Evaluation of Eco-Friendly Consolidating Treatments in Pugliese Tuff (Gravina Calcarenite) Used in Italian Heritage Buildings
Abstract
1. Introduction
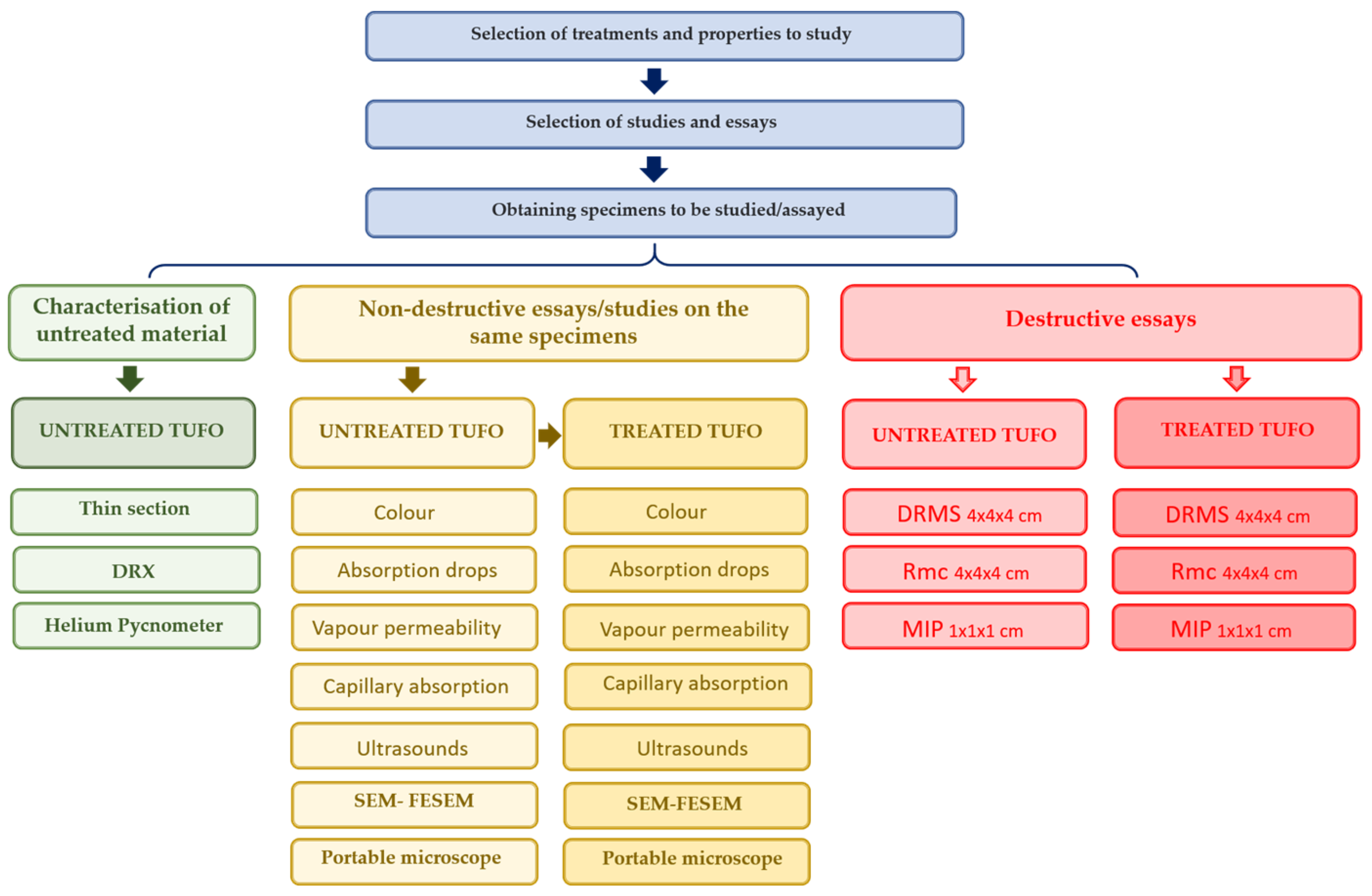
2. Materials and Methods
2.1. Materials and Treatments
- Bioconsolidant: Mixostone M3P from the company KBYO Biological S.L. Product patented by the University of Granada (Spain) [59]. The composition of the M3P nutritional solution includes 1% Bacto-Casitone (a hydrolyzed casein), 1% Ca(CH3COO)2·4H2O (total calcium: 43.44 mM), 0.2% K2CO3·1/2H2O (total potassium: 35.6 mM; total carbonate: 17.8 mM), and 10 mM phosphate buffer in distilled water (pH 8).
- Lime water enriched with lime putty with a concentration of 20 g/L from the company “Cales Pascual” was used. This product consolidates by the crystallization of calcite on the treated surface.
- Nanolime: Nanorestore from CTS was used. The product is based on nanophase calcium hydroxide dispersed in isopropyl alcohol that is capable of generating calcium carbonate nanocrystals.
- Nanosilica: Nano Estel from CTS is an aqueous colloidal silica dispersion of nanometric dimensions (~10–20 nm).
2.2. Methods and Essays
2.2.1. Instruments
- Scanning electron microscope (SEM)
- Scanning electron microscope (FE-SEM)
- Polarizing petrographic microscope (MOP):
- X-ray diffractometers (DRX)
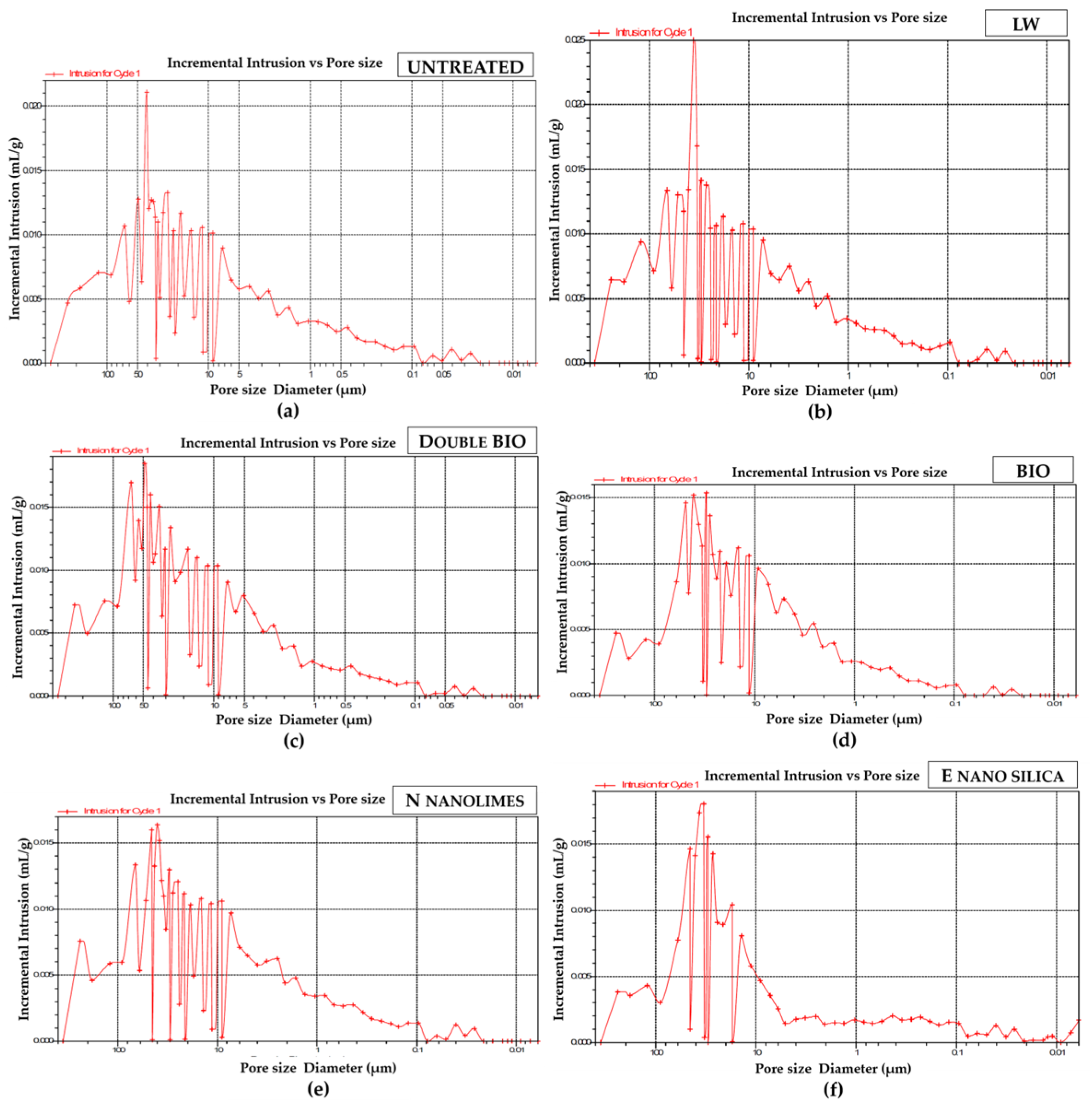
- Mercury intrusion porosity (MIP):
- Handheld digital microscope
- Compression testing machine
- DRMS
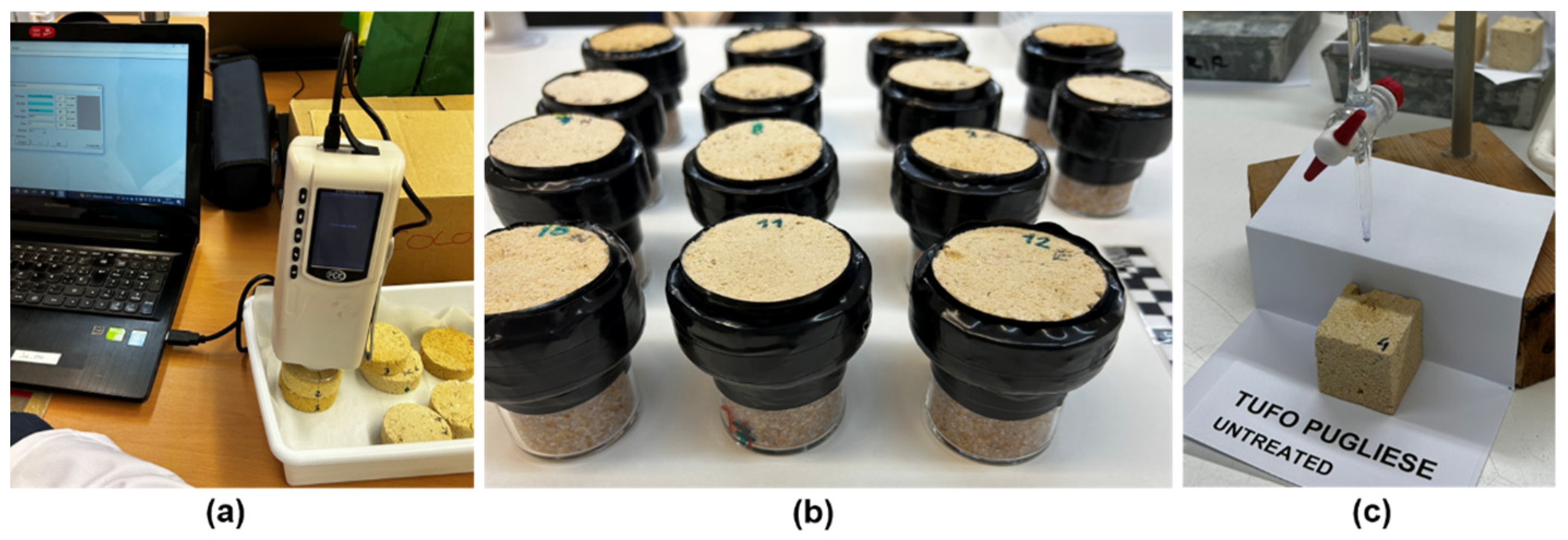
- Colorimeter
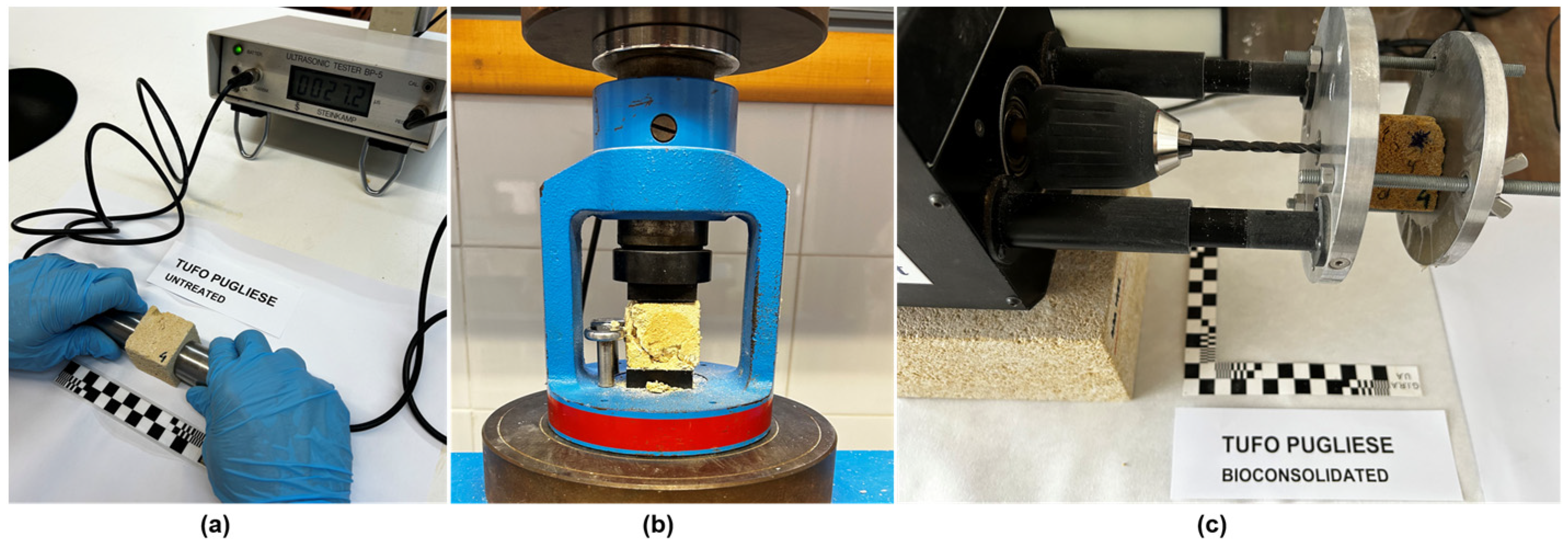
- Ultrasonic tester.
2.2.2. Test Descriptions for Treatment Evaluation
- DRMS
- Compressive strength
- Ultrasonic pulse velocity test.
- Study of color
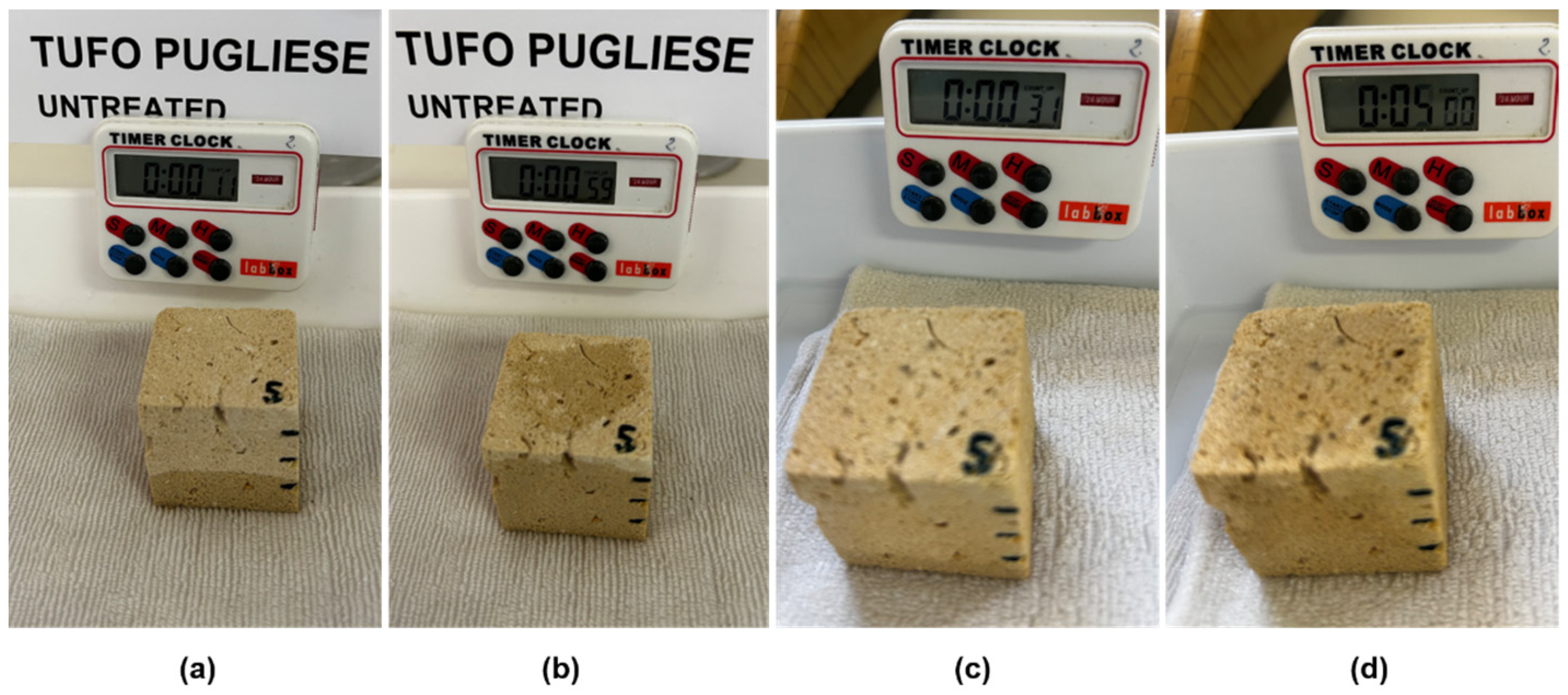
- Water absorptivity
- Vapor permeability
- Capillary absorption
3. Results
3.1. Gravina Calcarenite Characterization
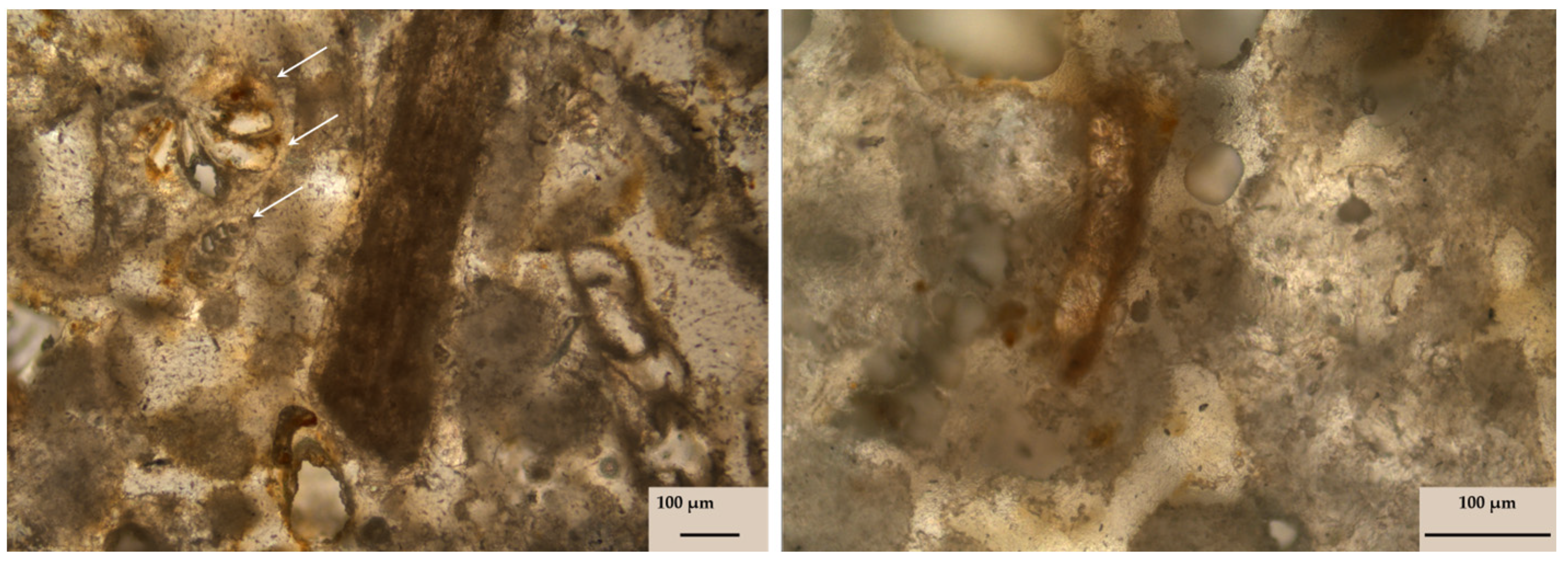
3.1.1. Petrographic Description
3.1.2. Petrophysical Properties
- Color (C)
- Densities (D) and porosity (P):
- Droplet absorption
- Capillary absorption
- Water vapor permeability
3.1.3. Mechanical Properties
- Ultrasonic testing (Rmu) (Figure 5a)
- Compressive strength (Rmc) (Figure 5b)
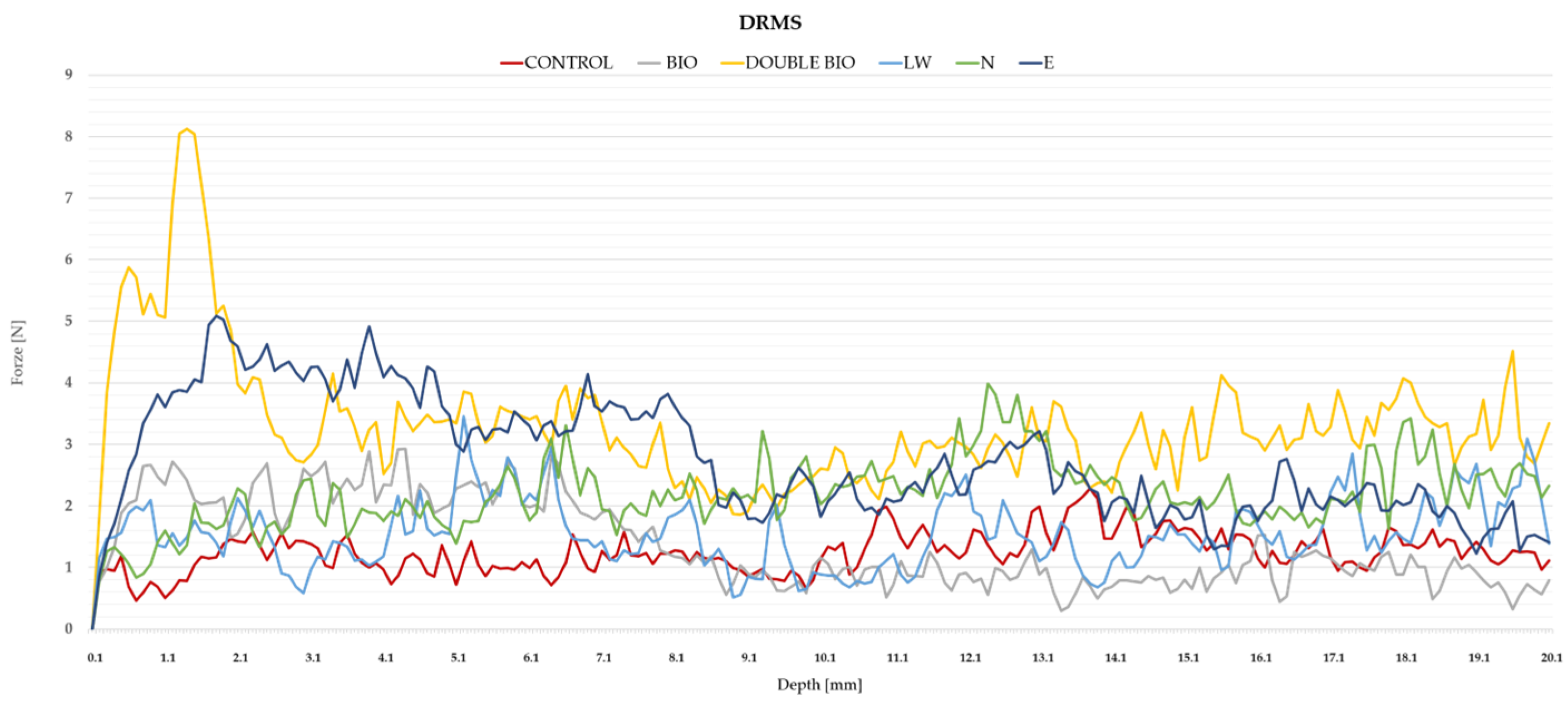
- Drilling resistance measuring system (DRMS) (Figure 5c)
3.2. Treated Gravina Calcarenite
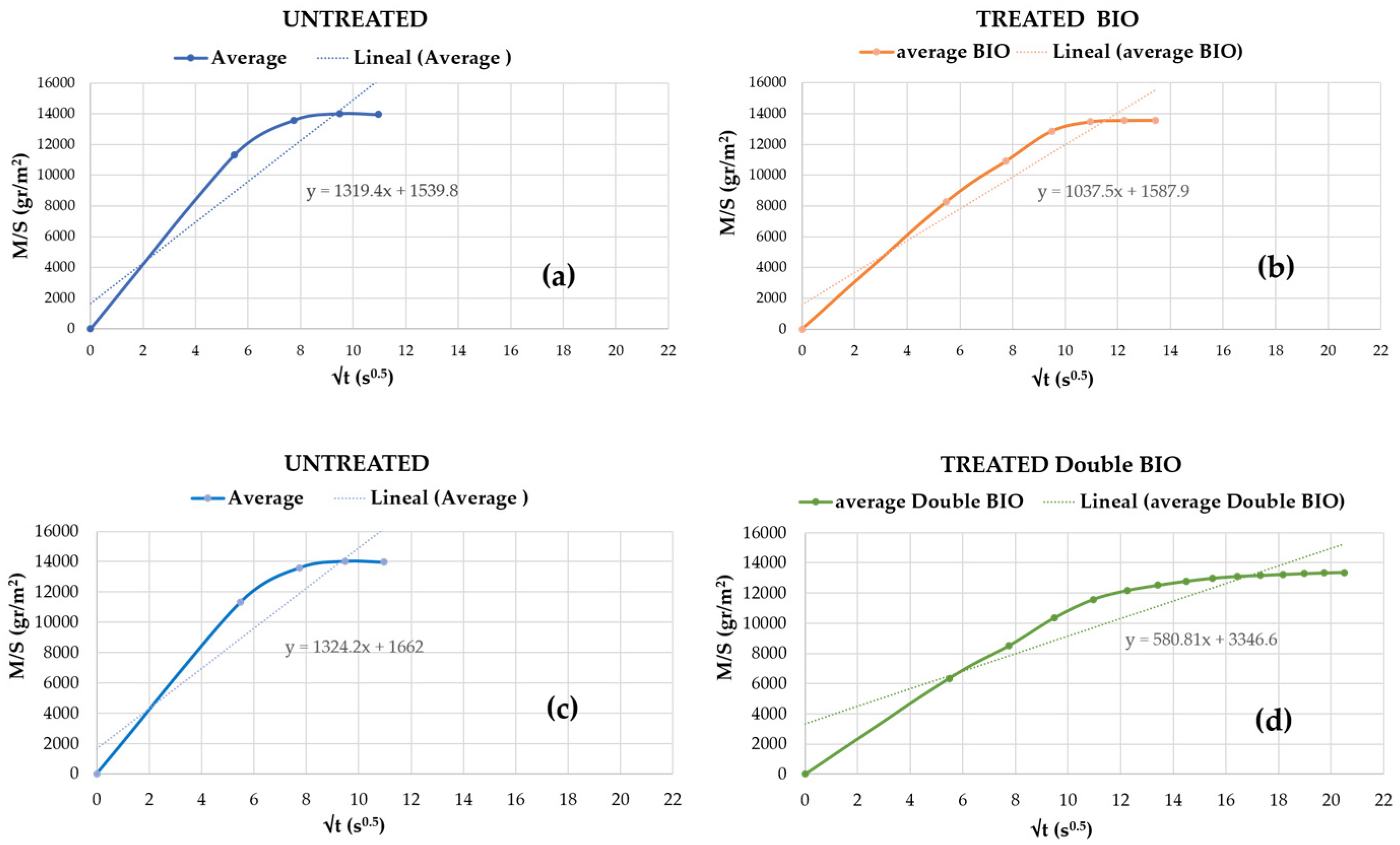
3.2.1. Treated with Bioconsolidant “Bio”
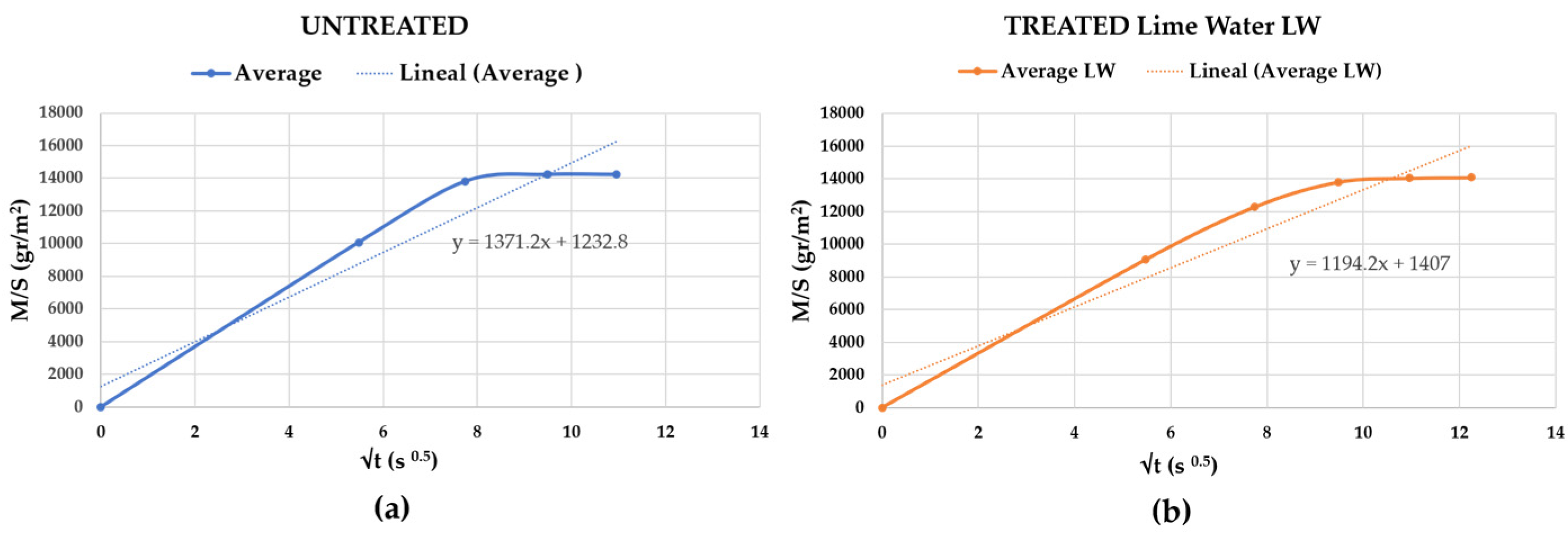
3.2.2. Treated with Lime Water “LW”
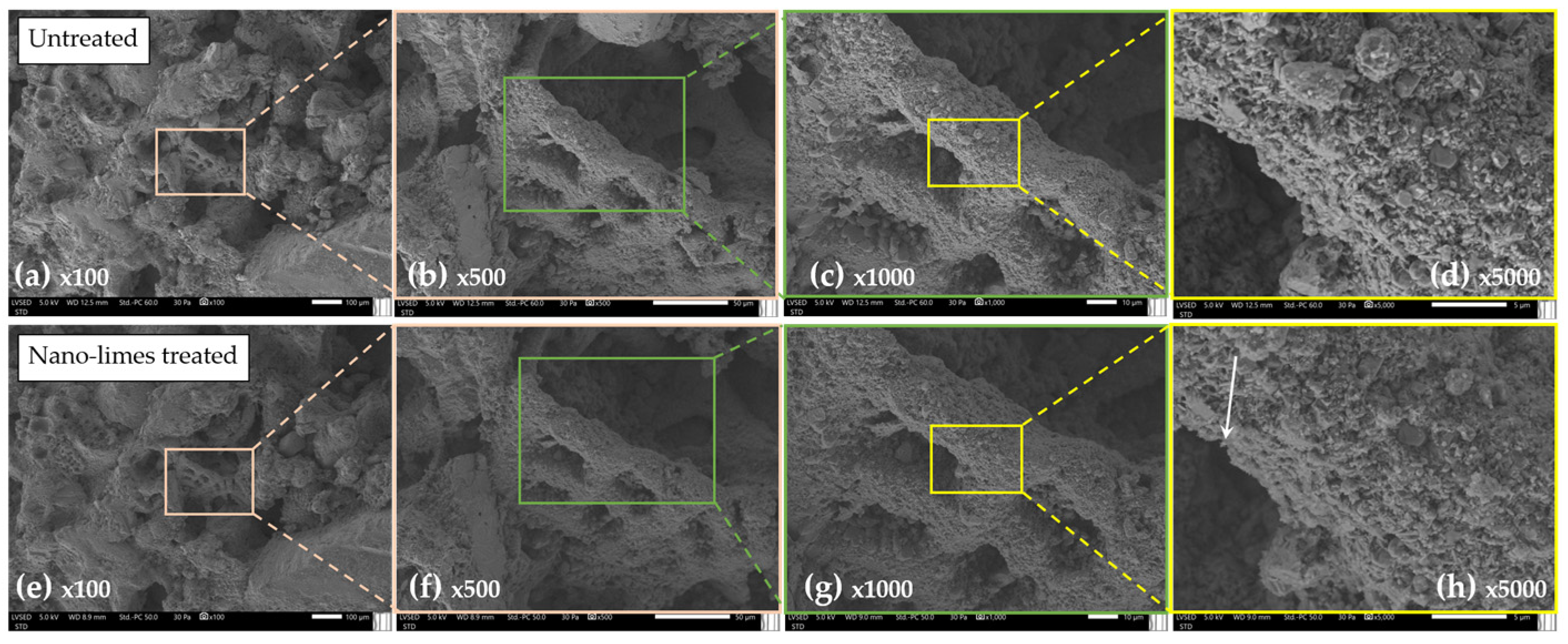
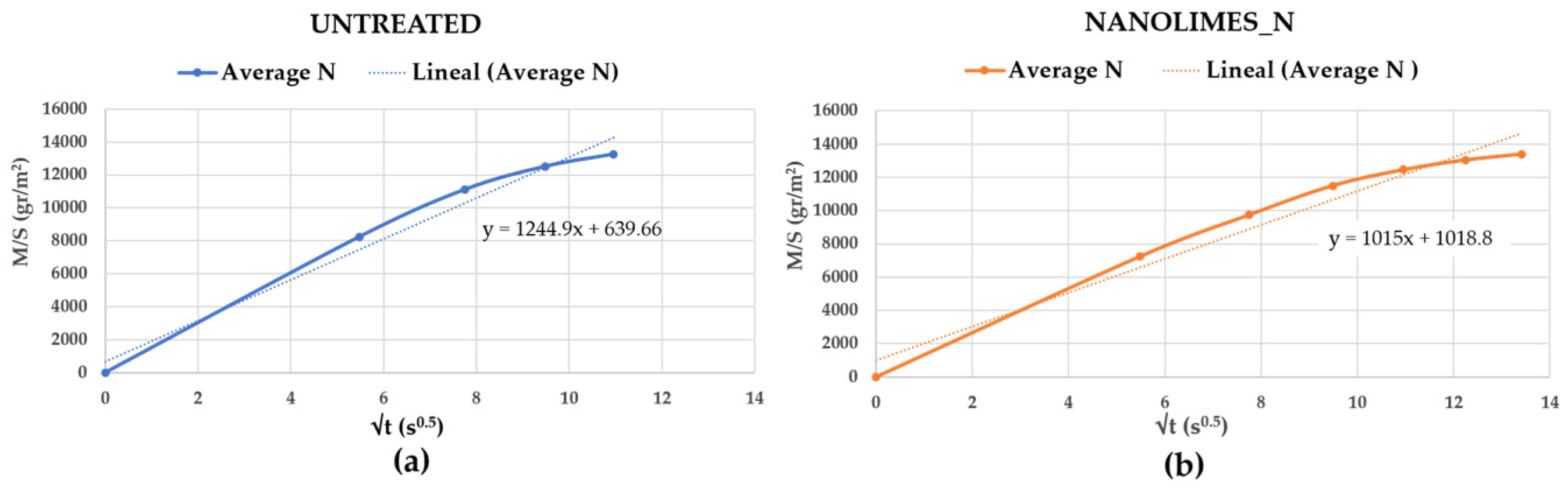
3.2.3. Treated with Nanolimes “N”
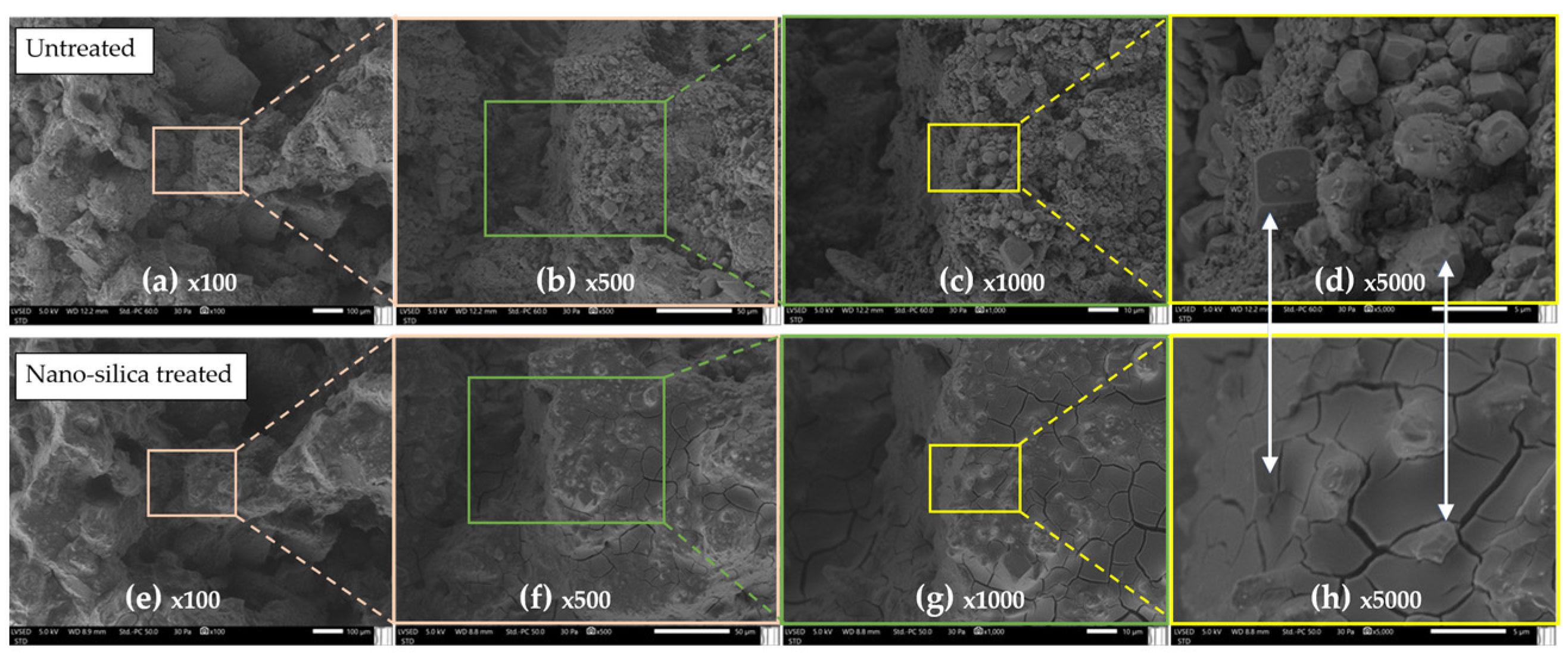
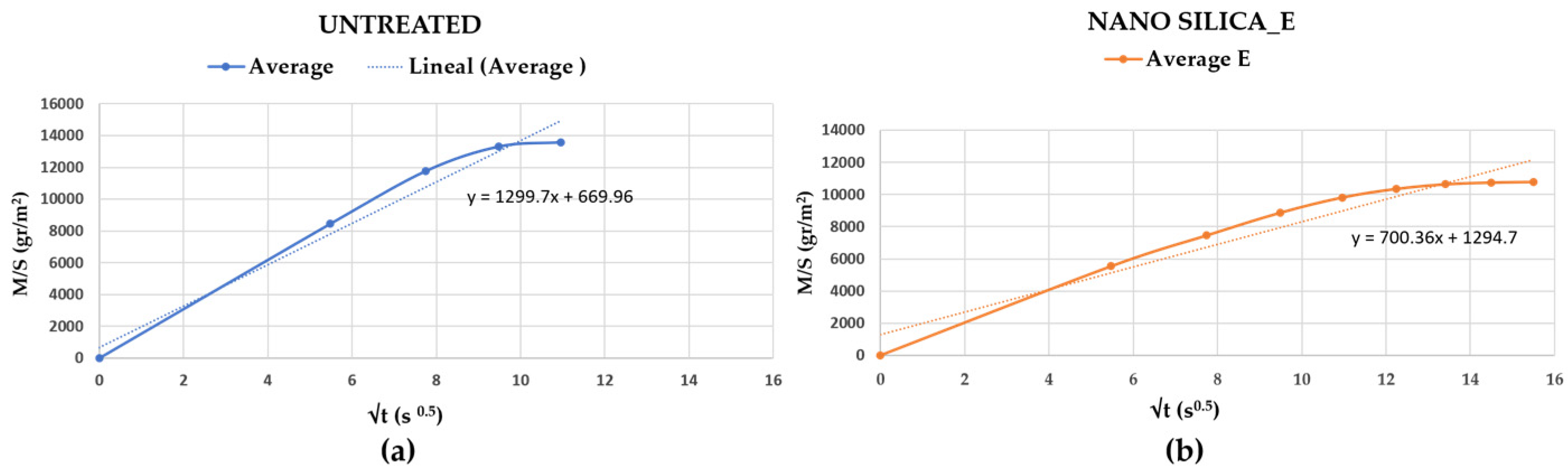
3.2.4. Treated with Nanosilica “E”
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitiello, V.; Castelluccio, R.; Ramondini, M. Conservation of Cultural Heritage. Evaluation of Archaeological Digs Effect on the Degradation of the Structures Found. In Proceedings of the 4th International Conference on Protection of Historical Constructions (PROHITECH 2020/21), Athens, Greece, 25–27 October 2021. [Google Scholar] [CrossRef]
- Bonomo, A.; Acito, A.; Prosser, G.; Rizzo, G.; Munnecke, A.; Koch, R.; Bentivenga, M. Matera’s Old Quarries: Geological and Historical Archives That Need Protection and Valorization. Geoheritage 2019, 11, 1603–1619. [Google Scholar] [CrossRef]
- Artiola, V.; La Verde, G.; D’Avino, V.; Pugliese, M. Sassi of Matera Building Material: High-Resolution Gamma-Ray Spectroscopy Characterization for Radioprotection. Buildings 2021, 11, 258. [Google Scholar] [CrossRef]
- Corbi, I.; Baratta, A.; Corbi, O.; Li, H. Existing Mono-Cell and Multi-Cell Low-Rise Dry, Hollowed Constructions. Buildings 2023, 13, 340. [Google Scholar] [CrossRef]
- Bonomo, A.E.; Rizzo, G.; Prosser, G. Calcarenite di Gravina formation a row material for the lime production. In Proceedings of the International Conference on Metrology for Archaeology and Cultural Heritage, Florence, Italy, 4–6 December 2019; pp. 84–89. [Google Scholar]
- Andriani, G.F.; Walsh, N. Petrophysical and mechanical properties of soft and porous building rocks used in Apulian monuments (south Italy). Geol. Soc. Lond. Spec. Publ. 2010, 333, 129–141. [Google Scholar] [CrossRef]
- Martínez Martínez, J.; Berrezueta, E.; Aguilera, H.; Fusi, N.; Gómez Heras, M. Cálculo de la (micro)tasa de alteración en calcarenitas expuestas en clima semiárido costero. Geogaceta 2023, 74, 27–30. [Google Scholar] [CrossRef]
- Fort, R.; Varas, M.J.; Alvarez de Buergo, M.; Martin-Freire, D. Determination of anisotropy to enhance the durability of natural stone. J. Geophys. Eng. 2011, 8, S132–S144. [Google Scholar] [CrossRef]
- Bonomo, A.; Amodio, A.; Prosser, G.; Sileo, M.; Rizzo, G. Evaluation of soft limestone degradation in the Sassi UNESCO site (Matera, Southern Italy): Loss of material measurement and classification. J. Cult. Herit. 2020, 42, 191–201. [Google Scholar] [CrossRef]
- Weiss, T.; Siegesmund, S.; Kirchner, D.; Sippel, J. Insolation weathering and hygric dilatation: Two competitive factors in stone degradation. Environ. Geol. 2004, 46, 402–413. [Google Scholar] [CrossRef]
- Borea, E. (Coord). Materiali Lapidei. Problemi Relativi Allo Studio del Degrado e Della Conservazione. I-II. Bolletino D’arte (supplementi al n 41). Ministero per I Beni Culturali e Ambientali, Italia. 1987. Available online: http://www.bollettinodarte.beniculturali.it/opencms/multimedia/BollettinoArteIt/documents/1580393362188_volume_I_intero.pdf (accessed on 25 March 2024).
- Lupo, M.; Genevois, R.; Tecca, P.R. Physical and mechanical characteristics of the Gravina Calcarenite (southern Italy). Ital. J. Eng. Geol. Environ. 2019, 2, 37–54. [Google Scholar] [CrossRef]
- Caputo, M.C.; De Carlo, L.; Masciale, R.; Perkins, K.; Turturro, A.C.; Nimmo, J.R. Detection and quantification of preferential flow using artificial rainfall with multiple experimental approaches. Hydrogeol. J. 2023, 32, 467–485. [Google Scholar] [CrossRef]
- Tropeano, M.; Caldara, M.A.; De Santis, V.; Festa, V.; Parise, M.; Sabato, L.; Spalluto, L.; Francescangeli, R.; Iurilli, V.; Mastronuzzi, G.A. Geological Uniqueness and Potential Geotouristic Appeal of Murge and Premurge, the First Territory in Puglia (Southern Italy) Aspiring to Become a UNESCO Global Geopark. Geosciences 2023, 13, 131. [Google Scholar] [CrossRef]
- Moretti, M.; Owen, G.; Tropeano, M. Soft-sediment deformation induced by sinkhole activity in shallow marine environments: A fossil example in the Apulian Foreland (Southern Italy). Sediment. Geol. 2011, 235, 331–342. [Google Scholar] [CrossRef]
- Grassi, D.; Grimaldi, S.; Simeone, V. On the causes of the instability affecting rupestrian urban centres of Apulia Region (Southerrn Italy). In 32° Intentional Geological Congress Firenze, Paper A32IGC9KF3, Session T16.06—Geoscience for Cultural Heritage; Natural Hazard and Cultural Heritages: Firenze, Italy, 2004. [Google Scholar]
- D’Angeli, I.M.; Lacalamita, M.; Sasso, C.; Schingaro, E.; Parise, M. Morphological and mineralogical characterization of surficial weathering on calcarenite rocks in the rupestrian system of “San Michele delle Grotte” at Gravina in Puglia (Bari, Apulia). CATENA 2022, 216, 106382. [Google Scholar] [CrossRef]
- Simeone, V.; Doglioni, A.; Lacertosa, R.M.; Sdao, F. Environmental and Geological Characters and Stability Problems in the Historic Centre of Matera (South Italy). In IAEG/AEG Annual Meeting Proceedings; Shakoor, A., Cato, K., Eds.; Springer: San Francisco, CA, USA, 2019; Volume 2, pp. 161–168. [Google Scholar] [CrossRef]
- Siegesmund, S.; Gross, C.J.; ·Dohrmann, R.; Marler, B.; Ufer, K.; Koch, T. Moisture expansion of tuf stones and sandstones. Environ. Earth Sci. 2023, 82, 146. [Google Scholar] [CrossRef]
- Mecca, I. In situ experimentations for the compatibility and durability of the restorations: The case study of the Sassi of Matera. VITRUVIO—Int. J. Archit. Technol. Sustain. 2016, 1, 35–48. [Google Scholar] [CrossRef]
- Benavente, D.; Martinez-Martinez, J.; Cueto, N.; Ordoñez, S.; García del Cura, M.A. Impact of salt and frost weathering on the physical and durability properties of travertines and carbonate tuffs used as building material. Environ. Earth Sci. 2018, 77, 147. [Google Scholar] [CrossRef]
- Benavente, D.; De Jongh, M.; Cañaveras, J.C. Weathering processes and mechanisms caused by capillary waters and pigeon droppings on porous limestones. Minerals 2021, 11, 18. [Google Scholar] [CrossRef]
- Smith, B.; Gómez-Heras, M.; McCabe, S. Understanding the decay of stone-built cultural heritage. Prog. Phys. Geogr. Earth Environ. 2008, 32, 439–461. [Google Scholar] [CrossRef]
- Alonso, F.J.; Alonso, L.; Vázquez, P. Hydric properties and anisotropy of porous sedimentary rocks. In Proceedings of the VIII Congreso Geológico de España, Oviedo, España, 17–19 July 2012. [Google Scholar]
- Toniolo, L.; Gherardi, F. The protection of marble surfaces: The challenge to develop suitable nanostructured treatments. In Advanced Materials for the Conservation of Stone; Springer: Cham, Switzerland, 2018; pp. 57–78. [Google Scholar] [CrossRef]
- Lazzarini, L.; Tabasso, M. Il Restauro Della Pietra; CEDAM: Padova, Italy, 1980. [Google Scholar]
- Zornoza-Indart, A.; López-Arce, P.; Leal, N.; Simão, J.; Zoghlami, K. Consolidation of a Tunisian bioclastic calcarenite: From conventional ethyl silicate products to nanostructured and nanoparticle based consolidants. Constr. Build. Mater. 2016, 116, 188–202. [Google Scholar] [CrossRef]
- Gherardi, F. Current and Future Trends in Protective Treatments for Stone Heritage. In Conserving Stone Heritage. Cultural Heritage Science; Gherardi, F., Maravelaki, P.N., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- ICOMOS-ISCS: Illustrated Glossary on Stone Deterioration Patterns 2008. Available online: http://www.international.icomos.org/publications/monuments_and_sites/15/pdf/Monuments_and_Sites_15_ISCS_Glossary_Stone.pdf (accessed on 25 March 2024).
- Jimenez-Lopez, C.; Rodriguez-Navarro, C.; Piñar, G.; Carrillo-Rosúa, F.J.; Rodriguez-Gallego, M.; Gonzalez-Muñoz, M.T. Consolidation of degraded ornamental porous limestone stone by calcium carbonate precipitation induced by the microbiota inhabiting the stone. Chemosphere 2007, 68, 1929–1936. [Google Scholar] [CrossRef]
- Ripoll, A.; Rojo, A.; Ruiz de Argandoña, V.G. Evaluation of nanoparticulate consolidants applied to Novelda Stone (Spain). Mater. Constr. 2022, 72, e294. [Google Scholar] [CrossRef]
- Wen, Y.; Qing, H.; Shu, H.; Liu, Q. Evaluating the Protective Effects of Calcium Carbonate Coating on Sandstone Cultural Heritage. Coatings 2021, 11, 1534. [Google Scholar] [CrossRef]
- Fernandez, F.; Germinario, S.; Montagno, R.; Basile, R.; Borgioli, L.; Laviano, R. Experimental Procedures of Accelerated Aging and Evaluation of Effectiveness of Nanostructured Products for the Protection of Volterra (Italy) Panchina Stone. Buildings 2022, 12, 1685. [Google Scholar] [CrossRef]
- Chen, W.; Dai, P.; Yuan, P.; Zhang, J. Effect of inorganic silicate consolidation on the mechanical and durability performance of sandstone used in historical sites. Constr. Build. Mater. 2016, 121, 445–452. [Google Scholar] [CrossRef]
- Koestler, R. Polymers and resins as food for microbes. In Of Microbes and Art: The Role of Microbial Communities in the Degradation and Protection of Cultural Heritage; Ciferri, O., Tiano, P., Mastromei., G., Eds.; Springer: Boston, MA, USA, 2000; pp. 153–167. [Google Scholar] [CrossRef]
- Melo, M.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in the conservation of stone. Polym. Degrad. Stab. 1999, 66, 23–30. [Google Scholar] [CrossRef]
- Lombardini, N. Piero Sanpaolesi e il restauro della facciata della basílica di San Michele Maggiore a Pavia. In Piero Sanpaolesi. Restauro e Método; Tampone, G., Gurrieri, F., Girgi, L., Eds.; Nardini: Firenze, Italy, 2012; pp. 325–352. [Google Scholar]
- Fort González, R.; Pérez-Monserrat, E.M.; Layuno Rosas, M.d.l.Á.; Varas, M.J.; Álvarez de Buergo, M.; Martínez-Ramírez, S.; Blanco-Varela, M.T.; Villegas Broncano, M.Á.; García Heras, M.; López-Arce, P.; et al. La Con-servación de Los Geomateriales Utilizados en el Patrimonio, Universidad Complutense de Madrid. 2012. Available online: http://hdl.handle.net/10261/46731 (accessed on 25 March 2024).
- UNE 41810; Conservación del Patrimonio Cultural. Criterios de Intervención en Materiales Pétreos. AENOR: Madrid, Spain, 2007.
- Clifton, J. Stone Consolidating Materials: A Status Report; National Bureau of Standards: Washington, DC, USA, 1980. [Google Scholar] [CrossRef]
- Hansen, E.; Doehne, E.; Fidler, J.; Larson, J.; Martin, B.; Matteini, M.; Rodriguez-Navarro, C.; Sebastian Pardo, E.; Price, C.; de Tagle, A.; et al. A review of selected inorganic consolidants and protective treatments for porous calcareous materials. Estud. Conserv. 2003, 48 (Suppl. 1), 13–25. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.; Arizzi, A.; Benavente, D. The Role of Calcite Dissolution and Halite Thermal Expansion as Secondary Salt Weathering Mechanisms of Calcite-Bearing Rocks in Marine Environments. Minerals 2021, 11, 911. [Google Scholar] [CrossRef]
- Benavente, D. Why Pore Size Is Important in the Deterioration of Porous Stones Used in the Built Heritage. Macla 2011, 15, 41–42. Available online: http://hdl.handle.net/10045/19869 (accessed on 25 March 2024).
- Rodriguez-Navarro, C.; Doehne, E. Salt weathering: Influence of evaporation rate, supersaturation and crystallization pattern. Earth Surf. Process. Landf. 1999, 24, 191–209. [Google Scholar] [CrossRef]
- Yu, S.; Oguchi, C. Role of pore size distribution in salt uptake, damage, and predicting salt susceptibility of eight types of Japanese building stones. Eng. Geol. 2010, 115, 226–236. [Google Scholar] [CrossRef]
- Coviello, C.G.; Lassandro, P.; Sabbà, M.F.; Foti, D. Mechanical and Thermal Effects of Using Fine Recycled PET Aggregates in Common Screeds. Sustainability 2023, 15, 16692. [Google Scholar] [CrossRef]
- Lerna, M.; Foti, D.; Petrella, A.; Sabbà, M.F.; Mansour, S. Effect of the Chemical and Mechanical Recycling of PET on the Thermal and Mechanical Response of Mortars and Premixed Screeds. Materials 2023, 16, 3155. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J. Lime treatments: An overview of lime watering and shelter coating of friable historical limestone masonries. In Stone: Stone Building Materials, Construction and Associated Component Systems: Their Decay and Treatment; English Heritage Research Transactions: London, UK, 2002; pp. 19–28. [Google Scholar]
- Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Elert, K.; Martín-Sánchez, I.; González-Muñoz, M.T.; Rodriguez-Navarro, C. Protection and consolidation of stone heritage by self-inoculation with indigenous carbonatogenic bacterial communities. Nat. Commun. 2017, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Spairani-Berrio, Y.; Huesca-Tortosa, J.A.; Rodriguez-Navarro, C.; Gonzalez-Muñoz, M.T.; Jroundi, F. Bioconsolidation of Damaged Construction Calcarenites and Evaluation of the Improvement in Their Petrophysical and Mechanical Properties. Materials 2023, 16, 6043. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E. Nanolimes: From synthesis to application. Pure Appl. Chem. 2018, 90, 523–550. [Google Scholar] [CrossRef]
- Slížková, Z.; Drdácký, M. Effects of Various Chemical Agents on Mechanical Characteristics of Weak Lime Mortar. In Historic Mortars; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- D’Armada, P.; Hirst, E. Nano-Lime for Consolidation of Plaster and Stone. J. Archit. Conserv. 2012, 18, 63–80. [Google Scholar] [CrossRef]
- Borsoi, G.; Lubelli, B.; Hees, R.; Veiga, R.; Silva, A. Application Protocol for the Consolidation of Calcareous Substrates by the Use of Nanolimes: From Laboratory Research to Practice. Restor. Build. Monum. 2018, 22, 109–199. [Google Scholar] [CrossRef]
- Navarro-Moreno, D.; Martínez-Arredondo, A.; García-Vera, V.E.; Lanzón, M. Uso de nanomateriales para la conservación de la piedra del Teatro Romano de Cartagena. Loggia Arquit. Restauración 2023, 36, 106–119. [Google Scholar] [CrossRef]
- Dziadkowiec, J.; Cheng, H.; Ludwig, M.; Ban, M.; Tausendpfund, T.; Klitzing, R.; Mezger, M.; Valtiner, M. Cohesion Gain Induced by Nanosilica Consolidants for Monumental Stone Restoration. Langmuir 2022, 38, 6949–6958. [Google Scholar] [CrossRef] [PubMed]
- Iucolano, F.; Colella, A.; Liguori, B.; Calcaterra, D. Suitability of silica nanoparticles for tuff consolidation. Constr. Build. Mater. 2019, 202, 73–81. [Google Scholar] [CrossRef]
- Gomez-Villalba, L.S.; Salcines, C.; Fort, R. Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures. Nanomaterials 2023, 13, 1454. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Muñoz, M.T.; Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Gallego, M. Method and Product for Protecting and Reinforcing Construction and Ornamental Materials. Spanish Patent WO 2008/009771 A1, January 2008. [Google Scholar]
- Delgado-Rodrigues, J.; Ferreira Pinto, A.; da Costa, D.R. Tracing of decay profiles and evaluation of stone treatments by means of microdrilling techniques. J. Cult. Herit. 2002, 3, 117–125. [Google Scholar] [CrossRef]
- Fratini, F.; Rescic, S.; Tiano, P. A new portable system for determining the state of conservation of monumental stones. Mater. Struct. 2005, 39, 139–147. [Google Scholar] [CrossRef]
- Pamplona, M.; Kocher, M.; Snethlage, R.; Aires Barros, L. Drilling resistance: Overview and Outlook. Z. Der. Dtsch. Ges. Für. Geowissenschaften. J. Appl. Reg. Geol. 2007, 158, 665–679. [Google Scholar] [CrossRef]
- UNE-EN 1926:2007; Natural Stone Test Methods-Determination of Uniaxial Compressive Strength. AENOR: Madrid, Spain, 2007.
- Eljufout, T.; Alhomaidat, F. Evaluation of natural building stones’ characterizations using ultrasonic testing technique. Arab. J. Sci. Eng. 2021, 46, 11415–11424. [Google Scholar] [CrossRef]
- UNE-EN ISO 16809:2020; Non-Destructive Testing-Ultrasonic Thickness Measurement (ISO 16809:2017). AENOR: Madrid, Spain, 2020.
- UNE-EN 15886:2011; Conservation of Cultural Property-Test Methods-Colour Measurement of Surfaces. AENOR: Madrid, Spain, 2011.
- Pérez-Ema, N.; Buergo, M.; Bustamante, R.; Gómez-Heras, M. Changes in Petrophysical Properties of the Stone Surface due to Past Conservation Treatments in Archaeological Sites of Merida (Spain). In Engineering Geology for Society and Territory; Lollino, G., Giordan, D., Marunteanu, C., Christaras, B., Yoshinori, I., Margottini, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 8. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Noya, D.; Montojo, C. Aesthetic Effects on Granite of Adding Nanoparticle TiO2 to Si-Based Consolidants (Ethyl Silicate or Nano-Sized Silica). Coatings 2020, 10, 215. [Google Scholar] [CrossRef]
- RILEM. Commission 25-PEM Protection et érosion des Monuments. Recomandations provisoires. Mater. Constr. 1980, 13, 175–252. [Google Scholar] [CrossRef]
- UNE-EN 15803:2010; Conservation of Cultural Property-Test Methods-Determination of Water Vapour Permeability (dp). AENOR: Madrid, Spain, 2010.
- UNE-EN 15801:2010; Conservation of Cultural Property-Test Methods-Determination of Water Absorption by Capillarity. AENOR: Madrid, Spain, 2010.
- Ciantia, M.O.; Castellanza, R.; Crosta, G.B.; Hueckel, T. Effects of mineral suspension and dissolution on strength and compressibility of soft carbonate rocks. Eng. Geol. 2014, 184, 1–18. [Google Scholar] [CrossRef]
- Ciantia, M.O.; Castellanza, R.; di Prisco, C. Experimental Study on the Water-Induced Weakening of Calcarenites. Rock. Mech. Rock. Eng. 2015, 48, 441–461. [Google Scholar] [CrossRef]
- de Jongh, M.; Benavente, D.; Young, M.; Graham, C.; Lee, M. The Long-Term Efficiency and Compatibility of Hydrophobic Treatments in Protecting Vulnerable Sandstone at Arbroath Abbey (Scotland). Heritage 2023, 6, 4864–4885. [Google Scholar] [CrossRef]
- Benavente, D.; García del Cura, M.A.; Fort, R.; Ordóñez, S. Durability estimation of porous building stones from pore structure and strength. Eng. Geol. 2004, 74, 113–127. [Google Scholar] [CrossRef]
- Spairani, Y.; Cisternino, A.; Foti, D.; Lerna, M.; Ivorra, S. Study of the Behavior of Structural Materials Treated with Bioconsolidant. Materials 2021, 14, 5369. [Google Scholar] [CrossRef] [PubMed]
- Reinemann, D.; Parlange, J.; Timmons, M. Theory of small-diameter airlift pumps. Int. J. Multiph. Flow. 1990, 16, 113–122. [Google Scholar] [CrossRef]
- Ban, M.; Aliotta, L.; Gigante, V.; Mascha, E.; Sola, A.; Lazzeri, A. Distribution depth of stone consolidants applied on-site: Analytical modelling with field and lab cross-validation. Constr. Build. Mater. 2020, 259, 120394. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jroundi, F.; Gonzalez-Muñoz, M. Stone Consolidation by Bacterial Carbonatogenesis: Evaluation of in situ Applications. Restor. Build. Monum. 2015, 21, 9–20. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Gonzalez-Muñoz, M.T. Influence of Substrate Mineralogy on Bacterial Mineralization of Calcium Carbonate: Implications for Stone Conservation. Appl. Environ. Microbiol. 2012, 78, 4017–4029. [Google Scholar] [CrossRef]
- Elert, K.; Ruiz-Agudo, E.; Jroundi, F.; Gonzalez-Muñoz, M.T.; Fash, B.W.; Fash, W.L.; Valentin, N.; de Tagle, A.; Rodriguez-Navarro, C. Degradation of ancient Maya carved tuff stone at Copan and its bacterial bioconservation. NPJ Mater. Degrad. 2021, 5, 44. [Google Scholar] [CrossRef]
- Di Benedetto, C.; Cappelletti, P.; Favaro, M.; Graziano, S.F.; Langella, A.; Calcaterra, D.; Colella, A. Porosity as key factor in the durability of two historical building stones: Neapolitan yellow tuff and vicenza stone. Eng. Geol. 2015, 193, 310–319. [Google Scholar] [CrossRef]
- Ellwood, B.; Burkart, B.; Rajeshwar, K.; Darwin, R.; Neeley, R.; McCall, A.; Long, G.; Buhl, M.; Hickcox, C. Are the iron carbonate minerals, ankerite and ferroan dolomite, like siderite, important in paleomagnetism? J. Geophys. Res. 1989, 94, 7321–7331. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]

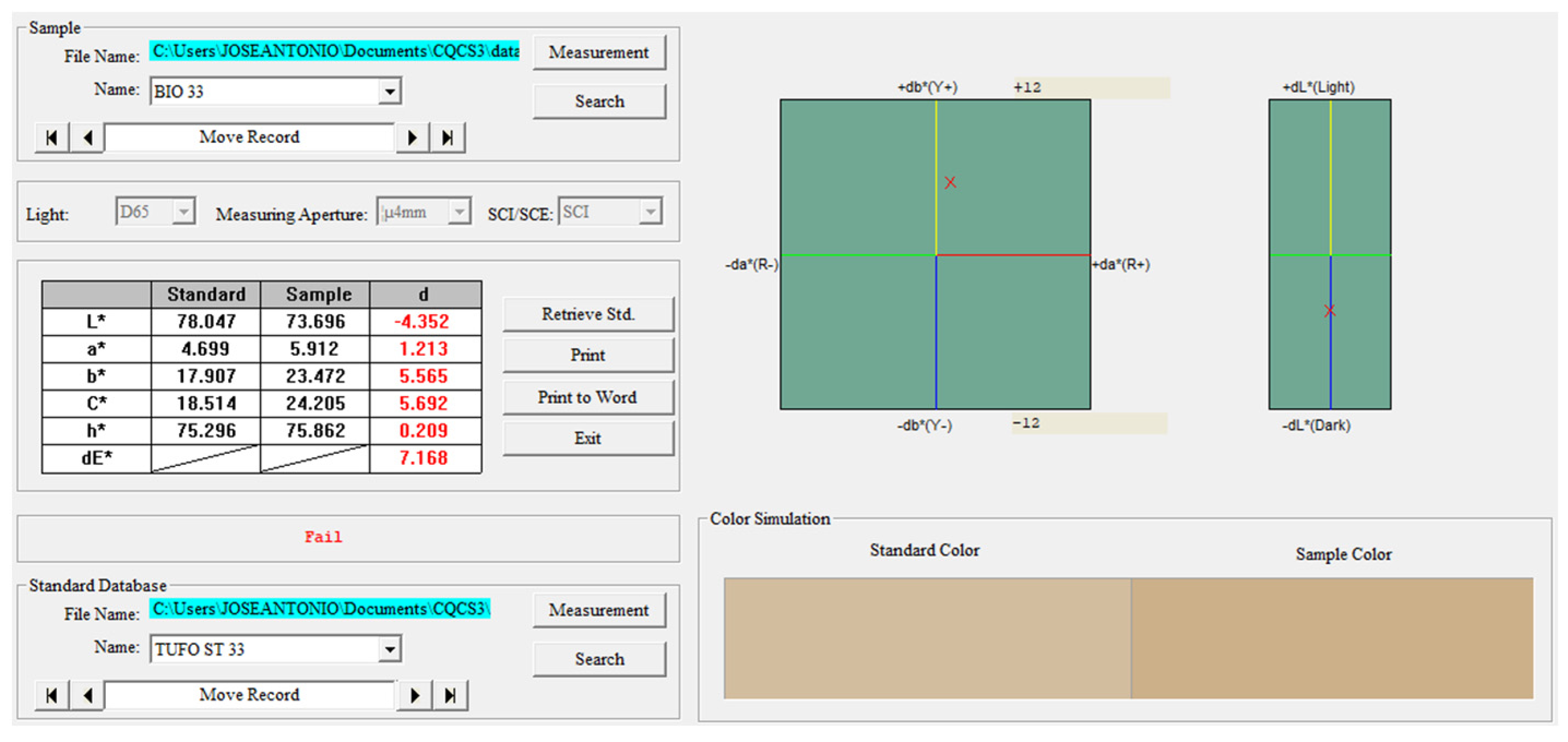




| Treatment | Middle Value | Min. Value | Max. Value | Difference from Untreated | Conclusions Behavior | |
|---|---|---|---|---|---|---|
| Bio | Color ΔE* | 10.090 | 7.168 | 13.609 | 10.090 | Modification. Obscured * |
| Ap. Density g/cm3) | 1.29 | 1.26 | 1.32 | 0.8% | Same density * | |
| Porosity % | 39 | - | - | −4.5% | Slight reduction ** | |
| Ø Pore (µm) | 2.71 | - | - | +36% | No reduction ** | |
| Absorption drops (s) | ≤2 | ≤1 | ≤2 | ≤2 | Increased absorption time * | |
| Capillary (g/m2 120 s0.5) | 1226 | 1202 | 1250 | −2% | Low reduced * | |
| Permeability (g/10 days) | 2.69 | 1.97 | 2.1 | +4% | Increased Permeability * | |
| Ultrasounds (m/s) | 1.817 | 1.694 | 1.977 | +0.6% | Same speed * | |
| Rmc (Mp) | 2.13 | 1.92 | 2.24 | +29% | Increased Resistances ** | |
| DRMS (N) | 1.36 | 1.14 | 1.58 | +9.7% | Increased Resistances ** |
| Treatment | Middle Value | Min. Value | Max. Value | Difference from Untreated | Conclusions Behavior | |
|---|---|---|---|---|---|---|
| Bio Double application | Color ΔE* | 10.258 | 8.807 | 11.710 | 10.258 | Modification * Obscured |
| Ap. Density (g/cm3) | 1.34 | 1.25 | 1.43 | +2.3% | Increased density * | |
| Porosity (MIP) | 42% | - | - | −2% | Slight reduction ** | |
| Ø Pore (µm) | 2.70 | - | - | +36% | No reduction ** | |
| Absorption drops (s) | ≤3 | ≤1 | ≤4 | +3 | Increased absorption time * | |
| Capillary (g/m2 120 s0.5) | 1042 | 845 | 1239 | −16% | Reduced capillary * | |
| Permeability (g/10 days) | 3.05 | 2.1 | 2.5 | +18% | Increased Permeability * | |
| Ultrasounds (m/s) | 1.860 | 1.718 | 1.951 | +4% | Increased speed * | |
| Rmc (Mp) | 3.45 | 3.12 | 3.75 | +109% | Increased Resistances ** | |
| DRMS (N) | 3.30 | 2.58 | 4.02 | +166% | Increased Resistances ** |
| Treatment | Middle Value | Min. Value | Max. Value | Difference from Untreated | Conclusions Behavior | |
|---|---|---|---|---|---|---|
| LW | Color (ΔE*) | 1.855 | 0.651 | 3.369 | 1.855 | Low Modification * Obscured * |
| Ap. Density (g/cm3) | 1.295 | 1.255 | 1.332 | 0.1% | Same density * | |
| Porosity (%) | 44 | - | - | +1% | No reduction ** | |
| Ø Pore (µm) | 1.91 | - | - | −3% | Low reduced ** | |
| Absorption drops (s) | ≤1 | ≤1 | ≤1 | 0 | No absorption time modification * | |
| Capillary (g/m2 120 s0.5) | 1042 | 1228 | 1282 | −1% | Low reduced * | |
| Permeability (g/10 days) | 2.36 | 2.31 | 2.41 | −8.5% | Reduced Permeability * | |
| Ultrasounds (m/s) | 1.670 | 1511 | 1821 | −3% | Reduced speed * | |
| Rmc (Mp) | 1.65 | 1.28 | 1.90 | 0 | No increased Resistances ** | |
| DRMS (N) | 1.55 | 1.72 | 1.38 | +25% | Increased DRMS ** |
| Treatment | Middle Value | Min. Value | Max. Value | Difference from Untreated | Conclusions Behavior | |
|---|---|---|---|---|---|---|
| Nanolimes | Color (ΔE*) | 0.993 | 0.250 | 2.340 | 0.993 | No modification * Whitish * |
| Ap. Density (g/cm3) | 1.295 | 1.255 | 1.332 | +0.1% | Same density * | |
| Porosity (%) | 44 | - | - | +1% | No reduction ** | |
| Ø Pore (µm) | 1.87 | - | - | −5% | Low reduced ** | |
| Absorption drops (s) | ≤1 | ≤1 | ≤1 | 0 | No modification * | |
| Capillary (g/m2 120 s0.5) | 1126 | 1008 | 1284 | −6% | Low reduced * | |
| Permeability (g/10 days) | 2.37 | 2.24 | 2.54 | −8% | Low reduced * | |
| Ultrasounds (m/s) | 1776 | 1567 | 2027 | +3% | Increased speed * | |
| Rmc (Mp) | 2.35 | 1.56 | 3.32 | +48% | Increased ** | |
| DRMS (N) | 2.18 | 1.26 | 3.36 | +76% | Increased ** |
| Treatment | Middle Value | Min. Value | Max. Value | Difference from Untreated | Conclusions Behavior | |
|---|---|---|---|---|---|---|
| Nanosilica E | Color ΔE* | 4.093 | 3.068 | 4.713 | 4.093 | Low modification * |
| Ap. Density (g/cm3) | 1.44 | 1.42 | 1.48 | +9.5% | Increased density * | |
| Porosity (%) | 32% | - | - | −25% | High reduction ** | |
| Ø Pore (µm) | 0.34 | −83% | High reduction ** | |||
| Absorption drops (s) | ≤1 | ≤1 | ≤1 | 0 | No modification time * | |
| Capillary (g/m2 120 s0.5) | 878 | 805 | 982 | −28% | Reduced capillary * | |
| Permeability (g/10 days) | 3.14 | 3.08 | 3.19 | +23% | Increased Permeability * | |
| Ultrasounds (m/s) | 1.809 | 1671 | 1.929 | +2% | Increased speed * | |
| Rmc (Mp) | 3.45 | 3.12 | 3.75 | +109% | Increased Rmc ** | |
| DRMS (N) | 2.76 | 2.31 | 3.21 | +123% | Increased DRMS ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huesca-Tortosa, J.A.; Spairani-Berrio, Y.; Coviello, C.G.; Sabbà, M.F.; Rizzo, F.; Foti, D. Evaluation of Eco-Friendly Consolidating Treatments in Pugliese Tuff (Gravina Calcarenite) Used in Italian Heritage Buildings. Buildings 2024, 14, 940. https://doi.org/10.3390/buildings14040940
Huesca-Tortosa JA, Spairani-Berrio Y, Coviello CG, Sabbà MF, Rizzo F, Foti D. Evaluation of Eco-Friendly Consolidating Treatments in Pugliese Tuff (Gravina Calcarenite) Used in Italian Heritage Buildings. Buildings. 2024; 14(4):940. https://doi.org/10.3390/buildings14040940
Chicago/Turabian StyleHuesca-Tortosa, Jose Antonio, Yolanda Spairani-Berrio, Cristiano Giuseppe Coviello, Maria Francesca Sabbà, Fabio Rizzo, and Dora Foti. 2024. "Evaluation of Eco-Friendly Consolidating Treatments in Pugliese Tuff (Gravina Calcarenite) Used in Italian Heritage Buildings" Buildings 14, no. 4: 940. https://doi.org/10.3390/buildings14040940
APA StyleHuesca-Tortosa, J. A., Spairani-Berrio, Y., Coviello, C. G., Sabbà, M. F., Rizzo, F., & Foti, D. (2024). Evaluation of Eco-Friendly Consolidating Treatments in Pugliese Tuff (Gravina Calcarenite) Used in Italian Heritage Buildings. Buildings, 14(4), 940. https://doi.org/10.3390/buildings14040940











