Gypsum-Enhanced Red Mud Composites: A Study on Strength, Durability, and Leaching Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Red Mud
2.1.2. Titanium Gypsum
2.1.3. Phosphogypsum
2.1.4. Cement
2.2. Specimen Preparation
2.3. Experimental Methods
2.3.1. Compaction Test
2.3.2. Unconfined Compressive Strength
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Continuous Flume Leaching Test
- : the amount of a component leached in the leaching stage , mg/kg;
- : concentration of a component in the leach solution at leaching stage , mg/L;
- : mass of sample dry matter in the column, kg.
- : leaching concentration of a component at leaching stage , mg/m2;
- : volume of leach solution at leaching stage , L;
- : concentration of a component in the leach solution at leaching stage , μg/L;
- : a conversion factor, 1000 μg/mg;
- : surface area of the sample.
- : cumulative leaching of a component in stages 1–8, mg/kg;
- : the amount of a component leached at the leaching stage , mg/kg.
3. Results and Analysis
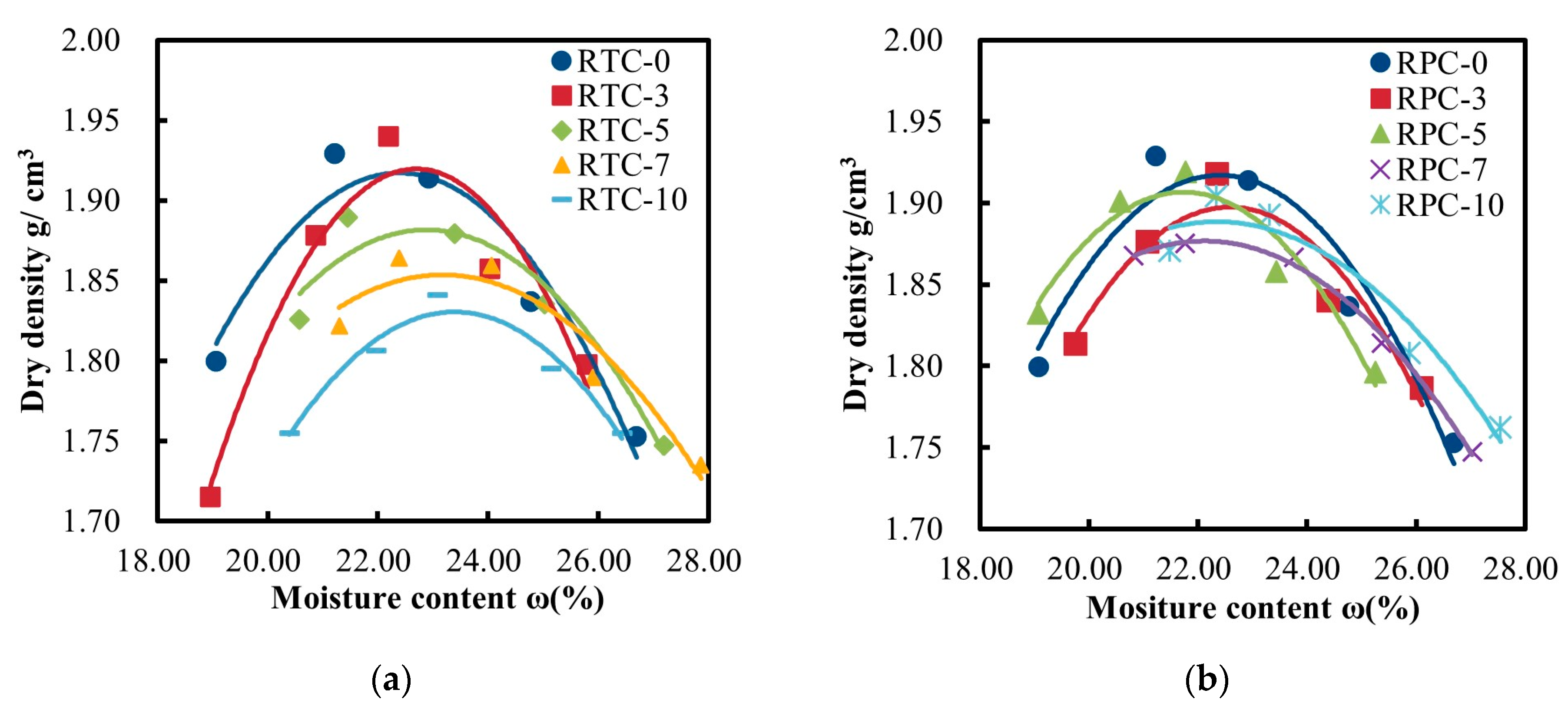
3.1. Comparative Analysis of Optimum Moisture Content and Maximum Dry Density
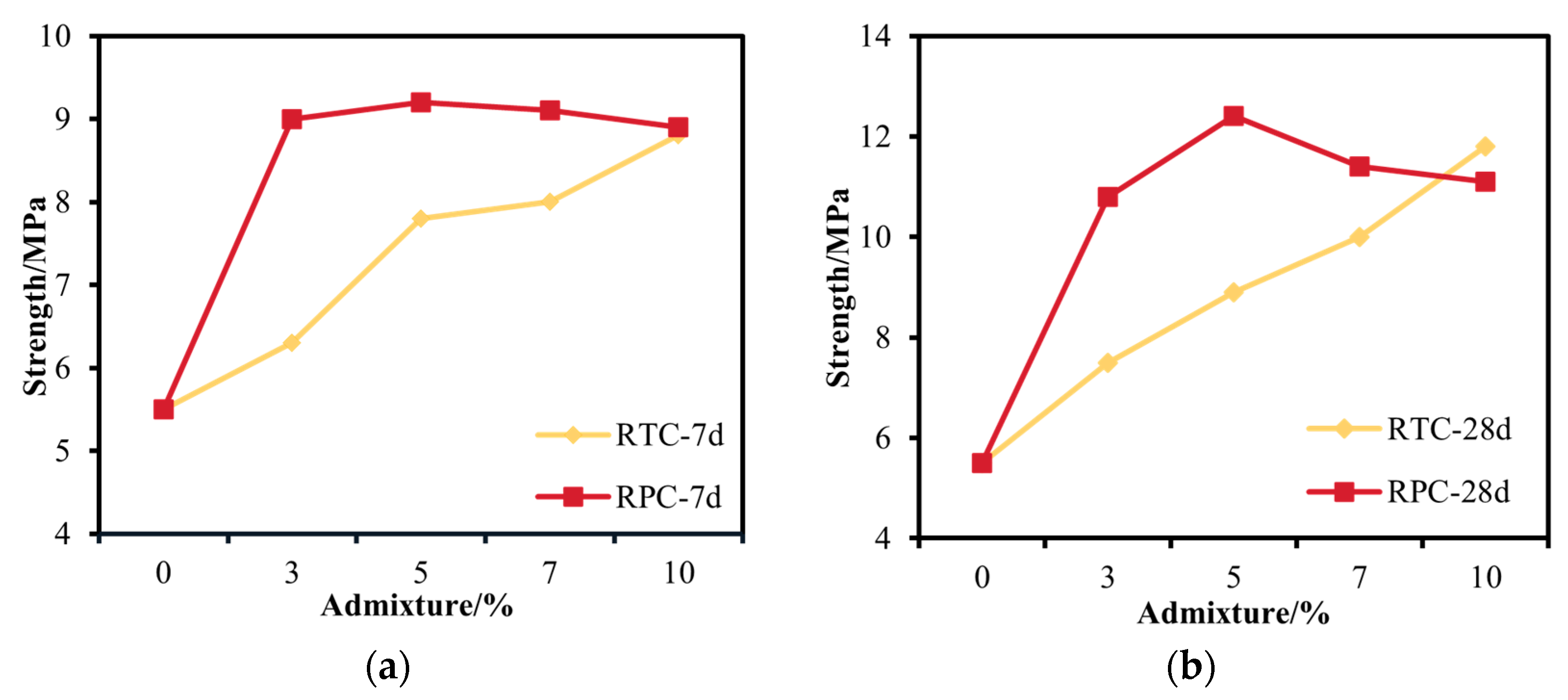
3.2. Unconfined Compressive Strength Analysis
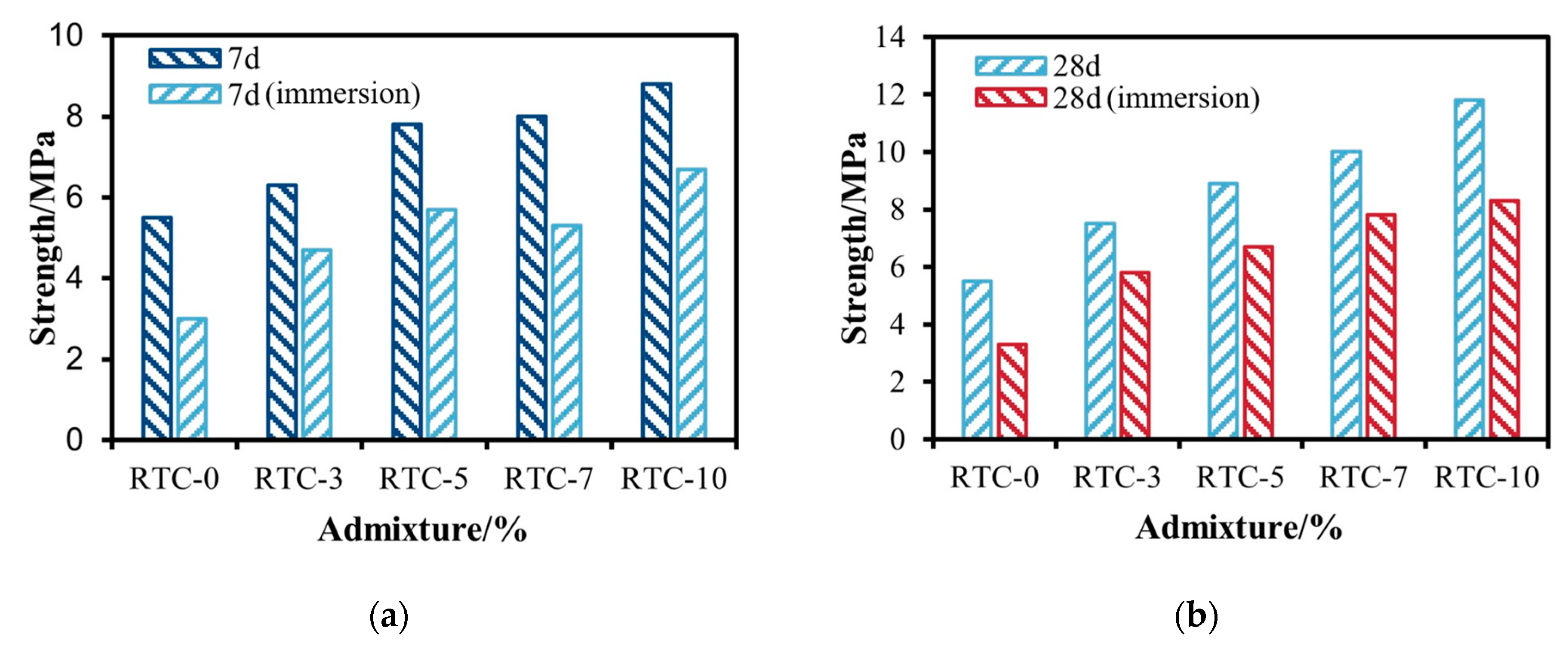
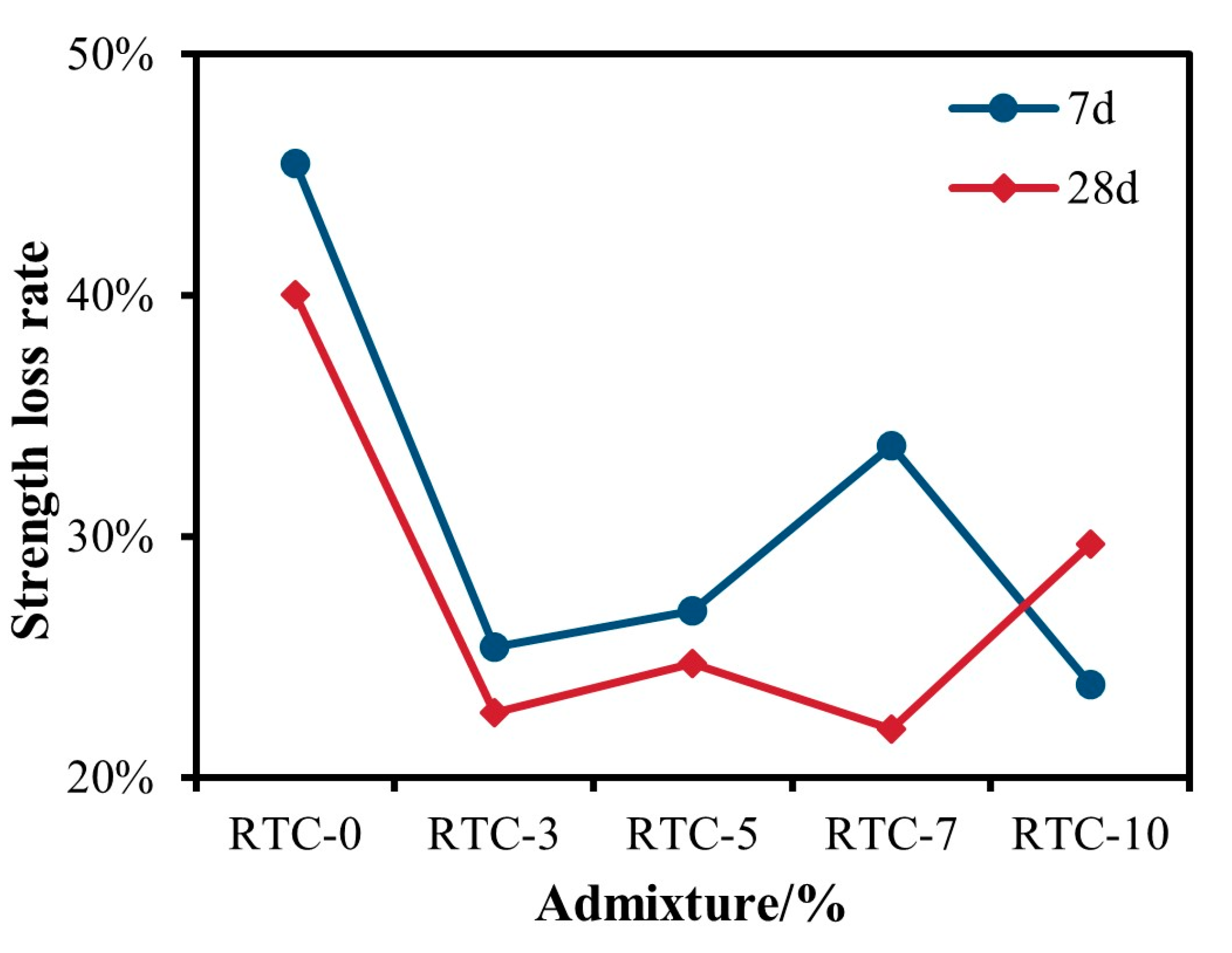
3.2.1. RTC Unconfined Compressive Strength Analysis
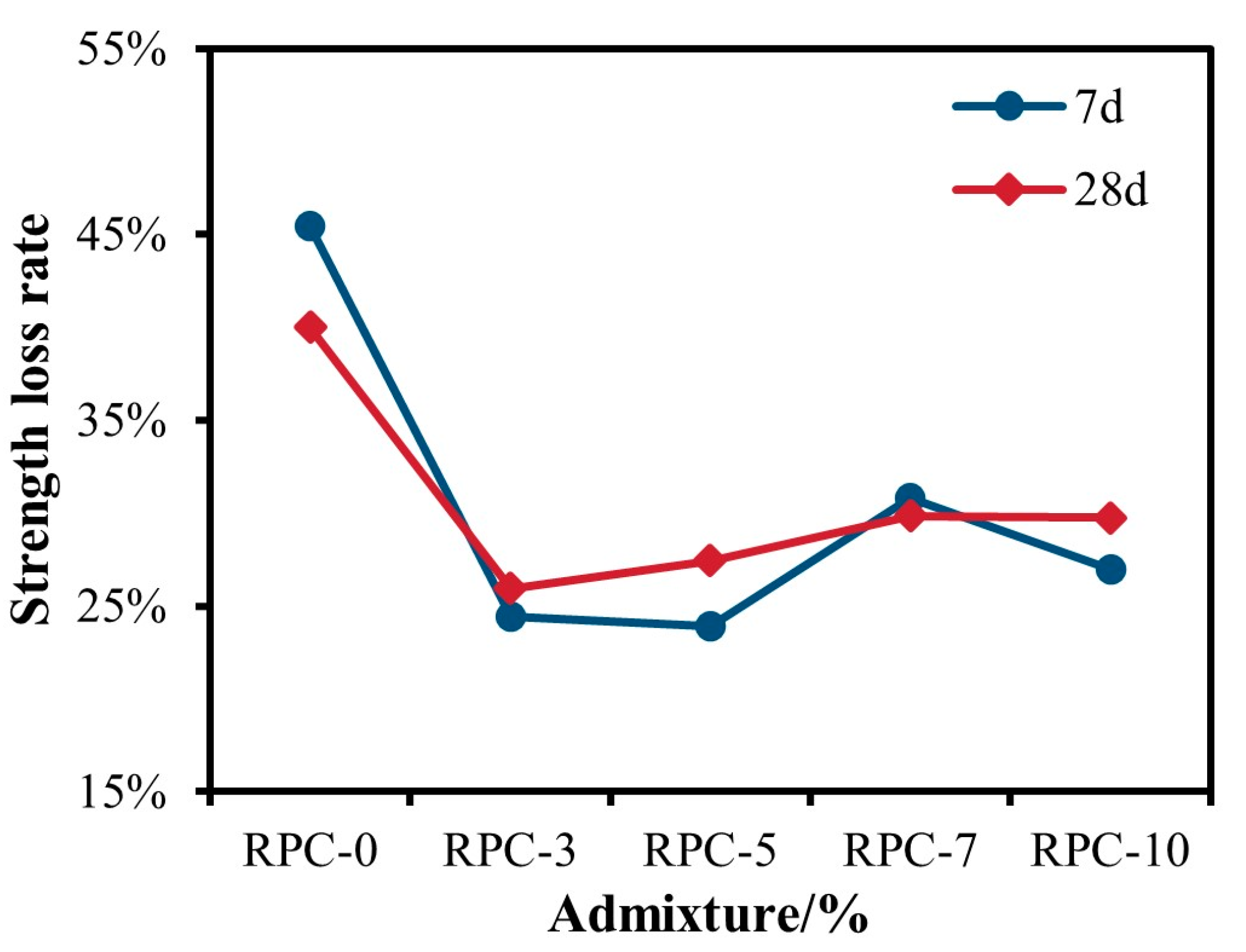
3.2.2. RPC Unconfined Compressive Strength Analysis
3.2.3. Comparative Analysis of RTC and RPC Unconfined Compressive Strength
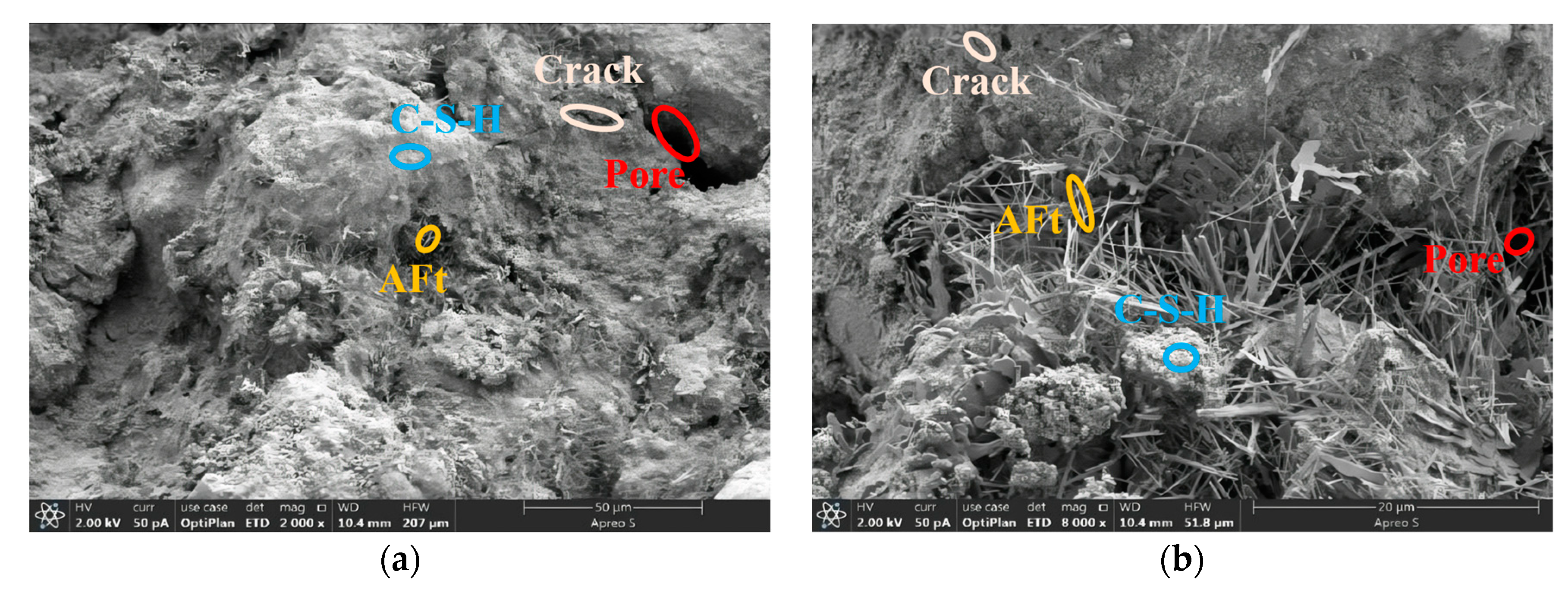
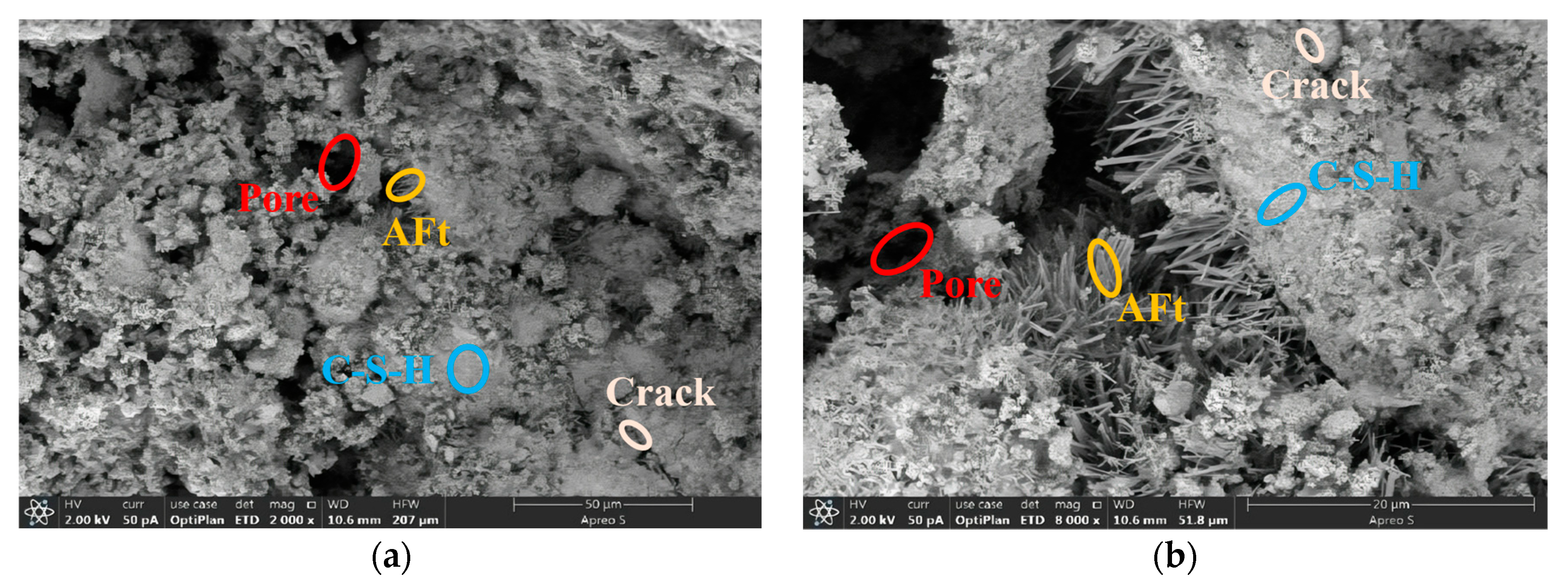
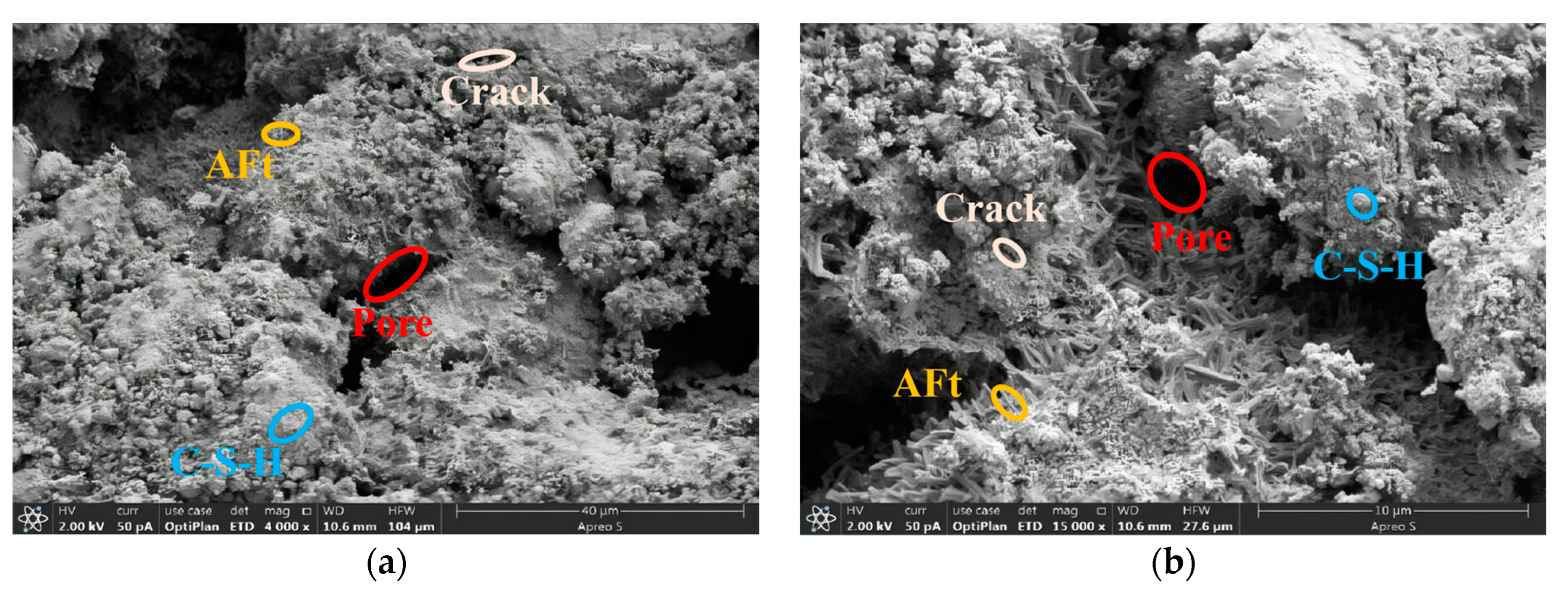
3.3. Microstructure Analysis
3.3.1. Comparative Analysis of RC and RTC Microstructures
3.3.2. RPC Microstructure Analysis
3.4. Analysis of Heavy Metal Leaching Pattern
3.4.1. Analysis of the Leaching Pattern of As
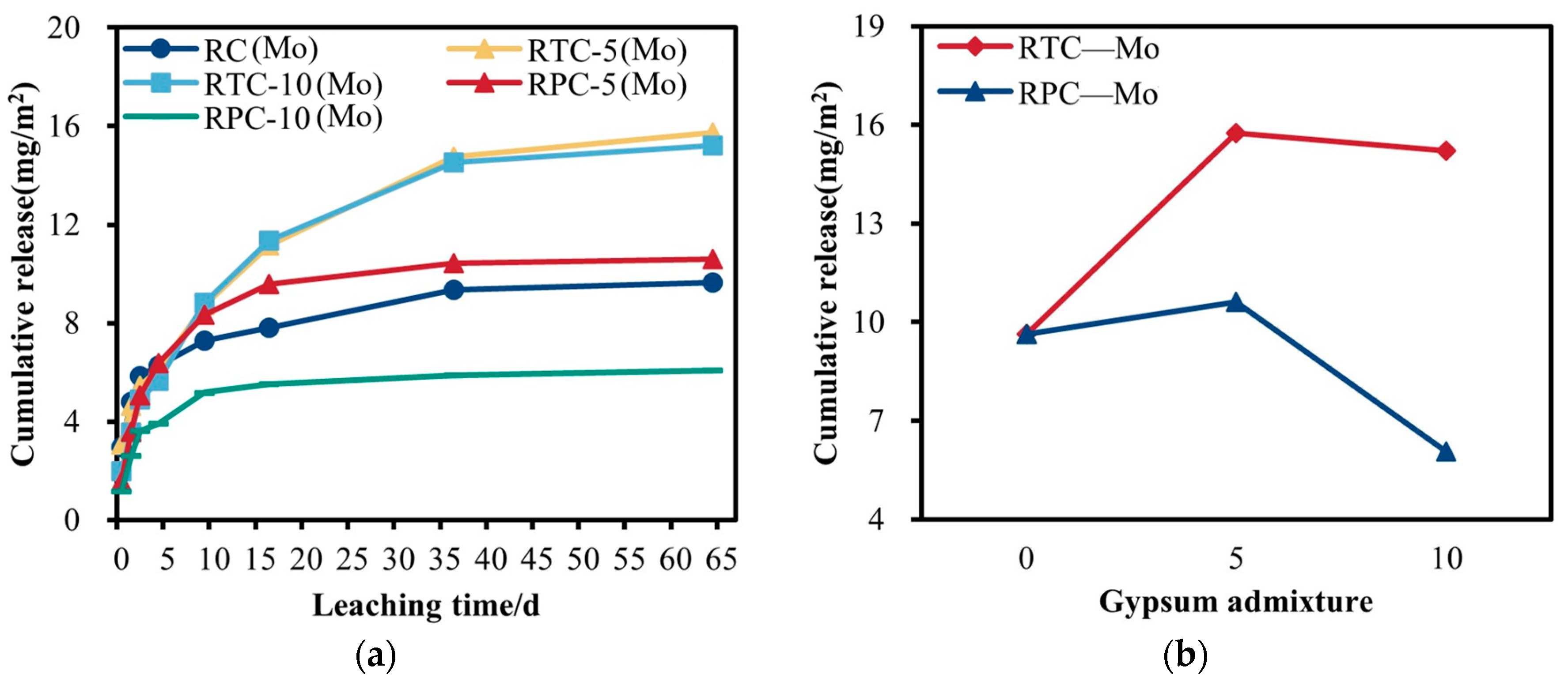
3.4.2. Analysis of the Leaching Pattern of Mo
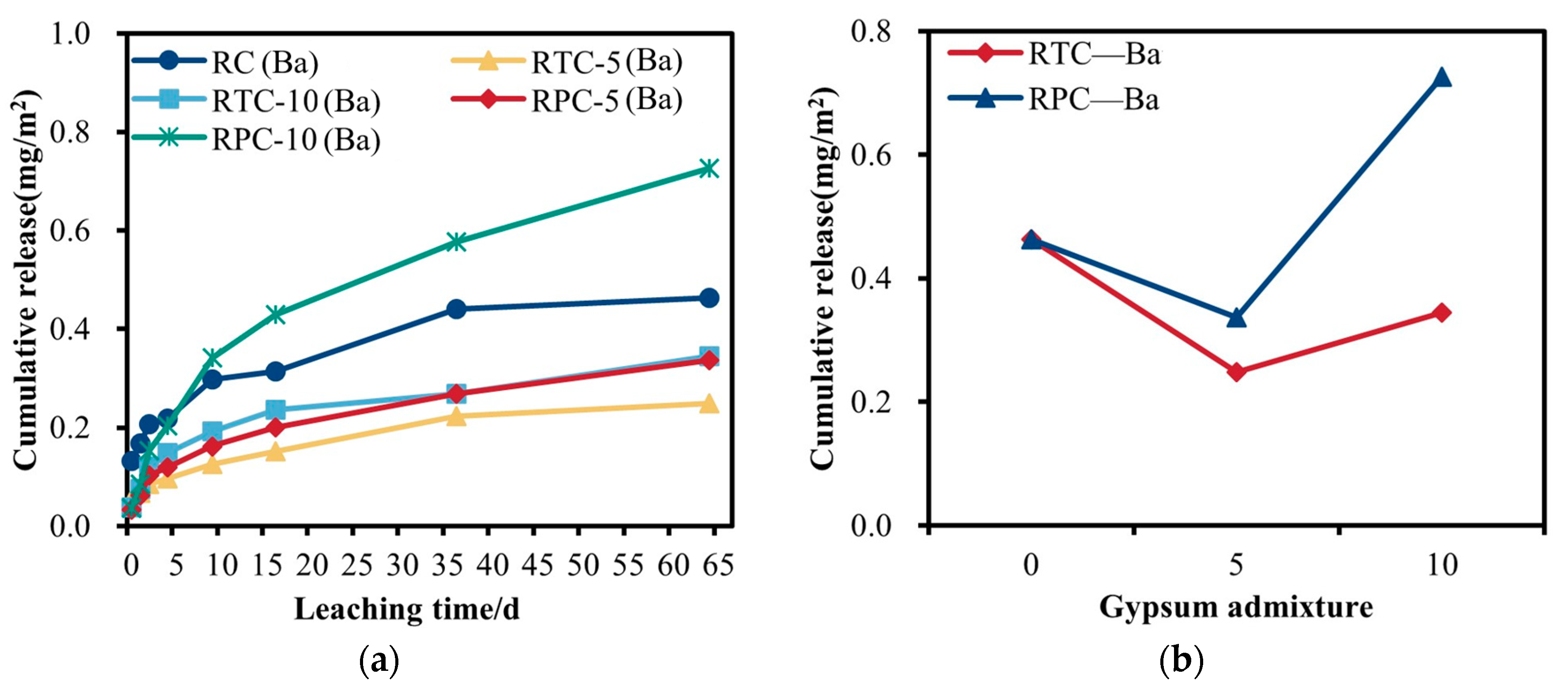
3.4.3. Analysis of the Leaching Pattern of Ba
4. Conclusions and Prospects
4.1. Conclusions
- (1)
- The results of the compaction test showed that there were minor differences in the optimal moisture content between RTC and RPC, while the maximum dry densities of the two were very close to each other, at 1.91 g/cm³ and 1.93 g/cm³, respectively.
- (2)
- Based on the unconfined compressive strength at 7 d and 28 d, when the admixture of gypsum was controlled to be less than 10%, the RPC was better than the RTC. When the phosphogypsum admixture was 5%, the strength reached a maximum of 12.4 MPa. RPC-5 showed a 125.5% increase in strength compared to RC.
- (3)
- Based on the 28 d microstructure of the specimens, phosphogypsum cured better at the optimum mixing ratio. Among them, RPC-5 had the best curing effect, with a denser structure with fewer pores.
- (4)
- The cumulative releases of As, Mo, Zn, and Cr from the 8% cement-stabilized red mud material in the continuous flume leaching experiments were larger. The cumulative releases were 0.09978 mg/L, 0.05653 mg/L, 0.02643 mg/L, and 0.014419 mg/L, respectively. The two gypsum additions had the same effect on the leaching of the As and different effects on the leaching of Mo and Ba.
- (5)
- Summarizing all the test results, the optimal modified red mud material that met both the mechanical requirements of road subgrade materials and the requirements of groundwater standards was the RPC, and the optimal mix ratio was red mud/phosphogypsum/cement = 87:5:8.
4.2. Prospects and Limitations
- (1)
- In this paper, only the effects of two gypsums on the mechanical properties and heavy metal leaching of red mud-based composites were investigated when the cement was 8%. The effects of the two gypsums on the composites were not investigated when the cement dosage was less than 8% or more than 8%. In addition, only 96% compaction was used to prepare the specimens in this paper. The effects of other compaction levels on the material performance characteristics were not investigated.
- (2)
- The research in this paper only carried out indoor tests, and no work was carried out on the experimental sections of the modified red mud. The large-scale application of modified red mud for road construction requires long-term stability testing of gypsum–cement-stabilized composites. The results of the heavy metal leaching tests likewise indicate the need to analyze groundwater and soil hazards through long-term monitoring of the cumulative releases of heavy metals from the materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Editorial Board of the Dictionary of Environmental Science. Dictionary of Environmental Science, 2nd ed.; China Environmental Science Press: Beijing, China, 2008; pp. 60–61. [Google Scholar]
- Zhang, Y.; Wang, Y.; Wang, K.; Dou, Z.; Liu, Y.; Zhao, Q.; Niu, L.; Zhang, Z.; Han, J.; Guo, J. A Method of Iron Extraction and Direct Cementation of High Iron Red Mud. China Patent No. 201910291219.4, 12 October 2021. [Google Scholar]
- Liang, G.; Chen, W.; Nguyen, A.; Nguyen, T. Red mud carbonation using carbon dioxide: Effects of carbonate and calcium ions on goethite surface properties and settling. J. Colloid Interface Sci. 2018, 517, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Khairul, M.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Wang, Y. Strength Characteristics and Micro-Mechanism of Red Mud-Based Modified Silt Loam. Master’s Thesis, Zhongyuan University of Technology, Zhengzhou, China, 2022. [Google Scholar]
- Xie, W.; Zhang, N.; Li, J.; Zhou, F.; Ma, X.; Gu, G.; Zhang, W. Optimization of condition for extraction of aluminum and iron from red mud by hydrochloric acid leaching. Chin. J. Environ. Eng. 2017, 11, 5677–5682. [Google Scholar] [CrossRef]
- Ning, L.; He, D.; Chen, W.; Zhou, K.; Peng, C.; Zhang, X.; Teng, C. Sulfuric Acid Leaching and Kinetics Study for Separation of Iron and Scandium from Red Mud. Min. Metall. Eng. 2019, 39, 81–84+88. [Google Scholar] [CrossRef]
- Luo, S.; Liu, M.; Yang, L.; Chang, J.; Yang, W.; Yan, X.; Yu, H.; Shen, Y. Utilization of waste from alumina industry to produce sustainable cement-based materials. Constr. Build. Mater. 2019, 229, 116795. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Xie, J.; He, B.; Chen, T.; Zhang, N.; Peng, X.; Nie, X.; Ma, F. Characterization of red mud and fly ash based for co-curing of Cu2+-contaminated soil. Case Stud. Constr. Mater. 2024, 20, e02769. [Google Scholar] [CrossRef]
- Santona, L.; Castaldi, P.; Melis, P. Evaluation of the interaction mechanisms between red muds and heavy metals. J. Hazard. Mater. 2006, 136, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Peng, X.; Luan, Z. Immobilization of Cd, Zn and Pb in sewage sludge using red mud. Environ. Earth Sci. 2012, 66, 1321–1328. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, S.C.; Sahoo, S.K.; Chakraverty, A.P.; Maharana, H.S. Progress of Red Mud Utilization: An Overview. Am. Chem. Sci. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Sarath Chandra, K.; Krishnaiah, S. Strength and leaching characteristics of red mud (bauxite residue) as a geomaterial in synergy with fly ash and gypsum. Transp. Res. Interdiscip. Perspect. 2022, 13, 100566. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Wang, X.; Tang, H. Effects of the position and chloride-induced corrosion of strand on bonding behavior between the steel strand and concrete. Structures 2023, 58, 105500. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Li, Y.; Zhou, R.; Yu, H.; Zhu, X.; Quan, H.; Li, Y. Risk assessment for the long-term stability of fly ash-based cementitious material containing arsenic: Dynamic and semidynamic leaching. Environ. Pollut. 2024, 345, 123361. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Lei, Z.; Tong, L.; Sun, J.; Wang, Y.; Wang, X.; Jiang, X. Ternary cementless composite based on red mud, ultra-fine fly ash, and GGBS: Synergistic utilization and geopolymerization mechanism. Case Stud. Constr. Mater. 2023, 19 Pt A, e02410. [Google Scholar] [CrossRef]
- Deelwal, K. Evaluation of characteristic properties of red mud for possible use as a geotechnical material in civil construction. Int. J. Adv. Eng. Technol. 2014, 7, 1053–1059. [Google Scholar]
- Shi, M.; Tian, X.; Wang, H.; Yu, C.; Du, X.; Zhang, R. Performance of Cement-Lime-Phosphogypsum Solidified Red Mud as Road Base Material. J. Changjiang River Sci. Res. Inst. 2024, 41, 114–120. [Google Scholar] [CrossRef]
- Yu, C. Feasibility Study on Phosphogypsum Modified Cement-Lime Stabilized Red Cement Road Base Material. Master’s Thesis, Southeast University, Nanjing, China, 2021. [Google Scholar]
- Li, C. Study on the Basic Performance and Engineering Application of Bayer Red Mud Used in Roadbed Filling Applications. Master’s Thesis, Shandong Jianzhu University, Jinan, China, 2018. [Google Scholar]
- Zeng, H.; Jin, M.; Li, W.; Gao, C.; Ma, Y.; Guan, Q.; Liu, J. Performance evolution of low heat cement under thermal cycling fatigue: A comparative study with moderate heat cement and ordinary Portland cement. Constr. Build. Mater. 2024, 412, 134863. [Google Scholar] [CrossRef]
- Pu, S.; Zhu, Z.; Huo, W. Evaluation of engineering properties and environmental effect of recycled gypsum stabilized soil in geotechnical engineering: A comprehensive review. Resour. Conserv. Recycl. 2021, 174, 105780. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, S.; Gao, Y.; Liu, C.; Qi, Y. Effect of different gypsums on the workability and mechanical properties of red mud-slag based grouting materials. J. Clean. Prod. 2020, 245, 118759. [Google Scholar] [CrossRef]
- Suo, C.; Wen, H.; Cao, J.; Dong, X. Performance of a Composite Soil Prepared with Red Mud and Desulfurized Gypsum. Ksce J. Civ. Eng. 2022, 26, 47–56. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, J.; Lai, Z.; Guo, Q.; Wan, X. Mechanical and microstructural properties of silt roadbed filling improved with cement, red mud and desulfurization gypsum. Eur. J. Environ. Civ. Eng. 2024, 28, 176–196. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, G.; Bai, X.; Kong, S.; Li, J.; Li, G.; Ge, Z.; Huang, J. Resource Utilization Potential of Red Mud: A Study on the Micro-Mechanism of the Synergistic Effect of Multiple Solid Waste Filling Materials. Sustainability 2023, 15, 15532. [Google Scholar] [CrossRef]
- GB/T 14848-2017; Standard for Groundwater Quality. 2nd ed. Standards Press of China: Beijing, China, 2017.
- JTG 3430-2020; Test Methods of Soils for Highway Engineering. 1st ed. China Communications Press: Beijing, China, 2020; 122–127, 213–217.
- NEN 7375-2004; Leaching Characteristics—Determination of the Leaching of Inorganic Components from Moulded or Monolitic Materials with a Diffusion Test—Solid Earthy and Stony Materials. Milieukwaliteit: Amsterdam, The Netherlands, 2004.
- Huang, Z.; Su, X.; Zhang, J.; Luo, D.; Chen, Y.; Li, H. Study on leaching of heavy metals from red mud-phosphogypsum composite materials. Inorg. Chem. Ind. 2022, 54, 133–140. [Google Scholar] [CrossRef]
- Rong, S. Study on the Leaching Behavior of Rare Metals in Red Mud under pH-Influenced Conditions. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2020. [Google Scholar]
- Sun, Z. Study on the Engineering Technology and Environmental Impacts of the Bayer Method of Filling Roadbeds with Red Mud. Master’s Thesis, Shandong University, Jinan, China, 2017. [Google Scholar]
- Li, Y. Research on Road Use Technology of Industrial Waste Red Mud. Master’s Thesis, Chongqing Jiaotong University, Chongqing, China, 2021. [Google Scholar]
- Zhang, X.; Fu, L. Application of cement stabilized aggregates in municipal projects. Heilongjiang Sci. Technol. Inf. 2008, 10, 190. [Google Scholar]
- Zhao, Y. Experimental Study on Regeneration of Subgrade Phosphogypsum Lime Stabilized Soil. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2008. [Google Scholar]
- Lu, H. Development of New Soft Soil Curing Agent and Research on Its Reaction Mechanism. Master’s Thesis, Jinan University, Guangzhou, China, 2006. [Google Scholar]
- Zhang, J.; Huang, Z.; Su, X.; Chen, Y.; Li, H.; Li, W. Effect of phosphogypsum addition on properties of red mud-phosphogypsum composites. China Nonferrous Metall. 2023, 52, 121–130. [Google Scholar] [CrossRef]
- Cho, J.H.; Eom, Y.K.; Lee, T.G. Stabilization/solidification of mercury-contaminated waste ash using calcium sodium phosphate (CNP) and magnesium potassium phosphate (MKP) processes. J. Hazard. Mater. 2014, 278, 474–482. [Google Scholar] [CrossRef]










| Plastic Limit/% | Liquid Limit/% | The Natural Moisture Content/% | Optimum Moisture Content ω/% | Maximum Dry Density/g·cm−3 | pH |
|---|---|---|---|---|---|
| 27.4 | 17.21 | 35.9 | 24.1 | 1.82 | 10.49 |
| Composition | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | TiO2 | Na2O | K2O | Other |
|---|---|---|---|---|---|---|---|---|---|
| Content/wt% | 28.09 | 25.54 | 19.81 | 1.71 | 0.29 | 2.15 | 10.17 | 0.31 | 11.43 |
| Composition | CaO | SO3 | Fe2O3 | TiO2 | SiO2 | Na2O | Al2O3 | Other |
|---|---|---|---|---|---|---|---|---|
| Content/t% | 27.43 | 34.42 | 9.28 | 5.49 | 4.34 | 1.13 | 1.37 | 0.786 |
| Composition | SO3 | P2O5 | Al2O3 | SiO2 | CaO | Other |
|---|---|---|---|---|---|---|
| Content/% | 52.29 | 1.23 | 1.02 | 6.76 | 37.53 | 1.17 |
| Fineness 0.08/% 1 | Specific Surface Area m2/kg | Density g/cm3 | Solidification Time (min) | Flexural Strength (MPa) | Compressive Strength (MPa) | |||
|---|---|---|---|---|---|---|---|---|
| Initial Set | Final Set | 3 days | 7 days | 3 days | 7 days | |||
| 0.4 | 345 | 3.12 | 95 | 156 | 6.3 | 7.8 | 28.0 | 39.5 |
| Composition | SO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | f-CaO 1 | Cl− | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Content/% | 20.12 | 5.12 | 3.62 | 63.56 | 2.07 | 2.38 | 0.53 | 0.75 | 0.015 | 2.06 |
| Number | Admixture/% | Number | Admixture/% | ||||
|---|---|---|---|---|---|---|---|
| Red Mud | Titanium Gypsum | Cement | Red Mud | Phospho Gypsum | Cement | ||
| RTC-0 | 92 | 0 | 8 | RPC-0 | 92 | 0 | 8 |
| RTC-3 | 89 | 3 | 8 | RPC-3 | 89 | 3 | 8 |
| RTC-5 | 87 | 5 | 8 | RPC-5 | 87 | 5 | 8 |
| RTC-7 | 85 | 7 | 8 | RPC-7 | 85 | 7 | 8 |
| RTC-10 | 82 | 10 | 8 | RPC-10 | 82 | 10 | 8 |
| Leach Stage | Leach Solution Replacement Time | |
|---|---|---|
| Cumulative Time/d | Interval Time/h | |
| 1 | 0.25 | 6 |
| 2 | 1 | 18 |
| 3 | 2.25 | 30 |
| 4 | 4 | 42 |
| 5 | 9 | 120 |
| 6 | 16 | 168 |
| 7 | 36 | 480 |
| 8 | 64 | 672 |
| Number | Maximum Dry Density (g/cm3) | Optimum Moisture Content/% | Number | Maximum Dry Density (g/cm3) | Optimum Moisture Content/% |
|---|---|---|---|---|---|
| RTC-0 | 1.92 | 22.39 | RPC-0 | 1.92 | 22.39 |
| RTC-3 | 1.91 | 22.66 | RPC-3 | 1.90 | 22.59 |
| RTC-5 | 1.89 | 22.92 | RPC-5 | 1.93 | 21.85 |
| RTC-7 | 1.84 | 23.10 | RPC-7 | 1.89 | 22.26 |
| RTC-10 | 1.81 | 23.26 | RPC-10 | 1.88 | 22.24 |
| Group III Groundwater | RC | RTC-5 | RTC-10 | RPC-5 | RPC-10 | |
|---|---|---|---|---|---|---|
| Be (mg/L) | ≤0.0002 | — | — | — | — | — |
| Cr (mg/L) | ≤0.05 | 0.014419 | 0.02709 | 0.02572 | 0.01947 | 0.01051 |
| Mn (mg/L) | ≤0.1 | 0.00158 | 0.00014 | — | — | — |
| Co (mg/L) | ≤0.05 | 0.00020 | 0.00002 | 0.00003 | 0.00002 | — |
| Ni (mg/L) | ≤0.05 | 0.00034 | 0.00015 | 0.00039 | 0.00169 | — |
| Cu (mg/L) | ≤1.0 | 0.00273 | 0.00004 | 0.00085 | 0.00080 | 0.00044 |
| Zn (mg/L) | ≤1.0 | 0.02643 | 0.01017 | 0.0052 | 0.01736 | 0.01856 |
| As (mg/L) | ≤0.05 | 0.09978 | 0.04719 | 0.02151 | 0.03064 | 0.01073 |
| Se (mg/L) | ≤0.01 | 0.00078 | 0.00132 | 0.00141 | 0.00129 | 0.00133 |
| Mo (mg/L) | ≤0.1 | 0.05653 | 0.08971 | 0.08766 | 0.06412 | 0.03573 |
| Cd (mg/L) | ≤0.01 | 0.00013 | — | — | — | — |
| Ba (mg/L) | ≤1.0 | 0.00271 | 0.00144 | 0.00199 | 0.00204 | 0.00428 |
| Hg (mg/L) | ≤0.001 | — | — | — | — | — |
| Pb (mg/L) | ≤0.05 | 0.00767 | — | 0.00046 | 0.00013 | 0.00026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Cheng, Y.; Wang, W.; Jin, L.; Ding, Z. Gypsum-Enhanced Red Mud Composites: A Study on Strength, Durability, and Leaching Characteristics. Buildings 2024, 14, 1979. https://doi.org/10.3390/buildings14071979
Yan S, Cheng Y, Wang W, Jin L, Ding Z. Gypsum-Enhanced Red Mud Composites: A Study on Strength, Durability, and Leaching Characteristics. Buildings. 2024; 14(7):1979. https://doi.org/10.3390/buildings14071979
Chicago/Turabian StyleYan, Shiying, Yu Cheng, Wentong Wang, Lu Jin, and Ziyi Ding. 2024. "Gypsum-Enhanced Red Mud Composites: A Study on Strength, Durability, and Leaching Characteristics" Buildings 14, no. 7: 1979. https://doi.org/10.3390/buildings14071979
APA StyleYan, S., Cheng, Y., Wang, W., Jin, L., & Ding, Z. (2024). Gypsum-Enhanced Red Mud Composites: A Study on Strength, Durability, and Leaching Characteristics. Buildings, 14(7), 1979. https://doi.org/10.3390/buildings14071979







