Abstract
The circular economy transition encompasses the identification of various available and sustainable materials to replace traditional binders in the construction industry. The utilization of water sediments represents this point as a beneficial action that may provide synergy in terms of waste reduction and replacement of energy-intensive materials. To explore the potential of water sediments, this study contemplated the characterization of water sediments as precursors for the design of alkali-activated materials (AAMs). The experimental approach was based on the detailed characterization of raw materials’ chemical, mineralogical, and basic material properties and the assessment of the designed AAM paste and mortar samples. The results achieved revealed the capability of low amorphous water sediments to form dense structures with favorable mechanical performance, reaching up to 36.8 MPa in compressive strength. The microstructural and water sorption characteristics point to the applicability of such materials in the building practice and, thus, the valorization of water sediments into valuable material.
1. Introduction
The environmental impact of traditional Portland cement (PC) production has driven research into suitable alternatives. According to Li et al. [1], PC production contributes approximately 8% of annual CO2 emissions, contributing to greenhouse gas effects and global warming. Li et al. [2] added that 12–15% of global industrial energy is consumed in cement production, leading to high costs of the product. Cement production involves environmentally harmful practices, such as the extraction of limestone [3], so the increasing cement demand worsens environmental pollution [1]. Consequently, there is a growing need for eco-friendly cement materials to support construction while reducing environmental impact. In addition, the gradually increasing costs of carbon dioxide emission allowance pose another factor that creates pressure to search for a material alternative. Carbon tax and carbon emission trading system incentives are considered tools for reducing carbon emissions in the building industry.
To address these issues, researchers have explored cementless binders like alkali activation of industrial by-products and waste materials, offering a promising alternative to traditional cement [2,4]. Mostly, silica-alumina rich industrial by-products such as ground granulated blast furnace slag (GBFS), silica fume, fly ash (FA), red mud, agricultural waste ashes, e.g., rice husk ash, clay minerals like kaolin, soil, and sediments, can be used to produce cementitious materials (AAMs) through an alkali-activation process [5].
The chemical composition of these materials, particularly the silica (SiO2) and alumina (Al2O3) content, directly influences the properties of the resulting AAMs. The materials form a three-dimensional aluminosilicate binder gel in a geopolymerization reaction with an alkali activator to produce AAM material with physical properties similar to those of Portland cement [6,7]. High aluminosilicate content generally leads to better strength and durability due to the formation of more stable AAM gels by the dissolution of the material in an alkali activator to form monomers of silicate [SiO4]4− and aluminate [AlO4]5− and polymerization of the dissolved species to form oligomers, which further polymerize to form amorphous gels, “a three-dimensional network of Si–O–Al bonds”. The mechanical properties, such as strength and stiffness, as well as chemical resistance and durability of the resulting AAMs, are influenced by the degree of polymerization and cross-linking, type and amount of the alkali activator used, and the size and shape of the precursor [8,9].
In addition, alkaline activation may also lead to the formation of crystalline phases, such as zeolites and calcium silicates, depending on the composition of the precursors and the processing conditions [10]. These crystalline phases can contribute to the material’s properties by enhancing its thermal stability, modifying its pore structure, and providing additional mechanical reinforcement. Excessive crystallization may lead to reduced mechanical properties or undesired changes in material behavior, highlighting the importance of controlling the phase composition during the activation process [11,12].
However, the feasibility of alkali-activated materials (AAMs) lies significantly in the local availability of raw materials [13]. Many of the raw materials used to produce AAMs are also sought after for blending with ordinary Portland cement (OPC). This competition for raw materials is a crucial consideration in AAMs’ supply chains. For AAMs to be advocated as an environmentally friendly option, it is imperative to minimize the transport of bulk materials, which can increase production costs and, consequently, increase the carbon emissions footprint [5,14]. A key role in sustainable building material design is played by the valorization of locally available by-products into value-added materials. While the majority of the published works are conducted on conventional precursors (GGBS, FA), which face limited availability, the primary aim of this study lied in the utilization of abundant material.
Water sediments (WSs), a kaolinitic clayey characterized by high amounts of SiO2, Al2O3, and CaO, possess pozzolanic properties, making them potentially suitable for alkaline activation. WS composition relies on the rock from which sediments originated and subsequent reactions with fluids that convert these source rocks into solutes and minerals. It is of particular importance to distinguish the high-organic content sediments with a significant portion of phosphorus and nitrogen, sandy sediments, and fine-grain sediments with clay and silt content that require further treatment [15]. Unlike traditional precursors such as GGBS or FA, WS represents material that is abundant and produced locally with regard to the number of reservoirs or other water bodies. For example, Beddaa et al. [16] estimated yearly global dredging activities to yield about 600 million m3 of WS, with Europe accounting for about 300 million tons. Therefore, repurposing this volume into AAM production may reduce demand for traditional cement and, at the same time, enhance safe sediment disposal. Hence, the utilization of sediment in AAMs will signify a substantial move toward the principles of circular economy, promoting sustainability and efficient resource management [8,17].
Notwithstanding, only a few studies have explored the activation of WS through alkali activation. For instance, Fort et al. [18] investigated alkali-activated WS calcinated at 900 °C by employing a mixture of sodium hydroxide (NaOH) and potassium silicate solution (K2SiO3) as an activator. The compressive strength across the samples ranged from 14.59 MPa to 37.09 MPa, with the enhanced strength attributed to effective aluminosilicate dissolution during the alkali activation. WS with lower Si/Al ratios exhibited higher porosity in the 0.01–0.1 µm range, with scanning electron microscopy (SEM) analysis and mercury intrusion porosimetry (MIP) results indicating the formation of denser structures for sediments with fine particles [19]. In another study, Jiang et al. [20] prepared artificial stone through the alkali activation of yellow river silt using Ca (OH)2 as an activator. The compressive strength and splitting tensile strength of the stone were determined under various conditions, such as alkali dosages, fly ash contents, and curing ages. Results indicate that the compressive strengths of heat-treated specimens increased by 60% with an increase in curing age. Furthermore, the addition of 10% fly ash significantly enhanced the mechanical properties of the mixture, with compressive strengths and splitting tensile strengths of specimens, with fly ash being 2 to 3 times greater than those without. The highest compressive strength of heat-treated specimens was recorded as 16.6 MPa at 90 days of curing with a Ca(OH)2 dosage of 15%, while the highest splitting tensile strength was 1.4 MPa with a Ca(OH)2 dosage of 10%. Zibret et al. [21] analyzed clay-rich river WS as potential precursors for AAMs, calcinating two samples, fresh sediment and old sediment, to compare the material reactivity. As found, only limited mechanical performance was achieved, and FA and slag need to be applied to increase the compressive strength up to 32.2 and 29.3 MPa, respectively. Similarly, Komnitsas [22] conducted experimental studies on two Greek marine WS from Souda and Patras. The research concluded that Souda WS exhibited limited alkali activation due to their low SiO2 content, resulting in compressive strength reaching only 5 MPa. Conversely, Patras WS, containing higher amounts of amorphous silica compounds, showed better mechanical performance, with compressive strengths increasing from 8.5 to 19 MPa as KOH molarity increased from 2 M to 4 M. The analogy within the design stage can be recognized in the use of crushed recycled glass for alkali-activated fly ash based geopolymer concrete and prediction of its capacity [23].
Based on the above-mentioned findings, utilizing sediment may offer a promising pathway for new construction material while, at the same time, promoting resource conservation, environmental protection, and sustainable treatment of sediment [24]. Instead of viewing sediments as waste to be discarded, it is important to prioritize their valorization into AAMs, thereby converting sediment into valuable construction material [25,26]. As evident from the literature, the transformation of WS into a building material with sufficient strength is still an unsolved task, especially due to the limited amorphous content. Despite the advances in the understanding of the alkaline activation process for the conventional precursors, the utilization of WS in a similar way struggles with limited mechanical performance and no clear design guidelines. This study focuses on the design of freshwater sediment-based AAMs and the characterization of the material properties. For this purpose, the different grades of WS are collected and consequently used for the design of paste samples. On the basis of analysis of mineralogical and chemical composition, supplemented by the determination of mechanical parameters and the development of reaction heat evolution, the most suitable WS are further utilized for mortar design. The accessed results provide advances in the field of valorization of WS based on the characterization of the microstructural and mechanical performance.
2. Materials and Methods
2.1. Materials
The freshwater sediments used for alkali activation were collected from eight different water dams in the Czech Republic and from S1 to S8 accordingly. Before the characterization, the sediments were naturally pre-dried to remove the excessive water content and consequently dried in the electric oven to achieve steady-state mass. The calcination of the WS is important for AAM production, as it transforms crystalline phases into amorphous phases, making enough aluminosilicate available for the polymerization reaction. The dehydroxylation carried out at calcination temperatures of 900 °C improves the reactivity, mechanical properties, and overall performance of the formed AAMs by leaching the available aluminosilicate in the alkaline solution. This process consequently forms an AAM network with a denser and more compact microstructure.
The XRF machine (ARL QUANT’X Energy Dispersive X-ray Fluorescence spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) determined the elemental composition of the collected samples with an adjustable X-ray beam size of 1 to 15 mm. The oven-dried samples were calcined at 900 °C (based on preliminary testing) in a muffle furnace (Classic Clare 4.0) at a heating rate of 5 °C/min for 3 h (peak temperature). The sediment samples were ground to a fine powder using a ball mill machine for 1 h and sieved with a 0.125 mm mesh to achieve a larger surface area. The phase compositing of the processed material was evaluated using X-ray powder diffractometer AERIS (XRD).
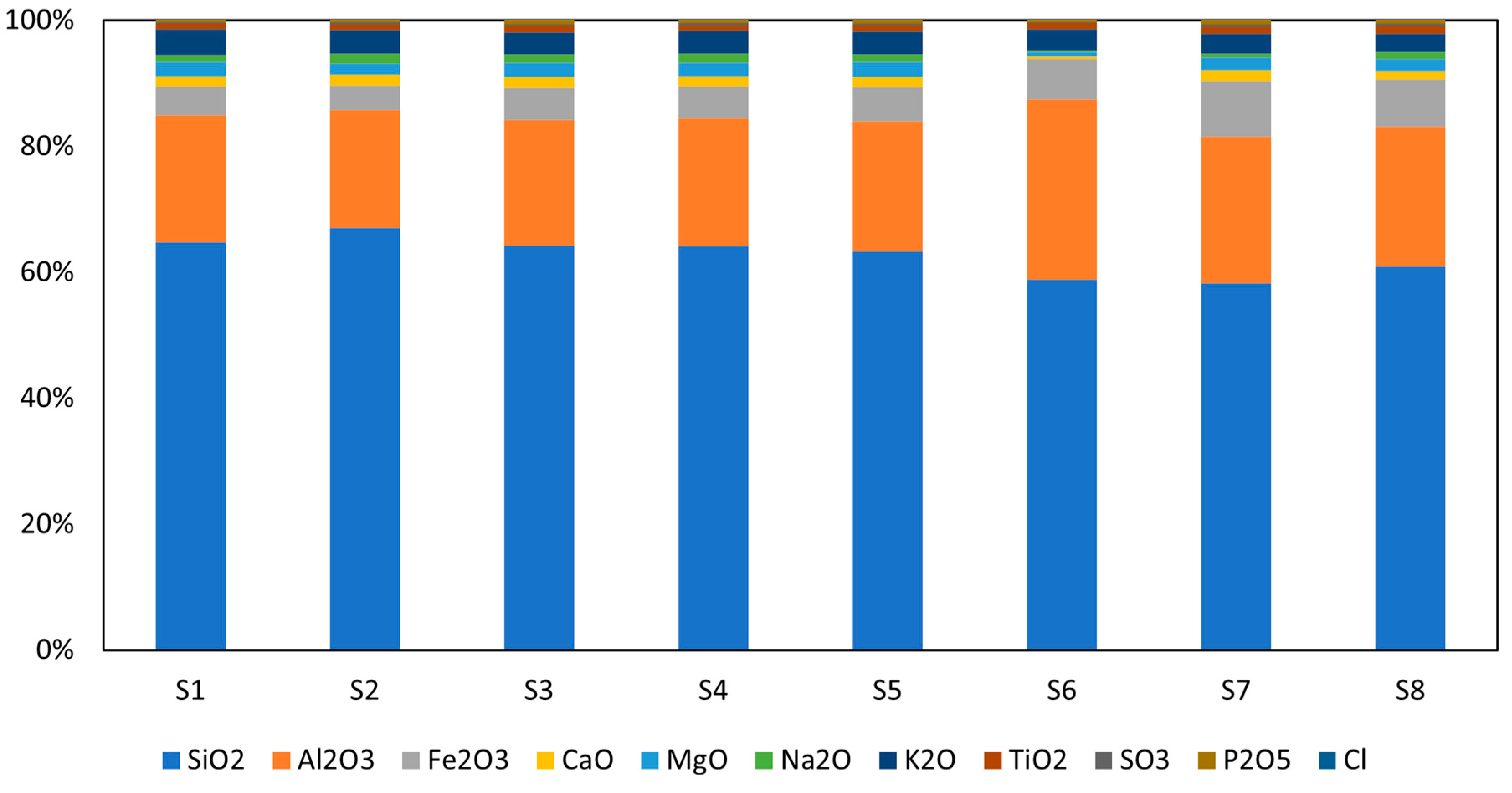
The chemical composition of the dry WS samples was determined by X-ray fluorescence (XRF) analysis, as shown in Figure 1. The analysis revealed that the samples were rich in silica (SiO2) and alumina (Al2O3), with combined contents of 81 to 86 wt.%. Also, there was a significant amount of iron oxide (4 to 9 wt.%) and a small quantity of other elements. The materials exhibited low levels of heavy metals, below the threshold value at which they are considered toxic. In comparison to the EU fly ash standards in terms of heavy metals, the revealed concentration was below despite the slightly increased concentrations of Mn, Zr, and Ba in the materials [27,28]. Oxide composition corresponds well to the rice husk ash studied by Alvarado et al. [29]; here, only variations in Al2O3 can be recognized. The analogy with the fly ash (Class-F) is more complicated, as a significantly lower share of the amorphous reactive phase can be recognized. This finding makes the polycondensation process more complicated, and less favorable results can be expected without additional treatment.
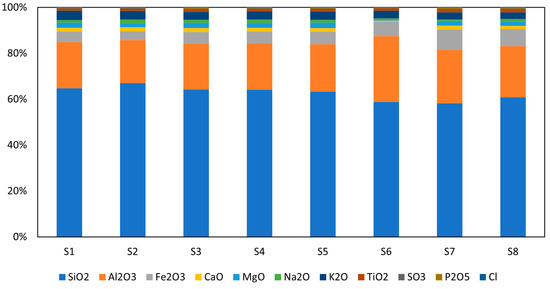
Figure 1.
Chemical composition of studied raw WS.
Mineralogical Composition (XRD)
The mineralogical phases present in the WS include kaolinite (Al2Si2O5(OH)4), chlorite (ClO−2), albite (NaAlSi3O8), quartz (SiO2), microcline (KAlSi3O8), and illite (K, H3O) (Al, Mg, Fe)2(Si, Al)4O10[(OH)2·(H2O)] were determined using XRD, as shown in Table 1.

Table 1.
Phase composition of the raw WS.
The mineralogical compositions are generally similar, but some differences are notable. All samples contain an average amount of clay minerals in varying proportions (chlorite, illite, kaolinite), feldspar (albite, plagioclase, microcline), tectosilicates such as quartz, and small amounts of hornblende and hillebrandite (hydrated calcium silicate Ca2(SiO3)(OH)2).
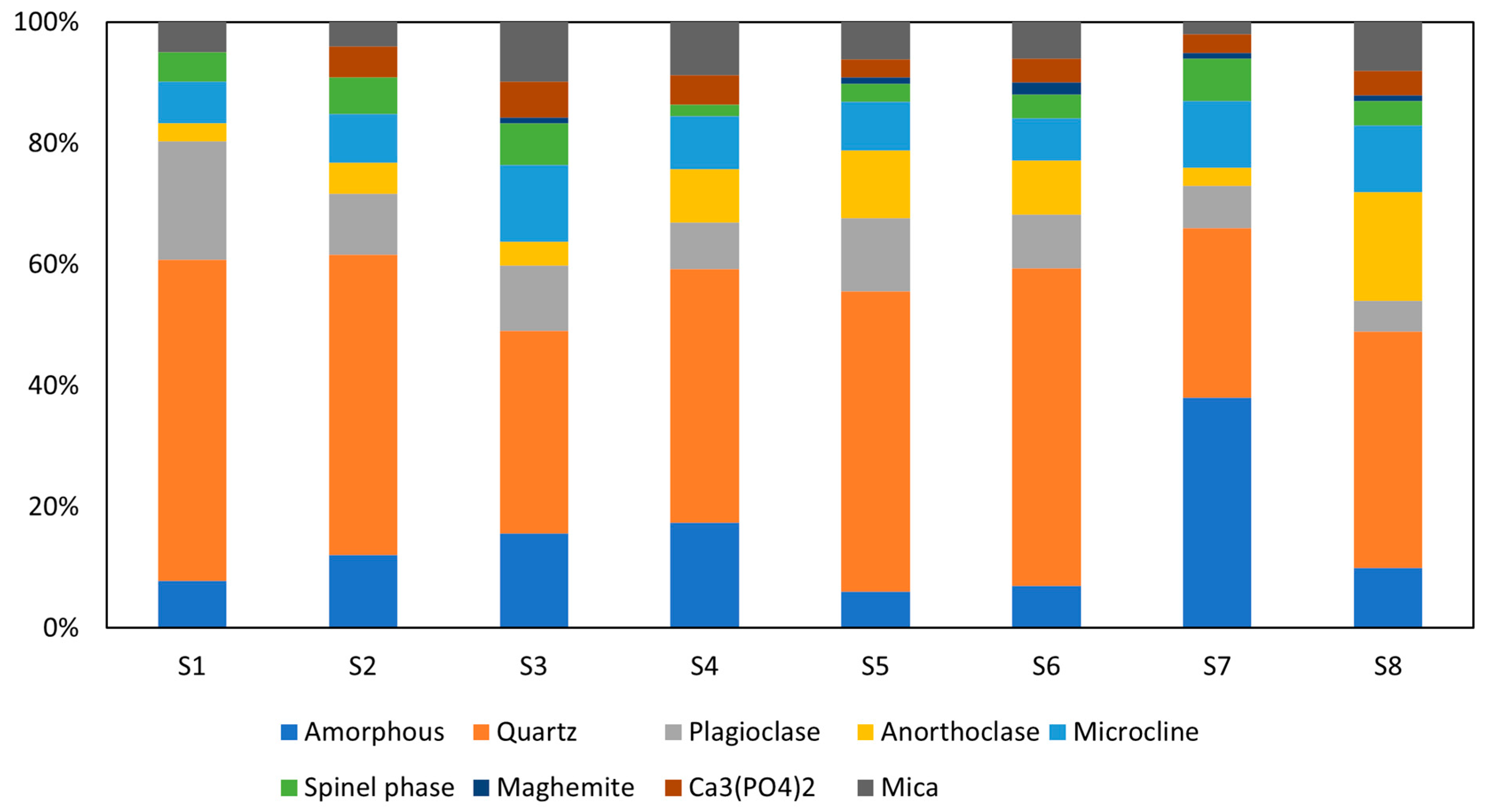
Notably, samples S6, S7, and S8 exhibit significant amounts of kaolinite, while samples S1 and S2 show a high content of illite, indicating their potential for geopolymerization reactions after thermal treatment [30]. Negligible amounts of microclines and varying amounts of amorphous albite, which can be made reactive by calcination, were also detected.
2.2. Sample Preparations
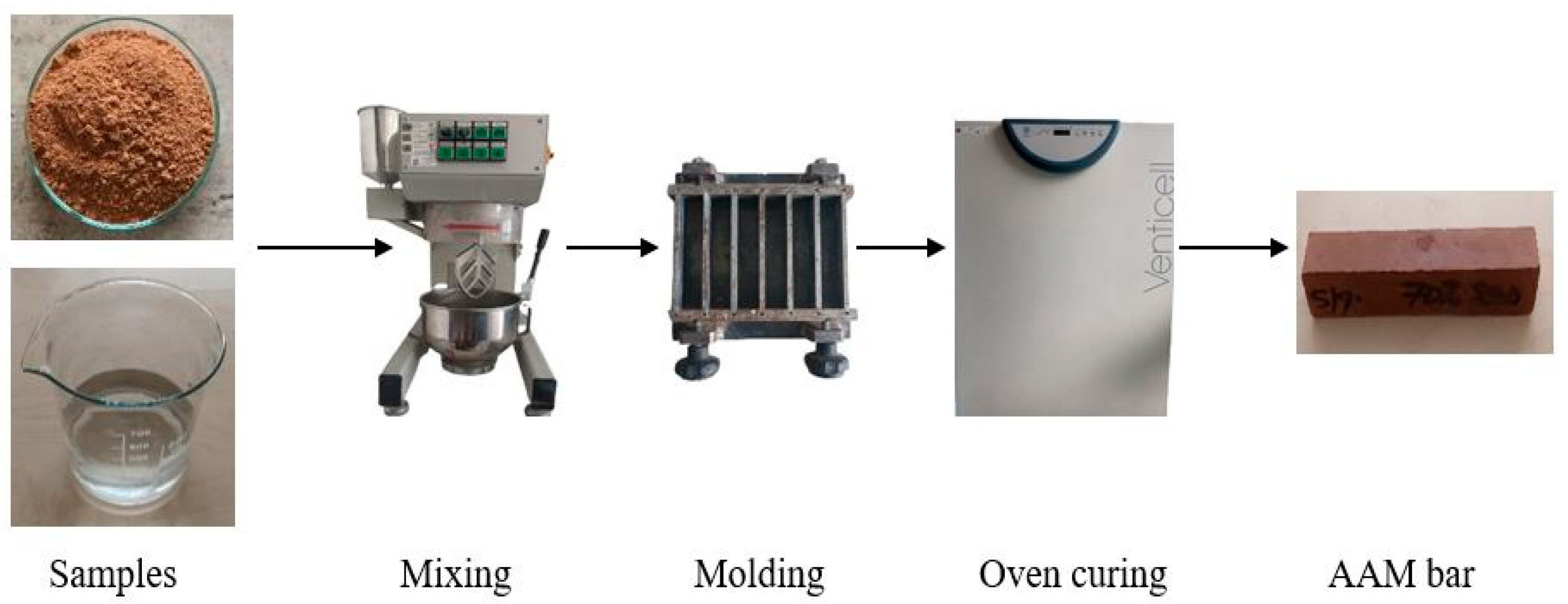
First, the paste samples were prepared to identify the most suitable materials for detailed investigation. To synthesize the AAMs, the calcinated WS sample, water, and an alkali activator, potassium silicate, were blended into a homogeneous mixture using a FORM +TEST Prufsysteme standard cement/mortar mixer for 4 min in a liquid/solid ratio of 0.4 according to CSN EN 196 [31]. Designed paste samples were denoted S1-P to S8-P according to the type of raw sediment used (S1–S8). The mixture was then poured into a 40 mm × 40 mm × 160 mm steel mold. After vibrating the mold on a vibration table to remove air bubbles, it was covered with polyethylene film and cured at ambient temperature for 2 days. The samples were then de-molded, wrapped in polyethylene film, and left in a natural environment for an additional 26 days. Subsequently, they were oven-cured to a constant weight at 80 °C for 2 days. The preparation process is depicted in Figure 2. After the first round of mixing performed on the pasta samples, the most promising materials were selected based on the reactivity parameters, and the mortars (S1-M, S4-M, S7-M) were designed according to the ratios presented in Table 2.
Figure 2.
AAM preparation process.

Table 2.
Experimental design for mortar mix.
2.3. AAM Characterization
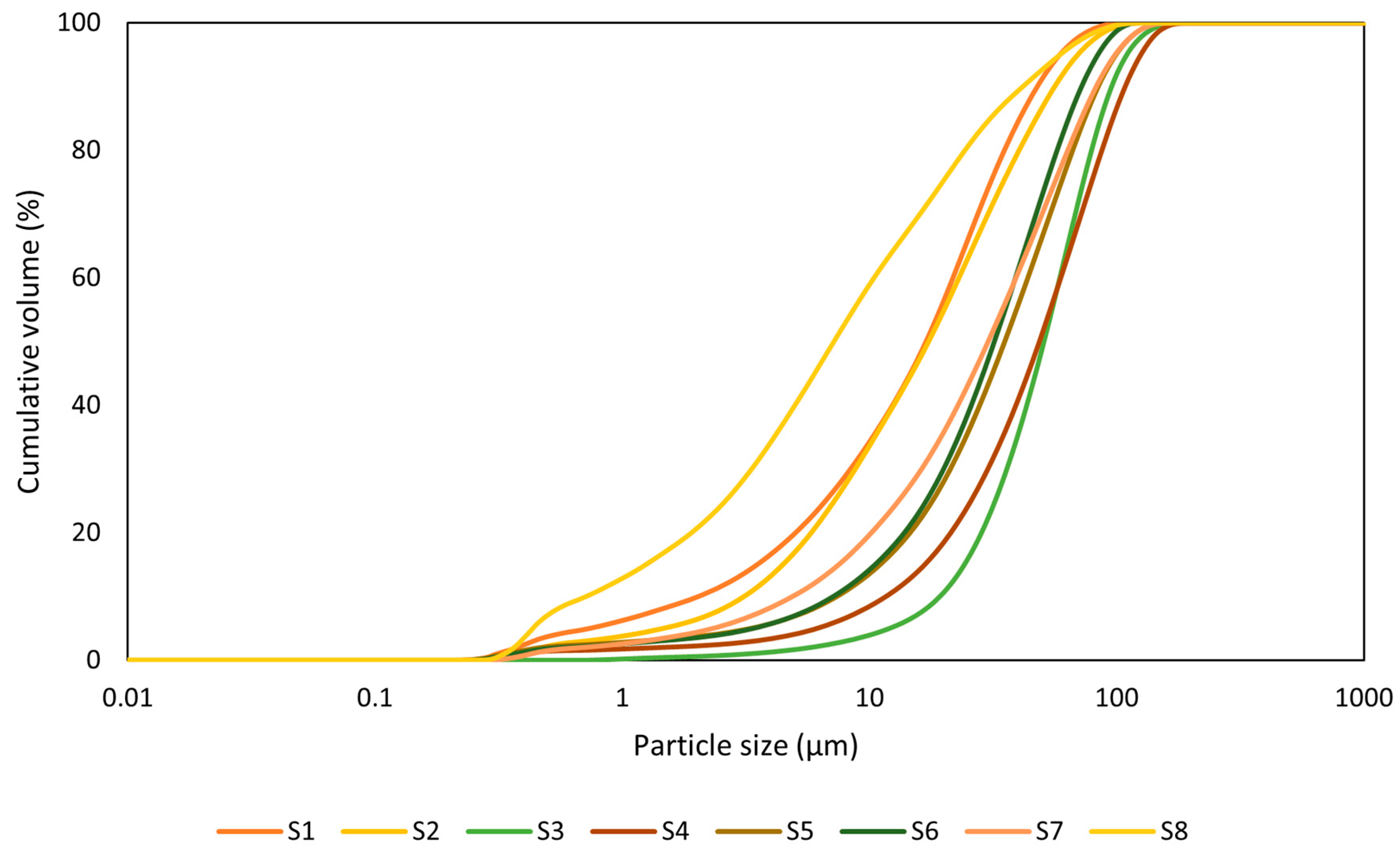
The Bettersizer S3 Plus laser diffraction particle size and shape analyzer, equipped with 0.5× and 10× image magnification, was used to determine the particle size distribution of the FSW. The particle size distribution was determined by dry sieving, according to the European standard EN 933-1 [28]. During measurement, particles dispersed in the fluid were pumped through two sample cells. In the first cell, short-wave laser light (532 nm) illuminates the particles, causing them to scatter. The optical signals were detected at an angle range of 0.02° to 165°. In the second sample cell, cameras captured images of the particles to provide shape information within the range of 2 µm to 3500 µm. The particle size distribution (PSD) of WS indicates average size distributions ranging from 7.198 µm to 50.78 µm at D50.
The chemical analysis of the raw WS was analyzed at a time range of 10 to 60 s per element. The resulting WS composes a good amount of silica-alumina, which indicates its suitability for AAM production.
The mineralogy was characterized using a AERIS XRD (Malvern PANalytical, Malvern, UK) with Cu-Kα wavelength radiation with a 600 W X-ray generation tube set at 30 kV. The qualitative and quantitative phases, as well as the amorphous content in the samples, were determined. The onboard RoboRiet 4.9 software was used for automated analysis, and the quantification of different phases was determined by the Rietveld method. For this purpose, the powder sample was mounted on an aluminum slide and scanned from 5° to 45° 2θ with a scan rate of 0.1 steps per minute.
A TAM Air eight-channel calorimeter (TA Instruments, New Castle, DE, USA) was employed for recording the reaction heat evolution during the alkaline reaction. Each calorimetric channel is a twin type, consisting of a reference chamber and a sample. The ampoules of the calorimeter have a volume of 20 mL operated at the temperature range from 5 to 90 °C with a detection limit of about 4 W.
Physical properties of the formulated AAM composites such as the bulk density, matrix density, and total open porosity were analyzed. The bulk density was obtained by determining the dimensions and weight of the samples, the matrix density was calculated using Pycnomatic ATC helium pycnometer manufactured by Thermo Fisher Scientific, and the total open porosity was calculated from the bulk and matrix density values.
A VEB WPM Leipzig mechanical device with a loading capacity of 3000 kN was used to examine the mechanical performance of the produced AAMs following the European standard EN 12390-2 guideline [32]. The flexural strength was determined by a three-point bending test on bar samples with dimensions of 40 mm × 40 mm × 160 mm (three specimens), following the EN 12390-5 procedure [33]. Compressive strength was determined using the broken piece (six specimens) from the flexural strength test, following the EN 12390-3 procedure [34].
Pore distribution analyses were carried out using Mercury Intrusion Porosimetry (MIP), Pascal 140 and Pascal 440 (Thermo Fisher Scientific, Prague, Czech Republic), with high pressurization speed, pressure capacity up to 400 MPa, and pore size range of 3 nm to 100 µm. During the evaluation, gases in the AAMs pores were removed at low pressure. The pores were subsequently filled with mercury at a contact angle of 140°.
The microstructure of the AAMs was analyzed using a JSM 6510 LV-Jeol scanning electron microscope, with a magnification capacity of 5 to 300,000 times. The samples were examined at magnifications between 250× and 2000×, using a 5 kV image with a spot size of 1.07 mm to 1.34 µm on the x-axis and 300 µm to 30 µm on the y-axis.
Sorptivity (S) is a term used for water ingress into pores of concrete; it is essentially a determination of the rate of water absorption due to capillary suction in a porous solid over a period of time. The sorptivity study was carried out by coating cut AAM cubes of 40 × 40 × 40 mm dimensions with epoxy resin on four sides while exposing one face of the cube to water for unidirectional water absorption. Before coating, the cubes were dried in an oven at 80 °C for 2 days. The dried samples were weighed to determine the initial mass before they were exposed to 10 mm water height at immersion times of 30, 60, and 120 s. The difference between the initial and final weight was compared to determine the water absorption. Using this experiment, the water absorption of the materials was characterized by sorptivity (S) and water absorption coefficient.
Sorptivity (S) is derived using Equation (1).
where is the square root of time (s).
I is the cumulative water absorption (mm), which is determined from Equation (2).
where Δm is the increase in weight of the sample due to water absorption (g), A is the cross-sectional surface area of the sample (mm2), and is the density of water (g/mm3).
When “I” is plotted against the square root of time (), data are represented by a straight line, and water sorptivity in can be determined as the slope of the line [35].
3. Results
3.1. Precusor Characterization
3.1.1. Particle Size Distribution
The particle size distribution of the studied WS is shown in (Figure 3). The obtained distribution curves are further extended by d50 values presented in Table 3. Here, the values range between 16.93 µm and 50.78 µm except for sample S7, which exhibits the smallest particle size distribution (PSD) of 7.198 µm. The particle size distribution significantly influences the reactivity and strength development of the AAMs [18]. Reactivity is proportional to particle size; smaller particles (less than 10 µm) have better reactivity due to their larger surface area compared with larger particles (greater than 45 µm), resulting in increased compressive strength [36]. Furthermore, small particle size positively affects the packing density [37].
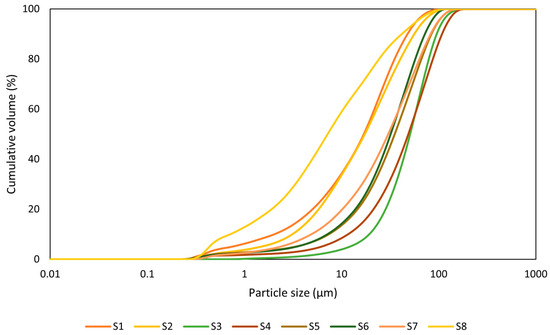
Figure 3.
Particle size distribution plot (PSD).

Table 3.
Average particle size distribution (d50).
The morphology of the materials is also influenced by particle size, with smaller particles having smoother surfaces.
Figure 3 presents the PSD plot of cumulative volume versus particle size; the result demonstrates that a larger percentage of the materials have particle sizes below 100 µm, which translates to better workability and reactivity.
3.1.2. Chemical Composition
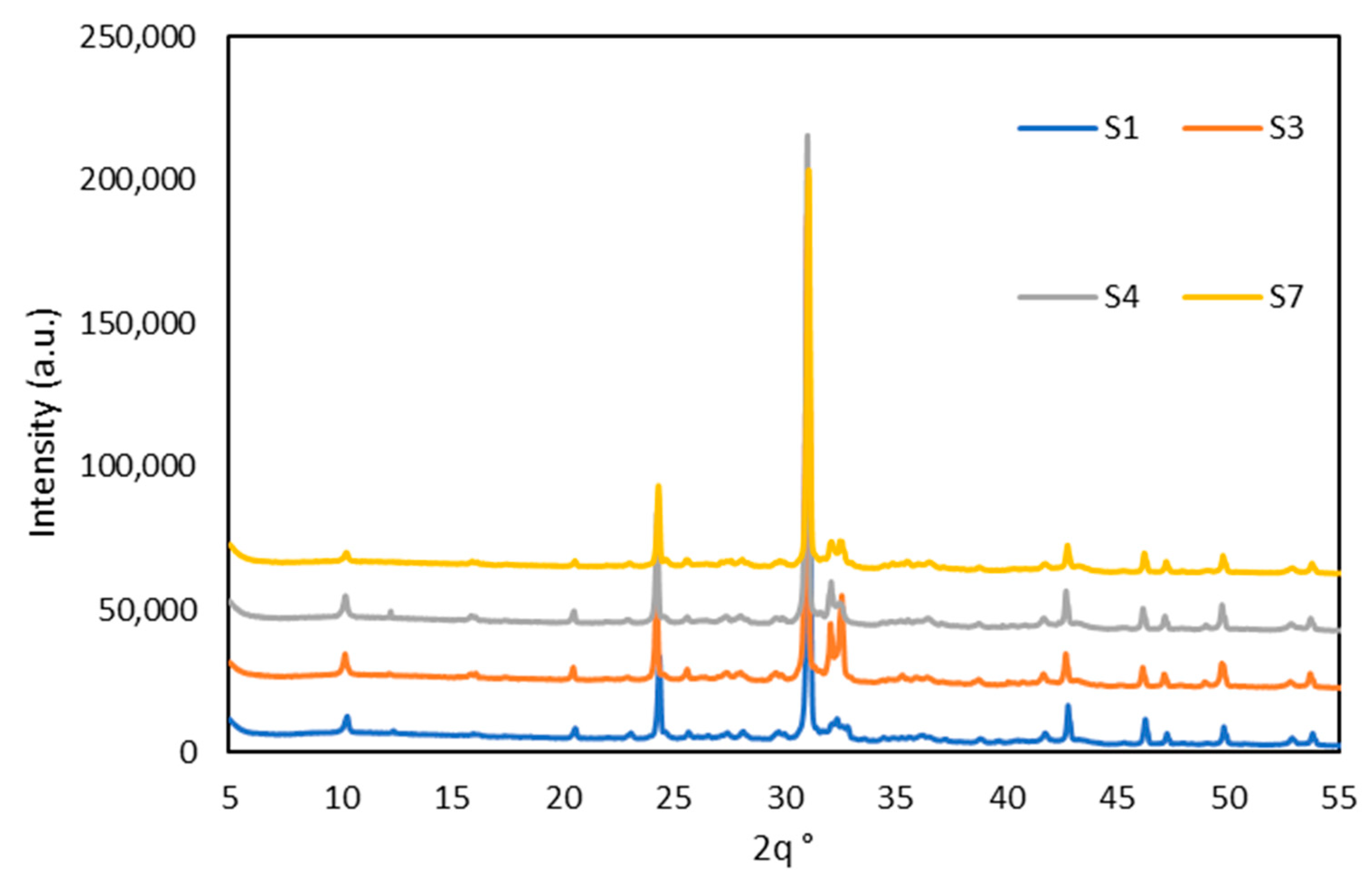
The quantified results of the mineralogical composition of calcined WS are depicted in Figure 4, while diffractograms of calcined WS are plotted in Figure 5 (only four WS are present for the clarity of the presentation). Before the calcination, quartz, illites, and kaolinites were the most dominant phases, with peaks observed at diffraction angles of 25°, 30°, and 40° for quartz and 10°, 21°, 42°, and 15°, 24° for illites and kaolinites, respectively, with low content of other minerals such as chlorite, albite, hornblende, and hillebrandite. After calcination, the clay minerals kaolinite, chlorite, and hillebrandite largely decomposed, causing the disappearance of their peaks and leading to a significant increase in the amorphous phase. However, the complete decomposition of illite was not achieved, as its peaks remain at the same diffraction angles but at a lower intensity. The increase in the amorphous phase suggests higher reactivity when in contact with alkaline solutions [38]. The major persistent peaks at 25°, 30°, and 42° are attributed to crystalline phases such as quartz, which are more difficult to decompose. These crystalline phases can reinforce the resulting consolidated materials. The calcination process also promotes the formation of new phases, including tricalcium phosphate Ca3(PO4)2), iron as maghemite Mag (γ-Fe2O3), and spinel phases Sp, which are reactive in alkaline media. The phase compositions of the materials are quite similar; thus, the four materials were selected for clarity in the presentation.
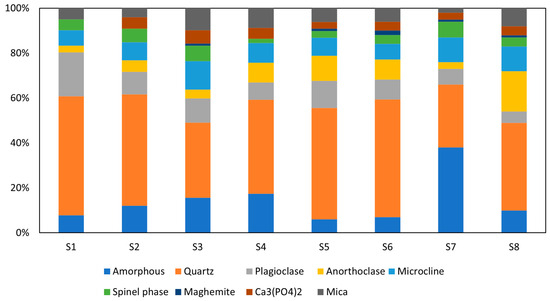
Figure 4.
Mineralogical composition of calcined WS.
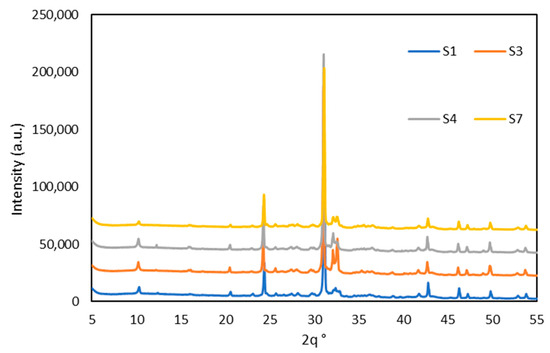
Figure 5.
Diffractograms of calcined WS.
3.2. Paste Samples Characterization
3.2.1. Calorimetry Analysis
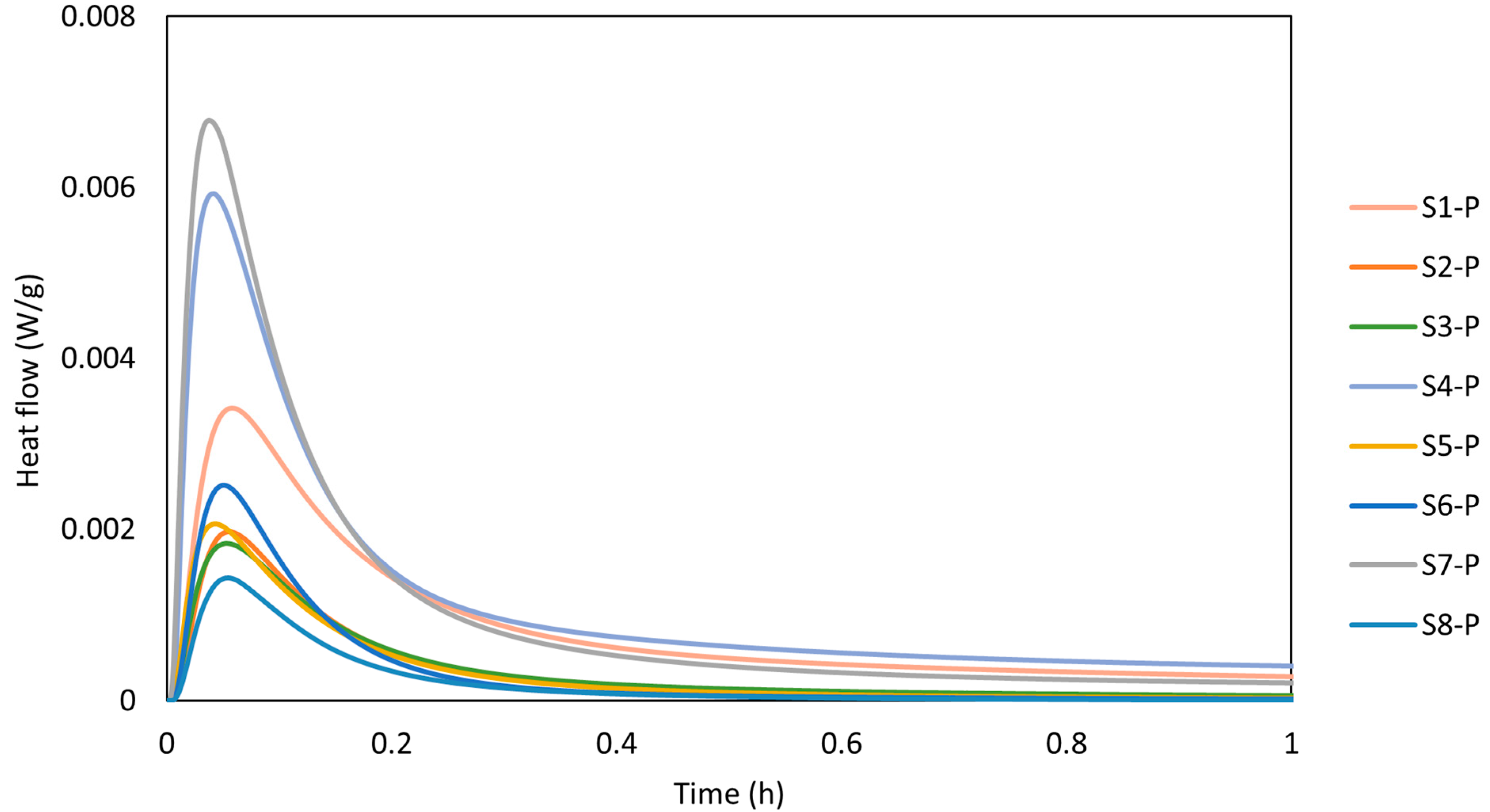
The calorimetry curves plotted in Figure 6 show the reaction kinetics of the calcinated WSs within the first hour of the reaction, while Figure 7 depicts the cumulative heat evolution over 168 h. A sudden temperature rise was observed when the calcinated WS powder reacted with the alkali solution, which absorbed the solution on the powder surface. The heat was released in the process due to wetting and dissolution of the reacting cations Si and Al, which resulted in a sharp exothermic peak in the first few minutes [2]. This phenomenon is linked with the initial adsorption of the alkaline solution on the surface of sediment particles and the following breakdown of the Si-O and Al-O bonds. The results of heat evolution well correspond to the presence of the mineralogical phases, whereas the positive correlation between the amorphous phase and the heat release [39]. Here, the highest rate of heat release was reached for the S7 precursor. Thus, the rate of dissolution depends on was positively affected by the presence of the Si and Al ions in the reactive form. Al and Si react slower as opposed to Ca2+ ions. Thus, the exothermic peak usually reaches lower values for Ca-low precursors. A beneficial effect can be noted for the finer particles as the higher contact area between the precursor and the liquid activator plays an important role and, consequently, contributes to the more rapid heat release. Such findings are accompanied by the setting time, whereas the polycondensation phase was delayed by the limited presence of Ca2+ [40].
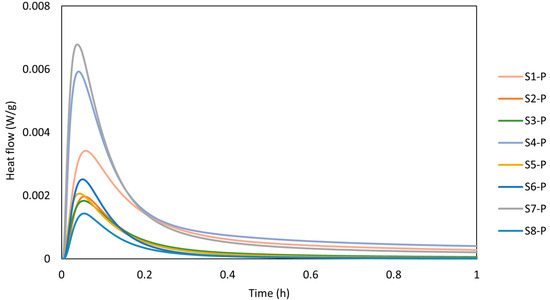
Figure 6.
Calorimetry plot of AAMs-WS.
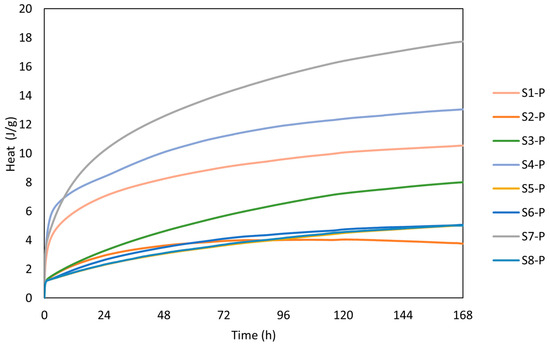
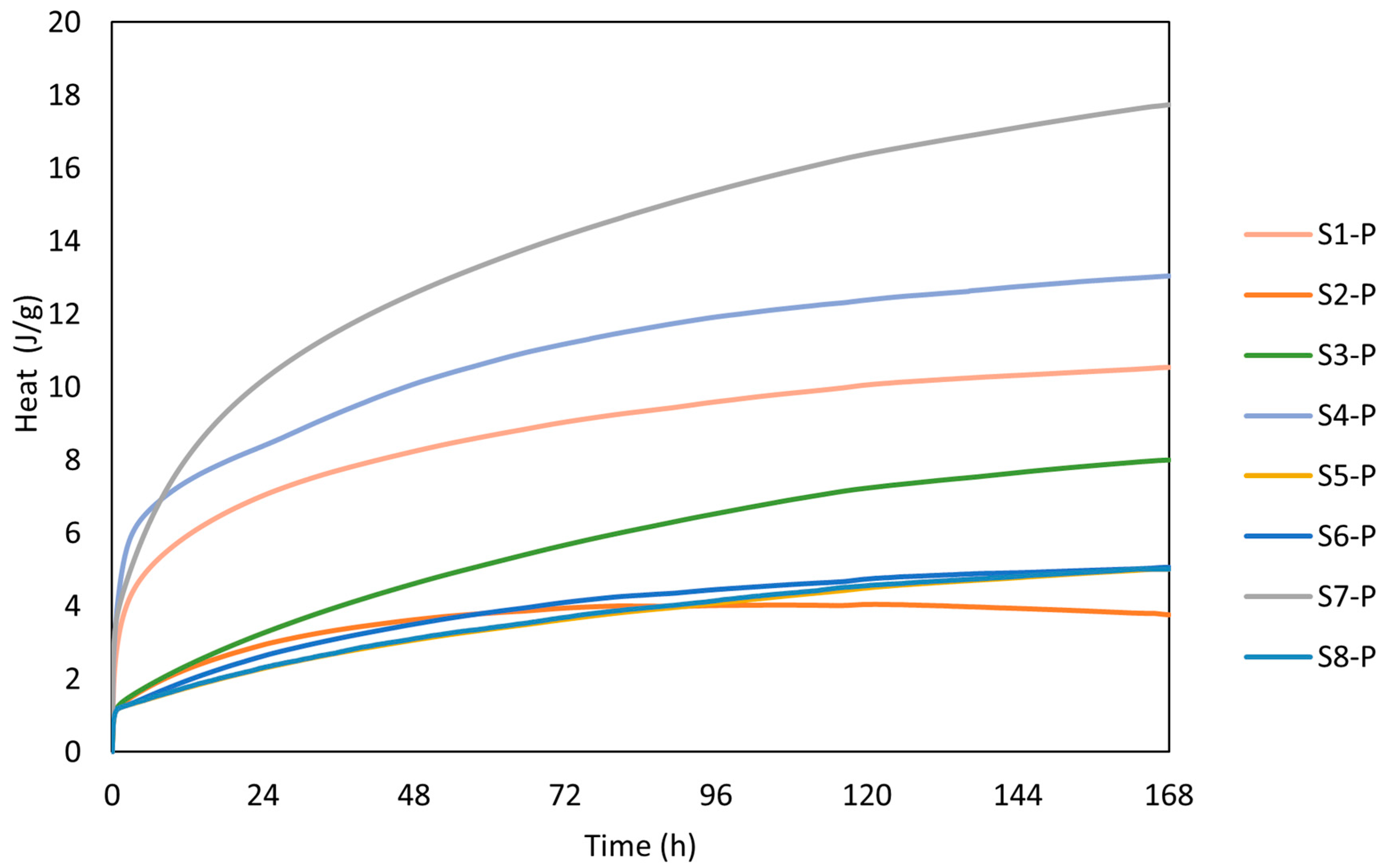
Figure 7.
Cumulative heat evolution.
Besides the characteristics of the precursor, the peak’s magnitude depends on the concentration of the alkali activator; high alkali concentrations promote the rapid dissolution of soluble species and the formation of new reaction products [41,42]. The activation process is usually followed by an endothermic reaction, which is indicated by a sharp decline in the peak that suggests the continuation of the dissolution process. Improvements in heat evolution can be secured by the more advanced selection of the used activators or, eventually, suitable admixture that promotes the dissolution process [43]. Namely, the tailored Na/Al ratio was found to be an efficient tool for improvements in mechanical strength. On the other hand, a too rapid setting time was accompanied by consequent shrinkage and deterioration at later ages.
Notwithstanding, the calorimetry record of WS presents a limited ability to release heat as the heat flow drops to the barest minimum, indicating a low reaction rate and reduced gel formation compared with other precursors [44]. Despite the role of the alkali concentration in the geopolymerisation reaction process, reaction time also plays a significant role. Low heat release implies slow setting time and strength development, which may be beneficial for applications requiring longer workability. Other authors have previously mentioned the correlation between the calorimetry and the setting time [45,46] and found that the initial setting time corresponds to the end of the exothermic peak. This finding can be attributed to the beginning of the main reaction phase, while the final setting time corresponds to the point where the rate of heat release decreases significantly (almost to zero), indicating the completion of the reaction where the mortar becomes hardened. Respectively marks the end of the exothermic peaks, which correspond to the initial setting time of 6 min, while the final setting time corresponds to the point where the rate of heat release decreases significantly. This is more obvious for S7, which shows a more visible continuous setting for the prolonged time of 16 h, similar to S1 and S4, though at a very low insignificant heat release close to zero.
Since the studied WS exhibits slow setting characteristics (see Table 4) for over 16 h and prolonged reaction time (S2), the results correspond well to the observed findings. The recorded heat flow of the samples was 0.0029 W/g, 0.006 W/g, and 0.0063 W/g for S1, S4, and S7. The cumulative heat evolution within the first 168 h was significantly lower compared with results published by Stefanini et al. [47], who recorded significantly higher total heat evolution reaching 160 J/g after 150 h of experiment. However, this shift can be assigned to a high content of slag applied between 70 and 80 wt.%, with better 396 solubility in an alkaline environment. Considering the evolution of the reaction heat for low amorphous precursor, Fořt et al. [48], by using brick powder, ranged between 0.002 W/g and 0.008 W/g for the initial peak.

Table 4.
Initial and final setting time of designed paste samples.
The initial setting time of designed mixtures varied from 140 min to 290 min, and the final setting time was in the range of 220 to 1040 min. Both parameters partially reflect the precursors’ mineralogical composition, namely the share of the amorphous phase. In this sense, the S2 sample exceeded the average final setting time significantly, and this sample was excluded from most of the performed experiments due to its limited capability to form a dense structure. The apparent explanation of the findings consists in the limited share of the amorphous content, as well as the undesired molar ratio that limited the polycondensation of dissolved species. As the setting time of AAMs often results in highly variable intervals, the comparison is more complicated. Taking into account the results published by Hou et al. [49], the achieved results fit well with the intervals, taking into account the stable dosage of the alkaline activator. The effect of the particle size cannot be confirmed due to the diverse mineralogical composition of precursors.
3.2.2. The Basic Material Properties of Paste Samples
The basic properties of AAMs, including bulk density, matrix density, and total open porosity (TOP), provide valuable insights into the performance of WS-based AAMs. From the paste samples results in Table 5, bulk density values from 1560 to 1880 kg/m3 and matrix density of 2430 to 2560 kg/m3 were recorded. The TOP values ranged from 0.24 to 0.38, according to the changes in the material microstructure. Typically, values of the bulk density varied significantly across different used precursors; thus, no clear relationship can be formulated as described for ordinary concrete. In general, TOP below 20% typically signifies a material with relatively dense and compact characteristics, with limited interconnected voids. As TOP surpasses this threshold, the material becomes more porous, allowing for greater moisture ingress or gas exchange and potentially impacting mechanical strength and durability [18]. Opposed to the results of Vasic et al. [50], only limited cracking was observed. Notwithstanding, the level of the porosity exceeded values reported by Lv et al. [51], who studied the suitability of river sediment in a mixture with red mud and slag as precursors. As concluded, the increased level of sediments shifted the level of the porosity due to the roughness of the particle surface and the compaction process. Gharzouni et al. [52] elucidated the different behaviors of AAMs activated by potassium-based solutions compared with sodium-based solutions. Here, the higher porosity level was measured for potassium-based alkaline activators according to lower polarizing power and consequent reaction of aluminum with calcium. A slightly lower level of porosity was reported in the study by Zibret et al. [21], with values ranging from 14% to 24%. Notwithstanding, the level of porosity does not correlate with the mechanical performance, as samples activated by potassium solution achieved higher compressive strength after 7 days and 28 days of curing. This finding can be assigned to the formation of several networks and the precipitation of different compounds despite the increased pore size.

Table 5.
Basic material properties of paste samples.
3.2.3. Compressive Strength
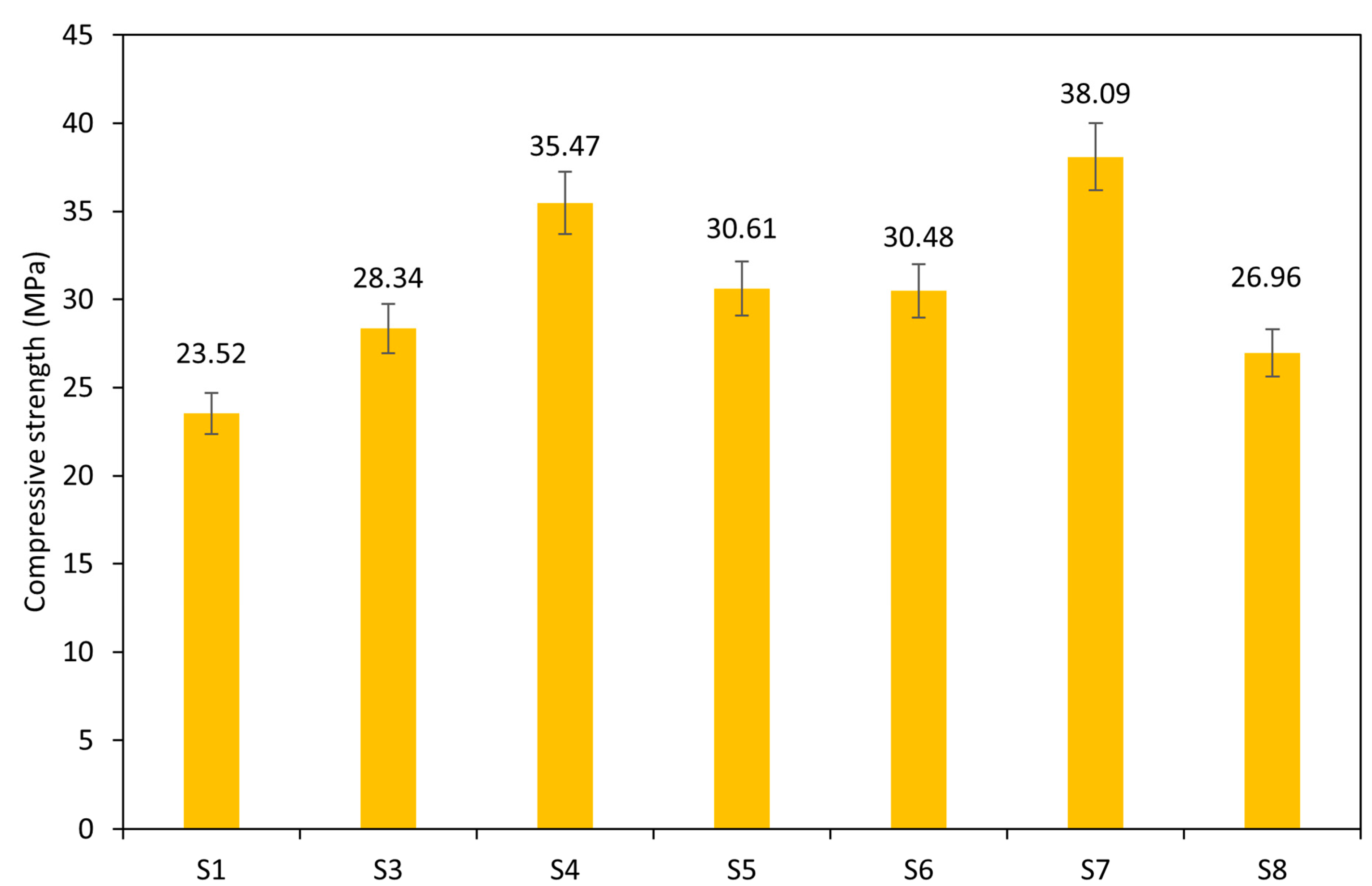
Figure 8 illustrates the compressive strength of the AAMs, which ranges from 23.52 MPa to 38.09 MPa after 28 days of curing at ambient temperature. Sample S2 was excluded from the analysis due to its high unreactive content, which hindered alkali activation. The results highlight the performance of pure sediment samples for future valorization in the construction industry. Notably, samples S7 and S4 achieved the highest strengths of 38.09 MPa and 35.47 MPa, respectively. The observed shift in the compressive strength can be attributed to the increased content of the amorphous phase in the precursor. As reported, the content of the amorphous phase enhances the strength and durability of AAMs due to the greater dissolution of aluminosilicate in the presence of an alkali solution [53]. The geopolymerization reaction between the dissolved aluminosilicate and alkali solution forms interconnected structures that result in more stable, stronger gel phases and a more cohesive polymer network that translates to higher compressive strength [54]. The effect of the particle size on the mechanical properties was not confirmed, as the highest compressive strength was achieved by both precursors with fine and coarser particles. Here, the reaction heat evolution corresponds well to the development of the compressive strength after 28 days.
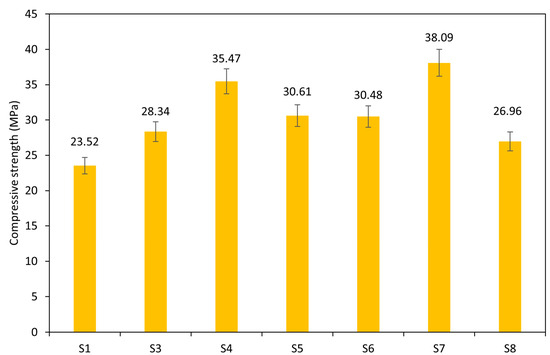
Figure 8.
Compressive strength of paste samples.
The observation in the result obtained is comparable to the work of Komnitsas et al. [22], who activated marine sediments (Patras sediments) containing higher amounts of amorphous silica in KOH to obtain better mechanical performance (compressive strengths from 8.5 to 19 MPa). Messin et al. [55] synthesized geopolymer bricks based on a mixture of sediment and water potabilization sludge to obtain low-carbon construction material. Despite the different proportions between both precursors, very similar levels close to 20 MPa in compressive strength were achieved. Slightly better mechanical performance was reached by Alloul et al. [56], who supplemented the minor content of flash-calcined sediment with metakaolin and slag. Specifically, the mechanical strength was improved by the addition of slag about 20% despite the observed negative effects of K+ ions on strength development. The study by Zibret et al. [21] explored the difference between the use of fresh and aged calcined sediments activated by various activator solutions. The results from experimental testing reveal that the strength varies between 7.94 MPa and 32.18 MPa in accordance with the additives and sediment types used. More favorable results can be assigned to the use of fresh calcined sediment activated by the mixture of sodium hydroxide and potassium water glass. As concluded by Belayali et al. [57,58] and Castillo et al. [57,58], further improvements in the mechanical performance can be secured by the addition of FA or slag to increase the amorphous content and balance the atomic ratios. The beneficial effect could be attributed to the release of a large amount of Ca+ during the slag hydration process. Small amounts of calcium have been proven to be good for strength development due to the formation of calcium-alumino-silicate-hydrate gel (C-A-S-H) that coexists with the AAMs gel. In contrast, high amounts of calcium have proven detrimental to AAMs’ strength development [57,58]. The flexural strength of pastes varied from 1.4 MPa to 3.8 MPa as shrinkage of materials resulted in minor microcracking and negatively affected this parameter. Results were reduced compared with the findings by Zhou et al. [59], which reached higher values of flexural strength.
Additionally, the particle size of the WS significantly influences the strength of the FSW-AAMs. Sample S7, with a median particle size (d50) of 7.2 µm, is about five times finer than other samples whose particle sizes range from 16.93 µm to 50.78 µm. Particles with larger surface areas promote the dissolution rate of the reactive aluminosilicate and accelerate the alkali activation process and gel formation, thereby improving the overall mechanical performance of hardened pastes [53,60].
3.3. Mortars Characterization
3.3.1. The Basic Material Properties of Mortar Samples
The samples exhibited acceptable density values, with bulk densities ranging from 2015 kg/m3 to 2056 kg/m3 and matrix densities from 2580 kg/m3 to 2630 kg/m3, as shown in Table 6. The samples displayed low TOP values from 20% to 23%, indicating relatively compact and dense AAMs with fewer open pores. The positive influence of aggregate on AAM density, which accounts for about 70% of the total mass, contributes to these favorable properties. It should be noted that the duality of the low-amorphous precursors represents a more challenging task to understand, as precursors act as both binders and microfillers [61]. Despite the limited variations in the open porosity, the mechanical strength differs more distinctively; therefore, the link between the microstructural and mechanical performance is not evident. This finding contrasts with the cement-based composites and points to a more complex structure of AAMs and the formation of diverse reaction products.

Table 6.
Basic material properties of mortar samples.
3.3.2. Porosimetry Analysis
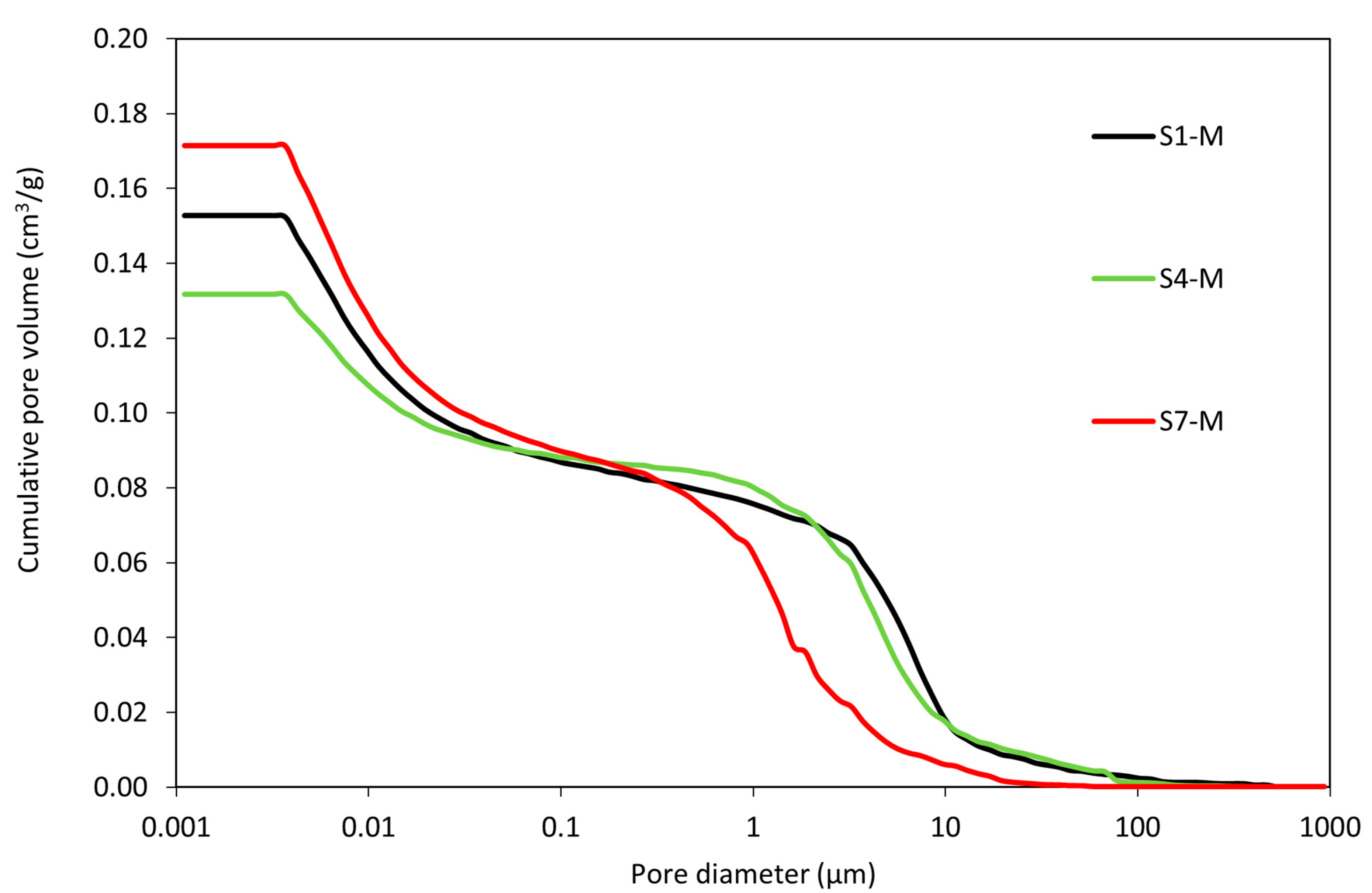
The mercury intrusion porosimetry analysis was conducted to determine the pore distribution in the WS-AAMs and to understand its role in material performance. Figure 9 presents the development of pore structure in terms of cumulative pore volumes. Insights into the pore structure reveal that a larger percentage of the pores are smaller, with a gradual shift toward larger pore sizes. The presence of larger pores, though fewer in number, can be attributed to the total consumption of some reacting species during the geopolymerization process.
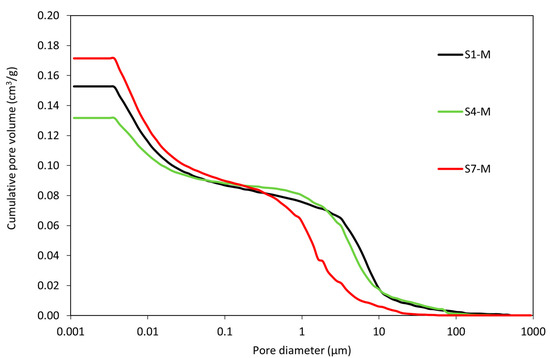
Figure 9.
Porosimetry analysis of mortar samples.
The results show a steep decrease in pore diameter between 0.001 µm and 10 µm. Beyond this range, the pore volume approaches zero, and all samples displayed flat slopes, indicating the absence of pore volume. Based on this, the pore types in the material can be categorized as micro, macro, and mesopores, with some amount of air voids [62].
The micropores contribute significantly to the pore formation; the microstructure of the WS-AAMs is dominated by fine pores, which account for about 75% of the total porosity. Samples with micropore volumes normally exhibit better compressive strength because of their compact nature [63]. Samples S7-M, S1-M, and S4-M recorded cumulative pore volumes of 0.17 cm3/g, 0.15 cm3/g, and 0.13 cm3/g, respectively. S7-M exhibited the highest micropore volume and S4 the lowest, which aligns with the results of the total open porosity determined by the knowledge of the bulk and matrix density. As previously mentioned, the total open porosity level cannot be clearly defined as a threshold parameter for mechanical strength. Here, rather, the presence of pores in specific regions well fits the development of the compressive strength after 28 days. A higher share of pores above 1 µm resulted in the reduction of strength and a higher probability of material cracking.
In general, the AAMs contain highly interconnected nanopores (2 to 100 nm), which are difficult to characterize, whereas mesopores and micropores around 100 nm to 10,000 nm are related to isolated interstices between individual particles of used mixture constituent. Therefore, the nanopores are deemed too tiny to contribute to the crack development [64]. Larger pores in the meso and micro range are dispersed in the matrix randomly and interconnected by the porosity of the AAM gel [21]. The utilization of sediments as a precursor increases the pore volume in mortars, as confirmed in the study of Zouch et al. [65].
3.3.3. Microstructure
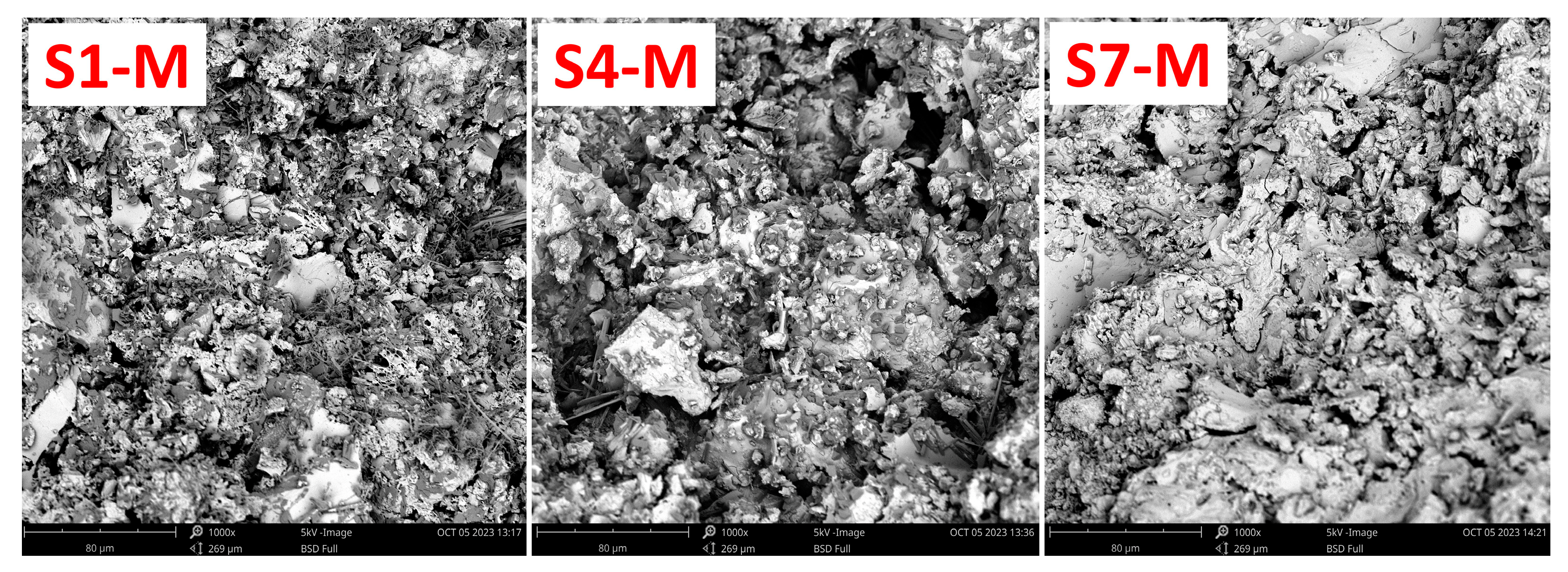
The SEM analysis investigated the morphological and microstructural characteristics of the alkali-activated WS-AAMs. The micrograph images from this analysis are shown in Figure 10. Notably, sample S7-M exhibited a well-dispersed and compact morphology with an integrated texture, in contrast with samples S1-M and S4-M. The matrix of S7-M is more homogeneous, indicating a higher reactivity of the material. Additionally, S7-M has fewer cracks and fissures, while the morphologies of S1-M and S4-M are more rugged, with visible cracks and some fragmentation. The observed cracks in S1-M and S4-M can be attributed to water loss during the hardening process and subsequent shrinkage [18]. In contrast, the fewer cracks in S7-M suggest a material with reduced permeability, resulting in better mechanical strength [53,60]. The apparent differences between particular SEM images are in good agreement with MIP curves, as more distinct pores can be recognized for S1-M and S4-M, while S7-M exhibited a higher share of very fine pores in the range from 0.001 µm to 0.1 µm. On the contrary, an opposite situation can be recognized in the area from 1 to 10 µm.
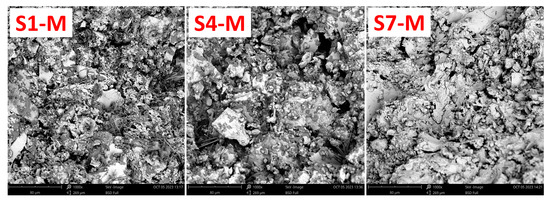
Figure 10.
Microstructure of the AAM samples S1-M, S4-M, and S7-M.
The obtained findings are consistent with other authors who dealt with using sediments as precursors for alkali activation. In this regard, incorporating slag or fly ash may positively contribute to the densification of the matrix through the improved reactivity of the constituents [66].
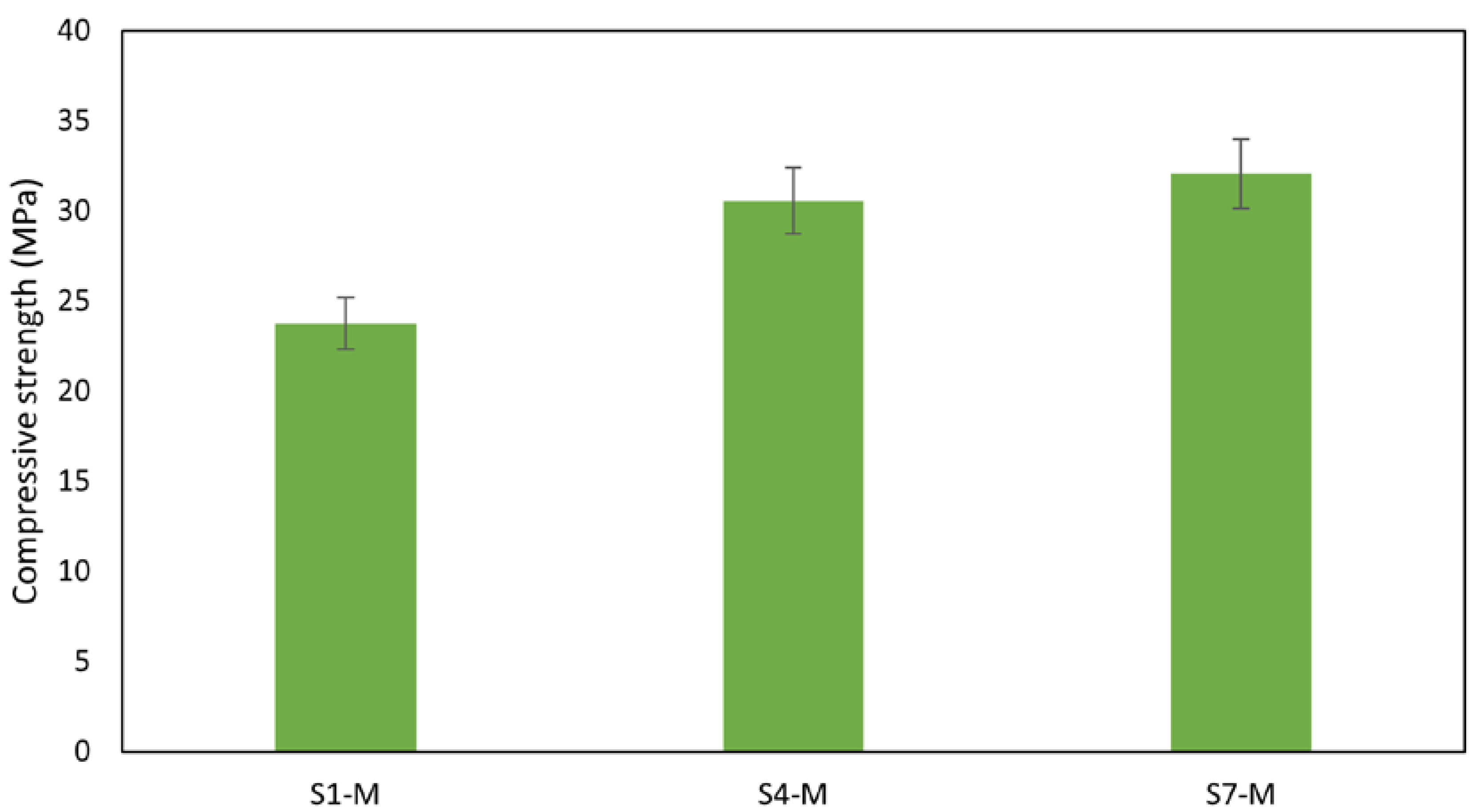
3.3.4. Mechanical Strength
The promising performance of WS suggests that further investigation into the mechanical behavior of WS as alkali-activated materials (AAMs) is of particular concern. On this note, mortar mixes were prepared with selected WS precursors S1, S4, and S7, as presented in Figure 11 and Figure 12. The results obtained show no significant difference in the strength of the mortars compared with the paste sample. A reduction in strength of about 5% was observed in S4-M and S7-M, likely due to little fluctuations in measurement. Despite this slight reduction, the strength obtained is still suitable for structural applications. The strength developed by these materials can be attributed to the high dissolution of the aluminosilicate in the alkali solution, which was further promoted by the curing temperature, which leads to the formation of a stronger AAM network. In addition, the role of the liquid/solid ratio may be of particular concern since the insufficient or too excessive alkali content usually hinders the condensation reaction [59].
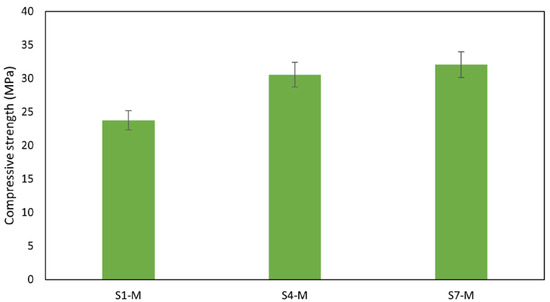
Figure 11.
Compressive strength of mortars.
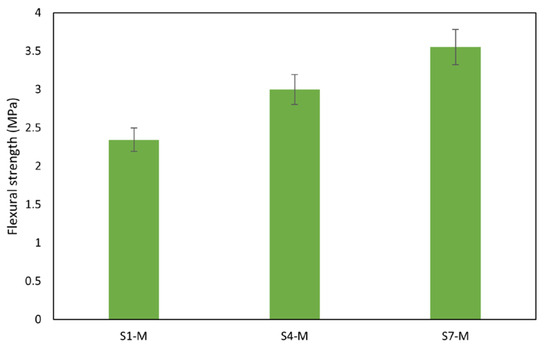
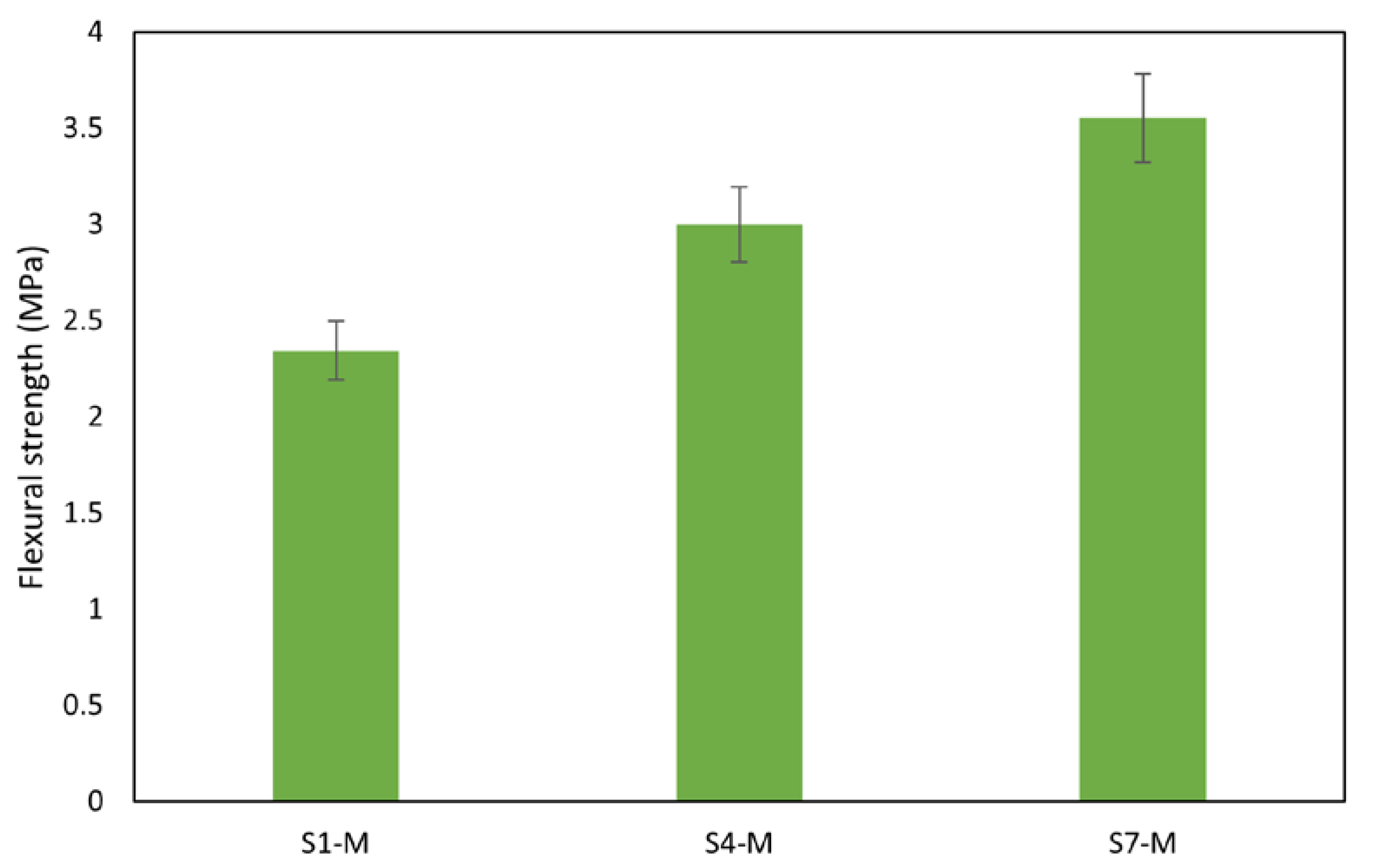
Figure 12.
Flexural strength of mortars.
Notably, the flexural strength of the mortars also performed well, falling within acceptable limits for concrete [67]. The aggregates’ rough surface texture, shape, and size contribute to better sediment bonding. Rough surfaces form bonds with binders to create resistance against applied loads, thereby enhancing overall strength [68,69]. Mortar sample S7-M exhibited the highest flexural strength at 3.6 MPa, while S1-M had the lowest at 2.3 MPa, as shown in Figure 12. With compressive strengths of 30 MPa and flexural strengths of 3.0 MPa and above, AAMs S4-M and S7-M are suitable for pavement and other applications that meet the mechanical performance requirements, in contrast with results reported by Zouch et al. [65], who reached 30 to 39 MPa, a much higher portion of WS can be utilized. Zibret et al. [21] needed the addition of more reactive material to secure the same strength level (approx. 30 MPa) and highlighted requirements on sufficient solubility and amorphous content. Considering the use of pure sediments, the compressive strength achieved was significantly improved compared with the performance reported by Komnitsas [22].
3.3.5. Moisture Sorptivity
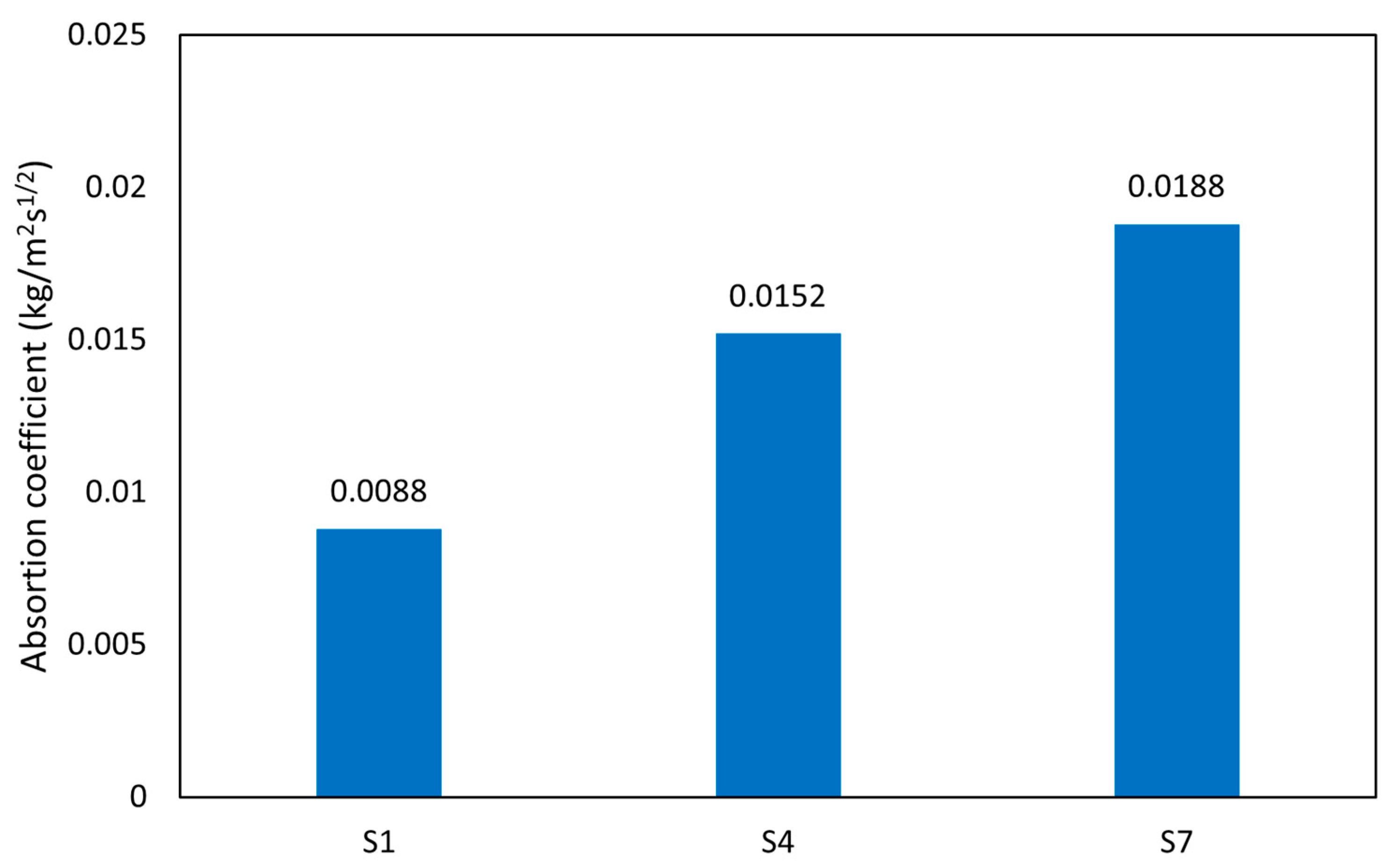
The results of sorptivity tests that were performed on all hardened AAM mortars after 28 days of curing are presented by plots of cumulative water absorption against the square root of time. The relationships were fitted by linear regression, and their slope describes the sorptivity according to the literature [70,71,72]. The water sorptivity links the material durability, as higher water sorption corresponds to increased material deterioration. As shown in Figure 13, the moisture absorption coefficient (A) for samples S7-M, S1-M, and S4-M are 0.0188, 0.0088, and 0.0152 kg/m2 s½, which corresponds to values determined for the calcined clay bricks (0.011–0.045 kg/m2 s½) [70,72]. These low values are mainly due to the influence of porosity on moisture absorption of the material. The results obtained in the study correspond well with those of Ismail et al. [73], who investigated the different ratios of slag and FA. AAMs are mostly porous materials, and the variation in internal pore structure directly affects water absorption. Low porosity increases the matrix structure’s compactness, subsequently reducing the AAMs’ absorption coefficient. This assertion is supported by the TOP results, which coincide with the reported moisture absorption coefficient values. The chemical composition affects the pore network’s tortuosity, therefore playing an important role in the water uptake. C-A-S-H gel exhibits a higher level of tortuosity compared with N-A-S-H gel, and the water uptake of C-A-S-H-based AAMs is reduced despite the higher level of total porosity. In a nutshell, the dissolution of the samples generates adequate gelling composition, which leads to gradual pores filling and a more compact structure, thereby reducing the moisture absorption rate of the materials [71]. The treatment conditions were found to be another influencing factor for evaluating the water absorption coefficient. The higher drying temperature results in more distinct shrinkage and formation of cracks that accelerate the water uptake compared with samples dried at a lower temperature (60 °C) [73].
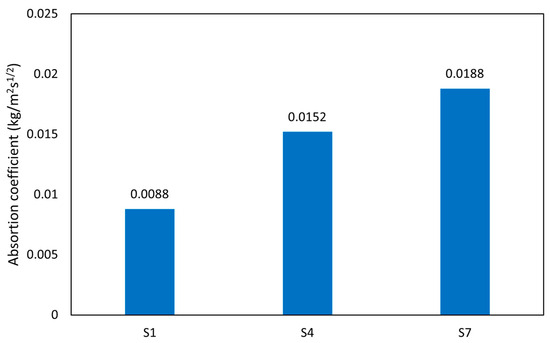
Figure 13.
Absorption coefficient.
4. Conclusions
This work contemplated the utilization of calcined freshwater sediments for sustainable building material and the consequent characterization of designed composites obtained through alkaline activation. The calcinated FSWs indicate a content of reactive aluminosilicate clay mineral that can be accepted as the precursor.
- The calorimetry of WS presents a low rate of reaction after the initial exothermic peak observed during wetting and dissolution of the reacting species. This low and slow reaction rate is obvious in the setting characteristics of the materials over a prolonged period of 16 h.
- The positive influence of aggregate on AAM density was observed with samples exhibiting acceptable density values; the bulk densities ranged from 2060 kg/m3 to 2110 kg/m3, and matrix densities varied from 2580 kg/m3 to 2630 kg/m3. The mortar samples S1-M, S4-M, and S7-M achieved low TOP values of 20% to 23%, indicating a relatively compact and dense structure suitable for structural applications.
- The microstructure of the AAMs is dominated by fine pores with compact morphology and homogenous texture, indicating high reactivity of the WS. S4-M exhibited the lowest micropore volume of 0.13 cm3/g, while S7-M presented a more homogeneous matrix with fewer cracks and fissures.
- Additionally, the low porosity increases the structure’s compactness, subsequently reducing the AAMs’ absorption coefficient. The moisture absorption coefficient (A) for samples S7-M, S1-M, and S4-M were 0.0188, 0.0088, and 0.0152 (g/m2 s ½), respectively.
- Notably, the AAMs demonstrate suitability for structural applications, with pure sediments displaying high compressive strength values between 23.52 MPa and 38.09 MPa. Mortar samples maintained similar strength to the paste, exhibiting a compressive strength of 23.75 MPa for S1, 30.56 MPa for S4, and 32.06 MPa for S7. A slight decrease in strength observed in S4 and S7 was due to measurement fluctuation; the strength performance is attributed to the effective dissolution of aluminosilicate during the polymerization reaction process. A similar observation was made in the flexural strength, which is generally low in most of the paste samples; however, a notable improvement was recorded with the addition of aggregates thanks to the mitigation of the shrinkage. The mortar samples exhibited flexural strengths of 3.55 MPa for S7-M, 3.00 MPa for S4-M, and 2.34 MPa for S1-M.
As revealed, utilization of WS or other types of WS may provide several synergic effects, including the reduction in the generated waste material from dredging operations, establishment of a new type of precursor with good availability, and consequent application of such designed materials as replacement of the traditional binders. However, the nature of the sediment materials requires further research aimed at understanding the solubility of the particular minerals, optimization of the treatment processes to increase the material reactivity and the coherent guidelines for the mixture design according to the sediment type. Follow-up research focusing on these areas should be of particular importance as the composition of the sediments is highly correlated with the locations; thus, such guidelines can be applicable to different regions with similar geological bedrock compositions.
Author Contributions
Conceptualization, J.F. and A.A.; methodology, J.F. and M.K.; software, J.F.; validation, J.F., A.A. and M.K.; formal analysis, J.F.; investigation, M.M., P.H., A.A., M.K. and J.F.; resources, J.F.; data curation, A.A.; writing—original draft preparation, J.F. and A.A.; writing—review and editing, J.F. and R.Č; visualization, A.A.; supervision, J.F.; project administration, R.Č.; funding acquisition, J.F. and R.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Czech Science Foundation, under project No 22-04726S, and by Czech Technical University in Prague, under project No SGS23/149/OHK1/3T/11.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Y.; Wu, B.R.; Wang, R.J. Critical review and gap analysis on the use of high-volume fly ash as a substitute constituent in concrete. Constr. Build. Mater. 2022, 341, 127889. [Google Scholar] [CrossRef]
- Li, L.M.; Xie, J.H.; Zhang, B.F.; Feng, Y.; Yang, J. A state-of-the-art review on the setting behaviours of ground granulated blast furnace slag- and metakaolin-based alkali-activated materials. Constr. Build. Mater. 2023, 368, 130389. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Tuyan, M.; Nehdi, M.L. Alkali-activated and geopolymer materials developed using innovative manufacturing techniques: A critical review. Constr. Build. Mater. 2021, 303, 124483. [Google Scholar] [CrossRef]
- He, J.; Jie, Y.X.; Zhang, J.H.; Yu, Y.Z.; Zhang, G.P. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. An overview of geopolymers derived from industrial by-products. Constr. Build. Mater. 2016, 127, 183–198. [Google Scholar] [CrossRef]
- Wongsa, A.; Sata, V.; Nematollahi, B.; Sanjayan, J.; Chindaprasirt, P. Mechanical and thermal properties of lightweight geopolymer mortar incorporating crumb rubber. J. Clean. Prod. 2018, 195, 1069–1080. [Google Scholar] [CrossRef]
- Capasso, I.; Liguori, B.; Ferone, C.; Caputo, D.; Cioffi, R. Strategies for the valorization of soil waste by geopolymer production: An overview. J. Clean. Prod. 2021, 288, 125646. [Google Scholar] [CrossRef]
- El Hafid, K.; Hajjaji, M.; El Hafid, H. Influence of NaOH concentration on microstructure and properties of cured alkali-activated calcined clay. J. Build. Eng. 2017, 11, 158–165. [Google Scholar] [CrossRef]
- Palacios, M.; Alonso, M.M.; Varga, C.; Puertas, F. Influence of the alkaline solution and temperature on the rheology and reactivity of alkali-activated fly ash pastes. Cem. Concr. Compos. 2019, 95, 277–284. [Google Scholar] [CrossRef]
- Alastair, M.; Andrew, H.; Pascaline, P.; Mark, E.; Pete, W. Alkali activation behaviour of un-calcined montmorillonite and illite clay minerals. Appl. Clay Sci. 2018, 166, 250–261. [Google Scholar] [CrossRef]
- Ye, H.L.; Radlinska, A. Fly ash-slag interaction during alkaline activation: Influence of activators on phase assemblage and microstructure formation. Constr. Build. Mater. 2016, 122, 594–606. [Google Scholar] [CrossRef]
- Kanagaraj, B.; Anand, N.; Raj, R.S.; Lubloy, E. Techno-socio-economic aspects of Portland cement, Geopolymer, and Limestone Calcined Clay Cement (LC3) composite systems: A-State-of-Art-Review. Constr. Build. Mater. 2023, 398, 132484. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, J.X.; Song, Y.B.; Xu, Y. Alkali-activated fly ash foam concrete with Yellow River silt: Physico-mechanical and structural properties. Constr. Build. Mater. 2023, 373, 130879. [Google Scholar] [CrossRef]
- Beddaa, H.; Ben Fraj, A.; Ducléroir, S. Experimental study on river sediment incorporation in concrete as a full aggregate replacement: Technical feasibility and economic viability. Constr. Build. Mater. 2021, 313, 125425. [Google Scholar] [CrossRef]
- Du, H.J.; Pang, S.D. Value-added utilization of marine clay as cement replacement for sustainable concrete production. J. Clean. Prod. 2018, 198, 867–873. [Google Scholar] [CrossRef]
- Fort, J.; Afolayan, A.; Mildner, M.; Hotek, P.; Keppert, M.; Cerny, R.; Huang, B.T. Assessment of Clayey Freshwater Sediments as Suitable Precursors for Alkaline Activation. Polymers 2024, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, B.A.; Barbu, A.M.; Lazarescu, A.V.; Rada, S.; Gabor, T.; Florean, C. The Influence of Substitution of Fly Ash with Marble Dust or Blast Furnace Slag on the Properties of the Alkali-Activated Geopolymer Paste. Coatings 2023, 13, 403. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, J.X.; Song, Y.B.; Gong, W.J.; Wang, L.M.; Yu, Z.H.; Wang, G.L. Effect of calcination pretreatment on mechanical properties of alkali-activated artificial stone incorporating Yellow River silt. J. Clean. Prod. 2022, 364, 132682. [Google Scholar] [CrossRef]
- Zibret, L.; Wisniewski, W.; Horvat, B.; Bozic, M.; Gregorc, B.; Ducman, V. Clay rich river sediments calcined into precursors for alkali activated materials. Appl. Clay Sci. 2023, 234, 106848. [Google Scholar] [CrossRef]
- Komnitsas, K. Co-valorization of marine sediments and construction & demolition wastes through alkali activation. J. Environ. Chem. Eng. 2016, 4, 4661–4669. [Google Scholar] [CrossRef]
- Özkiliç, Y.; Çelik, A.; Tunç, U.; Karalar, M.; Deifalla, A.; Alomayri, T.; Althoey, F. The use of crushed recycled glass for alkali activated fly ash based geopolymer concrete and prediction of its capacity. J. Mater. Res. Technol. 2023, 24, 8267–8281. [Google Scholar] [CrossRef]
- Crocetti, P.; Gonzalez-Camejo, J.; Li, K.; Foglia, A.; Eusebi, A.L.; Fatone, F. An overview of operations and processes for circular management of dredged sediments. Waste Manag. 2022, 146, 20–35. [Google Scholar] [CrossRef]
- Kubeneck, L.J.; Lenstra, W.K.; Malkin, S.Y.; Conley, D.J.; Slomp, C.P. Phosphorus burial in vivianite-type minerals in methane-rich coastal sediments. Mar. Chem. 2021, 231, 103948. [Google Scholar] [CrossRef]
- Shen, R.C.; Huang, X.Y.; Wen, X.T.; Liu, J.; Song, H.C.; Weihrauch, C.; Rinklebe, J.; Yang, H.; Yuan, Z.F.; Zheng, B.F.; et al. The determining factors of sediment nutrient content and stoichiometry along profile depth in seasonal water. Sci. Total Environ. 2023, 856, 158972. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.; Querol, X.; Andrés, J.M.; Stanton, K.; Towler, M.; Nugteren, H.; Janssen-Jurkovicová, M.; Jones, R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- Beddaa, H.; Tchiotsop, J.; Ben Fraj, A.; Somé, C. Reuse of river sediments in pervious concrete: Towards an adaptation of concrete to the circular economy and climate change challenges. Constr. Build. Mater. 2023, 368, 130443. [Google Scholar] [CrossRef]
- Alvarado, C.; Martínez-Cerna, D.; Alvarado-Quintana, H. Geopolymer Made from Kaolin, Diatomite, and Rice Husk Ash for Ceiling Thermal Insulation. Buildings 2024, 14, 112. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, O.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- CSN EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. Czech Standardization Institute: Prague, Czech Republic, 2011.
- EN 12390-2; Testing Hardened Concrete—Part 2: Making and Curing Specimens for Strength Tests. Czech Standardization Institute: Prague, Czech Republic, 2002.
- EN 12390-5; Testing of Hardened Concrete—Part 5: Flexural Strength. Czech Standardization Institute: Prague, Czech Republic, 2007.
- EN 12390e3; Testing of Hardened Concrete—Part 3: Compressive Strength. Czech Standardization Institute: Prague, Czech Republic, 2002.
- Yang, L.; Gao, D.Y.; Zhang, Y.S.; Tang, J.Y.; Li, Y. Relationship between sorptivity and capillary coefficient for water absorption of cement-based materials: Theory analysis and experiment. R. Soc. Open Sci. 2019, 6, 190112. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.G.; Nazari, A. Suitability of Sarawak and Gladstone fly ash to produce geopolymers: A physical, chemical, mechanical, mineralogical and microstructural analysis. Ceram. Int. 2016, 42, 9613–9620. [Google Scholar] [CrossRef]
- Trejo, D.; Prasittisopin, L. Chemical Transformation of Rice Husk Ash Morphology. Aci. Mater. J. 2015, 112, 385–392. [Google Scholar] [CrossRef]
- Mostefa, F.; Bouhamou, N.E.; Mesbah, H.A.; Aggoune, S.; Mekhatria, D. Sedimentary Clays as Geopolymer Precursor. Int. J. Eng. Res. Afr. 2018, 39, 97–111. [Google Scholar] [CrossRef]
- Prasittisopin, L.; Sereewatthanawut, I. Effects of seeding nucleation agent on geopolymerization process of fly-ash geopolymer. Front. Struct. Civ. Eng. 2018, 12, 16–25. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zhu, H.; Wu, Q. Reaction kinetics and mechanical properties of a mineral-micropowder/ metakaolin-based geopolymer. Ceram. Int. 2022, 48, 14173–14181. [Google Scholar] [CrossRef]
- Shi, Z.G.; Shi, C.J.; Wan, S.; Li, N.; Zhang, Z.H. Effect of of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Hu, Z.L.; Wyrzykowski, M.; Lura, P. Estimation of reaction kinetics of geopolymers at early ages. Cem. Concr. Res. 2020, 129, 105971. [Google Scholar] [CrossRef]
- Çelik, A.; Tunç, U.; Kayabasi, R.; Acar, M.; Sener, A.; Özkiliç, Y. Influence of aluminum waste, hydrogen peroxide, and silica fume ratios on the mechanical and thermal characteristics of alkali-activated sustainable raw perlite based geopolymer paste. Sustain. Chem. Pharm. 2024, 41, 101727. [Google Scholar] [CrossRef]
- Liang, G.W.; Yao, W.; She, A.M. New insights into the early-age reaction kinetics of metakaolin geopolymer by 1H low-field NMR and isothermal calorimetry. Cem. Concr. Compos. 2023, 137, 104932. [Google Scholar] [CrossRef]
- Kaze, C.; Alomayri, T.; Hasan, A.; Tome, S.; Lecomte-Nana, G.; Nemaleu, J.; Tchakoute, H.; Kamseu, E.; Melo, U.; Rahier, H. Reaction kinetics and rheological behaviour of meta-halloysite based geopolymer cured at room temperature: Effect of thermal activation on physicochemical and microstructural properties. Appl. Clay Sci. 2020, 196, 105773. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, M.; Yang, K.; Yu, L.; Yang, C. Setting behaviours and early-age microstructures of alkali-activated ground granulated blast furnace slag (GGBS) from different regions in China. Cem. Concr. Compos. 2020, 114, 103782. [Google Scholar] [CrossRef]
- Stefanini, L.; Ansari, D.; Walkley, B.; Provis, J. Characterisation of calcined waste clays from kaolinite extraction in alkali-activated GGBFS blends. Mater. Today Commun. 2024, 38, 107777. [Google Scholar] [CrossRef]
- Fořt, J.; Novotny, R.; Vejmelkova, E.; Trnik, A.; Rovnanikova, P.; Keppert, M.; Pommer, V.; Cerny, R. Characterization of geopolymers prepared using powdered brick. J. Mater. Res. Technol. 2019, 8, 6253–6261. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.; Lu, Z. Effect of Na/Al on formation, structures and properties of metakaolin based Na-geopolymer. Constr. Build. Mater. 2019, 226, 250–258. [Google Scholar] [CrossRef]
- Vasic, M.V.; Terzic, A.; Radovanovic, Z.; Radojevic, Z.; Warr, L.N. Alkali-activated geopolymerization of a low illitic raw clay and waste brick mixture. An alternative to traditional ceramics. Appl. Clay Sci. 2022, 218, 106410. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, Y.; Dai, W.; Yang, H.; Jiang, L.; Li, K.; Jin, W. Preparation and Properties of Porous Concrete Based on Geopolymer of Red Mud and Yellow River Sediment. Materials 2024, 17, 923. [Google Scholar] [CrossRef]
- Gharzouni, A.; Ouamara, L.; Sobrados, I.; Rossignol, S. Alkali-activated materials from different aluminosilicate sources: Effect of aluminum and calcium availability. J. Non-Cryst. Solids 2018, 484, 14–25. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yehualaw, M.D.; Vo, D.H.; Huyn, T.P. Development of high-strength alkali-activated pastes containing high volumes of waste brick and ceramic powders. Constr. Build. Mater. 2019, 218, 519–529. [Google Scholar] [CrossRef]
- González-García, D.M.; Téllez-Jurado, L.; Jiménez-Alvarez, F.J.; Zarazua-Villalobos, L.; Balmori-Ramírez, H. Evolution of a natural pozzolan-based geopolymer alkalized in the presence of sodium or potassium silicate/hydroxide solution. Constr. Build. Mater. 2022, 321, 126305. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Molino, A.; Roviello, G.; Colangelo, F.; Molino, B.; Cioffi, R. Synergistic recycling of calcined clayey sediments and water potabilization sludge as geopolymer precursors: Upscaling from binders to precast paving cement-free bricks. Constr. Build. Mater. 2017, 133, 14–26. [Google Scholar] [CrossRef]
- Alloul, A.; Amar, M.; Benzerzour, M.; Abriak, N.E. Geopolymer mortar with flash-calcined sediments cured under ambient conditions. Constr. Build. Mater. 2023, 391, 131809. [Google Scholar] [CrossRef]
- Belayali, F.; Maherzi, W.; Benzerzour, M.; Abriak, N.E.; Senouci, A. Compressed Earth Blocks Using Sediments and Alkali-Activated Byproducts. Sustainability 2022, 14, 3158. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Sanchez, S.; Vesely, M.; Garrido, P.; Palma, S. Factors Affecting the Compressive Strength of Geopolymers: A Review. Minerals 2021, 11, 11317. [Google Scholar] [CrossRef]
- Zhou, A.; Li, K.X.; Liu, T.J.; Zou, D.J.; Peng, X.; Lyu, H.X.; Xiao, J.D.; Luan, C.C. Recycling and optimum utilization of engineering sediment waste into low-carbon geopolymer paste for sustainable infrastructure. J. Clean. Prod. 2023, 383, 135549. [Google Scholar] [CrossRef]
- Pachideh, G.; Gholhaki, M.; Ketabdari, H. Effect of pozzolanic wastes on mechanical properties, durability and microstructure of the cementitious mortars. J. Build. Eng. 2020, 29, 101178. [Google Scholar] [CrossRef]
- Fort, J.; Vejmelkova, E.; Keppert, M.; Rovnanikova, P.; Bezdicka, P.; Cerny, R. Alkaline activation of low-reactivity ceramics: Peculiarities induced by the precursors’ dual character. Cem. Concr. Compos. 2020, 105, 103440. [Google Scholar] [CrossRef]
- Rao, S.M.; Acharya, I.P. Mercury Intrusion Porosimetry Studies with Geopolymers. Indian Geotech. J. 2017, 47, 495–502. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 27. [Google Scholar] [CrossRef]
- Chen, S.; Ruan, S.; Zeng, Q.; Liu, Y.; Zhang, M.; Tian, Y.; Yan, D. Pore structure of geopolymer materials and its correlations to engineering properties: A review. Constr. Build. Mater. 2022, 328, 127064. [Google Scholar] [CrossRef]
- Zouch, A.; Mamindy-Pajany, Y.; Bouchikhi, A.; Abriak, N.; Ksibi, M. Valorization of marine sediments in geopolymer mortars: Physico-mechanical, microstructural and environmental investigations at laboratory scale. J. Mater. Cycles Waste Manag. 2022, 24, 1109–1123. [Google Scholar] [CrossRef]
- Lirer, S.; Liguori, B.; Capasso, I.; Flora, A.; Caputo, D. Mechanical and chemical properties of composite materials made of dredged sediments in a fly-ash based geopolymer. J. Environ. Manag. 2017, 191, 1–7. [Google Scholar] [CrossRef]
- Grubesa, I.N.; Barisic, I.; Ducman, V.; Korat, L. Draining capability of single-sized pervious concrete. Constr. Build. Mater. 2018, 169, 252–260. [Google Scholar] [CrossRef]
- Wu, F.; Yu, Q.L.; Liu, C.W.; Brouwers, H.J.H.; Wang, L.F. Effect of surface treatment of apricot shell on the performance of lightweight bio-concrete. Constr. Build. Mater. 2019, 229, 116859. [Google Scholar] [CrossRef]
- Fort, J.; Afolayan, A.; Medved, I.; Scheinherrová, L.; Cerny, R. A review of the role of lightweight aggregates in the development of mechanical strength of concrete. J. Build. Eng. 2024, 89, 109312. [Google Scholar] [CrossRef]
- Liu, J.X.; Wang, Y.Z. Performance degradation mechanism of adobe materials under freeze-thaw cycles based on moisture transportation characteristics and microstructural analysis. Constr. Build. Mater. 2024, 411, 134464. [Google Scholar] [CrossRef]
- Zhu, Z.; Huo, W.; Sun, H.; Ma, B.; Yang, L. Correlations between unconfined compressive strength, sorptivity and pore structures for geopolymer based on SEM and MIP measurements. J. Build. Eng. 2023, 67, 106011. [Google Scholar] [CrossRef]
- Hall, M.; Djerbib, Y. Moisture ingress in rammed earth: Part 3—Sorptivity, surface receptiveness and surface inflow velocity. Constr. Build. Mater. 2006, 20, 384–395. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.; Provis, J.; Nicolas, R.; Brice, D.; Kilcullen, A.; Hamdan, S.; van Deventer, J. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).