Analysis of Hydration Mechanism of Steel Slag-Based Cementitious Materials under Saline–Alkaline-Coupled Excitation
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Raw Materials
2.2. Test Methods
2.2.1. Sample Mixing Ratios
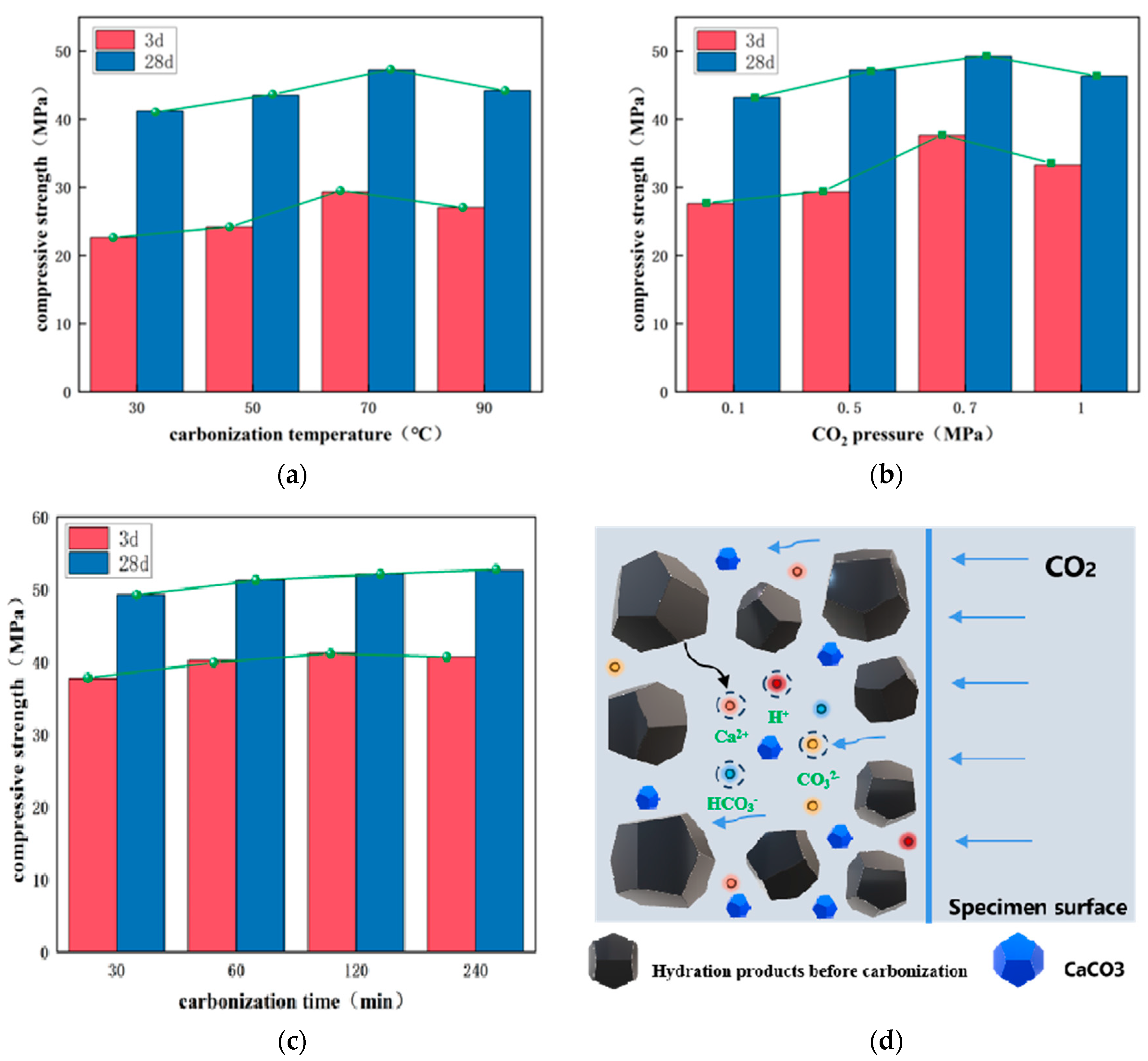
2.2.2. Carbonation Conservation
2.2.3. Strength Testing
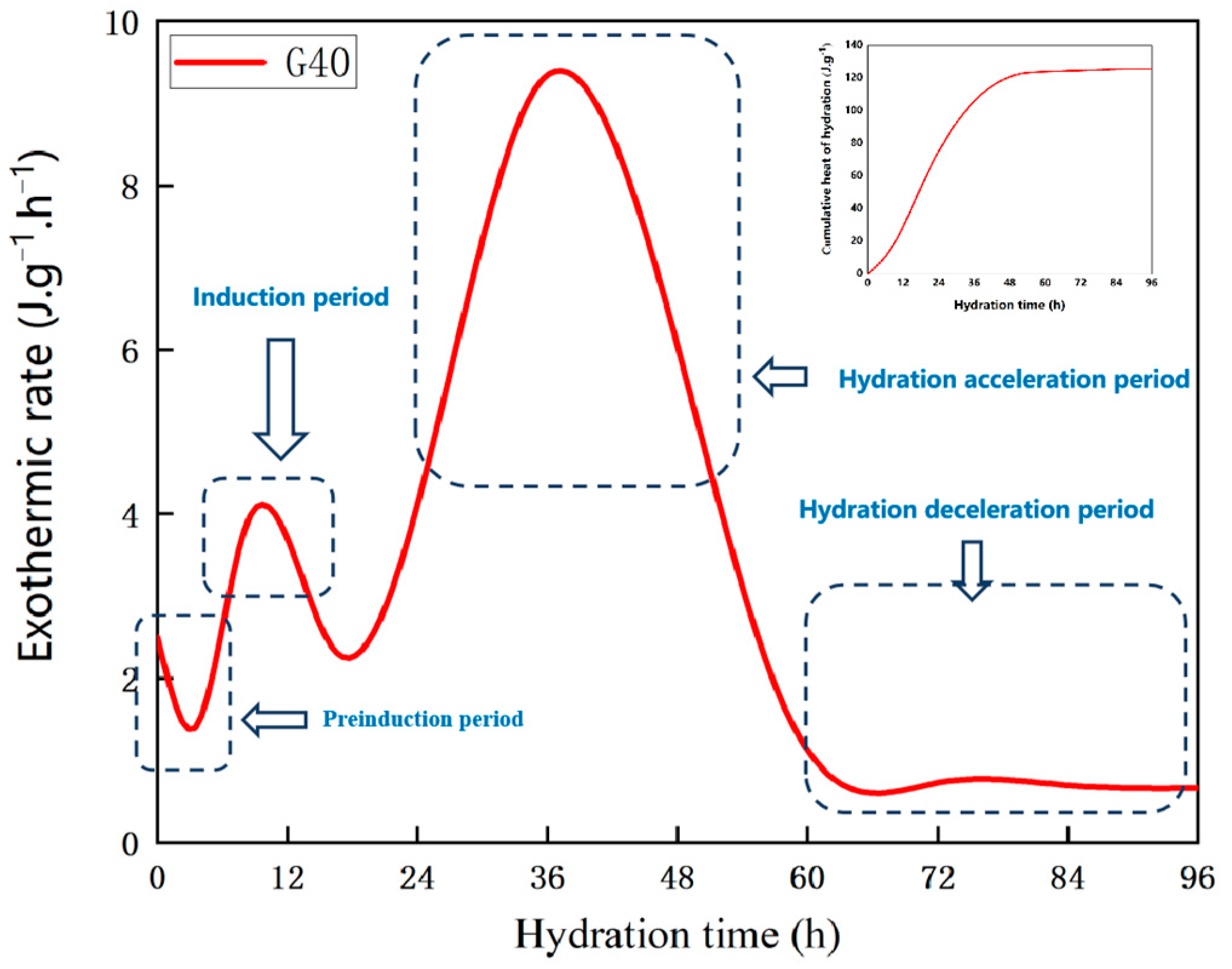
2.2.4. Exothermic Hydration Test
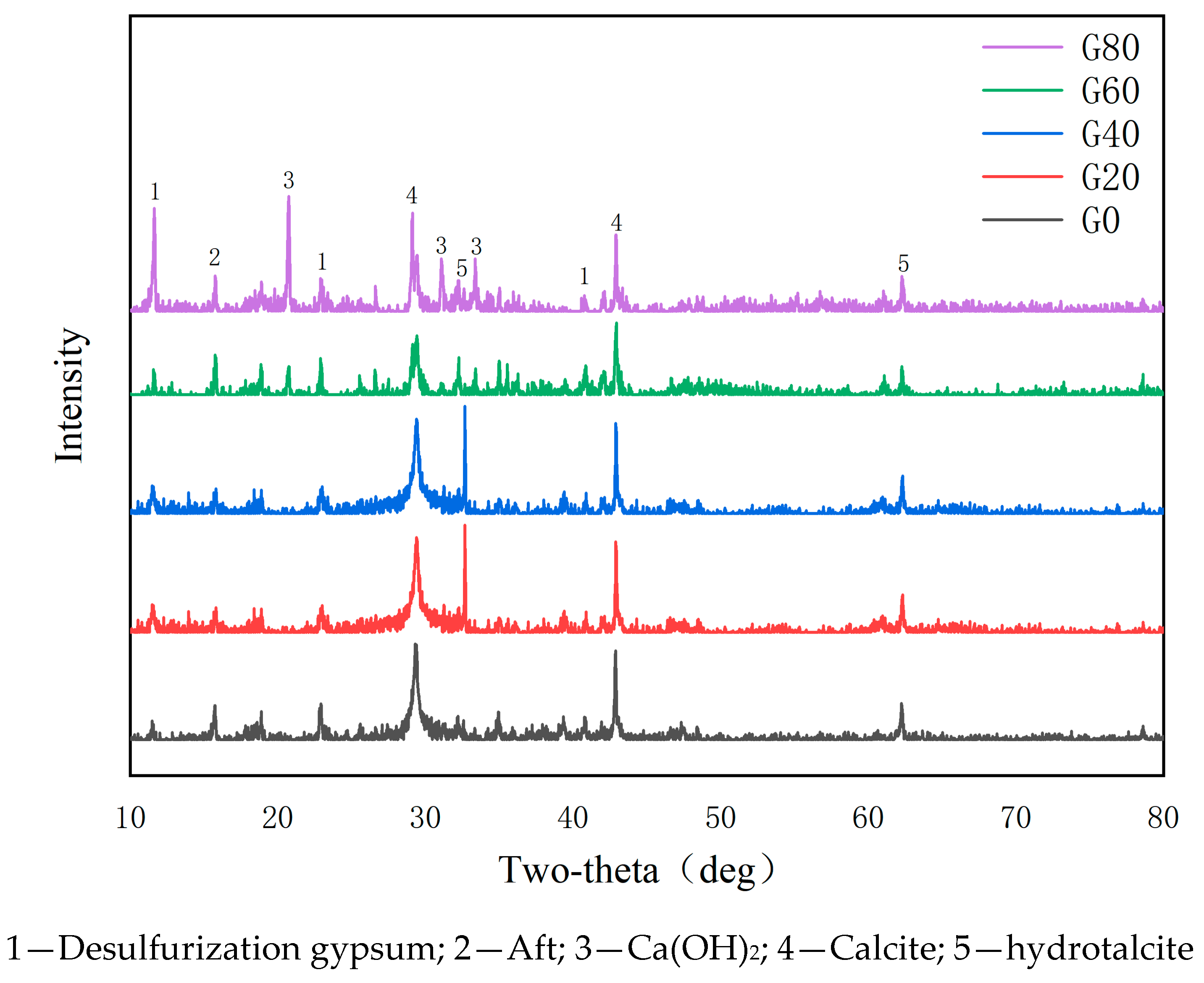
2.2.5. XRD Analysis
2.2.6. SEM Analysis
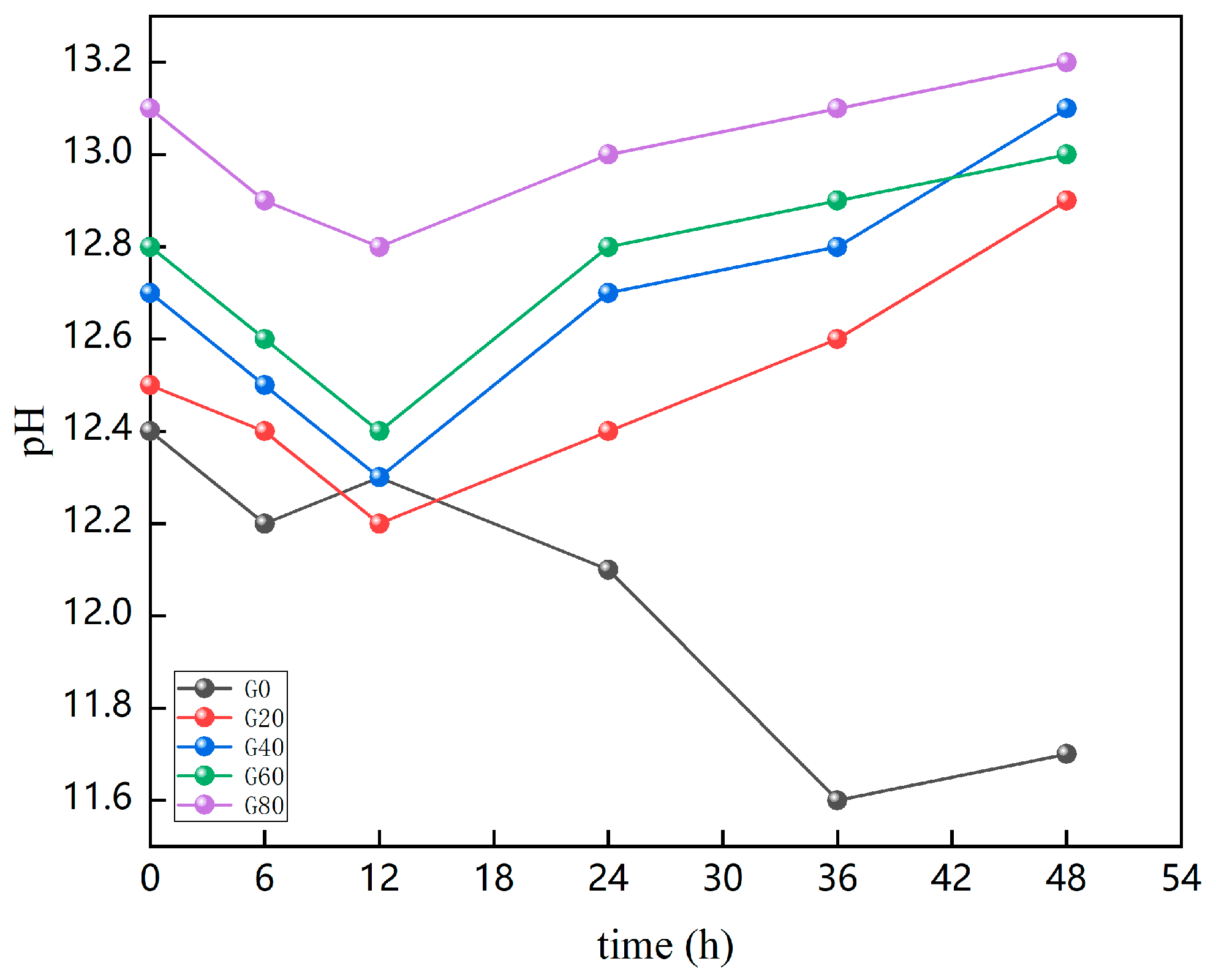
2.2.7. Determination of the pH of the Pore Solution
3. Strength Development and Carbonation Effect of Steel Slag-Based Cementitious Materials
3.1. Strength Development
3.2. Carbonization Conservation Effect
4. Hydration Process of Steel Slag-Based Cementitious Materials
4.1. Heat of Hydration
4.2. Pore Solution Analysis
5. Hydration Products of Steel Slag-Based Cementitious Materials
5.1. XRD Analysis
5.2. SEM Analysis
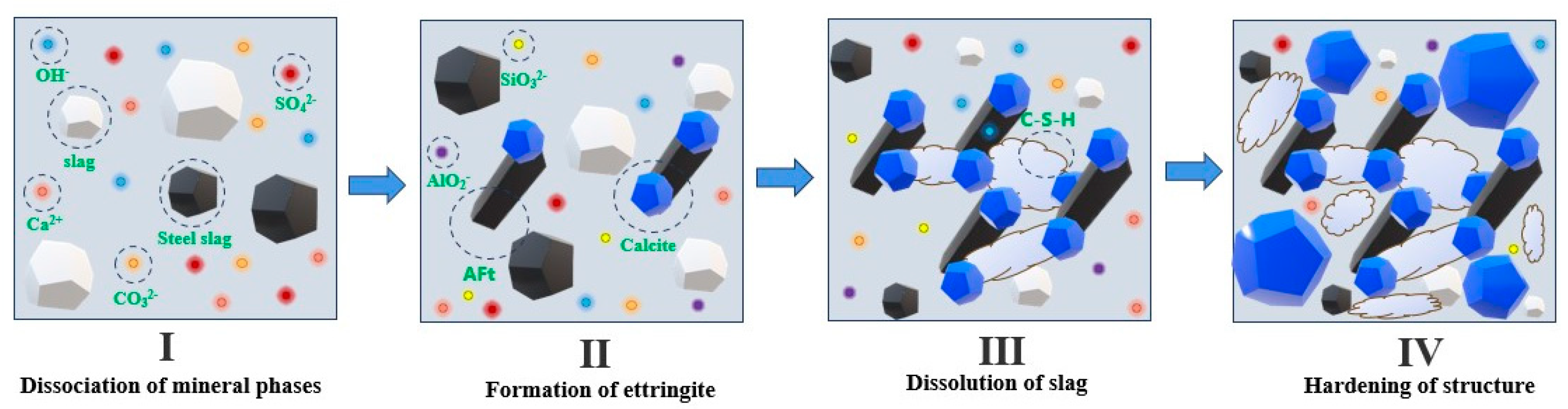
6. Hydration Mechanism Analysis
7. Conclusions
- (1)
- Under the condition of saline–alkaline-coupled excitation, the ratio of slag, steel slag, and desulfurization gypsum was 53:40:7, and the late mechanical properties of the cementitious material decreased the least (only 5.67%, and the 28 d compressive strength was 40.44 MPa). This indicates that a steel slag dosing of 40% can produce the best hydration synergy with the slag, offsetting some of the effects of the steel slag in the RO phase and the low-activity γ-C2S on the mechanical properties of the cementitious materials;
- (2)
- When the steel slag-based cementitious materials were standardized to 28 d and then placed at a carbonization temperature of 70 °C, a CO2 pressure 0.7 MPa, and a carbonization time of 30 min, the carbonization effect was the most appropriate, with a strength of up to 51.22 MPa;
- (3)
- The addition of desulfurization gypsum enables the system to generate AFt to provide sites for the subsequent generation of C-S-H gels with calcite, while the addition of low-activity magnesium oxide can generate a hydrotalcite-like phase. The latter can play a role in accelerating the transformation of the steel carbonate phase;
- (4)
- For the initial hydration mechanism of the gelling system, first, the hydrolysis of the chemical exciters provides an alkaline environment and associated ions for the gelling system, which promotes the generation of the AFt and hydrotalcite phases. Subsequently, AFt provides a site for the generation of C-S-H gels and calcite, while the hydrotalcite phase accelerates the transformation of the carbonate phase in the gelling system, promoting a synergistic effect on the hydration of the steel slag and slag. Eventually, under the synergistic effect of steel slag and slag hydration, a large number of C-S-H gels, calcites, and various other hydration products are generated in the gelling system, thereby improving the mechanical properties of the gelling materials.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; Ling, T.C.; Shi, C.; Pan, S.Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, P.; Wang, D. Research on mineral characteristics of converter steel slag and its comprehensive utilization of internal and external recycle. J. Clean. Prod. 2017, 156, 50–61. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q. Inhibition mechanisms of steel slag on the early-age hydration of cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- Gu, X.; Wang, H.; Liu, J.; Zhu, Z.; Wang, S.; Xu, X. Synergistic effects of steel slag and metakaolin in cementitious systems: Packing properties, strength, and microstructure. Constr. Build. Mater. 2024, 411, 134395. [Google Scholar] [CrossRef]
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef]
- Brand, A.S.; Roesler, J.R. Steel furnace slag aggregate expansion and hardened concrete properties. Cem. Concr. Compos. 2015, 60, 1–9. [Google Scholar] [CrossRef]
- Gao, T.; Dai, T.; Shen, L.; Jiang, L. Benefits of using steel slag in cement clinker production for environmental conservation and economic revenue generation. J. Clean. Prod. 2021, 282, 124538. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, S.; Zhao, X.; He, T. Cementitious property modification of basic oxygen furnace steel slag. Constr. Build. Mater. 2013, 48, 575–579. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Yan, P. Cementitious properties of super-fine steel slag. Powder Technol. 2013, 245, 35–39. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Liu, J. Hydration activity and expansibility model for the RO phase in steel slag. Metall. Mater. Trans. B 2020, 51, 1697–1704. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Z.; Wang, D.; Yan, P. Hydration heat evolution and kinetics of blended cement containing steel slag at different temperatures. Thermochim. Acta 2015, 605, 43–51. [Google Scholar] [CrossRef]
- Pal, S.C.; Mukherjee, A.; Pathak, S.R. Investigation of hydraulic activity of ground granulated blast furnace slag in concrete. Cem. Concr. Res. 2003, 33, 1481–1486. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.İ. Utilization and efficiency of ground granulated blast furnace slag on concrete properties–A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutiérrez, R.M.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Jin, F.; Abdollahzadeh, A.; Al-Tabbaa, A. Effect of different MgO on the hydration of MgO-activated granulated ground blastfurnace slag paste. In Proceedings of the 3rd International Conference on Sustainable Construction Materials and Technologies, Kyoto, Japan, 18–21 August 2013. [Google Scholar]
- Shen, W.; Zhou, M.; Ma, W.; Hu, J.; Cai, Z. Investigation on the application of steel slag–fly ash–phosphogypsum solidified material as road base material. J. Hazard. Mater. 2009, 164, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wu, Y. Studies on some factors affecting CO2 curing of lightweight concrete products. Resour. Conserv. Recycl. 2008, 52, 1087–1092. [Google Scholar] [CrossRef]
- Ghouleh, Z.; Guthrie, R.I.L.; Shao, Y. High-strength KOBM steel slag binder activated by carbonation. Constr. Build. Mater. 2015, 99, 175–183. [Google Scholar] [CrossRef]
- Liu, J.; Ge, X.; Liu, P.; Song, G.; Hu, Z. Experimental study on the preparation of cementitious materials from iron ore tailings by activation. Constr. Build. Mater. 2023, 385, 131409. [Google Scholar] [CrossRef]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.; Milestone, N.B.; Collier, N.C. Hydration and properties of sodium sulfate activated slag. Cem. Concr. Compos. 2013, 37, 20–29. [Google Scholar] [CrossRef]
- Zhu, X.; Hou, H.; Huang, X.; Zhou, M.; Wang, W. Enhance hydration properties of steel slag using grinding aids by mechanochemical effect. Constr. Build. Mater. 2012, 29, 476–481. [Google Scholar] [CrossRef]
- Shi, C. Characteristics and cementitious properties of ladle slag fines from steel production. Cem. Concr. Res. 2002, 32, 459–462. [Google Scholar] [CrossRef]
- Yan, J.; Wu, S.; Yang, C.; Zhao, Z.; Xie, J. Influencing mechanisms of RO phase on the cementitious properties of steel slag powder. Constr. Build. Mater. 2022, 350, 128926. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Jia, X.; Mo, K.H.; Ling, T.C. Effect of water-to-cement ratio induced hydration on the accelerated carbonation of cement pastes. Environ. Pollut. 2021, 280, 116914. [Google Scholar] [CrossRef]
- Zhong, X.; Li, L.; Jiang, Y.; Ling, T.C. Elucidating the dominant and interaction effects of temperature, CO2 pressure and carbonation time in carbonating steel slag blocks. Constr. Build. Mater. 2021, 302, 124158. [Google Scholar] [CrossRef]
- Gu, X.; Ge, X.; Liu, J.; Song, G.; Wang, S.; Hu, Z.; Wang, H. Study on the synergistic effect of calcium carbide residue-fly ash enhanced desulphurisation gypsum under high temperature maintenance condition. Constr. Build. Mater. 2024, 412, 134706. [Google Scholar] [CrossRef]
- Liu, J.; Song, G.; Ge, X.; Liu, B.; Liu, K.; Tian, Y.; Wang, X.; Hu, Z. Experimental Study on the Properties and Hydration Mechanism of Gypsum-Based Composite Cementitious Materials. Buildings 2024, 14, 314. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, D.; Yan, P.; Zhang, D.; Wang, H. Self-cementitious property of steel slag powder blended with gypsum. Constr. Build. Mater. 2016, 113, 835–842. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, H. Characterization and optimization of eco-friendly cementitious materials based on titanium gypsum, fly ash, and calcium carbide residue. Constr. Build. Mater. 2022, 349, 128635. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, H. The role of CaO and MgO incorporation in chloride resistance of sodium carbonate-activated slag. Cem. Concr. Compos. 2022, 132, 104625. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Characterisation of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr. Build. Mater. 2015, 93, 506–513. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Wang, D.; Yan, P.; Luo, L.; Zhang, H.; Zhang, H.; Gu, X. Hydration superposition effect and mechanism of steel slag powder and granulated blast furnace slag powder. Constr. Build. Mater. 2023, 366, 130101. [Google Scholar] [CrossRef]
- Duan, S.; Liao, H.; Cheng, F.; Song, H.; Yang, H. Investigation into the synergistic effects in hydrated gelling systems containing fly ash, desulfurization gypsum and steel slag. Constr. Build. Mater. 2018, 187, 1113–1120. [Google Scholar] [CrossRef]
- Han, F.; Zhou, Y.; Zhang, Z. Effect of gypsum on the properties of composite binder containing high-volume slag and iron tailing powder. Constr. Build. Mater. 2020, 252, 119023. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Cui, X.; Ni, W. Hydration behavior of cementitious materials with all solid waste based of steel slag and blast furnace slag. Rev. Fac. Ing. 2016, 31, 172–181. [Google Scholar]





| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | FeO | P2O5 | |
|---|---|---|---|---|---|---|---|
| Cement | 62–68 | 20–24 | 4–7 | 2.5–6.5 | 1–2 | — | trace |
| Steel slag | 30–55 | 8–20 | 1–6 | 3–9 | 3–13 | 7–20 | 1–4 |
| Material Science | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | SO3 |
|---|---|---|---|---|---|---|
| Steel slag | 16.75 | 3.87 | 20.12 | 7.35 | 41.74 | |
| Slag | 34.5 | 17.7 | 1.03 | 6.01 | 34 | 1.64 |
| Magnesium oxide | 7.09 | 0.38 | 0.28 | 85.09 | 2.35 | |
| Desulfurization gypsum | 4.3 | 3.1 | 31.97 | 0.18 | 0.33 | 51.84 |
| Number | Steel Slag | Slag | Gypsum | CaCO3 | Mgo | Water Reducer | Water-to-Gel Ratio |
|---|---|---|---|---|---|---|---|
| G0 | 0 | 93% | 7% | 2% | 7% | 0.30% | 0.32 |
| G20 | 20% | 73% | 7% | 2% | 7% | 0.30% | 0.32 |
| G40 | 40% | 53% | 7% | 2% | 7% | 0.30% | 0.32 |
| G60 | 60% | 33% | 7% | 2% | 7% | 0.30% | 0.32 |
| G80 | 80% | 13% | 7% | 2% | 7% | 0.30% | 0.32 |
| Number | Temp (°C) | CO2 Pressure (MPa) | Time (min) |
|---|---|---|---|
| T-30 | 30 | 0.5 | 30 |
| T-50 | 50 | 0.5 | 30 |
| T-70 | 70 | 0.5 | 30 |
| T-90 | 90 | 0.5 | 30 |
| P-0.1 | 70 | 0.3 | 30 |
| P-0.5 | 70 | 0.5 | 30 |
| P-0.7 | 70 | 0.7 | 30 |
| P-1.0 | 70 | 1 | 30 |
| t-30 | 70 | 0.7 | 30 |
| t-60 | 70 | 0.7 | 60 |
| t-120 | 70 | 0.7 | 120 |
| t-240 | 70 | 0.7 | 240 |
| Steel Slag Content | Flexural Strength (Mpa) | Compressive Strength (Mpa) | ||
|---|---|---|---|---|
| 3 d | 28 d | 3 d | 28 d | |
| 0 | 7.31 | 11.23 | 24.72 | 47.47 |
| 20% | 7.69 | 10.68 | 20.22 | 42.77 |
| 40% | 6.08 | 10.03 | 19.28 | 40.44 |
| 60% | 5.52 | 7.25 | 13.21 | 21.22 |
| 80% | 1.99 | 6.11 | 8.62 | 11.22 |
| Number | Temperature (°C) | CO2 Pressure (MPa) | Time (min) | Compressive Strength (MPa) | |
|---|---|---|---|---|---|
| 3 d | 28 d | ||||
| T-30 | 30 | 0.5 | 30 | 22.64 | 41.22 |
| T-50 | 50 | 0.5 | 30 | 24.21 | 43.52 |
| T-70 | 70 | 0.5 | 30 | 29.32 | 47.25 |
| T-90 | 90 | 0.5 | 30 | 27.04 | 44.21 |
| P-0.1 | 70 | 0.3 | 30 | 27.63 | 43.21 |
| P-0.5 | 70 | 0.5 | 30 | 29.32 | 47.25 |
| P-0.7 | 70 | 0.7 | 30 | 37.63 | 49.24 |
| P-1.0 | 70 | 1 | 30 | 33.27 | 46.31 |
| t-30 | 70 | 0.7 | 30 | 37.63 | 49.24 |
| t-60 | 70 | 0.7 | 60 | 40.22 | 51.22 |
| t-120 | 70 | 0.7 | 120 | 41.21 | 52.05 |
| t-240 | 70 | 0.7 | 240 | 40.68 | 52.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, B.; Ge, X.; Tian, Y.; Song, G.; Liu, K.; Wang, Y. Analysis of Hydration Mechanism of Steel Slag-Based Cementitious Materials under Saline–Alkaline-Coupled Excitation. Buildings 2024, 14, 597. https://doi.org/10.3390/buildings14030597
Liu J, Liu B, Ge X, Tian Y, Song G, Liu K, Wang Y. Analysis of Hydration Mechanism of Steel Slag-Based Cementitious Materials under Saline–Alkaline-Coupled Excitation. Buildings. 2024; 14(3):597. https://doi.org/10.3390/buildings14030597
Chicago/Turabian StyleLiu, Jianping, Bing Liu, Xiaowei Ge, Yulin Tian, Ge Song, Kaixin Liu, and Yilin Wang. 2024. "Analysis of Hydration Mechanism of Steel Slag-Based Cementitious Materials under Saline–Alkaline-Coupled Excitation" Buildings 14, no. 3: 597. https://doi.org/10.3390/buildings14030597





