Abstract
The escalating occurrence of hospital-associated infections globally, compounded by the ongoing pandemic, has spurred researchers to delve into innovative approaches for combating pathogens and overcoming their resistance to commonly used materials. One of the most important concerns is frequently touched building components in public places and hospitals, which serve as potential sources of infection transmission, prompting a pressing need for effective antimicrobial solutions. This research developed antimicrobial polymeric composites comprising Copper (Cu), Aluminum (Al), and Stainless Steel (SS) particles incorporated into Polylactic Acid (PLA) via injection molding as a commercial method for the production of building components, to investigate the antimicrobial properties. The study aims at increasing the antimicrobial efficiency of polymeric composites with different metallic particles and tests the prepared polymeric composites (two sets of Cu-enriched composites, i.e., Cu–PLA–SS, by mixing Al–PLA with Cu–PLA, and Cu–PLA–Al, by mixing SS–PLA with Cu–PLA) against various bacteria. The results demonstrate that the samples prepared with Cu-PLA mixed with SS and Al exhibited the best antibacterial activity (98.6%) after 20 min of exposure to all bacteria, notably against Staphylococcus aureus and Enterococci. In addition, the hybrid composites Cu–PLA–SS and Cu–PLA–Al, prepared using injection molding, showed similar antimicrobial activity against all bacteria compared to those prepared using 3D printing. Therefore, polymeric composites enriched with metallic particles such as Cu, Al, and SS prepared via injection molding show potential in biomedical applications, food packaging, tissue engineering, and various technological industries, offering viable solutions for environments where risks from contact with infected surfaces are a concern.
1. Introduction
In recent decades, the development of polymeric composites has garnered much attention from researchers globally because of environmental concerns, their properties (thermal, chemical, and mechanical), which the addition of fillers has further enhanced, and the potential of their application in different fields like manufacturing and packaging [1,2]. The most prevalent method to improve composite material’s antiviral and antibacterial properties is to incorporate minerals as fillers. Consequently, polymer composites have been used instead of pure polymers for various applications based on their cost-effectiveness, durability, and environmental friendliness. Microbiological pollution can impact the degradation of civil engineering structures, and understanding the impact and challenges associated with this is essential to adopting appropriate measures to exterminate them. Also, investigations during the pandemic have demonstrated that because of the indoor transmission of the virus through air, people contracted COVID-19, particularly in crowded and poorly ventilated environments [3]. The COVID-19 pandemic influenced building construction techniques by forcing changes to the architectural design of the buildings. Our existing buildings are not designed or built to effectively reduce the spread of communicable diseases, especially regarding hospitals and healthcare settings [4]. Thus, it is critical to consider new solutions to overcome such a situation through adaptive reuse. This can be accomplished in various ways, including engineering controls and retrofitting existing structures with the help of antimicrobial materials [5]. In response to the Coronavirus Disease 2019 (COVID-19) pandemic, novel and effective virus-resistance strategies have been implemented worldwide. Globally, COVID-19 has affected the health and economies of nations, causing 33.3 million infections and nearly one million deaths [6,7]. The influenza virus that caused the Spanish flu pandemic in the early 20th century led to over 50 million deaths and 500 million infections [8]. In addition, many pathogens, particularly viruses, are known to survive for a long time on surfaces, subject to weather conditions [9]. Thus, one of the main ways viruses spread in public spaces is by contact with infected surfaces [10]. Thus, contaminated surfaces are crucial in the spread of viruses to a great degree in hospitals, clinics, and diagnostic facilities, where frontline staff have frequent contact with patients and objects [11]. In addition, the increased use of biocides and antibiotics for disinfection during the pandemic may have played a role in the increase in antimicrobial resistance [12]. Antimicrobial resistance and hospital-associated infections are key global health concerns today [13]. According to the World Health Organization, for every 100 patients admitted to acute-care medical facilities, seven individuals from high-income nations and fifteen individuals from middle- and low-income nations will develop at least one healthcare-associated infection while staying in the hospital. In addition, medical facilities are responsible for 63.5% of antibiotic-resistant bacterial infections, resulting in 72.4% of antibiotic-resistance-related deaths [14]. To prevent the transmission of viruses and pathogenic microbes in the environment and reduce the number of infections, antimicrobial polymeric composites that are affordable, biodegradable, and environmentally safe and that can be used on a broad range of surfaces must be urgently developed [15]. Because of their antiviral and antibacterial attributes, various metal and metal oxide nanoparticles (e.g., copper (Cu), silver (Ag), gold (Au), zinc (Zn), titanium (Ti), and aluminum (Al)) have been utilized in many forms (soluble and insoluble) [16]. Nonetheless, medical equipment and products are generally subjected to Cu and Ag. These metal-free ions can break down many virus membranes and eradicate them from the host cell [17]. Metal-free ions refer to ions that do not contain metal atoms in their structure. In the context of antiviral properties, metal-free ions like copper ions are considered effective in breaking down virus membranes and eradicating them from host cells [18]. Despite being called metal-free, copper ions are derived from copper, a metal known for its antimicrobial properties (copper ions can generate ROS, highly reactive molecules that cause oxidative stress and damage microbial cells; this leads to membrane damage, protein denaturation, and ultimately, cell death in microbes and bacteria). These ions interact with viral membranes, disrupting their structure and leading to the destruction of the virus. The ability of copper ions to generate reactive oxygen species (ROS) contributes to their toxic effect on viruses, ultimately resulting in the virus’s breakdown of viral genomic DNA and inactivation [15]. Therefore, when discussing metal-free ions in the context of antiviral activity, we refer to ions like copper ions that, despite not being metal-free, play a crucial role in combating viruses by breaking down their membranes and eliminating them from host cells [15]. Copper ions follow a similar mechanism to eliminate microbes and bacteria [18].
Some metal ions only possess antibacterial properties like SS and Al when treated. Still, some metal ions possess antiviral properties like copper, which is crucial when referring to HAI and the pandemic, in addition to antimicrobial properties and antibacterial properties and are effective against a wide range of pathogens including fungi, algae, and bacteria; thus, antimicrobial and antibacterial are commonly used when referring to copper. Copper ions can inhibit and kill a wide range of microorganisms, including bacteria, fungi, algae, and viruses [19]. Copper surfaces have been shown to inactivate various viruses, including influenza, poliovirus, and the COVID-19 virus [20,21].
In a recent study, it was found that Copper ArmourTM, a novel composite coating made from copper, has bactericidal activity against a wide range of human pathogens [22]. After contact for 1 h, the coating demonstrated solid antimicrobial activity, removing over 99.9% of microorganisms [22]. Reactive oxygen species, which lead to irreversible membrane damage, have been associated with Cu’s inherent ability to fight against bacteria, viruses, and fungi. This suggests the size of copper nanoparticles (CuNPs) determines their activity far more than their concentration, with smaller nanoparticles being more efficient [23]. A study by Di Cerebro et al. [24] revealed that anodized Al surfaces covered with a unique titanium oxide-based coating (DURALTI®) have a bactericidal effect on Gram-negative and Gram-positive bacteria, regardless of the surface texture and treatment (i.e., roughness or sanitizing treatment) [24]. Aluminum oxide nanoparticles were synthesized using Lyngbya majuscula extract, showing that aluminum oxide is a powerful antibacterial agent that could be utilized for treating a wide range of human illnesses [25]. Aluminum targets bacterial membranes by increasing their permeability, jeopardizing bacteria’s ability to control metal influx, such as E. coli [26]. Metal nanoparticle’s antibacterial activity heavily relies on their stability, size, shape, and capping agent. Because of their high reactivity, metal nanoparticles may agglomerate, decreasing their activity [27]. To address this issue, polymeric composites can be developed using other organic and inorganic compounds as fillers, and the stability of their surface structure can be further enhanced [28].
PLA (Polylactic acid) is an attractive injection molding material because of its eco-friendly and biodegradable nature. It can be produced from renewable resources like maize starch or sugarcane [29,30]. PLA can be used to produce high-quality plastic parts with precise tolerances and complicated designs. PLA is used across multiple automotive, medical, and components industries [29]. PLA has a lower melting point and can be brittle, rendering it unsuitable for projects that require outstanding durability. PLA can be combined with different substances to enhance its properties. PLA cannot endure hot liquids or other applications that require high temperatures. Although PLA is a versatile and environmentally friendly material for injection molding, it has a few drawbacks [30]. Stainless steel (SS) is the most widely utilized metal in healthcare settings owing to its clean appearance, better mechanical strength, efficient biocompatibility, and corrosion-resistant nature. However, using this metal has no inherent antibacterial properties. Although SS has limited inherent antimicrobial properties, these can be improved via surface treatment and antimicrobial agents [24]. Modifying the surface chemistry via coating with antimicrobial peptides, silver nanoparticles, essential oils, light-activated antimicrobials, cationic molecules, antibiotics, enzymes, and nanotechnological coatings has been used to improve the antimicrobial properties of SS [31,32]. With the help of nanofiltration and ultrafiltration, many methods, such as spray coating, dip coating, etc., have been employed to develop antimicrobial materials. This study uses injection molding to develop antimicrobial materials for biomedical applications due to its high precision and accuracy, material compatibility, cost-effectiveness, corrosion resistance, improved safety, and hygiene [33,34,35]. Injection molding is an excellent manufacturing process for producing antimicrobial materials for biomedical applications. This is due to its precision, uniformity, design flexibility, production efficiency, cost-effectiveness, regulation compliance, and biological compatibility [36,37]. Adding antimicrobial agents to materials fabricated using injection molding offers both challenges and possibilities. Some of the challenges include the commercialization of antimicrobial materials, the stability of the materials, and manufacturing on a large scale.
Antimicrobial agents may be added to polymeric materials in several ways, such as via direct incorporation and chemical deposition on the surface of the polymer, providing various possibilities for developing new antimicrobial material [38]. Emerging trends in the worldwide antimicrobial agent market involve the development of new medications and agents and more robust resistance to current ones.
Advanced molding technologies are crucial to manufacturing complicated medical device components developed from high-performance materials, particularly those with antimicrobial characteristics. While there are constraints, there are also ongoing advancements and the potential for developing antimicrobial agents using the injection molding method [39,40]. Altan et al. [41] found that Propylenene/TiO2 nanoparticles prepared from injection molding showed a high antimicrobial activity due to their photocatalytic nature. In a study by Heidari et al. [42], the method of injection molding for three distinct polylactic acid (PLA)-based bone screws was simulated and altered shrinkage and warpage. The results indicated that the PLA9 screw showed more water resistance and was extremely durable when compared to higher loads as compared to the other versions. Based on the outcomes of simulations of injection molding, the design of experiments (DOE), and evaluation of the structure, PLA9 is the most suitable option for biomedical material production among the three types of bone screws studied [42]. While advancements in injection molding and the development of antimicrobial polymeric composites have been explored, there has been little research about the use of injection molding to develop novel metal-enriched polymeric composites for biomedical applications. Moreover, aluminum and stainless steel do not have effective antimicrobial activity unless their surface is treated for antimicrobial properties [43]. Copper enhances the antimicrobial properties of stainless steel in a PLA composite without needing surface treatment. Research indicates that incorporating metallic particles like copper into polymeric materials such as PLA significantly boosts the antimicrobial activity of the composite. The authors obtained excellent antimicrobial efficacy from the composite by mixing copper and aluminum as well as copper and stainless steel [43]. The above is patented research conducted with 3D printing [44,45]. Here, we use injection molding to compare the results with 3D printing to study the effect of aluminum and stainless steel in conjunction with copper to make it more budget-friendly and to make the large-scale production of the composites practical and usable in daily life. In this study, copper, stainless steel, and aluminum are used as antimicrobial agents, as the antimicrobial properties of these metals are surface properties, and metals should retain their antimicrobial properties in the polymeric composite.
The study aims to employ injection molding to create innovative metal-enriched hybrid polymeric composites tailored for building components in public places and evaluate them in comparison to materials produced using 3D printing technology. Injection molding, known for its economic efficiency and rapid mass production capabilities, is utilized. The resulting polymeric hybrid composite holds promise for various applications, including food packaging, water treatment, construction, and biomedical purposes. The use of injection molding and multi-metals in a composite effectively increased the antimicrobial attributes of stainless steel and aluminum surfaces and is a better alternative for developing aluminum and steel surfaces with antimicrobial properties to combat the spread of infection in public places and buildings.
2. Material and Methods
2.1. Materials
The Cu–PLA pellets comprised 90% Cu and 10% PLA by weight and had a 4700 kg/m3 density. The Al 6061/PLA pellets contained 65% Al 6061 metal by weight and had a 1540 kg/m3 density. The SS 17-4/PLA pellets contained around 85% SS 17-4 metal by weight and had a 3000 kg/m3 density. For the composite used in the study, Copper-PLA, Al-PLA, and SS-PLA pellets were procured from Virtual Foundry LLC (Stoughton, WI, USA).
A known number of target bacteria was challenged against submitted samples and a standard surface, such as plastic/steel (control surface), to ascertain the bacteriological reduction after introducing the bacteria to the antimicrobial strip. The recovery of the bacteria on both surfaces after the agreed time of action (5 min, 10 min, 20 min, 1 h, 8 h, and 24 h) was estimated and compared. PLA materials were selected for developing the antimicrobial sheets, as they are easy to use, cost-effective, environmentally friendly, and biodegradable, demonstrating fewer warping issues.
The target bacteria and quality control measured used were
- E. coli—ATCC 10536;
- Pseudomonas aeruginosa (P. aeruginosa)—NCTC 10662;
- Staphylococcus aureus (S. aureus)—NCTC 6571;
- Salmonella poona—NCTC 4840;
- Enterococcus faecalis—NCTC 12697;
- Quality control measures according to ISO 22196:2011 [46], CCFRA 1.1.4:2003 [47].
2.2. Injection Molding
- A.
- Size of the pellets used for injection molding
ASTM B214 provides comprehensive instructions for conducting sieve analysis of metal powders. The standard outlines the process from sample preparation to reporting results, ensuring accuracy and reliability. It specifies the types and sizes of sieves, the duration and method of shaking, and the calculations required for determining particle size distribution [48]. Quality control measures are also emphasized to maintain the integrity of the analysis, including equipment calibration and sample handling. By following the guidelines outlined in ASTM B214, manufacturers and researchers can effectively assess the quality and consistency of metal powders, which is crucial for various industrial applications, such as powder metallurgy and additive manufacturing [48]. The sieve analysis is illustrated in Table 1, Table 2 and Table 3 for Copper-PLA, Al-PLA, and SS-PLA composite pellets respectively.

Table 1.
Particle size for Copper-PLA pellet.

Table 2.
Particle size for Aluminum-PLA pellet.

Table 3.
Particle size for Stainless Steel-PLA pellet.
- B.
- Ratio of pellets used for injection molding
The ratio of the components of the mixture was selected to compare with the results of the composites the authors obtained with the same composites prepared from 3D printing, in which the authors obtained excellent antimicrobial properties from a patented formula [44,45]. The aim of the paper was to make the composites more budget-friendly and make the large-scale production of these composites more practical, ensuring they are ready to use and accessible, through the application of injection molding.
- C.
- Process of injection molding
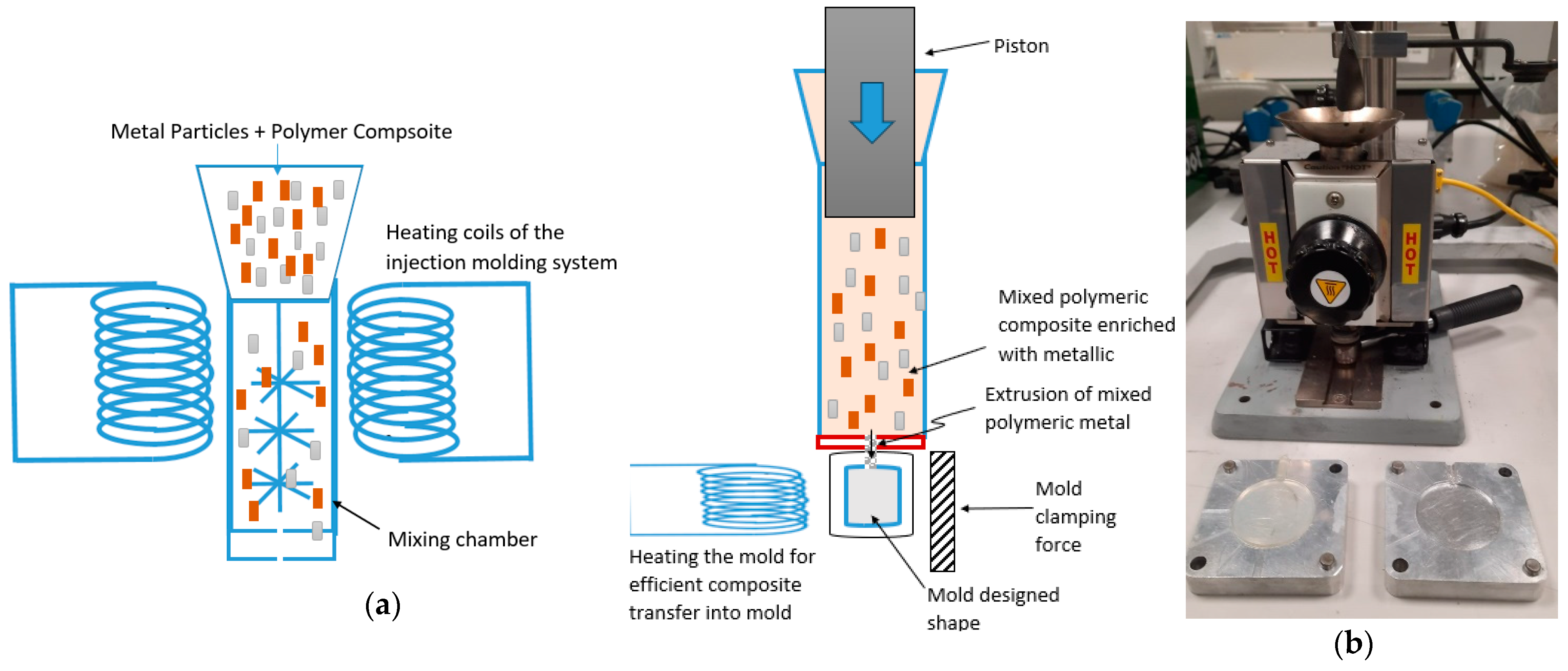
The mixed metal particles were molded using the Benchtop Model B-100 injection molder to obtain the mechanical and antimicrobial test specimens, as shown in Figure 1. The ratio of the samples for mixing Cu–PLA and SS–PLA for the first composite was 5:8, and the ratio was 11:4 for Cu–PLA and PLA-Al for the second composite. Figure 2 shows microcopic image of the composites. A known amount of pellets of Cu-PLA and Al-PLA were taken in the ratio of 5:8; the mixture was then added to the mixing chamber of the injection molder (at 446°F) and left for 5 min for the pellets to melt before mixing the metals in the chamber for another 5 min at 12 rpm. The mixture was then shifted to the molding chamber of the injection molder, which was maintained at a temperature of 446°F, left there for 3–4 min to ensure the sample was maintained at the temperature, and then injected into the mold (before injecting the samples in the mold, the molds were heated in the oven at 80°F for 7–8 min to ensure the smooth flow of the samples into the mold; a heat gun can also be used to heat the molds before injection). The mold was later left to cool down before collecting the samples from the mold. A circular steel mold with a diameter of 40 mm was used for the samples. The same method was followed for the production of Cu-PLA-SS composite, where the ratio of Cu-PLA and SS-PLA was maintained at 11:4. The mold of 40 mm was selected because of the 40 mm diameter sample requirement for the antimicrobial testing.
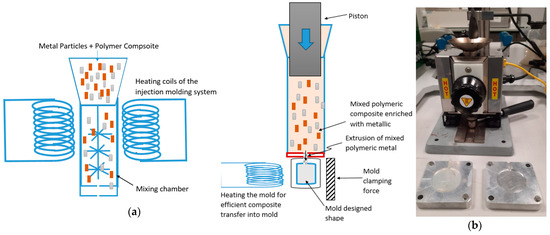
Figure 1.
Schematic of injection molding in two stages: (a) mixing metals and polymeric material and (b) transferring mixed metal–polymeric materials into desired molds.
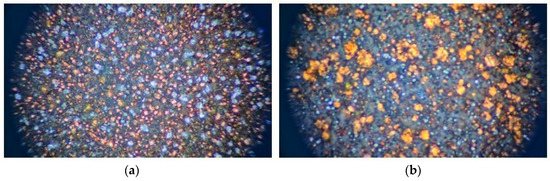
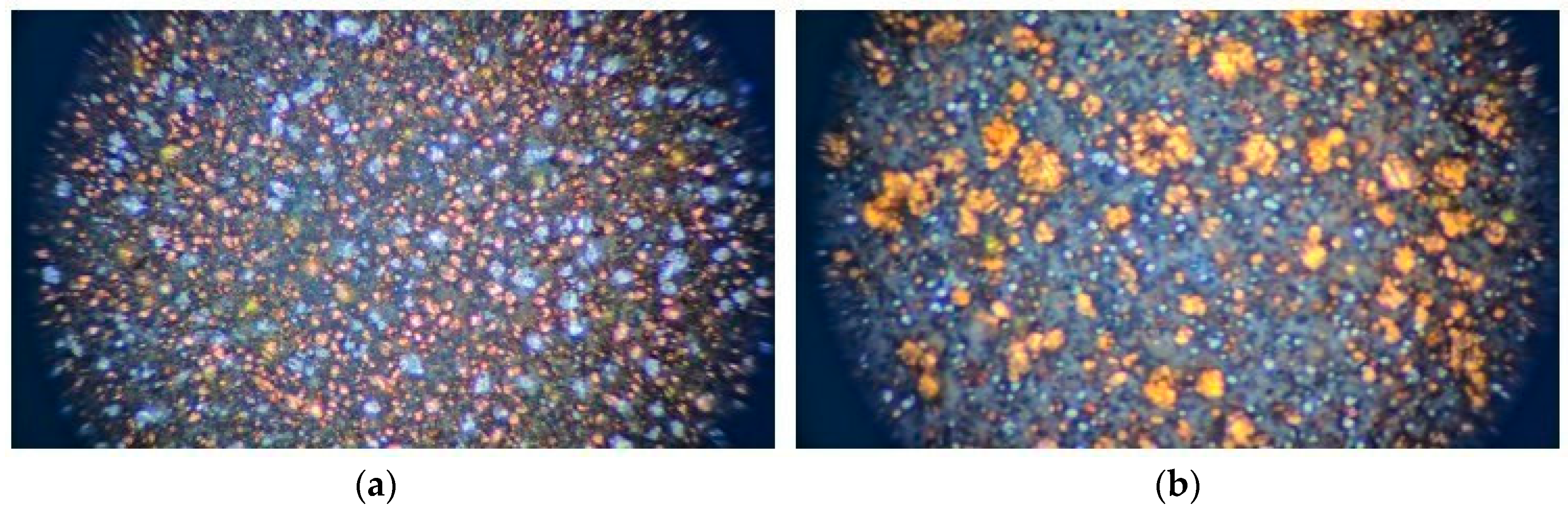
Figure 2.
Microscopic surface image of injection-molded metal sheets: (a) Cu–PLA–Al and (b) Cu–PLA–SS.
2.3. Physical Properties: Composite Density
The material density can affect the antimicrobial properties of composites such as PLA metal composites. Research on Metal–Organic Frameworks (MOFs) and polymer-MOF composites has shown that the structure and properties of the composite, including density, play a role in its antimicrobial effectiveness [49,50]. For example, in the case of PolyCu-MOF@AgNPs, the composite displayed antimicrobial activity by releasing copper and silver ions, which damaged bacterial cells and disrupted their metabolism, leading to the killing of bacteria [49]. The density of the material can influence factors like porosity, surface area, and the release rate of antimicrobial agents, all of which can impact the composite’s effectiveness against bacteria [49,50]. Therefore, when considering a PLA metal composite for antimicrobial applications [51], it is essential to consider the density of the material, as it can affect the composite’s antimicrobial properties by influencing factors like the release of antimicrobial agents and the interaction with bacterial cells.
The experimental densities of the prepared samples were measured by calculating the volume of the mold (using a Vernier caliper, the radius of the mold was calculated), and the weights of the prepared composite disks were measured using a weighing machine.
The density of each sample was calculated, and for the value of the density of the Cu–SS–PLA material, the average of all the densities of individual samples was considered. A similar method was used for the experimental density of the Cu–Al–PLA material. For the theoretical density, the reference densities of Cu–PLA, Al–PLA, and SS–PLA are 4.8, 1.54, and 3 gm/cc, respectively. The theoretical density of the CU–SS–PLA material is as follows:
- Density of PLA-Cu-SS composite
- Mass of the Cu–PLA material = MCu
- Volume of the Cu–PLA material = Vcu
- Mass of the SS–PLA material = Mss
- Volume of the Cu–PLA material = Vss
Assuming that Vcu = Vss, (Density) cu = 4.8; MCu/Vcu = 4.8; MCu = 4.8Vcu;
(Density) ss = 3; MCu/Vcu = 3; Mss = 3Vcu (Vcu = Vss).
Volume of the composite Vc = Vcu + Vss = 2Vcu (Vcu = Vss).
The theoretical density of the Cu–PLA–SS composite = MCu + Mss/Vc.= (4.8 + 3) Vcu/2Vcu = 3.9.
- Density of PLA-Cu-Al composite:
- Mass of the Cu–PLA material = MCu
- Volume of the Cu–PLA material = Vcu
- Mass of the Al–PLA material = Mss
- Volume of the Cu–PLA material = Vss
Assuming that Vcu = Vss, (Density) cu = 4.8; MCu/Vcu = 4.8; MCu = 4.8Vcu;
(Density) Al = 1.54; MCu/Vcu = 1.54; MAl = 1.54Vcu (Vcu = Vss).
Volume of the composite Vc = Vcu + VAl = 2Vcu (Vcu = Vss).
The theoretical density of the Cu–PLA–Al composite = MCu + MAl/Vc.= (4.8 + 1.54) Vcu/2Vcu= 3.17.
2.4. Antimicrobial Testing and Standards
The agar disk diffusion method is one of the oldest methods utilized to estimate the minimum inhibition concentration on media, plates of agar, and broth dilution for a wide range of bacteria, except for fastidious microbes. Many modifications have been made to this method using different culture conditions, media, and criteria for inhibition zones [52]. The antimicrobial activity of bacteria was measured using an agar pour plate diffusion technique in compliance with the protocol devised in CCFRA 1.1.4.2003 [47]. The complete experimental setup and methodology were developed in-house based on general microbiology practices and referring to ISO 22196. Familiar sources of bacteria referenced via certified materials were evaluated for their antimicrobial activity and later stored as quality control measures in the laboratory.
First, the laboratory used NCTC/ATCC-type cultures to prepare a bacterial solution (A). Before the test began, the number of bacteria in a designated volume of solution (A) was calculated. This count is referred to as “Inoculum” in the report (please see the bacterial count calculation section for the calculation criteria)
The test materials and a neutral surface were exposed to this predetermined volume of known-count bacterial solution. The number of bacterial counts that survived was estimated and compared from each surface.
Bacterial count calculation:
After serial dilution, a total bacterial count test was run on each dilution of the original bacterial suspension (A). The countable dilution (ideally with less than 300 bacterial colonies) was measured, and the formula below was applied, where we used the report’s control surface result table as an example.
The inoculum column shows that there are 9600 E. coli.
It is impossible to count 9600 colonies on a plate manually. Because of this, we chose a dilution with fewer than 300 bacterial colonies in a plate.
In the 10-2, we had 96 colonies. So, the calculation is as follows.
Count = 9600
We could not choose the 10-1 dilution because the colonies were too numerous to count (More than 1000).
We could not choose the 10-3 dilution because the number of colonies was too small to be accurate.
The bacteria were measured using the pour plate technique. The standard used for the method was CCFRA 1.1.4:2003. With the help of guidelines from ISO 22196:2011 and CCFRA 1.1.4:2003, the testing protocol and the microbiology analytical methods were devised [46,47,53,54]. The results were further analyzed using Minitab software (Release 21.3.1) and a detailed statistical analysis was performed using a statistical t-test and the ANOVA method.
2.5. Hypotheses
The hypotheses cover two main research areas: optimizing injection molding processes for plastic disc production and testing antimicrobial properties in materials. Injection molding hypotheses aimed to achieve consistent dimensions and material properties through parameters such as pressure, temperature, and cooling time. On the other hand, antimicrobial property hypotheses explored the effectiveness of materials against common bacteria, the stability over time, and the compatibility with the desired material traits. These investigations sought to enhance manufacturing processes and develop materials for various applications. The following are the considered hypotheses in this study:
- Consistent injection pressure and temperature settings during injection molding can reduce defects in plastic discs, while adjusting cooling time ensures consistent crystallinity and thermal stability. Optimizing parameters like melt temperature and injection speed maximizes efficiency and quality.
- Incorporating metallic particles in the material will enhance antimicrobial activity against various pathogens.
- The antimicrobial efficacy of the material will remain stable over time, demonstrating long-term protection against microbial contamination.
- The antimicrobial properties of the material will not adversely affect its mechanical strength, durability, or other desired characteristics.
- Comparing different antimicrobial metallic particles incorporated into the material will reveal variations in their effectiveness against specific types of bacteria.
- The antimicrobial material’s antimicrobial activity remains unaffected by environmental conditions, and its practical efficacy can be assessed under simulated real-world conditions.
3. Results and Discussion
3.1. Density
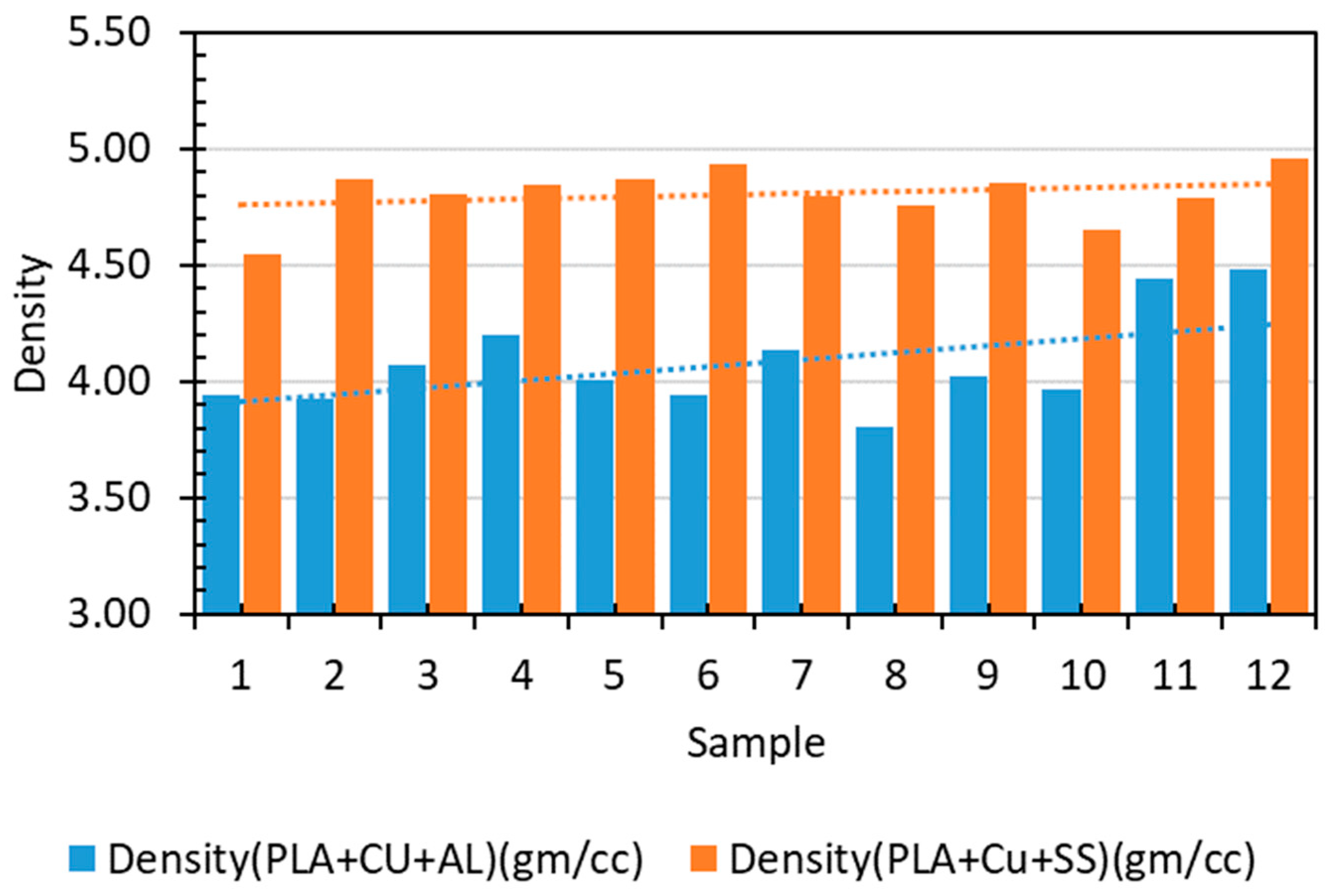
Table 4 presents the experimental densities of Cu–PLA–SS and Cu–PLA–Al composites, indicating detailed testing for accuracy. The mass of injection-molded disc samples was measured using a digital scale, with dimensions precisely determined for volume calculation. Densities were computed using fundamental physics principles, reflecting mass per unit volume of the composites. These measurements offer insights into material composition, structural integrity, and potential performance. The accurate density determination emphasizes research consistency and strengthens the credibility of the findings for antimicrobial applications. Figure 3 depicts the densities of the composites produced for different samples.

Table 4.
Experimental densities of Cu–PLA–SS and Cu–PLA–Al composites.
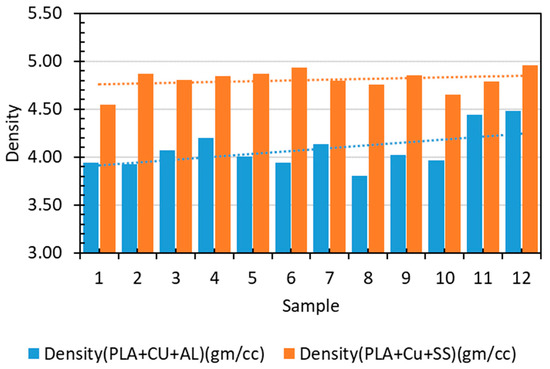
Figure 3.
Experimental densities of Cu–PLA–SS and Cu–PLA–Al composites.
The experimental densities of Cu–PLA–Al and Cu–PLA–SS composites were 4.078 gm/cc and 4.806 gm/cc, respectively, with corresponding standard deviations of 0.206 and 0.114. These densities were higher than their theoretical values of 3.9 gm/cc and 3.17 gm/cc, respectively. This difference is likely due to the incorporation of denser metals in composites compared to PLA. While theoretical density assumes perfect mixing and no voids, the actual distribution and potential imperfections can lead to higher experimental densities.
When applied in injection molding processes, the Clements method offers practical advantages for manufacturers working with PLA-based composites containing various metallic particles. Manufacturers can gauge the uniformity and consistency of the injection molding process by assessing statistical parameters like average values, standard deviations, skewness, and kurtosis. Variations in average values hint at disparities in metallic particle dispersion within the PLA matrix, impacting material properties. Skewness and kurtosis analysis helps pinpoint issues like non-uniform melting and cooling rates, which are crucial for preventing defects in final products. The method also enables the creation of prediction intervals, aiding in production outcome forecasting and quality control management. Through systematic application, manufacturers can track production trends, identify improvement areas, and optimize the injection molding process for better efficiency and performance in PLA-based composites. Employing the Clements method ensures data-driven reliability, quality, and consistency in PLA-based composite manufacturing.
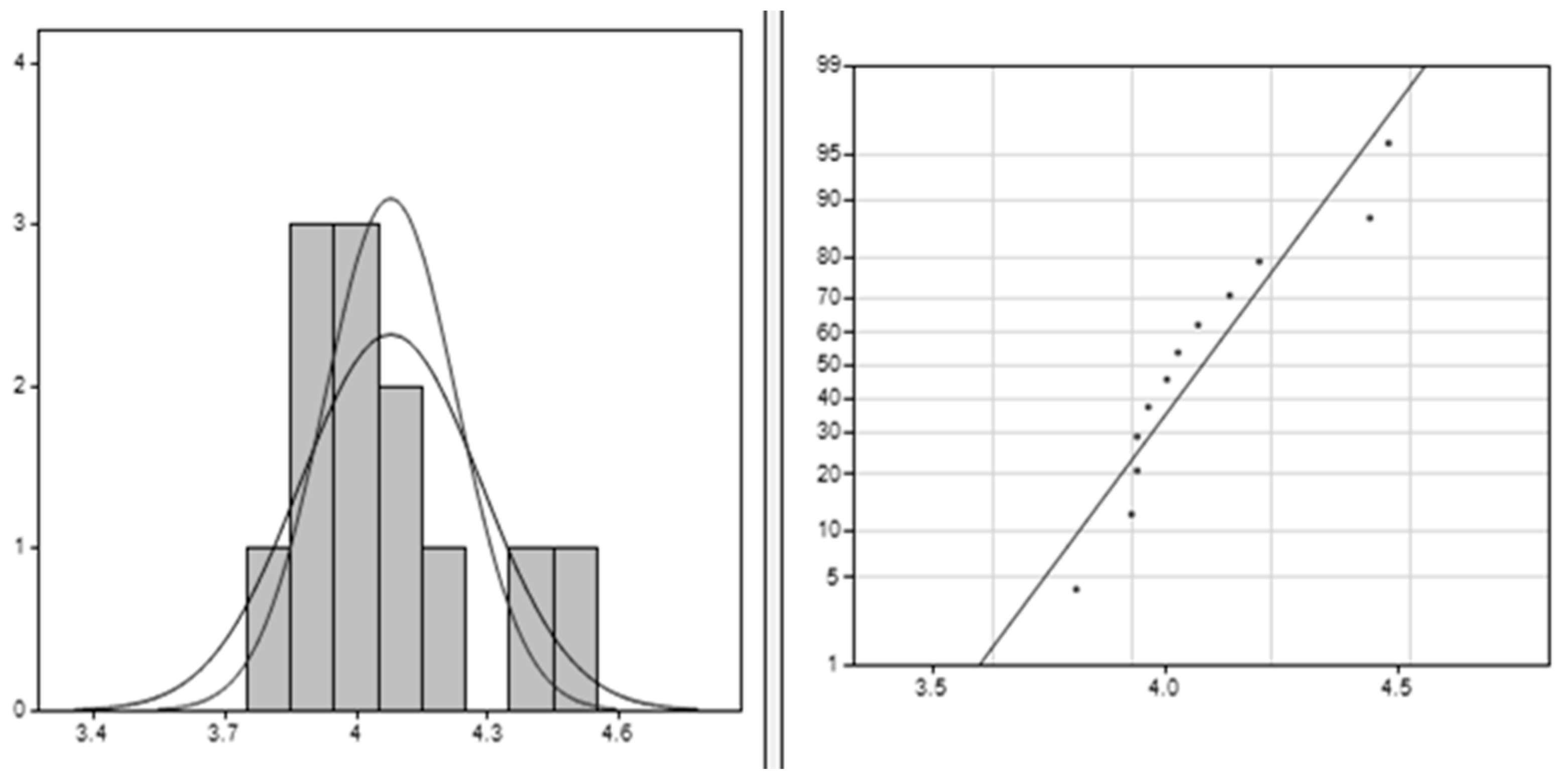
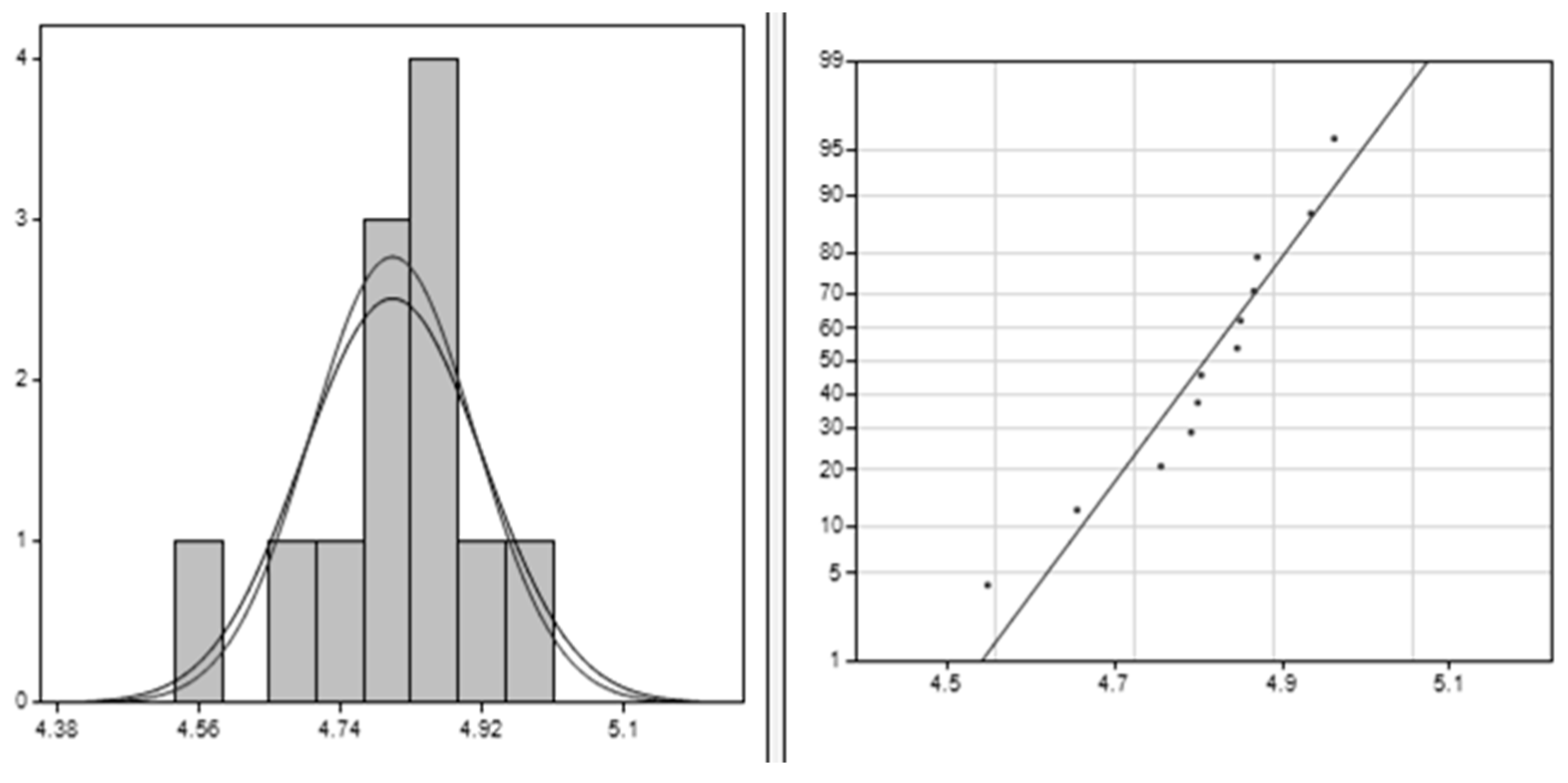
The results from the Clements method, illustrated in Figure 4, provide significant insights into the dataset characteristics. The average value of approximately 4.078 indicates the central tendency, suggesting clustering around this value. With a standard deviation of around 0.206, there is low variability in the data points around the mean. A positive skewness value of 1.045 indicates a moderate right skew, implying higher values than lower ones. The kurtosis value of 0.358 suggests a slightly peaked distribution compared to normal, indicating clustering around the mean. These findings collectively enhance understanding of the dataset’s shape, spread, and central tendency, facilitating better interpretation for analytical purposes.
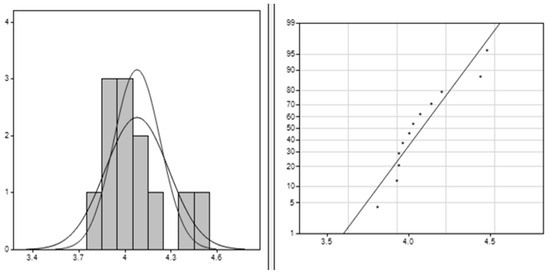
Figure 4.
Clements method applied for PLA–Cu–Al: the right image is the data histogram; the left is the normal probability.
The results obtained for PLA–Cu–SS using the Clements method, depicted in Figure 5, provide a comprehensive understanding of the dataset’s key characteristics. The average value of around 4.80658 represents the central tendency, indicating where most observations cluster. The standard deviation of approximately 0.114494 indicates low variability in data points around the mean, offering insights into the spread of the dataset. A negative skewness value of −1.053 suggests a moderate left skew, indicating more low values than high ones. Additionally, a positive kurtosis value of 1.366 signifies a more peaked distribution than usual, implying a higher frequency of extreme values relative to the tails. Together, these findings enhance comprehension of the dataset shape, spread, and central tendency, facilitating better analysis for practical purposes.
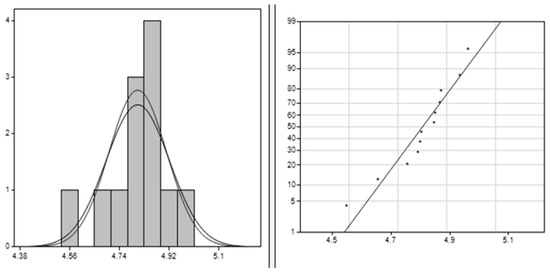
Figure 5.
Clements method applied for PLA–Cu–SS: the right image is the data histogram; the left is the normal probability.
Comparing density measurements between PLA–Cu–Al and PLA–Cu–SS provides valuable insights into injection molding production processes and material characteristics. The lower average density in PLA–Cu–Al suggests lighter structural compositions during molding, while the higher density in PLA–Cu–SS indicates a denser structure, possibly due to varied molding parameters or material compositions. Density variation within PLA–Cu–Al, reflected in higher standard deviation, may result from molding conditions or material property variations. Skewness and kurtosis values indicate distinct density distributions, likely influenced by material composition, cooling rates, or mold design. Precise control over molding parameters and material compositions is crucial to achieving the desired density, tailored to specific applications and performance requirements.
3.2. Optimization of Injection Molding Parameters
The temperature for the preparation of the mold was kept consistent at 446F for consistent results in samples. On decreasing the temperature to 405F, sufficient for PLA, we were not able to obtain a complete disc in the mold for either Cu-PLA-Al or PLA-Cu-SS. This might be because the higher melting point of metals when mixed with PLA causes the reinforcement of the PLA matrix, making it more resistant to deformation and requiring a higher temperature to melt; the reinforcement force is similar to the strengthening observed in composite materials. On gradually increasing the temperature from 405F to 446F (the maximum melting point for PLA), heating the mold to up to 90 degrees Fahrenheit using a mold release spray, and maintaining the injection speed with multiple trials and errors. we were able to obtain smooth surfaces for the samples without any air bubbles; the smoothness of the samples were further enhanced by polishing them with sandpaper with grain size 180. The mixing speed of the samples was kept at 70 rpm for 5 min to ensure there were no bubbles during injection; on decreasing the speed of the mixer and increasing the time to 8–9 min, we were able to obtain smooth samples, but some very small bubbles were still seen. The cooling time for the mold was kept consistent at 8–10 min until the surface of the mold was lukewarm, and we were able to obtain a smooth surface for the samples without defects. When trying to decrease the cooling time and using water to cool the surface of the mold, we were still able to obtain a complete disc but with many surface deformations, which might be because the rapid cooling can induce higher levels of stress within the molded part, so when the surface of the mold is cooled quickly, it can cause differential contraction between the surface and inner parts of the mold, leading to warpage or distortion.
3.3. Antimicrobial Testing
Two sets of antimicrobial polymeric composites were developed by mixing a known composition using injection molding and were labeled as samples 1 and 2. A commercially available nylon sheet was used as a control to examine the antimicrobial results of the two antimicrobial polymeric composites. The antimicrobial activity was measured over five different periods, i.e., 5 min, 10 min, 20 min, 1 h, 8 h, and 24 h, against five types of Gram-positive and Gram-negative bacteria: E. coli, S. aureus, P. aeruginosa, Salmonella Poona, and Enterococci.
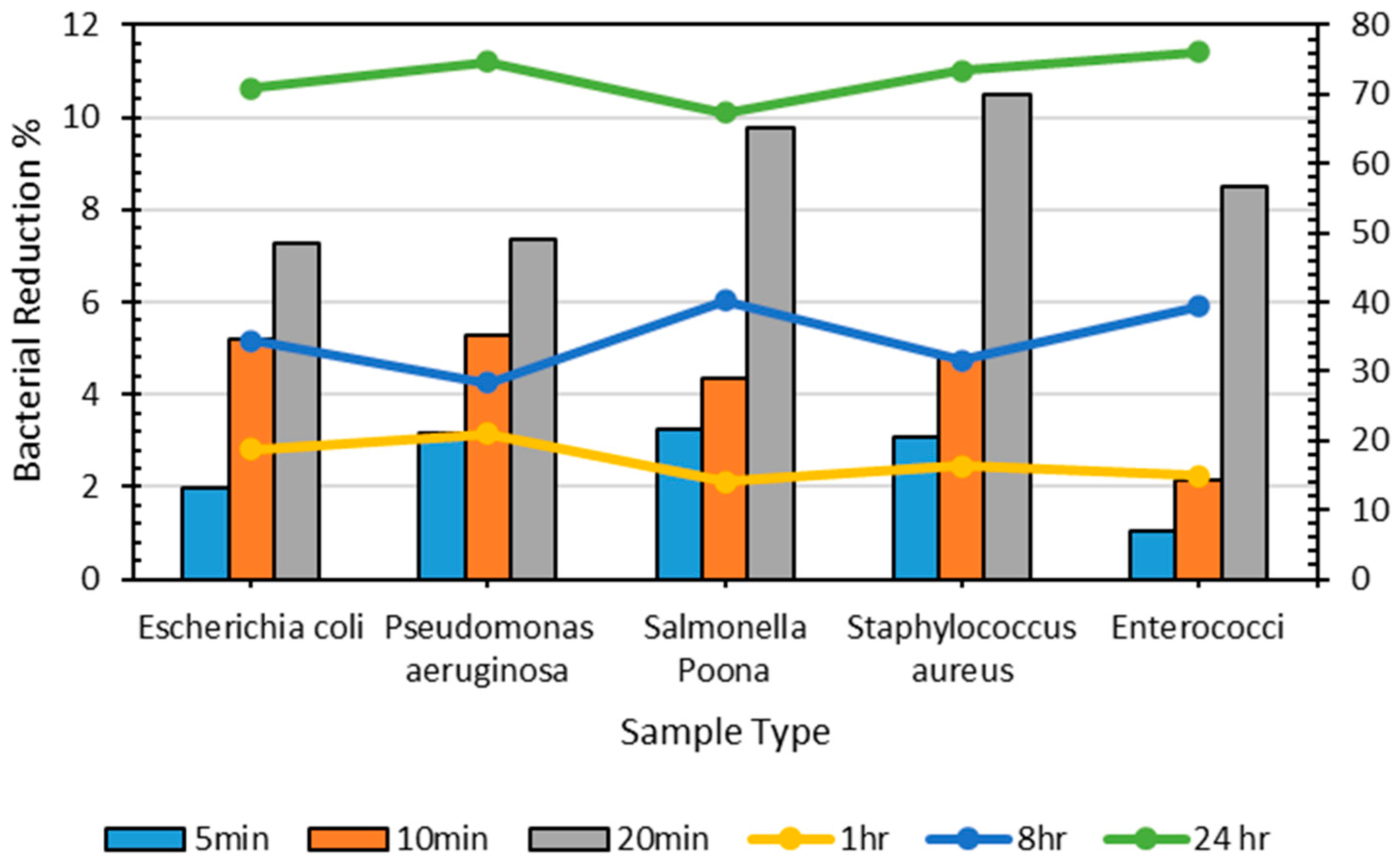
Figure 6 and Table 5 demonstrate that the reduction in the number of bacterial percentages does not substantially increase until 8 h, when >70% of P. aeruginosa was present on the plastic surfaces; however, after 24 h, the most significant decrease in the number of bacterial percentages was observed for all bacteria (i.e., greater than 70%). Table 5 shows the bacterial count of all microorganisms on the control sheet at different periods. The bacterial counts for S. aureus and P. aeruginosa were minimal after 24 h (2250 and 2400, respectively); nonetheless, E. coli, S. Poona, and Enterococci were available in considerable quantities on the surface of the control, even after 24 h. The decline in bacterial count on a plastic sheet after a specific period can be explained by environmental conditions, the type of microbes, and bacterial colonization, reducing bacterial diversity and population over time. Regardless, considerable numbers of bacteria were present at the end of 24 h [55,56].
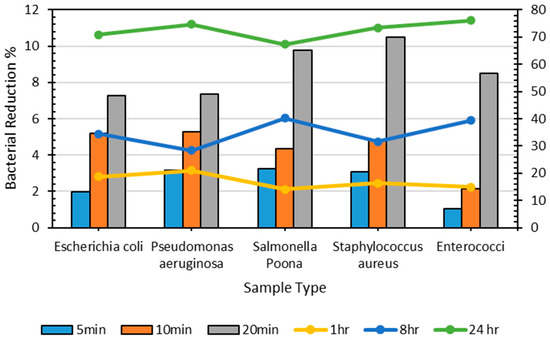
Figure 6.
Percentage of decrease in bacterial count on the control sheet over different periods.

Table 5.
Number of bacteria on the reference control sheet across different periods.
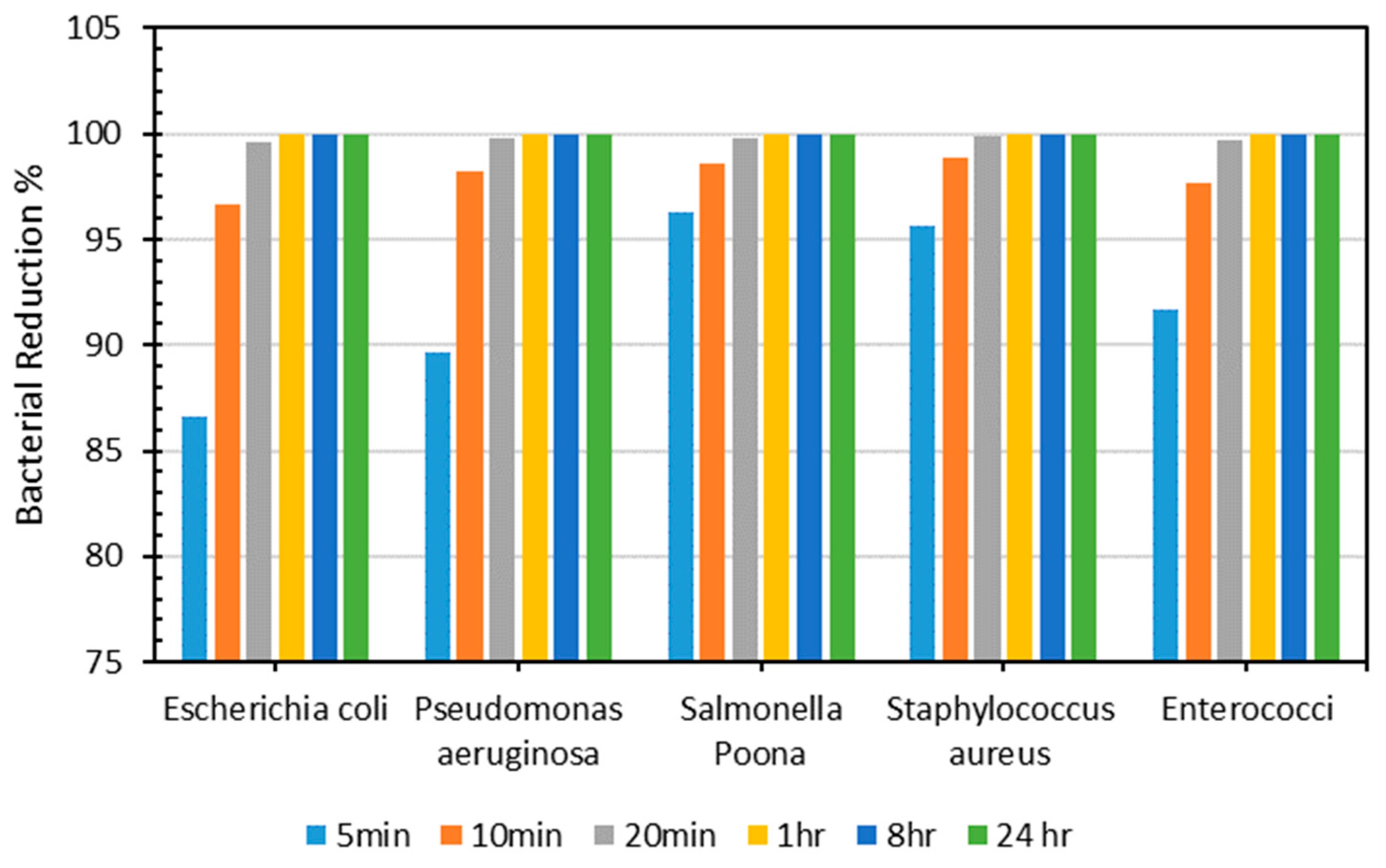
As demonstrated in Figure 7, the resultant sheet showed outstanding antimicrobial efficiency, reducing bacterial activity by more than 96% for all types in under 10 min. Next, antimicrobial efficiency was evaluated for 20 min, 1 h, 8 h, and 24 h, and the outcomes were consistent, with more than 99% antimicrobial efficiency achieved for all bacteria after 20 min. The polymeric composite Cu and Al ions caused a decline in microorganisms [57,58,59,60]. Antimicrobial composites produce metal ions, which interact with bacteria cells, causing cell damage and death [61]. Therefore, incorporating Cu and Al into the PLA-based composite is responsible for the material’s reduced bacterial count. Table 6 shows the count of all microorganisms tested on PLA–Cu–Al sheets at various periods.
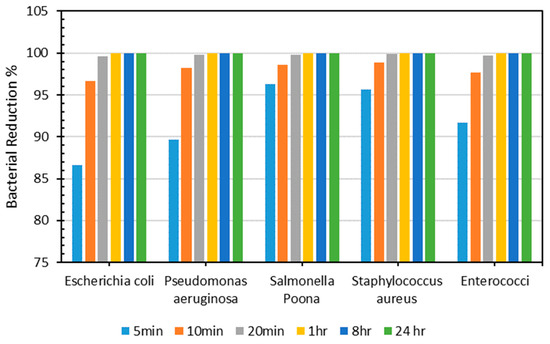
Figure 7.
Percentage of bacterial count reduction on Cu–PLA–Al sheets for different intervals.

Table 6.
Number of bacteria on Cu–PLA–Al sheets at various periods.
Valerini [62] studied the antimicrobial properties of Al-doped ZnO PLA coatings and found that they efficiently eliminated the bacteria in 5 h, making them suitable for use in food packaging applications. Most prior studies evaluated the composite antibacterial activity against a specific type of bacterial strain (Gram-positive or Gram-negative) [63]. Chu et al. [64] modified PLA by incorporating Ag and Zn nanoparticles using solvent volatilization to study the antimicrobial activity of the modified active PLA films against E. coli. It was found that the metal nanoparticle-infused PLA films were highly effective against E. coli. In this work, the efficacy of the developed samples was assessed against five strains of bacteria. In another study, Podstawczyk et al. [65] used 3D printing to produce an antimicrobial PLA–Ag nanoparticle material, and its antimicrobial efficiency was determined against two strains of bacteria (both Gram-positive and -negative). The results demonstrated the excellent antimicrobial efficacy of the material against these bacteria and its potential use in developing customized antimicrobial objects [65].
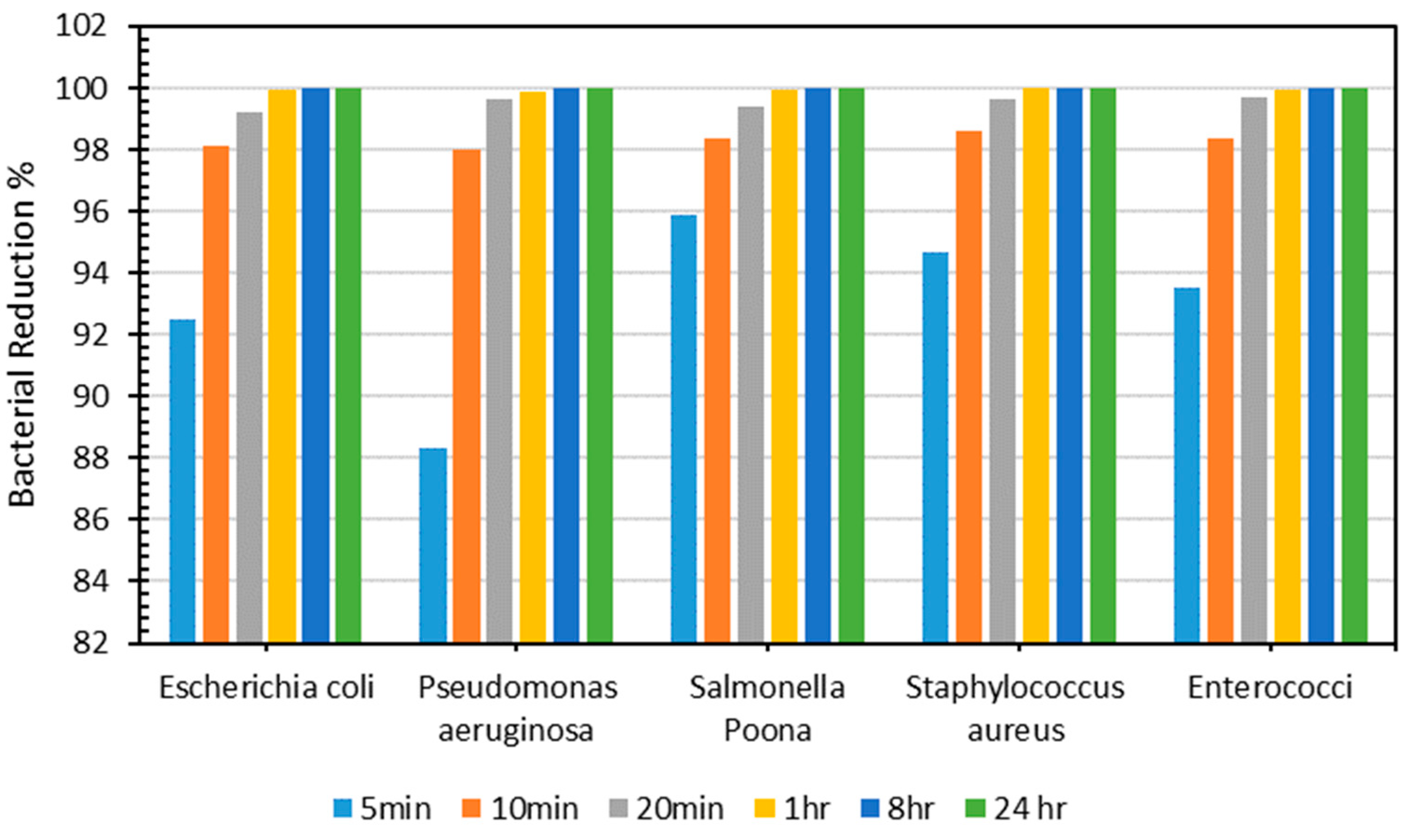
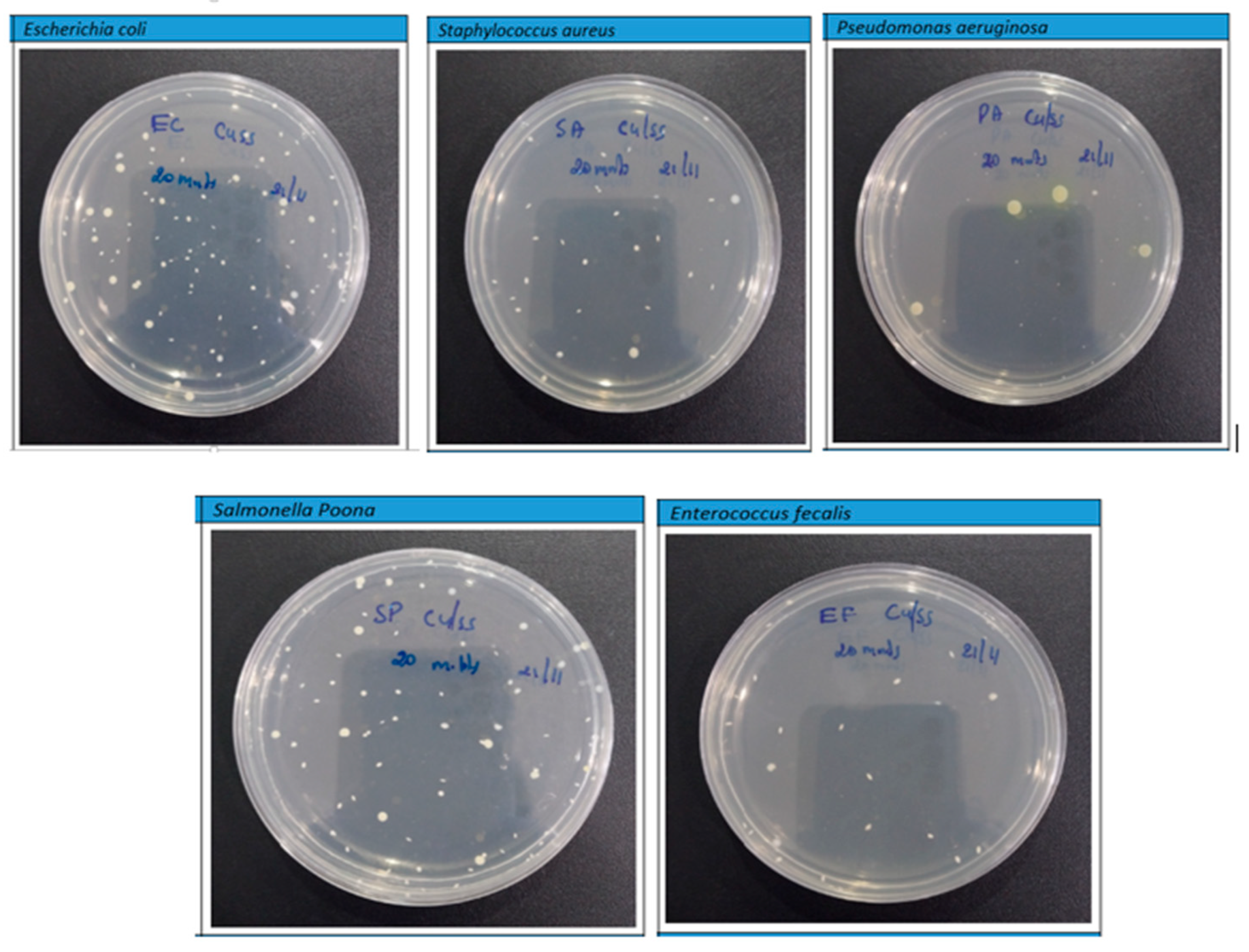
Figure 8 illustrates the decrease in the bacterial count (%) of each kind of bacteria across different periods for the produced PLA–Cu–SS sheet. As demonstrated in Figure 8, the produced sheet exhibited exceptional antibacterial efficacy, with a >90% decrease in bacterial count for each type of bacteria obtained after just 5 min. Antibacterial efficacy was evaluated for 10 min, 20 min, 1 h, 8 h, and 24 h, and outcomes were consistent, with a >98% antibacterial efficiency obtained for different types of bacteria within just 10 min. The decrease in the number of bacteria could be because of the antimicrobial action of Cu and SS particles or the increased interaction between the metallic particles [16,18,39]. These findings are akin to other research found in the literature [65]. Kudzin et al. [66] studied the antibacterial activity of polylactide–Cu alginate composite fibers against various bacteria (E. coli and S. aureus) and fungi (Aspergillus niger). It was found that the composite fibers demonstrated excellent antimicrobial activity and exhibited immense potential for use in biomedical applications. Table 7 shows the bacterial counts of all microorganisms tested on the PLA–Cu–SS sheets at various time intervals.
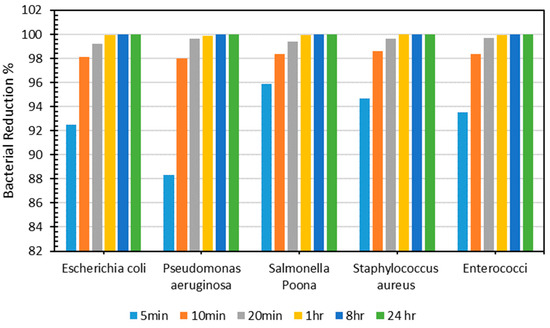
Figure 8.
Percentage of bacterial count reduction on Cu–PLA–SS sheets for different intervals.

Table 7.
Number of bacteria on Cu–PLA–SS sheets across different periods.
The current study found that PLA-Cu-SS composite sheets have high antibacterial efficacy against five investigated microorganisms. In comparison to the previously published work by Maslana et al. [67], the antimicrobial efficacy of PLA-Cu-SS sheets against Escherichia coli and Staphylococcus aureus is higher [67], with only 60% antimicrobial efficiency after 24 h using a cellulose-based composite. It has been shown that antibacterial bimetallic polymer-based composites have the property of reducing bacteria on surfaces quickly [68]. Using a bimetallic non-conductive composite constructed of polymer-based components resulted in outstanding antibacterial characteristics. The voltage difference (electromotive force) between at least two distinct non-conductive metallic composites was associated with the efficiency of the antibacterial properties [69].
As demonstrated in Figure 7 and Figure 8, little variation existed between the two produced sheets regarding antibacterial effectiveness against microorganisms. Table 5 shows the bacterial counts of all microorganisms on PLA–Cu–Al sheets at different intervals. The decline in the number of bacteria from the initial count in the inoculum was observed between the 5 and 20 min periods; however, a considerable decrease in the number of bacteria was obtained in the period between 20 min and 1 h, with no substantial decrease or variation for several bacteria from 1 to 24 h (Table 2 and Table 3). The two tables show that PLA–Cu–Al showed better efficiency in comparison to that of PLA–Cu–SS; however, the difference in efficacy was not noteworthy. This might be because of the increased interaction between the metal particles Cu and Al vs. Cu and SS, leading to better antimicrobial action for Cu–PLA–Al [16,18,39].
Previous research studied self-disinfecting films of PLA incorporated with Cu nanoparticles; the developed novel nanofilm was highly effective against E. coli [70]. Most previous research has focused on the antimicrobial efficacy of samples against E. coli and S. aureus. On the contrary, our work evaluated materials’ effectiveness against five types of bacteria at different periods. The results indicate that the antibacterial effectiveness of the developed samples was much more significant in terms of time when compared with previous findings, where the developed materials were tested against only two microorganisms.
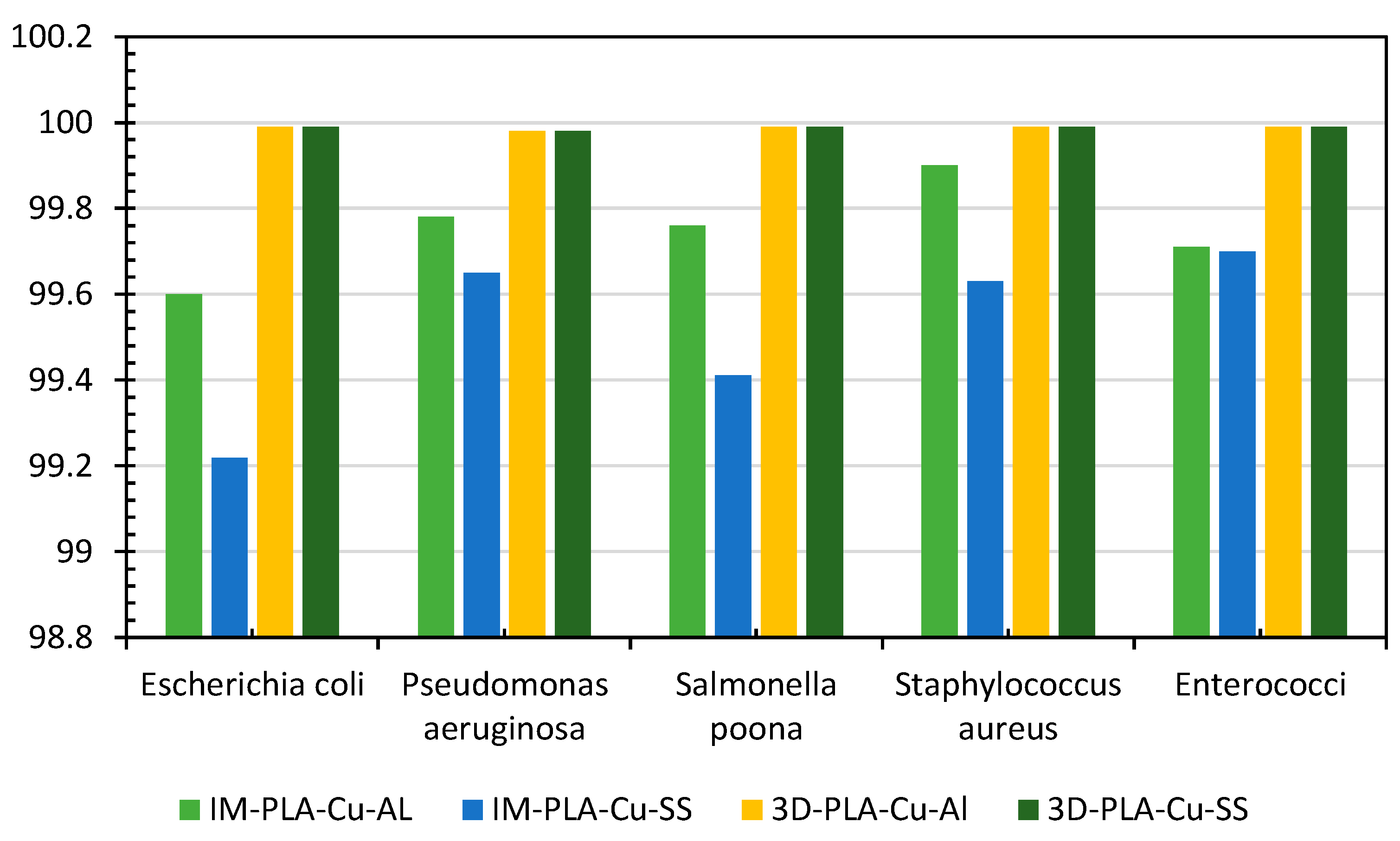
The antimicrobial efficacy of the produced polymeric composites (PLA–Cu–Al and PLA–Cu–SS) using injection molding (IM) vs. 3D printing (reported previously) was compared (Figure 9). At the end of 20 min, for the different bacteria, the antimicrobial efficacy of PLA–Cu–Al developed using 3D printing was more significant than that produced using injection molding, except for S. aureus, and the antimicrobial efficiency of PLA–Cu–SS developed using 3D printing was more significant than that fabricated using injection molding. This might be because 3D printing allows for better dispersion and homogeneity of the bimetal composite within the polymer matrix, leading to more consistent antimicrobial properties throughout the printed object [71,72]. In addition, the layer-by-layer construction in 3D printing might create a layered structure that enhances the overall antimicrobial performance. This layered structure could influence the diffusion of antimicrobial agents and their interaction with microbes [73,74].
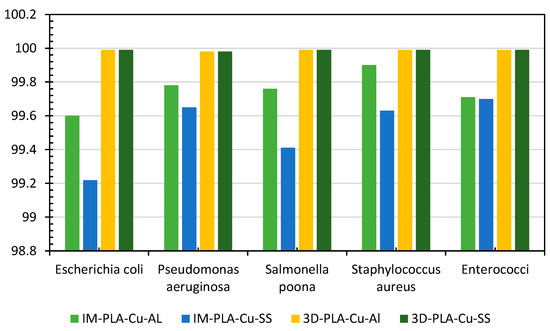
Figure 9.
Comparison of antimicrobial efficiency of injection-molding (IM) mixed PLA–Cu–SS and PLA–Cu–Al sheets with 3D-printed PLA–Cu–SS and PLA–Cu–Al sheets against different bacteria for 20 min.
3.4. Agar Plate Diffusion Tests
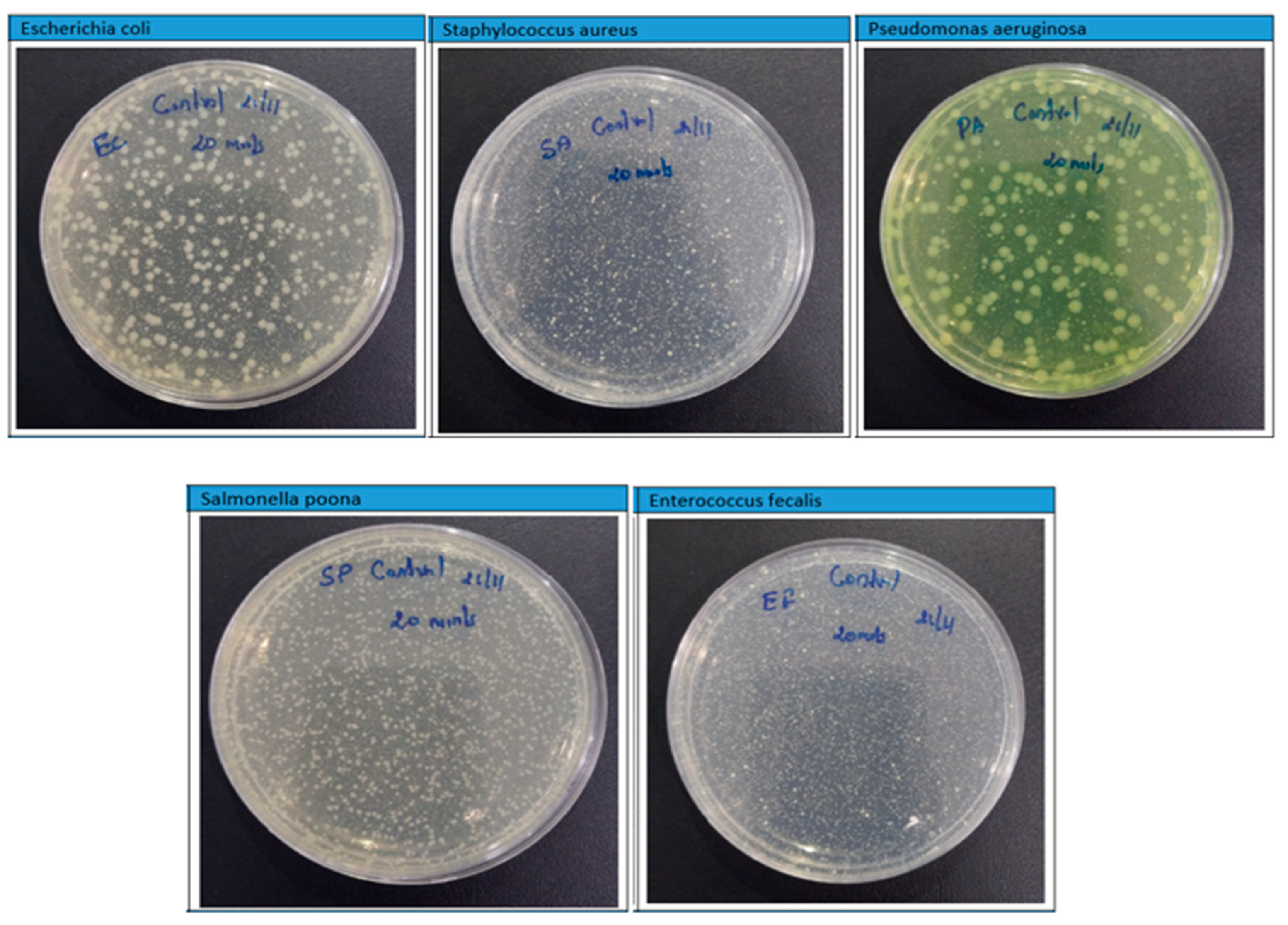
The agar plate test allows for rapid susceptibility identification while providing adequate findings and measures of growth inhibition caused by a tested microorganism [56]. Figure 10 shows the photographs of Petri plates used in the agar diffusion method for the “control sheet” against E. coli, S. aureus, P. aeruginosa, Salmonella Poona, and Enterococci at different intervals (5 min, 10 min, 20 min, 1 h, 8 h, and 24 h). The results showed that the plastic control sheet had no notable antibacterial efficacy against any tested microorganisms, since levels were still high after 24 h.
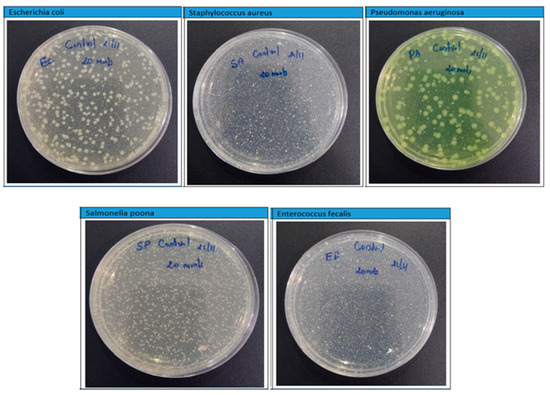
Figure 10.
Photographs of Petri plates used in the agar diffusion method measuring control of different bacteria after 8 h.
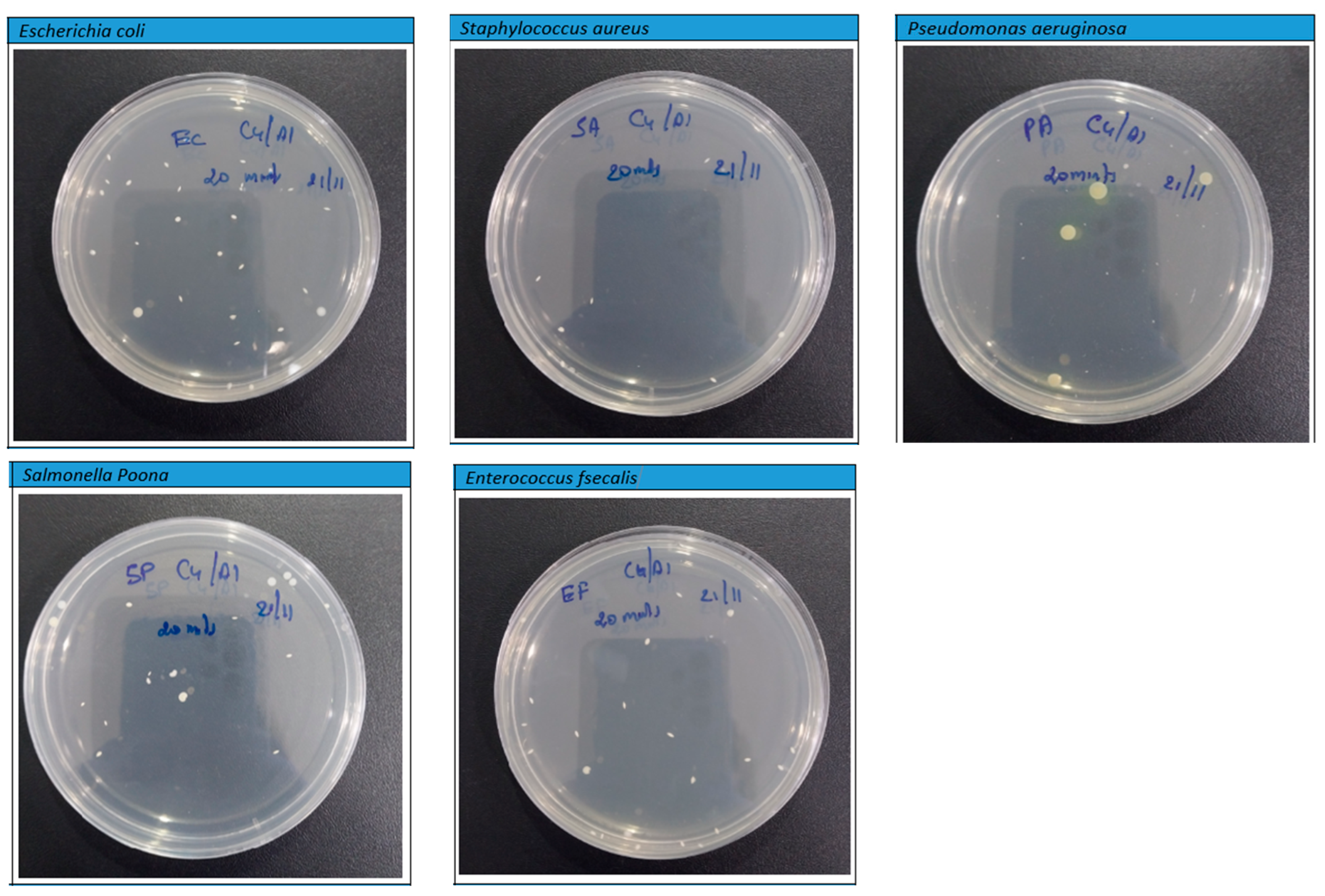
However, the findings depicted in Figure 11 and Figure 12 disclose a notable trend of a substantial reduction in bacterial population upon exposure to the PLA–Cu–SS and PLA–Cu–Al samples, featuring their remarkable antibacterial efficacy. This significant decline is convincing evidence of the impressive antimicrobial properties inherent within these composite materials. The effectiveness of these composites in combating bacterial proliferation can be primarily attributed to the presence of copper (Cu) and aluminum (Al) nanoparticles embedded within their structure. These particles exhibit a complicated antibacterial mechanism under their ability to coagulate proteins and impede bacterial growth [16,18]. The integration of Cu and Al particles into the composite matrix engenders a potent antibacterial effect, precipitating a notable reduction in bacterial viability. Notably, the protein-coagulating property of these particles disrupts critical cellular processes within bacteria, precipitating their functional destruction and subsequent death. Furthermore, the inhibitory effect exerted on bacterial growth highlights the remarkable capacity of these particles to inhibit replication and increase microbial populations. This interactive antibacterial action features the promising potential of PLA–Cu–SS and PLA–Cu–Al composite materials across a spectrum of biomedical, environmental, and industrial applications. Leveraging the intrinsic antimicrobial properties of metal particles within polymer matrices represents a significant step toward developing advanced materials endowed with enhanced antibacterial efficacy and versatile applicability in diverse settings.
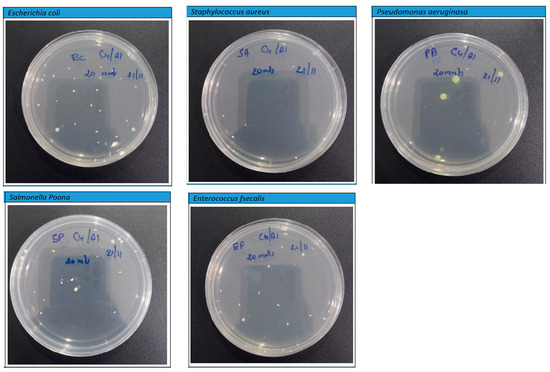
Figure 11.
Photographs of Petri plates used in the agar diffusion method for Cu–PLA–Al against different bacteria after 20 min.
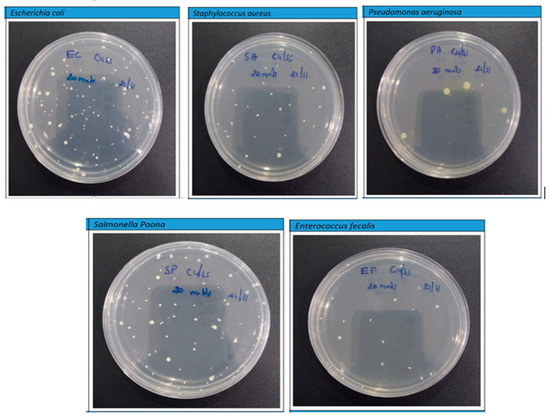
Figure 12.
Photographs of Petri plates used in the agar diffusion method for Cu–PLA–SS against various bacteria after 20 min.
On the other hand, after 20 min, the PLA–Cu–Al sheet showed the highest antibacterial efficacy (99.60%) against all microorganisms tested. After 20 min, similar effects were observed in the PLA–Cu–Al sheet against the tested microorganisms. Figure 11 shows the photographs of Petri plates used in the agar diffusion method for the PLA–Cu–Al sheet against E. coli, S. aureus, P. aeruginosa, Salmonella Poona, and Enterococci at 20 min. Figure 7 shows that few bacteria were left on the Petri dish after 20 min, and no further growth was observed even after 24 h, demonstrating that the PLA–Cu–Al sheet had outstanding effectiveness against all tested microorganisms.
Cresnar et al. [75] modified the PLA films via the addition of different metal nanoparticles (varying amounts of Ag, ZnO, and TiO2 nanoparticles, 0–3 wt%) for industrial applications using melt extrusion and film development methods, and the mechanical and antimicrobial properties (for E. coli and S. aureus) of the modified films were studied. The modified nanofilms were effective against only E. coli, with minimal antimicrobial efficiency against S. aureus; the Ag-nanoparticle-modified films showed the highest antimicrobial activity against both microbes after 6 h. Based on previously published studies, along with the outcomes of this study, we deduce that antimicrobial polymeric composites fabricated via injection molding, such as PLA–Cu–SS and PLA–Cu–Al composites, can be efficiently utilized as antimicrobial agents against both Gram-positive and Gram-negative bacteria.
4. Potential Applications of Antimicrobial Materials in Construction
It is well established in the literature that microbes affect the durability of construction materials through a range of biodeterioration mechanisms [76]. The effect of microbes depends on the type and extent of microbial growth, the type of colonized material, the level of pollution, and the kind of material [77]. For example, in concrete sewer pipes, large amounts of sulfuric acid are produced, resulting in the colonization of sulfur-oxidizing bacteria, which leads to the microbiological corrosion of concrete [78,79]. The presence of microbes in construction materials can accelerate their deterioration. It is, therefore, essential to investigate the physio-chemical changes found in different construction materials, such as stone, wood, concrete, bricks, cement, tiles, glass, structural steel, reinforcing steel bars, etc. The harmful effect of the presence of microbes in construction materials highlights the need to use antimicrobial material to prevent or eliminate microbial formation on the surface of this material
Antimicrobial protection of construction materials can be done using any of the following three methods: (1) applying paint or coating on the finished surface after the material is constructed; (2) the mixing of inorganic additives, such as metallic nano-powders (e.g., copper), into mortar or concrete during construction; (3) mixing in antimicrobial agents during the fabrication of construction materials such as cement, mortar, blocks, tiles, bricks, paint, precast slabs, and wall panels during factory production. Method 1 (painting or coating of construction materials) is applied to a wide range of finished surfaces such as tiles, walls, doors, windows, wall and floor tiles, door and window hardware, partition walls, taps, wash basins, sinks, and urinals. Antimicrobial coatings eliminate the possibility of bacteria staying on the surfaces of these products and, therefore, help avoid biofilm formation [80]. Three types of antimicrobial coating are used: (1) bacterial-resistant coating; (2) bactericidal coatings, which can kill the bacteria landing on the surface; and (3) smart coatings using advancing technologies [81,82,83]. While method 1 is the most popular one, providing a protective layer by painting or coating the finished surfaces of constructed materials incurs high initial expense and additional recurring costs resulting from recoating these surfaces [84,85]. Method 2, using metallic nanoparticles as an antimicrobial protection, involves the use of metal and metal oxide nanoparticles, such as silver, titanium dioxide, or zinc oxide, as additives to bulk materials (such as concrete or cement) or to coatings and paints used in construction to improve the resistance of building materials to microbes [86]. Despite its perceived benefits, this antimicrobial protection may result in issues such as inhalation, causing serious health issues or agricultural contamination if released into soil or water [87].
5. Conclusions
We investigated the bacteriological reduction over time upon introducing bacteria to the polymeric composites comprising PLA infused with known quantities of Cu, SS, and Al particles using injection molding commercial technology to produce antimicrobial components used for buildings in public places to combat microbes. The experiments involved the injection molding of mixed metal particles and polymeric materials to create mechanical and antimicrobial test specimens. The measured densities of the Cu–PLA–SS and Cu–PLA–Al composites were higher than their theoretical densities, likely due to the distribution of the metallic components in the composites. The meticulous optimization of injection molding processes ensured the production of uniform, high-quality composites with consistent material properties. Incorporating copper, aluminum, and stainless steel particles substantially improved antimicrobial activity against various bacteria, underscoring the potential of these composites in infection control. Antimicrobial testing was conducted using the agar disk diffusion method based on established protocols and standards. The results demonstrated substantial bacteriological reduction over time for both types of antimicrobial sheets, with >98% reduction observed in just 10 min for all types of bacteria tested. Little variation was observed in the antimicrobial activity of both polymeric PLA sheets. The antimicrobial efficiency of the composites was attributed to the release of Cu and Al ions, which interacted with bacterial cells, causing damage and death. The agar plate diffusion tests further confirmed the antimicrobial effectiveness of the PLA–Cu–SS and PLA–Cu–Al sheets, showing a substantial reduction in bacterial growth compared to the control. The comparison with previous findings in the literature revealed that the developed polymeric composites exhibited superior antimicrobial efficacy against a broader range of bacteria in shorter periods. In addition, the study highlighted the potential advantages of 3D printing in achieving better dispersion and homogeneity of bimetallic composites within the polymer matrix, leading to enhanced antimicrobial properties. Overall, the findings indicate that injection-molded polymeric composites containing Cu, SS, and Al nanoparticles offer promising antimicrobial applications against Gram-positive and Gram-negative bacteria. Thus, the use of these antimicrobial composites in buildings and construction can reduce the spread of infectious diseases, especially in hospitals, by using these composites as a coating on high-touch surfaces such as walls, doors, and window handles, which will disinfect the pathogenic microbes and germs in shorter periods to reduce transmission. Further research will optimize composite materials and manufacturing processes to enhance antimicrobial performance and suitability for various biomedical and food packaging applications.
Author Contributions
Conceptualization, W.A., A.H.A.-M. and T.A.R.; Methodology, W.A., T.A.R. and M.K.; Validation, T.A.R.; Formal analysis, A.H.A.-M. and E.Z.; Investigation, A.A., W.A., A.H.A.-M., E.Z. and M.K.; Resources, A.H.A.-M.; Data curation, A.A., E.Z. and M.K.; Writing – original draft, A.A.; Visualization, A.A.; Project administration, W.A.; Funding acquisition, W.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UAEU, grant number 12R109.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Attaran, S.A.; Hassan, A.; Wahit, M.U. Materials for food packaging applications based on bio-based polymer nanocomposites: A review. J. Thermoplast. Compos. Mater. 2017, 30, 143–173. [Google Scholar] [CrossRef]
- Corrêa, A.C.; de Santi, C.R.; Manrich, S. December. Synthetic paper from plastic waste: The effect of CaCO3 on physical, surface properties and printability. In Macromolecular Symposia; WILEY-VCH Verlag: Weinheim, Germany, 2006; Volume 245, pp. 611–620. [Google Scholar]
- Kirthika, S.K.; Goel, G.; Matthews, A.; Goel, S. Review of the untapped potentials of antimicrobial materials in the construction sector. Prog. Mater. Sci. 2023, 133, 101065. [Google Scholar] [CrossRef]
- Ahmed, W.; Al-Marzouqi, A.; Rizvi, T.; Khan, M.; Zaneldin, E.; Nazir, M. Antibacterial Efficacy of Non-Copper Polymer Based Composite Enhanced with Metallic Particles Using Fused Deposition Modeling. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2023; Volume 1090, pp. 9–15. [Google Scholar]
- Navaratnam, S.; Nguyen, K.; Selvaranjan, K.; Zhang, G.; Mendis, P.; Aye, L. Designing post-COVID-19 buildings: Approaches for achieving healthy buildings. Buildings 2022, 12, 74. [Google Scholar] [CrossRef]
- Koven, S. They call us and we go. N. Engl. J. Med. 2020, 382, 1978–1979. [Google Scholar] [CrossRef]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef]
- Johnson, N.P.; Mueller, J. Updating the accounts: Global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull. Hist. Med. 2002, 76, 105–115. [Google Scholar] [CrossRef]
- Fong, M.W.; Leung, N.H.; Xiao, J.; Chu, D.K.; Cheng, S.M.; So, H.C.; Cowling, B.J. Presence of influenza virus on touch surfaces in kindergartens and primary schools. J. Infect. Dis. 2020, 222, 1329–1333. [Google Scholar] [CrossRef]
- Scully, J.R. The COVID-19 pandemic, Part 1: Can antimicrobial copper-based alloys help suppress infectious transmission of viruses originating from human contact with high-touch surfaces? Corrosion 2020, 76, 523–527. [Google Scholar] [CrossRef]
- Das Jana, I.; Kumbhakar, P.; Banerjee, S.; Gowda, C.C.; Kedia, N.; Kuila, S.K.; Mondal, A. Copper nanoparticle–graphene composite-based transparent surface coating with antiviral activity against influenza virus. ACS Appl. Nano Mater. 2020, 4, 352–362. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Anand, U.; González-Acosta, S.; López, M.R.; Dey, A.; Bontempi, E.; Morales delaNuez, A. Antimicrobial resistance in the COVID-19 landscape: Is there an opportunity for anti-infective antibodies and antimicrobial peptides? Front. Immunol. 2022, 13, 921483. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Barber, R.M.; Sorensen, R.J.; Pigott, D.M.; Bisignano, C.; Carter, A.; Amlag, J.O.; Murray, C.J. Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: A statistical analysis. Lancet 2022, 399, 2351–2380. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Fan, C.; Li, M.; Nie, H.L.; Wang, F.B.; Wang, H.; Huang, J. COVID-19: A call for physical scientists and engineers. ACS Nano 2020, 14, 3747–3754. [Google Scholar] [CrossRef] [PubMed]
- Olmos, D.; González-Benito, J. Polymeric materials with antibacterial activity: A Review. Polymers 2021, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Rani, I.; Goyal, A.; Bhatnagar, M.; Manhas, S.; Goel, P.; Pal, A.; Prasad, R. Potential molecular mechanisms of zinc-and copper-mediated antiviral activity on COVID-19. Nutr. Res. 2021, 92, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity mechanisms of copper nanoparticles and copper surfaces on bacterial cells and viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, A.; Ahmed, W.; Al-Marzouqi, A.H.; Rizvi, T.A.; Khan, M.; Zaneldin, E. Characteristics and Key Features of Antimicrobial Materials and Associated Mechanisms for Diverse Applications. Molecules 2023, 28, 8041. [Google Scholar] [CrossRef] [PubMed]
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.R.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral properties of copper and its alloys to inactivate COVID-19 virus: A review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Arendsen, L.P.; Thakar, R.; Sultan, A.H. The use of copper as an antimicrobial agent in health care, including obstetrics and gynecology. Clin. Microbiol. Rev. 2019, 32, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Vidal, R.M. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob. Resist. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Mescola, A.; Iseppi, R.; Canton, R.; Rossi, G.; Stocchi, R.; Sabia, C. Antibacterial Effect of Aluminum Surfaces Untreated and Treated with a Special Anodizing Based on Titanium Oxide Approved for Food Contact. Biology 2020, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Londono, S.C.; Hartnett, H.E.; Williams, L.B. Antibacterial activity of aluminum in clay from the Colombian Amazon. Environ. Sci. Technol. 2017, 51, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Manogar, P.; Morvinyabesh, J.E.; Ramesh, P.; Jeyaleela, G.D.; Amalan, V.; Ajarem, J.S.; Vijayakumar, N. Biosynthesis and antimicrobial activity of aluminum oxide nanoparticles using Lyngbya majuscula extract. Mater. Lett. 2022, 311, 131569. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Xiong, Z.; Yong, Y.; Zhao, X.S. Preparation, characterization, and antibacterial properties of silver-modified graphene oxide. J. Mater. Chem. 2011, 21, 3350–3352. [Google Scholar] [CrossRef]
- Perdikaki, A.; Galeou, A.; Pilatos, G.; Prombona, A.; Karanikolos, G.N. Ion-based metal/graphene antibacterial agents comprising mono-ionic and bi-ionic silver and copper species. Langmuir 2018, 34, 11156–11166. [Google Scholar] [CrossRef]
- Lay, M.; Thajudin, N.L.N.; Hamid, Z.A.A.; Rusli, A.; Abdullah, M.K.; Shuib, R.K. Comparison of physical and mechanical properties of PLA, ABS, and nylon 6 fabricated using fused deposition modeling and injection molding. Compos. Part B Eng. 2019, 176, 107341. [Google Scholar] [CrossRef]
- Pivsa-Art, S.; Kord-Sa-Ard, J.; Pivsa-Art, W.; Wongpajan, R.; Narongchai, O.; Pavasupree, S.; Hamada, H. Effect of compatibilizer on PLA/PP blend for injection molding. Energy Procedia 2016, 89, 353–360. [Google Scholar] [CrossRef]
- Ojeil, M.; Jermann, C.; Holah, J.; Denyer, S.P.; Maillard, J.Y. Evaluation of new in vitro efficacy test for antimicrobial surface activity reflecting UK hospital conditions. J. Hosp. Infect. 2013, 85, 274–281. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A mini-review of antibacterial properties of Al2O3 nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef]
- Saadi, N.; Alotaibi, K.; Hassan, L.; Smith, Q.; Watanabe, F.; Khan, A.A.; Karabacak, T. Enhancing the antibacterial efficacy of aluminum foil by nanostructuring its surface using hot water treatment. Nanotechnology 2021, 32, 325103. [Google Scholar] [CrossRef]
- Choudhury, M.; Bindra, H.S.; Singh, K.; Singh, A.K.; Nayak, R. Antimicrobial polymeric composites in consumer goods and healthcare sector: A healthier way to prevent infection. Polym. Adv. Technol. 2022, 33, 1997–2024. [Google Scholar] [CrossRef]
- Alkarri, S.; Sharma, D.; Bergholz, T.M.; Rabnawaz, M. Fabrication methodologies for antimicrobial polypropylene surfaces with leachable and nonleachable antimicrobial agents. J. Appl. Polym. Sci. 2024, 141, e54757. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-based polymers with antimicrobial properties towards sustainable development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Ghamrawi, S.; Bouchara, J.P.; Tarasyuk, O.; Rogalsky, S.; Lyoshina, L.; Bulko, O.; Bardeau, J.F. Promising silicones modified with cationic biocides for the development of antimicrobial medical devices. Mater. Sci. Eng. C 2017, 75, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Yoo, J.; Jang, Y. Engineering approaches to create antibacterial surfaces on biomedical implants and devices. In Racing for the Surface: Pathogenesis of Implant Infection and Advanced Antimicrobial Strategies; Springer: Cham, Switzerland, 2020; pp. 313–340. [Google Scholar]
- Francolini, I.; Donelli, G.; Crisante, F.; Taresco, V.; Piozzi, A. Antimicrobial polymers for anti-biofilm medical devices: State-of-art and perspectives. In Biofilm-Based Healthcare-Associated Infections: Volume II; Springer International Publishing: Cham, Switzerland, 2014; pp. 93–117. [Google Scholar]
- Sidambe, A.T.; Figueroa, I.A.; Hamilton, H.G.C.; Todd, I. Metal injection molding of CP-Ti components for biomedical applications. J. Mater. Process. Technol. 2012, 212, 1591–1597. [Google Scholar] [CrossRef]
- Altan, M.; Yildirim, H. Mechanical and antibacterial properties of injection molded polypropylene/TiO2 nano-composites: Effects of surface modification. J. Mater. Sci. Technol. 2012, 28, 686–692. [Google Scholar] [CrossRef]
- Heidari, B.S.; Oliaei, E.; Shayesteh, H.; Davachi, S.M.; Hejazi, I.; Seyfi, J.; Rashedi, H. Simulation of mechanical behavior and optimization of simulated injection molding process for PLA-based antibacterial composite and nanocomposite bone screws using central composite design. J. Mech. Behav. Biomed. Mater. 2017, 65, 160–176. [Google Scholar] [CrossRef]
- Jardón-Maximino, N.; Cadenas-Pliego, G.; Ávila-Orta, C.A.; Comparán-Padilla, V.E.; Lugo-Uribe, L.E.; Pérez-Alvarez, M.; Tavizón, S.F.; Santillán, G.D.J.S. Antimicrobial property of polypropylene composites and functionalized copper nanoparticles. Polymers 2021, 13, 1694. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Al-Marzouqi, A.H.; Nazir, M.H.; Rizvi, T.A.; Zaneldin, E.; Khan, M.; Aziz, M. Investigating the properties and characterization of a hybrid 3D printed antimicrobial composite material using FFF process: Innovative and swift. Int. J. Mol. Sci. 2023, 24, 8895. [Google Scholar] [CrossRef]
- Ahmed, W.K.; Al-Marzouqi, A.H.; Aziz, M.A.; Rizvi, T.A.; Zaneldin, E.; Khan, M.A. Antimicrobial Bi-Metallic Polymeric Composite. U.S. Patent US11866545B1, 9 January 2024. [Google Scholar]
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/standard/54431.html (accessed on 19 November 2023).
- CCFRA 1.1.4:2003; Manual of Microbiological Methods for the Food and Drink Industry. Campden & Chorleywood Food Research Association: Chipping Campden, UK, 2007.
- ASTM International. Standard Specification for Aluminum and Aluminum-Alloy Drawn Seamless Tubes (B0214—22). ASTM International. Available online: https://www.astm.org/b0214-22.html (accessed on 5 June 2024).
- Livesey, T.C.; Mahmoud, L.A.; Katsikogianni, M.G.; Nayak, S. Metal–organic frameworks and their biodegradable composites for controlled delivery of antimicrobial drugs. Pharmaceutics 2023, 15, 274. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Liebscher, M.; Tzounis, L. Three-dimensional printed antimicrobial objects of polylactic acid (PLA)-silver nanoparticle nanocomposite filaments produced by an in-situ reduction reactive melt mixing process. Biomimetics 2020, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Siraj, S.; Al-Marzouqi, A.H.; Iqbal, M.Z.; Ahmed, W. Impact of micro silica filler particle size on mechanical properties of polymeric based composite material. Polymers 2022, 14, 4830. [Google Scholar] [CrossRef] [PubMed]
- Tolba, S.; El Shatoury, E.H.; Abo AlNasr, N.M. Prevalence of carbapenem-resistant acinetobacter baumannii (CRAB) in some Egyptian hospitals: Evaluation of the use of blaOXA-51-like gene as a species-specific marker for CRAB. Egypt. J. Bot. 2019, 59, 723–733. [Google Scholar] [CrossRef]
- Hartman, P.E. Manual of Microbiological Methods; McGraw-Hill: New York, NY, USA, 1957. [Google Scholar]
- Perez-Gavilan, A.; de Castro, J.V.; Arana, A.; Merino, S.; Retolaza, A.; Alves, S.A.; Marimón, J.M. Antibacterial activity testing methods for hydrophobic patterned surfaces. Sci. Rep. 2021, 11, 6675. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Al-Marzouqi, A.H.; Nazir, M.H.; Rizvi, T.A.; Zaneldin, E.; Khan, M. Comparative Experimental Investigation of Biodegradable Antimicrobial Polymer-Based Composite Produced by 3D Printing Technology Enriched with Metallic Particles. Int. J. Mol. Sci. 2022, 23, 11235. [Google Scholar] [CrossRef]
- Kampf, G. How long can nosocomial pathogens survive on textiles? A systematic review. GMS Hyg. Infect. Control 2020, 15, Doc10. [Google Scholar]
- Krause, S.; Molari, M.; Gorb, E.V.; Gorb, S.N.; Kossel, E.; Haeckel, M. Persistence of plastic debris and its colonization by bacterial communities after two decades on the abyssal seafloor. Sci. Rep. 2020, 10, 9484. [Google Scholar] [CrossRef]
- Bharadishettar, N.; Bhat, K.U.; Bhat Panemangalore, D. Coating technologies for copper-based antimicrobial active surfaces: A perspective review. Metals 2021, 11, 711. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Hu, Y.; Wang, G.Q.; Gui, Y.; Wang, D.F.; Huang, J.Q.; Jie, X.H. Preparation and antimicrobial properties of the surface antibacterial layer of aluminum. Adv. Mater. Res. 2014, 941, 1659–1663. [Google Scholar] [CrossRef]
- Ahmed, W.; Siraj, S.; Al-Marzouqi, A.H. 3D Printing PLA Waste to Produce Ceramic Based Particulate Reinforced Composite Using Abundant Silica-Sand: Mechanical Properties Characterization. Polymers 2020, 12, 2579. [Google Scholar] [CrossRef]
- Dawari, C.K.; Gunell, M.; Mönkkönen, K.; Suvanto, M.; Saarinen, J.J. Antibacterial Activity of Electrodeposited Copper and Zinc on Metal Injection Molded (MIM) Micropatterned WC-CO Hard Metals. Coatings 2022, 12, 485. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Villani, F.; Rizzo, A.; Caputo, I.; Paolella, G.; Vigliotta, G. Antibacterial Al-doped ZnO coatings on PLA films. J. Mater. Sci. 2020, 55, 4830–4847. [Google Scholar] [CrossRef]
- Al Zahmi, S.; Alhammadi, S.; ElHassan, A.; Ahmed, W. Carbon fiber/PLA recycled composite. Polymers 2022, 14, 2194. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of antimicrobial poly (lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Podstawczyk, D.; Skrzypczak, D.; Połomska, X.; Stargała, A.; Witek-Krowiak, A.; Guiseppi-Elie, A.; Galewski, Z. Preparation of antimicrobial 3D printing filament: In situ thermal formation of silver nanoparticles during the material extrusion. Polym. Compos. 2020, 41, 4692–4705. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Boguń, M.; Mrozińska, Z.; Kaczmarek, A. Physical properties, chemical analysis, and evaluation of the antimicrobial response of new polylactide/alginate/copper composite materials. Mar. Drugs 2020, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Maślana, K.; Żywicka, A.; Wenelska, K.; Mijowska, E. Boosting of antibacterial performance of cellulose-based paper sheet via TiO2 nanoparticles. Int. J. Mol. Sci. 2021, 22, 1451. [Google Scholar] [CrossRef] [PubMed]
- Kasi, G.; Gnanasekar, S.; Zhang, K.; Kang, E.T.; Xu, L.Q. Polyurethane-based composites with promising antibacterial properties. J. Appl. Polym. Sci. 2022, 139, 52181. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, G.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; Qian, G. A strawberry-like Ag-decorated barium titanate enhances the piezoelectric and antibacterial activities of the polymer scaffold. Nano Energy 2020, 74, 104825. [Google Scholar] [CrossRef]
- Duvanova, E.; Krasnou, I.; Krumme, A.; Mikli, V.; Radio, S.; Rozantsev, G.M.; Karpichev, Y. Development of functional composite Cu (II)-Polyoxometalate/PLA with antimicrobial properties. Molecules 2022, 27, 2510. [Google Scholar] [CrossRef]
- Ahmed, W.; Siraj, S.; Al-Marzouqi, A.H. Embracing additive manufacturing technology through fused filament fabrication for antimicrobial with enhanced formulated materials. Polymers 2021, 13, 1523. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Vahabi, H.; Kumaravel, V. Antimicrobial and Biodegradable 3D Printed Scaffolds for Orthopedic Infections. ACS Biomater. Sci. Eng. 2023, 9, 4020–4044. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, M.I.A.; Ahamad, I.; Iqbal, S.; Malik, M.A.; Dar, O.A.; Akram, M.K.; Hashmi, A.A. Fabrication of metal incorporated polymer composite: An excellent antibacterial agent. J. Mol. Struct. 2021, 1225, 129091. [Google Scholar] [CrossRef] [PubMed]
- Pušnik Črešnar, K.; Aulova, A.; Bikiaris, D.N.; Lambropoulou, D.; Kuzmič, K.; Fras Zemljič, L. Incorporation of metal-based nano additives into the pla matrix: Effect of surface properties on antibacterial activity and mechanical performance of pla nano additive films. Molecules 2021, 26, 4161. [Google Scholar] [CrossRef] [PubMed]
- Pourchez, J.; Peyron, A.; Sarry, G.; Leclerc, L.; Verhoeven, P.O.; Choi, P.; Dumitrescu, C. Antimicrobial performance of an innovative technology of atmospheric plasma reactors against bioaerosols: Effectiveness in removing airborne viable viruses. Buildings 2022, 12, 1587. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Komar, M.; Gutarowska, B. Towards understanding the link between the deterioration of building materials and the nature of aerophytic green algae. Sci. Total Environ. 2022, 802, 149856. [Google Scholar] [CrossRef]
- Grengg, C.; Mittermayr, F.; Baldermann, A.; Böttcher, M.E.; Leis, A.; Koraimann, G.; Dietzel, M. Microbiologically induced concrete corrosion: A case study from a combined sewer network. Cem. Concr. Res. 2015, 77, 16–25. [Google Scholar] [CrossRef]
- Strigáč, J.; Števulová, N.; Mikušinec, J.; Varečka, L.; Hudecová, D. Antimicrobial efficiency of metallurgical slags for application in building materials and products. Buildings 2018, 8, 33. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Chen, H. Responsive and synergistic antibacterial coatings: Fighting against bacteria in a smart and effective way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef]
- Tiwari, A.; Chaturvedi, A.; Ganesan, N. Smart antimicrobial coating with endless applications. Adv. Mater. TechConnect Briefs 2018, 1, 236–239. [Google Scholar]
- Zhou, C.; Song, H.; Zhang, F.; Liu, J.; Li, J.; Liu, B.; Liang, J. A facile method to fabricate an antimicrobial coating based on poly (1-vinyl-3-allylimidazolium iodide)(PAVI) and poly (ethylene glycol) dimethyl acrylate (PEGDMA). Polym. Bull. 2019, 76, 5433–5449. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Olmo, J.A.D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial coatings for improving the performance of biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Klapiszewska, I.; Skowrońska, D.; Janczarek, M.; Jesionowski, T.; Klapiszewski, Ł. A comprehensive review of building materials modified with metal and metal oxide nanoparticles against microbial multiplication and growth. Chem. Eng. J. 2023, 466, 143276. [Google Scholar] [CrossRef]
- Kujawa, W.; Didyk-Mucha, A.; Olewnik-Kruszkowska, E.; Gierszewska, M.; Rudawska, A. Synergistic Effect of Combined Polymorphs Anatase-Rutile Nano-Modified Lightweight Concrete on Photocatalytic Reduction of NOx, Self-Cleaning Performance, and Antimicrobial Properties. Buildings 2023, 13, 1736. [Google Scholar] [CrossRef]
- Bersch, J.D.; Flores-Colen, I.; Masuero, A.B.; Dal Molin, D.C. Photocatalytic TiO2-based coatings for mortars on facades: A review of efficiency, durability, and sustainability. Buildings 2023, 13, 186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).