the specified or desired range for a good brick quality. Apparent porosity increases linearly with DOL amount, but the M1 sample with a 90/10 clay/DOL ratio was a perfectly acceptable range for it. ASTM C62 recommends WA values less than 17% and 22% for different conditions, and the WA values obtained in this study were less than 17% for all mixtures of any DOL value. Similarly, the generally accepted range for linear shrinkage is below 8% and the addition of DOL in clay bricks did not exceed the limit.
- A negative effect of dolomite residue was seen in the physical properties of bricks. Efflorescence was observed in the fired bricks, and the percentage of white dots increased with the increment of DOL ratio in the bricks. This can be attributed to the high amount of CaO in the DOL as a raw material. However, bricks can be used in internal applications other than façades in order to avoid this aesthetic deficiency.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valášková, M. Clays, clay minerals and cordierite ceramics—A review. Orig. Pap. Ceram. 2015, 59, 331–340. [Google Scholar]
- Zhang, J.; Srinivasan, R.S.; Peng, C. Ecological Assessment of Clay Brick Manufacturing in China Using Emergy Analysis. Buildings 2020, 10, 190. [Google Scholar] [CrossRef]
- Korpayev, S.; Bayramov, M.; Kandymov, N.; Durdyev, S. Recycling of agricultural irrigation canal sludge and mirror factory residue in green brick production. Constr. Build. Mater. 2022, 346, 128474. [Google Scholar] [CrossRef]
- Korpayev, S.; Bayramov, M.; Durdyev, S.; Hamrayev, H. Characterization of three amu-darya basin clays in ceramic brick industry and their applications with brick waste. Materials 2021, 14, 7471. [Google Scholar] [CrossRef]
- Kurmus, H.; Mohajerani, A. Recycling of cigarette butts in fired clay bricks: A new laboratory investigation. Materials 2020, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Velasco, P.; Morales Ortíz, M.P.; Mendívil Giró, M.A.; Muñoz Velasco, L. Fired clay bricks manufactured by adding wastes as sustainable construction material—A review. Constr. Build. Mater. 2014, 63, 97–107. [Google Scholar] [CrossRef]
- Bilgin, N.; Yeprem, H.A.; Arslan, S.; Bilgin, A.; Günay, E.; Maroglu, M. Use of waste marble powder in brick industry. Constr. Build. Mater. 2012, 29, 449–457. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Eliche-Quesada, D.; Iglesias-Godino, F.J.; Martínez-García, C.; Corpas-Iglesias, F.A. Recycling of ash from biomass incinerator in clay matrix to produce ceramic bricks. J. Environ. Manag. 2012, 95, S349–S354. [Google Scholar] [CrossRef]
- Kandymov, N.; Korpayev, S.; Bayramov, M.; Durdyev, S. Sustainable Use of Raw Sand Residue in Production of Fired Clay Bricks. Arab. J. Sci. Eng. 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Sakhare, V.V.; Ralegaonkar, R.V. Use of bio-briquette ash for the development of bricks. J. Clean. Prod. 2016, 112, 684–689. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Munir, M.J.; Wu, Y.F.; Hanif, A.; Patnaikuni, I. Thermal performance evaluation of eco-friendly bricks incorporating waste glass sludge. J. Clean. Prod. 2018, 172, 1867–1880. [Google Scholar] [CrossRef]
- Isa, N.F.; Muhamad, K.; Yahya, N.; Ahmad, M.M.; Ab Manaf, M.B.H.; Abdul Rahim, M.; Khair Ishak, M.; Hashim, N.H.; Mansor, A.F.; Ahmad Naspu, S.N. Dolomite quarry waste as sand replacement in sand brick. Mater. Sci. Forum 2016, 857, 319–322. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, F.; Luo, W.; You, L. Recycling the waste dolomite powder with excellent consolidation properties: Sample synthesis, mechanical evaluation, and consolidation mechanism analysis. Constr. Build. Mater. 2021, 290, 123198. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Chen, Q.; Lawi, A.S.; Chen, J. Effect of partial substitution of cement with Dolomite powder on Glass-Fiber-Reinforced mortar. Constr. Build. Mater. 2022, 344, 128201. [Google Scholar] [CrossRef]
- Agrawal, Y.; Gupta, T.; Siddique, S.; Sharma, R.K. Potential of dolomite industrial waste as construction material: A review. Innov. Infrastruct. Solut. 2021, 6, 205. [Google Scholar] [CrossRef]
- Agrawal, Y.; Gupta, T.; Sharma, R.K. Strength and durability assessment of concrete containing dolomite quarry waste as fine aggregate. J. Mater. Cycles Waste Manag. 2022, 24, 268–286. [Google Scholar] [CrossRef]
- Monteiro, R.C.C.; Mota, C.S.; Lima, M.M.R.A. Effect of Dolomite Addition on the Densification of Fly Ash Based Ceramics. Mater. Sci. Forum 2006, 514–516, 1711–1715. [Google Scholar] [CrossRef]
- Kizinievic, O.; Kizinievic, V.; Skamat, J. Characteristics of Fired Clay Brick with Added Dolomite Powder. Glas. Ceram. 2023, 80, 22–24. [Google Scholar] [CrossRef]
- Kizinievič, O.; Gencel, O.; Kizinievič, V.; Sutcu, M.; Skamat, J. Recycling of dolomite powder in clay bricks: Effects on characteristics and gas release. Constr. Build. Mater. 2023, 404, 133217. [Google Scholar] [CrossRef]
- ISO 13320:2020; Particle Size Analysis—Laser Diffraction Methods. ISO: Geneva, Switzerland, 2020.
- ASTM C20-00; Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. ASTM: West Conshohocken, PN, USA, 2015.
- ASTM C67/C67M-18; ASTM International Standard Test Methods for Sampling and Testing Brick and Structural Clay Tile. ASTM: West Conshohocken, PN, USA, 2018.
- Kazanskaya, L.F.; Smirnova, O.M.; Palomo, Á.; Pidal, I.M.; Romana, M. Supersulfated Cement Applied to Produce Lightweight Concrete. Materials 2021, 14, 403. [Google Scholar] [CrossRef]
- Ministry of Economy of The Republic of Armenia. National Body for Standards and Metrology. Available online: https://www.armstandard.am/en/standart/5531 (accessed on 4 December 2023).
- Diko, M.L.; Ekosse, G.E.; Ayonghe, S.N.; Ntasin, E.B. Note. Appl. Clay Sci. 2011, 3, 380–384. [Google Scholar] [CrossRef]
- Sutcu, M.; Alptekin, H.; Erdogmus, E.; Er, Y.; Gencel, O. Characteristics of fired clay bricks with waste marble powder addition as building materials. Constr. Build. Mater. 2015, 82, 1–8. [Google Scholar] [CrossRef]
- Sutcu, M.; Erdogmus, E.; Gencel, O.; Gholampour, A.; Atan, E.; Ozbakkaloglu, T. Recycling of bottom ash and fly ash wastes in eco-friendly clay brick production. J. Clean. Prod. 2019, 233, 753–764. [Google Scholar] [CrossRef]
- Gencel, O.; Erdugmus, E.; Sutcu, M.; Oren, O.H. Effects of concrete waste on characteristics of structural fired clay bricks. Constr. Build. Mater. 2020, 255, 119362. [Google Scholar] [CrossRef]
- Litvan, G.G. Determination of the firing temperature of clay brick. Natl. Res. Counc. Canada 1984, 63, 617–627. [Google Scholar]
- Ajam, L.; Ben Ouezdou, M.; Felfoul, H.S.; Mensi, R. El Characterization of the Tunisian phosphogypsum and its valorization in clay bricks. Constr. Build. Mater. 2009, 23, 3240–3247. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Abbas, S.; Saleem, M.A.; Munir, M.J.; Khitab, A. Manufacturing of sustainable clay bricks: Utilization of waste sugarcane bagasse and rice husk ashes. Constr. Build. Mater. 2016, 120, 29–41. [Google Scholar] [CrossRef]
- Elert, K.; Cultrone, G.; Navarro, C.R.; Pardo, E.S. Durability of bricks used in the conservation of historic buildings-influence of composition and microstructure. J. Cult. Herit. 2003, 4, 91–99. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Leite-Costa, J. Use of bottom ash from olive pomace combustion in the production of eco-friendly fired clay bricks. Waste Manag. 2016, 48, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Gencel, O.; Sutcu, M.; Erdogmus, E.; Koc, V.; Cay, V.V.; Gok, M.S. Properties of bricks with waste ferrochromium slag and zeolite. J. Clean. Prod. 2013, 59, 111–119. [Google Scholar] [CrossRef]
- ASTM C90; ASTM C90 Standard Specification for Loadbearing Concrete Masonry Units. ASTM: West Conshohocken, PN, USA, 2022.
- Taurino, R.; Ferretti, D.; Cattani, L.; Bozzoli, F.; Bondioli, F. Lightweight clay bricks manufactured by using locally available wine industry waste. J. Build. Eng. 2019, 26, 100892. [Google Scholar] [CrossRef]
- Yaras, A. Combined effects of paper mill sludge and carbonation sludge on characteristics of fired clay bricks. Constr. Build. Mater. 2020, 249, 118722. [Google Scholar] [CrossRef]
- Benlalla, A.; Elmoussaouiti, M.; Dahhou, M.; Assafi, M. Utilization of water treatment plant sludge in structural ceramics bricks. Appl. Clay Sci. 2015, 118, 171–177. [Google Scholar] [CrossRef]
- Cai, L.; Ma, B.; Li, X.; Lv, Y.; Liu, Z.; Jian, S. Mechanical and hydration characteristics of autoclaved aerated concrete (AAC) containing iron-tailings: Effect of content and fineness. Constr. Build. Mater. 2016, 128, 361–372. [Google Scholar] [CrossRef]
- Muttashar, H.L.; Ali, N.B.; Mohd Ariffin, M.A.; Hussin, M.W. Microstructures and physical properties of waste garnets as a promising construction materials. Case Stud. Constr. Mater. 2018, 8, 87–96. [Google Scholar] [CrossRef]
- Phonphuak, N.; Kanyakam, S.; Chindaprasirt, P. Utilization of waste glass to enhance physical–mechanical properties of fired clay brick. J. Clean. Prod. 2016, 112, 3057–3062. [Google Scholar] [CrossRef]
- Abbas, S.; Saleem, M.A.; Kazmi, S.M.S.; Munir, M.J. Production of sustainable clay bricks using waste fly ash: Mechanical and durability properties. J. Build. Eng. 2017, 14, 7–14. [Google Scholar] [CrossRef]
- ASTM C62; Standard Specification for Building Brick (Solid Masonry Units Made from Clay or Shale). ASTM: West Conshohocken, PN, USA, 2012; pp. 12959–19428.
- Phonphuak, N. Effects of Additive on the Physical and Thermal Conductivity of Fired Clay Brick Types of waste, properties, and durability of pore-forming waste-based fired masonry bricks View project Effects of Additive on the Physical and Thermal Conductivity of Fired Clay Brick. J. Chem. Sci. Technol. 2013, 2, 95–99. [Google Scholar]
- More, A.S.; Tarade, A.; Anant, A. Assessment of suitability of Fly Ash and Rice Husk Ash burnt clay bricks. Int. J. Sci. Res. Publ. 2014, 4, 1–6. [Google Scholar]
- Karaman, S.; Gunal, H.; Ersahin, S. Assesment of clay bricks compressive strength using quantitative values of colour components. Constr. Build. Mater. 2006, 20, 348–354. [Google Scholar] [CrossRef]
- Chidiac, S.E.; Federico, L.M. Effects of waste glass additions on the properties and durability of fired clay brick. Can. J. Civ. Eng. 2007, 34, 1458–1466. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, D.F.; Chiang, P.C. Utilization of sludge as brick materials. Adv. Environ. Res. 2003, 7, 679–685. [Google Scholar] [CrossRef]
- Tiffo, E.; Elimbi, A.; Manga, J.D.; Tchamba, A.B. Red ceramics produced from mixtures of kaolinite clay and waste glass. Brazilian J. Sci. Technol. 2015, 2, 4. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Abbas, S.; Nehdi, M.L.; Saleem, M.A.; Munir, M.J. Feasibility of Using Waste Glass Sludge in Production of Ecofriendly Clay Bricks. J. Mater. Civ. Eng. 2017, 29, 04017056. [Google Scholar] [CrossRef]
- Leiva, C.; Arenas, C.; Alonso-Fariñas, B.; Vilches, L.F.; Peceño, B.; Rodriguez-Galán, M.; Baena, F. Characteristics of fired bricks with co-combustion fly ashes. J. Build. Eng. 2016, 5, 114–118. [Google Scholar] [CrossRef]







| Sample | Clay % | DOL % | Total % | Added Water % |
|---|---|---|---|---|
| M1 | 90 | 10 | 100 | 19 |
| M2 | 87.5 | 12.5 | 100 | 19.5 |
| M3 | 85 | 15 | 100 | 20 |
| M4 | 82.5 | 17.5 | 100 | 20.5 |
| Constituents | Clay | Dolomite Residue |
|---|---|---|
| SiO2 | 57.61 | 1.59 |
| Al2O3 | 17.54 | 0.25 |
| Na2O | 1.9 | 0.030 |
| K2O | 4.37 | 0.084 |
| MgO | 2.46 | 17.03 |
| CaO | 0.59 | 35.01 |
| Ti2O | 0.77 | 0.014 |
| Fe2O3 | 6.51 | 0.12 |
| L.O.I | 7.59 | 45.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandymov, N.; Korpayev, S.; Durdyev, S.; Myratberdiyev, R.; Gurbanmyradova, L. Manufacturing of Fired Clay Bricks for Internal Walls with Dolomite Residue as a Secondary Material. Buildings 2023, 13, 3065. https://doi.org/10.3390/buildings13123065
Kandymov N, Korpayev S, Durdyev S, Myratberdiyev R, Gurbanmyradova L. Manufacturing of Fired Clay Bricks for Internal Walls with Dolomite Residue as a Secondary Material. Buildings. 2023; 13(12):3065. https://doi.org/10.3390/buildings13123065
Chicago/Turabian StyleKandymov, Nurmurat, Serdar Korpayev, Serdar Durdyev, Rejepmyrat Myratberdiyev, and Leyla Gurbanmyradova. 2023. "Manufacturing of Fired Clay Bricks for Internal Walls with Dolomite Residue as a Secondary Material" Buildings 13, no. 12: 3065. https://doi.org/10.3390/buildings13123065
APA StyleKandymov, N., Korpayev, S., Durdyev, S., Myratberdiyev, R., & Gurbanmyradova, L. (2023). Manufacturing of Fired Clay Bricks for Internal Walls with Dolomite Residue as a Secondary Material. Buildings, 13(12), 3065. https://doi.org/10.3390/buildings13123065







