Feasibility Study of Applying Enzyme-Induced Carbonate Precipitation (EICP) without Calcium Source for Remediation of Lead-Contaminated Loess
Abstract
1. Introduction
2. Materials and Methods
2.1. Testing Materials
2.1.1. Tested Soil
2.1.2. Chemical Agents
2.2. Specimen Preparation
2.3. Experimental Methods
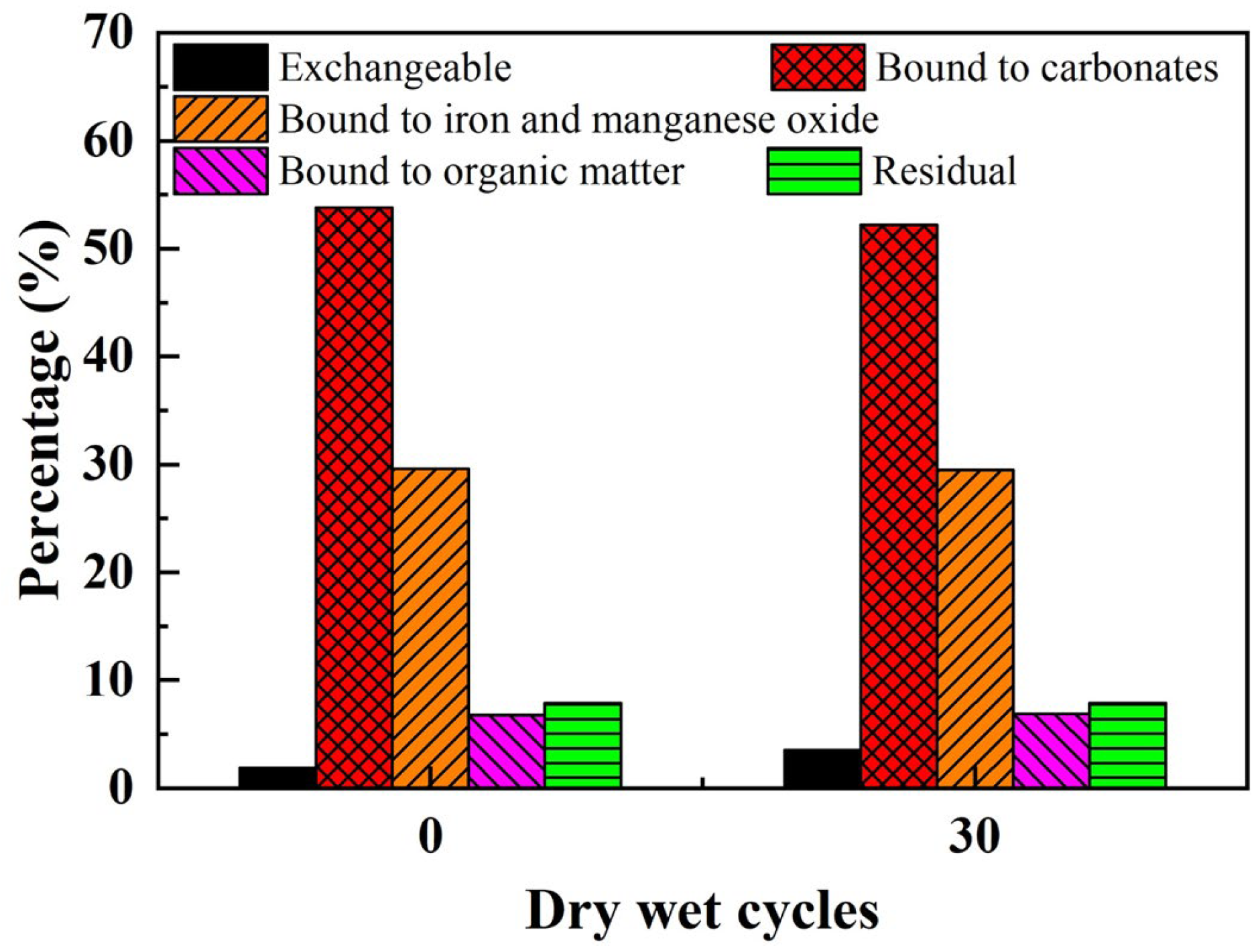
2.3.1. Analysis of Speciation Forms
2.3.2. Surface Strength Test
2.3.3. Seepage Test
2.3.4. Dry–Wet Cycle Test
2.3.5. Acid Rain Leaching Filtration Test
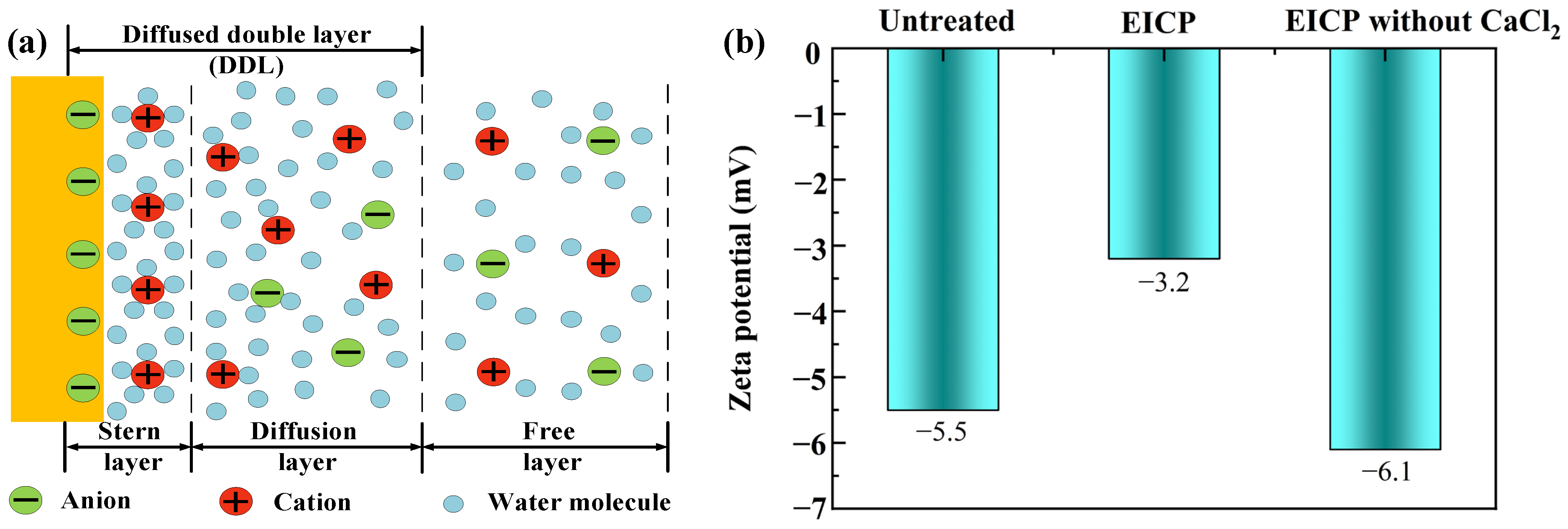
2.3.6. Zeta Potential Measurement
2.3.7. SEM Tests
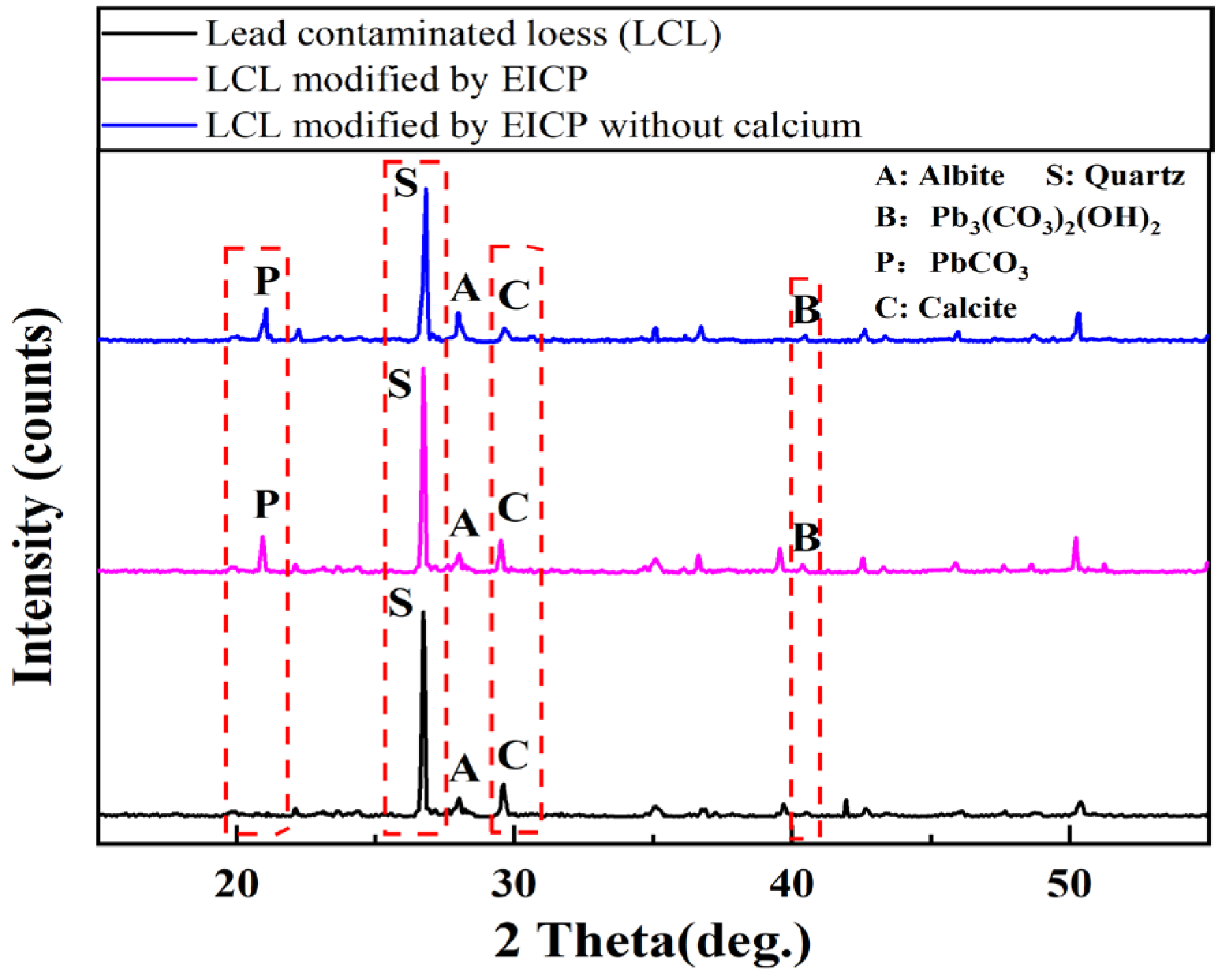
2.3.8. XRD Tests
3. Results
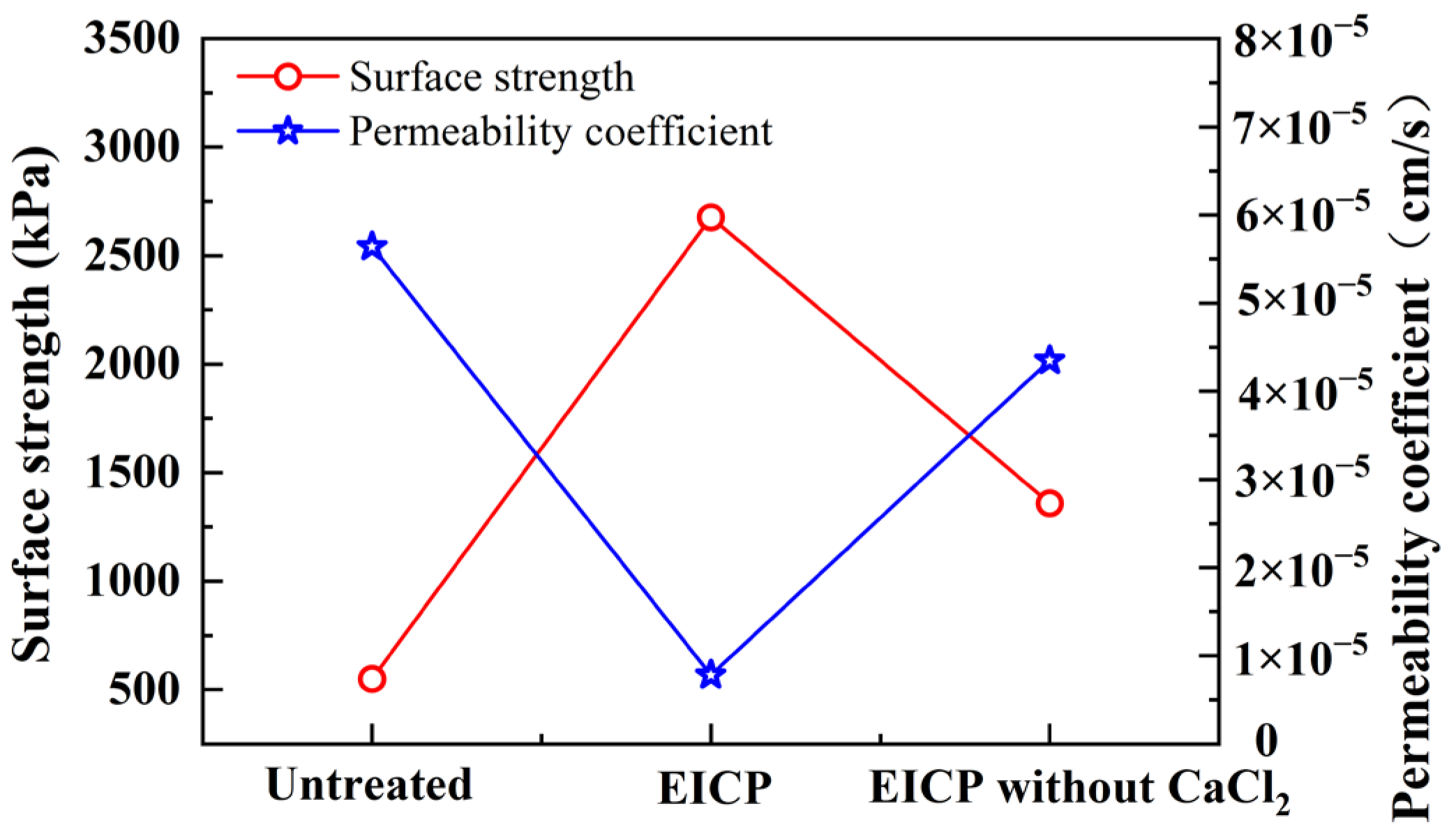
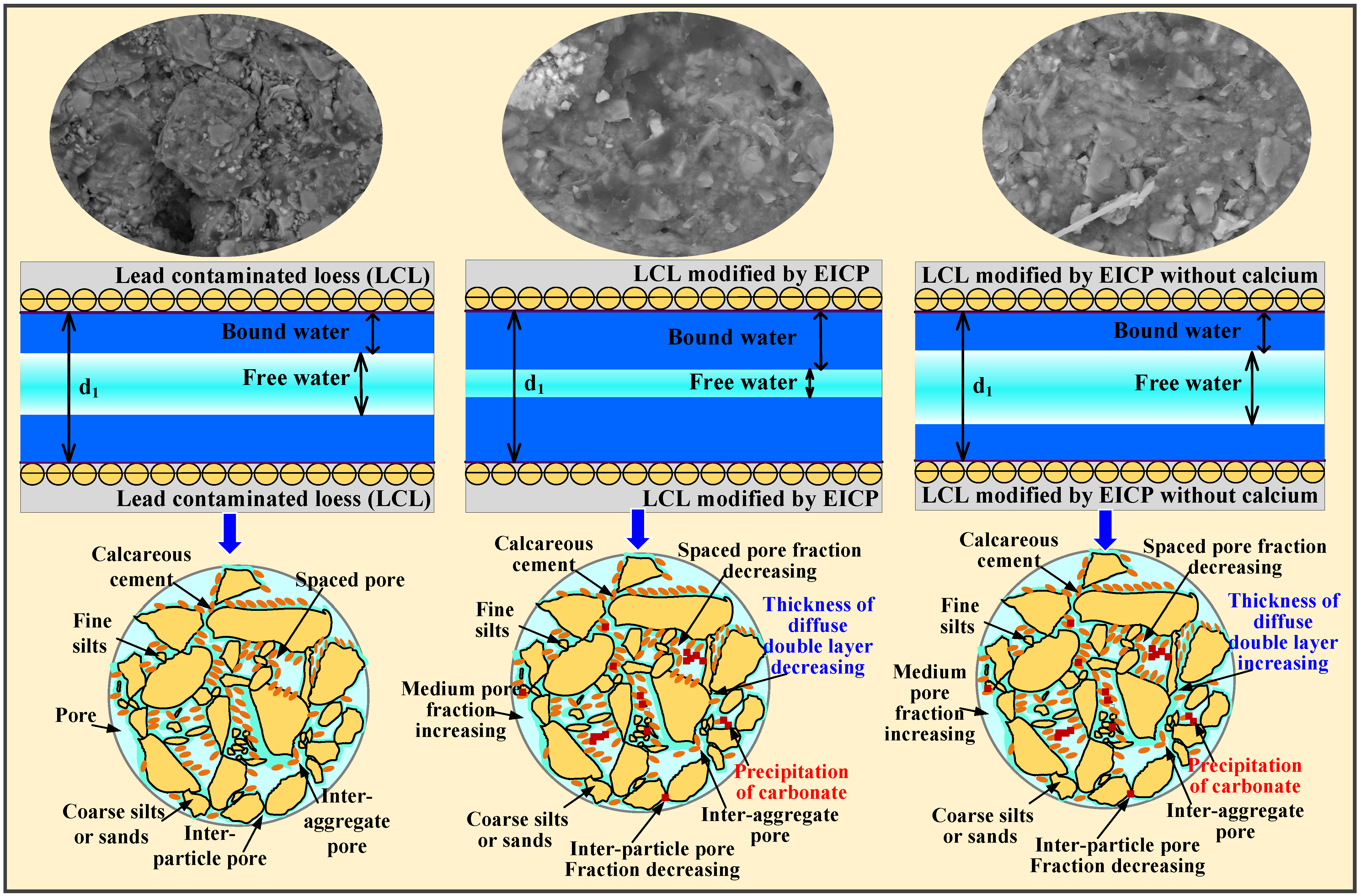
3.1. Comparison of EICP Technology and EICP Technology without Calcium Source
3.2. Stability Analysis under Dry–Wet Cycles
3.3. Stability Analysis under Acid Rain Leaching
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Ministry of Ecology and Environment of China. 2021 Ecological Environment Bulletin [EB/OL]. [26 May 2022]. Available online: https://www.mee.gov.cn/hjzl/sthjzk/zghjzkgb/202205/P020220608338202870777.pdf (accessed on 26 May 2020). (In Chinese)
- Zhao, B.; Wang, L.; Wei, Y.Q.; Zhu, W.B.; Cao, H.L.; Zhang, H. Status and prospect of standard systems for agricultural soil heavy metal contamination prevention and control in China. Res. Environ. Sci. 2024, 37, 169–180. [Google Scholar] [CrossRef]
- Rehman, Z.; Junaid, M.F.; Ijaz, N.; Khalid, U.; Ijaz, Z. Remediation methods of heavy metal contaminated soils from environmental and geotechnical standpoints. Sci. Total Environ. 2023, 867, 161468. [Google Scholar] [CrossRef] [PubMed]
- Won, C.; Hanlie, H.; Cheng, F.; Fang, Q.; Wang, C.; Zhao, L.; Churchman, J. Clay mineralogy and its palaeoclimatic significance in the Luochuan loess-palaeosols over ~1.3 ma, Shaanxi, northwestern China. Front. Earth Sci. 2017, 12, 134–147. [Google Scholar] [CrossRef]
- Chen, P.; Jia, S.G.; Wei, X.Q.; Sun, P.P.; Yi, P.P.; Wei, C.F. Hydraulic path dependence of shear strength for compacted loess. J. Rock Mech. Geotech. Eng. 2023, 7, 1872–1882. [Google Scholar] [CrossRef]
- Cui, M.J.; Zheng, J.J.; Zhang, R.J.; Miao, C.X.; Zhang, J.J. Study of effect of chemical treatment on strength of bio-cemented sand. Yantu Lixue/Rock Soil Mech. 2015, 36, 392–396. [Google Scholar]
- Adimalla, N. Heavy metals pollution assessment and its associated human health risk evaluation of urban soils from Indian cities: A review. Environ. Geochem. Health 2020, 42, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Bu, C.M.; Lu, X.Y.; Zhu, D.X.; Liu, L.; Sun, Y.; Wu, Q.T.; Zhang, W.T.; Wei, Q.K. Soil improvement by microbially induced calcite precipitation (MICP): A review about mineralization mechanism, factors, and soil properties. Arab. J. Geosci. 2022, 15, 863. [Google Scholar] [CrossRef]
- Kang, B.; Zha, F.; Deng, W.; Wang, R.; Sun, X.; Lu, Z. Biocementation of pyrite tailings using microbially induced calcite carbonate precipitation. Molecules 2022, 27, 3608. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gao, Y.F.; Paassen, L.A.V.; He, J.; Wang, L.Y.; Li, C. Microbially induced carbonate precipitation for improving the internal stability of silty sand slopes under seepage conditions. Acta Geotech. 2023, 18, 2719–2732. [Google Scholar] [CrossRef]
- Choi, S.G.; Chang, I.; Lee, M.; Lee, J.H.; Han, J.T.; Kwon, T.H. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Fu, T.Z.; Saracho, A.C.; Haigh, S.K. Microbially induced carbonate precipitation (MICP) for soil strengthening: A comprehensive review. Biogeotechnics 2023, 1, 100002. [Google Scholar] [CrossRef]
- He, J.; Fang, C.H.; Hang, L.; Qi, Y.S.; Mao, X.Y.; Yan, B.Y.; Zhou, Y.D.; Gao, Y.F. Enzyme induced carbonate precipitation for soil internal erosion control under water seepage. Geomech. Eng. 2021, 26, 289–299. [Google Scholar]
- Wang, Y.; Sun, X.H.; Miao, L.C.; Wang, H.X.; Wu, L.Y.; Shi, W.B.; Kawasaki, S. State-of-the-art review of soil erosion control by MICP and EICP techniques: Problems, applications, and prospects. Sci. Total Environ. 2024, 912, 169016. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, D.K.; Saraf, M.; Aeron, A. Beneficial effects of plant growth-promoting rhizobacteria on improved crop production. In Bacteria in Agrobiology: Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2013; pp. 46–63. [Google Scholar]
- Kumari, D.; Pan, X.L.; Lee, D.J. Immobilization of cadmium in soil by microbially induced carnonate precipitation with Exiguobacterium undae at low temperature. Int. Biodeterior. Biodegrad. 2014, 94, 98–102. [Google Scholar] [CrossRef]
- Wu, L.Y.; Miao, L.C.; Sun, X.H.; Wang, H.G. Enzyme-induced carbonate precipitation combined with polyvinyl alcohol to solidify Aeolian sand. J. Mater. Civ. Eng. 2021, 33, 04021373. [Google Scholar] [CrossRef]
- Saif, A.; Cuccurullo, A.; Gallipoli, D.; Perlot, C.; Bruno, A.W. Advances in enzyme induced carbonate precipitation and application to soil improvement: A review. Materials 2022, 15, 950. [Google Scholar] [CrossRef] [PubMed]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Enzyme induced calcium carbonate precipitation and its engineering application: A systematic review and metal-analysis. Constr. Build. Mater. 2021, 308, 125000. [Google Scholar] [CrossRef]
- He, P.L.; Guo, J.J.; Zhang, S.X. Feasibility of microbially induced carbonate precipitation to enhance the internal stability of loess under Zn-contaminated seepage conditions. Buildings 2024, 14, 1230. [Google Scholar] [CrossRef]
- Xu, P.P.; Zhang, Q.Y.; Qian, H.; Li, M.N.; Yang, F.X. An investigation into the relationship between saturated permeability and microstructure of remolded loess: A case study from Chinese Loess Plateau. Geoderma 2021, 382, 114774. [Google Scholar] [CrossRef]
- Xu, P.P.; Qian, H.; Li, W.Q.; Ren, W.H.; Yang, F.X.; Wang, L.B. New insights into the seepage behavior of heavy metal-contaminated loess and its underlying geochemical mechanism. J. Hydrol. 2023, 620 Pt B, 129476. [Google Scholar] [CrossRef]
- Hou, X.K.; Qi, S.W.; Li, T.L.; Guo, S.F.; Wang, Y.; Li, Y.; Zhang, L.X. Microstructure and soil-water retention behavior of compacted and intact silt loess. Eng. Geol. 2020, 277, 105814. [Google Scholar] [CrossRef]
- ASTM D2487; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM (American Society for Testing and Materials): West Conshohocken, PA, USA, 2011.
- GB/T 50123-2019; Standard for Geotechnical Test Methods. China Planning Press: Beijing, China; Ministry of Water Resources of the People’s Republic of China: Beijing, China, 2019.
- GB 15618-2018; Soil Environmental Quality: Risk Control Strandard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment: Beijing, China, 2018.
- Kidanemariam, T.G.; Gebru, K.A.; Gebretinsae, H.K. A mimi review of enzyme-induced calcite precipitation (EICP) technique for eco-friendly bio-cement production. Environ. Sci. Pollut. Res. 2024, 31, 17033–17051. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Uday, K.V. A comparative study of various parameters influencing biocalcification via ureolysis mediated by enzyme and microbe: A comprehensive review. Geomicrobiol. J. 2024, 41, 17–34. [Google Scholar] [CrossRef]
- Arab, M.G.; Refaei, M.; Alotaibi, E.; Omar, M.; Almajed, A.; Haridy, S. Optimizing the compressive strength of sodium alginate-modified EICP-treated sand using design of experiments. J. Mater. Civ. Eng. 2024, 36, 04024017. [Google Scholar] [CrossRef]
- He, J.; Huang, A.G.; Ji, J.F.; Qu, S.Y.; Hang, L. Enzyme induced carbonate precipitation with fibers for the improvement of clay soil slopes against rainfall and surface runoff erosions. Transp. Geotech. 2023, 42, 101074. [Google Scholar] [CrossRef]
- Sun, X.H.; Miao, L.C.; Wang, H.X.; Yuan, J.H.; Wu, L.Y. Research on freeze-thaw and dry-wet durability of enzymatic calcification for surface protection. Environ. Sci. Pollut. Res. 2022, 29, 16762–76771. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.L.; Ji, P.R.; Wang, J.L.; Zhang, X.G.; Xu, X.C. Study on the environmental durability of heavy metal contaminated soil remediated by enzyme induced carbonate precipitation. Rock Soil Mech. 2023, 44, 2779–2788. [Google Scholar]
- Zhang, S.Q.; Zhang, X.; Zhang, K.N.; Yuan, B.Y.; Ren, D.J.; Zhang, X.Q. Comparison of remediation mechanism of heavy metal–contaminated soil by combined leaching and two-step leaching. Water Air Soil Pollut. 2023, 234, 387. [Google Scholar] [CrossRef]
- Hong, M.F.; Zhang, L.M.; Tan, Z.X.; Huang, Q.Y. Effect mechanism of biochar’s zeta potential on farmland soil’s cadmium immobilization. Environ. Sci. Pollut. Res. 2019, 26, 19738–19748. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.K.; Qian, H.; Gao, Y.Y.; Li, Y.B. Classification and physical characteristics of bound water in loess and its main clay minerals. Eng. Geol. 2020, 265, 105394. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Lateef, M.A.; Mohammed, S.A. Heavy metal immobilization studies and enhancement in geotechnical properties of cohesive soils by EICP technique. Appl. Sci. 2020, 10, 7568. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Lateef, M.A.; Mohammed, S.A. Efficacy of enzymatically induced calcium carbonate precipitation in the retention of heavy metal ions. Sustainability 2020, 12, 7019. [Google Scholar] [CrossRef]
- Liu, J.D.; Feng, C.; Li, J.S.; Wang, K.K. Soil-water characteristic and microscopic mechanism of arsenic and cadmium composite heavy metal contaminated soil. Rock Soil Mechnics 2022, 43, 2841–2851. [Google Scholar]
- Gat, D.; Ronen, Z.; Tsesarsky, M. Long-term sustainability of microbial-induced CaCO3 precipitation in aqueous media. Chemosphere 2017, 184, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Liu, X.D.; Yin, H.Q.; Liang, Y.L.; Liu, H.W.; Miao, B.; Peng, Q.Q.; Meng, D.L.; Wang, S.Q.; Yang, J.J. The utilization of biomineralization technique based on microbial induced phosphate precipitation in remediation of potentially toxic ions contaminated soil: A review. Ecotoxicol. Environ. Saf. 2020, 191, 110009. [Google Scholar] [CrossRef] [PubMed]
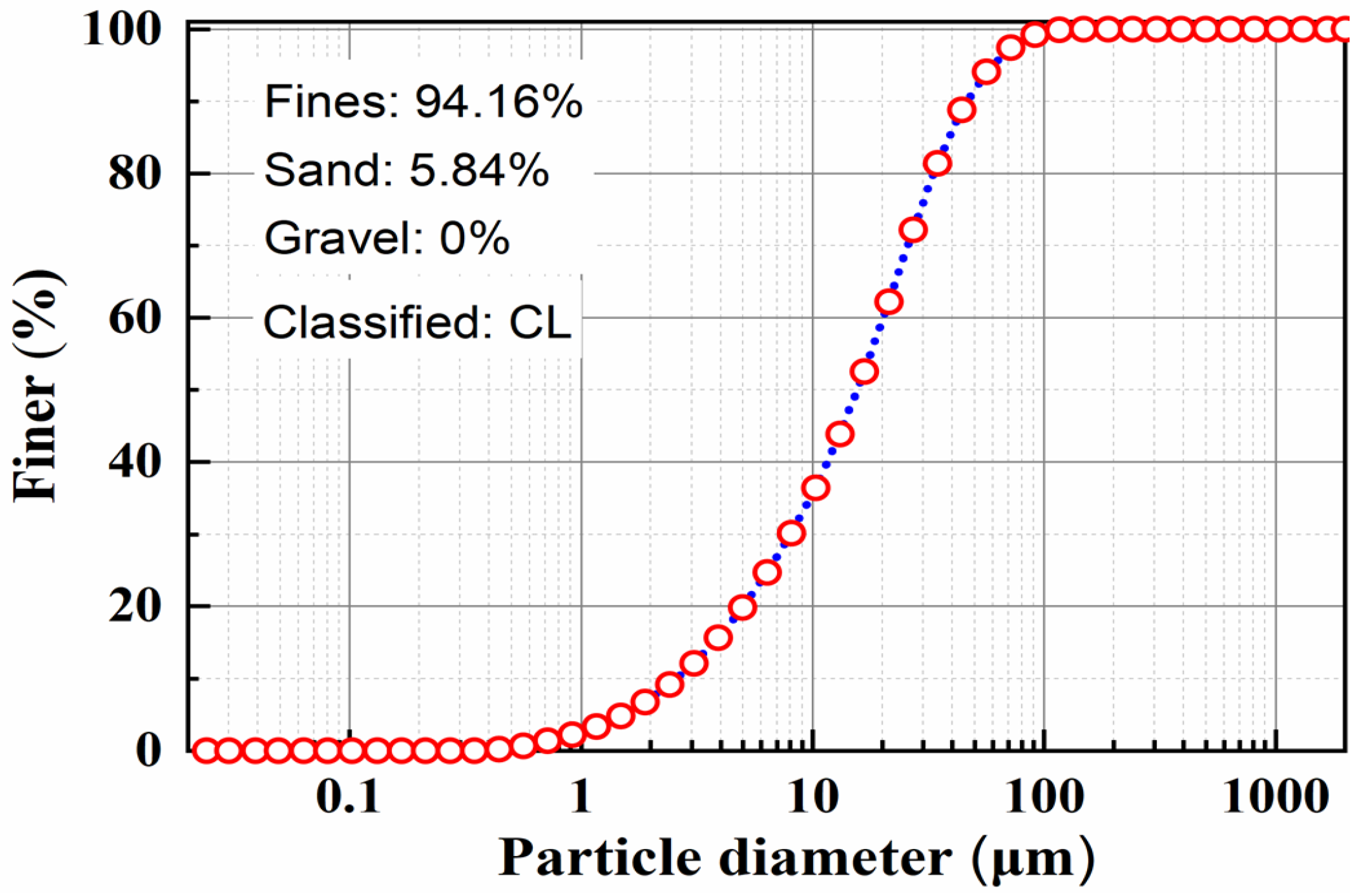







| Physical Index. | Data |
|---|---|
| Fines (%) | 94.16 |
| Sand (%) | 5.84 |
| Gravel (%) | 0 |
| Specific gravity, Gs | 2.70 |
| Void ratio, e | 0.86 |
| Dry density, ρdmax/(g/cm3) | 1.73 |
| Initial water content (%) | 16.6 |
| The Atterberg limit | |
| Liquid limit (%) | 32.52 |
| Plastic limit (%) | 18.68 |
| Soil classification | CL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhang, S. Feasibility Study of Applying Enzyme-Induced Carbonate Precipitation (EICP) without Calcium Source for Remediation of Lead-Contaminated Loess. Buildings 2024, 14, 1810. https://doi.org/10.3390/buildings14061810
Zhang K, Zhang S. Feasibility Study of Applying Enzyme-Induced Carbonate Precipitation (EICP) without Calcium Source for Remediation of Lead-Contaminated Loess. Buildings. 2024; 14(6):1810. https://doi.org/10.3390/buildings14061810
Chicago/Turabian StyleZhang, Kun, and Shixu Zhang. 2024. "Feasibility Study of Applying Enzyme-Induced Carbonate Precipitation (EICP) without Calcium Source for Remediation of Lead-Contaminated Loess" Buildings 14, no. 6: 1810. https://doi.org/10.3390/buildings14061810
APA StyleZhang, K., & Zhang, S. (2024). Feasibility Study of Applying Enzyme-Induced Carbonate Precipitation (EICP) without Calcium Source for Remediation of Lead-Contaminated Loess. Buildings, 14(6), 1810. https://doi.org/10.3390/buildings14061810





