Preliminary Reactivity Test for Precursors of Alkali-Activated Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Acid Attack—Method
2.2.2. Structural Analysis—Method
3. Results
3.1. Acid Attack—Results
3.2. Structural Analysis—Results
4. Discussion
5. Conclusions
- The surface area is not relevant when the materials exhibit particles size medium smaller than 23 µm.
- The amorphous area is only relevant if the material exhibits the optimal chemical composition.
- The chemical composition is a crucial parameter of alkali activation.
- Potential precursors for effective alkali activation should exhibit significant amorphous halo and SiO2/Al2O3 proportion from 2 to 5.
- Silica fume is not viable as a single-precursor for alkali-activation due to the absence of aluminum on its chemical composition.
- Sugarcane bagasse ash mechanically and heat-treated is not a suitable precursor for alkali-activation due to its crystalline character and lower aluminum content.
- Sugarcane bagasse ash mechanically-treated, blast furnace slag, and metakaolin are viable precursors for alkali-activation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WBCSD. Guidelines for Co-Processing Fuels and Raw Materials in Cement Manufacturing; World Business Council for Sustainable Development: Geneva, Switzerland, 2014. [Google Scholar]
- United Nations. World Population Prospects 2019; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Sanjuán, M.Á.; Andrade, C.; Mora, P.; Zaragoza, A. Carbon Dioxide Uptake by Cement-Based Materials: A Spanish Case Study. Appl. Sci. 2020, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.L.; Sairam, V.; Srinivasan, K.; Muruganandam, L. Synthesis and characterization of geopolymer from metakaolin and sugarcane bagasse ash. Constr. Build. Mater. 2020, 258, 119231. [Google Scholar] [CrossRef]
- Gunasekara, C.; Law, D.W.; Setunge, S.; Sanjayan, J.G. Zeta potential, gel formation and compressive strength of low calcium fly ash geopolymers. Constr. Build. Mater. 2015, 95, 592–599. [Google Scholar] [CrossRef]
- Srividhya, S.; Vidjeapriya, R.; Neelamegam, M. Enhancing the performance of hyposludge concrete beams using basalt fiber and latex under cyclic loading. Comput. Concr. 2021, 28, 93–105. [Google Scholar] [CrossRef]
- Prakash, R.; Thenmozhi, R.; Raman, S.N.; Subramanian, C.; Divyah, N. An investigation of key mechanical and durability properties of coconut shell concrete with partial replacement of fly ash. Struct. Concr. 2021, 22, E985–E996. [Google Scholar] [CrossRef]
- Prakash, R.; Raman, S.N.; Subramanian, C.; Divyah, N. Eco-friendly fiber-reinforced concretes. In Handbook of Sustainable Concrete and Industrial Waste Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 109–145. [Google Scholar]
- Prakash, R.; Thenmozhi, R.; Raman, S.N.; Subramanian, C.; Divyah, N. Mechanical characterisation of sustainable fibre-reinforced lightweight concrete incorporating waste coconut shell as coarse aggregate and sisal fibre. Int. J. Environ. Sci. Technol. 2021, 18, 1579–1590. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Prakash, R.; Thenmozhi, R.; Raman, S.N.; Subramanian, C. Characterization of eco-friendly steel fiber-reinforced concrete containing waste coconut shell as coarse aggregates and fly ash as partial cement replacement. Struct. Concr. 2020, 21, 437–447. [Google Scholar] [CrossRef]
- Prakash, R.; Raman, S.N.; Divyah, N.; Subramanian, C.; Vijayaprabha, C.; Praveenkumar, S. Fresh and mechanical characteristics of roselle fibre reinforced self-compacting concrete incorporating fly ash and metakaolin. Constr. Build. Mater. 2021, 290, 123209. [Google Scholar] [CrossRef]
- ABNT NBR 12653; Materiais Pozolânicos—Requisitos. Associação Brasileira de Normas Técnicas (ABNT): Rio de Janeiro, Brazil, 2014.
- ASTM C618-19; Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- ABNT NBR 5752; Materiais Pozolânicos—Determinação do Índice de Desempenho Com Cimento Portland Aos 28 Dias. Associação Brasileira de Normas Técnicas (ABNT): Rio de Janeiro, Brazil, 2014.
- ASTM C311/C311M-18; Test Methods for Sampling and Testing Fly Ash or Natural Pozzolans for Use in Portland-Cement Concrete. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- ABNT NBR 15895; Materiais Pozolânicos—Determinação do Teor de Hidróxido de Cálcio Fixado—Método Chapelle Modificado. Associação Brasileira de Normas Técnicas (ABNT): Rio de Janeiro, Brazil, 2010.
- AFNOR NF P 18-513; Pozzolanic Addition for Concrete—Metakaolin—Definitions, Specifications and Compliance Criteria. AFNOR: Paris, France, 2012.
- Luxán, M.P.D.; Madruga, F.; Saavedra, J. Rapid evaluation of pozzolanic activity of natural products by conductivity measurement. Cem. Concr. Res. 1989, 19, 63–68. [Google Scholar] [CrossRef]
- Yasaswini, K.; Rao, A.V. Behaviour of geopolymer concrete at elevated temperature. Mater. Today Proc. 2020, 33, 239–244. [Google Scholar] [CrossRef]
- Akbar, A.; Farooq, F.; Shafique, M.; Aslam, F.; Alyousef, R.; Alabduljabbar, H. Sugarcane bagasse ash-based engineered geopolymer mortar incorporating propylene fibers. J. Build. Eng. 2021, 33, 101492. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, R.; Jiang, X.; Li, W.; Zhu, X.; Huang, B. Effect of particle size and curing temperature on mechanical and microstructural properties of waste glass-slag-based and waste glass-fly ash-based geopolymers. J. Clean. Prod. 2020, 273, 122970. [Google Scholar] [CrossRef]
- Zhou, S.; Ma, C.; Long, G.; Xie, Y. A novel non-Portland cementitious material: Mechanical properties, durability and characterization. Constr. Build. Mater. 2020, 238, 117671. [Google Scholar] [CrossRef]
- Cai, J.; Li, X.; Tan, J.; Vandevyvere, B. Thermal and compressive behaviors of fly ash and metakaolin-based geopolymer. J. Build. Eng. 2020, 30, 101307. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodríguez, E.D.; Walkley, B.; Zhang, Z.; Kirchheim, A.P. Metakaolin-based geopolymers: Relation between formulation, physicochemical properties and efflorescence formation. Compos. Part B Eng. 2020, 182, 107671. [Google Scholar] [CrossRef]
- Longhi, M.A.; Walkley, B.; Rodríguez, E.D.; Kirchheim, A.P.; Zhang, Z.; Wang, H. New selective dissolution process to quantify reaction extent and product stability in metakaolin-based geopolymers. Compos. Part B Eng. 2019, 176, 107172. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Ma, X.; Reid, A.; Wang, H. Efflorescence and subflorescence induced microstructural and mechanical evolution in fly ash-based geopolymers. Cem. Concr. Compos. 2018, 92, 165–177. [Google Scholar] [CrossRef]
- Nmiri, A.; Duc, M.; Hamdi, N.; Yazoghli-Marzouk, O.; Srasra, E. Replacement of alkali silicate solution with silica fume in metakaolin-based geopolymers. Int. J. Miner. Metall. Mater. 2019, 26, 555–564. [Google Scholar] [CrossRef]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, X.; Zhu, H.; Chen, Y. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- Palomo, A.; Fernández-Jiménez, A.; Criado, M. «Geopolimeros»: Una única base química y diferentes microestructuras. Mater. Constr. 2004, 54, 77–91. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Skibsted, J.; Fernández-Jiménez, A.; Palomo, A. Alkaline solution/binder ratio as a determining factor in the alkaline activation of aluminosilicates. Cem. Concr. Res. 2012, 42, 1242–1251. [Google Scholar] [CrossRef]
- Kuenzel, C.; Neville, T.P.; Donatello, S.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Influence of metakaolin characteristics on the mechanical properties of geopolymers. Appl. Clay Sci. 2013, 83–84, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Jimenez, A.; De La Torre, A.G.; Palomo, A.; López-Olmo, G.; Alonso, M.M.; Aranda, M.A.G. Quantitative determination of phases in the alkali activation of fly ash. Part I. Potential ash reactivity. Fuel 2006, 85, 625–634. [Google Scholar] [CrossRef]
- Lancellotti, I.; Ponzoni, C.; Barbieri, L.; Leonelli, C. Alkali activation processes for incinerator residues management. Waste Manag. 2013, 33, 1740–1749. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.-H.; Ong, D.E.L.; Liu, Z.; Hadi, M.N.S. Methods to evaluate and quantify the geopolymerization reactivity of waste-derived aluminosilicate precursor in alkali-activated material: A state-of-the-art review. Constr. Build. Mater. 2023, 362, 129784. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Barroso, T.R.; Toledo Filho, R.D. Enhancement the Properties of Sugar Cane Bagasse Ash with High Carbon Content by a Controlled Re-calcination Process. KSCE J. Civ. Eng. 2018, 22, 1250–1257. [Google Scholar] [CrossRef]
- Ríos-Parada, V.; Jiménez-Quero, V.G.; Valdez-Tamez, P.L.; Montes-García, P. Characterization and use of an untreated Mexican sugarcane bagasse ash as supplementary material for the preparation of ternary concretes. Constr. Build. Mater. 2017, 157, 83–95. [Google Scholar] [CrossRef]
- Cordeiro, G.C.C.; Tavares, L.M.M.; Toledo Filho, R.D.D. Improved pozzolanic activity of sugar cane bagasse ash by selective grinding and classification. Cem. Concr. Res. 2016, 89, 269–275. [Google Scholar] [CrossRef]
- Bahurudeen, A.; Santhanam, M. Influence of different processing methods on the pozzolanic performance of sugarcane bagasse ash. Cem. Concr. Compos. 2015, 56, 32–45. [Google Scholar] [CrossRef]
- Maldonado-García, M.A.; Hernández-Toledo, U.I.; Montes-García, P.; Valdez-Tamez, P.L. The influence of untreated sugarcane bagasse ash on the microstructural and mechanical properties of mortars. Mater. Constr. 2018, 68, 148. [Google Scholar] [CrossRef] [Green Version]
- Arenas-Piedrahita, J.C.; Montes-García, P.; Mendoza-Rangel, J.M.; López Calvo, H.Z.; Valdez-Tamez, P.L.; Martínez-Reyes, J. Mechanical and durability properties of mortars prepared with untreated sugarcane bagasse ash and untreated fly ash. Constr. Build. Mater. 2016, 105, 69–81. [Google Scholar] [CrossRef]
- de Soares, M.M.N.S.; Garcia, D.C.S.; Figueiredo, R.B.; Aguilar, M.T.P.; Cetlin, P.R. Comparing the pozzolanic behavior of sugar cane bagasse ash to amorphous and crystalline SiO2. Cem. Concr. Compos. 2016, 71, 20–25. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Andreão, P.V.; Tavares, L.M. Pozzolanic properties of ultrafine sugar cane bagasse ash produced by controlled burning. Heliyon 2019, 5, e02566. [Google Scholar] [CrossRef]
- Shcherban’, E.M.; Stel’makh, S.A.; Beskopylny, A.; Mailyan, L.R.; Meskhi, B. Influence of Mechanochemical Activation of Concrete Components on the Properties of Vibro-Centrifugated Heavy Concrete. Appl. Sci. 2021, 11, 10647. [Google Scholar] [CrossRef]
- Mancini, A.; Lothenbach, B.; Geng, G.; Grolimund, D.; Sanchez, D.F.; Fakra, S.C.; Dähn, R.; Wehrli, B.; Wieland, E. Iron speciation in blast furnace slag cements. Cem. Concr. Res. 2021, 140, 106287. [Google Scholar] [CrossRef]
- Mehta, A.; Ashish, D.K. Silica fume and waste glass in cement concrete production: A review. J. Build. Eng. 2020, 29, 100888. [Google Scholar] [CrossRef]
- Raheem, A.A.; Abdulwahab, R.; Kareem, M.A. Incorporation of metakaolin and nanosilica in blended cement mortar and concrete—A review. J. Clean. Prod. 2021, 290, 125852. [Google Scholar] [CrossRef]
- Pal, S.C.; Mukherjee, A.; Pathak, S.R. Investigation of hydraulic activity of ground granulated blast furnace slag in concrete. Cem. Concr. Res. 2003, 33, 1481–1486. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; De Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernández-Jimenez, A.; Pena, P.; Palomo, A. Alkaline activation of synthetic aluminosilicate glass. Ceram. Int. 2014, 40, 5547–5558. [Google Scholar] [CrossRef]
- Kovalchuk, G.; Fernández-Jiménez, A.; Palomo, A. Alkali-activated fly ash. Relationship between mechanical strength gains and initial ash chemistry. Mater. Constr. 2008, 58, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash. Part III: Effect of curing conditions on reaction and its graphical description. Fuel 2010, 89, 3185–3192. [Google Scholar] [CrossRef]
- Pimraksa, K.; Chindaprasirt, P.; Rungchet, A.; Sagoe-Crentsil, K.; Sato, T. Lightweight geopolymer made of highly porous siliceous materials with various Na2O/Al2O3 and SiO2/Al2O3 ratios. Mater. Sci. Eng. A 2011, 528, 6616–6623. [Google Scholar] [CrossRef]
- Barreto, I.A.R.; Costa, M.L.D. Use of the clayey cover of bauxite deposits of the Amazon region for geopolymer synthesis and its application in red ceramics. Constr. Build. Mater. 2021, 300, 124318. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Wang, S.D.; Pu, X.C.; Scrivener, K.L.; Pratt, P.L. Alkali-activated slag cement and concrete: A review of properties and problems. Adv. Cem. Res. 1995, 7, 93–102. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; San Nicolas, R.; Provis, J.L. Generalized Structural Description of Calcium–Sodium Aluminosilicate Hydrate Gels: The Cross-Linked Substituted Tobermorite Model. Langmuir 2013, 29, 5294–5306. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, A.; Puertas, F.; Sobrados, I.; Sanz, J. Structure of Calcium Silicate Hydrates Formed in Alkaline-Activated Slag: Influence of the Type of Alkaline Activator. J. Am. Ceram. Soc. 2003, 86, 1389–1394. [Google Scholar] [CrossRef]
- Pommer, V.; Vejmelková, E.; Černý, R.; Keppert, M. Alkali-activated waste ceramics: Importance of precursor particle size distribution. Ceram. Int. 2021, 47, 31574–31582. [Google Scholar] [CrossRef]
- Barbosa, W.; Ramalho, R.D.P.; Portella, K.F. Influence of gypsum fineness in the first hours of cement paste: Hydration kinetics and rheological behaviour. Constr. Build. Mater. 2018, 184, 304–310. [Google Scholar] [CrossRef]
- Scrivener, K.; Ouzia, A.; Juilland, P.; Kunhi Mohamed, A. Advances in understanding cement hydration mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Role of particle fineness on engineering properties and microstructure of fly ash derived geopolymer. Constr. Build. Mater. 2020, 233, 117294. [Google Scholar] [CrossRef]
- Yaseri, S.; Hajiaghaei, G.; Mohammadi, F.; Mahdikhani, M.; Farokhzad, R. The role of synthesis parameters on the workability, setting and strength properties of binary binder based geopolymer paste. Constr. Build. Mater. 2017, 157, 534–545. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y.; Wei, Q.; Cui, S.; Hao, L. Effect of the Formation of Amorphous Networks on the Structure and Hydration Characteristics of Granulated Blast Furnace Slag. Materials 2020, 13, 1462. [Google Scholar] [CrossRef] [Green Version]
- Setayesh Gar, P.; Suresh, N.; Bindiganavile, V. Sugar cane bagasse ash as a pozzolanic admixture in concrete for resistance to sustained elevated temperatures. Constr. Build. Mater. 2017, 153, 929–936. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Kurtis, K.E. Effect of mechanical processing on sugar cane bagasse ash pozzolanicity. Cem. Concr. Res. 2017, 97, 41–49. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Toledo Filho, R.D.; Tavares, L.M.; Fairbairn, E.M.R. Pozzolanic activity and filler effect of sugar cane bagasse ash in Portland cement and lime mortars. Cem. Concr. Compos. 2008, 30, 410–418. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef] [Green Version]
- Tchakouté, H.K.; Rüscher, C.H.; Hinsch, M.; Djobo, J.N.Y.; Kamseu, E.; Leonelli, C. Utilization of sodium waterglass from sugar cane bagasse ash as a new alternative hardener for producing metakaolin-based geopolymer cement. Geochemistry 2017, 77, 257–266. [Google Scholar] [CrossRef]
- García-Delgado, C.; Cala, V.; Eymar, E. Influence of chemical and mineralogical properties of organic amendments on the selection of an adequate analytical procedure for trace elements determination. Talanta 2012, 88, 375–384. [Google Scholar] [CrossRef]
- Zhou, F.; Hu, B.; Cui, B.; Liu, F.; Liu, F.; Wang, W.; Liu, Y.; Lu, R.; Hu, Y.-M.; Zhang, Y.; et al. Preparation and Characteristics of Polyaluminium Chloride by Utilizing Fluorine-Containing Waste Acidic Mother Liquid from Clay-Brine Synthetic Cryolite Process. J. Chem. 2014, 2014, 274126. [Google Scholar] [CrossRef] [Green Version]
- Milonjić, S.K.; Čerović, L.S.; Čokeša, D.M.; Zec, S. The influence of cationic impurities in silica on its crystallization and point of zero charge. J. Colloid Interface Sci. 2007, 309, 155–159. [Google Scholar] [CrossRef]
- Castaldelli, V.N.; Akasaki, J.L.; Melges, J.L.P.; Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J.; Castaldelli, V.N.; Monzó, J.; et al. Use of Slag/Sugar Cane Bagasse Ash (SCBA) Blends in the Production of Alkali-Activated Materials. Materials 2013, 6, 3108–3127. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, J.T.; Babafemi, A.J.; Fanijo, E.; Chandra Paul, S.; Combrinck, R. State-of-the-art review on the use of sugarcane bagasse ash in cementitious materials. Cem. Concr. Compos. 2021, 118, 103975. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.-K.; Hilonga, A.; Kim, H.T. Recovery of high surface area mesoporous silica from waste hexafluorosilicic acid (H2SiF6) of fertilizer industry. J. Hazard. Mater. 2010, 173, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Irfan Khan, M.; Khan, H.U.; Azizli, K.; Sufian, S.; Man, Z.; Siyal, A.A.; Muhammad, N.; Faiz ur Rehman, M. The pyrolysis kinetics of the conversion of Malaysian kaolin to metakaolin. Appl. Clay Sci. 2017, 146, 152–161. [Google Scholar] [CrossRef]
- Ansari, A.A.; Parchur, A.K.; Labis, J.P.; Shar, M.A.; Khan, A. Highly hydrophilic CaF2:Yb/Er upconversion nanoparticles: Structural, morphological, and optical properties. J. Fluor. Chem. 2021, 247, 109820. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. One year geopolymerisation of sodium silicate activated fly ash and metakaolin geopolymers. Cem. Concr. Compos. 2019, 95, 98–110. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Zhang, Z.; Huang, J.; Zhang, P.; Ma, Y.; Wang, H. Study of acidic degradation of alkali-activated materials using synthetic C-(N)-A-S-H and N-A-S-H gels. Compos. Part B Eng. 2022, 230, 109510. [Google Scholar] [CrossRef]
- Jain, B.; Sancheti, G.; Jain, V. FTIR analysis of silica fume and iron dust added concrete. Mater. Today Proc. 2022, 60, 777–781. [Google Scholar] [CrossRef]
- Reig, F. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Comparative study on flame retardancy of silica fume-based geopolymer activated by different activators. J. Alloys Compd. 2018, 743, 108–114. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Tchakoute Kouamo, H.; Elimbi, A.; Mbey, J.A.A.; Ngally Sabouang, C.J.J.; Njopwouo, D. The effect of adding alumina-oxide to metakaolin and volcanic ash on geopolymer products: A comparative study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Nasab, G.M.; Golestanifard, F.; MacKenzie, K.J.D. The effect of the SiO2/Na2O ratio in the structural modification of metakaolin-based geopolymers studied by XRD, FTIR and MAS-NMR. J. Ceram. Sci. Technol. 2014, 5, 185–191. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; MacPhee, D.E.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía De Gutierrez, R. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Kaze, C.R.; Jiofack, S.B.K.; Cengiz, Ö.; Alomayri, T.S.; Adesina, A.; Rahier, H. Reactivity and mechanical performance of geopolymer binders from metakaolin/meta-halloysite blends. Constr. Build. Mater. 2022, 336, 127546. [Google Scholar] [CrossRef]
- Rodrigue Kaze, C.; Adesina, A.; Alomayri, T.; Assaedi, H.; Kamseu, E.; Chinje Melo, U.; Leonelli, C. Characterization, reactivity and rheological behaviour of metakaolin and Meta-halloysite based geopolymer binders. Clean. Mater. 2021, 2, 100025. [Google Scholar] [CrossRef]
- Azevedo, A.R.G.; Vieira, C.M.F.; Ferreira, W.M.; Faria, K.C.P.; Pedroti, L.G.; Mendes, B.C. Potential use of ceramic waste as precursor in the geopolymerization reaction for the production of ceramic roof tiles. J. Build. Eng. 2020, 29, 101156. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Shi, C.; Li, N.; Jiao, D.; Yuan, Q. Rheology of alkali-activated materials: A review. Cem. Concr. Compos. 2021, 121, 104061. [Google Scholar] [CrossRef]

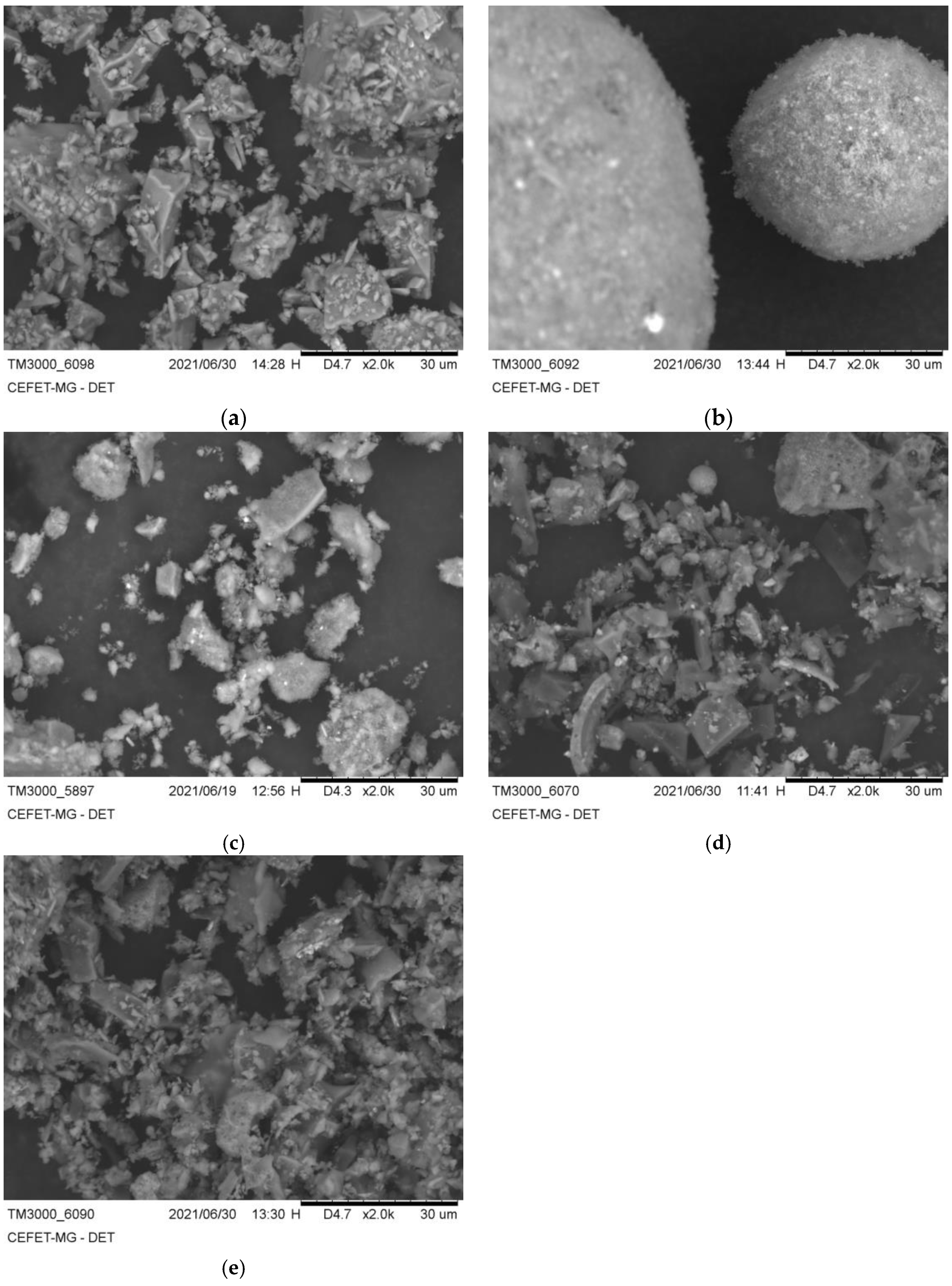

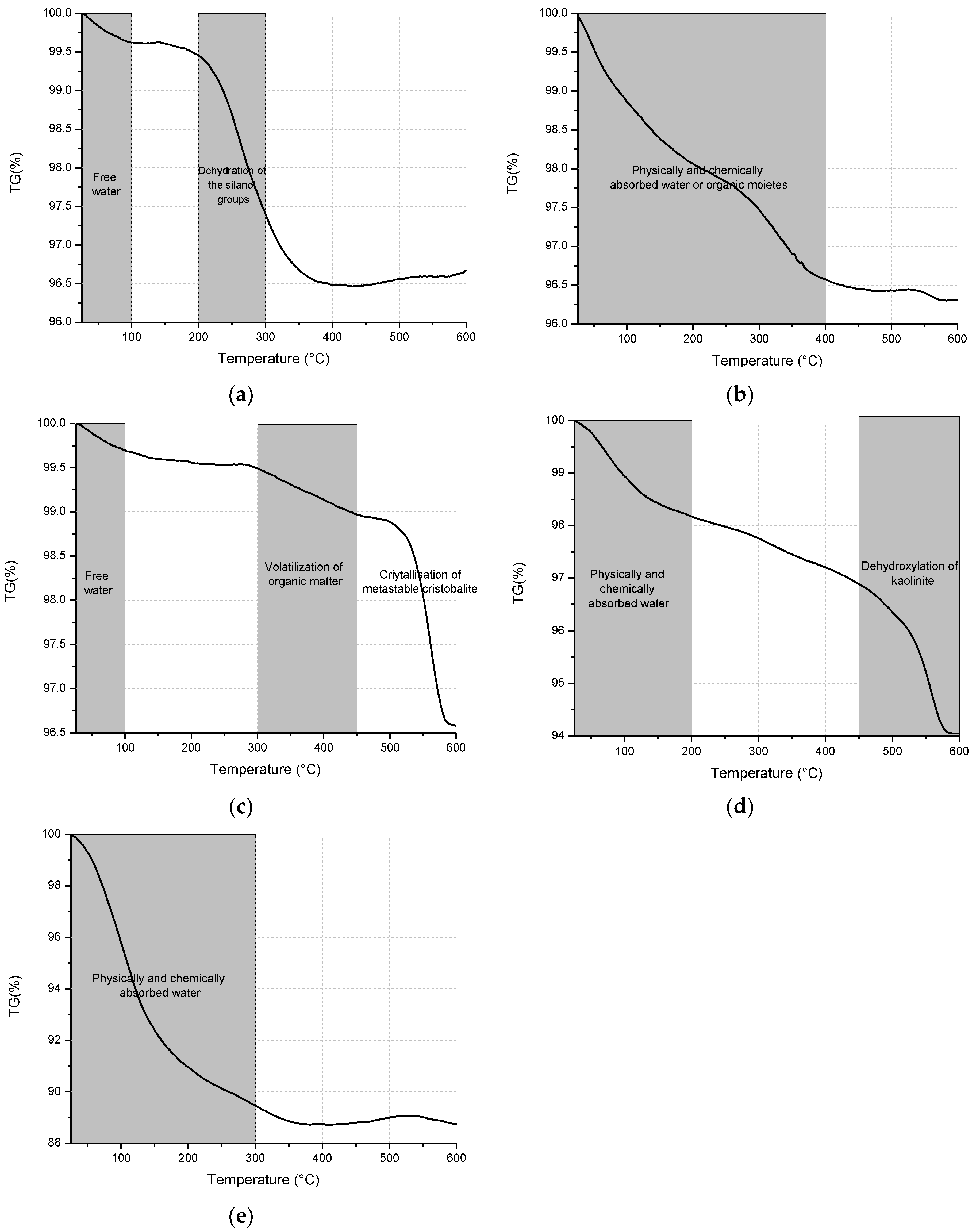
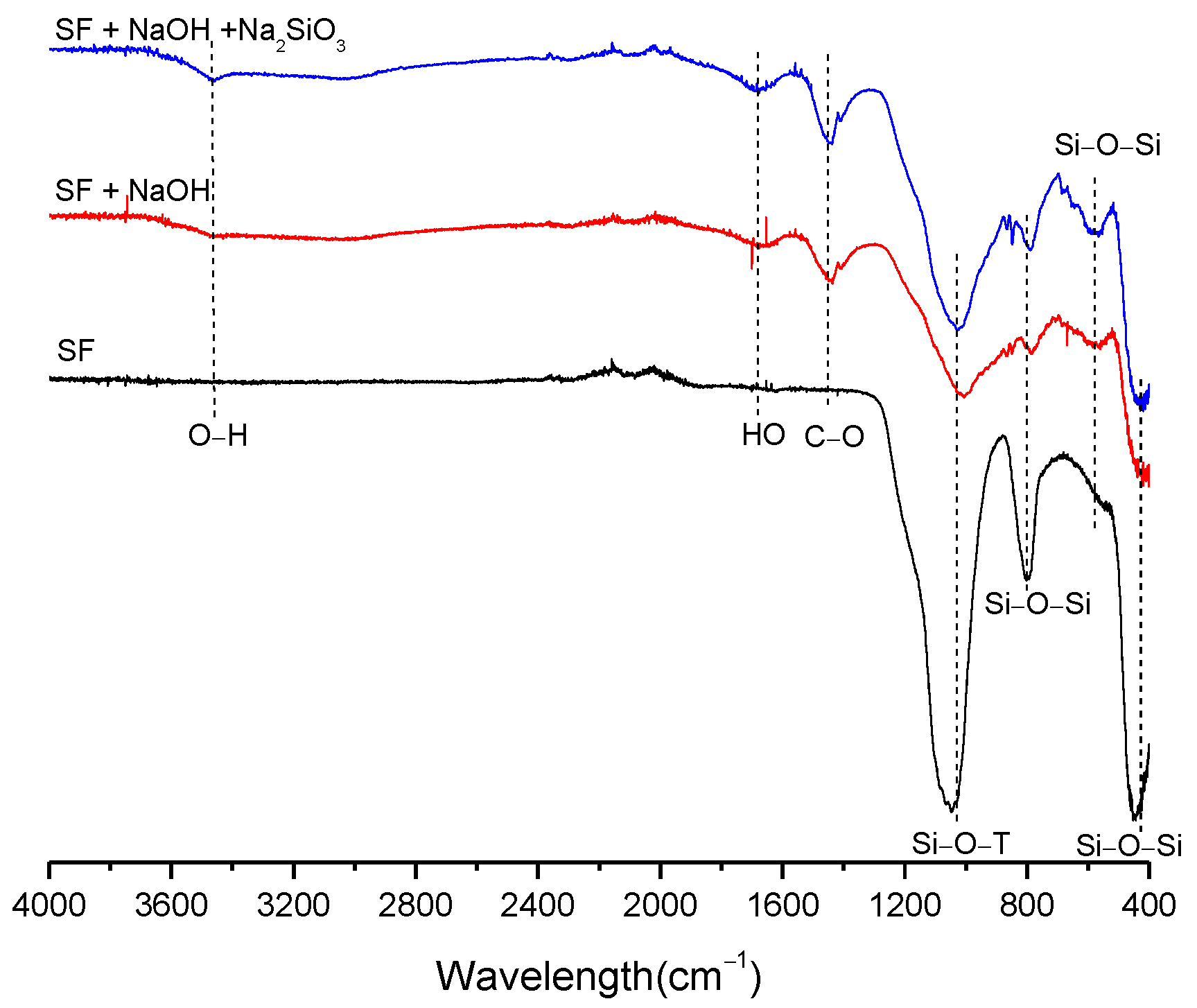
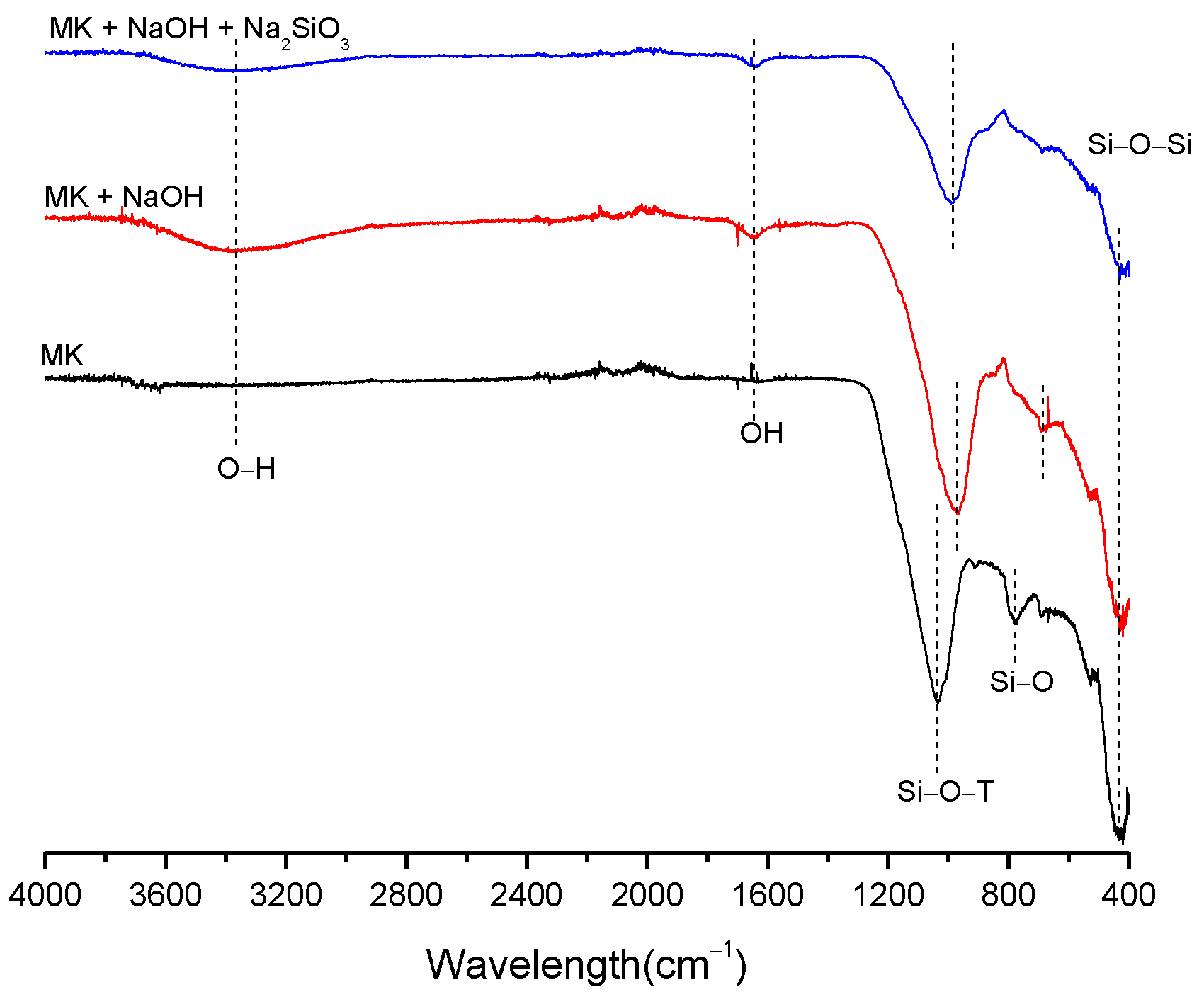
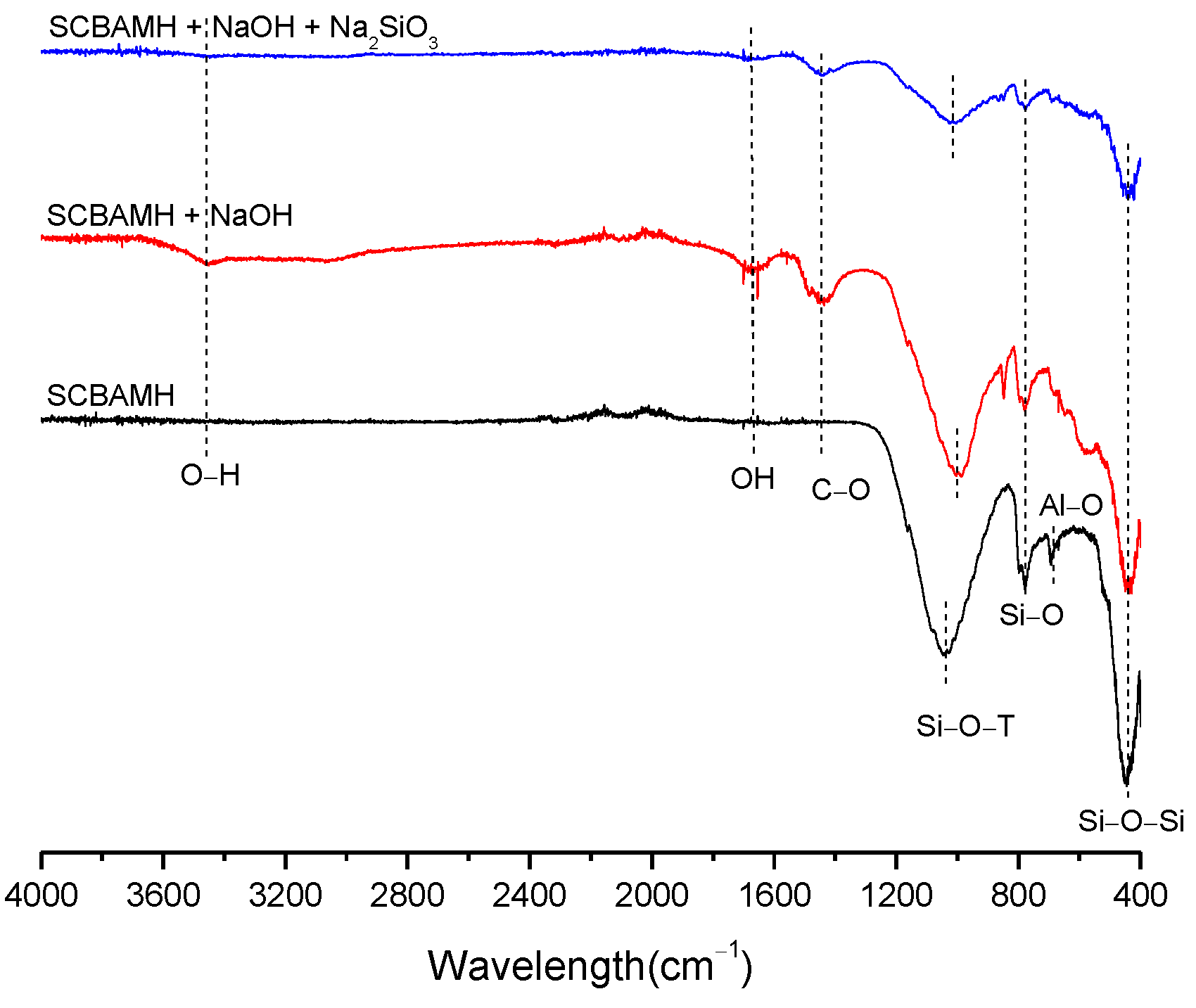
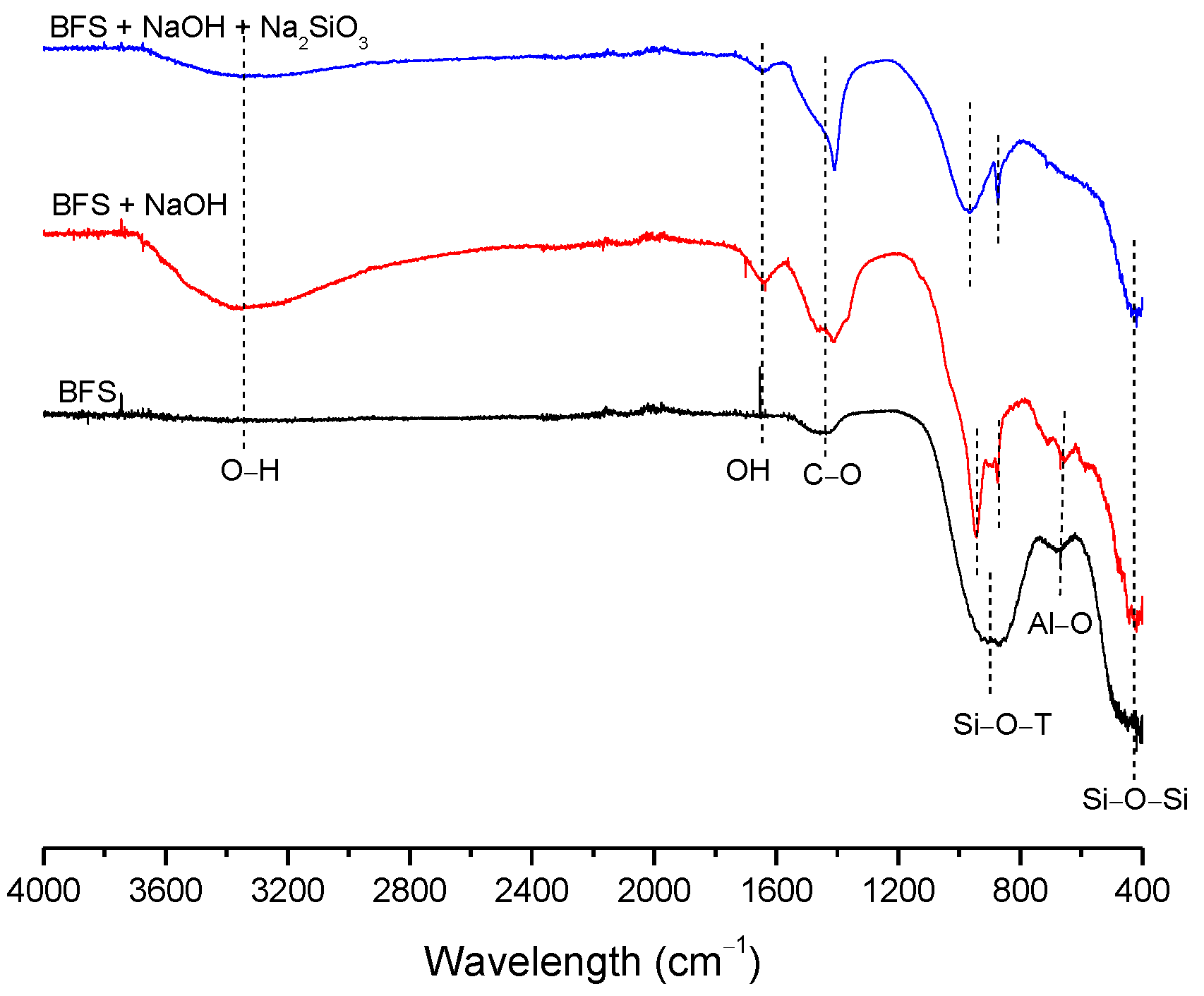
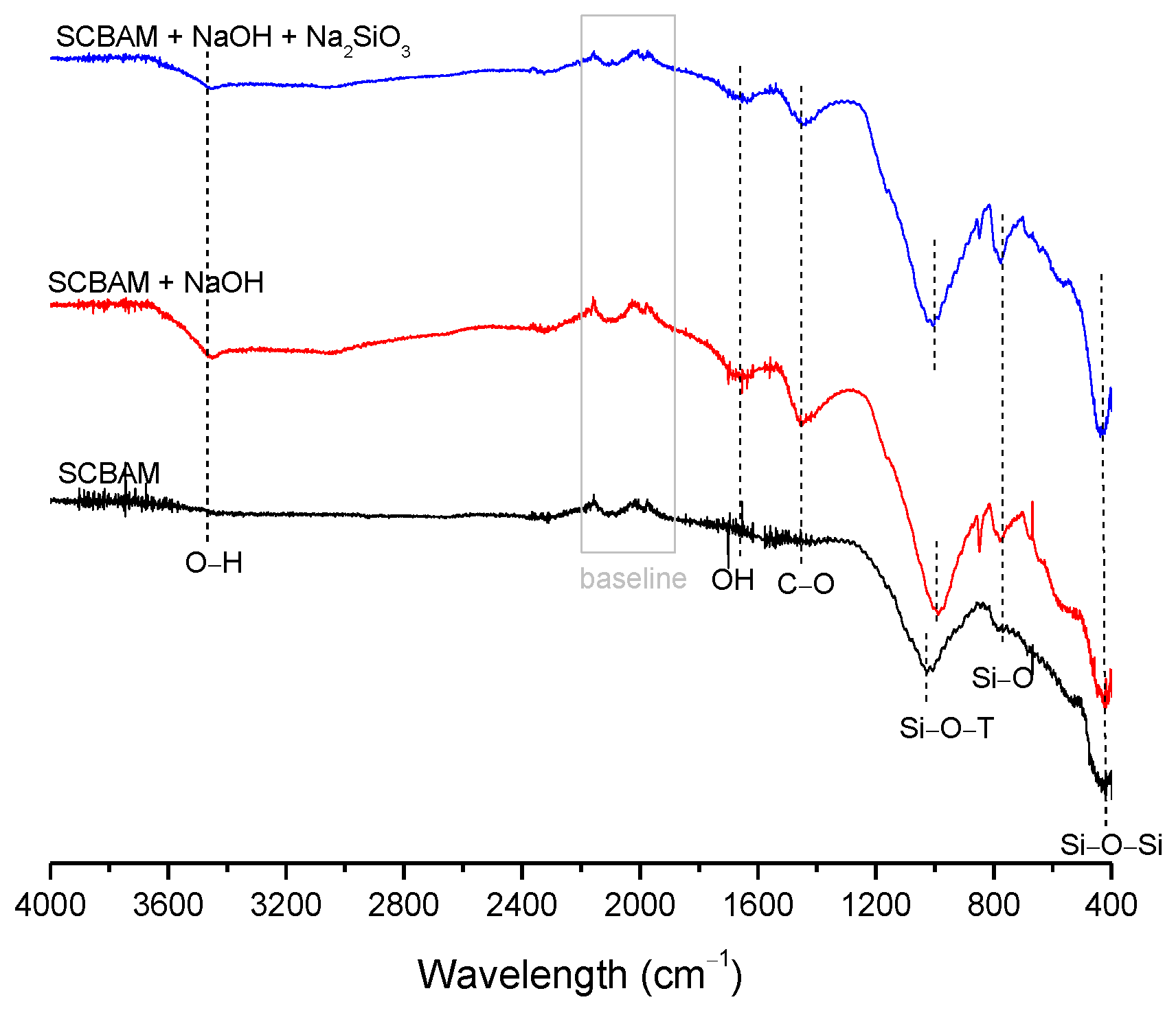
| BFS | SF | MK | SCBAM | SCBAMH | |
|---|---|---|---|---|---|
| SiO2 | 32.05 | 95.94 | 62.00 | 32.89 | 73.53 |
| Al2O3 | 14.33 | 0.21 | 30.55 | 11.72 | 10.65 |
| Fe2O3 | 1.27 | 0.12 | 2.51 | 18.26 | 6.14 |
| CaO | 39.46 | 0.35 | 0.04 | 2.90 | 2.39 |
| MgO | 8.77 | 0.41 | 0.25 | 2.82 | 1.39 |
| TiO2 | 0.53 | 0.01 | 1.45 | 5.90 | 0.95 |
| K2O | 0.17 | 0.4 | 0.49 | 2.84 | 2.22 |
| MnO | 0.63 | 0.02 | 0.01 | 0.25 | 0.13 |
| P2O5 | <0.01 | <0.01 | <0.01 | 0.93 | 1.14 |
| ZrO2 | - | <0.01 | 0.06 | 0.04 | 0.04 |
| SO3 | 1.43 | <0.01 | - | <0.01 | 0.02 |
| Na2O | 0.96 | <0.1 | <0.1 | <0.1 | <0.1 |
| Cr2O3 | 0.11 | <0.01 | 0.02 | 0.27 | 0.20 |
| Sum of pozzolanic oxides | 47.65 | 96.27 | 95.06 | 62.87 | 90.32 |
| Loss on ignition (LOI) | 0.18 | 2.15 | 2.44 | 20.68 | 0.98 |
| SiO2/Al2O3 | 3.80 | 776.66 | 3.45 | 4.77 | 11.74 |
| Particle size distribution | |||||
| DM (μm) | 22.92 | 21.91 | 22.79 | 17.27 | 17.77 |
| D10 (µm) | 1.75 | 5.12 | 3.08 | 2.56 | 2.20 |
| D50 (µm) | 15.78 | 20.52 | 19.64 | 13.34 | 12.73 |
| D90 (µm) | 55.10 | 40.86 | 46.84 | 38.26 | 41.68 |
| Precursor (g) | Paste 01 | Paste 02 | |
|---|---|---|---|
| H2O/Na2O | H2O/Na2O | SiO2/Na2O | |
| 30 | 10.44 | 11.38 | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
França, S.; Sousa, L.N.; Silva, M.V.d.M.S.; Borges, P.H.R.; Bezerra, A.C.d.S. Preliminary Reactivity Test for Precursors of Alkali-Activated Materials. Buildings 2023, 13, 693. https://doi.org/10.3390/buildings13030693
França S, Sousa LN, Silva MVdMS, Borges PHR, Bezerra ACdS. Preliminary Reactivity Test for Precursors of Alkali-Activated Materials. Buildings. 2023; 13(3):693. https://doi.org/10.3390/buildings13030693
Chicago/Turabian StyleFrança, Sâmara, Leila Nóbrega Sousa, Marcos Vinicio de Moura Solar Silva, Paulo Henrique Ribeiro Borges, and Augusto Cesar da Silva Bezerra. 2023. "Preliminary Reactivity Test for Precursors of Alkali-Activated Materials" Buildings 13, no. 3: 693. https://doi.org/10.3390/buildings13030693





