Mechanical Properties and Hydration Mechanism of Iron Tailings–Cement-Based Supplementary Cementitious Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Specimens
2.2.1. Preparation of Blend Mortar
2.2.2. Preparation of Blend Paste
2.3. Test Procedure
2.3.1. Compressive Strength
2.3.2. X-ray Diffraction
2.3.3. Thermogravimetric Analysis
2.3.4. Fourier Transform Infrared
2.3.5. Scanning Electron Microscopy
2.3.6. Inductively Coupled Plasma–Optical Emission Spectroscopy
3. Results
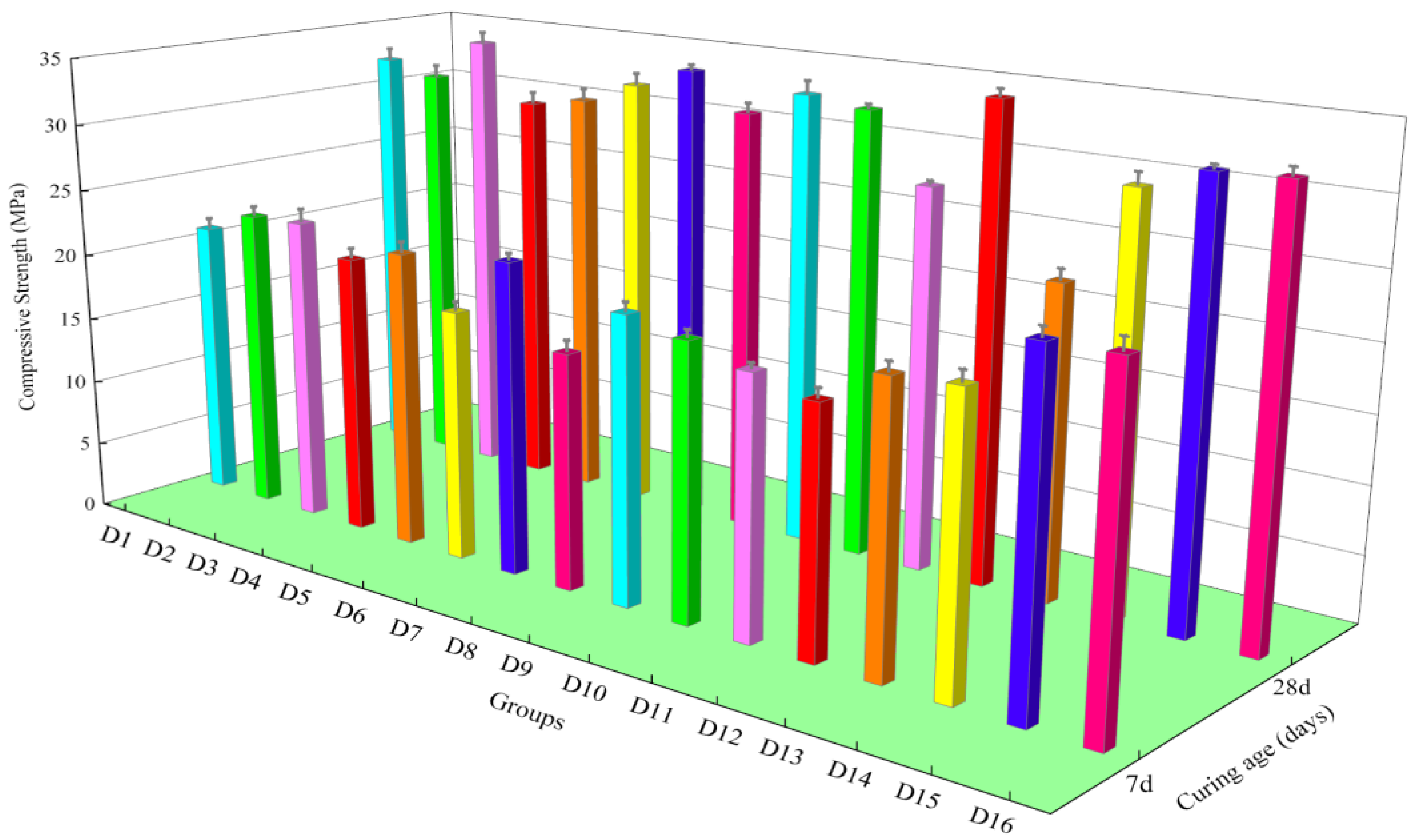
3.1. Mechanical Properties
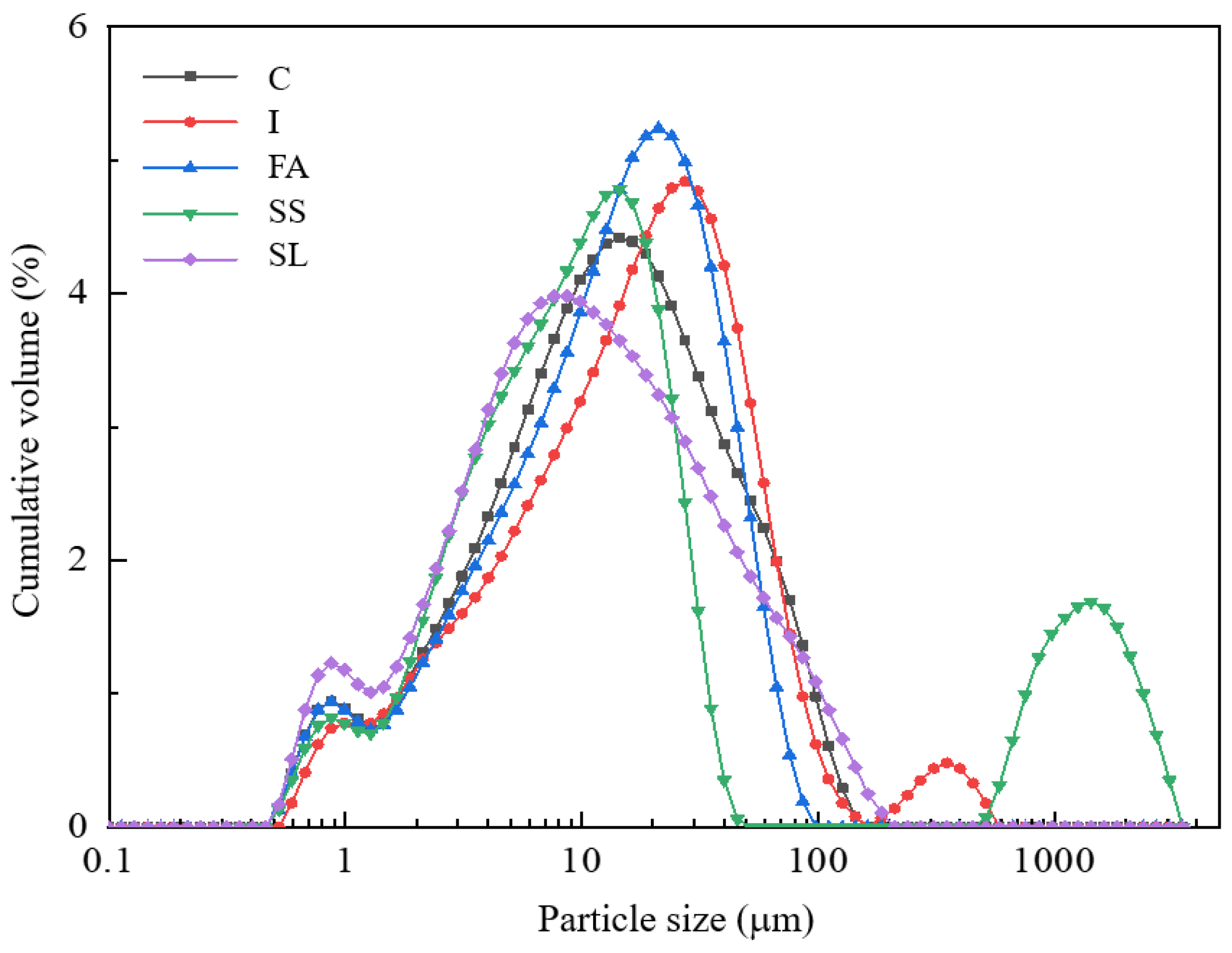
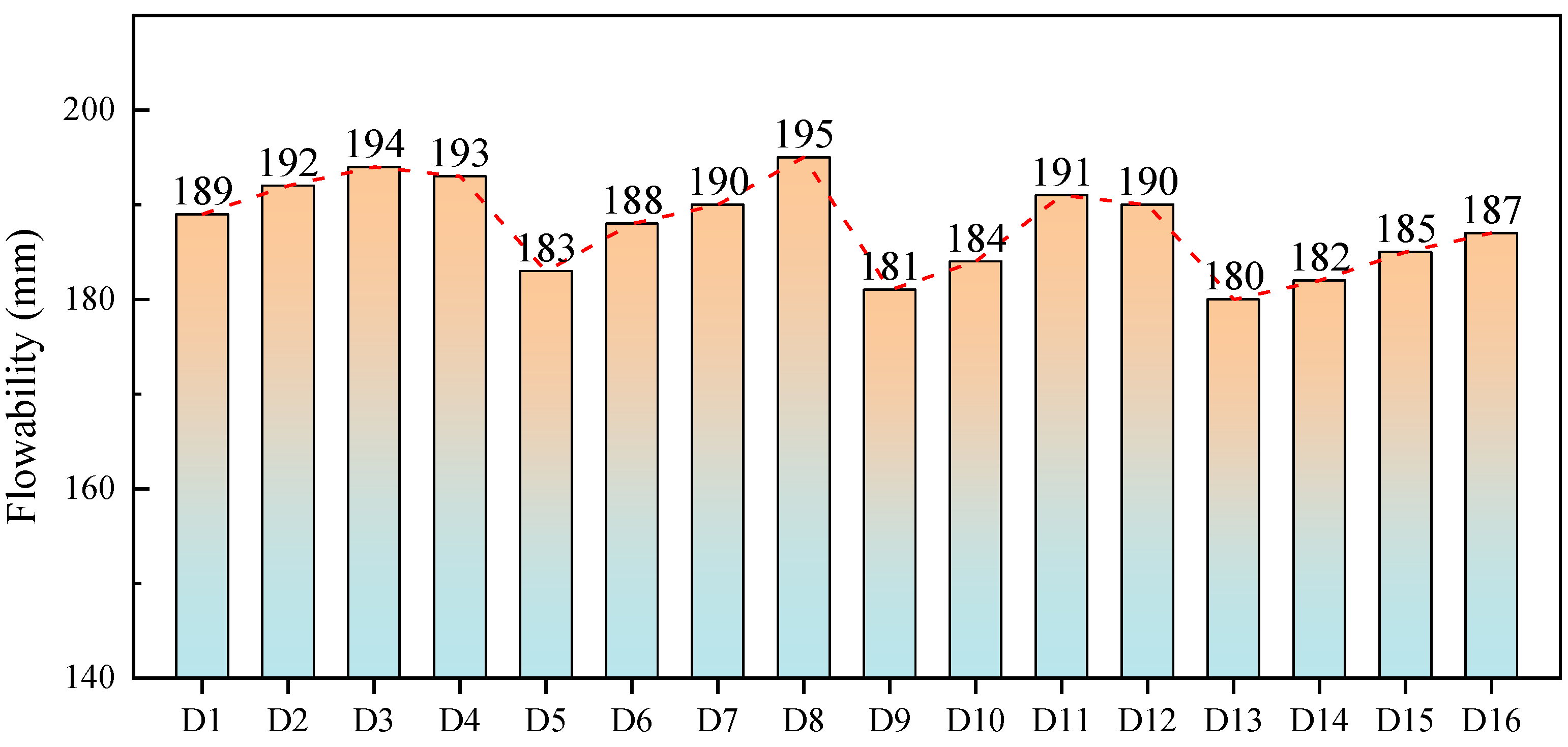
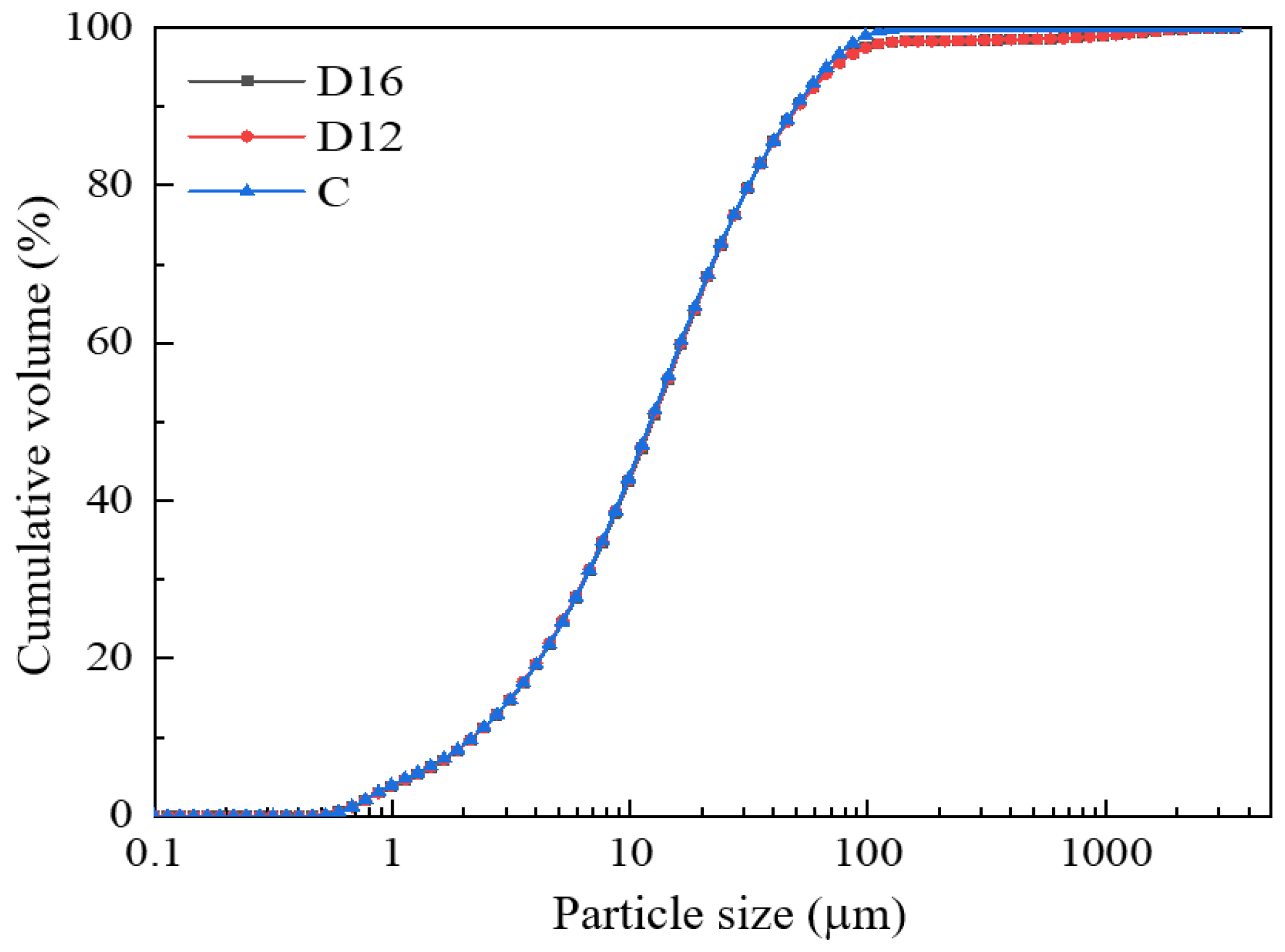
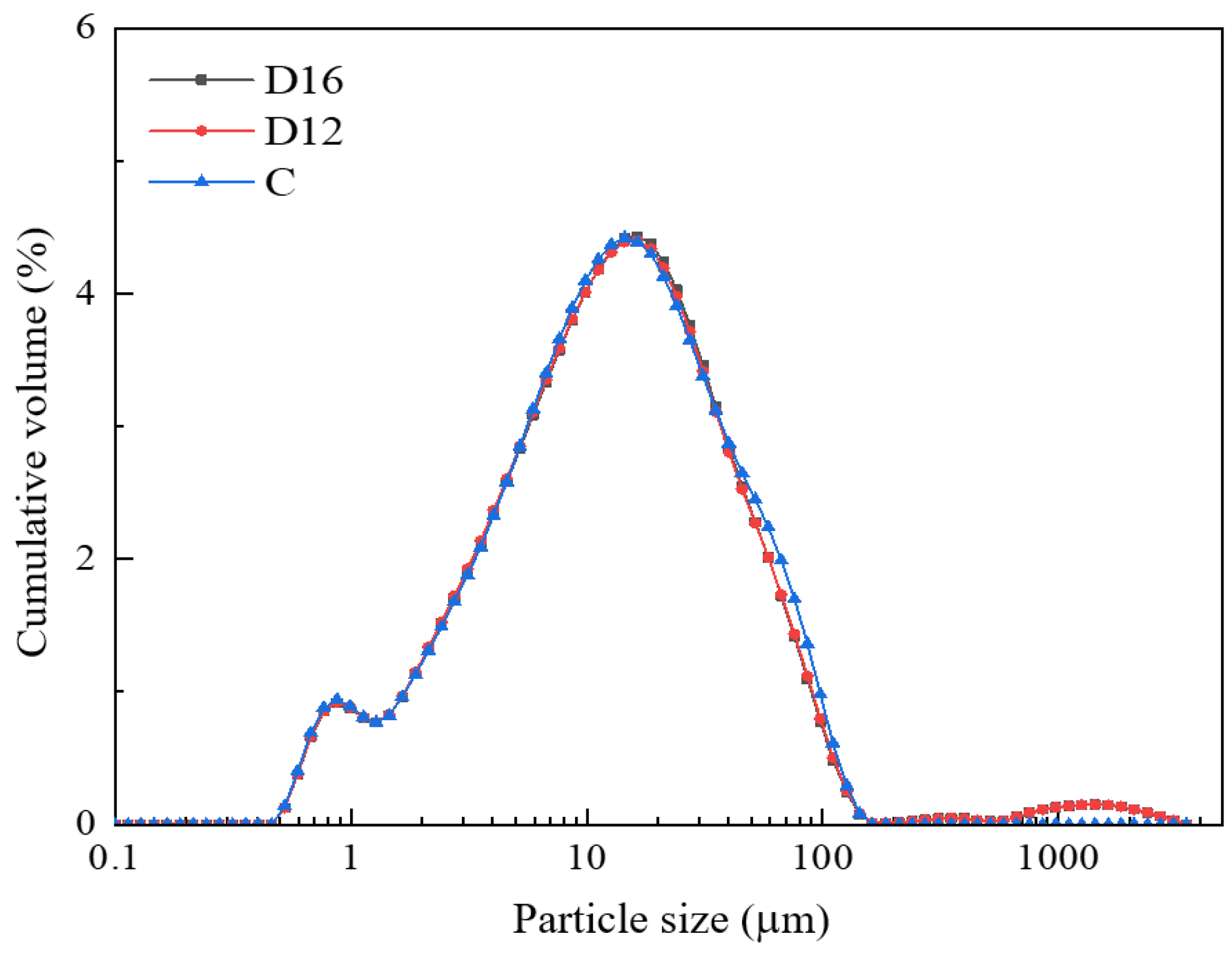
3.2. Particle Characteristic
3.3. Hydration Product-Type Test Results
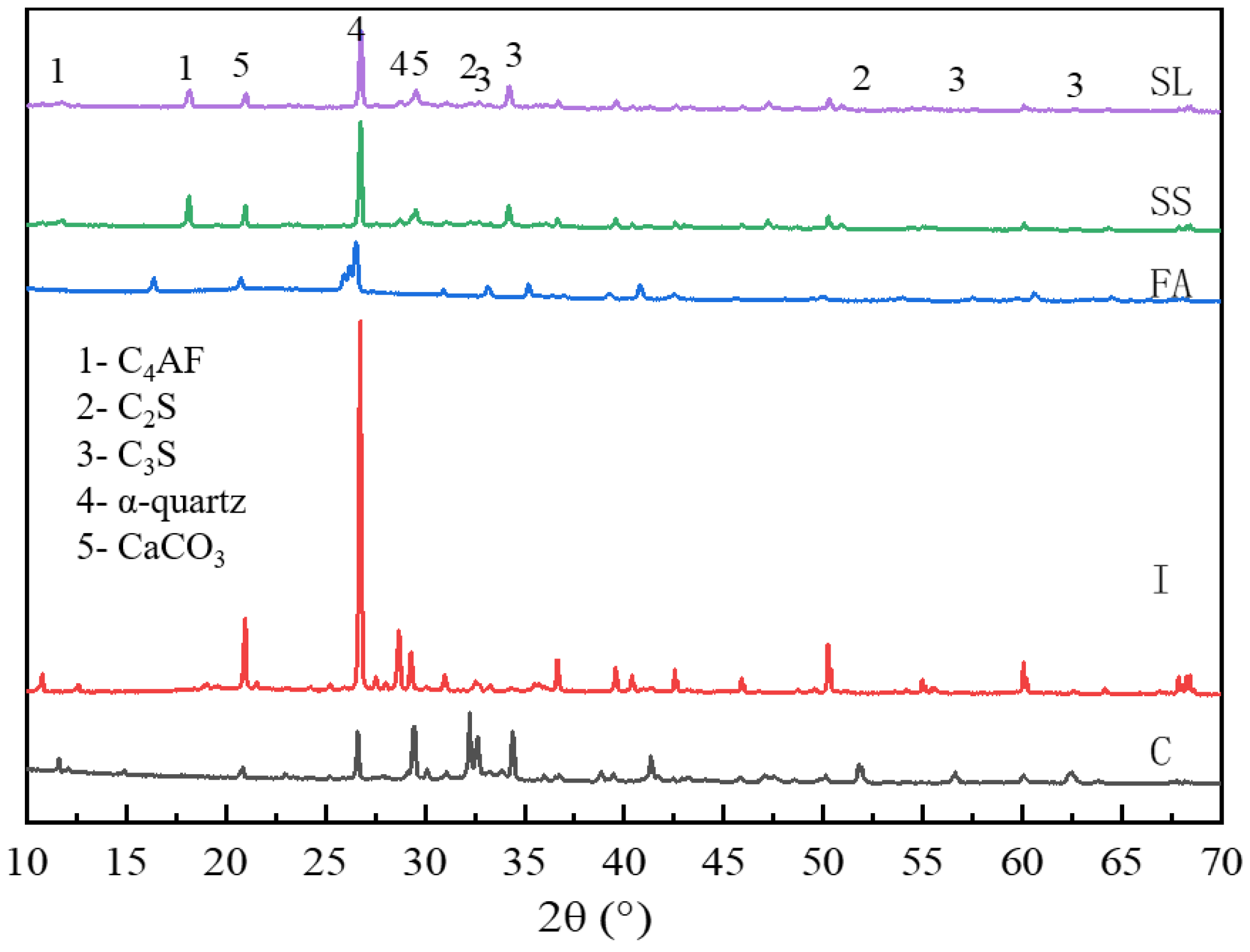
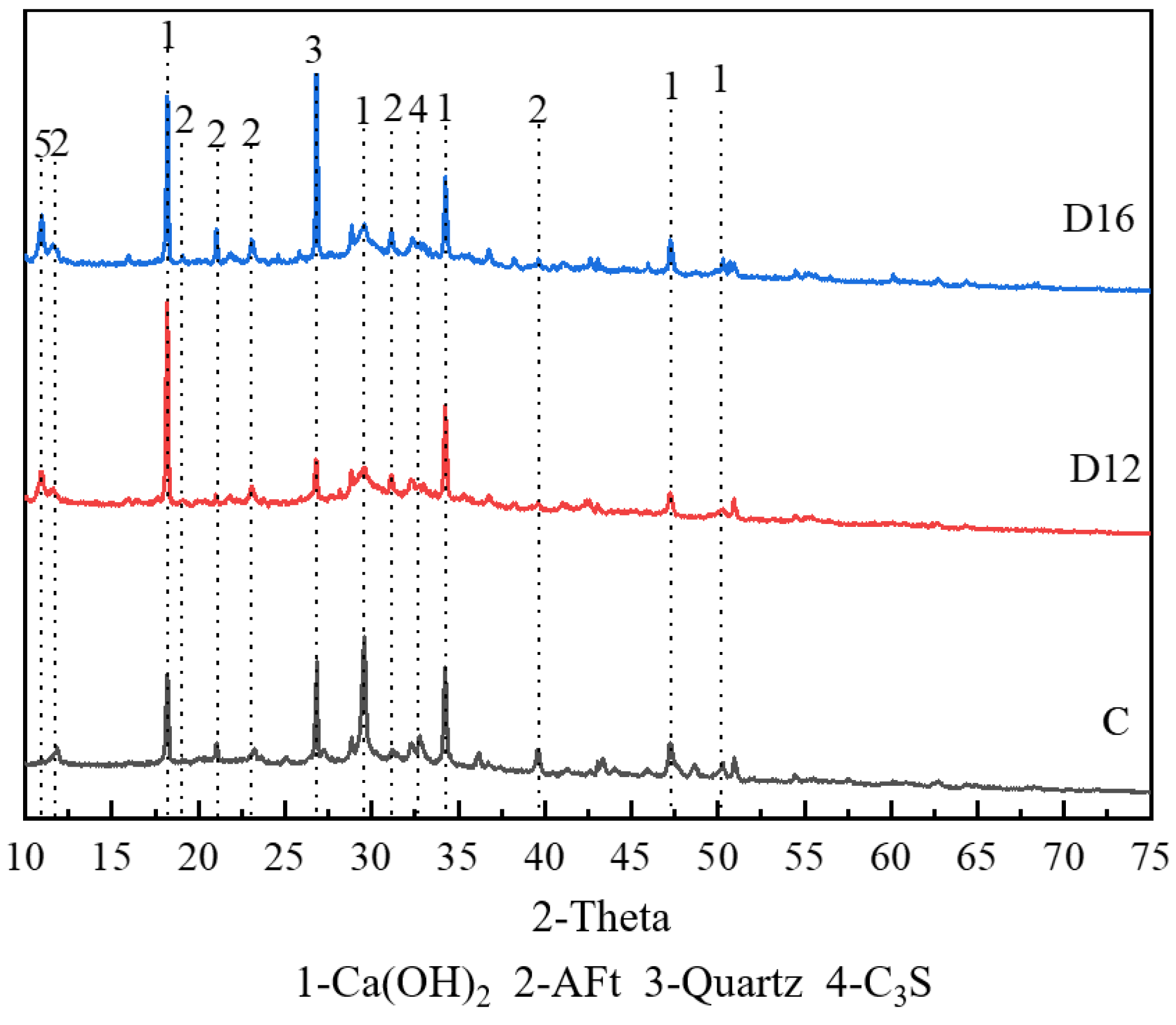
3.3.1. XRD Results
3.3.2. FTIR Spectroscopy Results
3.4. Hydration Products’ Quantitative Analysis Test Results
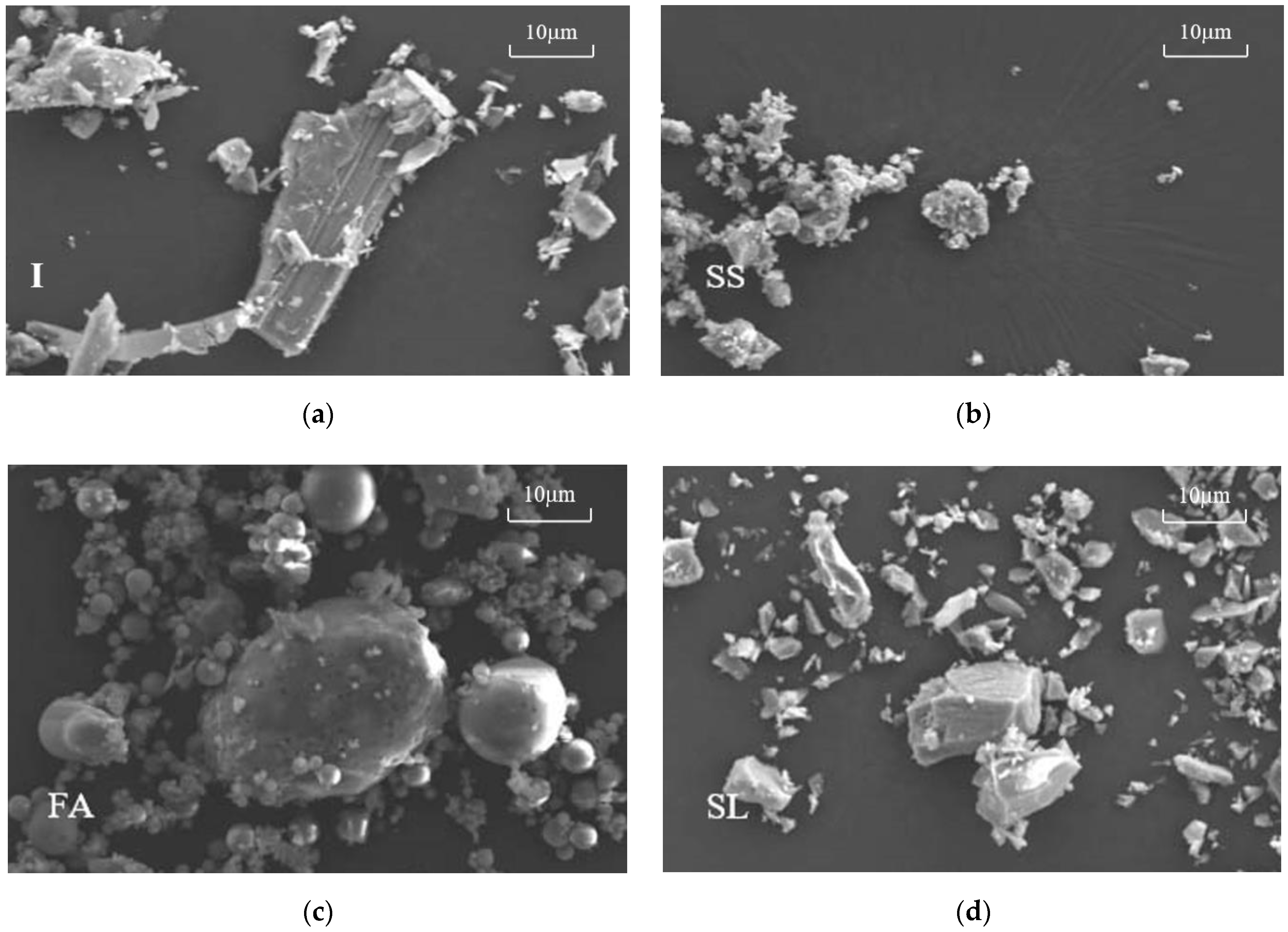
3.5. Micromorphological Analysis of Hydration Products’ Test Results
4. Discussion
5. Conclusions
- When the ratio of IOTs:SS:FA:SL was 9:8:8:2, the 7 d compressive strength of iron tailings–cement-based supplementary cementitious materials was the highest. When the ratio of IOTs:SS:FA:SL was 9:6:8:4, the 28 d compressive strength of iron tailings–cement-based supplementary cementitious materials was the highest. The quantity of raw materials in the mixture affected the 28 d compressive strength of cementitious materials, and the degree of influence was SS > SL > FA. In addition, the raw materials in the composite system exhibited synergistic hydration.
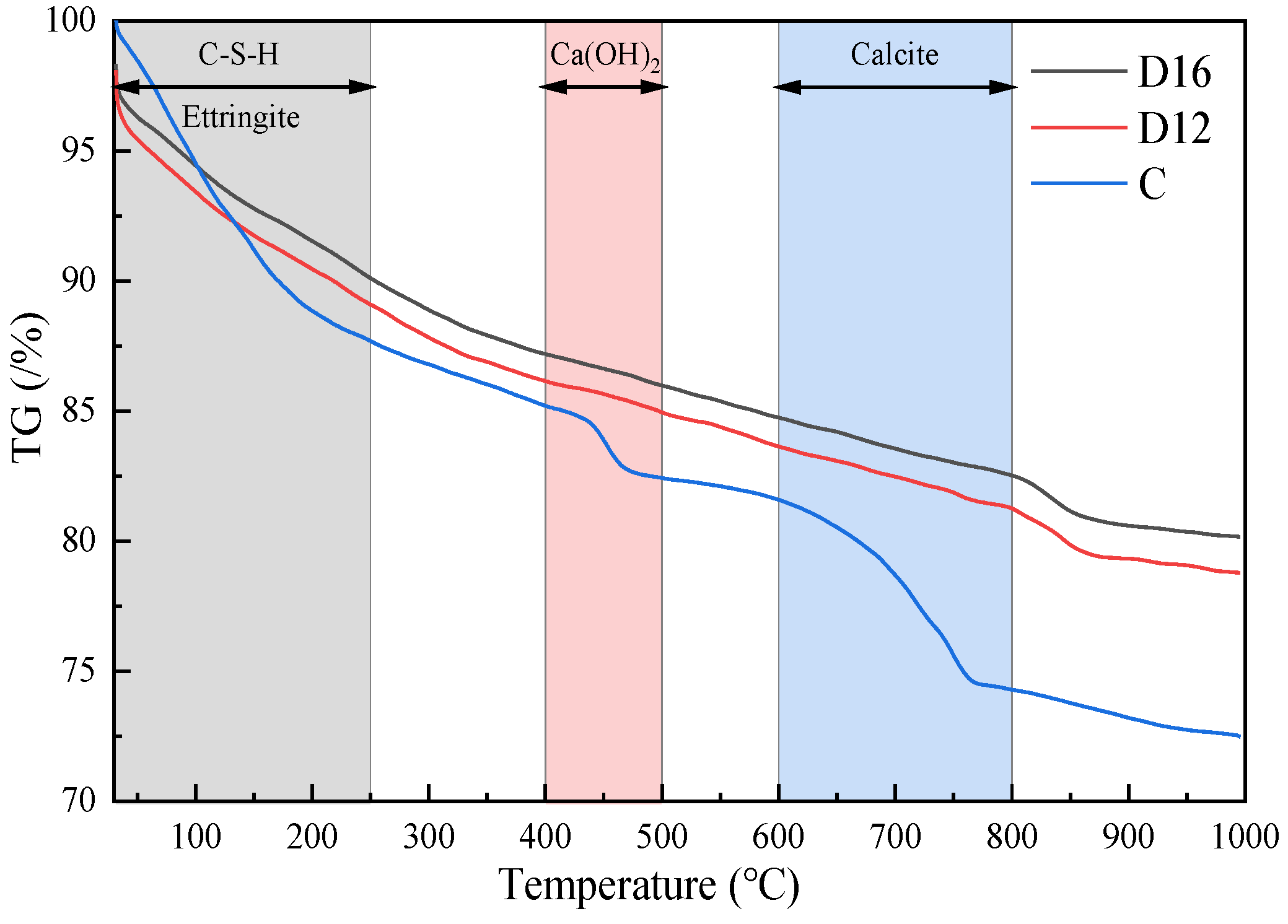
- Based on the results of the hydration product-type tests, it was found that the main hydration products in the iron tailings–cement-based supplementary cementitious materials were ettringite and C-(A)-S-H gel. In the reaction system, SL and FA provided aluminium, the calcium carbonate in SS provided carbonate, and the sulphate in monosulphoaluminate was replaced by carbonate. As the SS:SL ratio decreased, the degree of polymerisation of the silica–oxygen tetrahedra increased, producing a more stable ettringite, which improved the strength and microstructural densification of the cementitious material.
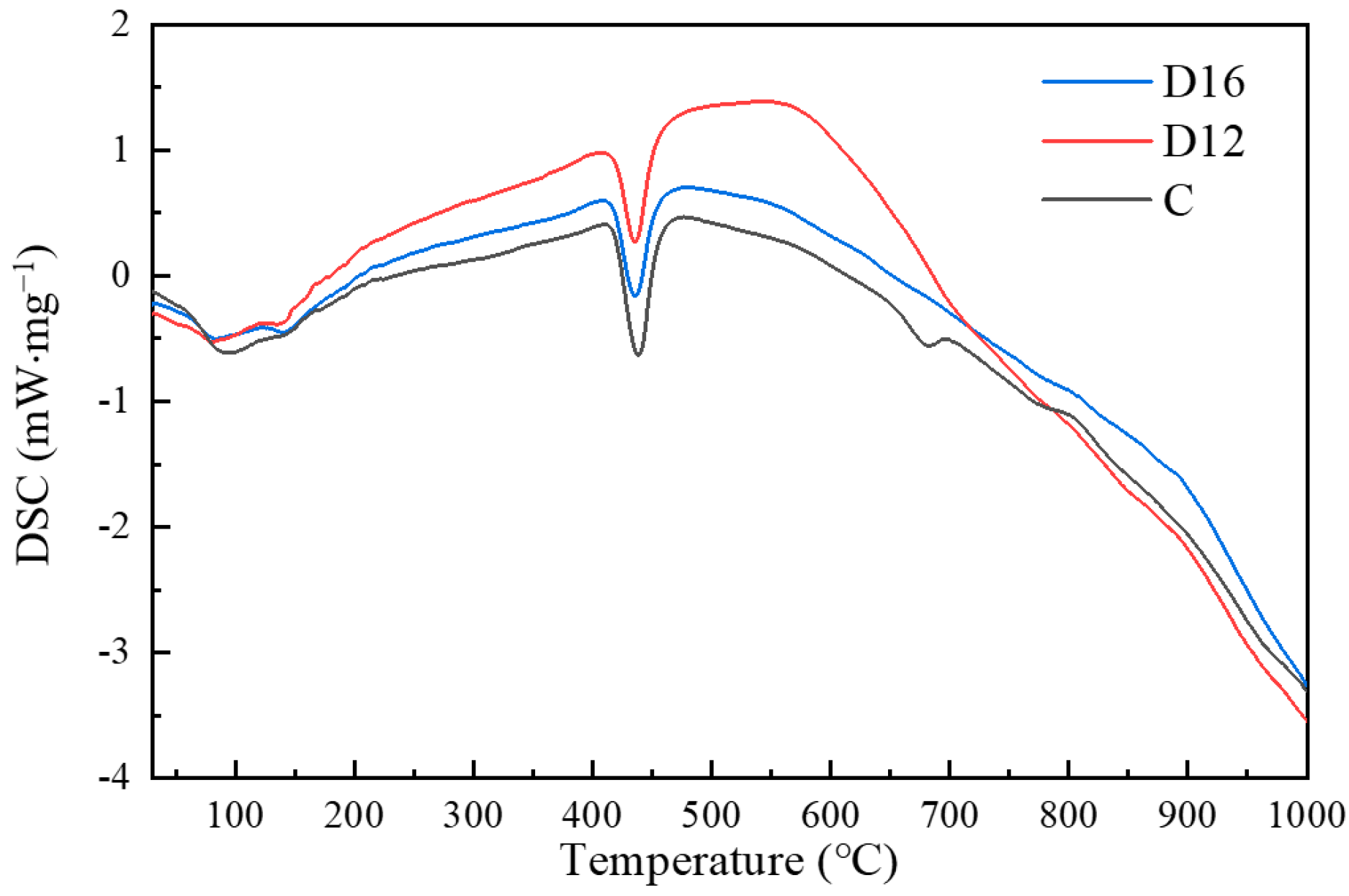
- Based on the results of the quantitative analysis tests of the hydration products, it was found that there are two exothermic peaks and three heat-absorbing peaks in the iron tailings–cement-based supplementary cementitious materials (SCMs). When the test was carried out from room temperature to 1000 °C, the mass loss of sample C and the SCM samples D12 and D16 was 27.5%, 21.2%, and 19.8%, respectively; more hydration products existed in sample D12, the content of ettringite and C-(A)-S-H gel accounted for 10.9%, and the appropriate ratio of SL and SS provided the SCM system with more active components, which in turn promoted the improvement in strength.
- Based on the synergistic effect analysis results, it was found that the raw materials play their respective roles in the hybrid system and jointly promote the hydration reaction continuously. After the introduction of SL into the SS system, the hydration reaction in the SS and SL system was actively promoted by the increase in Al3+. Combined with the changes in the hornblende diffraction peaks, it can be seen that the IOTs provided part of the Si4+ for the hybrid system, and together with the other three solid wastes, they promoted the continuation of the hydration reaction and the production of C-(A)-S-H gel.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghasemi, S.; Behnamfard, A.; Arjmand, R. Iron ore tailings valorization through separate characterization and upgradation of different tailings streams of an Iranian iron ore processing plant. Environ. Sci. Pollut. Res. 2023, 30, 115448–115460. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.D.; Shen, Y.; Liu, J.H.; Cheng, L.N.; Zhang, G.T.; Zhang, Y.Y. Effect of Iron Tailings and Slag Powders on Workability and Mechanical Properties of Concrete. Front. Mater. 2021, 8, 723119. [Google Scholar]
- Segui, P.; Safhi, A.E.; Amrani, M.; Benzaazoua, M. Mining Wastes as Road Construction Material: A Review. Minerals 2023, 13, 90. [Google Scholar] [CrossRef]
- Zuccheratte, A.C.V.; Freire, C.B.; Lameiras, F.S. Synthetic gravel for concrete obtained from sandy iron ore tailing and recycled polyethyltherephtalate. Constr. Build. Mater. 2017, 151, 859–865. [Google Scholar] [CrossRef]
- Ma, B.G.; Cai, L.X.; Li, X.G.; Jian, S.W. Utilization of iron tailings as substitute in autoclaved aerated concrete: Physico-mechanical and microstructure of hydration products. J. Clean. Prod. 2016, 127, 162–171. [Google Scholar] [CrossRef]
- Fei, A.P.; Zhang, T.Z. Research on Performance of Pavement Cement Concrete Mixed with Iron Tailings and Fibers. In Proceedings of the 3rd International Forum on Energy, Environment Science and Materials (IFEESM), Shenzhen, China, 25–26 November 2017; pp. 348–354. [Google Scholar]
- Panda, L.; Biswal, S.K.; Venugopal, R.; Mandre, N.R. Recovery of Ultra-Fine Iron Ore from Iron Ore Tailings. Trans. Indian Inst. Met. 2018, 71, 463–468. [Google Scholar] [CrossRef]
- Zhu, Q.; Yuan, Y.X.; Chen, J.H.; Fan, L.; Yang, H. Research on the high-temperature resistance of recycled aggregate concrete with iron tailing sand. Constr. Build. Mater. 2022, 327, 126889. [Google Scholar] [CrossRef]
- Galvao, J.L.B.; Andrade, H.D.; Brigolini, G.J.; Peixoto, R.A.F.; Mendes, J.C. Reuse of iron ore tailings from tailings dams as pigment for sustainable paints. J. Clean. Prod. 2018, 200, 412–422. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Shaikh, F.; Krishna, R.S.; Mishra, J. Utilization potential of mine tailings in geopolymers: Physicochemical and environmental aspects. Process Saf. Environ. Protect. 2021, 147, 559–577. [Google Scholar] [CrossRef]
- Huang, X.Y.; Ranade, R.; Li, V.C. Feasibility Study of Developing Green ECC Using Iron Ore Tailings Powder as Cement Replacement. J. Mater. Civ. Eng. 2013, 25, 923–931. [Google Scholar] [CrossRef]
- Fontes, W.C.; Mendes, J.C.; Da Silva, S.N.; Peixoto, R.A.F. Mortars for laying and coating produced with iron ore tailings from tailing dams. Constr. Build. Mater. 2016, 112, 988–995. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Ramos, F.C.R.; Caetano, T.R.; Panzerra, T.H.; Santos, H. Reuse of iron ore tailings in the production of geopolymer mortars. REM-Int. Eng. J. 2019, 72, 581–587. [Google Scholar] [CrossRef]
- Fontes, W.C.; de Carvalho, J.M.F.; Defaveri, K.; Brigolini, G.J.; Segadaes, A.M.; Peixoto, R.A.F. Hydraulic Tiles Produced with Fine Aggregates and Pigments Reclaimed from Iron Ore Tailings. J. Sust. Metall. 2021, 7, 151–165. [Google Scholar] [CrossRef]
- Gu, X.W.; Zhang, W.F.; Zhang, X.L.; Li, X.H.; Qiu, J.P. Hydration characteristics investigation of iron tailings blended ultra high performance concrete: The effects of mechanical activation and iron tailings content. J. Build. Eng. 2022, 45, 103459. [Google Scholar] [CrossRef]
- Duarte, M.S.; Almada, B.S.; Santos, W.J.D.; Bessa, S.A.L.; Bezerra, A.C.D.; Aguilar, M.T.P. Influence of mechanical treatment and magnetic separation on the performance of iron ore tailings as supplementary cementitious material. J. Build. Eng. 2022, 59, 105099. [Google Scholar] [CrossRef]
- Bezerra, C.G.; Rocha, C.A.A.; de Siqueira, I.S.; Toledo, R.D. Feasibility of iron-rich ore tailing as supplementary cementitious material in cement pastes. Constr. Build. Mater. 2021, 303, 124496. [Google Scholar] [CrossRef]
- de Magalhaes, L.F.; França, S.; Oliveira, M.D.; Peixoto, R.A.F.; Bessa, S.A.L.; Bezerra, A.C.D. Iron ore tailings as a supplementary cementitious material in the production of pigmented cements. J. Clean. Prod. 2020, 274, 123260. [Google Scholar] [CrossRef]
- Kwon, E.; Ahn, J.; Cho, B.; Park, D. A study on development of recycled cement made from waste cementitious powder. Constr. Build. Mater. 2015, 83, 174–180. [Google Scholar] [CrossRef]
- Li, J.S.; Ren, W.Y.; Zhang, A.J.; Li, S.C.; Tan, J.P.; Liu, H.T. Mechanical Properties and Microstructure Analysis of Cement Mortar Mixed with Iron Ore Tailings. Buildings 2023, 13, 149. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Yang, D.K.; Gu, X.W.; Chen, H.; Li, Z.J. Application of Iron Tailings-Based Composite Supplementary Cementitious Materials (SCMs) in Green Concrete. Materials 2022, 15, 3866. [Google Scholar] [CrossRef]
- Zhang, Y.N.A.; Zhang, X.K.; Gu, X.W.; Wang, T.; Liu, B.A. Study on Properties and Optimization of Ternary Auxiliary Cementing Materials for IOTs. Materials 2022, 15, 3851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Luo, X.C.; Sun, M.K.; Shen, W.G.; Cao, B.; Li, X.L. Utilization of Steel Slags in Cement Industry. In Proceedings of the Annual Meetings of Chinese-Society’s-Building-Materials, Professional Committees of Stone and Aggregate and Utilization of Solid Waste, Wuhan, China, 28 November–1 December 2013; p. 98. [Google Scholar]
- Wang, Q.; Wang, Z.M.; Su, Y.W.; Wu, P.; Hu, S.G.; Chen, P.; Lyu, X.J. Application of calcareous iron ore tailings in the production of cement. Energy Sources Part A-Recovery Util. Environ. Eff. 2020, 1–10. [Google Scholar] [CrossRef]
- Singh, S.K.; Jyoti; Vashistha, P. Development of newer composite cement through mechano-chemical activation of steel slag. Constr. Build. Mater. 2021, 268, 121147. [Google Scholar] [CrossRef]
- Gao, D.; Wang, F.P.; Wang, Y.T.; Zeng, Y.N. Sustainable Utilization of Steel Slag from Traditional Industry and Agriculture to Catalysis. Sustainability 2020, 12, 9295. [Google Scholar] [CrossRef]
- Xiang, X.D.; Xi, J.C.; Li, C.H.; Jiang, X.W. Preparation and application of the cement-free steel slag cementitious material. Constr. Build. Mater. 2016, 114, 874–879. [Google Scholar] [CrossRef]
- Zhou, M.K.; Cheng, X.; Chen, X. Studies on the Volumetric Stability and Mechanical Properties of Cement-Fly-Ash-Stabilized Steel Slag. Materials 2021, 14, 495. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Hou, H.M.; Su, L.J.; Guo, D.F.; Xu, H. Resource utilization of solid waste for the collaborative reduction of pollution and carbon emissions: Case study of fly ash. J. Clean. Prod. 2023, 383, 135449. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, J.X.; Song, W.D.; Du, C.F.; Fu, H.T. High-volume ultrafine fly ash-cement slurry mechanical properties and strength development model establishment. Constr. Build. Mater. 2021, 277, 122350. [Google Scholar] [CrossRef]
- Yang, J.; Hu, H.C.; He, X.Y.; Su, Y.; Wang, Y.B.; Tan, H.B.; Pan, H. Effect of steam curing on compressive strength and microstructure of high volume ultrafine fly ash cement mortar. Constr. Build. Mater. 2021, 266, 120894. [Google Scholar] [CrossRef]
- Sun, C.Y.; Zhang, J.; Yan, C.W.; Yin, L.Q.; Wang, X.X.; Liu, S.G. Hydration characteristics of low carbon cementitious materials with multiple solid wastes. Constr. Build. Mater. 2022, 322, 126366. [Google Scholar] [CrossRef]
- Yang, W.H.; Hwang, J.S.; Jeon, C.; Lee, S.-H. An Experimental Study on the Chloride Attack Resistibility of Alkali-Activated Ternary Blended Cement Concrete. J. Korea Inst. Build. Constr. 2016, 16, 321–329. [Google Scholar] [CrossRef]
- Song, R.J.; Zhao, Q.X.; Zhang, J.R.; Liu, J.Z. Microstructure and Composition of Hardened Paste of Soda Residue-Slag-Cement Binding Material System. Front. Mater. 2019, 6, 211. [Google Scholar] [CrossRef]
- Cheng, Y.Z.; Bai, L.; Du, W.J.; Chen, L.J.; Yan, Y.L. Engineered cementitious composites using powder regenerated from municipal solid waste incineration bottom ash as supplementary cementitious material. Constr. Build. Mater. 2023, 394, 132224. [Google Scholar] [CrossRef]
- Jin, R.Y.; Chen, Q.; Soboyejo, A. Survey of the current status of sustainable concrete production in the US. Resour. Conserv. Recycl. 2015, 105, 148–159. [Google Scholar] [CrossRef]
- Carette, J.; Staquet, S. Monitoring and modelling the early age and hardening behaviour of eco-concrete through continuous non-destructive measurements: Part II. Mechanical behaviour. Cem. Concr. Compos. 2016, 73, 1–9. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, C.; Liu, X.M.; Zhang, Z.Q. Effect of Phosphorus Slag Admixture on the Properties and Hydration Mechanism of Circulating Fluidized Bed Fly Ash-Based Multi-Solid Waste Cementitious Material. Materials 2022, 15, 6774. [Google Scholar] [CrossRef] [PubMed]
- He, Z.H.; Du, S.G.; Chen, D. Microstructure of ultra high performance concrete containing lithium slag. J. Hazard. Mater. 2018, 353, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, G.; Zhang, W.Z.; Li, Z.L.; Xing, F.; Tang, L.P. Application potential analysis of biochar as a carbon capture material in cementitious composites: A review. Constr. Build. Mater. 2022, 350, 128715. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Wu, X.; Li, B.; Wei, D.B.; Guo, F.C.; Ji, H.D. Investigation of Preparation and Shrinkage Characteristics of Multi-Source Solid Waste-Based Cementitious Materials. Materials 2023, 16, 7522. [Google Scholar] [CrossRef] [PubMed]
- Shmlls, M.; Abed, M.A.; Horvath, T.; Bozsaky, D. Sustainability framework of recycled aggregate concrete produced with supplementary cementitious materials. Ain Shams Eng. J. 2023, 14, 102036. [Google Scholar] [CrossRef]
- Shen, L.L.; Zhang, J.X.; Lai, W.A.; Li, M.; Huo, B.B. Microstructure and mechanical behaviors of coal gangue-Coal slime water backfill cementitious materials. J. Mater. Res. Technol. 2022, 20, 3772–3783. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, C.X.; Ma, Z.Y.; Li, F. Study on preparation of supplementary cementitious material using microbial CO2 fixation of steel slag powder. Constr. Build. Mater. 2022, 326, 126864. [Google Scholar] [CrossRef]
- Tao, M.; Lu, D.M.; Shi, Y.; Wu, C.Q. Utilization and life cycle assessment of low activity solid waste as cementitious materials: A case study of titanium slag and granulated blast furnace slag. Sci. Total Environ. 2022, 849, 157797. [Google Scholar] [CrossRef]
- Khaidarov, B.B.; Suvorov, D.S.; Lysov, D.V.; Abramov, A.K.; Luchnikova, G.G.; Khaidarov, T.B.; Kuznetsov, D.V.; Bychkov, A.V.; Burmistrov, I.N.; Mamulat, S.L. Production and Investigation of a Finely Dispersed Fraction of Blast-Furnace Granulated Slag for Use as Components of Clinker-Free Binders. Refract. Ind. Ceram. 2021, 62, 347–354. [Google Scholar] [CrossRef]
- Pinto, P.S.; Milagre, L.E.; Moreira, L.C.M.; Rocha, H.P.; Salviano, A.B.; Ardisson, J.D.; Parreira, F.V.; Teixeira, A.P.C.; Lago, R.M. Iron Recovery from Iron Ore Tailings by Direct Hydrogen Reduction at Low Temperature and Magnetic Separation. J. Braz. Chem. Soc. 2022, 33, 969–977. [Google Scholar] [CrossRef]
- Wu, S.Z.; Zhou, Y.; Li, R.F.; Liu, X.Q.; Li, C.W.; Huang, Z.Y. Reaction Sintered Porous Ceramics Using Iron Tailings: Preparation and Properties. J. Inorg. Mater. 2023, 38, 1193–1199. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Lian, F.; Ma, L.J.; Jiang, Y.S. Effects of Quicklime and Iron Tailings as Modifier on Composition and Properties of Steel Slag. J. Iron Steel Res. Int. 2015, 22, 15–20. [Google Scholar] [CrossRef]
- Adesina, A.; Das, S. Durability Evaluation of Green-Engineered Cementitious Composite Incorporating Glass as Aggregate. J. Mater. Civ. Eng. 2020, 32, 04020354. [Google Scholar] [CrossRef]
- Yao, G.; Wang, Q.; Wang, Z.M.; Wang, J.X.; Lyu, X.J. Activation of hydration properties of iron ore tailings and their application as supplementary cementitious materials in cement. Powder Technol. 2020, 360, 863–871. [Google Scholar] [CrossRef]
- Wei, J.Q.; Gencturk, B.; Jain, A.; Hanifehzadeh, M. Mitigating alkali-silica reaction induced concrete degradation through cement substitution by metakaolin and bentonite. Appl. Clay Sci. 2019, 182, 105257. [Google Scholar] [CrossRef]
- Choi, Y.-W.; Rok, O.S.; Seok, P.M.; Choi, B.-K. The Experimental Study on the Fluidity Properties of Mortar Using Basalt Fiber and High Volume Fly Ash. J. Korean Recycl. Constr. Resour. Inst. 2014, 2, 345–353. [Google Scholar]
- Libre, N.A.; Khoshnazar, R.; Shekarchi, M. Relationship between fluidity and stability of self-consolidating mortar incorporating chemical and mineral admixtures. Constr. Build. Mater. 2010, 24, 1262–1271. [Google Scholar] [CrossRef]
- Gu, X.W.; Wang, H.Y.; Zhu, Z.G.; Liu, J.P.; Xu, X.C.; Wang, Q. Synergistic effect and mechanism of lithium slag on mechanical properties and microstructure of steel slag-cement system. Constr. Build. Mater. 2023, 396, 131768. [Google Scholar] [CrossRef]
- Xu, W.X.; Chen, H.S. Numerical investigation of effect of particle shape and particle size distribution on fresh cement paste microstructure via random sequential packing of dodecahedral cement particles. Comput. Struct. 2013, 114, 35–45. [Google Scholar] [CrossRef]
- Wang, X.Y. Analysis of hydration kinetics and strength progress in cement-slag binary composites. J. Build. Eng. 2021, 35, 101810. [Google Scholar] [CrossRef]
- Suraneni, P.; Weiss, J. Examining the pozzolanicity of supplementary cementitious materials using isothermal calorimetry and thermogravimetric analysis. Cem. Concr. Compos. 2017, 83, 273–278. [Google Scholar] [CrossRef]




| Chemical Composition (wt. %) | Materials | ||||
|---|---|---|---|---|---|
| PC | IOTs | SS | FA | GBFS | |
| SiO2 | 28.16 | 70.46 | 15.20 | 60.20 | 34.50 |
| Al2O3 | 7.44 | 0.91 | 2.53 | 29.39 | 17.70 |
| CaO | 54.86 | 2.68 | 42.65 | 2.49 | 34.00 |
| Fe2O3 | 2.76 | 14.54 | 27.54 | 3.78 | 1.03 |
| MgO | 2.37 | 11.29 | 6.05 | 0.51 | 6.01 |
| SO3 | 2.32 | 0.12 | 0.12 | 0.26 | 1.64 |
| Specific surface area (m2/g) | 1.01 | 0.83 | 0.97 | 1.02 | 1.23 |
| Mixture | Mix Proportion | Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC | IOTs | SS | FA | GBFS | Sand | Water | SS | FA | GBFS | |
| D1 | 315 | 45 | 30 | 30 | 30 | 1350 | 225 | 1 | 1 | 1 |
| D2 | 45 | 18 | 36 | 36 | 1 | 2 | 2 | |||
| D3 | 45 | 13 | 39 | 39 | 1 | 3 | 3 | |||
| D4 | 45 | 10 | 40 | 40 | 1 | 4 | 4 | |||
| D5 | 45 | 36 | 18 | 36 | 2 | 1 | 2 | |||
| D6 | 45 | 36 | 36 | 18 | 2 | 2 | 1 | |||
| D7 | 45 | 20 | 30 | 40 | 2 | 3 | 4 | |||
| D8 | 45 | 20 | 40 | 30 | 2 | 4 | 3 | |||
| D9 | 45 | 39 | 13 | 39 | 3 | 1 | 3 | |||
| D10 | 45 | 30 | 20 | 40 | 3 | 2 | 4 | |||
| D11 | 45 | 39 | 39 | 13 | 3 | 3 | 1 | |||
| D12 | 45 | 30 | 40 | 20 | 3 | 4 | 2 | |||
| D13 | 45 | 40 | 10 | 40 | 4 | 1 | 4 | |||
| D14 | 45 | 40 | 20 | 30 | 4 | 2 | 3 | |||
| D15 | 45 | 40 | 30 | 20 | 4 | 3 | 2 | |||
| D16 | 45 | 40 | 40 | 10 | 4 | 4 | 1 | |||
| Mixture | Levels of the Composite SCMs | Compressive Strength | |||
|---|---|---|---|---|---|
| SS | FA | GBFS | 7 d | 28 d | |
| D1 | 1 | 1 | 1 | 21.0 | 32.2 |
| D2 | 1 | 2 | 2 | 22.7 | 31.3 |
| D3 | 1 | 3 | 3 | 22.9 | 34.5 |
| D4 | 1 | 4 | 4 | 20.9 | 30.2 |
| D5 | 2 | 1 | 2 | 22.1 | 31.0 |
| D6 | 2 | 2 | 1 | 18.6 | 32.7 |
| D7 | 2 | 3 | 4 | 23.1 | 34.3 |
| D8 | 2 | 4 | 3 | 17.4 | 31.7 |
| D9 | 3 | 1 | 3 | 21.1 | 33.6 |
| D10 | 3 | 2 | 4 | 20.2 | 33.1 |
| D11 | 3 | 3 | 1 | 19.0 | 28.3 |
| D12 | 3 | 4 | 2 | 18.0 | 35.0 |
| D13 | 4 | 1 | 4 | 20.7 | 23.1 |
| D14 | 4 | 2 | 3 | 21.0 | 30.3 |
| D15 | 4 | 3 | 2 | 24.7 | 32.0 |
| D16 | 4 | 4 | 1 | 24.8 | 32.2 |
| Age | Factor | SS | FA | GBFS |
|---|---|---|---|---|
| 7 d | 21.875 | 21.225 | 20.850 | |
| 20.300 | 20.625 | 21.925 | ||
| 19.575 | 22.475 | 20.600 | ||
| 22.850 | 20.275 | 21.225 | ||
| 3.275 | 2.200 | 1.325 | ||
| 28 d | 32.050 | 29.975 | 31.350 | |
| 32.425 | 31.850 | 32.325 | ||
| 32.500 | 32.275 | 32.525 | ||
| 29.400 | 32.275 | 30.175 | ||
| 3.100 | 2.300 | 2.350 |
| NO. | Ettringite and C-S-H Gel Dehydration Weight Loss | Ca(OH)2 Decomposition Weight Loss | CaCO3 Decomposition Weight Loss | Total Loss | H2O | Ca(OH)2 |
|---|---|---|---|---|---|---|
| D16 | 9.9 | 1.2 | 2.2 | 19.8 | 12.00 | 5.83 |
| D12 | 10.9 | 1.1 | 2.4 | 21.2 | 12.98 | 5.50 |
| C | 12.3 | 2.7 | 7.3 | 27.5 | 17.99 | 14.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Gu, X.; Liu, J.; Ge, X.; Wang, S.; Yin, S. Mechanical Properties and Hydration Mechanism of Iron Tailings–Cement-Based Supplementary Cementitious Materials. Buildings 2024, 14, 1044. https://doi.org/10.3390/buildings14041044
Hu Z, Gu X, Liu J, Ge X, Wang S, Yin S. Mechanical Properties and Hydration Mechanism of Iron Tailings–Cement-Based Supplementary Cementitious Materials. Buildings. 2024; 14(4):1044. https://doi.org/10.3390/buildings14041044
Chicago/Turabian StyleHu, Ziyang, Xiaowei Gu, Jianping Liu, Xiaowei Ge, Shenyu Wang, and Shiqi Yin. 2024. "Mechanical Properties and Hydration Mechanism of Iron Tailings–Cement-Based Supplementary Cementitious Materials" Buildings 14, no. 4: 1044. https://doi.org/10.3390/buildings14041044






