Mutual Activation Mechanism of Cement–GGBS–Steel Slag Ternary System Excited by Sodium Sulfate
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Cement Pastes
2.3. Test Methods
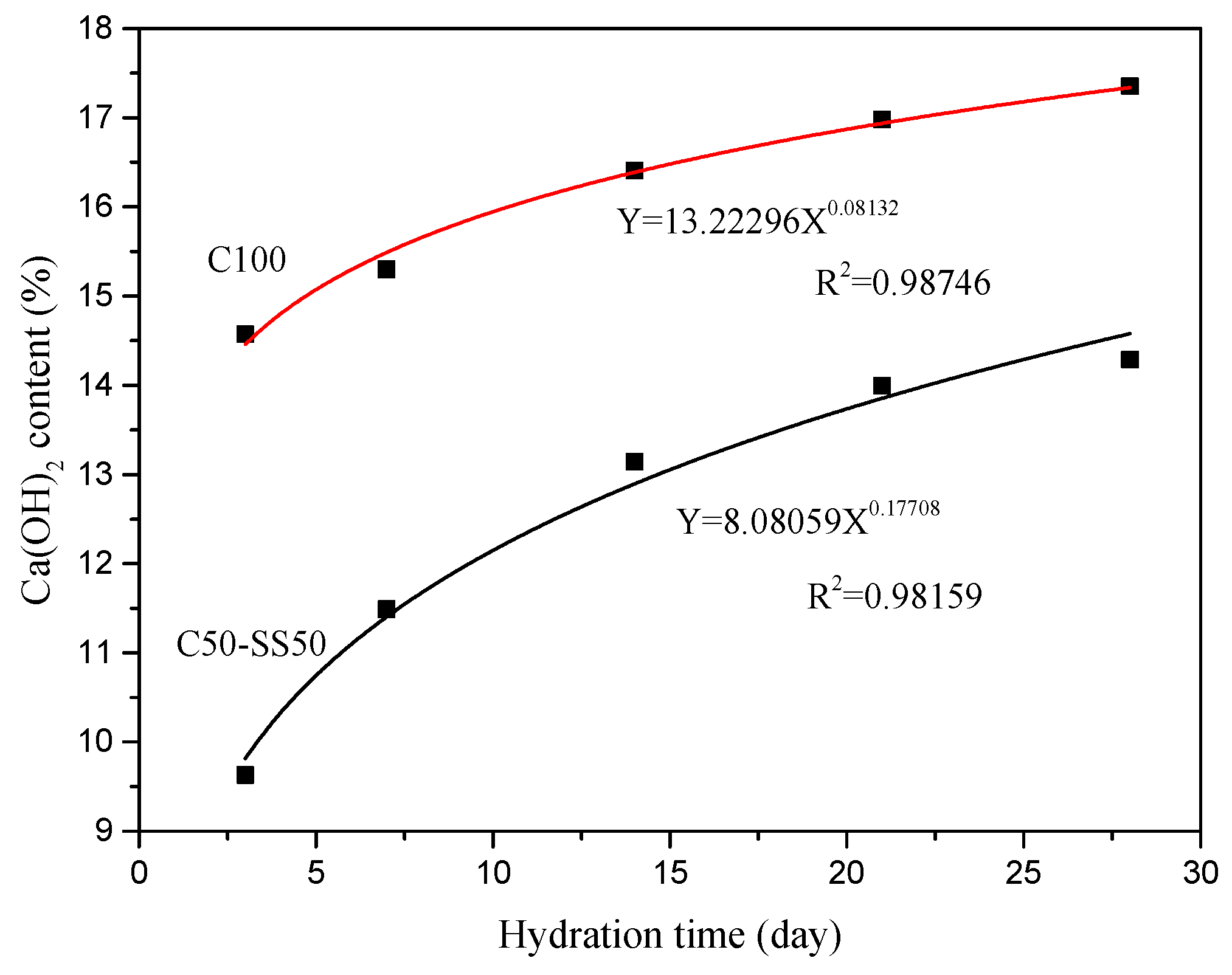
3. Results and Discussion
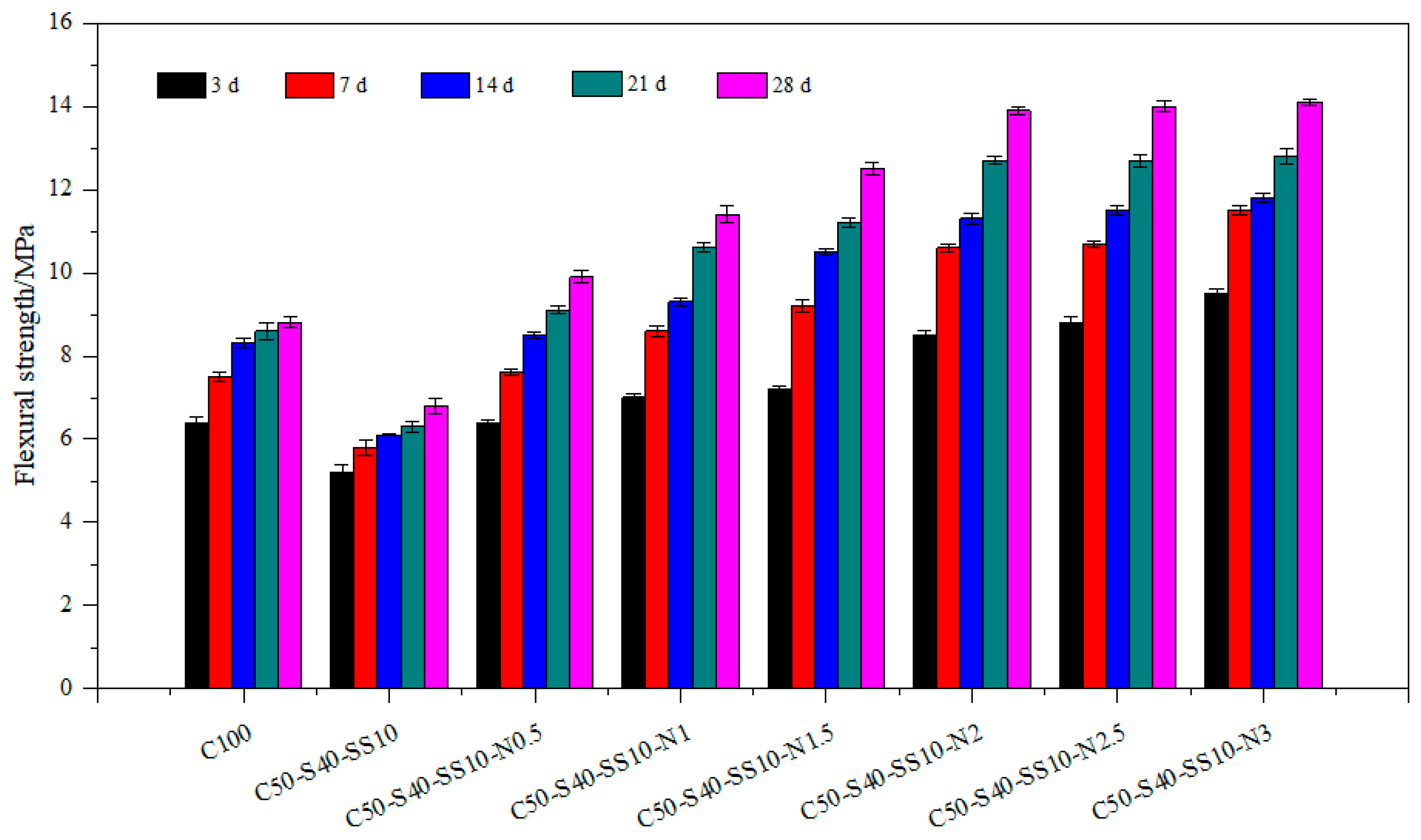
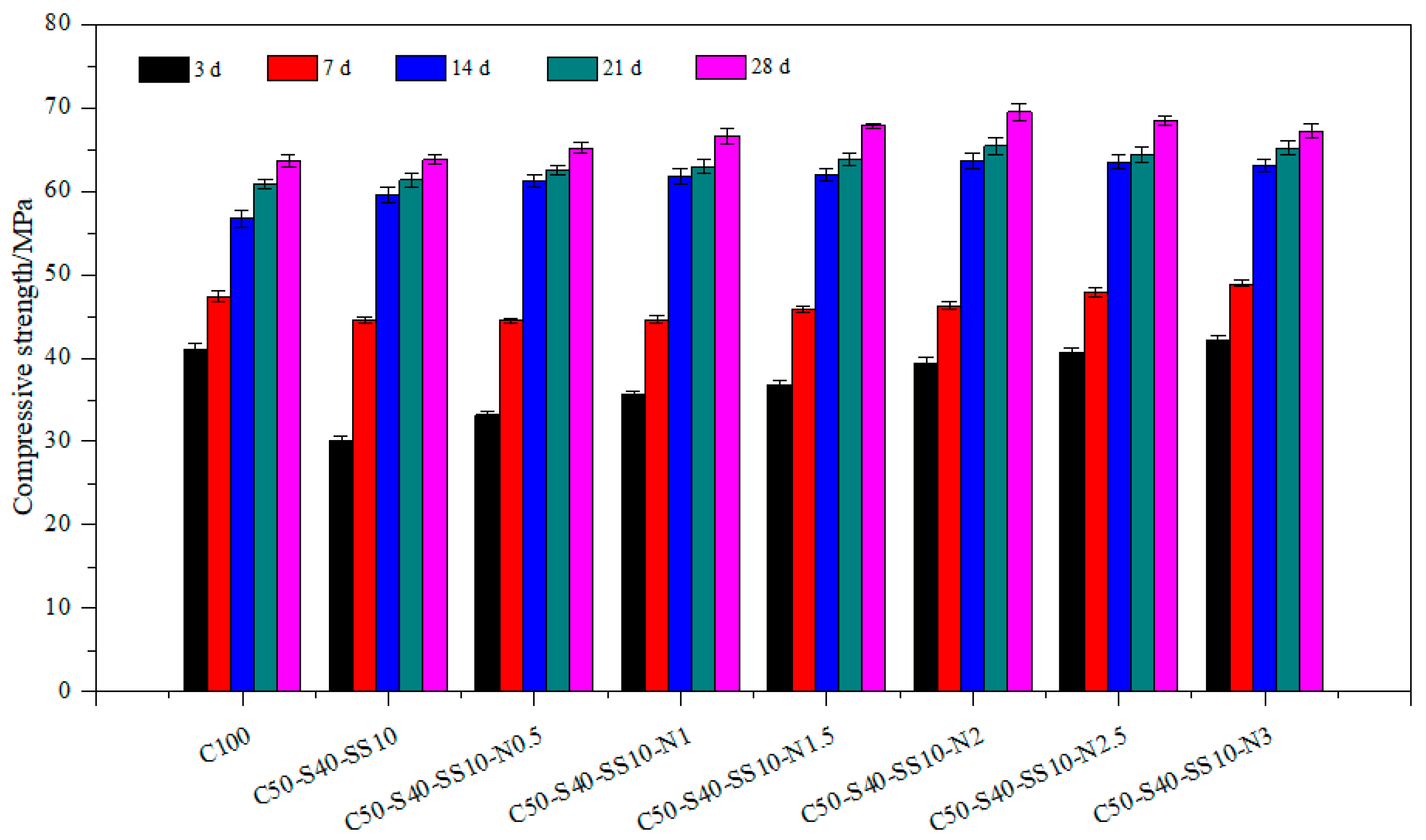
3.1. Mechanical Properties of Composite Cement
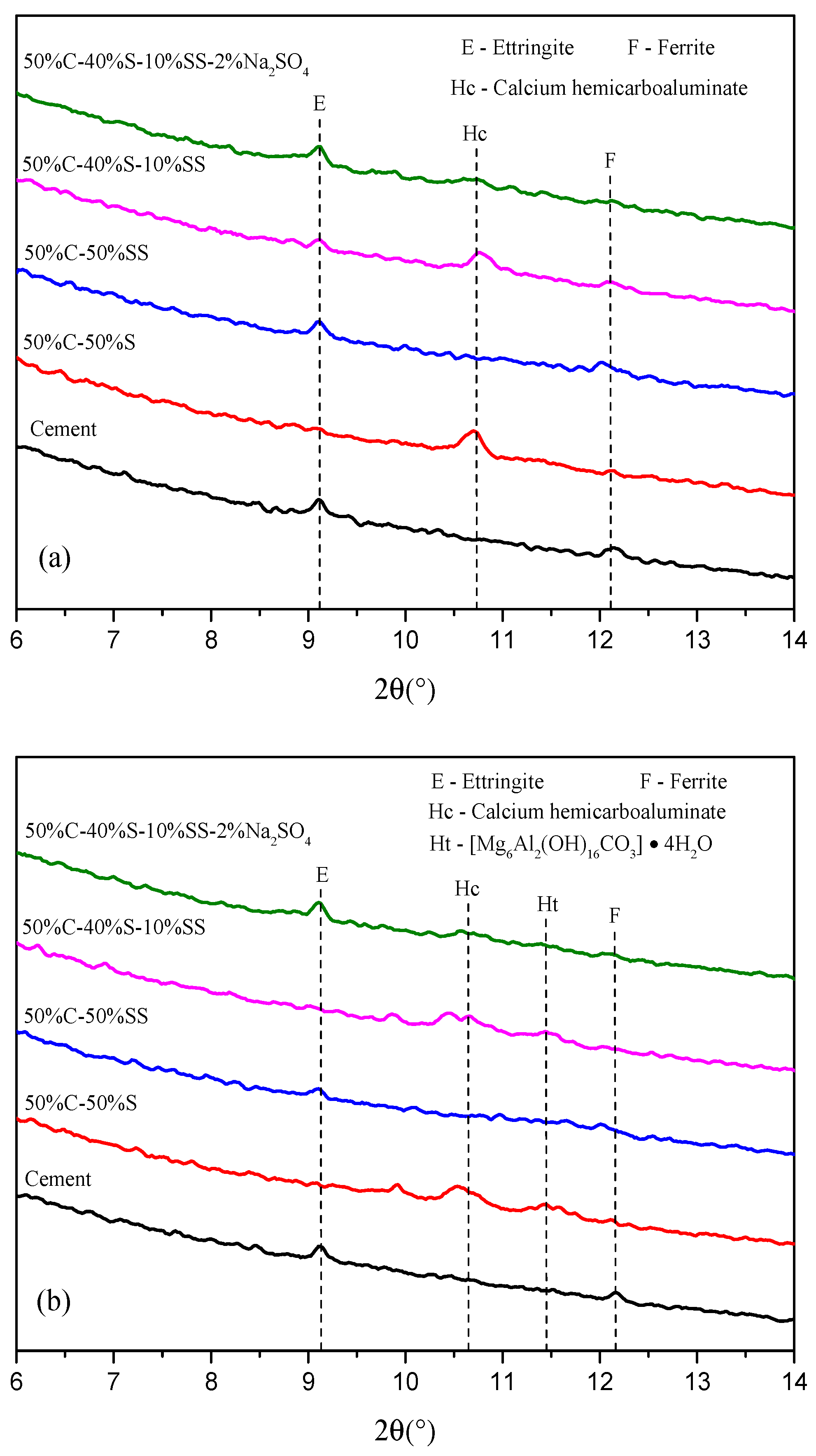
3.2. XRD Results of Hydration Products
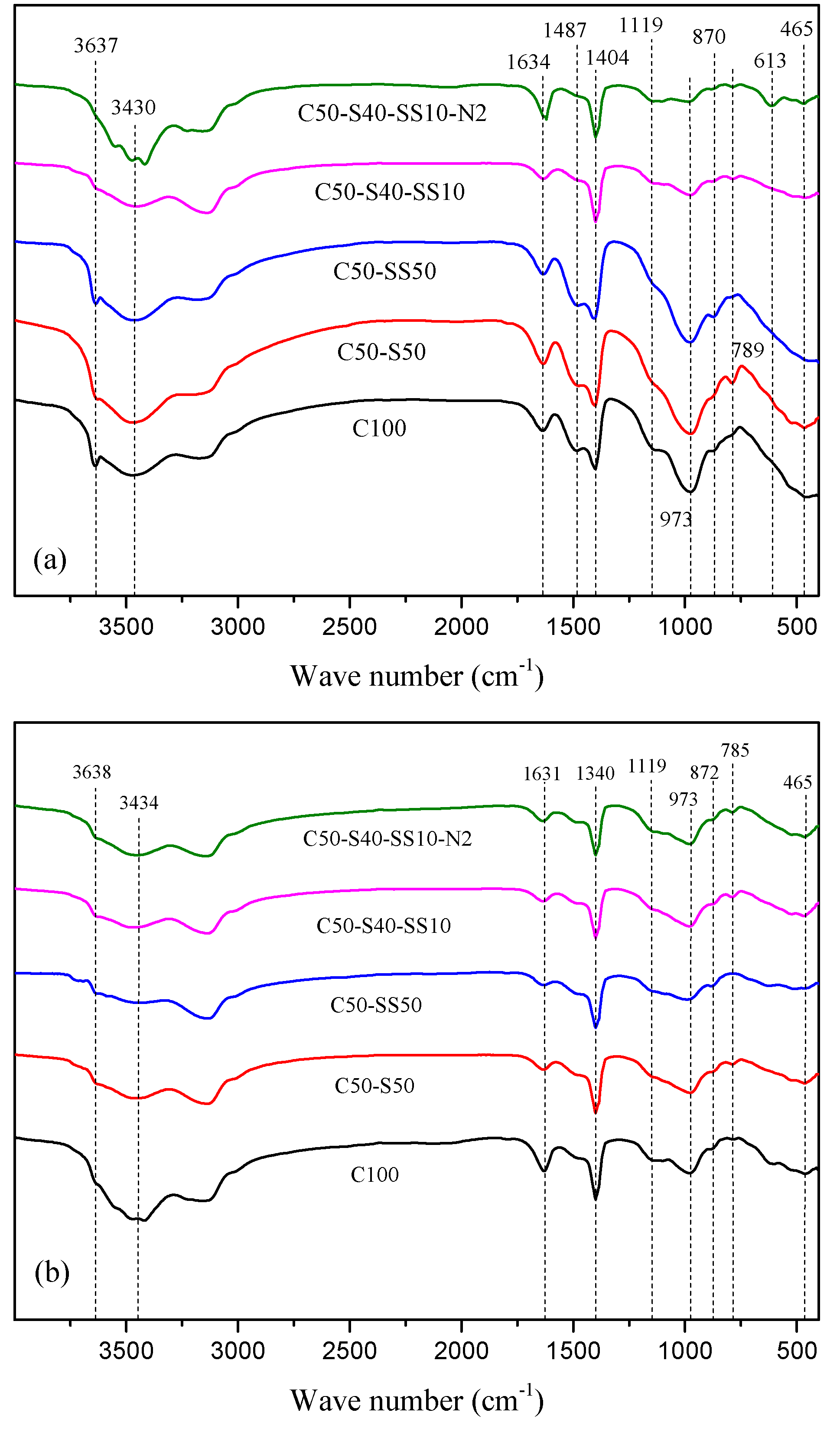
3.3. FT-IR Analysis of Hydration Products
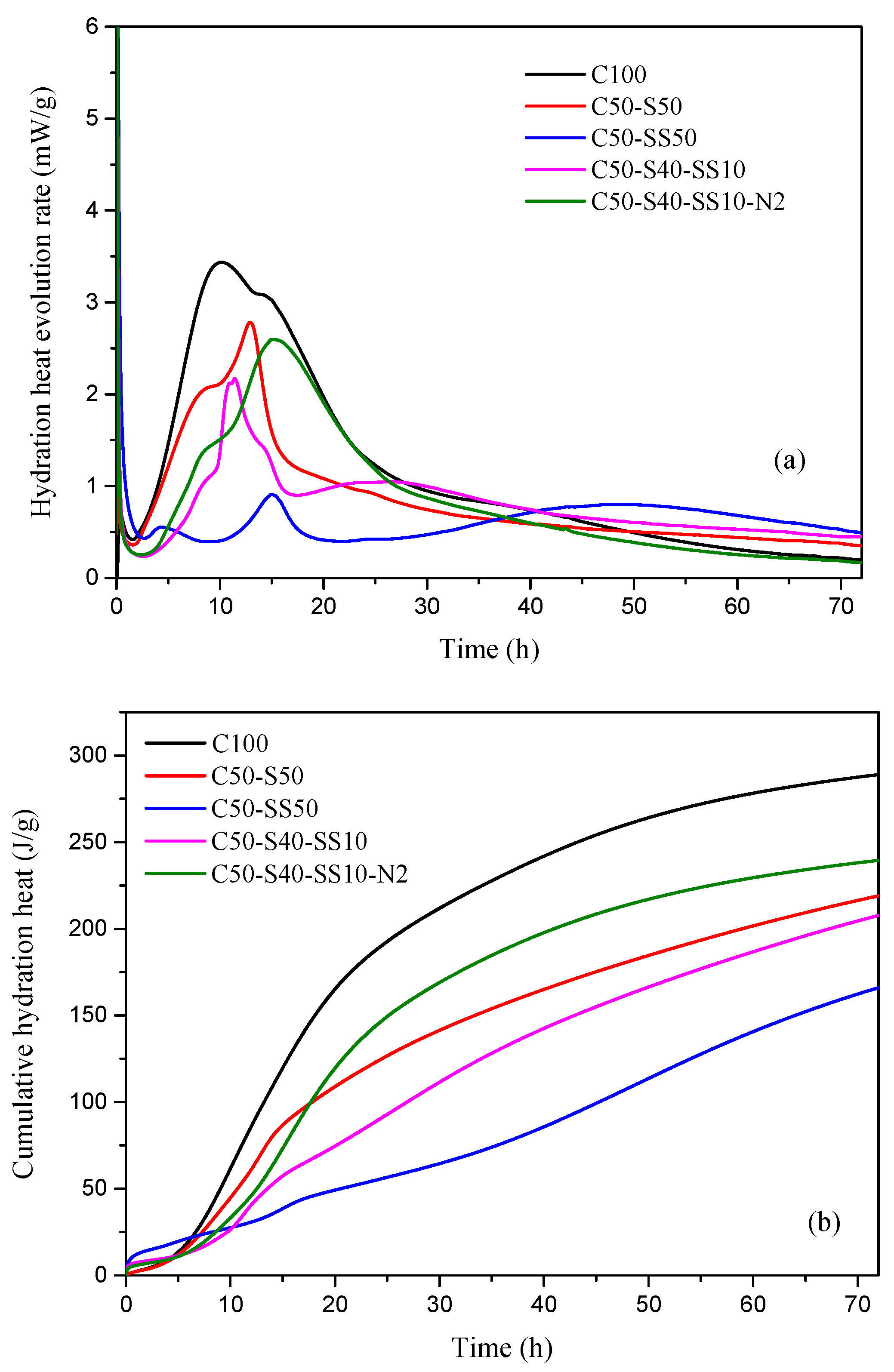
3.4. Hydration Heat Evolution
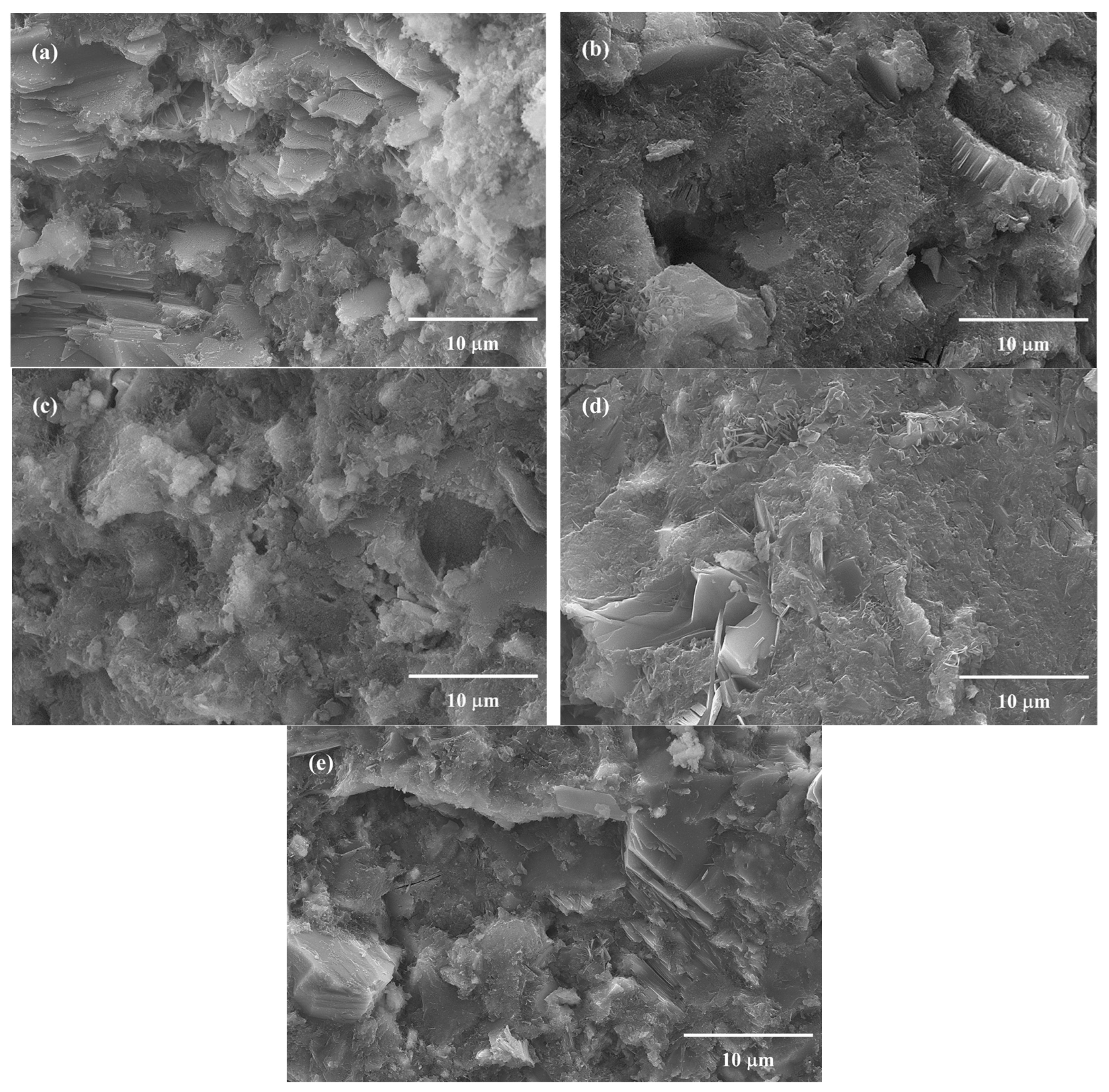
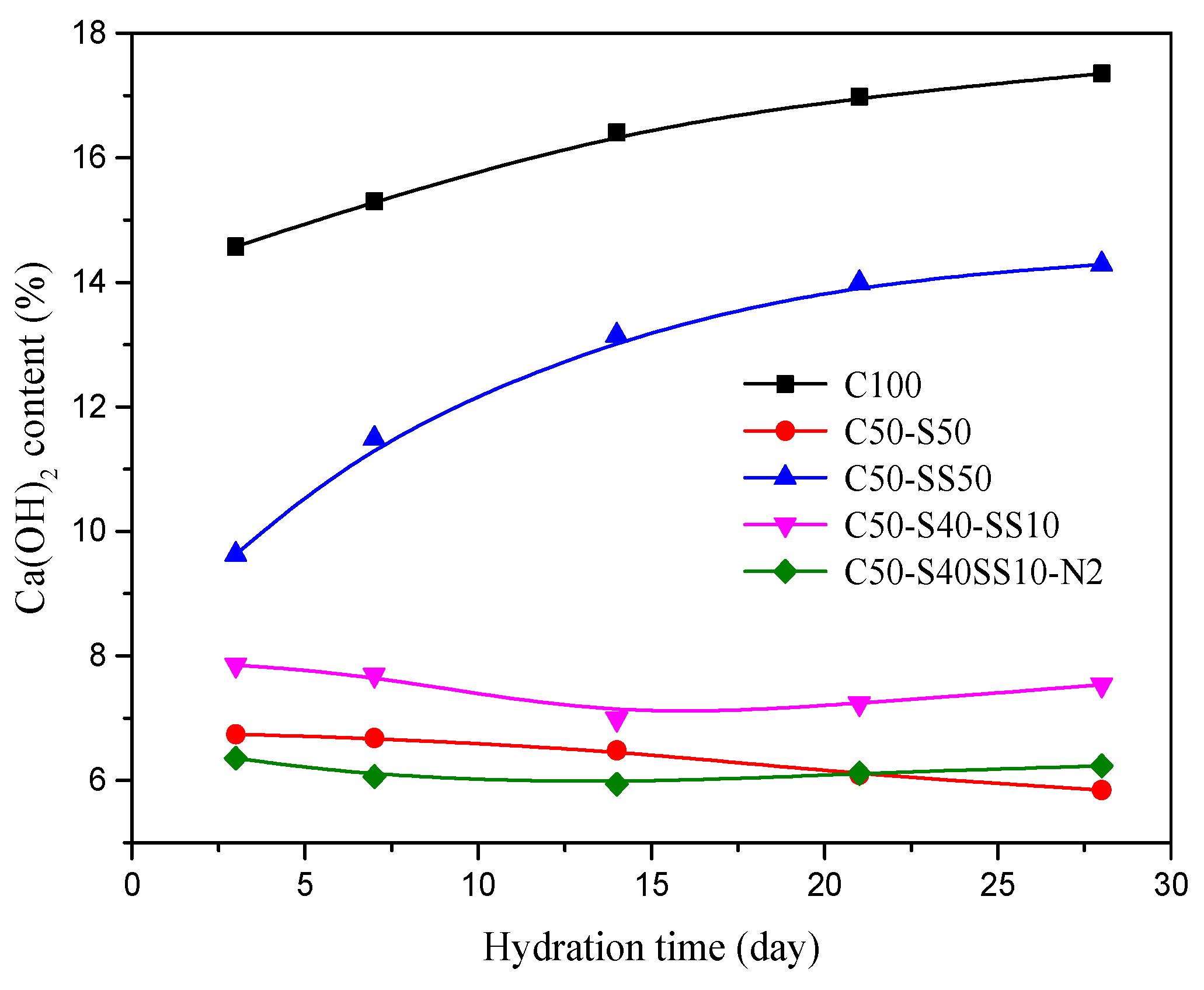
3.5. SEM and TG Analysis of Hydration Products
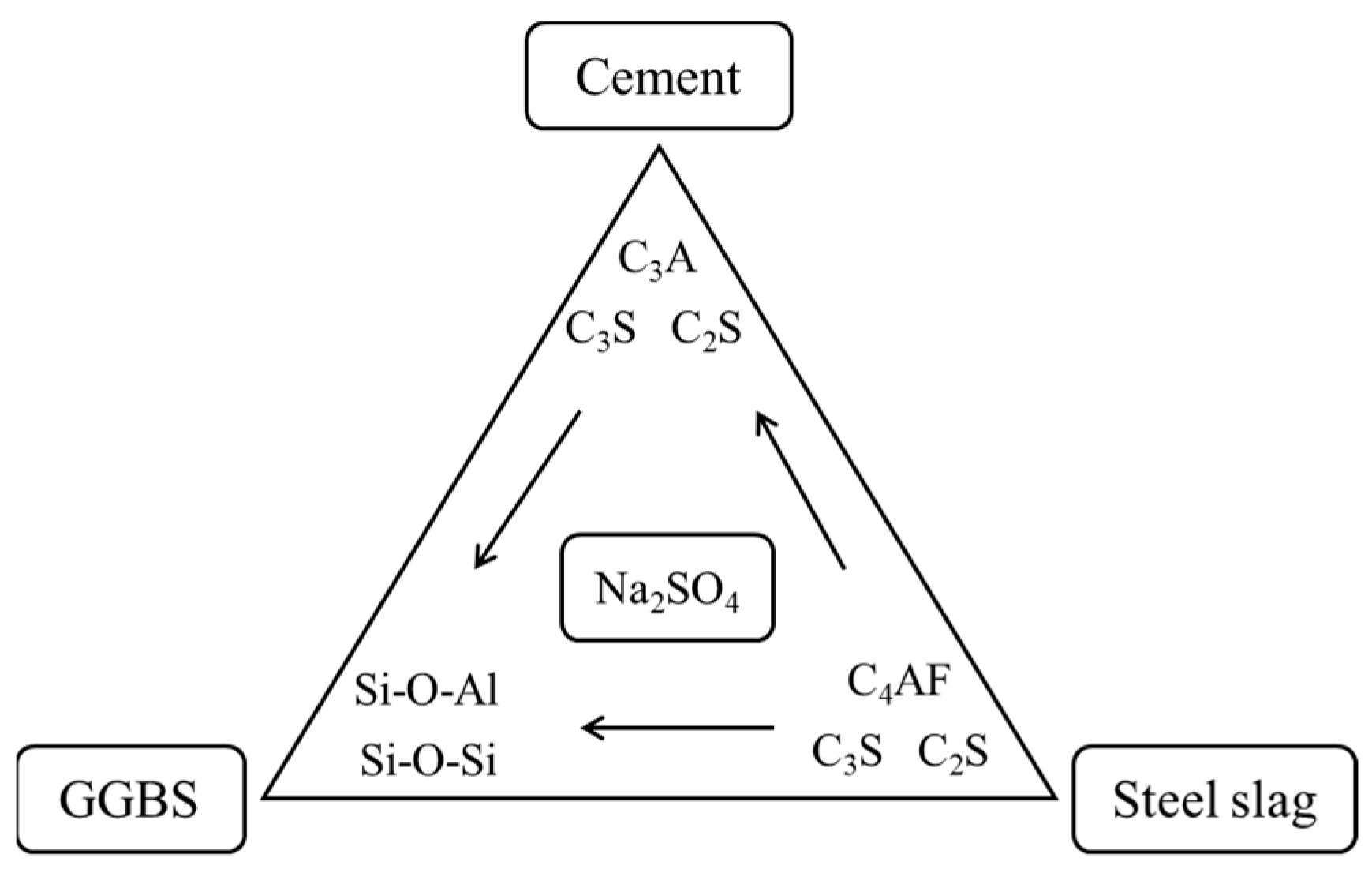
3.6. Hydration Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gong, K.; White, C.E. Impact of chemical variability of ground granulated blast-furnace slag on the phase formation in alkali-activated slag pastes. Cem. Concr. Res. 2016, 89, 310–319. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Kovtun, M.; Kearsley, E.P.; Shekhovtsova, J. Chemical acceleration of a neutral granulated blast-furnace slag activated by sodium carbonate. Cem. Concr. Res. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Ogirigbo, O.R.; Black, L. Influence of slag composition and temperature on the hydration and microstructure of slag blended cements. Constr. Build. Mater. 2016, 126, 496–507. [Google Scholar] [CrossRef]
- Çetin, C.; Erdoğan, S.T.; Tokyay, M. Effect of particle size and slag content on the early hydration of interground blended cements. Cem. Concr. Compos. 2016, 67, 39–49. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Zuo, Z.; Yang, F.; Duan, W.; Qin, Q. Blast furnace slag obtained from dry granulation method as a component in slag cement. Constr. Build. Mater. 2017, 131, 381–387. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía de Gutierrez, R. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Day, R.L.; Shi, C. Early strength development and hydration of alkali-activated blast furnace slag/fly ash blends. Adv. Cem. Res. 1999, 11, 189–196. [Google Scholar]
- Gesoğlu, M.; Güneyisi, E.; Özbay, E. Properties of self-compacting concretes made with binary, ternary, and quaternary cementitious blends of fly ash, blast furnace slag, and silica fume. Constr. Build. Mater. 2009, 23, 1847–1854. [Google Scholar] [CrossRef]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Hydration development of mineral additives blended cement using thermogravimetric analysis (TGA): Methodology of calculating the degree of hydration. Constr. Build. Mater. 2017, 146, 687–701. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Adu-Amankwah, S.; Zajac, M.; Stabler, C.; Lothenbach, B.; Black, L. Influence of limestone on the hydration of ternary slag cements. Cem. Concr. Res. 2017, 100, 96–109. [Google Scholar] [CrossRef]
- Shen, W.; Zhou, M.; Ma, W.; Hu, J.; Cai, Z. Investigation on the application of steel slag-fly ash-phosphogypsum solidified material as road base material. J. Hazard. Mater. 2009, 164, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, Q.; Wei, J.; Li, J.; Zhang, P. Preparation of high performance blended cements and reclamation of iron concentrate from basic oxygen furnace steel slag. Resour. Conserv. Recycl. 2011, 56, 48–55. [Google Scholar] [CrossRef]
- Kourounis, S.; Tsivilis, S.; Tsakiridis, P.E.; Papadimitriou, G.D.; Tsibouki, Z. Properties and hydration of blended cements with steelmaking slag. Cem. Concr. Res. 2007, 37, 815–822. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Wei, J.; Zhang, T. Structural characteristics and hydration kinetics of modified steel slag. Cem. Concr. Res. 2011, 41, 324–329. [Google Scholar] [CrossRef]
- Qiang, W.; Mengxiao, S.; Jun, Y. Influence of classified steel slag with particle sizes smaller than 20μm on the properties of cement and concrete. Constr. Build. Mater. 2016, 123, 601–610. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Agrela, F. Effect of stainless steel slag waste as a replacement for cement in mortars. Mechanical and statistical study. Constr. Build. Mater. 2017, 142, 444–458. [Google Scholar] [CrossRef]
- Yılmaz, I.A.A.I.s. Study on steel furnace slags with high MgO as additive in Portland cement. Cem. Concr. Res. 2002, 32, 1247–1249. [Google Scholar]
- Liu, Q.; Liu, J.; Qi, L. Effects of temperature and carbonation curing on the mechanical properties of steel slag-cement binding materials. Constr. Build. Mater. 2016, 124, 999–1006. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Li, X. Long-term properties of concrete containing ground granulated blast furnace slag and steel slag. Mag. Concr. Res. 2014, 66, 1095–1103. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Mi, G. Effect of blended steel slag–GBFS mineral admixture on hydration and strength of cement. Constr. Build. Mater. 2012, 35, 8–14. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Yang, J. Comparison of hydration properties between cement-GGBS-fly ash blended binder and cement-GGBS-steel slag blended binder. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2014, 29, 273–277. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Z.; Wang, D.; Yan, P. Hydration heat evolution and kinetics of blended cement containing steel slag at different temperatures. Thermochim. Acta 2015, 605, 43–51. [Google Scholar] [CrossRef]
- Shi, C.J.; Day, R.L. Some factors affecting early hydration of alkali-slag cements. Cem. Concr. Res. 1996, 26, 439–447. [Google Scholar] [CrossRef]
- Kozhukhova, N.I.; Alfimova, N.I.; Kozhukhova, M.I.; Nikulin, I.S.; Glazkov, R.A.; Kolomytceva, A.I.J.R. The Effect of Recycled Citrogypsum as a Supplementary Mineral Additive on the Physical and Mechanical Performance of Granulated Blast Furnace Slag-Based Alkali-Activated Binders. Recycling 2023, 8, 22. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Early age strength and workability of slag pastes activated by NaOH and Na2Co3. Cem. Concr. Res. 1998, 28, 655–664. [Google Scholar] [CrossRef]
- Kozhukhova, N.; Shurakov, I.; Alfimova, N.; Zhernovskaya, I.; Kozhukhova, M. Using of Citrogypsum in Alkali Activated Systems. In International Scientific Conference on Innovations and Technologies in Construction; Springer: Cham, Switzerland, 2023; pp. 17–22. [Google Scholar]
- Adesina, A.; Kaze, C.R. Physico-mechanical and microstructural properties of sodium sulfate activated materials: A review. Constr. Build. Mater. 2021, 295, 123668. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C.J. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- GB 8076-2008; Concrete Admixtures. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- ASTM C150-12; Standard Specification for Portland Cement. American Society for Testing Materials: Philadelphia, PA, USA, 2012.
- Scrivener, K.; Capmas, A. Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier Butterworth-Heinmann: Oxford, UK, 2004. [Google Scholar]
- DBJ/T 13-196-2014; Specification for Mix Proportion Design and Test of Cement Paste. Fujian Provincial Institute of Building Research, Cscec Straits Construction Development Co., Ltd.: Fuzhou, China, 2014.
- GB/T 17671-1999; Method of Testing Cements—Determination of Strength. National Cement Standardization Technical Committee, China Building Materials Federation. China Building Materials Academy Co., Ltd.: Beijing, China, 1999.
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Douglas, E.; Brandstetr, J. A preliminary study on the alkali activation of ground granulated blast-furnace slag. Cem. Concr. Res. 1990, 20, 746–756. [Google Scholar] [CrossRef]
- Fraay, A.; Bijen, J.M.; Vogelaar, P. Cement-stabilized fly ash base courses. Cem. Concr. Compos. 1990, 12, 279–291. [Google Scholar] [CrossRef]
- Douglas, E.; Bilodeau, A.; Brandstetr, J.; Malhotra, V.M. Alkali activated ground granulated blast-furnace slag concrete: Preliminary investigation. Cem. Concr. Res. 1991, 21, 101–108. [Google Scholar] [CrossRef]
- Wilson, W.; Rivera-Torres, J.M.; Sorelli, L.; Durán-Herrera, A.; Tagnit-Hamou, A. The micromechanical signature of high-volume natural pozzolan concrete by combined statistical nanoindentation and SEM-EDS analyses. Cem. Concr. Res. 2017, 91, 1–12. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Blanco, M.T.; Palomo, A. FTIR study of the sol–gel synthesis of cementitious gels: C–S–H and N–A–S–H. J. Sol-Gel Sci. Technol. 2007, 45, 63–72. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.J.; Provis, J.L. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Nasrazadani, S.; Springfield, T. Application of Fourier transform infrared spectroscopy in cement Alkali quantification. Mater. Struct. 2013, 47, 1607–1615. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Lee, H.-S. Modeling the hydration of concrete incorporating fly ash or slag. Cem. Concr. Res. 2010, 40, 984–996. [Google Scholar] [CrossRef]
- Langan, B.W.; Weng, K.; Ward, M.A. Effect of silica fume and fly ash on heat of hydration of Portland cement. Cem. Concr. Res. 2002, 32, 1045–1051. [Google Scholar] [CrossRef]




| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | MnO | P2O5 | TiO2 | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 18.7 | 4.22 | 3.33 | 66.33 | 1.97 | 0.854 | —— | 0.11 | 0.27 | 2.77 |
| GGBS | 35.8 | 14.4 | 0.69 | 34.9 | 6.57 | 1.01 | 0.24 | —— | 0.66 | 1.86 |
| Steel slag | 10.78 | 2.32 | 30.22 | 43.6 | 6.55 | 0.024 | 2.01 | 1.76 | 0.5 | 0.25 |
| Cement (%) | Slag (%) | Steel Slag (%) | |
|---|---|---|---|
| C100 | 100 | 0 | 0 |
| C50-S50 | 50 | 50 | 0 |
| C50-S45-SS5 | 50 | 45 | 5 |
| C50-S40-SS10 | 50 | 40 | 10 |
| C50-S35-SS15 | 50 | 35 | 15 |
| C50-S30-SS20 | 50 | 30 | 20 |
| C50-S25-SS25 | 50 | 25 | 25 |
| C50-S20-SS30 | 50 | 20 | 30 |
| C50-S15-SS35 | 50 | 15 | 35 |
| C50-S10-SS40 | 50 | 10 | 40 |
| C50-S5-SS45 | 50 | 5 | 45 |
| C50-SS50 | 50 | 0 | 50 |
| Cement (%) | GGBS (%) | Steel Slag (%) | Na2SO4 (%) | |
|---|---|---|---|---|
| C100 | 100 | 0 | 0 | 0 |
| C50-S40-SS10 | 50 | 40 | 10 | 0 |
| C50-S40-SS10-N0.5 | 50 | 40 | 10 | 0.5 |
| C50-S40-SS10-N1 | 50 | 40 | 10 | 1 |
| C50-S40-SS10-N1.5 | 50 | 40 | 10 | 1.5 |
| C50-S40-SS10-N2 | 50 | 40 | 10 | 2 |
| C50-S40-SS10-N2.5 | 50 | 40 | 10 | 2.5 |
| C50-S40-SS10-N3 | 50 | 40 | 10 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Cui, H.; Cui, L.; Yang, S.; Zhang, C.; Liu, W.; Zheng, D. Mutual Activation Mechanism of Cement–GGBS–Steel Slag Ternary System Excited by Sodium Sulfate. Buildings 2024, 14, 631. https://doi.org/10.3390/buildings14030631
Zhu J, Cui H, Cui L, Yang S, Zhang C, Liu W, Zheng D. Mutual Activation Mechanism of Cement–GGBS–Steel Slag Ternary System Excited by Sodium Sulfate. Buildings. 2024; 14(3):631. https://doi.org/10.3390/buildings14030631
Chicago/Turabian StyleZhu, Jiuwen, Hongzhi Cui, Lingzhi Cui, Shuqing Yang, Chaohui Zhang, Wei Liu, and Dapeng Zheng. 2024. "Mutual Activation Mechanism of Cement–GGBS–Steel Slag Ternary System Excited by Sodium Sulfate" Buildings 14, no. 3: 631. https://doi.org/10.3390/buildings14030631




