Performance and Mechanism of Zn-Contaminated Soil through Microbe-Induced Calcium Carbonate Precipitation
Abstract
1. Introduction
| Methods | Principle | Application | Disadvantages | |
|---|---|---|---|---|
| Physical methods | Heat treatment | The contaminated soil is heated to high temperature, causing the heavy metals to evaporate. | This method was used to degrade heavy metals from wastewater sludge [18]. | Such high temperatures lead to more leaching of the metals and also greater loss of humus. |
| Vitrification technology | Heating contaminated soil to a high temperature and then rapidly freeze, to form solids through glass transition. | In Japan, this method has been used to reduce radioactive waste from its nuclear power plants [19]. | It is generally applied to a small, heavily contaminated area. | |
| Chemical methods | Chemical extraction and oxidation | Using chelating agents to extract heavy metals from soil. | Application of such method in a wastewater plant in Henan Province, China, resulted in increased efficiency of heavy metal solidification [20]. | The transport of the chelating agents in the fine-grained soil is hindered. |
| Soil amendments (chemical fixation) | Use of chemical additives to reduce cation charged metals to an acceptable limit of discharge. | Four arctic and five subarctic sites with different soil characteristics were selected for the study, and the associated organic contaminants were subjected to alkaline hydrolysis [21]. | Generation of secondary waste and slow degradation of sludge. | |
| Biological methods | Phytoremediation | Based on the plant’s ability to degrade pollutants. | Soil samples were collected from Ogun State and the Mn, Zn, Cu ions in soils were removed [22]. | It is a slower process and plants need to be treated centrally. |
2. Materials and Methods
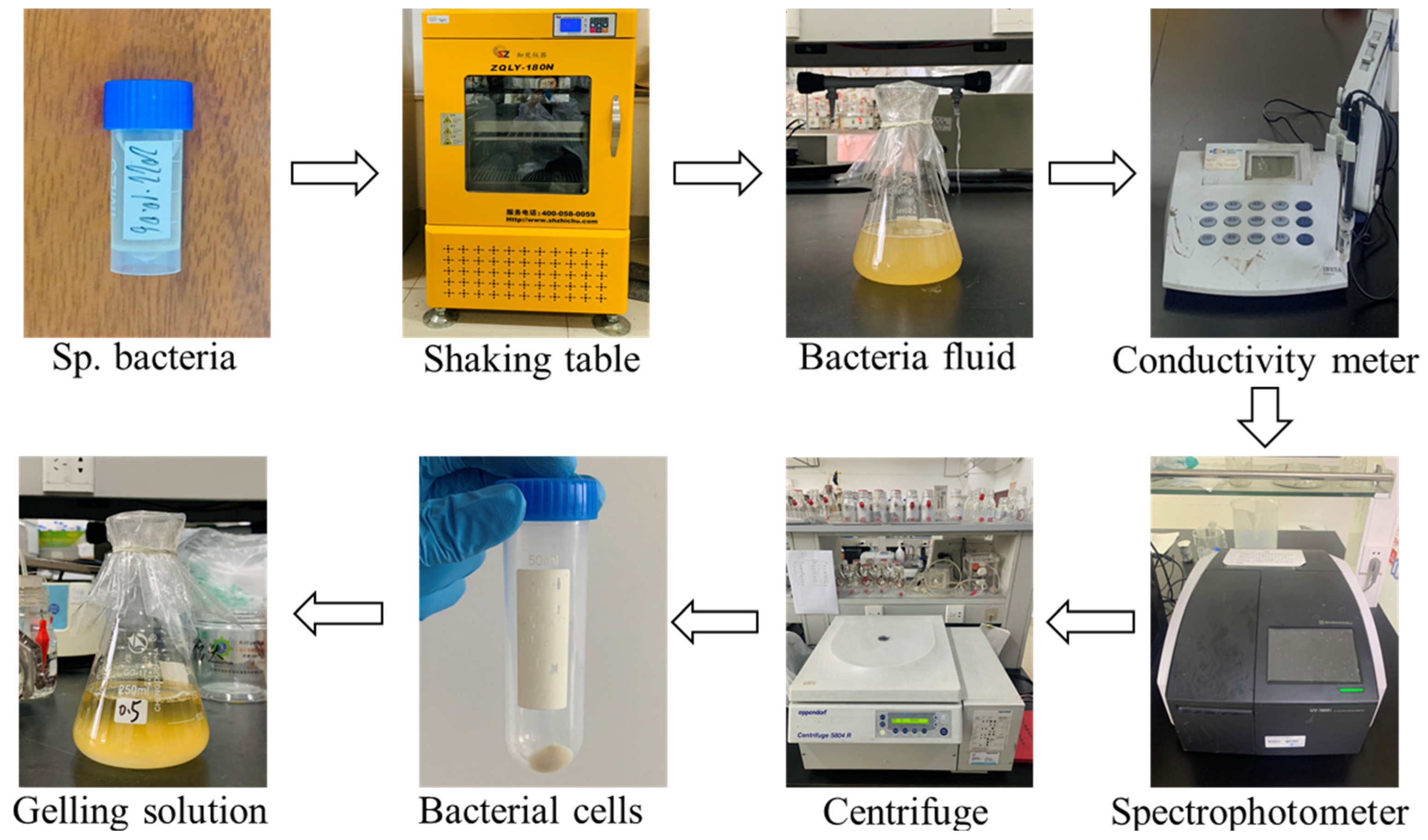
2.1. Test Materials
2.2. Specimen Preparation
- (1)
- Contaminant incorporation
- (2)
- MICP gelling solution
- (3)
- MICP treatment
2.3. Test Methods
3. Test Results
3.1. Mechanical Properties of Zn-Contaminated Soil
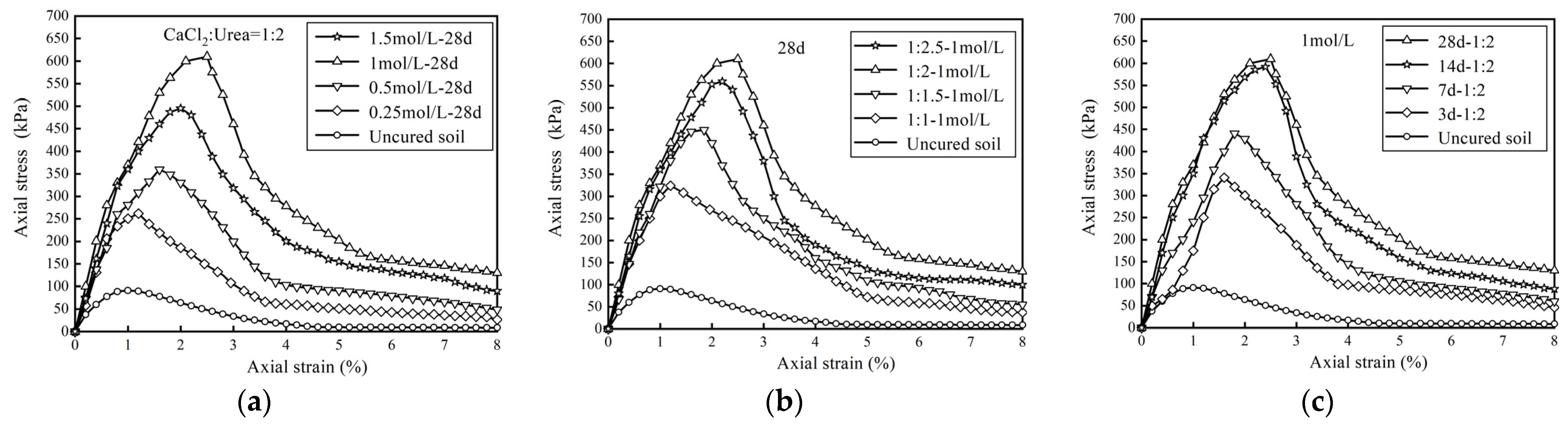
3.1.1. Stress–Strain Relationship
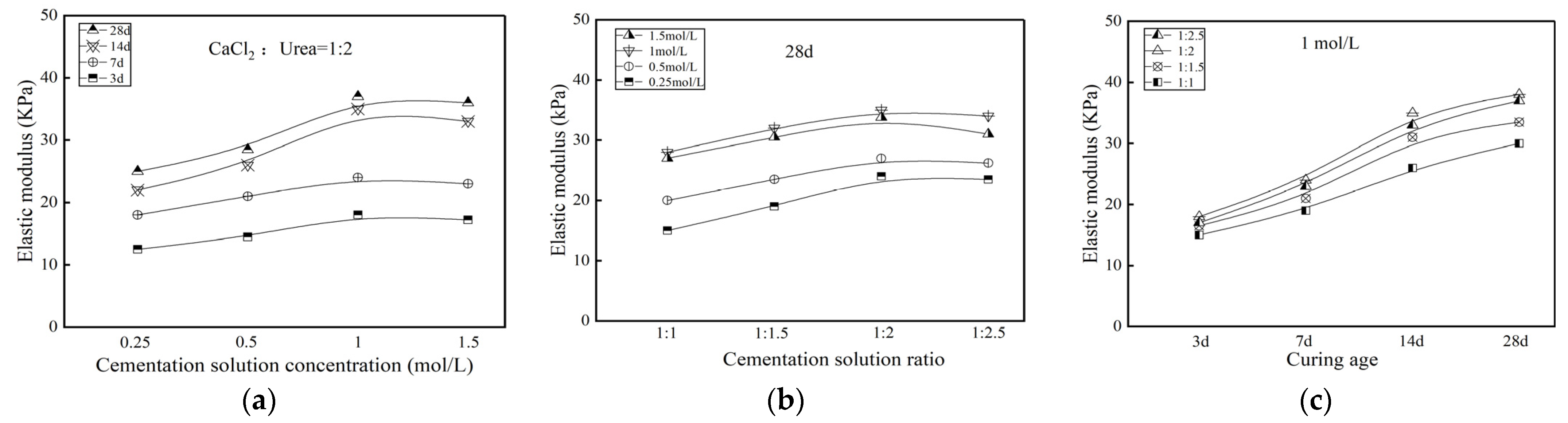
3.1.2. Elastic Modulus
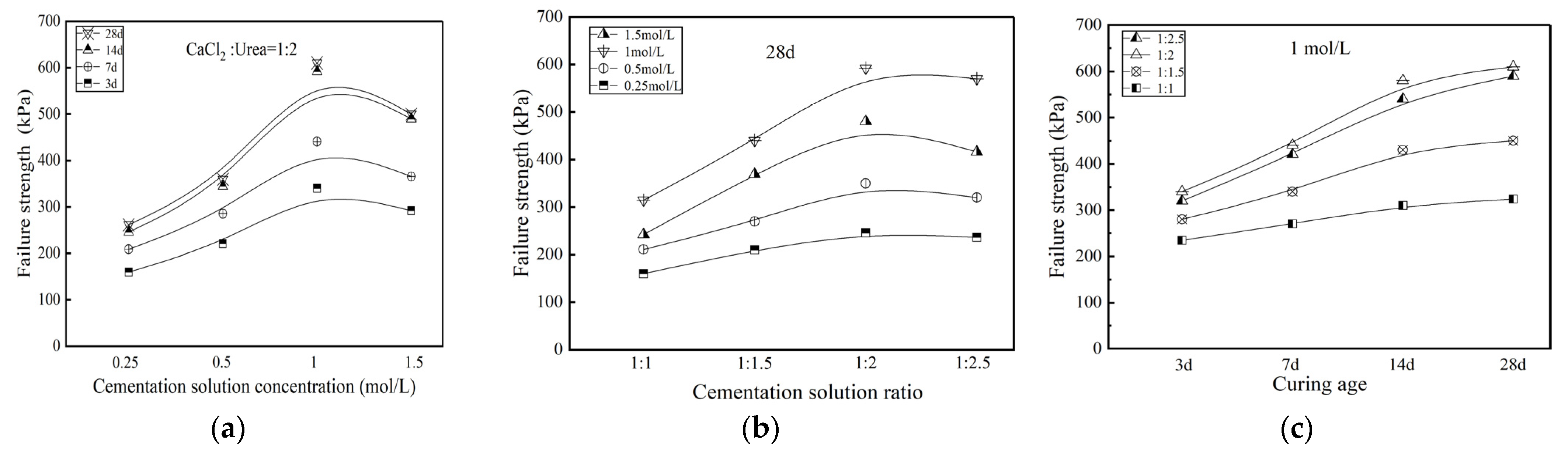
3.1.3. Failure Strength
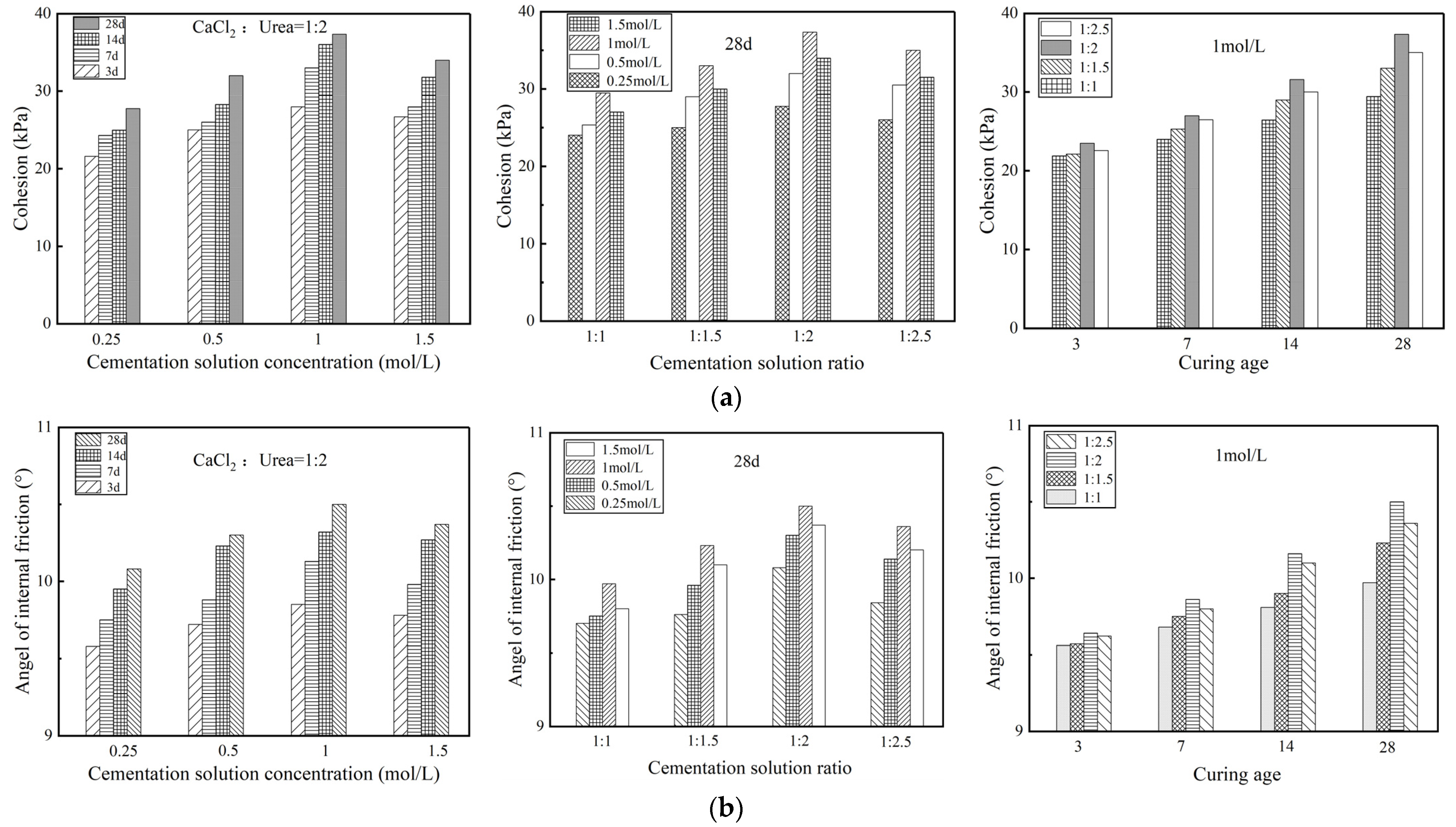
3.1.4. Shear Strength
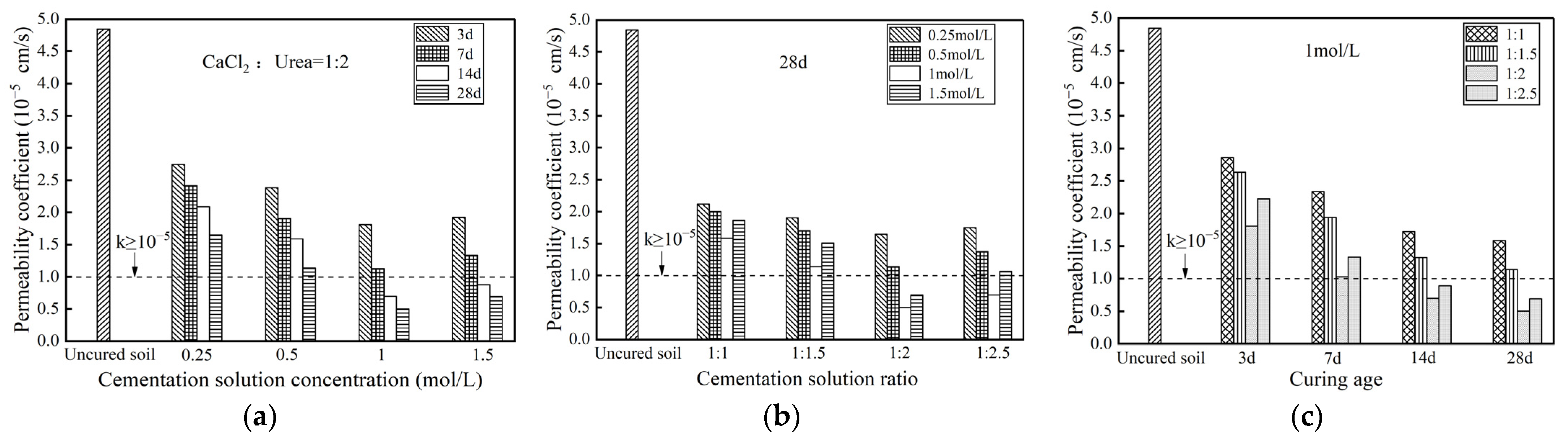
3.2. Permeability Properties of Zn-Contaminated Soil
Permeability Coefficient
3.3. The Stability Characteristics of Zn-Contaminated Soil
3.3.1. Zn Chemical Forms
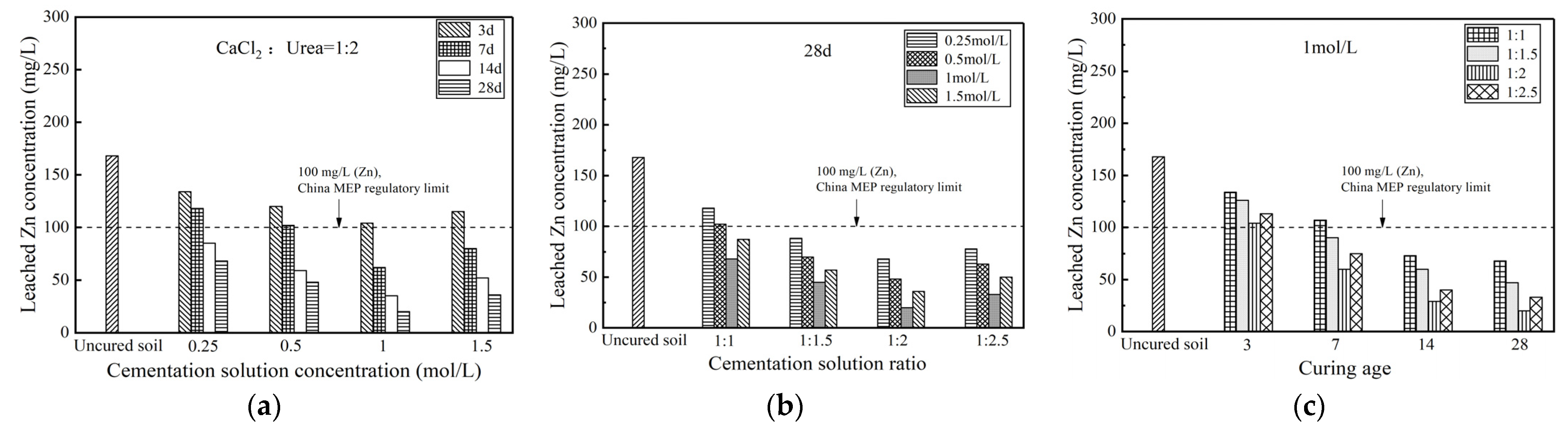
3.3.2. Toxic Leaching Characteristics
4. Mechanism Analysis
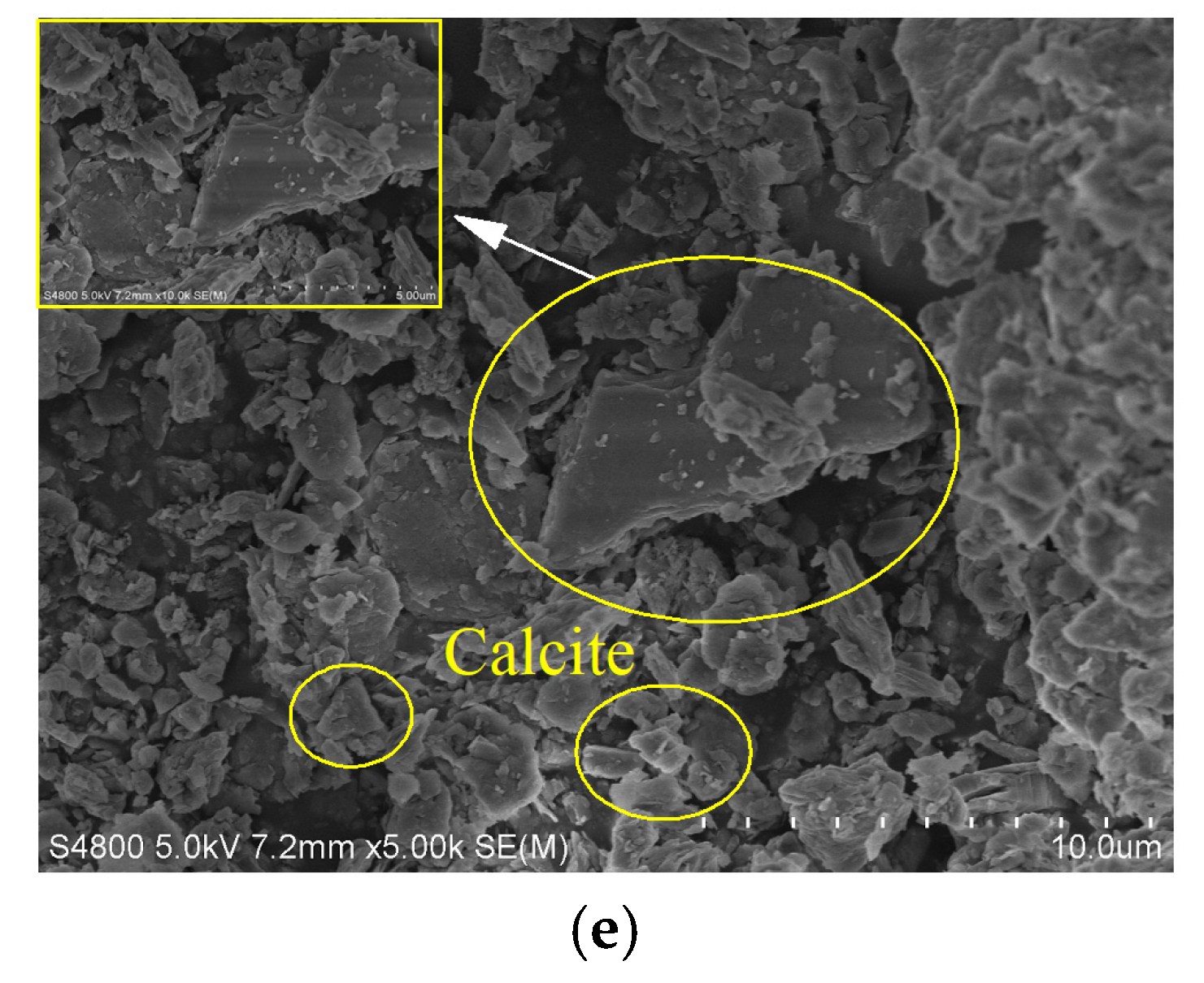
4.1. Scanning Electron Microscope
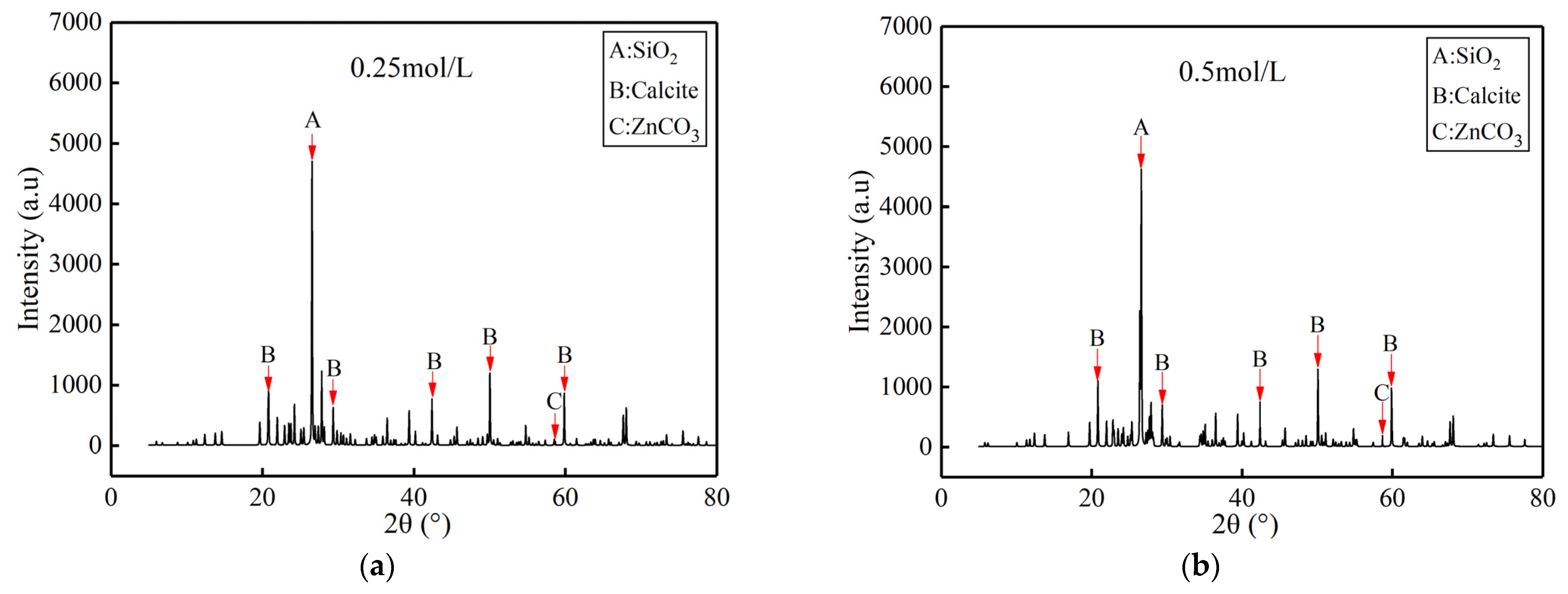
4.2. X-ray Diffraction
5. Conclusions
- (1)
- Microbial-induced calcium carbonate precipitation treatment changes the stress–strain relationship of Zn-contaminated soil from weakly softened type to strongly softened type. The unconfined compressive strength of the treated specimens increased by 187.2%~550.5%. The cohesion of treated specimens presented a significant upward trend while the internal friction angle keeps relatively stable. Microbial-induced calcium carbonate precipitation treatment could strengthen the mechanical properties of Zn-contaminated soils.
- (2)
- The permeability coefficient can be reduced by at least one order of magnitude. Microbial-induced calcium carbonate precipitation treatments significantly reduced the leaching concentration of zinc ions in Zn-contaminated soils to about 20 mg/L, which was lower than the limit (100 mg/L) in the standard. The mobility of heavy metal Zn was significantly reduced, and the proportion of exchangeable Zn content substantially declined. Microbial-induced calcium carbonate precipitation treatment could enhance permeability properties and reduce toxic leaching capacity for Zn-contaminated soils.
- (3)
- For mechanical properties, impermeability properties, and toxic leaching capacity, the stabilization effect of contaminated soil treated by MICP would be most significant when the specimens had a curing age of 28 d, a cementation solution concentration of 1 mol/L and a cementation solution ratio of 1:2.
- (4)
- The main microbial mineralization product of Zn-contaminated soil treated by MICP is calcite. The increase in cementation degree is the main reason for the improvement of the physical and mechanical properties of treated Zn-contaminated soil. At the same time, the exchangeable zinc ions in the specimen were removed and stabilized in the carbonate-bound during the MICP process, which made the Zn-contaminated soil in compliance with environmental requirements.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, S.; Wang, Y.W.; Lai, J.L.; Zhang, Y.L.; Xue, G. Effects of long-term herbaceous plant restoration on microbial communities and metabolic profiles in coal gangue-contaminated soil. Environ. Res. 2023, 234, 116491. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.M.; Li, Y.L.; Yu, C.; Chen, P.; Chen, J.K.; Gao, G.; Wang, X.F.; Xiong, H.P.; Zhu, A.G. Systematic evaluation of ramie (Boehmeria nivea L.) for phytoremediation of cadmium contaminated soil and the mechanism of microbial regulation. Chemosphere 2023, 337, 139298. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Song, Y.; Wei, J.; Mao, W.; Ju, J.; Zheng, S.Y.; Zhao, H.T. Synergistic Effects of Earthworms and Plants on Chromium Removal from Acidic and Alkaline Soils: Biological Responses and Implications. Biology 2023, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Dejong, J.T.; Soga, K.; Kavazanjian, E. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Wang, X.N.; Wu, F.; Zhang, J.W.; Ai, S.H.; Liu, Z.T. Microbial community composition and degradation potential of petroleum-contaminated sites under heavy metal stress. J. Hazard. Mater. 2023, 457, 131814. [Google Scholar] [CrossRef] [PubMed]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Boil. Biochem. 1999, 31, 1563–1571. [Google Scholar]
- Wang, S.N.; Guo, S.F.; Gao, X.Q.; Zhang, P.; Li, G.Y. Effects of cement content and soil texture on strength, hydraulic, and microstructural characteristics of cement? Stabilized composite soils. Bull. Eng. Geol. Environ. 2022, 81, 264. [Google Scholar] [CrossRef]
- Luo, M.; Dai, J.J.; Ding, Z.Q. Properties of mortar containing recycled fine aggregate modified by microbial mineralization. Buildings 2022, 12, 2035. [Google Scholar] [CrossRef]
- Chen, H.M.; Jiang, H.F.; Nazhafati, M.; Li, L.L.; Jiang, J.Y. Biochar: An effective measure to strengthen phosphorus solubilizing microorganisms for remediation of heavy metal pollution in soil. Front. Bioeng. Biotechnol. 2023, 11, 1127166. [Google Scholar]
- Chen, H.M.; Min, F.F.; Hu, X.; Ma, D.H.; Huo, Z.L. Biochar assists phosphate solubilizing bacteria to resist combined Pb and Cd stress by promoting acid secretion and extracellular electron transfer. J. Hazard. Mater. 2023, 452, 131176. [Google Scholar] [CrossRef] [PubMed]
- Mwandira, W.; Nakashima, K.; Kawasaki, S. Bioremediation of lead-contaminated mine waste by Pararhodobacter sp. based on the microbially induced calcium carbonate precipitation technique and its effects on strength of coarse and fine grained sand. Ecol. Eng. 2017, 109, 57–64. [Google Scholar] [CrossRef]
- Song, M.Z.; Lan, T.; Meng, Y. Effect of microbially induced calcium carbonate precipitation treatment on the solidification and stabilization of municipal solid waste incineration fly ash (MSWI FA)—Based materials incorporated with metakaolin. Chemosphere 2022, 308, 136089. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, Y.; Chu, J. In-situ microbially induced Ca2+—Alginate polymeric sealant for seepage control in porous materials. Microb. Biotechnol. 2018, 12, 324–333. [Google Scholar] [CrossRef]
- Zhang, J.K. Environmental impact and mechanical improvement of MICP-treated coal fly ash-soil mixture. Environ. Geotech. 2020, 1, 1–11. [Google Scholar]
- Li, M.; Cheng, X.H.; Guo, H.X. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Zhu, X.J.; Li, W.L.; Zhan, L.; Huang, M.S.; Zhang, Q.Z.; Achal, V. The large-scale process of microbial carbonate precipitation for nickel remediation from an industrial soil. Environ. Pollut. 2016, 219, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, C.; Ding, D.; Lei, Z.; Yang, Y.; Feng, C.; Zhang, Z. Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour. Technol. 2013, 137, 18–24. [Google Scholar] [CrossRef]
- Mallampati, S.R.; Mitoma, Y.; Okuda, T.; Simion, C.; Lee, B.K. Dynamic immobilization of simulated radionuclide 133 Cs in soil by thermal treatment/vitrification with nanometallic Ca/CaO composites. J. Environ. Radioact. 2015, 139, 118–124. [Google Scholar] [CrossRef]
- Wu, Q.; Cui, Y.; Li, Q.; Sun, J. Effective removal of heavy metalsfrom industrial sludge with the aid of a biodegradable chelating ligand GLDA. J. Hazard. Mater. 2015, 283, 748–754. [Google Scholar] [CrossRef]
- Albers, C.N.; Jacobsen, O.S.; Flores, E.M.M.; Johnsen, A.R. Arctic and Subarctic Natural Soils Emit Chloroform and Brominated Analogues by Alkaline Hydrolysis of Trihaloacetyl Compounds. Environ. Sci. Technol. 2017, 51, 6131. [Google Scholar] [CrossRef]
- Taiwo, A.M.; Gbadebo, A.M.; Oyedepo, J.A.; Ojekunle, Z.O.; Alo, O.M.; Oyeniran, A.A.; Onalaja, O.J.; Ogunjimi, D.; Taiwo, O.T. Bioremediation of industrially contaminated soil using compost and plant technology. J. Hazard. Mater. 2016, 304, 166–172. [Google Scholar] [PubMed]
- GB/T 50123-2019; Standard for Geotechnical Testing Method. China Planning Press: Beijing, China, 2019.
- Du, Y.J.; Wei, M.L.; Reddy, K.R. Effect of carbonation on leachability, strength and microstructural characteristics of KMP binder stabilized Zn and Pb contaminated soils. Chemosphere 2016, 144, 1033–1042. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Ferris, F.G.; Fyfe, W.S.; Beveridge, T.J. Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chem. Geol. 1987, 63, 225–232. [Google Scholar] [CrossRef]
- Jane, E.H.; Robert, H.; Garth, D.R. Growing bio-tiles using microbially induced calcium carbonate precipitation. Sci. Total Environ. 2023, 895, 165050. [Google Scholar]
- Alireza, K.; Issa, S.; Ali, H.A. Effects of Sporosarcina Pasteurii’s on Curing Time and Strength of Silty Sand Soil. Geotech. Geol. Eng. 2023, 41, 3289–3304. [Google Scholar]
- Zhou, G.; Xu, Y.X.; Wang, Y.M.; Zheng, L.; Zhang, Y.L.; Li, L.; Sun, B.; Li, S.L.; Zhu, Y.C. Study on MICP dust suppression technology in open pit coal mine: Preparation and mechanism of microbial dust suppression material. J. Environ. Manag. 2023, 343, 118181. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.L.; Zhang, D.Y. Bioremediation of Pb-contaminated soil based on microbially induced calcite precipitation. J. Microbiol. Biotechn. 2012, 22, 244–247. [Google Scholar] [CrossRef]
- Gillman, E.; Mogran, M.A.; Sherwood, M. Urease activity in irish soils at 6 °C. Biol. Environ. 1995, 95, 19–26. [Google Scholar]
- Gorospe, C.M.; Han, S.H.; Kim, S.G. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Ma, G.; He, X.; Jiang, X.; Liu, H.; Xiao, Y. Strength and permeability of bentonite-assisted biocemented coarse sand. Can. Geotech. J. 2020, 58, 969–981. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Morduch University, Perth, Australia, 2004. [Google Scholar]
- Wang, Y.J.; Jiang, N.J.; Saracho, A.C.; Doygun, O.; Du, Y.J.; Han, X.L. Compressibility characteristics of bio-cemented calcareous sand treated through the bio-stimulation approach. J. Rock Mech. Geotech. Eng. 2023, 15, 510–522. [Google Scholar] [CrossRef]
- Zhu, R.; Huang, Y.H.; Zhang, C.; Guo, W.L.; Chen, H. Laboratory and centrifugal model tests on failure mechanism of canal slopes under cyclic action of wetting-drying. Eur. J. Environ. Civ. Eng. 2022, 26, 2819–2833. [Google Scholar] [CrossRef]
- Zhu, R.; Cai, Z.Y.; Huang, Y.H.; Zhang, C.; Guo, W.L.; Wang, Y. Effects of wetting-drying-freezing-thawing cycles on mechanical behaviors of expansive soil. Cold Reg. Sci. Technol. 2022, 193, 103422. [Google Scholar] [CrossRef]
- ASTM D4219-02; Standard Test Method for Unconfined Compressive Strength Index of Chemical-Grouted Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D3080-04; Standard Test Method for Standard Test Method for Direct Shear Test of Soils under Consolidated Drained Conditions. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D2434-19; Standard Test Method for Permeability of Granular Soils (Constant Head). ASTM International: West Conshohocken, PA, USA, 2019.
- Tessier, A.; Campbell, P.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- ASTM. Method for Toxicity Characteristic Leaching Produce (1311); ASTM International: West Conshohocken, PA, USA, 1992. [Google Scholar]
- GB 5085.3-2007; Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. China Planning Press: Beijing, China, 2007.
- Lee, W.; Bohra, N.C.; Altschaeffl, A.G. Resilient modulus of cohesive soils. J. Geotech. Geoenviron. 1997, 123, 131–136. [Google Scholar] [CrossRef]
- Kunst, F.; Rapoport, G. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J. Bacteriol. 1995, 177, 2403–2407. [Google Scholar] [CrossRef]
- Nemati, M.; Greene, E.A.; Voordouw, G. Permeability profile modification using bacterially formed calcium carbonate: Comparison with enzymic option. Process. Biochem. 2005, 40, 925–933. [Google Scholar] [CrossRef]
- Harkes, M.P.; Paassen, L.; Booster, J.L. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Joer, H.; Randolph, M.; Meritt, A. Cementation of porous materials using calcite. Géotechnique 2002, 52, 313–324. [Google Scholar]
- Pakbaz, M.S.; Behzadipour, H.; Ghezelbash, G.R. Evaluation of shear strength parameters of sandy soils upon microbial treatment. Geomicrobiol. J. 2018, 35, 721–726. [Google Scholar] [CrossRef]
- Qabany, A.A.; Soga, K.; Santamarina, C. Factors affecting efficiency of microbially induced calcite precipitation. J. Geotech. Geoenviron. 2012, 138, 992–1001. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X. Influence of calcium sources on microbially induced calcium carbonate precipitation by bacillus sp. CR2. Appl. Biochem. Biotech. 2014, 173, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Cussac, V.; Ferrero, R.L.; Labigne, A. Expression of helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. J. Bacteriol. 1992, 174, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Du, L.; Yuan, J.; Sun, X.; Pathirage, M.; Sun, W.; Feng, J. A Experimental study on Engineered Cementitious Composites (ECC) Incorporated with Sporosarcina pasteurii. Buildings 2022, 12, 691. [Google Scholar] [CrossRef]
- Al-Thawadi, S. High Strength In-Situ Biocementation of Soil by Calcite Precipitating Locally Isolated Ureolytic Bacteria. Ph.D. Thesis, Murdoch University, Perth, Australia, 2008. [Google Scholar]
- Meldrum, F.C.; Cölfen, H.H. Controlling mineral morphologies and structures in biological and synthetic systems. Chem. Rev. 2009, 40, 4332–4432. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.X.; Cheng, X.H. Influences of calcium sources on microbially induced carbonate precipitation in porous media. Mater. Res. Innov. 2014, 18, S2-79–S2-84. [Google Scholar] [CrossRef]







| Soil Properties | Silt |
|---|---|
| Specific gravity | 2.67 |
| Liquid limit | 18.4% |
| Plastic limit | 12.3% |
| Optimum moisture content | 12.8% |
| Maximum dry density | 1.80 Mg/m3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, W.; Zhou, F.; Zhu, R.; Wang, X.; Chen, T. Performance and Mechanism of Zn-Contaminated Soil through Microbe-Induced Calcium Carbonate Precipitation. Buildings 2023, 13, 1974. https://doi.org/10.3390/buildings13081974
Xing W, Zhou F, Zhu R, Wang X, Chen T. Performance and Mechanism of Zn-Contaminated Soil through Microbe-Induced Calcium Carbonate Precipitation. Buildings. 2023; 13(8):1974. https://doi.org/10.3390/buildings13081974
Chicago/Turabian StyleXing, Wei, Feng Zhou, Rui Zhu, Xudong Wang, and Tingzhu Chen. 2023. "Performance and Mechanism of Zn-Contaminated Soil through Microbe-Induced Calcium Carbonate Precipitation" Buildings 13, no. 8: 1974. https://doi.org/10.3390/buildings13081974
APA StyleXing, W., Zhou, F., Zhu, R., Wang, X., & Chen, T. (2023). Performance and Mechanism of Zn-Contaminated Soil through Microbe-Induced Calcium Carbonate Precipitation. Buildings, 13(8), 1974. https://doi.org/10.3390/buildings13081974




