The Usability of Metallurgical Production Waste as a Siliceous Component in Autoclaved Aerated Concrete Technology
Abstract
:1. Introduction
2. Materials and Methods
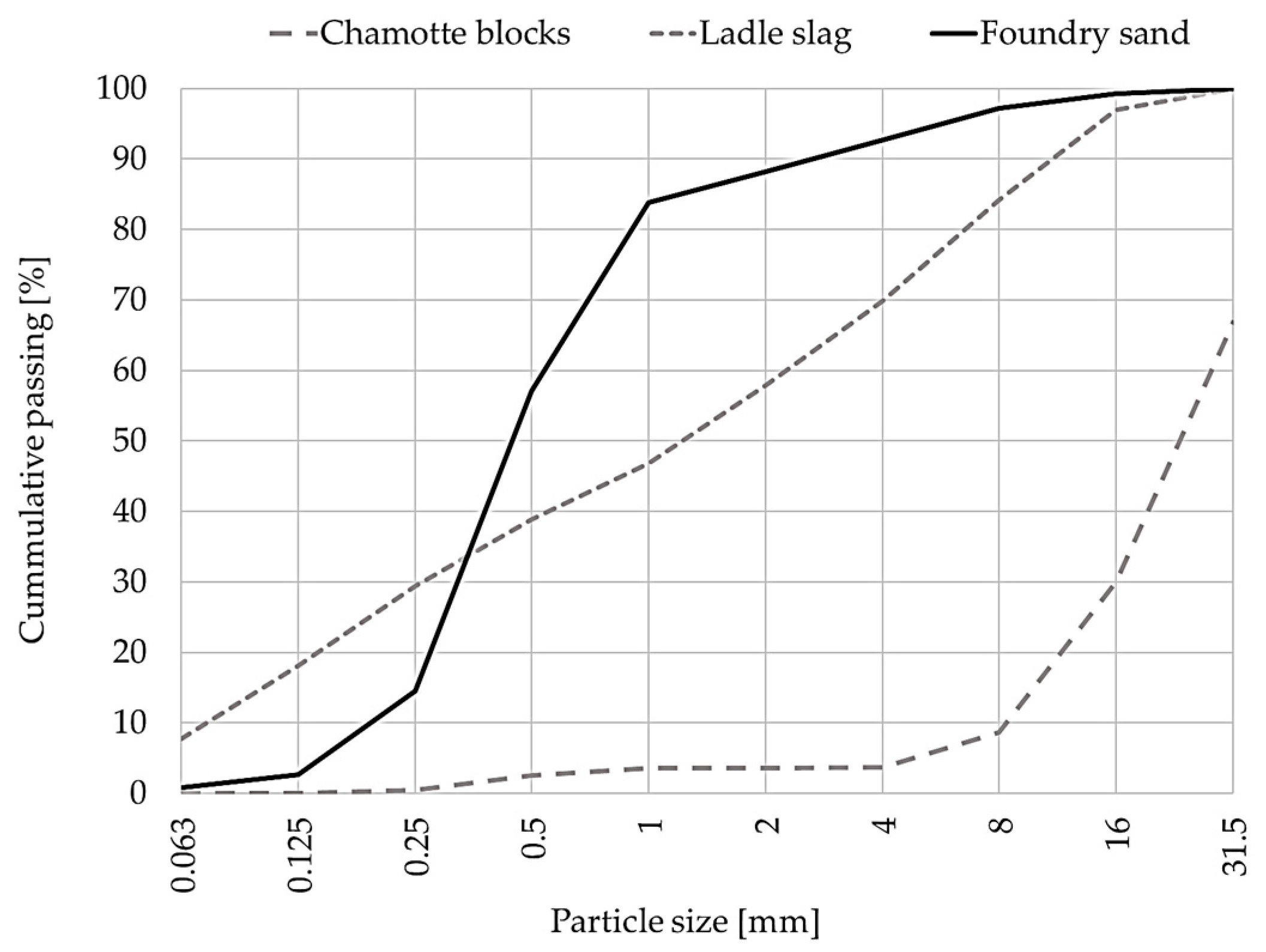
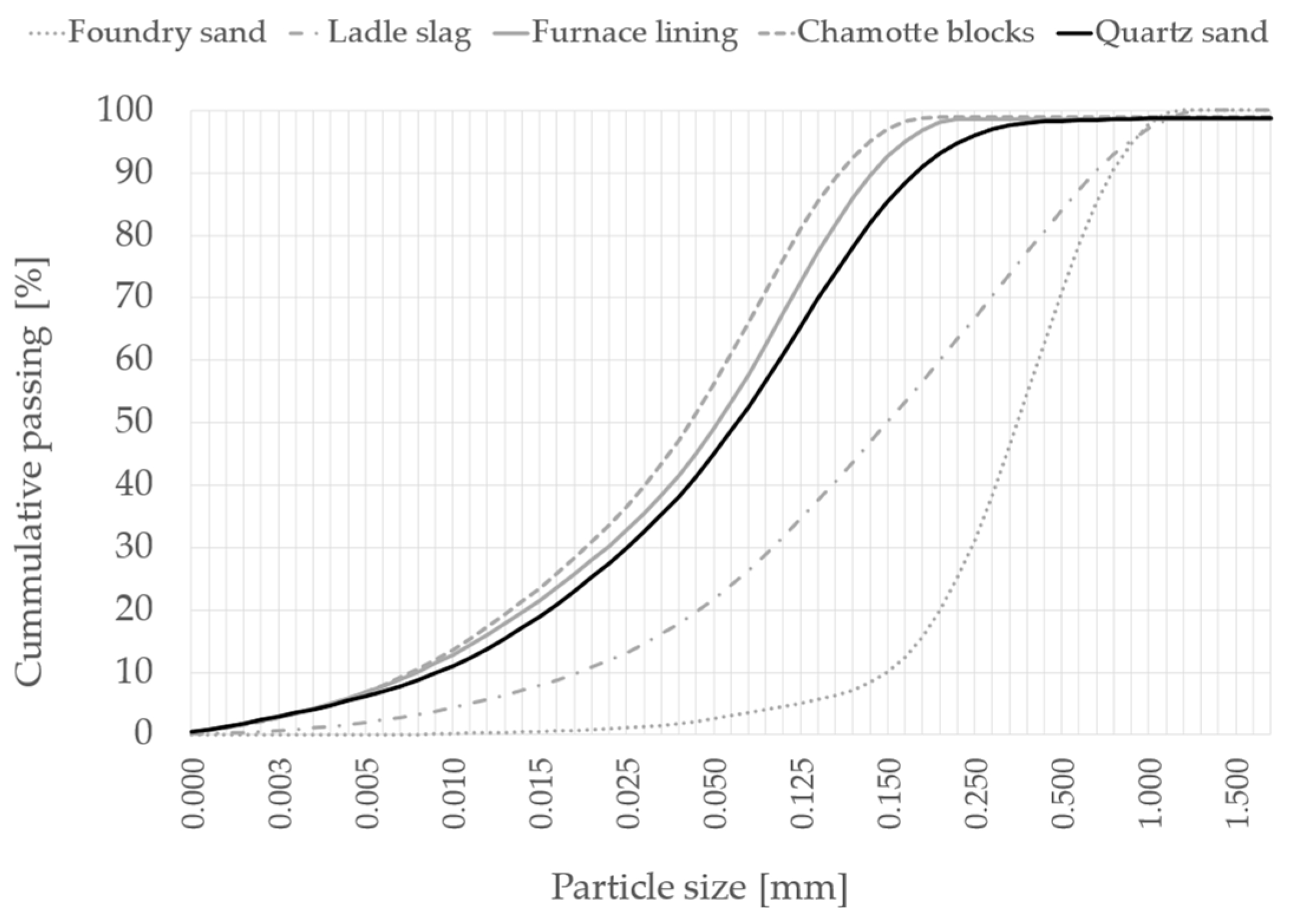
2.1. Material Properties
2.2. Material Processing
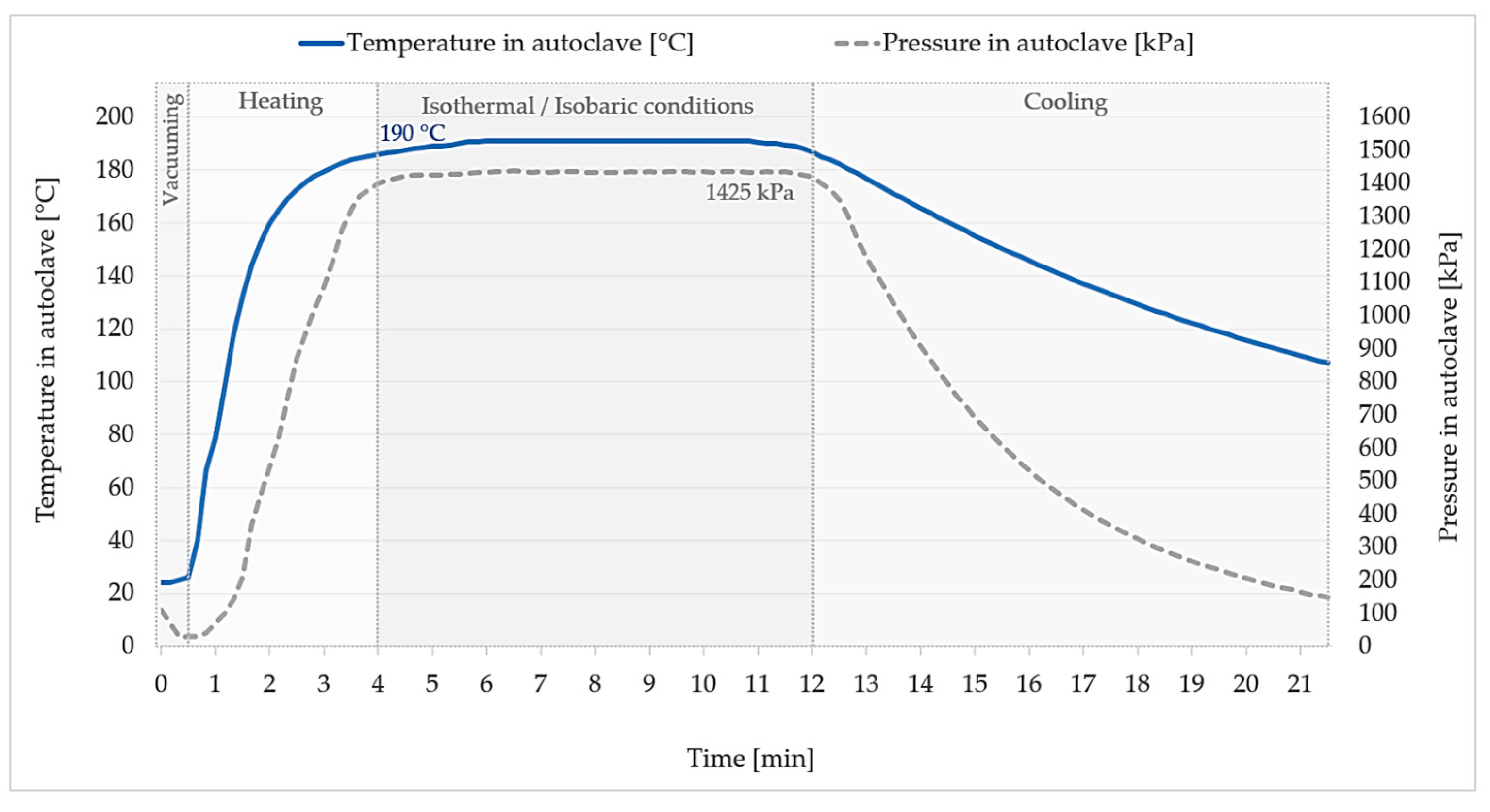
2.3. Sample Preparation and Treatment
2.4. Methods
3. Results and Discussion
3.1. Physical–Mechanical Properties
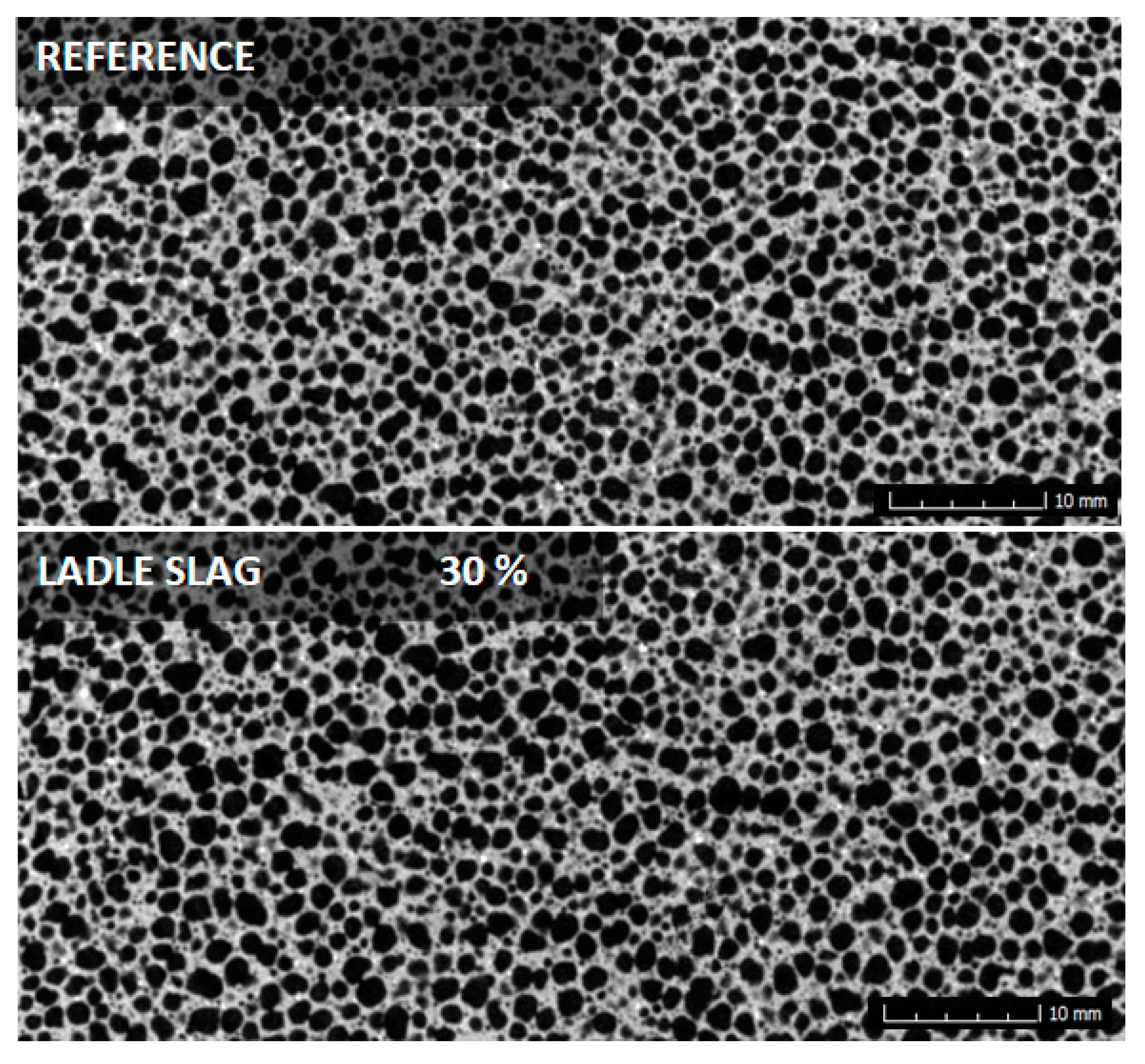
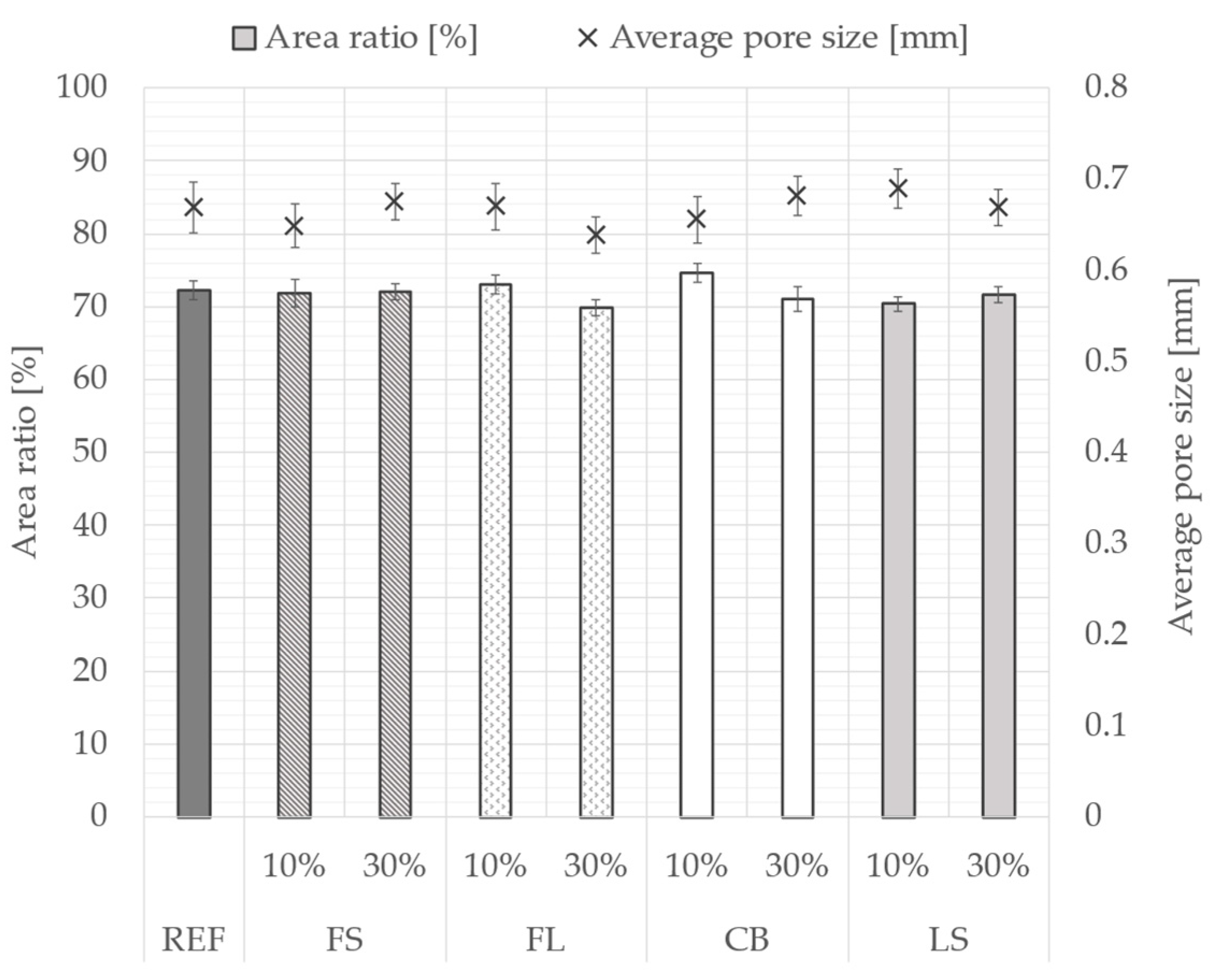
3.2. Distribution of Pores
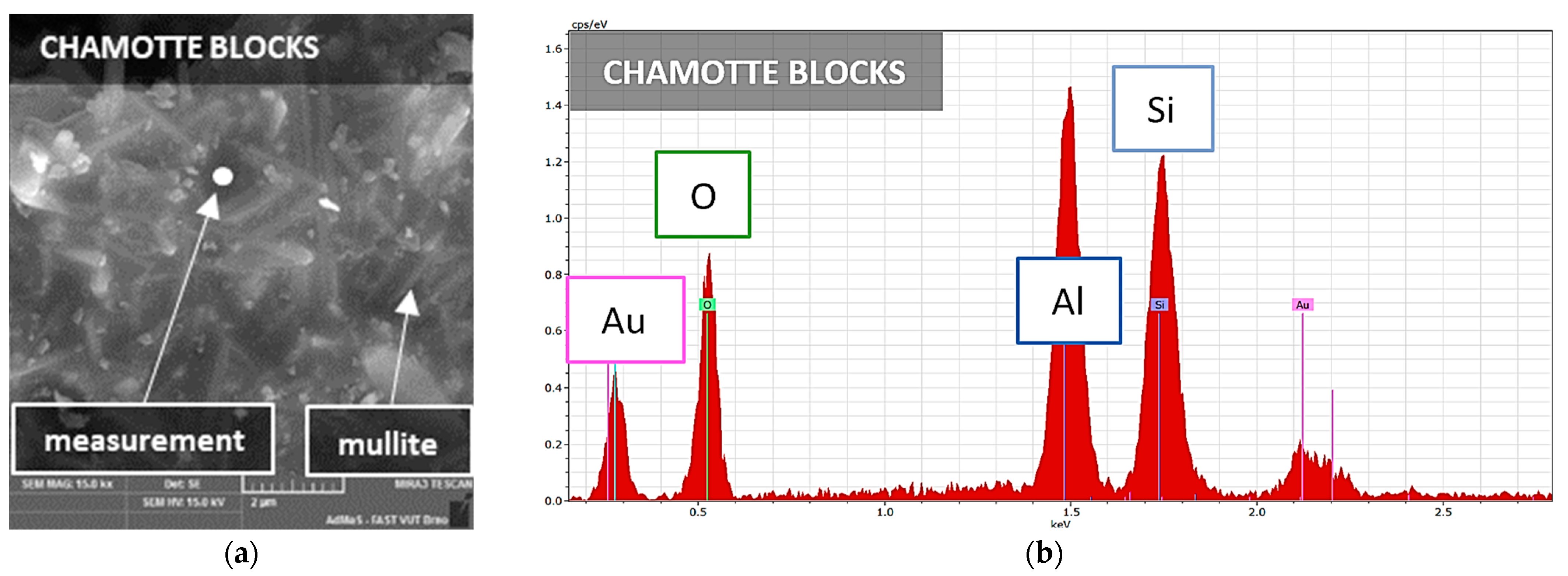
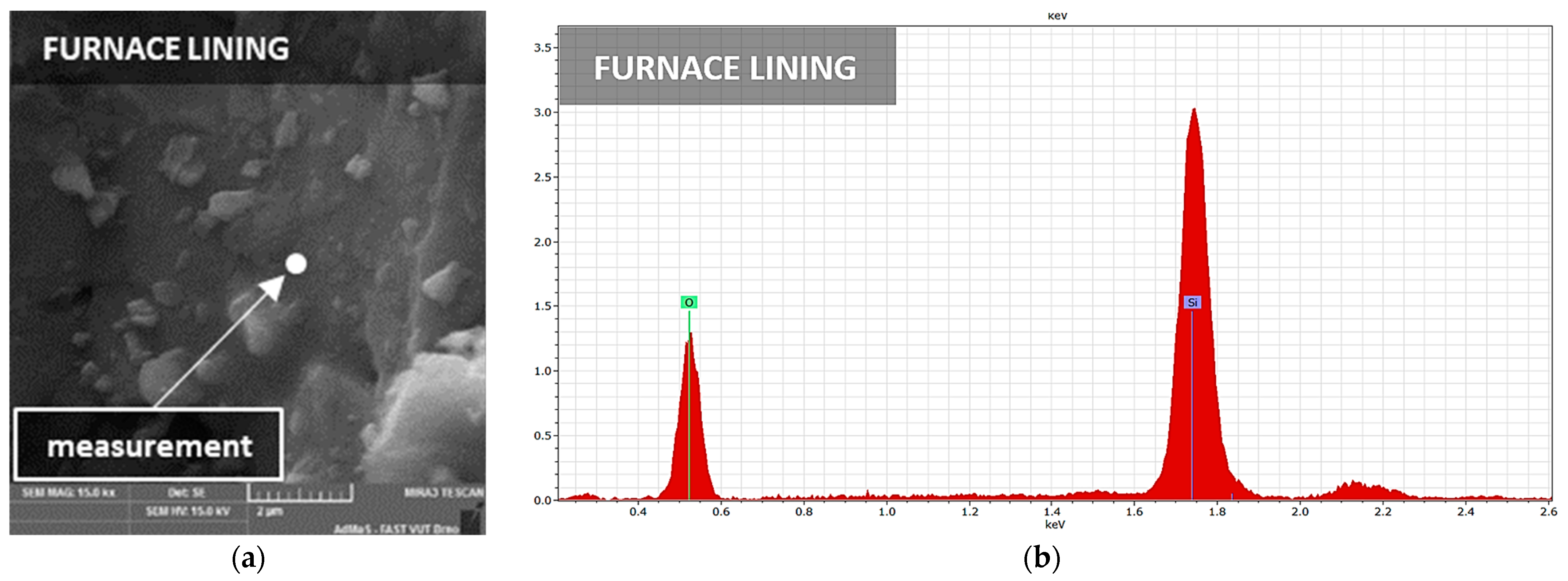
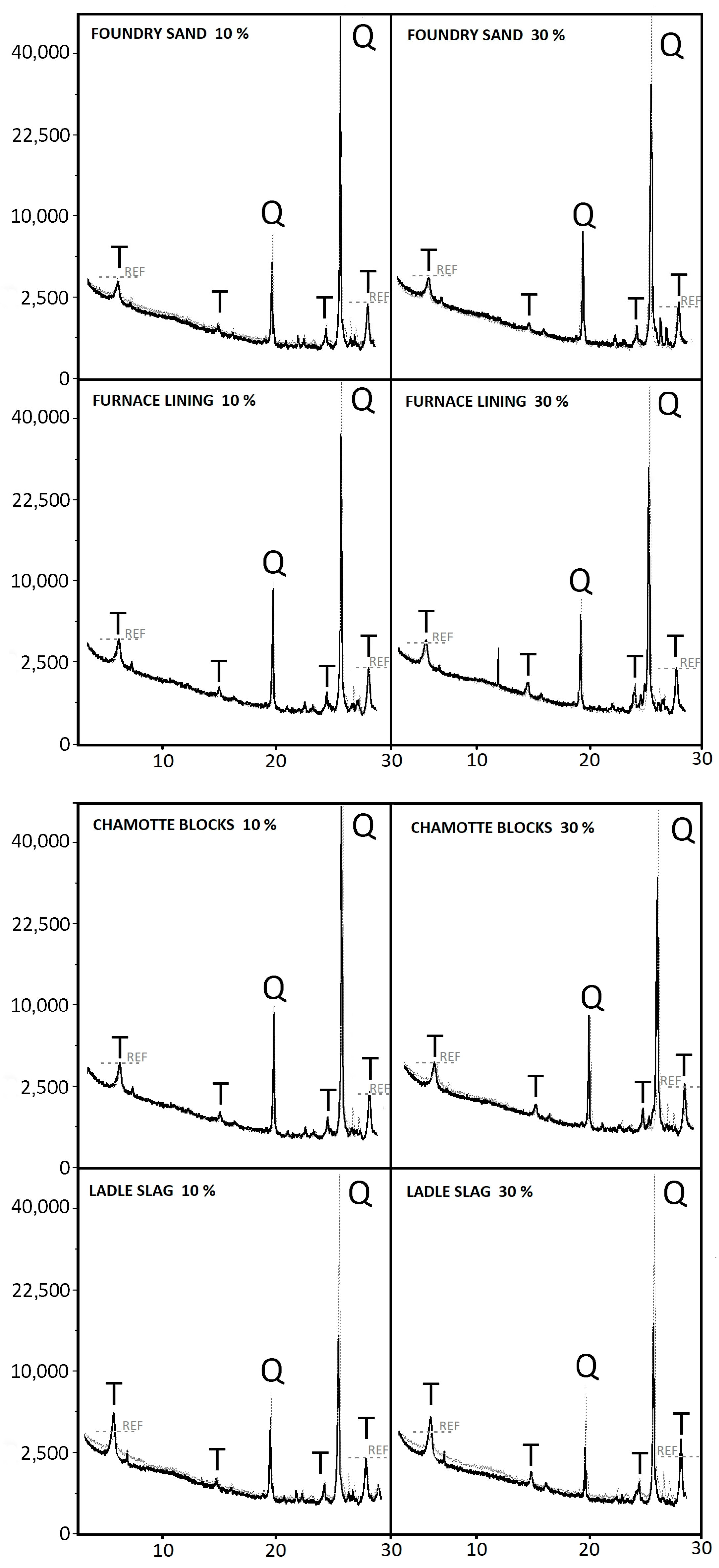
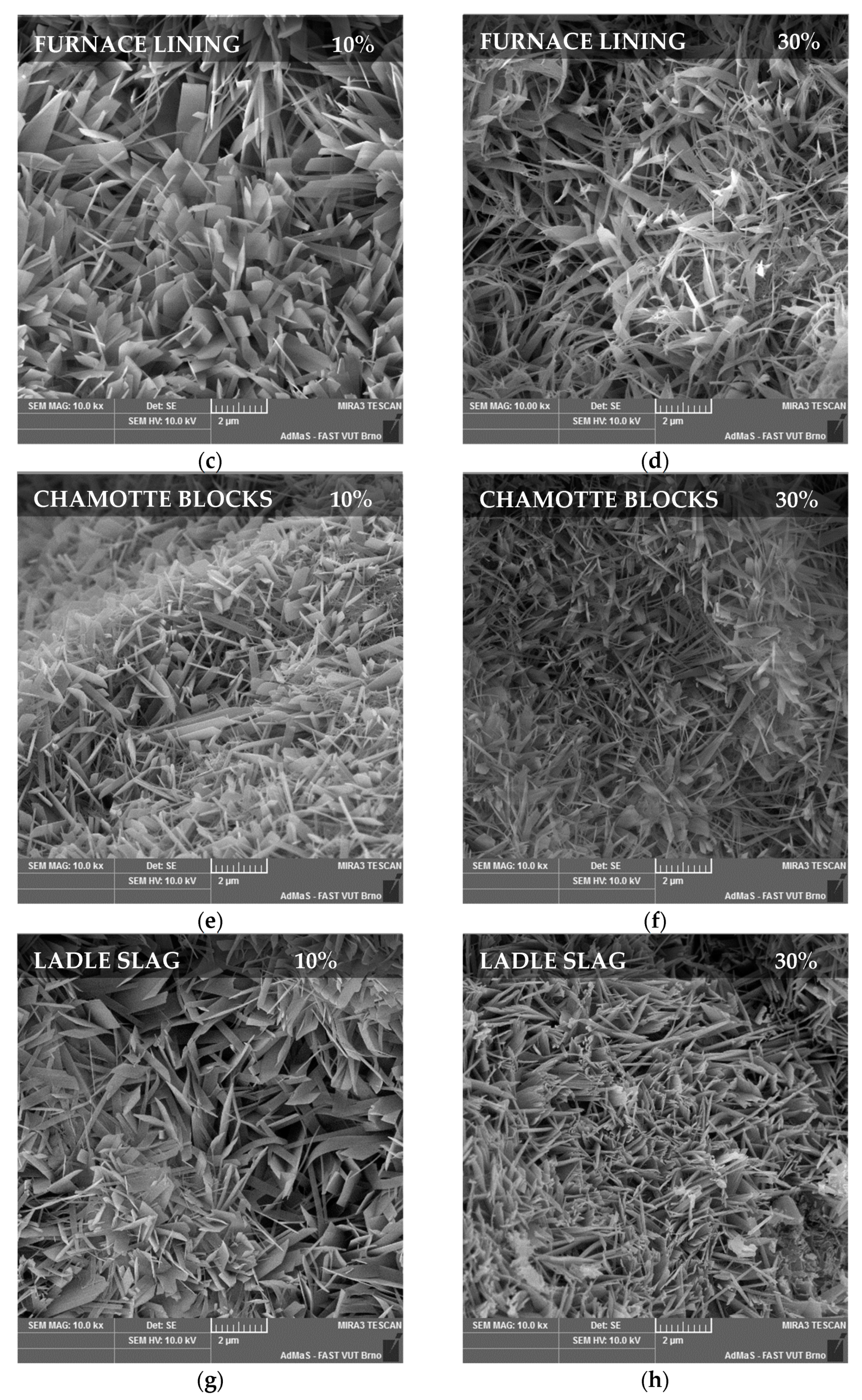
3.3. Microstructure
4. Conclusions
- Ladle slag increased the strength of the samples, even with 30% replacement, by up to 17.5% thanks to the content of CaO.
- Furnace lining increased the strength of the samples by up to 15% because of the high content of aluminum oxide, which has a positive effect on the microstructure of the samples. This positive trend was observed even in the case of a higher substitution rate of 30%.
- The influence of foundry sand on the strength and microstructure slightly deteriorated. This negative effect increased with an increasing amount of replacement.
- Using chamotte blocks to replace quartz sand had a slightly positive effect on the strength of the samples.
- Secondary raw materials from the metallurgical industry have a similar water absorbing capacity and therefore no impact on the distribution of pores.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanehira, S.; Kanamori, S.; Nagashima, K.; Saeki, T.; Visbal, H.; Fukui, T.; Hirao, K. Controllable hydrogen release via aluminum powder corrosion in calcium hydroxide solutions. J. Asian Ceram. Soc. 2013, 3, 296–303. [Google Scholar] [CrossRef]
- Mostafa, N.Y. Influence of air-cooled slag on physicochemical properties of autoclaved aerated concrete. Cem. Concr. Res. 2005, 35, 1349–1357. [Google Scholar] [CrossRef]
- Mitsuda, T.; Sasaki, K.; Ishida, H. Phase evolution during autoclaving process of aerated concrete. J. Am. Ceram. Soc. 1992, 75, 1858–1863. [Google Scholar] [CrossRef]
- Kikuma, J.; Tsunashima, M.; Ishikawa, T.; Matsuno, S.; Ogawa, A.; Matsui, K.; Sato, M. In situ time-resolved x-ray diffraction of tobermorite formation process under autoclave condition. J. Am. Ceram. Soc. 2010, 93, 2667–2674. [Google Scholar] [CrossRef]
- Matsui, K.; Kikuma, J.; Tsunashima, M.; Ishikawa, T.; Matsuno, S.; Ogawa, A.; Sato, M. In situ time-resolved x-ray diffraction of tobermorite formation in autoclaved aerated concrete: Influence of silica source reactivity and Al addition. Cem. Concr. Res. 2011, 41, 510–519. [Google Scholar] [CrossRef]
- Houston, J.R.; Maxwell, R.S.; Carroll, S.A. Transformation of meta-stable calcium silicate hydrates to tobermorite: Reaction kinetics and molecular structure from XRD and NMR spectroscopy. Geochem. Trans. 2009, 10, 1. [Google Scholar] [CrossRef]
- Al-Wakeel, E.I.; El-Korashy, S.A. Reaction mechanism of the hydrothermally treated CaO–SiO2–Al2O3 and CaO–SiO2–Al2O3–CaSO4 systems. J. Mater. Sci. 1996, 31, 1909–1913. [Google Scholar] [CrossRef]
- Dilnesa, B.Z.; Lothenbach, B.; Renaudin, G.; Wichser, A.; Kulik, D. Synthesis and characterization of hydrogarnet Ca3(AlxFe1−x)2(SiO4)y(OH)4(3−y). Cem. Concr. Res. 2014, 59, 96–111. [Google Scholar] [CrossRef]
- Siauciunas, R.; Smalakys, G.; Eisinas, A.; Prichockiene, E. Synthesis of High Crystallinity 1.13 nm Tobermorite and Xonotlite from Natural Rocks, Their Properties and Application for Heat-Resistant Products. Materials 2022, 15, 3474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melichar, T.; Bydžovský, J.; Dufka, Á. Composites based on alternative raw materials at high temperature conditions. Period. Polytech. Civ. Eng. 2017, 61, 911–919. [Google Scholar] [CrossRef]
- Melichar, T.; Bydžovský, J. Study of the parameters of lightweight polymer- cement repair mortars exposed to high temperatures. Appl. Mech. Mater. 2013, 395, 429–432. [Google Scholar] [CrossRef]
- Zach, J.; Sedlmajer, M.; Dufek, Z.; Bubeník, J. Development of light-weight concrete with utilization of foam glass based aggregate. Solid State Phenom. 2018, 276, 276–281. [Google Scholar] [CrossRef]
- Kunchariyakun, K.; Asavapisit, S.; Sombatsompop, K. Properties of autoclaved aerated concrete incorporating rice husk ash as partial replacement for fine aggregate. Cem. Concr. Compos. 2015, 55, 11–16. [Google Scholar] [CrossRef]
- Siddique, R.; Schutter, G.; Noumowe, A. Effect of used-foundry sand on the mechanical properties of concrete. Constr. Build. Mater. 2009, 23, 976–980. [Google Scholar] [CrossRef]
- Guney, Y.; Aydilek, A.H.; Demirkan, M.M. Geoenvironmental behavior of foundry sand amended mixtures for highway subbases. Waste Manag. 2006, 26, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.; Noumowe, A. Utilization of spent foundry sand in controlled low-strength materials and concrete. Resour. Conserv. Recycl. 2008, 53, 27–35. [Google Scholar] [CrossRef]
- Guney, Y.; Sari, Y.D.; Yalcin, M.; Tuncan, A.; Donmez, S. Re-usage of waste foundry sand in high-strength concrete. Waste Manag. 2010, 30, 1705–1713. [Google Scholar] [CrossRef]
- Aggarwal, Y.; Siddique, R. Microstructure and properties of concrete using bottom ash and waste foundry sand as partial replacement of fine aggregates. Constr. Build. Mater. 2014, 54, 210–223. [Google Scholar] [CrossRef]
- Jančara, D.; Tvardek, P.; Hašek, P. Pouring ladles in acelormital Ostrava steel plant utilization of new insulation layer. Met. Hradec Moravicí 2008, 6, 13–15. [Google Scholar]
- Adolf, Z.; Suchánek, P.; Husa, I. The infuence of carbon content on the corrosion of mgo-c refractory material caused by acid and alkaline ladle slag, SLAG. Mater. Technol. 2008, 42, 131–133. [Google Scholar]
- Wang, L.; Jin, M.; Zhou, S.; Tang, S.; Lu, X. Investigation of microstructure of C-S-H and micro-mechanics of cement pastes under NH4NO3 dissolution by 29Si MAS NMR and microhardness. Measurement 2021, 185, 110019. [Google Scholar] [CrossRef]
- Bernard, V.A.R.; Renuka, S.M.; Avudaiappan, S.; Umarani, C.; Amran, M.; Guindos, P.; Fediuk, R.; Vatin, N.I. Performance Investigation of the Incorporation of Ground Granulated Blast Furnace Slag with Fly Ash in Autoclaved Aerated Concrete. Crystals 2022, 12, 1024. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Wang, X.; Ren, Y.; Liu, X.; Shan, Q. Characteristics of inclusions in low carbon Al-killed steel during ladle furnace refining and calcium treatment. ISIJ Int. 2013, 53, 1401–1410. [Google Scholar] [CrossRef]
- Xusheng, D.; Zhe, X.; Junjiang, L.; Lei, W. Effects of lime content on properties of autoclaved aerated concrete made from circulating fluidized bed ash. Dev. Built Environ. 2024, 18, 100406. [Google Scholar] [CrossRef]
- Kurdowski, W. The Properties of Cement Paste. In Cement and Concrete Chemistry; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Tsuji, M.; Komarneni, S.; Malla, P. Substituted tobermorites: 27Al and 29Si MASNMR, cation exchange, and water sorption studies. J. Am. Ceram. Soc. 1991, 72, 274–279. [Google Scholar] [CrossRef]
- Rios, C.; Williams, C.; Fullen, M. Hydrothermal synthesis of hydrogarnet and tobermorite at 175 °C from kaolinite and metakaolinite in the CaO–Al2O3–SiO2–H2O system: A comparative study. Appl. Clay Sci. 2009, 43, 228–237. [Google Scholar] [CrossRef]
- Mostafa, N.Y.; Shaltout, A.; Omar, H.; Abo-El-Enein, S.A. Hydrothermal synthesis and characterization of aluminium and sulfate substituted 1.1nm tobermorites. J. Alloys Compd. 2009, 467, 332–337. [Google Scholar] [CrossRef]
- Isu, N.; Ishida, H.; Mitsuda, T. Influence of quartz particle size on the chemical and mechanical properties of autoclaved aerated concrete (I) tobermorite formation. Cem. Concr. Res. 1995, 25, 243–248. [Google Scholar] [CrossRef]
- Tchadjie, L.N.; Ekolu, S.O. Enhancing the reactivity of aluminosilicate materials toward geopolymer synthesis. J. Mater. Sci. 2018, 53, 4709–4733. [Google Scholar] [CrossRef]
- Melichar, J.; Černý, V.; Fleischhacker, J.; Drochytka, R. Content of Aluminium Hydroxide in Lime-Silica Composite and its Influence on Tobermorite Formation. In Materials Science Forum; Trans Tech Publications: Bäch, Switzerland, 2018; pp. 195–199. ISSN 0255-5476. [Google Scholar]
- Baltakys, K.; Siauciunas, R. The influence of γ-Al2O3 and Na2O on the formation of calcium silicate hydrates in the CaO-quartz-H2O system. Mater. Sci.-Pol. 2007, 25, 185–198. [Google Scholar]
- Oh, J.E.; Clark, S.M.; Monteiro, P.J.M. Does the Al substitution in C-S-H (I) change its mechanical property? Cem. Concr. Res. 2011, 41, 102–106. [Google Scholar] [CrossRef]
- Abhilasha; Kumar, R.; Lakhani, R.; Mishra, R.K.; Khan, S. Utilization of Solid Waste in the Production of Autoclaved Aerated Concrete and Their Effects on its Physio-mechanical and Microstructural Properties: Alternative Sources, Characterization, and Performance Insights. Int. J. Concr. Struct. Mater. 2023, 17, 6. [Google Scholar] [CrossRef]
- Fan, J.; Cao, D.; Jing, Z.; Zhang, Y.; Pu, L.; Jing, Y. Synthesis and microstructure analysis of autoclaved aerated concrete with carbide slag addition. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 1005–1010. [Google Scholar] [CrossRef]
- Alexanderson, J. Relations between structure and mechanical properties of autoclaved aerated concrete. Cem. Concr. Res. 1979, 9, 507–514. [Google Scholar] [CrossRef]
- Reinik, J.; Heinmaa, I.; Mikkola, J.P.; Kirso, U. Hydrothermal alkaline treatment of oil shale ash for synthesis of tobermorites. Fuel 2007, 86, 669–676. [Google Scholar] [CrossRef]
- Kalousek, G.L. Crystal chemistry of hydrous calcium silicates: I, substitution of aluminum in lattice of tobermorite. J. Am. Ceram. Soc. 1957, 40, 74–80. [Google Scholar] [CrossRef]
- Chucholowski, C.; Holger, M.; Thienel, K. Improving the recyclability, environmental compatibility, and CO2 balance of autoclaved aerated concrete by replacing sulfate carrier and cement with calcined clays. Ce/papers 2018, 4, 503–512. [Google Scholar] [CrossRef]
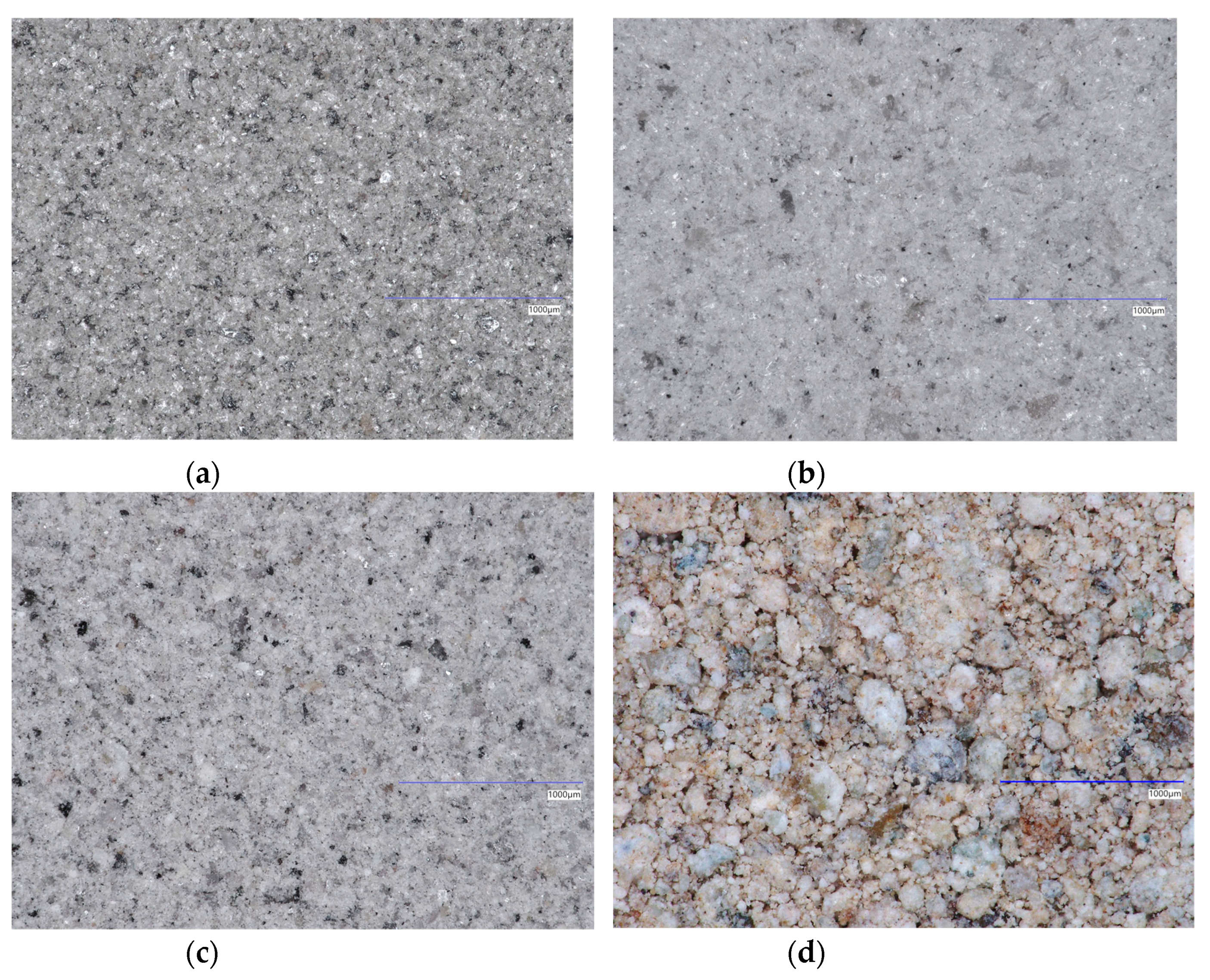





| Chemical Composition | Mineralogical Composition | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 [%] | SO4(2−) [%] | Al2O3 [%] | CaO [%] | Fe2O3 [%] | K2O [%] | Na2O [%] | ||
| Quartz sand | 92.91 | 0.02 | 2.53 | 0.23 | 0.84 | 1.53 | 0.7 | quartz |
| Cement | 19.67 | 2.66 | 5.4 | 64.25 | 3.06 | 3.32 | 0.36 | C3S, C2S, C3A, C4AF |
| Lime | - | 0.07 | - | 95.62 | - | - | - | lime |
| Chemical Composition | Mineralogical Composition | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 [%] | SO4(2−) [%] | Al2O3 [%] | CaO [%] | Fe2O3 [%] | K2O [%] | Na2O [%] | ||
| Foundry sand (FS) | 82.7 | 0.1 | 1.51 | 0.3 | 2.63 | 0.45 | 0.23 | quartz |
| Furnace lining (FL) | 56.1 | 0.17 | 32.4 | 0.4 | 2.38 | 1.14 | 0.12 | quartz, mullite, cristoballite |
| Chamotte blocks (CB) | 63.8 | 0.14 | 23.2 | 1.18 | 1.66 | 0.95 | 0.25 | quartz, mullite, cristobalite, corundum |
| Ladle slag (LS) | 15.8 | <0.10 | 13.4 | 30.7 | 2.2 | 0.24 | 0.19 | lime, C2S, calcite, magnetite, hematite, hydrocalumite |
| Physical Properties | |||
|---|---|---|---|
| Density [g·cm−3] | Specific Surface Area [cm2·g−1] | Water Absorbency [%] | |
| Primary raw materials | |||
| Quartz sand (QS) | 2.74 | 2820 | 31 |
| Secondary raw materials | |||
| Foundry sand (FS) | 2.86 | 2600 | 27 |
| Furnace lining (FL) | 2.73 | 3000 | 36 |
| Chamotte blocks (CB) | 2.75 | 2950 | 33 |
| Ladle slag (LS) | 2.92 | 2630 | 37 |
| Mix Designs [wt%] | |||
|---|---|---|---|
| Raw Materials | REF | Replacement of Siliceous Component | |
| 10% | 30% | ||
| Lime | 8 | 8 | 8 |
| Cement | 14 | 14 | 14 |
| Gypsum | 3 | 3 | 3 |
| Aluminum powder | 0.06 | 0.06 | 0.06 |
| Quartz sand | 74 | 67.3 | 51.8 |
| Secondary raw materials | 0 | 6.7 | 22.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mészárosová, L.; Černý, V.; Melichar, J.; Ondříčková, P.; Drochytka, R. The Usability of Metallurgical Production Waste as a Siliceous Component in Autoclaved Aerated Concrete Technology. Buildings 2024, 14, 3155. https://doi.org/10.3390/buildings14103155
Mészárosová L, Černý V, Melichar J, Ondříčková P, Drochytka R. The Usability of Metallurgical Production Waste as a Siliceous Component in Autoclaved Aerated Concrete Technology. Buildings. 2024; 14(10):3155. https://doi.org/10.3390/buildings14103155
Chicago/Turabian StyleMészárosová, Lenka, Vít Černý, Jindřich Melichar, Pavlína Ondříčková, and Rostislav Drochytka. 2024. "The Usability of Metallurgical Production Waste as a Siliceous Component in Autoclaved Aerated Concrete Technology" Buildings 14, no. 10: 3155. https://doi.org/10.3390/buildings14103155








