Passivation of Steel Reinforcement in Low Carbon Concrete
Abstract
1. Introduction
Investigated Binder Types Alternative to OPC
2. Materials and Methods
2.1. Materials and Mixes
2.2. Electrochemical Tests
3. Results and Discussion
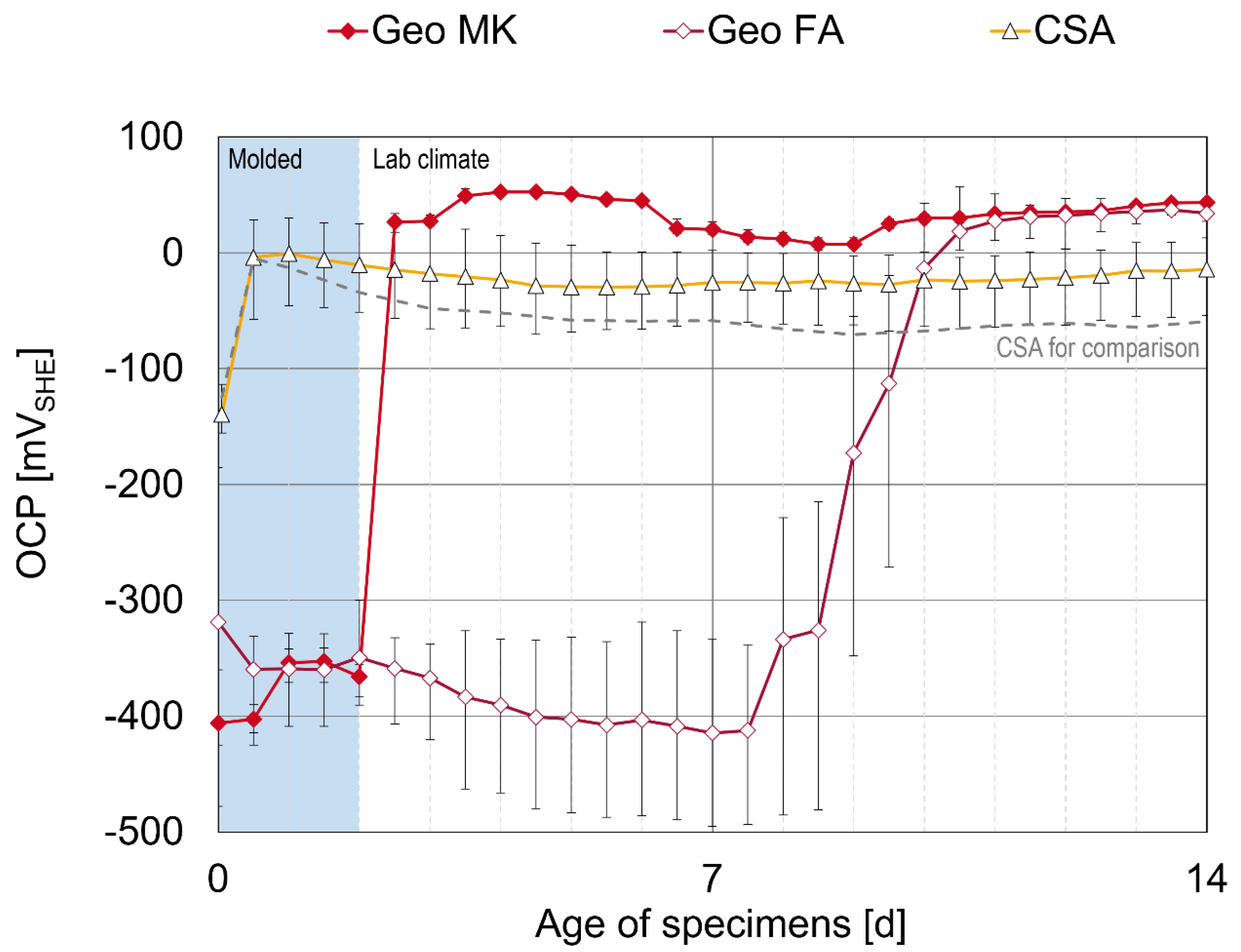
3.1. Open Circuit Potential and Polarization Curve
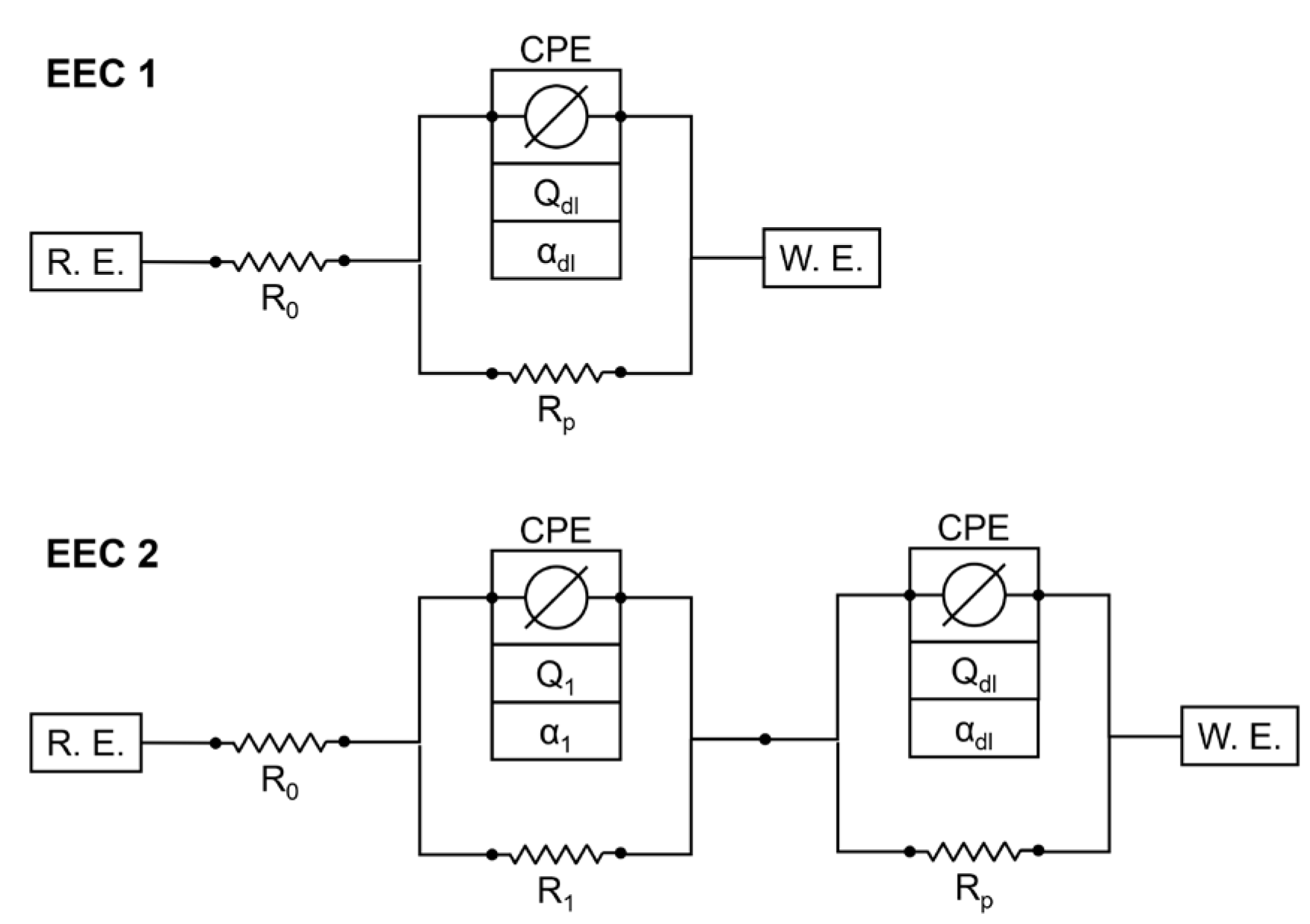
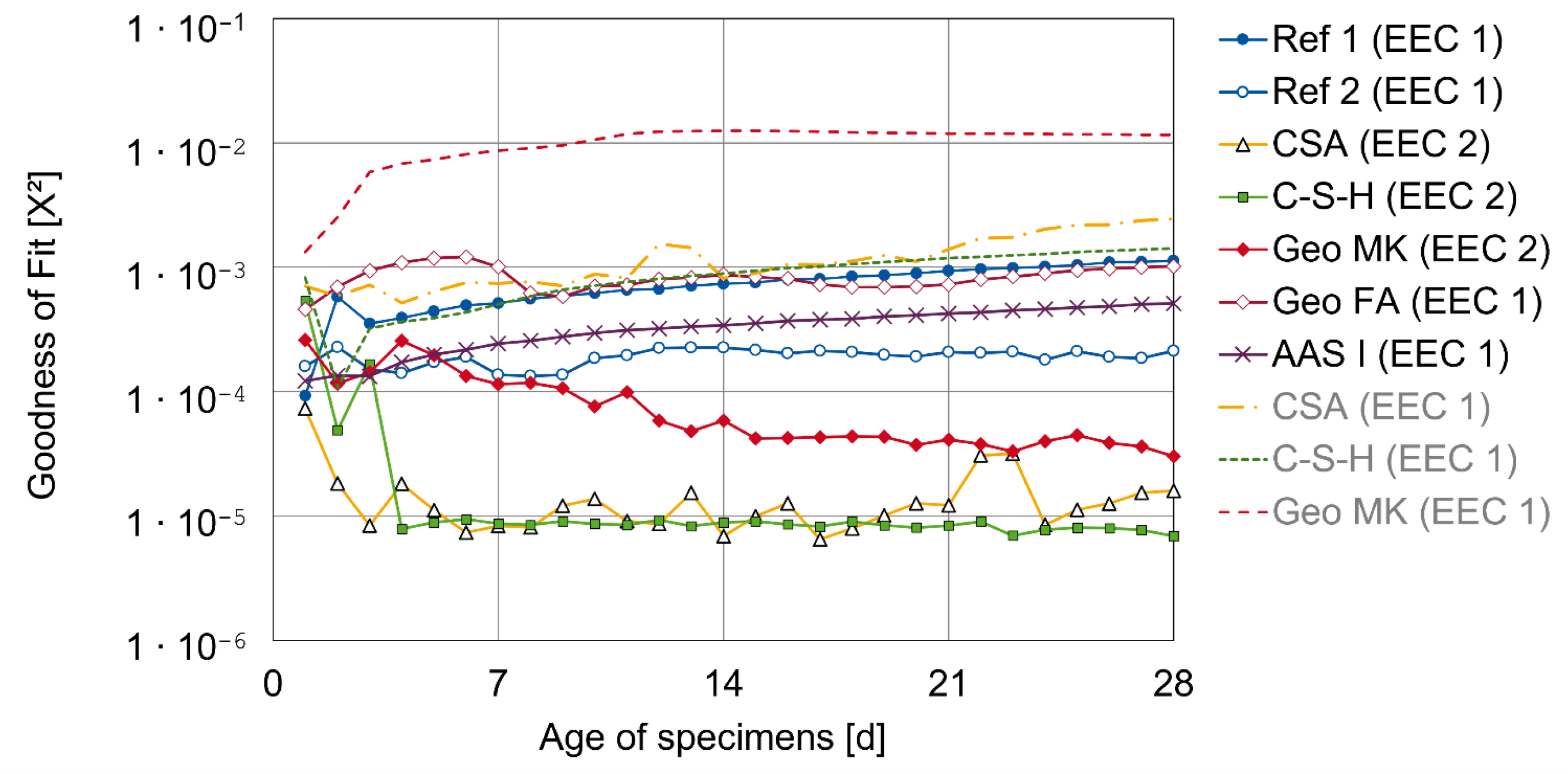
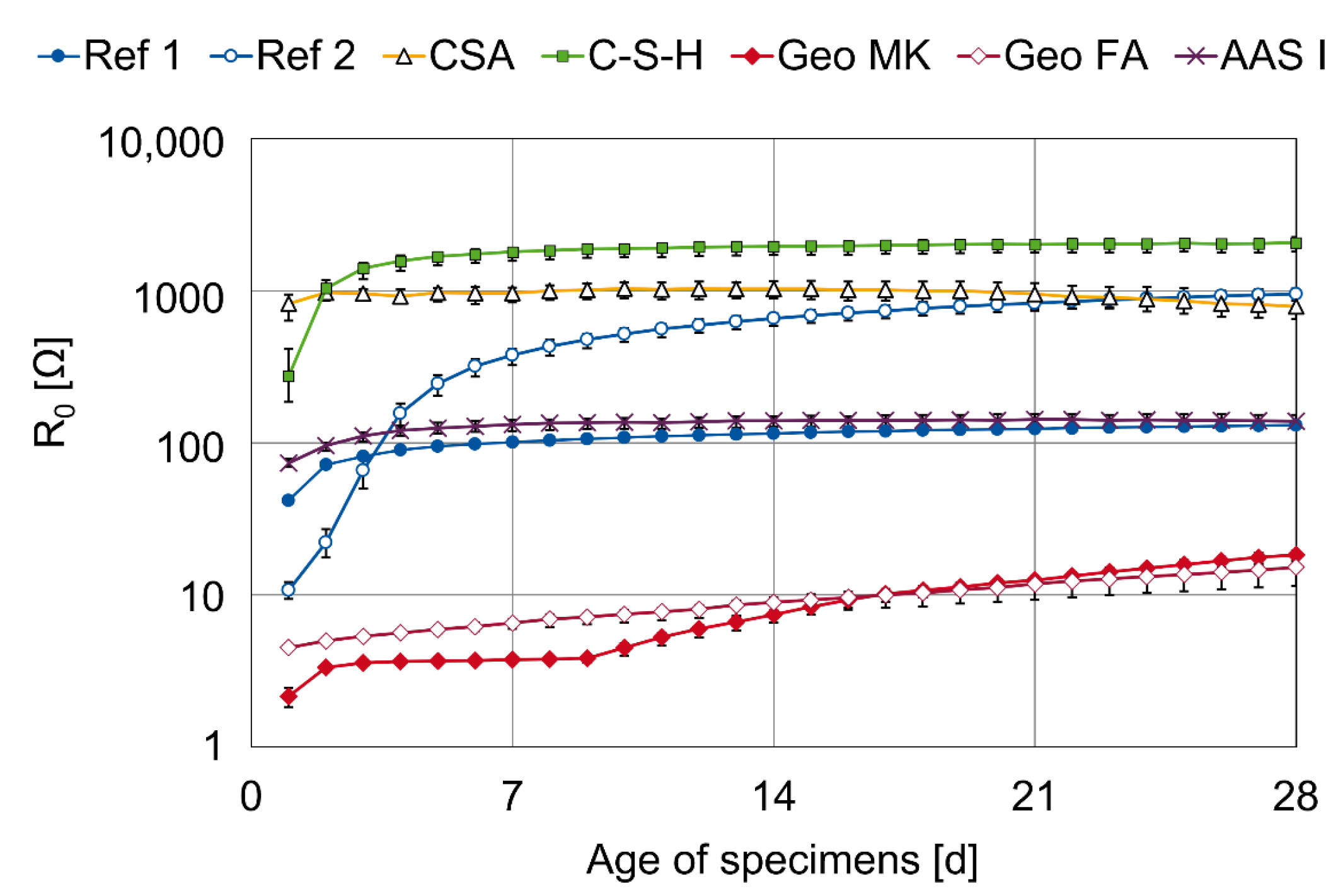
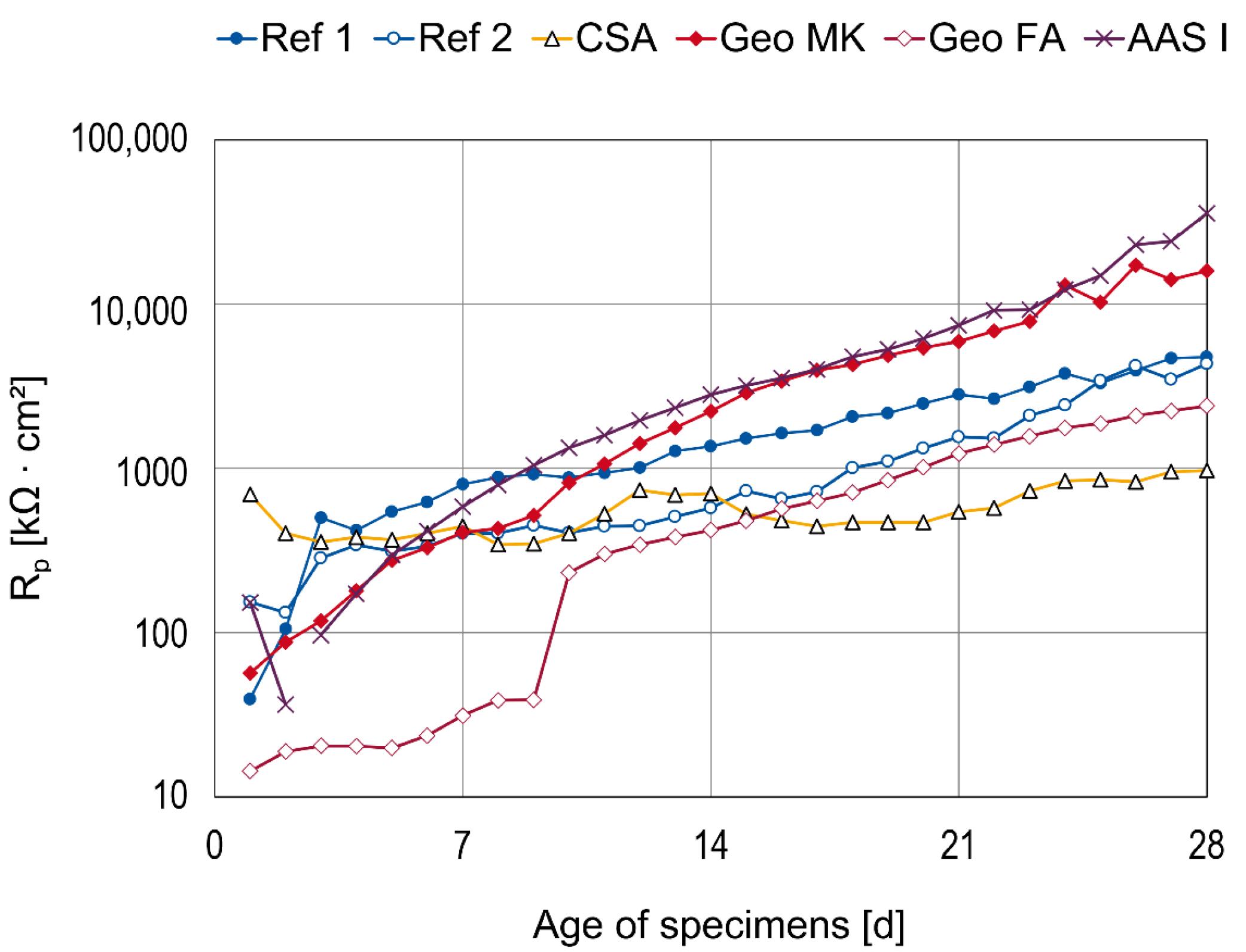
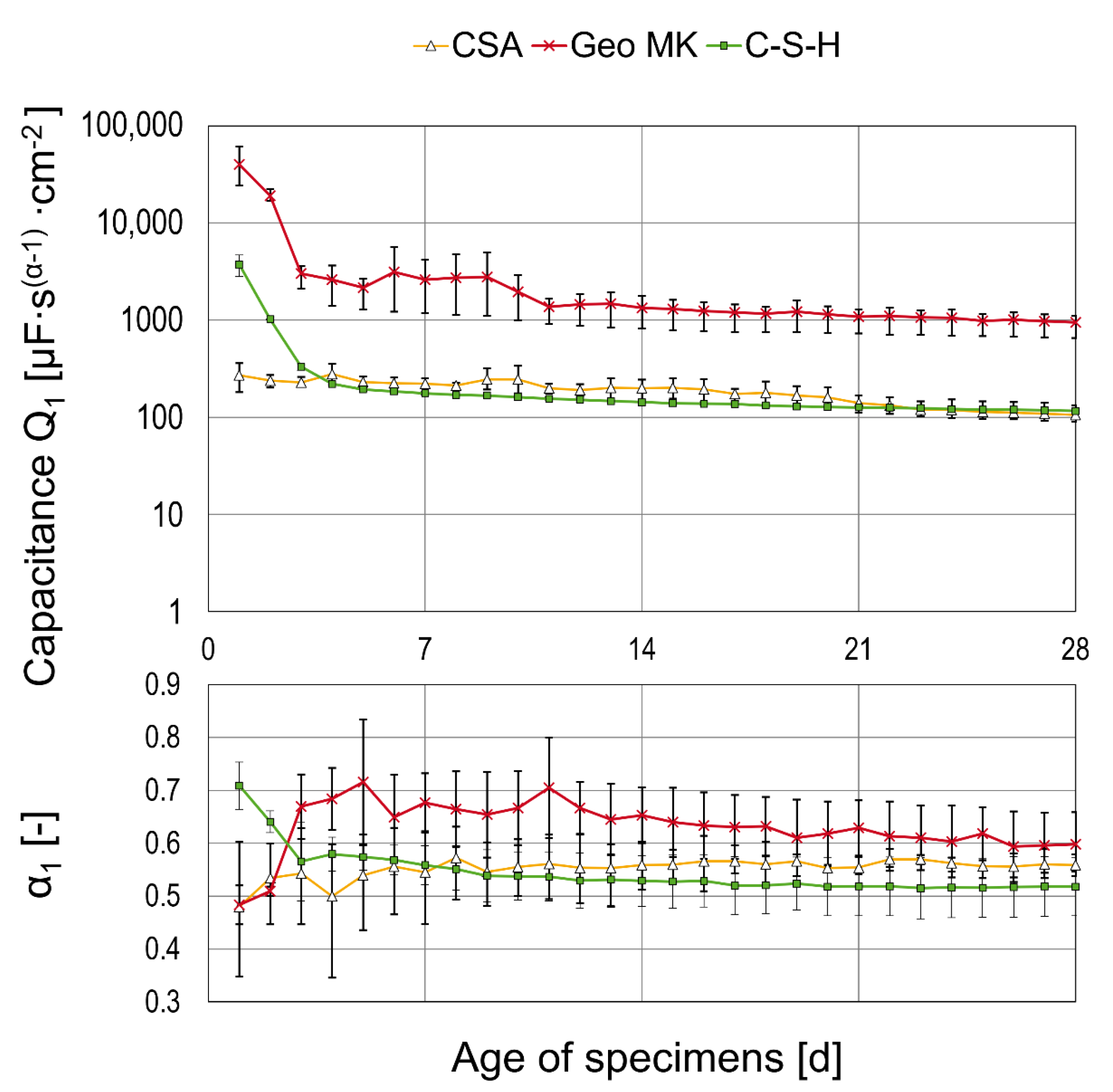
3.2. Results of AC Impedance Spectroscopy
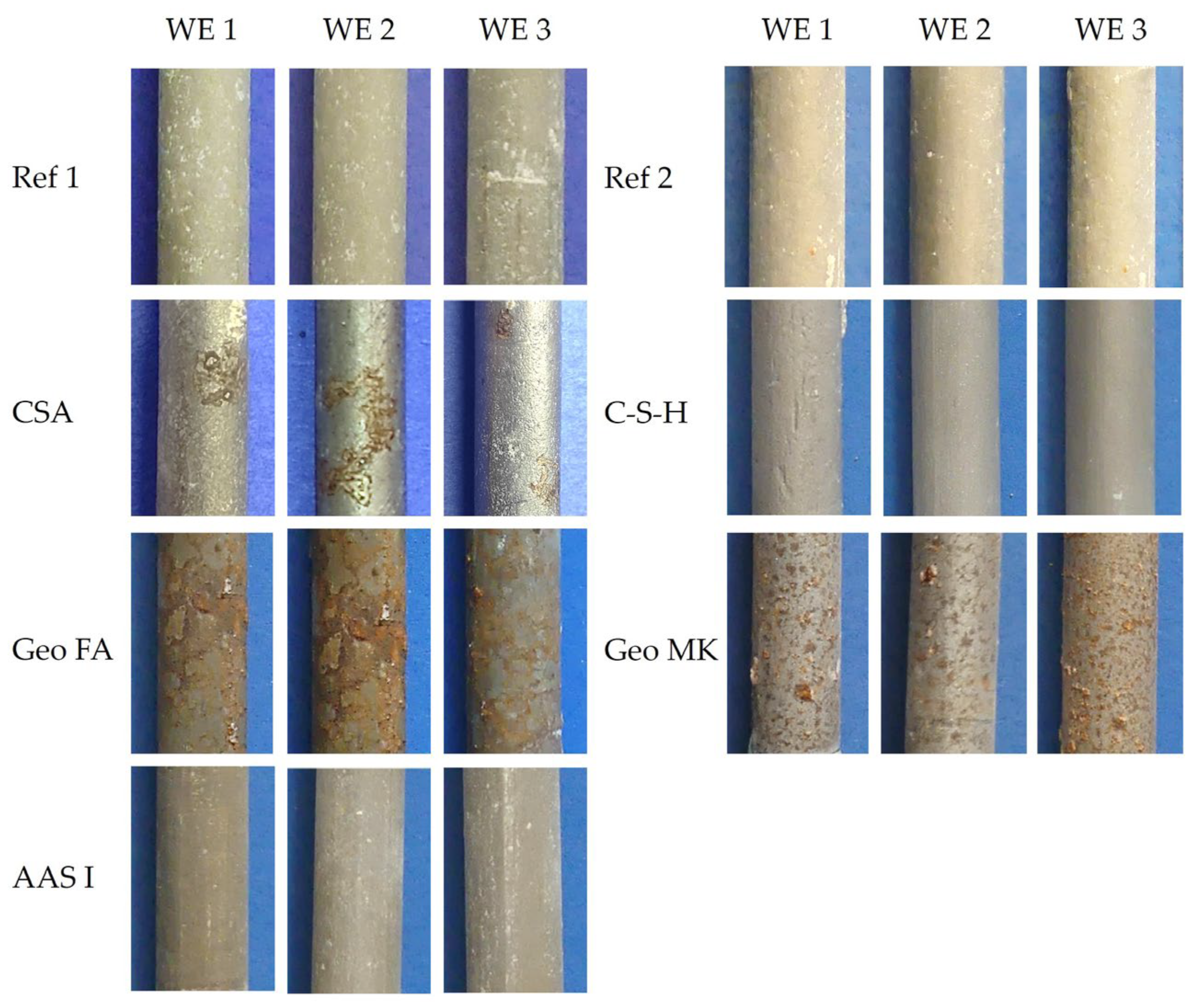
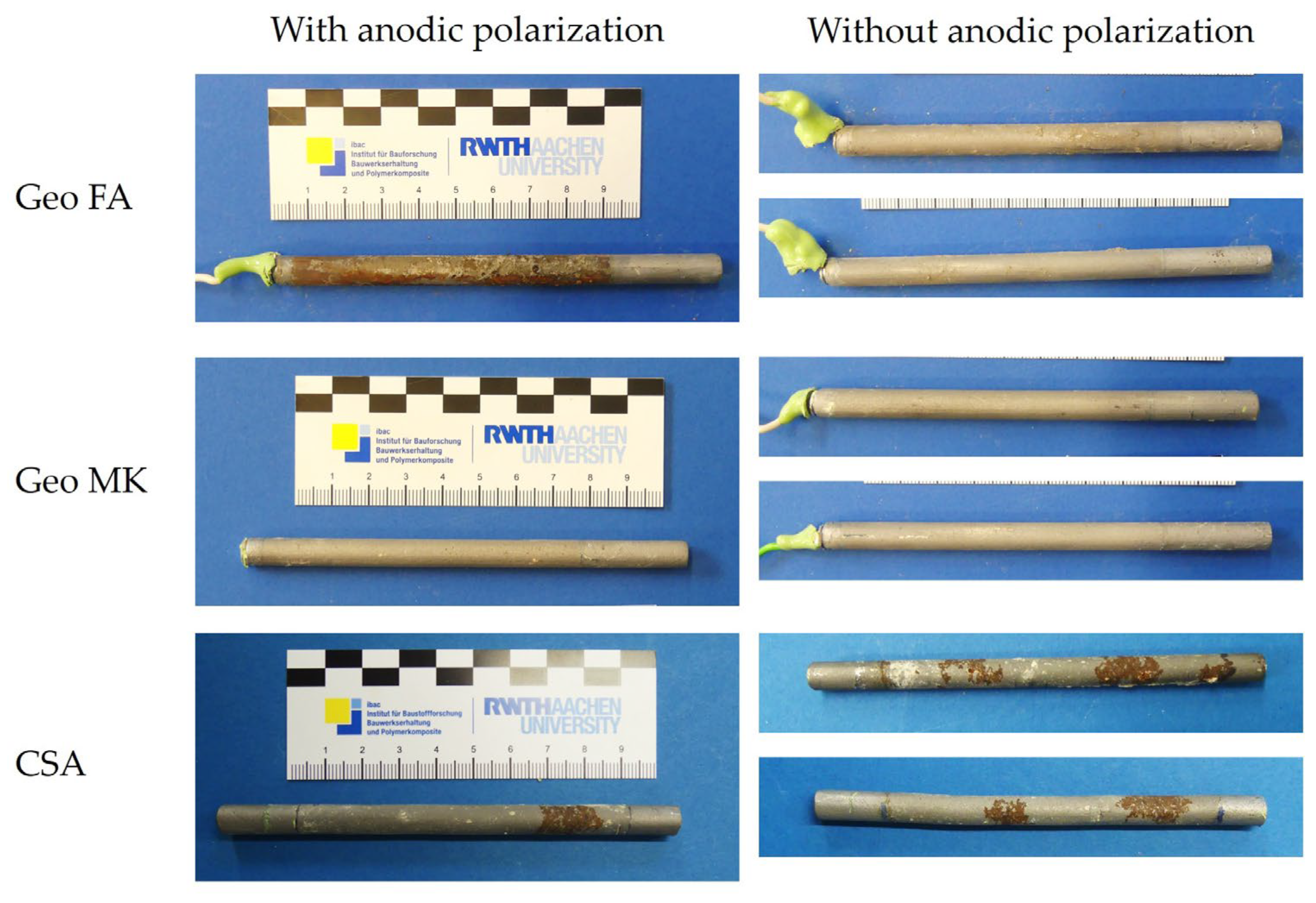
3.3. Working Electrodes after the Test
- Was the passivation influenced by the storage conditions, where the specimens were covered by plastic sheets for the first 7 days and stayed in their formwork for the whole 28 days?
- Has the corrosion occurred before or during the determination of the polarization curve?
4. Additional Tests on the Influence of Early Age Treatment on Geopolymers and CSA
5. Summary
6. Conclusions
- The CA mortar shows a weaker passivation during the observed period up to an age of 28 days, at least when desiccation is impeded. Lower and fluctuating OCP and polarization resistances are observed than in other binder types.
- The mortar based on Celitement (C-S-H) leads to passivation of the steel and reaches stable conditions within 5 to 7 days. The OCP was slightly more positive than for the other binder types investigated here, right from the beginning. Electrochemical measurements can be challenging because of the high resistivity of the mortar.
- The two low-Ca AAMs (geopolymers) based on fly ash or metakaolin showed very low electric resistances of the mortars. The onset of passivation was influenced by the storage condition of the specimens at a young age in the potassium silicate-activated metakaolin. The passivation of steel in alkali-activated fly ash (with sodium-based activator) was delayed and started approximately 7–9 days after casting. This effect was independent of the storage condition.
- The steel in the AAS mortar passivated within 5–7 days. However, the results differ from typical AASs in OCP values and polarization curve shape, which is attributed to the low sulfur content in the investigated AAS.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | TiO2 | K2O | Na2O | SO3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| CEM I (Ref 1) | 20.50 | 4.70 | 2.90 | 63.20 | 1.30 | 0.03 | 0.20 | 1.22 | 0.28 | 3.20 |
| CEM III/B (Ref 2) | 30.5 | 8.80 | 1.40 | 45.80 | 6.20 | 0.11 | 0.55 | 1.04 | 0.34 | 2.20 |
| A (CSA) | 6.70 | 24.00 | 3.30 | 43.30 | 1.10 | 0.01 | 1.09 | 0.27 | 0.09 | 17.40 |
| Celitement (C-S-H) | 40.90 | 2.00 | 1.20 | 43.10 | 1.20 | 0.01 | 0.03 | 0.86 | 0.29 | 0.00 |
| Metakaolin (Geo MK) | 65.80 | 21.20 | 4.30 | 2.20 | 0.50 | 0.01 | 0.80 | 0.49 | 0.16 | 0.10 |
| Fly ash (Geo FA) | 56.15 | 21.83 | 8.68 | 5.61 | 2.29 | 0.08 | 0.94 | 2.19 | 0.89 | 0.45 |
| Slag (AAS I) | 29.28 | 17.61 | 1.35 | 41.05 | 6.05 | 0.24 | 0.59 | 0.14 | 0.84 | 0.74 |
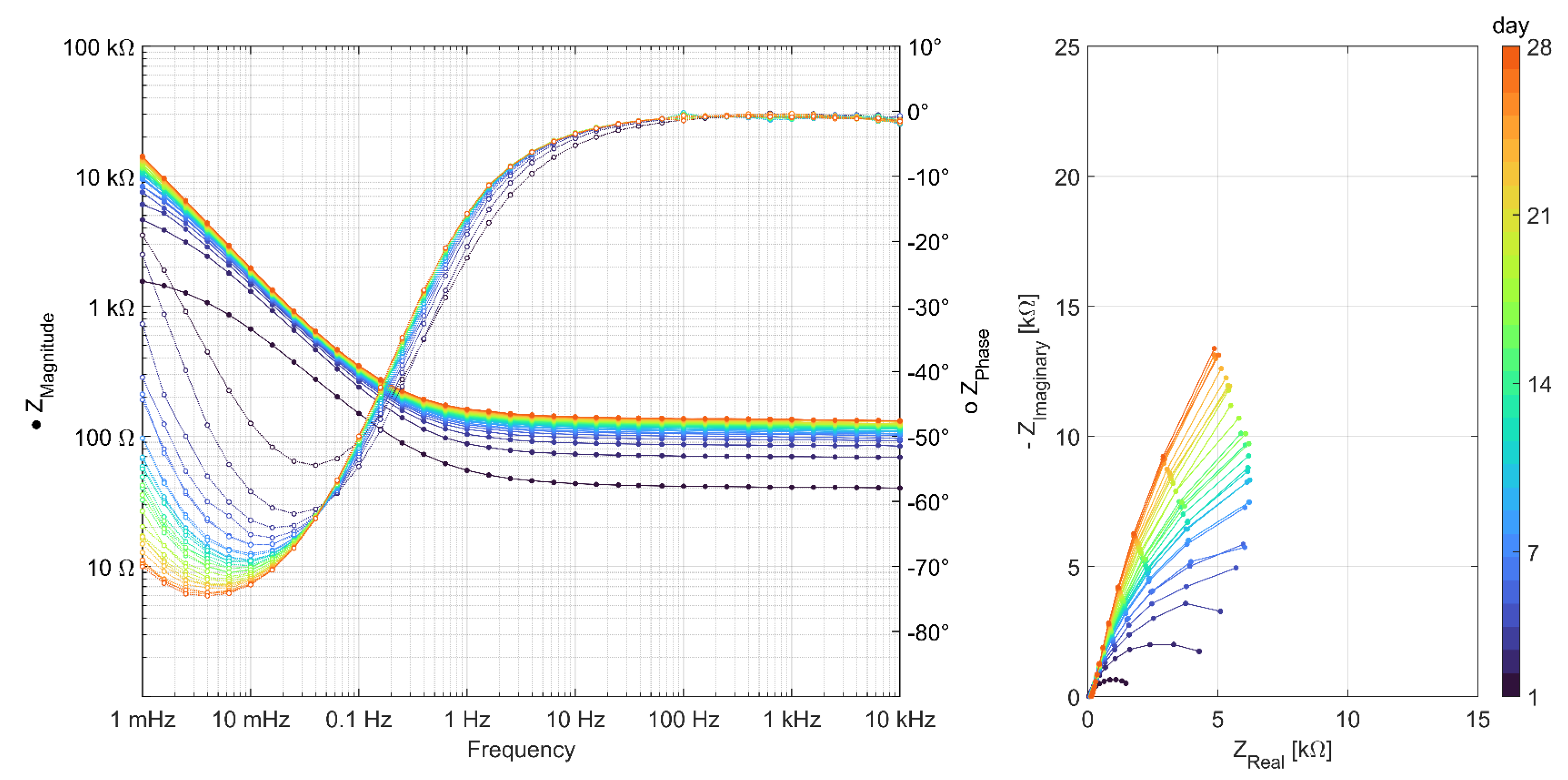
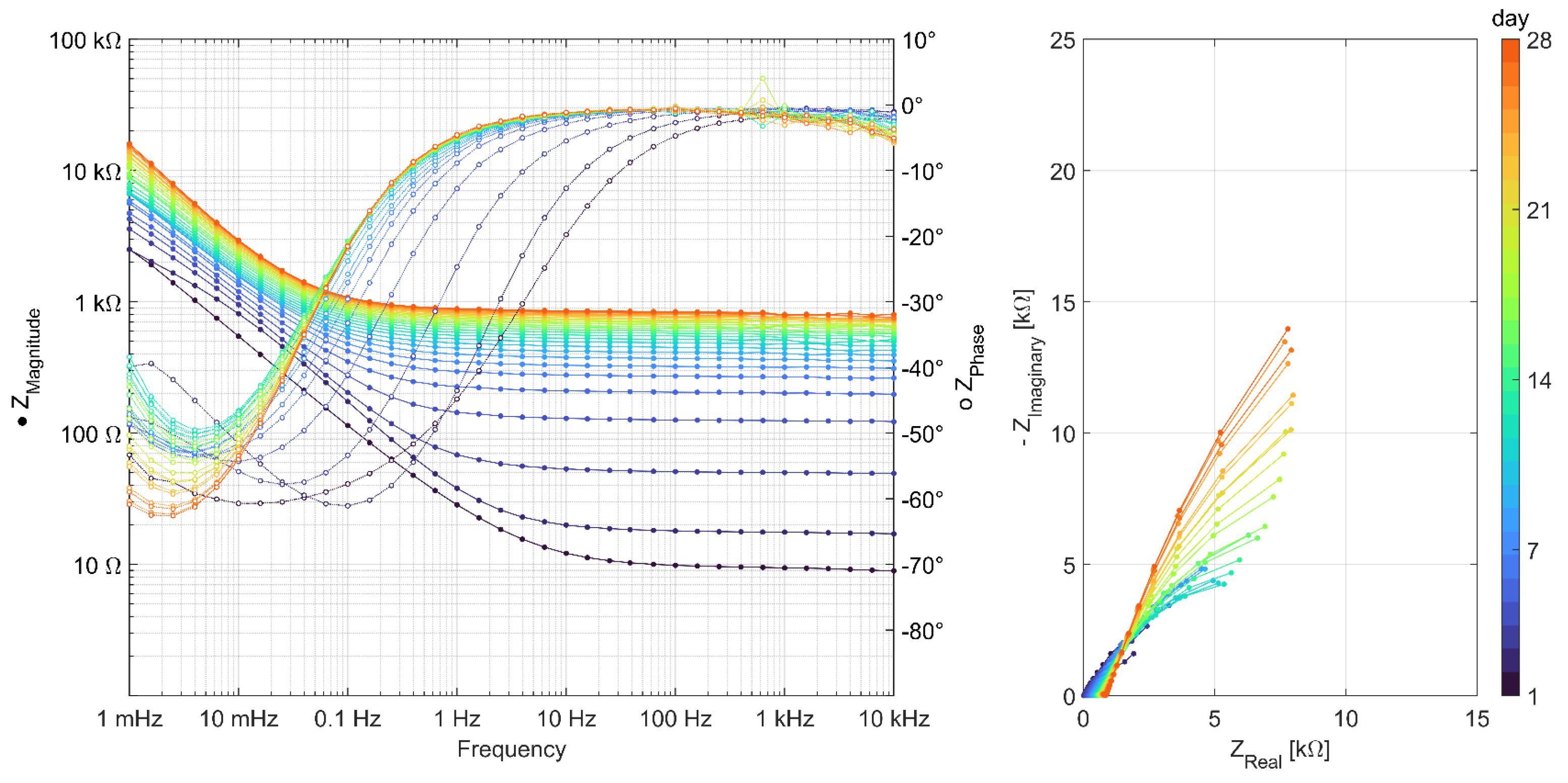


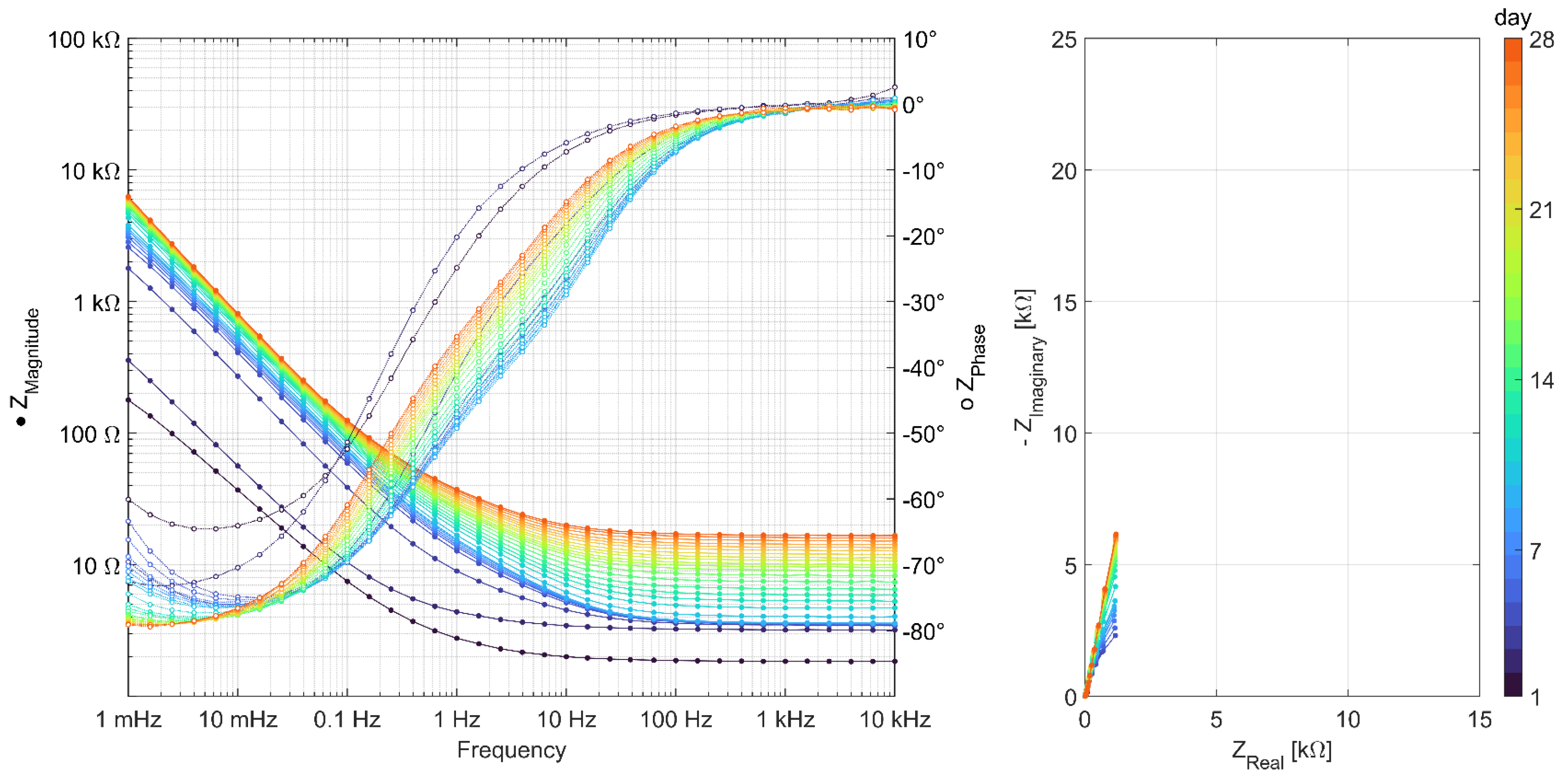

References
- Olivier, J.G.J.; Janssens-Maenhout, G.; Muntean, M.; Peters, J.A. Trends in Global CO2 Emissions: 2016 Report. 2016. Available online: https://www.pbl.nl/en/publications/trends-in-global-co2-emissions-2016-report (accessed on 1 February 2024).
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 74029. [Google Scholar] [CrossRef]
- Scrivener, K.; Ben Haha, M.; Juilland, P.; Levy, C. Research needs for cementitious building materials with focus on Europe. RILEM Tech. Lett. 2022, 7, 220–252. [Google Scholar] [CrossRef]
- Alhozaimy, A.; Hussain, R.R.; Al-Negheimish, A. Significance of oxygen concentration on the quality of passive film formation for steel reinforced concrete structures during the initial curing of concrete. Cem. Concr. Compos. 2016, 65, 171–176. [Google Scholar] [CrossRef]
- Poursaee, A.; Hansson, C.M. Reinforcing steel passivation in mortar and pore solution. Cem. Concr. Res. 2007, 37, 1127–1133. [Google Scholar] [CrossRef]
- Poursaee, A. Corrosion of Steel in Concrete Structures, 2nd ed.; Elsevier Science & Technology: San Diego, CA, USA, 2023; ISBN 978-0-12-821840-2. [Google Scholar]
- Sharp, J.H.; Lawrence, C.D.; Yang, R. Calcium sulfoaluminate cements—Low-energy cements, special cements or what? Adv. Cem. Res. 1999, 11, 3–13. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Kraft, B.; Achenbach, R.; Ludwig, H.-M.; Raupach, M. Hydration and Carbonation of Alternative Binders. CMD 2022, 3, 19–52. [Google Scholar] [CrossRef]
- Andac, M.; Glasser, F.P. Pore solution composition of calcium sulfoaluminate cement. Adv. Cem. Res. 1999, 11, 23–26. [Google Scholar] [CrossRef]
- Carsana, M.; Bianchi, M.; Canonico, F.; Buzzi, L.; Capelli, L.; Bertolini, L. Corrosion behaviour of steel embedded in calcium sulphoaluminate-cement concrete. In Proceedings of the 14th International Congress on the Chemistry of Cement, Beijing, China, 13–16 October 2015. [Google Scholar]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—Part 1. J. Appl. Biomater. Funct. Mater. 2018, 16, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhan, S.; Tang, X.; Xu, Q.; Qian, K. Pore Solution Chemistry of Calcium Sulfoaluminate Cement and Its Effects on Steel Passivation. Appl. Sci. 2019, 9, 1092. [Google Scholar] [CrossRef]
- Kalogridis, D.; Kostogloudis, G.; Ftikos, C.; Malami, C. A quantitative study of the influence of non-expansive sulfoaluminate cement on the corrosion of steel reinforcement. Cem. Concr. Res. 2000, 30, 1731–1740. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Wang, L.; Jian, Q.; Zhan, S.; Song, Y.; Ruan, S. Chloride-induced corrosion patterns of reinforcements in simulated pore solutions of calcium sulfoaluminate cement concrete: An analytical study. J. Build. Eng. 2024, 82, 108189. [Google Scholar] [CrossRef]
- Carsana, M.; Canonico, F.; Bertolini, L. Corrosion resistance of steel embedded in sulfoaluminate-based binders. Cem. Concr. Compos. 2018, 88, 211–219. [Google Scholar] [CrossRef]
- Yang, F. Corrosion Protection of Steel Embedded in New Sustainable Cementitious Materials. Ph.D. Thesis, Politecnico di Milano, Milan, Italy, 2017. [Google Scholar]
- Tan, B.; Okoronkwo, M.U.; Kumar, A.; Ma, H. Durability of calcium sulfoaluminate cement concrete. J. Zhejiang Univ. Sci. A 2020, 21, 118–128. [Google Scholar] [CrossRef]
- European Cement Research Academy. Development of State of the Art-Techniques in Cement Manufacturing: Trying to Look Ahead; CSI/ECRATechnology Papers 2017; European Cement Research Academy: Duesseldorf, Germany, 2017; Available online: https://ecra-online.org/research/technology-papers (accessed on 20 March 2024).
- Hinder, D.; Zimmermann, A.; Fylak, M. Mechanochemical Activation of the C-S-H binder Celitement. CE/Papers 2023, 6, 54–61. [Google Scholar] [CrossRef]
- Nolting, U.; Dehn, F.; Haist, M.; Link, J. Betone der Zukunft: Herausforderungen und Chancen. In Proceedings of the 14th Symposium Baustoffe und Bauwerkserhaltung, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 21 March 2018; Nolting, U., Dehn, F., Haist, M., Link, J., Eds.; KIT Scientific Publishing: Karlsruhe, Germany, 2018. ISBN 978-3-731507673. [Google Scholar]
- Mundra, S. Corrosion of Steel in Alkali-Activated Materials. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2018. [Google Scholar]
- Mundra, S.; Samson, G.; Masi, G.; Achenbach, R.; Bastidas, D.M.; Bernal, S.A.; Bignozzi, M.C.; Criado, M.; Cyr, M.; Gartner, N.; et al. Application of electrochemical methods for studying steel corrosion in alkali-activated materials. Mater. Corros. 2023, 74, 988–1008. [Google Scholar] [CrossRef]
- Mundra, S.; Provis, J.L. Mechanisms of passivation and chloride-induced corrosion of mild steel in sulfide-containing alkaline solutions. J. Mater. Sci. 2021, 56, 14783–14802. [Google Scholar] [CrossRef]
- Zuo, Y.; Nedeljković, M.; Ye, G. Pore solution composition of alkali-activated slag/fly ash pastes. Cem. Concr. Res. 2019, 115, 230–250. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Holzer, L.; Figi, R.; Winnefeld, F. Hydration of alkali-activated slag: Comparison with ordinary Portland cement. Adv. Cem. Res. 2006, 18, 119–128. [Google Scholar] [CrossRef]
- Tittarelli, F.; Mobili, A.; Giosuè, C.; Belli, A.; Bellezze, T. Corrosion behaviour of bare and galvanized steel in geopolymer and Ordinary Portland Cement based mortars with the same strength class exposed to chlorides. Corros. Sci. 2018, 134, 64–77. [Google Scholar] [CrossRef]
- Fan, L.F.; Zhong, W.L.; Zhang, Y.H. Effect of the composition and concentration of geopolymer pore solution on the passivation characteristics of reinforcement. Constr. Build. Mater. 2022, 319, 126128. [Google Scholar] [CrossRef]
- Mobili, A.; Belli, A.; Giosuè, C.; Bellezze, T.; Tittarelli, F. Metakaolin and fly ash alkali-activated mortars compared with cementitious mortars at the same strength class. Cem. Concr. Res. 2016, 88, 198–210. [Google Scholar] [CrossRef]
- DIN EN 197-1; Zement: Teil 1: Zusammensetzung, Anforderungen und Konformitätskriterien von Normalzement. Beuth Verlag GmbH: Berlin, Germany, 2011.
- DIN EN 10025-2; Warmgewalzte Erzeugnisse aus Baustählen: Teil 2: Technische Lieferbedingungen für Unlegierte Baustähle. Beuth Verlag GmbH: Berlin, Germany, 2019.
- Mundra, S.; Bernal, S.A.; Criado, M.; Hlaváček, P.; Ebell, G.; Reinemann, S.; Gluth, G.J.; Provis, J. Steel corrosion in reinforced alkali-activated materials. RILEM Tech. Lett. 2017, 2, 33–39. [Google Scholar] [CrossRef]
- Elsener, B.; Andrade, C.; Gulikers, J.; Polder, R.; Raupach, M. Recommendations of RILEM TC 154-EMC: Electrochemical techniques for measuring metallic corrosion Half-cell potential measurements-Potential mapping on reinforced concrete structures. Mater. Struct. 2003, 36, 461–471. [Google Scholar] [CrossRef]
- Oelßner, W.; Berthold, F.; Guth, U. The iR drop-well-known but often underestimated in electrochemical polarization measurements and corrosion testing. Mater. Corros. 2006, 57, 455–466. [Google Scholar] [CrossRef]
- Andrade, C. Propagation of reinforcement corrosion: Principles, testing and modelling. Mater. Struct. 2019, 52, 2. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Urquidi-Macdonald, M.; Real, S.; Macdonald, D.D. Application of Kramers-Kronig Transforms in the Analysis of Electrochemical Impedance Data: II. Transformations in the Complex Plane. J. Electrochem. Soc. 1986, 133, 2018–2024. [Google Scholar] [CrossRef]
- Herrera Hernández, H.; Ruiz Reynoso, A.M.; Trinidad González, J.C.; González Morán, C.O.; Miranda Hernández, J.G.; Mandujano Ruiz, A.; Morales Hernández, J.; Orozco Cruz, R. Electrochemical Impedance Spectroscopy (EIS): A Review Study of Basic Aspects of the Corrosion Mechanism Applied to Steels. In Electrochemical Impedance Spectroscopy; El-Azazy, M., Min, M., Annus, P., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-215-8. [Google Scholar]
- Sánchez, M.; Gregori, J.; Alonso, M.C.; García-Jareño, J.J.; Vicente, F. Anodic growth of passive layers on steel rebars in an alkaline medium simulating the concrete pores. Electrochim. Acta 2006, 52, 47–53. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Andrade, C.; Keddam, M.; Nóvoa, X.R.; Pérez, M.C.; Rangel, C.M.; Takenouti, H. Electrochemical behaviour of steel rebars in concrete: Influence of environmental factors and cement chemistry. Electrochim. Acta 2001, 46, 3905–3912. [Google Scholar] [CrossRef]
- Andrade, C.; Castelo, V.; Alonso, C.; González, J.A. The Determination of the Corrosion Rate of Steel Embedded in Concrete by the Polarization Resistance and AC Impedance Methods. In Corrosion Effect of Stray Currents and the Techniques for Evaluating Corrosion of Rebars in Concrete; Victor, C., Ed.; American Society for Testing and Materials: Philadelphia, PA, USA, 1985; pp. 43–63. ISBN 978-0-8031-0468-6. [Google Scholar]
- Achenbach, R.; Raupach, M.; Kraft, B.; Ludwig, H.-M. Comparative study on the electric resistance of mortars made of low carbon binders. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Achenbach, R.; Raupach, M. Applicability of the formation factor for different alternative binder types investigated on mortars. Corrosion 2023, 79, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Gluth, G.J.G.; Ebell, G.; Hlaváček, P.; Mietz, J. Chloride-induced steel corrosion in alkali-activated fly ash mortar: Increased propensity for corrosion initiation at defects. Mater. Corros. 2020, 71, 749–758. [Google Scholar] [CrossRef]
- Runci, A.; Provis, J.L.; Serdar, M. Revealing corrosion parameters of steel in alkali-activated materials. Corros. Sci. 2023, 210, 110849. [Google Scholar] [CrossRef]
- Benoit, M.; Bataillon, C.; Gwinner, B.; Miserque, F.; Orazem, M.E.; Sánchez-Sánchez, C.M.; Tribollet, B.; Vivier, V. Comparison of different methods for measuring the passive film thickness on metals. Electrochim. Acta 2016, 201, 340–347. [Google Scholar] [CrossRef]
- Sánchez, M.; Gregori, J.; Alonso, C.; García-Jareño, J.J.; Takenouti, H.; Vicente, F. Electrochemical impedance spectroscopy for studying passive layers on steel rebars immersed in alkaline solutions simulating concrete pores. Electrochim. Acta 2007, 52, 7634–7641. [Google Scholar] [CrossRef]
- Kurzweil, P. Angewandte Elektrochemie: Grundlagen, Messtechnik, Elektroanalytik, Energiewandlung, Technische Verfahren; Springer Vieweg: Wiesbaden/Heidelberg, Germany, 2020; ISBN 978-3-658-32420-9. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-0-470-04140-6. [Google Scholar]
- Volpi, E.; Olietti, A.; Stefanoni, M.; Trasatti, S.P. Electrochemical characterization of mild steel in alkaline solutions simulating concrete environment. J. Electroanal. Chem. 2015, 736, 38–46. [Google Scholar] [CrossRef]
- Feliu, V.; González, J.A.; Adrade, C.; Feliu, S. Equivalent circuit for modelling the steel-concrete interface. II. Complications in applying the stern-geary equation to corrosion rate determinations. Corros. Sci. 1998, 40, 995–1006. [Google Scholar] [CrossRef]
- Achenbach, R.; Kraft, B.I.E.; Raupach, M.; Ludwig, H.-M. Eignung des RCM-Versuchs zur Bestimmung des Chloridmigrationskoeffizienten in Mörteln aus alternativen Bindemitteln. CE/Papers 2023, 6, 1237–1245. [Google Scholar] [CrossRef]
- Moffatt, E.G.; Thomas, M.D.A. Durability of Rapid-Strength Concrete Produced with Ettringite-Based Binders. ACI Mater. J. 2018, 115, 105–115. [Google Scholar] [CrossRef]
- Mansfeld, F.; Kendig, M.W. Determination of the polarization resistance from impedance measurements. Mater. Corros. 1983, 34, 397–401. [Google Scholar] [CrossRef]
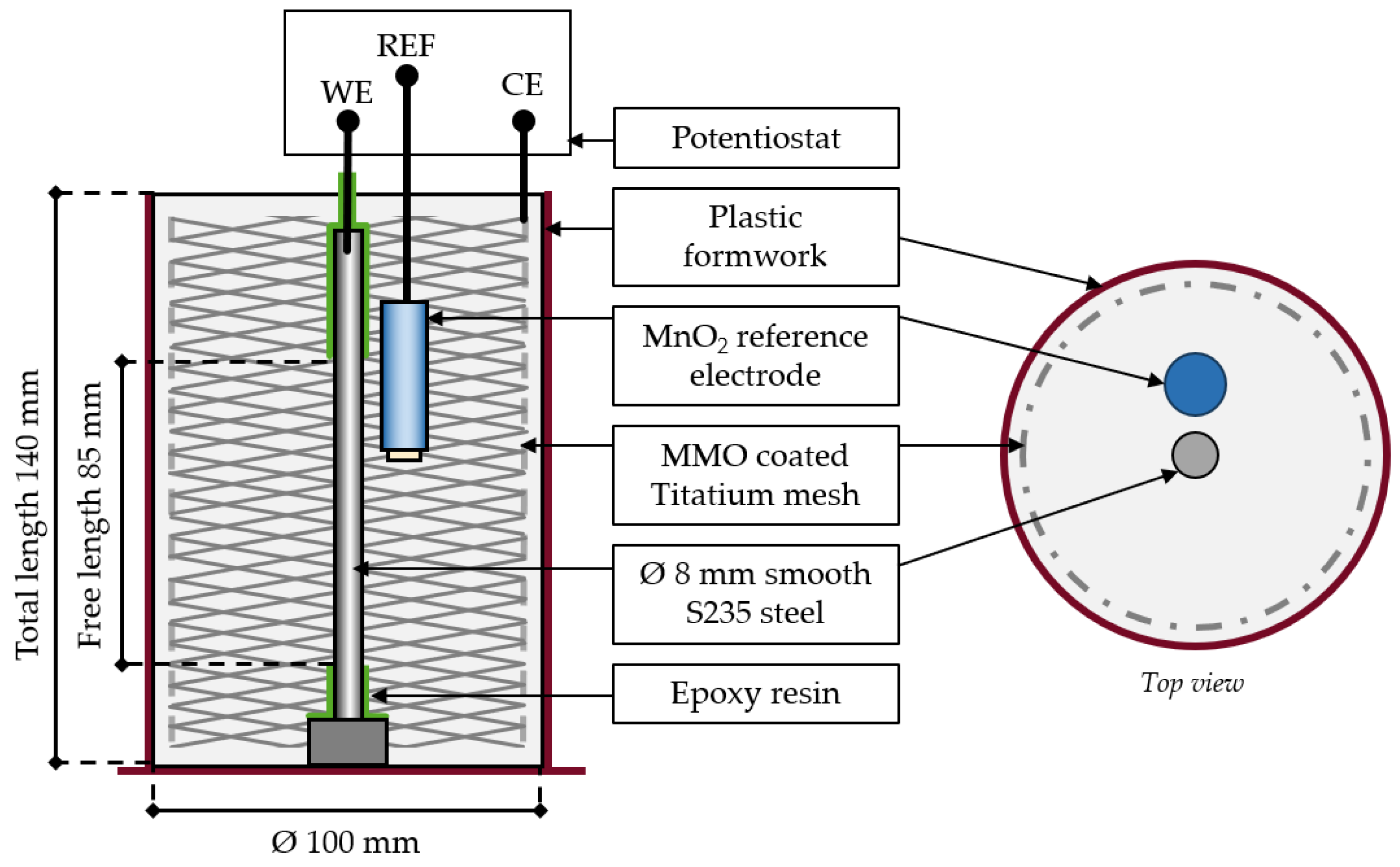




| Identifier | Material Composition | w/b |
|---|---|---|
| Ref 1 | CEM I 42.5 R | 0.5 |
| Ref 2 | CEM III/B CEM III/B 42.5 N-LH/SR | 0.5 |
| CSA | A (next Base) with 1% tartaric acid by weight of binder as retarder | 0.5 |
| C-S-H | Celitement, PCE-based superplasticizer (2% by weight of binder) | 0.4 |
| Geo MK | Metakaolin activated by potassium silicate (silicatemodulus 1.1) | 0.5 |
| Geo FA | Fly ash activated by NaOH (19.068 M) and sodium silicate (silicate modulus 2.0) | 0.34 |
| AAS I | Slag activated by sodium silicate (silicate modulus 2.0) | 0.38 |
| Fe | Mn | Cu | Si | Ni | C | Cr | Sb | Mo | S | Sn | As | Te | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 98.0 | 0.71 | 0.39 | 0.212 | 0.160 | 0.114 | 0.092 | 0.048 | 0.031 | 0.030 | 0.029 | 0.025 | 0.024 | 0.020 |
| Method | Measurement Parameters | Schedule |
|---|---|---|
| Open circuit potential (OCP) | Reference electrode: MnO2; Sample period: 1 s; Stability criterion: ±0.1 mV in 10 s | Daily from day 0 to 28 |
| Electrochemical impedance spectroscopy (EIS) | Frequency range: 0.001 Hz to 10 kHz; Voltage: ±10 mVrms vs. OCP; 5 points per decade | Daily from day 1 to 28 |
| Anodic polarization curve | Voltage: from OCP to ~1000 mVSHE (IR free); Feed rate: 2 mV/min; Sample period: 120 s | Once at the end (day 28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achenbach, R.; Raupach, M. Passivation of Steel Reinforcement in Low Carbon Concrete. Buildings 2024, 14, 895. https://doi.org/10.3390/buildings14040895
Achenbach R, Raupach M. Passivation of Steel Reinforcement in Low Carbon Concrete. Buildings. 2024; 14(4):895. https://doi.org/10.3390/buildings14040895
Chicago/Turabian StyleAchenbach, Rebecca, and Michael Raupach. 2024. "Passivation of Steel Reinforcement in Low Carbon Concrete" Buildings 14, no. 4: 895. https://doi.org/10.3390/buildings14040895
APA StyleAchenbach, R., & Raupach, M. (2024). Passivation of Steel Reinforcement in Low Carbon Concrete. Buildings, 14(4), 895. https://doi.org/10.3390/buildings14040895







