Experimental Investigation on the Influence of Strength Grade on the Surface Fractal Dimension of Concrete under Sulfuric Acid Attack
Abstract
:1. Introduction
2. Experimental Design and Evaluation Methods
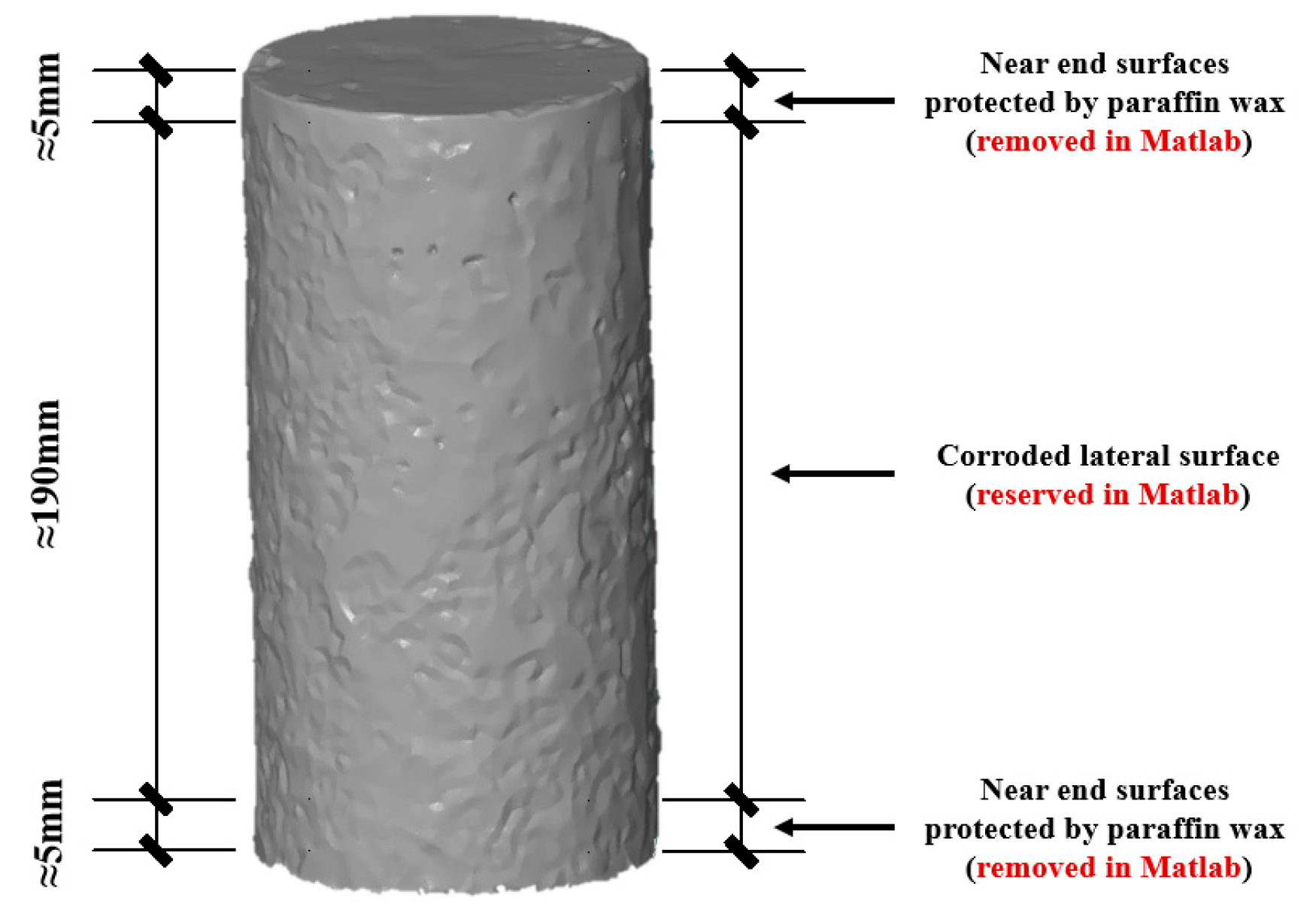
2.1. Experimental Design
2.2. Test Methodologies
2.2.1. The Calculation Method of Mass Loss
2.2.2. The Calculation Method of Surface Fractal Dimensions
3. Experimental Results and Analysis
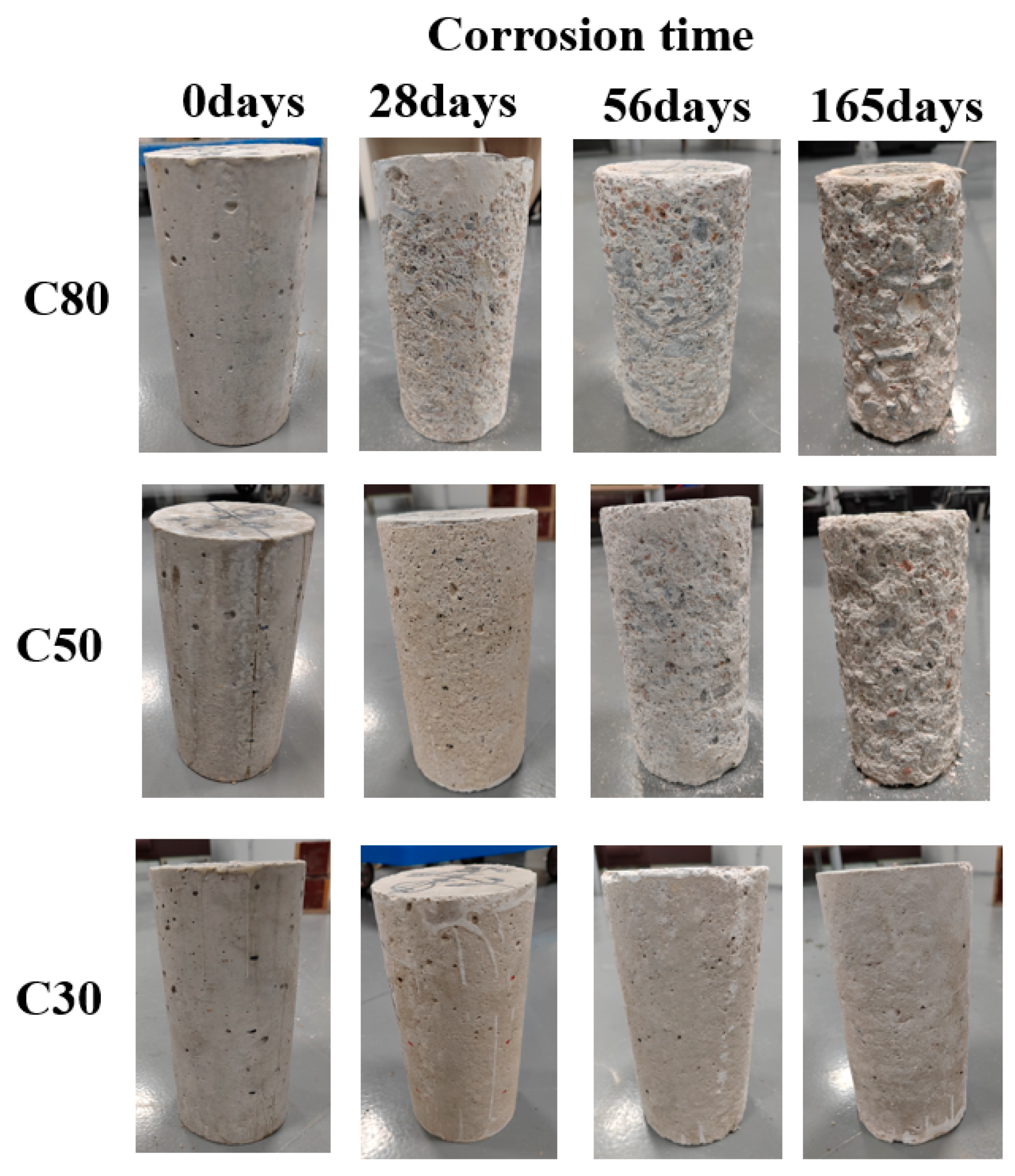
3.1. Visual Appearance
3.2. Mass Loss
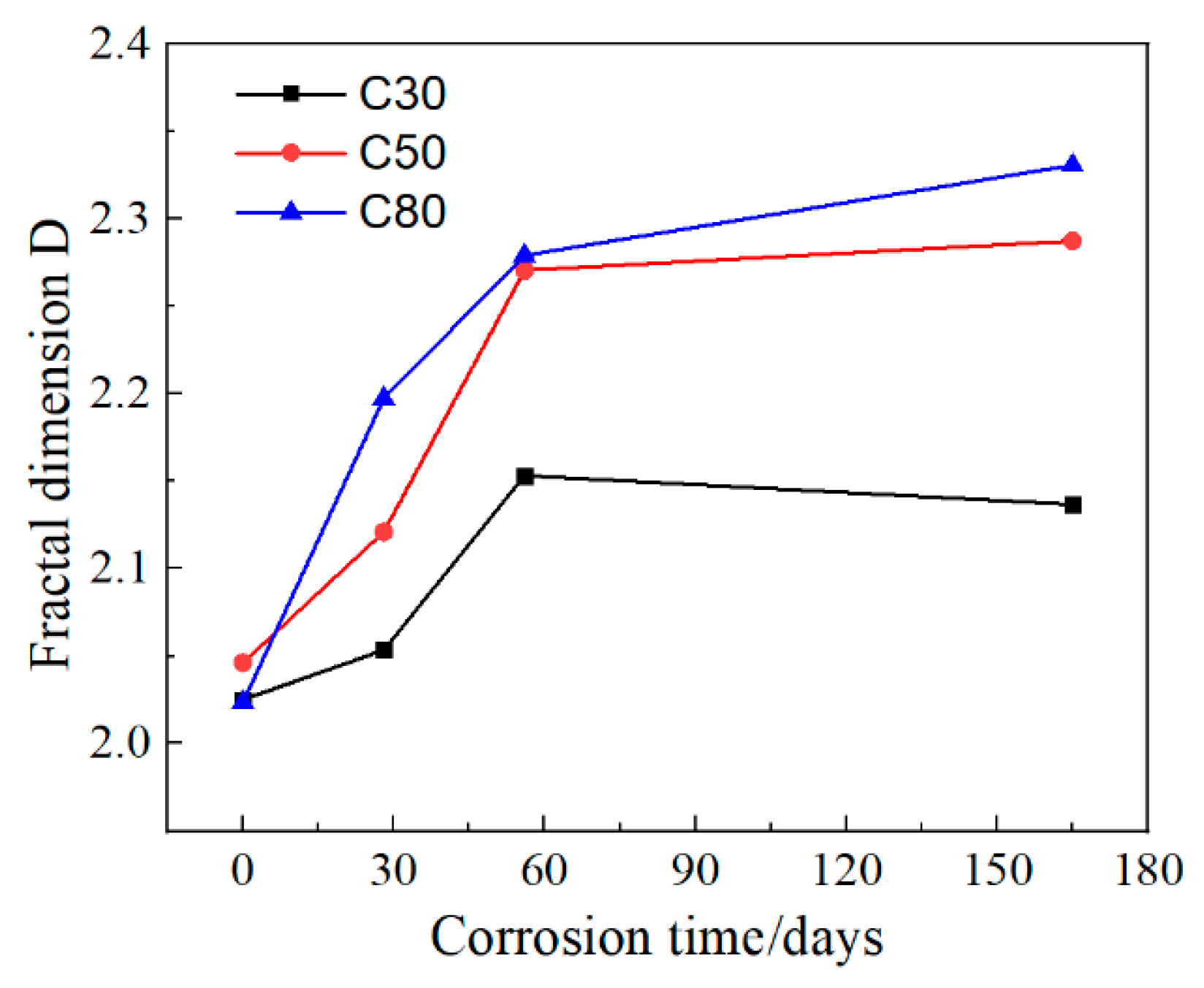
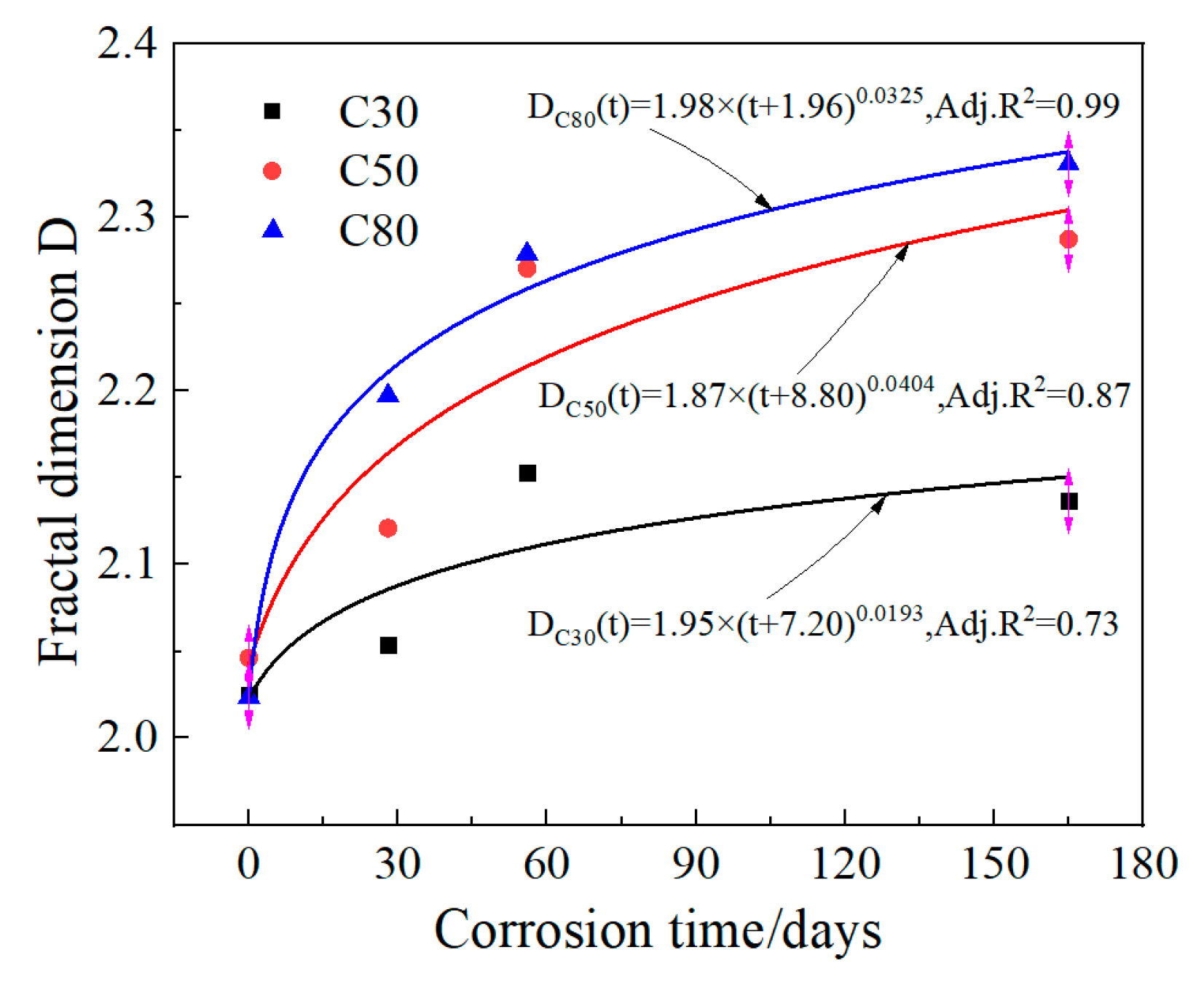
3.3. Fractal Dimension
4. Conclusions
- (1)
- Although previous studies have focused on the performance of concrete exposed to sulfuric acid corrosion under different water–cement ratios, usually using indicators such as corrosion depth, quality loss, and compressive strength to evaluate the resistance of concrete to sulfuric acid corrosion, the 3D fractal dimension in this paper provides a new method for characterizing the surface roughness of concrete specimens subjected to sulfuric acid corrosion through laser scanning technology.
- (2)
- From the perspective of appearance, in a sulfuric acid environment with pH ≈ 0.85, the degree of concrete corrosion deterioration in descending order is C80 > C50 > C30, indicating that the higher the strength grade of concrete, the greater the degree of concrete corrosion deterioration.
- (3)
- From the perspective of mass loss, in a sulfuric acid environment with pH ≈ 0.85, the order of concrete mass loss from high to low is C80 > C50 > C30, indicating that the higher the strength grade of concrete, the greater the quality loss of concrete.
- (4)
- The fractal dimension D of each strength shows an upward trend before corrosion for 56 days. Moreover, under the same pH solution, the higher the strength grade, the faster the fractal dimension D increases, indicating that the more surface products are generated, the more severe the corrosion is.
- (5)
- The widespread belief is that the higher the strength grade of concrete, the better its durability. However, the durability varies in sulfuric acid corrosive environments. Therefore, based on the above research, it is recommended that in extremely acidic environments (i.e., very low pH), more attention should be paid to high-strength grades of concrete. In our future work, we plan to further investigate the impact of commonly encountered low-concentration sulfuric acid in practical engineering on the performance degradation of concrete with different strength grades and analyze the mechanism of how the concentration of sulfuric acid affects the experimental results.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Z.; Wu, J.; Zhao, G.; Fang, S.; Ma, Y.; Jiang, H. Shear performance and design recommendations of single embedded nut bolted shear connectors in prefabricated steel–UHPC composite beams. Steel Compos. Struct. 2024, 50, 319–336. [Google Scholar]
- Feng, J.H.; Shao, X.D.; Qiu, M.H.; Li, H.H.; Gao, X.; Huang, Z.L. Reliability evaluation of flexural capacity design provision for UHPC beams reinforced with steel rebars/prestressing tendons. Eng. Struct. 2024, 300, 117160. [Google Scholar] [CrossRef]
- Jiang, H.B.; Hu, Z.B.; Cao, Z.P.; Gao, X.J.; Tian, Y.Q.; Sun, X.D. Experimental and numerical study on shear performance of externally prestressed precast UHPC segmental beams without stirrups br. Structures 2022, 46, 1134–1153. [Google Scholar] [CrossRef]
- Zhen, H.; Xiong, Z.; Song, Y.; Li, L.; Qiu, Y.; Zou, X.; Chen, B.; Chen, D.; Liu, F.; Ji, Y. Early mechanical performance of glass fibre-reinforced manufactured sand concrete. J. Build. Eng. 2024, 83, 108440. [Google Scholar] [CrossRef]
- Chen, M.; De Corte, W.; Jiang, H.; Taerwe, L. Experimental study on direct-shear behaviour of narrow joints in socket connections for precast pier-to-pile footing systems. Structures 2024, 61, 106006. [Google Scholar] [CrossRef]
- Prakash, R.; Raman, S.N.; Divyah, N.; Subramanian, C.; Vijayaprabha, C.; Praveenkumar, S. Fresh and mechanical characteristics of roselle fibre reinforced self-compacting concrete incorporating fly ash and metakaolin. Constr. Build. Mater. 2021, 290, 123209. [Google Scholar] [CrossRef]
- Jahan, A.; Mollazadeh, M.; Akbarpour, A.; Khatibinia, M. Health monitoring of pressurized pipelines by finite element method using meta-heuristic algorithms along with error sensitivity assessment. Struct. Eng. Mech. 2023, 87, 211–219. [Google Scholar] [CrossRef]
- Shao, W.; Li, Q.M.; Zhang, W.B.; Shi, D.D.; Li, H.H. Numerical modeling of chloride diffusion in cement-based materials considering calcium leaching and external sulfate attack. Constr. Build. Mater. 2023, 401, 132913. [Google Scholar] [CrossRef]
- Shao, W.; Qin, F.; Shi, D.; Soomro, M.A. Horizontal bearing characteristic and seismic fragility analysis of CFRP composite pipe piles subject to chloride corrosion. Comput. Geotech. 2024, 166, 105977. [Google Scholar] [CrossRef]
- Mehta, P.K. Durability of Concrete—Fifty Years of Progress? ACI Symp. Publ. 1991, 126, 1–32. [Google Scholar] [CrossRef]
- Prakash, R.; Thenmozhi, R.; Raman, S.N.; Subramanian, C.; Divyah, N. An investigation of key mechanical and durability properties of coconut shell concrete with partial replacement of fly ash. Struct. Concr. 2020, 22, E985–E996. [Google Scholar] [CrossRef]
- Yu, J.Q.; Qiao, H.X.; Zhu, F.F.; Wang, X.K. Research on Damage and Deterioration of Fiber Concrete under Acid Rain Environment Based on GM(1,1)-Markov. Materials 2021, 14, 6326. [Google Scholar] [CrossRef]
- Yin, Y.S.; Zhang, J.; Zhang, G.H. Effect of hygrothermal acid rain environment on the shear bonding performance of CFRP-concrete interface. Constr. Build. Mater. 2023, 364, 130002. [Google Scholar] [CrossRef]
- Xie, S.D.; Qi, L.; Zhou, D. Investigation of the effects of acid rain on the deterioration of cement concrete using accelerated tests established in laboratory. Atmos. Environ. 2004, 38, 4457–4466. [Google Scholar] [CrossRef]
- Xiao, J.; Qu, W.J.; Jiang, H.B.; Li, L.; Huang, J.; Chen, L. Fractal characterization and mechanical behavior of pile-soil interface subjected to sulfuric acid. Fractals 2021, 29, 2140010. [Google Scholar] [CrossRef]
- Xiao, J.; Qu, W.J.; Li, W.G.; Zhu, P. Investigation on effect of aggregate on three non-destructive testing properties of concrete subjected to sulfuric acid attack. Constr. Build. Mater. 2016, 115, 486–495. [Google Scholar] [CrossRef]
- Xiao, J.; Long, X.; Qu, W.J.; Li, L.; Jiang, H.B.; Zhong, Z.C. Influence of sulfuric acid corrosion on concrete stress-strain relationship under uniaxial compression. Measurement 2022, 187, 110318. [Google Scholar] [CrossRef]
- Monteny, J.; De Belie, N.; Vincke, E.; Verstraete, W.; Taerwe, L. Chemical and microbiological tests to simulate sulfuric acid corrosion of polymer-modified concrete. Cem. Concr. Res. 2001, 31, 1359–1365. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; Bassuoni, M.T. Response of Concrete to Incremental Aggression of Sulfuric Acid. J. Test. Eval. 2020, 48, 3220–3238. [Google Scholar] [CrossRef]
- Girardi, F.; Di Maggio, R. Resistance of concrete mixtures to cyclic sulfuric acid exposure and mixed sulfates: Effect of the type of aggregate. Cem. Concr. Compos. 2011, 33, 276–285. [Google Scholar] [CrossRef]
- Matalkah, F.; Salem, T.; Soroushian, P. Acid resistance and corrosion protection potential of concrete prepared with alkali aluminosilicate cement. J. Build. Eng. 2018, 20, 705–711. [Google Scholar] [CrossRef]
- Nnadi, E.O.; Lizarazo-Marriaga, J. Acid Corrosion of Plain and Reinforced Concrete Sewage Systems. J. Mater. Civ. Eng. 2013, 25, 1353–1356. [Google Scholar] [CrossRef]
- Pavlík, V. Corrosion of hardened cement paste by acetic and nitric acids part I: Calculation of corrosion depth. Cem. Concr. Res. 1994, 24, 551–562. [Google Scholar] [CrossRef]
- Bertron, A.; Duchesne, J.; Escadeillas, G. Accelerated tests of hardened cement pastes alteration by organic acids: Analysis of the pH effect. Cem. Concr. Res. 2005, 35, 155–166. [Google Scholar] [CrossRef]
- Bassuoni, M.T.; Nehdi, M.L. Resistance of self-consolidating concrete to sulfuric acid attack with consecutive pH reduction. Cem. Concr. Res. 2007, 37, 1070–1084. [Google Scholar] [CrossRef]
- Hasan, M.S.; Setunge, S.; Law, D.W.; Molyneaux, T.C.K. Predicting life expectancy of concrete septic tanks exposed to sulfuric acid attack. Mag. Concr. Res. 2013, 65, 793–801. [Google Scholar] [CrossRef]
- Cao, R.Z.; Yang, J.F.; Li, G.X.; Zhou, Q.; Niu, M.D. Durability performance of nano-SiO2 modified OPC-SAC composites subjected to sulfuric acid attack. Constr. Build. Mater. 2023, 371, 130802. [Google Scholar] [CrossRef]
- Khan, M.N.N.; Elahi, M.M.A.; Kuri, J.C.; Sarker, P.K.; Shaikh, F.U.A. Acid resistance of alkali-activated composites containing waste glass as fine aggregate. Adv. Cem. Res. 2022, 35, 248–257. [Google Scholar] [CrossRef]
- Shen, Y.J.; Wang, Y.Z.; Yang, Y.; Sun, Q.; Luo, T.; Zhang, H. Influence of surface roughness and hydrophilicity on bonding strength of concrete-rock interface. Constr. Build. Mater. 2019, 213, 156–166. [Google Scholar] [CrossRef]
- Chen, L.; Yan, J.; Wu, Z.G.; Yang, D.H.; Li, J.L.; Xiang, N.L. Experimental and numerical study on shear behavior of shear pockets between ultra-high-performance and normal concrete for precast girder bridges. Structures 2023, 55, 1645–1658. [Google Scholar] [CrossRef]
- Mohamad, M.E.; Ibrahim, I.S.; Abdullah, R.; Abd Rahman, A.B.; Kueh, A.B.H.; Usman, J. Friction and cohesion coefficients of composite concrete-to-concrete bond. Cem. Concr. Compos. 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Dai, M.L.; Wang, X.R.; Cheng, C.; Chen, Z.L.; Deng, J.Y. Efficient Evaluation of Concrete Fracture Surface Roughness Using Fringe Projection Technology. Materials 2023, 16, 4430. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhou, Z.G.; Chen, G.H. Effects of different types of acid rain on water stability of asphalt pavement. Constr. Build. Mater. 2022, 322, 126308. [Google Scholar] [CrossRef]
- Millman, L.R.; Giancaspro, J.W. Three-Dimensional Optical Profilometry Analysis of the International Concrete Repair Institute Concrete Surface Profiles (CSPs). ACI Mater. J. 2013, 110, 519–527. [Google Scholar]
- Sengoz, B.; Topal, A.; Tanyel, S. Comparison of pavement surface texture determination by sand patch test and 3D laser scanning. Period. Polytech.-Civ. Eng. 2012, 56, 73–78. [Google Scholar] [CrossRef]
- Courard, L.; Nelis, M. Surface analysis of mineral substrates for repair works: Roughness evaluation by profilometry and surfometry analysis. Mag. Concr. Res. 2003, 55, 355–366. [Google Scholar] [CrossRef]
- Liu, X.G.; Zhang, W.P.; Gu, X.L.; Ye, Z.W. Assessment of Fatigue Life for Corroded Prestressed Concrete Beams Subjected to High-Cycle Fatigue Loading. J. Struct. Eng. 2023, 149, 04022242. [Google Scholar] [CrossRef]
- Zhang, W.P.; Zhou, B.B.; Gu, X.L.; Dai, H.C. Probability Distribution Model for Cross-Sectional Area of Corroded Reinforcing Steel Bars. J. Mater. Civ. Eng. 2014, 26, 822–832. [Google Scholar] [CrossRef]
- Xue, D.J.; Liu, Y.T.; Zhou, H.W.; Wang, J.Q.; Liu, J.F.; Zhou, J. Fractal Characterization on Anisotropy and Fractal Reconstruction of Rough Surface of Granite Under Orthogonal Shear. Rock Mech. Rock Eng. 2020, 53, 1225–1242. [Google Scholar] [CrossRef]
- Issa, M.A.; Issa, M.A.; Islam, M.S.; Chudnovsky, A. Fractal dimension—A measure of fracture roughness and toughness of concrete. Eng. Fract. Mech. 2003, 70, 125–137. [Google Scholar] [CrossRef]
- Zhou, H.W.; Xie, H. Direct estimation of the fractal dimensions of a fracture surface of rock. Surf. Rev. Lett. 2003, 10, 751–762. [Google Scholar] [CrossRef]
- Zhou, H.W.; Xue, D.J.; Jiang, D.Y. On fractal dimension of a fracture surface by volume covering method. Surf. Rev. Lett. 2014, 21, 14500152. [Google Scholar] [CrossRef]
- Xiao, J.; Long, X.; Li, L.; Jiang, H.; Zhang, Y.; Qu, W. Study on the Influence of Three Factors on Mass Loss and Surface Fractal Dimension of Concrete in Sulfuric Acid Environments. Fractal Fract. 2021, 5, 146. [Google Scholar] [CrossRef]
- Xiao, J.; Xu, Z.; Murong, Y.; Wang, L.; Lei, B.; Chu, L.; Jiang, H.; Qu, W. Effect of Chemical Composition of Fine Aggregate on the Frictional Behavior of Concrete–Soil Interface under Sulfuric Acid Environment. Fractal Fract. 2022, 6, 22. [Google Scholar]
- Xiao, J.; Qu, W.; Jiang, H.; Dong, W. Three-Dimensional Fractal Characterization of Concrete Surface Subjected to Sulfuric Acid Attacks. J. Nondestruct. Eval. 2020, 39, 57. [Google Scholar] [CrossRef]
- Fattuhi, N.I.; Hughes, B.P. The performance of cement paste and concrete subjected to sulphuric acid attack. Cem. Concr. Res. 1988, 18, 545–553. [Google Scholar] [CrossRef]
- Pavlík, V. Corrosion of hardened cement paste by acetic and nitric acids Part III: Influence of water/cement ratio. Cem. Concr. Res. 1996, 26, 475–490. [Google Scholar] [CrossRef]
- Kawai, K.; Yamaji, S.; Shinmi, T. Concrete Deterioration Caused by Sulfuric Acid Attack. In Proceedings of the 10th DBMC International Conference on Durability of Building Materials and Component, Lyon, France, 17–20 April 2005. [Google Scholar]
- Hewayde, E.; Nehdi, M.; Allouche, E.; Nakha, G. Effect of mixture design parameters and wetting-drying cycles on resistance of concrete to sulfuric acid attack. J. Mater. Civ. Eng. 2007, 19, 155–163. [Google Scholar] [CrossRef]
- House, M.; Cheng, L.Q.; Banks, K.; Weiss, J. Concrete Resistance to Sulfuric Acid Immersion: The Influence of Testing Details and Mixture Design on Performance as It Relates to Microbially Induced Corrosion. Adv. Civ. Eng. Mater. 2019, 8, 544–557. [Google Scholar] [CrossRef]
- Witkowska-Dobrev, J.; Szlachetka, O.; Francke, B.; Chylinski, F.; Malek, M.; Sadzevieius, R.; Ramukevicius, D.; Frak, M.; Dzieciol, J.; Kruszewski, M.; et al. Effect of different water-cement ratios on the durability of prefabricated concrete tanks exposed to acetic acid aggression. J. Build. Eng. 2023, 78, 107712. [Google Scholar] [CrossRef]
- Capraro, A.P.B.; Cheremeta, M.A.; Gonçalves, M.P.G.; Cremonez, C.; de Medeiros, M.H.F. Influence of the cement type and water/cement ratio in concretes exposed in sewage treatment plants. Constr. Build. Mater. 2019, 229, 116842. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature; W.H. Freeman and Company: New York, NY, USA, 1982. [Google Scholar]
- Ai, T.; Zhang, R.; Zhou, H.W.; Pei, J.L. Box-counting methods to directly estimate the fractal dimension of a rock surface. Appl. Surf. Sci. 2014, 314, 610–621. [Google Scholar] [CrossRef]
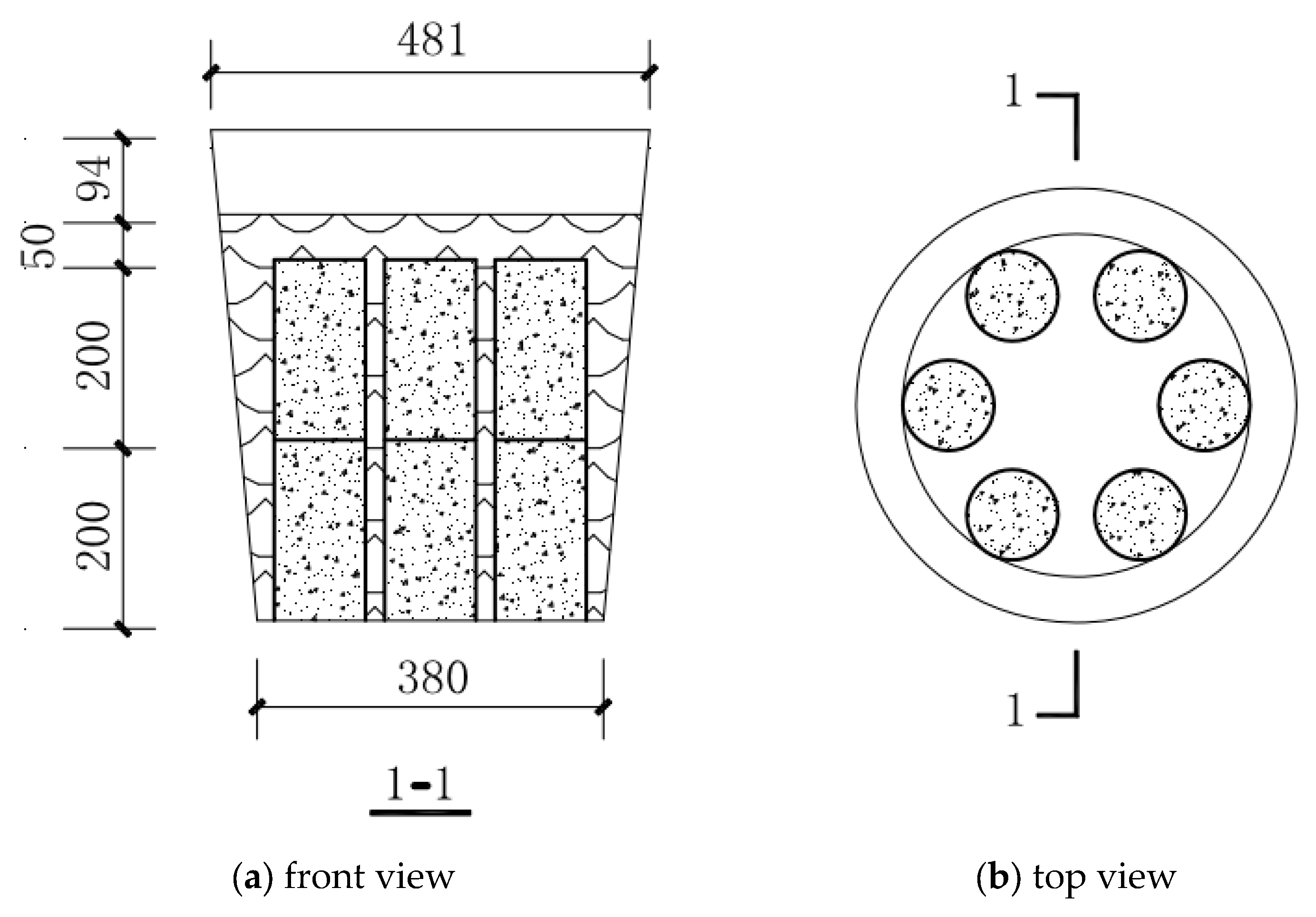
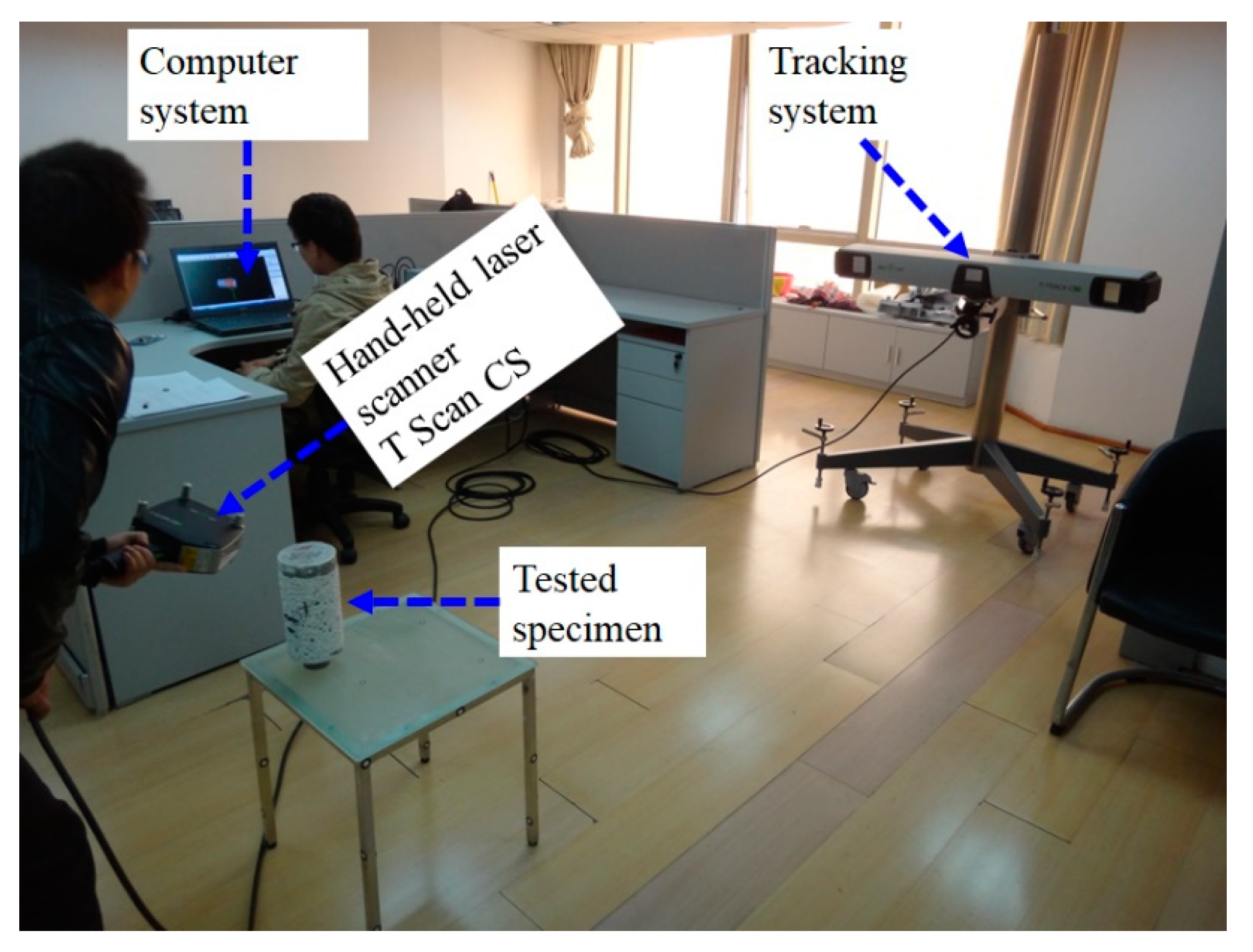
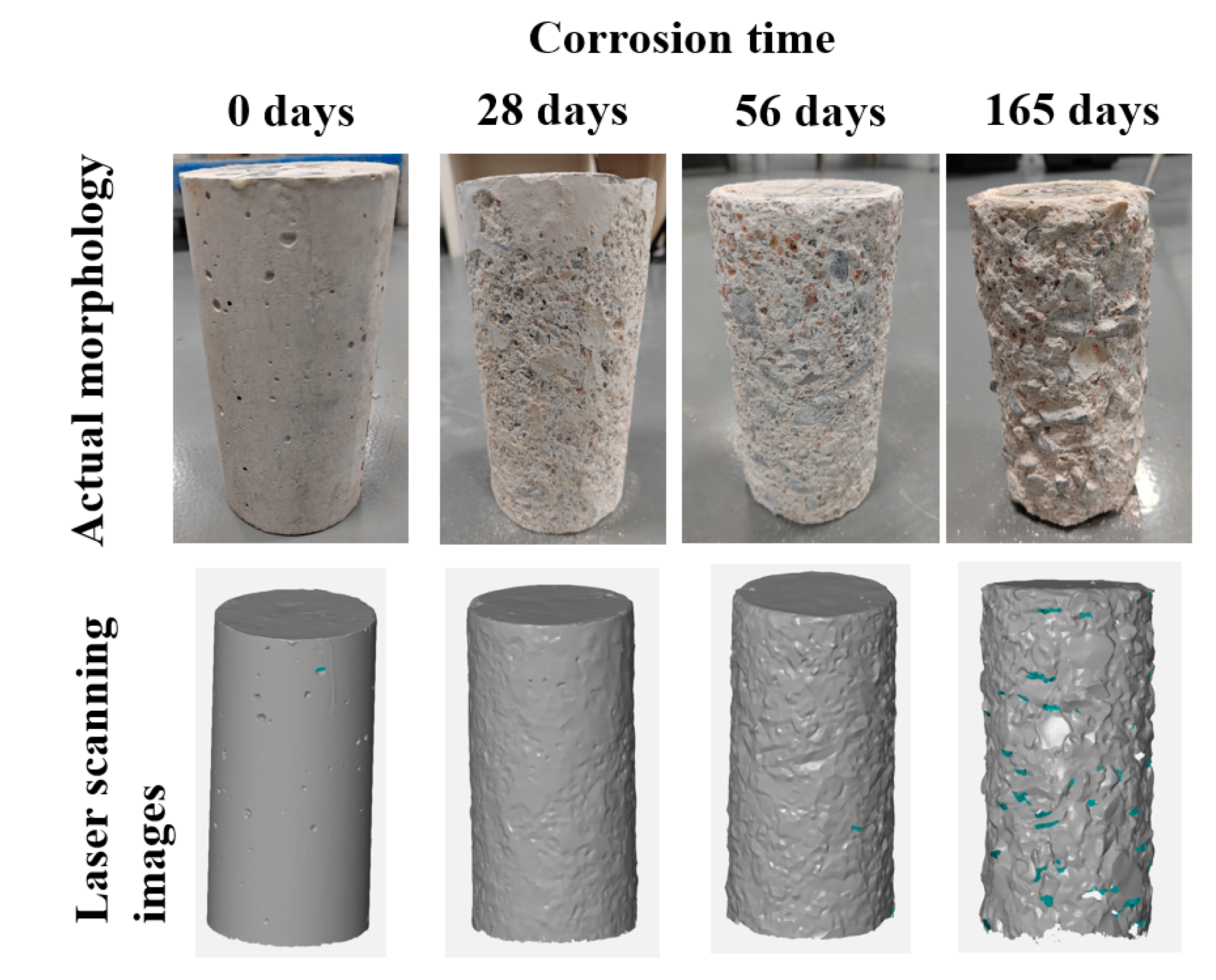
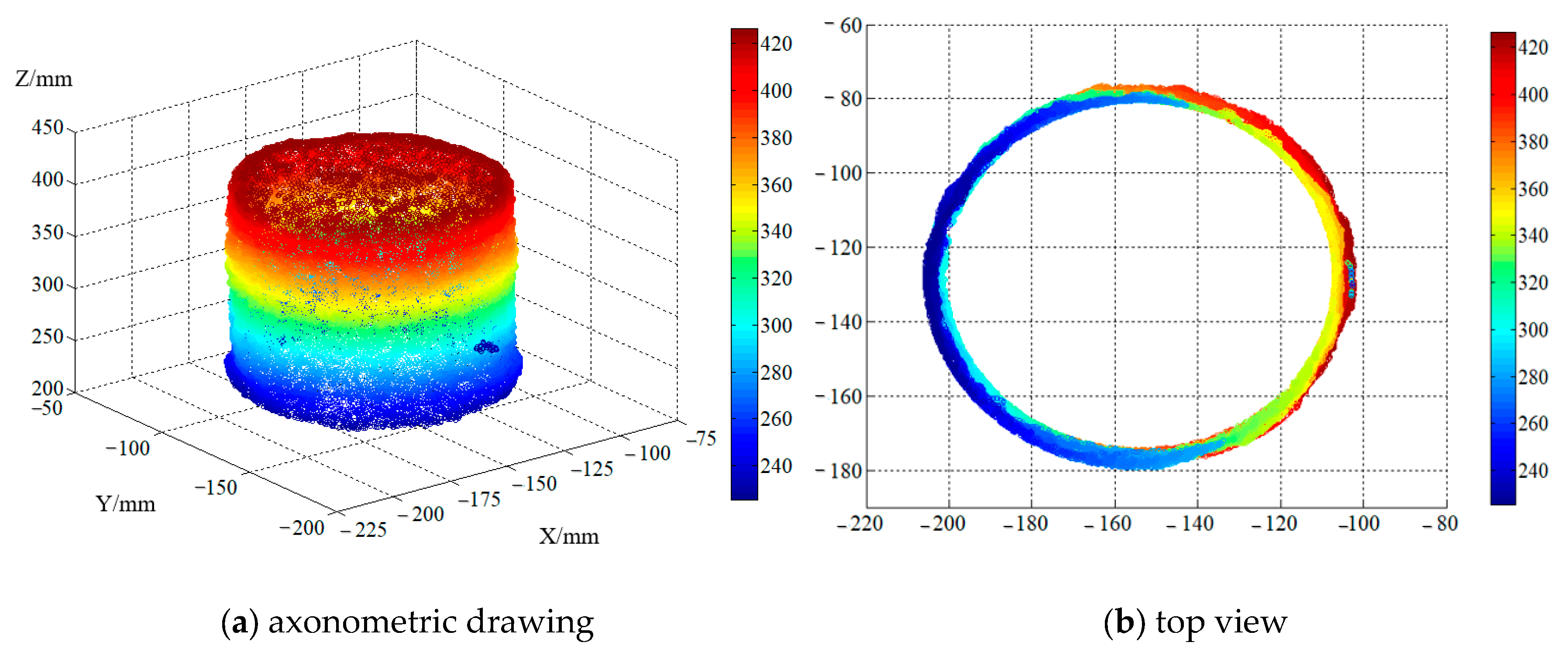





| Strength Grade | Cement | Fly Ash | Mineral Powder | Ground Sand | Sand | Gravel | Superplasticizer | Residual Slurry | Water |
|---|---|---|---|---|---|---|---|---|---|
| C30 | 198 | 66 | 66 | / | 780 | 1075 | 10.8 | / | 155.0 |
| C50 | 255 | / | / | 135 | 750 | 1300 | 9.5 | 150 | / |
| C80 | 255 | / | / | 135 | 720 | 1330 | 9.5 | 180 | / |
| Sieve size (mm) | 31.5 | 26.5 | 19.0 | 16.0 | 9.5 | 4.75 | 2.36 | <2.36 |
| Grader retained (%) | 0 | 2.5 | 18.0 | 46.7 | 24.5 | 5.0 | 2.8 | 0.5 |
| Accumulated retained (%) | 0 | 2.5 | 20.5 | 67.2 | 91.7 | 96.7 | 99.5 | 100 |
| Sieve size (mm) | 4.75 | 2.36 | 1.18 | 0.6 | 1.3 | 0.15 | <0.15 |
| Grader retained (%) | 0 | 21.6 | 20.0 | 16.8 | 19.6 | 9.6 | 12.4 |
| Accumulated retained (%) | 0 | 21.6 | 41.6 | 58.4 | 78.0 | 87.6 | 100 |
| Specimens Shape | Concrete Grade Strength | The Value of pH | Quantity | Immersion Method |
|---|---|---|---|---|
| cylinders | C30 | 0.85 | 12 | Total submersion |
| cylinders | C50 | 0.85 | 12 | Total submersion |
| cylinders | C80 | 0.85 | 12 | Total submersion |
| Corrosion Duration of Concrete with Strength Grade C80 | |||||||
|---|---|---|---|---|---|---|---|
| 0 Days | 28 Days | 56 Days | 165 Days | ||||
| δ/mm | N(δ) | δ/mm | N(δ) | δ/mm | N(δ) | δ/mm | N(δ) |
| 2.029 | 7315 | 2.011 | 7301 | 2.021 | 7539 | 1.992 | 9252 |
| 1.004 | 30,034 | 0.995 | 30,623 | 1.001 | 36,258 | 0.986 | 52,066 |
| 0.500 | 123,372 | 0.495 | 147,199 | 0.498 | 200,385 | 0.491 | 313,535 |
| 0.249 | 506,284 | 0.247 | 733,036 | 0.248 | 1,059,127 | 0.245 | 1,676,567 |
| 0.166 | 1,151,197 | 0.165 | 1,795,356 | 0.165 | 2,639,044 | 0.163 | 4,162,279 |
| 0.124 | 2,061,757 | 0.123 | 3,348,331 | 0.124 | 4,919,066 | 0.122 | 7,742,994 |
| 0.100 | 3,239,310 | 0.099 | 5,372,695 | 0.099 | 7,916,343 | 0.098 | 12,404,301 |
| 0.071 | 6,419,629 | 0.070 | 10,863,557 | 0.071 | 16,005,575 | 0.070 | 25,032,171 |
| 0.050 | 13,239,316 | 0.049 | 22,714,698 | 0.050 | 33,443,633 | 0.049 | 52,093,475 |
| Strength Grade | Corrosion Duration | |||
|---|---|---|---|---|
| 0 Days | 28 Days | 56 Days | 165 Days | |
| C30 | 2.0252 | 2.0535 | 2.1533 | 2.1368 |
| C50 | 2.0463 | 2.1210 | 2.2707 | 2.2875 |
| C80 | 2.0237 | 2.19744 | 2.2792 | 2.3308 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Zeng, H.; Huang, H.; Liu, L.; Li, L.; Yuan, B.; Zhong, Z. Experimental Investigation on the Influence of Strength Grade on the Surface Fractal Dimension of Concrete under Sulfuric Acid Attack. Buildings 2024, 14, 713. https://doi.org/10.3390/buildings14030713
Xiao J, Zeng H, Huang H, Liu L, Li L, Yuan B, Zhong Z. Experimental Investigation on the Influence of Strength Grade on the Surface Fractal Dimension of Concrete under Sulfuric Acid Attack. Buildings. 2024; 14(3):713. https://doi.org/10.3390/buildings14030713
Chicago/Turabian StyleXiao, Jie, Hehui Zeng, Huanqiang Huang, Lingfei Liu, Long Li, Bingxiang Yuan, and Zucai Zhong. 2024. "Experimental Investigation on the Influence of Strength Grade on the Surface Fractal Dimension of Concrete under Sulfuric Acid Attack" Buildings 14, no. 3: 713. https://doi.org/10.3390/buildings14030713






