High-Temperature Stirring Pretreatment of Waste Rubber Particles Enhances the Interfacial Bonding and Mechanical Properties of Rubberized Concrete
Abstract
:1. Introduction
2. Materials and Programs
2.1. Test Raw Materials
2.2. Test Fit Ratio
2.3. Specimen Preparation
2.3.1. Pretreatment Specimen of Waste Rubber Granules
2.3.2. Concrete Specimens
2.4. Test Methods
3. Results Analysis
3.1. Waste Rubber Particle Analysis
3.1.1. Composition Analysis of Waste Rubber Particles
3.1.2. Fourier-Transform Infrared (FT-IR) Spectral Analysis
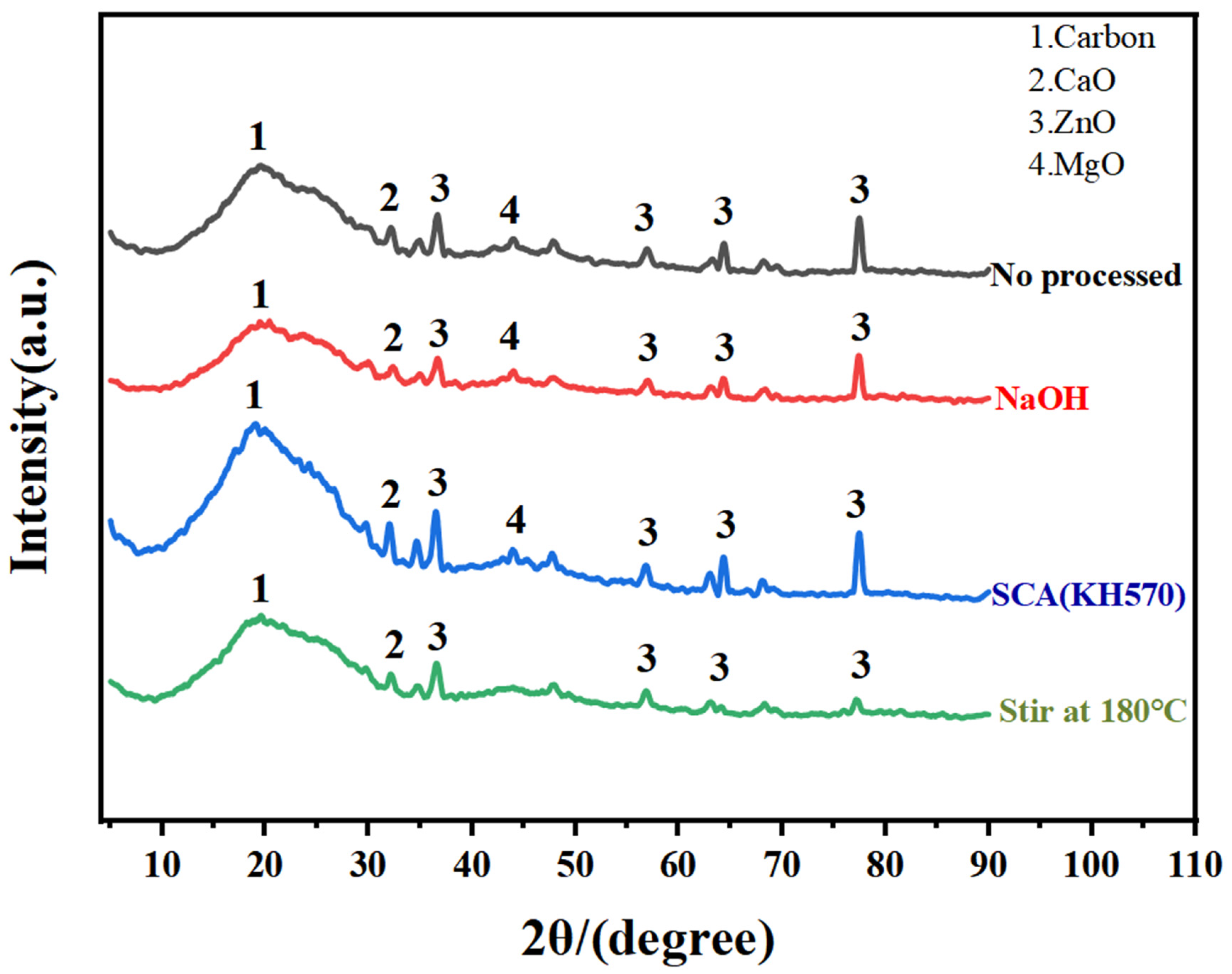
3.1.3. X-ray Diffraction Analysis Utilizes
3.1.4. Scanning Electron Microscopy (SEM) Analysis
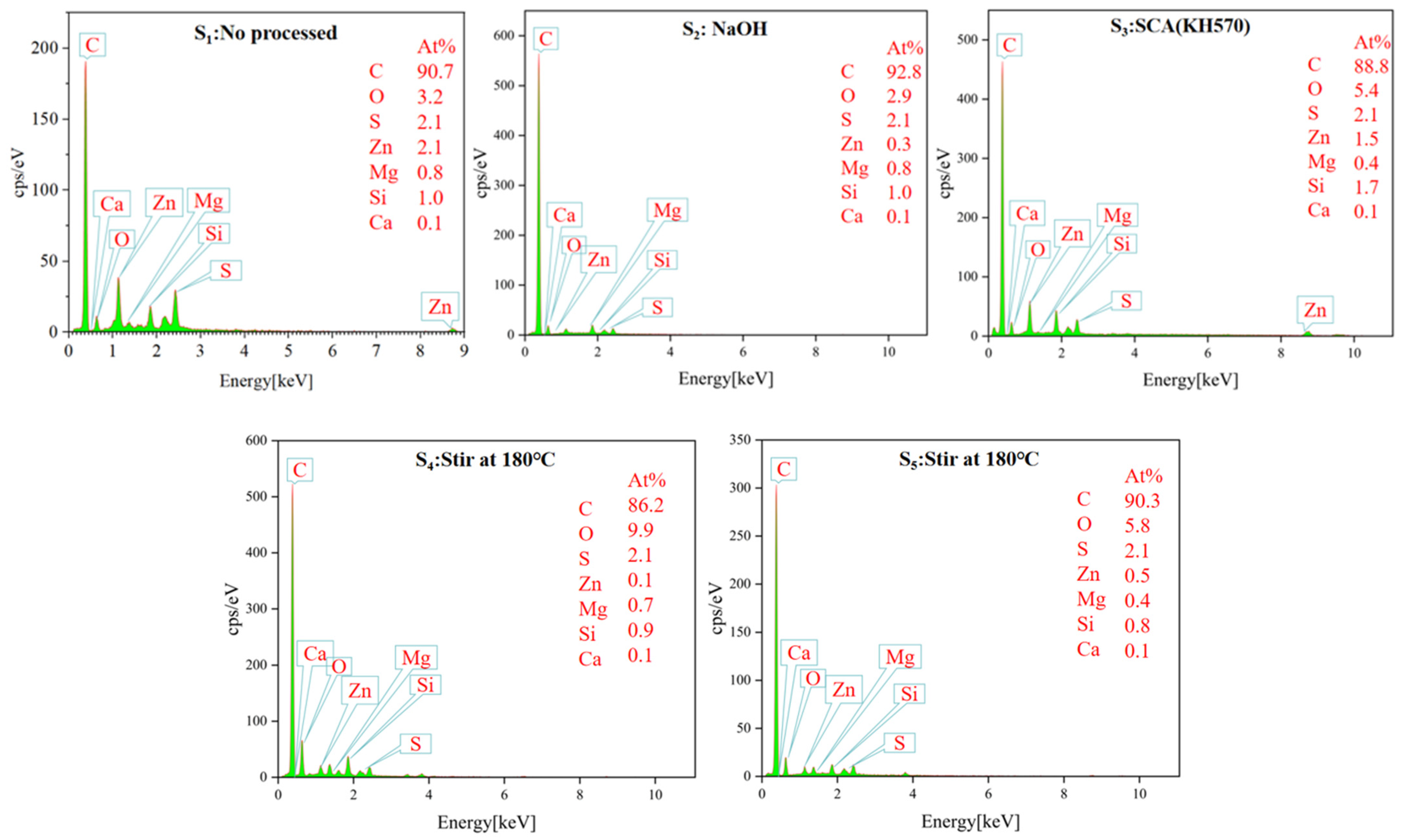
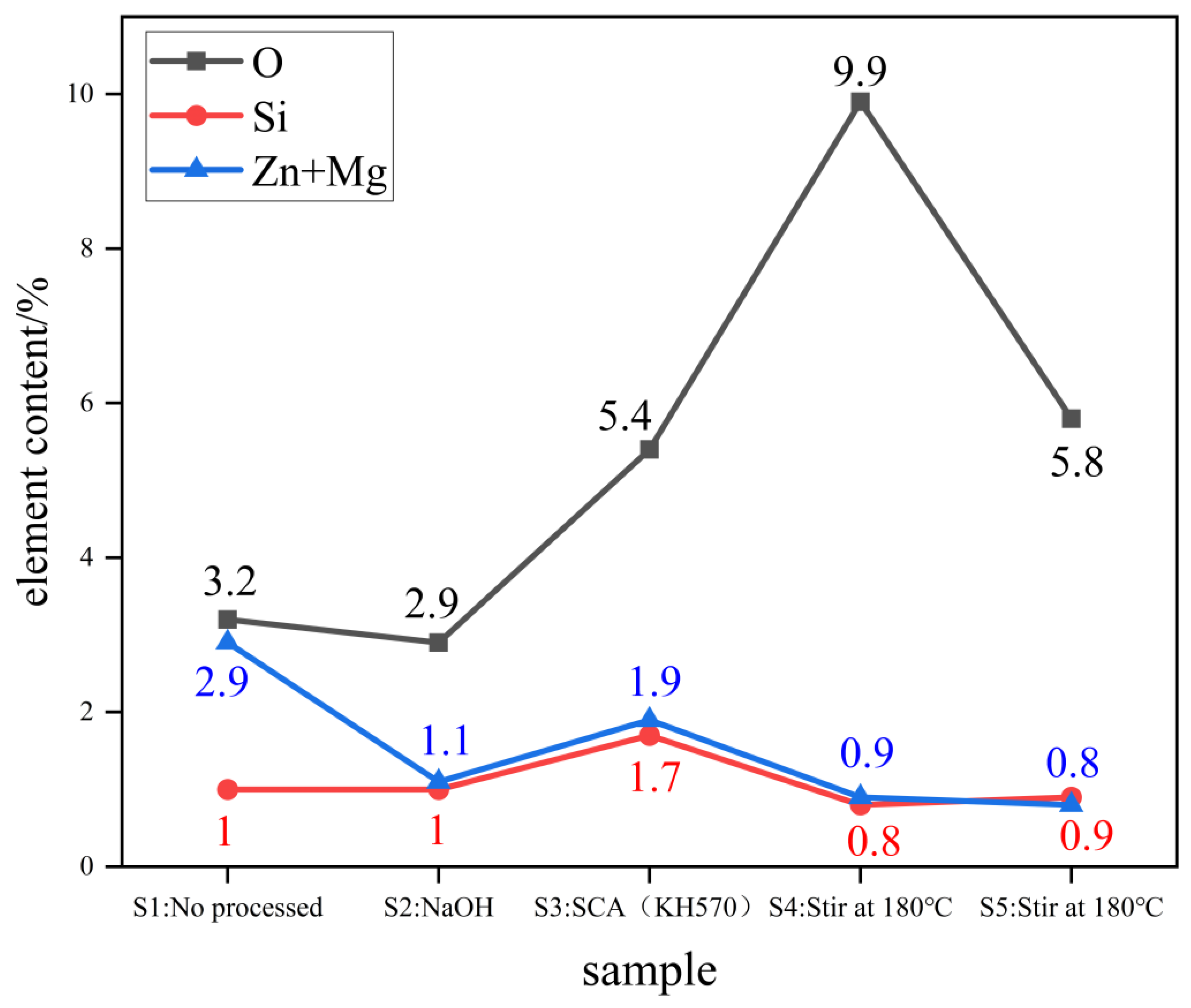
3.1.5. Energy-Dispersive X-ray (EDS) Analysis
3.1.6. Mechanistic Analysis
3.2. Analysis of Rubberized Concrete Performance
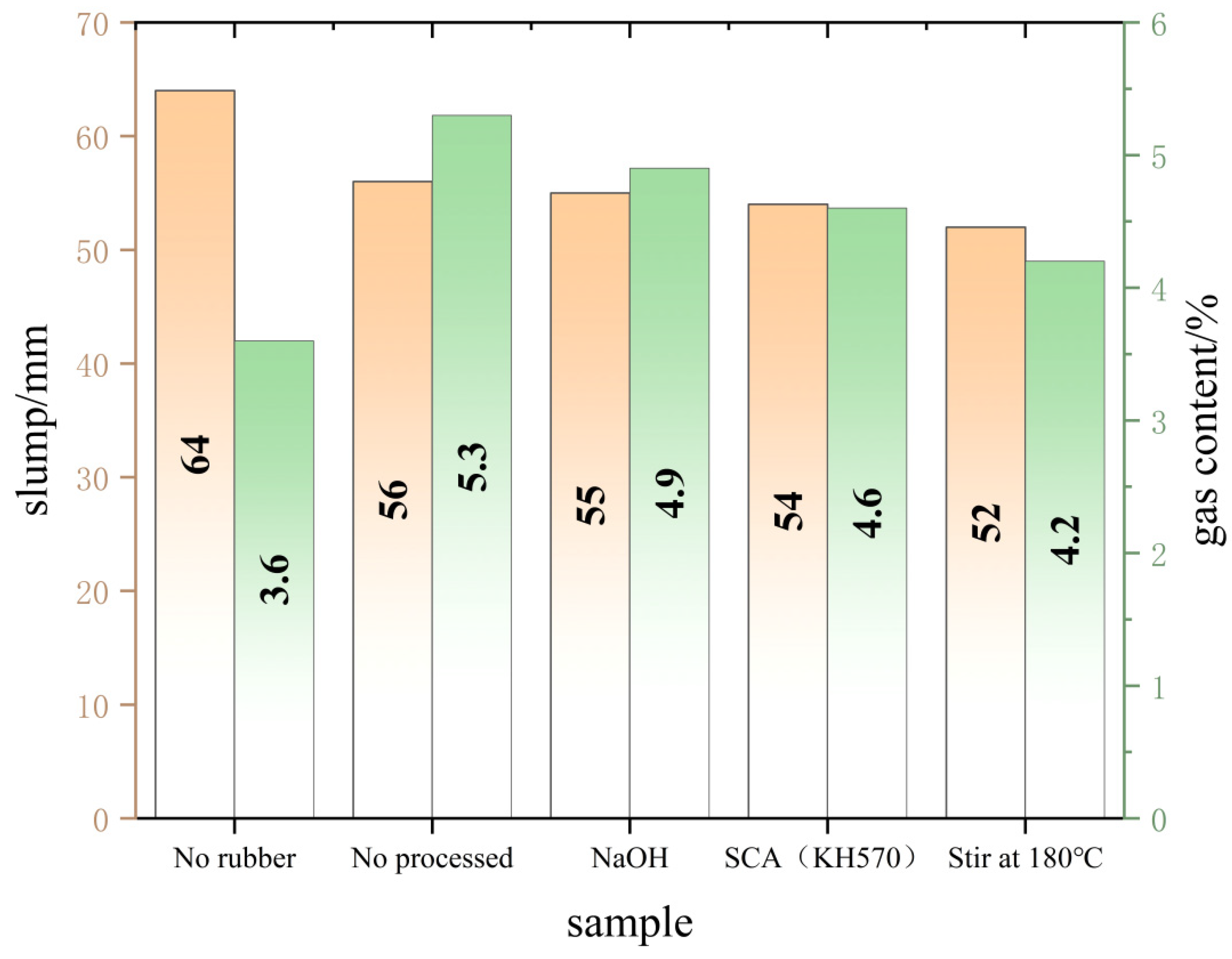
3.2.1. Working Performance Analysis
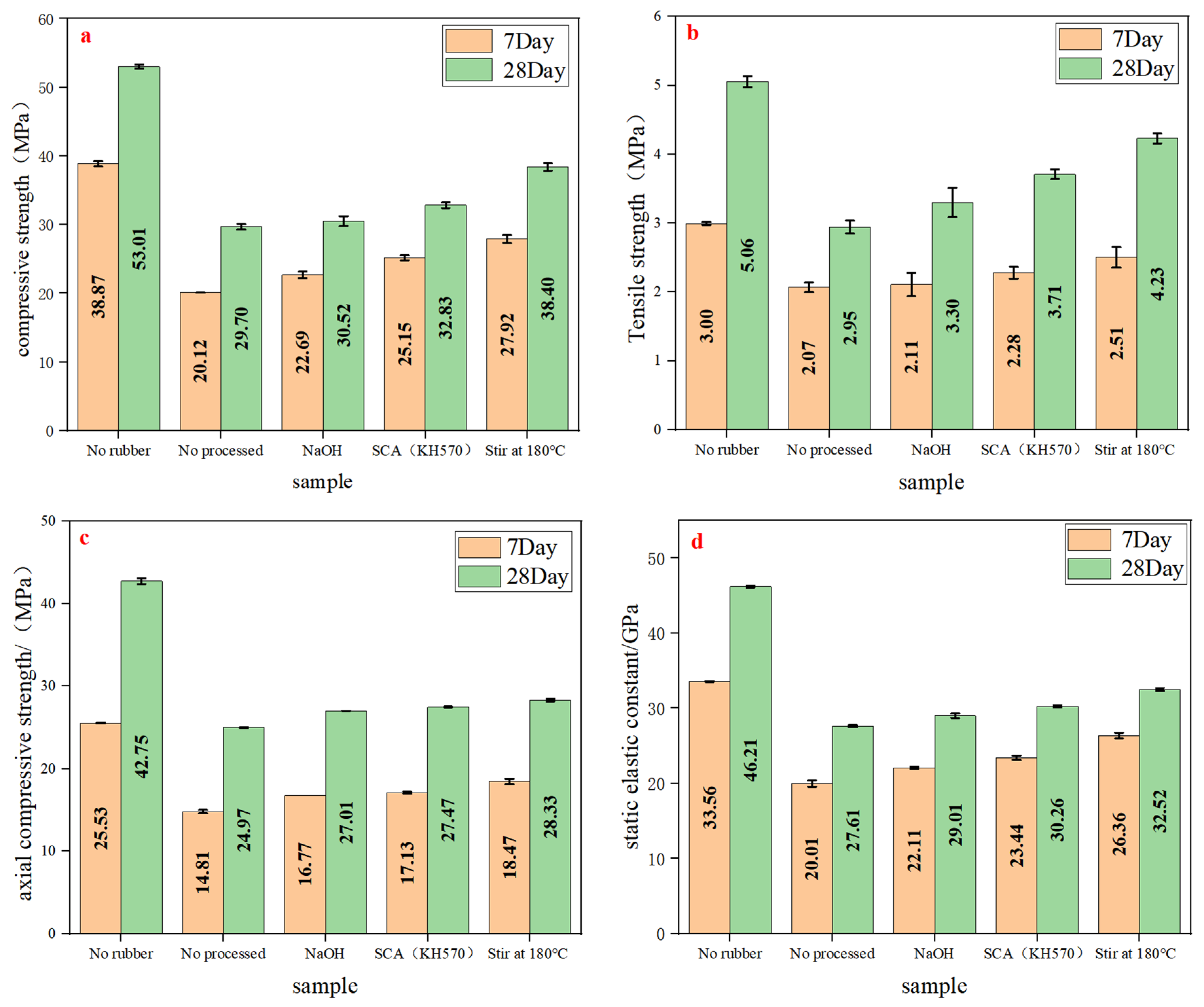
3.2.2. Analysis of Mechanical Properties
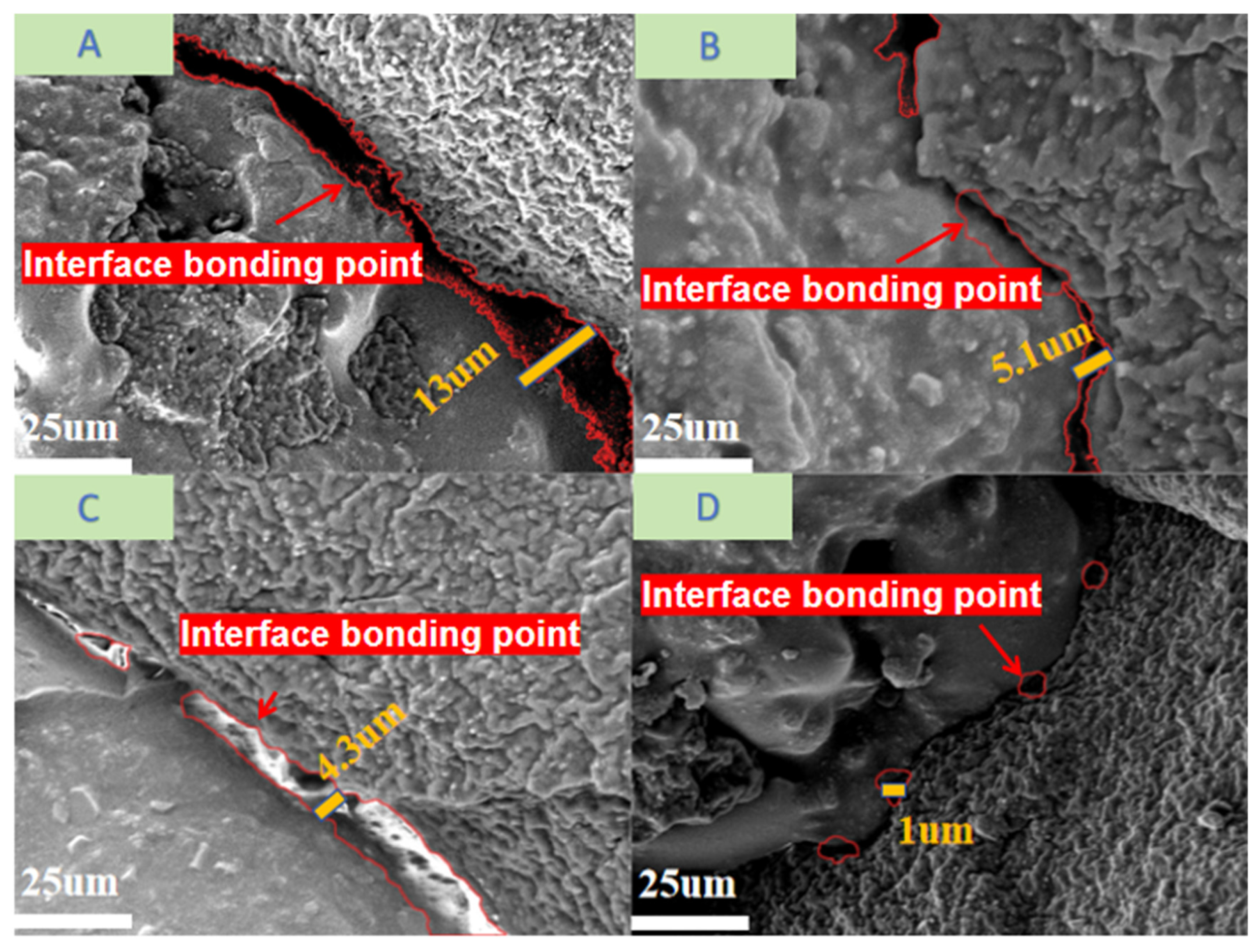
3.2.3. SEM Analysis
4. Conclusions
- The high-temperature stirring pretreatment of waste rubber particles at 180 °C removed surface impurities through friction and extrusion. Under the effect of temperature, the rubber hydrocarbons underwent oxidation reactions, forming an oxidation film, which enhanced the surface stiffness of the waste rubber particles and improved the strength and interfacial bonding performance of the rubberized concrete.
- Compared to rubberized concrete without added rubber, the rubberized concrete prepared by replacing fine aggregate with waste rubber particles experienced varying degrees of strength loss. This strength loss, from smallest to largest, was observed in concrete prepared with waste rubber particles that underwent high-temperature stirring pretreatment, KH570 pretreatment, NaOH pretreatment, and no treatment. The compressive strength recovery rates of the rubberized concrete prepared with high-temperature-stirred pretreated rubber particles were 41.6% at 7 days and 37.3% at 28 days, the split tensile strength recovery rates were 47.3% at 7 days and 60.6% at 28 days, the axial compressive strength recovery rates were 34.1% at 7 days and 18.8% at 28 days, and the static modulus of elasticity recovery rates were 46.8% at 7 days and 26.3% at 28 days. The strength recovery rate of the rubberized concrete obtained by this method is higher than that of rubberized concrete prepared using other pretreatment methods reported in most studies (such as water washing, acid–base solution, the two-stage method, etc.).
- Although the high-temperature stirring pretreatment of waste rubber particles could not completely compensate for the strength loss caused by the incorporation of waste rubber particles into the concrete, the process is simple, adaptable, and environmentally friendly and involves low strength loss. Therefore, it can be applied in scenarios that do not require high-strength concrete, such as civil construction and ground paving.
- Although the NaOH and KH570 pretreatment of waste rubber particles can improve the mechanical properties of rubberized concrete to a certain extent, the generation of wastewater and the complexity of the process due to the need for drying limit its application scope.
- The high-temperature stirring pretreatment of waste rubber particles generates no wastewater and does not require a drying step, so it has great potential for industrialization. However, waste gas is produced during the process. Moreover, although the oxide film formed on the surfaces of waste rubber particles by high-temperature stirring pretreatment can improve the strength of rubberized concrete to a certain extent, the oxidized waste rubber particles may have some impact on the durability of rubberized concrete. At the same time, fluctuations in pretreatment temperature and variations in stirring time may cause differences in the formation of oxide films on the surfaces of waste rubber particles, thereby affecting the effectiveness of the pretreatment. Therefore, future researchers can investigate the effects of different temperatures and stirring times on pretreated waste rubber particles to determine their optimal process conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El-Seidy, E.; Chougan, M.; Sambucci, M.; Al-Kheetan, M.J.; Biblioteca, I.; Valente, M.; Ghaffar, S.H. Lightweight alkali-activated materials and ordinary Portland cement composites using recycled polyvinyl chloride and waste glass aggregates to fully replace natural sand. Constr. Build. Mater. 2023, 368, 130399. [Google Scholar] [CrossRef]
- Tian, X.; Zhuang, Q.; Han, S.; Li, S.; Liu, H.; Li, L.; Bian, H. A novel approach of reapplication of carbon black recovered from waste tyre pyrolysis to rubber composites. J. Clean. Prod. 2021, 280, 124460. [Google Scholar] [CrossRef]
- Grammelis, P.; Margaritis, N.; Dallas, P.; Rakopoulos, D.; Mavrias, G. A review on management of end of life tires (ELTs) and alternative uses of textile fibers. Energies 2021, 14, 571. [Google Scholar] [CrossRef]
- Mutalib, N.A.; Mokhatar, S.N.; Budiea, A.M.; Jaini, Z.M.; Kamarudin, A.F.; Noh, M.M. A review: Study on waste rubber as construction material. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1144, 012003. [Google Scholar] [CrossRef]
- Thomas, B.S.; Gupta, R.C. Properties of high strength concrete containing scrap tire rubber. J. Clean. Prod. 2016, 113, 86–92. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Jiao, Y.; Sha, T. Experimental investigation of the mechanical and durability properties of crumb rubber concrete. Materials 2016, 9, 172. [Google Scholar] [CrossRef]
- Du, T.; Yang, Y.; Cao, H.; Si, N.; Kordestani, H.; Sktani, Z.D.I.; Arab, A.; Zhang, C. Rubberized Concrete: Effect of the Rubber Size and Content on Static and Dynamic Behavior. Buildings 2024, 14, 1541. [Google Scholar] [CrossRef]
- Jie, X.U.; Yao, Z.; Yang, G.; Han, Q. Research on crumb rubber concrete: From a multi-scale review. Constr. Build. Mater. 2020, 232, 117282. [Google Scholar]
- Chen, Z.; Li, L.; Xiong, Z. Investigation on the interfacial behaviour between the rubber-cement matrix of the rubberized concrete. J. Clean. Prod. 2019, 209, 1354–1364. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Feng, J. Frictional behaviour of the interface between concrete and rubber: Laboratory shear test and its elastoplastic model. Eng. Fract. Mech. 2018, 197, 192–205. [Google Scholar] [CrossRef]
- Roychand, R.; Gravina, R.J.; Zhuge, Y.; Ma, X.; Youssf, O.; Mills, J.E. A comprehensive review on the mechanical properties of waste tire rubber concrete. Constr. Build. Mater. 2020, 237, 117651. [Google Scholar] [CrossRef]
- Roychand, R.; Gravina, R.J.; Zhuge, Y.; Ma, X.; Mills, J.E.; Youssf, O. Practical rubber pre-treatment approch for concrete use—An experimental study. J. Compos. Sci. 2021, 5, 143. [Google Scholar] [CrossRef]
- Awan, H.H.; Javed, M.F.; Yousaf, A.; Aslam, F.; Alabduljabbar, H.; Mosavi, A. Experimental evaluation of untreated and pretreated crumb rubber used in concrete. Crystals 2021, 11, 558. [Google Scholar] [CrossRef]
- Mohammadi, I.; Khabbaz, H.; Vessalas, K. Enhancing mechanical performance of rubberised concrete pavements with sodium hydroxide treatment. Mater. Struct. 2016, 49, 813–827. [Google Scholar] [CrossRef]
- Youssf, O.; Mills, J.E.; Hassanli, R. Assessment of the mechanical performance of crumb rubber concrete. Constr. Build. Mater. 2016, 125, 175–183. [Google Scholar] [CrossRef]
- Najim, K.B.; Hall, M.R. Crumb rubber aggregate coatings/pre-treatments and their effects on interfacial bonding, air entrapment and fracture toughness in self-compacting rubberised concrete (SCRC). Mater. Struct. 2013, 46, 2029–2043. [Google Scholar] [CrossRef]
- Huang, B.; Shu, X.; Cao, J. A two-staged surface treatment to improve properties of rubber modified cement composites. Constr. Build. Mater. 2013, 40, 270–274. [Google Scholar] [CrossRef]
- Dong, Q.; Huang, B.; Shu, X. Rubber modified concrete improved by chemically active coating and silane coupling agent. Construct. Build. Mater. 2013, 48 (Suppl. C), 116–123. [Google Scholar] [CrossRef]
- Turatsinze, A.; Garros, M. On the modulus of elasticity and strain capacity of self-compacting concrete incorporating rubber aggregates. Resour. Conserv. Recycl. 2008, 52, 1209–1215. [Google Scholar]
- Kara De Maeijer, P.; Craeye, B.; Blom, J.; Bervoets, L. Crumb rubber in concrete—The barriers for application in the construction industry. Infrastructures 2021, 6, 116. [Google Scholar]
- Abd-Elaal, E.S.; Araby, S.; Mills, J.E.; Youssf, O.; Roychand, R.; Ma, X.; Zhuge, Y.; Gravina, R.J. Novel approach to improve crumb rubber concrete strength using thermal treatment. Constr. Build. Mater. 2019, 229, 116901. [Google Scholar] [CrossRef]
- National Standard of the People’s Republic of China. GB 50007-2011; Code for Design of Building Foundations and Basements. China Building Material Industry Publishing House: Beijing, China, 2011.
- National Standard of the People’s Republic of China. GB/T 3516-2006; Determination of Solvent Extracts in Rubber. Standards Press of China: Beijing, China, 2006.
- National Standard of the People’s Republic of China. GB/T 15904-2018; Determination of Polyisoprene Content in Rubber. Standards Press of China: Beijing, China, 2018.
- National Standard of the People’s Republic of China. GB/T 50080-2002; Standard for Test Methods of Performance on Ordinary Fresh Concrete. Standards Press of China: Beijing, China, 2002.
- National Standard of the People’s Republic of China. GB/T 50081-2016; Standard for Test Methods of Mechanical Properties of Ordinary Concrete. Standards Press of China: Beijing, China, 2016.
- Fazli, A.; Rodrigue, D. Recycling waste tires into ground tire rubber (GTR)/rubber compounds: A review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Feng, L.Y.; Chen, A.J.; Liu, H.D. Effect of waste tire rubber particles on concrete abrasion resistance under high-speed water flow. Int. J. Concr. Struct. Mater. 2021, 15, 37. [Google Scholar] [CrossRef]
- Medina, N.F.; Garcia, R.; Hajirasouliha, I.; Pilakoutas, K.; Guadagnini, M.; Raffoul, S. Composites with recycled rubber aggregates: Properties and opportunities in construction. Constr. Build. Mater. 2018, 188, 884–897. [Google Scholar] [CrossRef]
- Baptista, M.; Martins, I.; Conceição, T.; Reis, P. Multiple representations in the development of students’ cognitive structures about the saponification reaction. Chem. Educ. Res. Pract. 2019, 20, 760–771. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, R.; Lei, Y. Performance enhancement of rubberised concrete via surface modification of rubber: A review. Constr. Build. Mater. 2019, 227, 116691. [Google Scholar] [CrossRef]
- Taourit, S.; Le Gac, P.Y.; Bourdet, A.; Van Elslander, A.; Robin, C.; Fayolle, B. Changes in natural rubber mechanical behavior during oxidation: Relationship with oxygen consumption. Polym. Degrad. Stab. 2024, 225, 110787. [Google Scholar] [CrossRef]
- Li, G.Y.; Koenig, J.L. A review of rubber oxidation. Rubber Chem. Technol. 2005, 78, 355–390. [Google Scholar]
- Xiao, Y.; Huang, Y.; Li, B.; Xu, Y.; Wang, H.; Wang, C.; Bian, H. Wet continuous mixing technology and extrusion rheological properties of carbon black/natural rubber composites. Polym. Eng. Sci. 2024, 64, 1222–1234. [Google Scholar]
- Wattana, W.; Phetklung, S.; Jakaew, W.; Chumuthai, S.; Sriam, P.; Chanurai, N. Characterization of mixed biomass pellet made from oil palm and para-rubber tree residues. Energy Procedia 2017, 138, 1128–1133. [Google Scholar] [CrossRef]
- Wang, X.; Hong, L.; Wu, H.; Liu, H.; Jia, D. Grafting waste rubber powder and its application in asphalt. Constr. Build. Mater. 2021, 271, 121881. [Google Scholar] [CrossRef]
- Dong, R.; Zhao, M. Research on the pyrolysis process of crumb tire rubber in waste cooking oil. Renew. Energy 2018, 125, 557–567. [Google Scholar] [CrossRef]
- Sánchez-Olmos, L.A.; Medina-Valtierra, J.; Sathish-Kumar, K.; Sánchez Cardenas, M. Sulfonated char from waste tire rubber used as strong acid catalyst for biodiesel production. Environ. Prog. Sustain. Energy 2017, 36, 619–626. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Wan, C.; Zhang, Y.; Wang, S. Understanding H2O2-induced thermo-oxidative reclamation of vulcanized styrene butadiene rubber at low temperatures. ACS Sustain. Chem. Eng. 2021, 9, 2378–2387. [Google Scholar] [CrossRef]
- Williams, D. An application of infrared spectroscopy to rubber chemistry. Physics 1936, 7, 399–402. [Google Scholar] [CrossRef]
- Sattayanurak, S.; Sahakaro, K.; Kaewsakul, W.; Dierkes, W.K.; Reuvekamp, L.A.; Blume, A.; Noordermeer, J.W. Synergistic effect by high specific surface area carbon black as secondary filler in silica reinforced natural rubber tire tread compounds. Polym. Test. 2020, 81, 106173. [Google Scholar] [CrossRef]
- Grunert, F.; Wehmeier, A.; Blume, A. New insights into the morphology of silica and carbon black based on their different dispersion behavior. Polymers 2020, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.G.; Hardman, N.J. Nature of carbon black reinforcement of rubber: Perspective on the original polymer nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.Q.; Thomas, B.S.; Zhang, W.; Ji, B.; Li, S.; Brand, A.S. A comprehensive review on treatment methods for end-of-life tire rubber used for rubberized cementitious materials. Constr. Build. Mater. 2022, 359, 129365. [Google Scholar] [CrossRef]
- Wręczycki, J.; Bieliński, D.M.; Anyszka, R. Sulfur/organic copolymers as curing agents for rubber. Polymers 2018, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Kumar, V.; Potiyaraj, P.; Lee, D.J.; Choi, J. Synergistic activities of binary accelerators in presence of magnesium oxide as a cure activator in the vulcanization of natural rubber. J. Elastomers Plast. 2022, 54, 123–144. [Google Scholar] [CrossRef]
- Kopylov, V.M.; Kostyleva, E.I.; Kostylev, I.M.; Koviazin, A.V. Silica fillers for silicone rubber. Int. Polym. Sci. Technol. 2011, 38, 35–47. [Google Scholar] [CrossRef]
- Rácz, L.; Solti, S.; Gresits, I.; Tölgyesi, S.; Benedek, D.; Valentínyi, N.; Mizsey, P. Measurement of rarely investigated trace elements As, P, Sr, Zr, Rb and Y in waste tires. Period. Polytech. Chem. Eng. 2016, 60, 78–84. [Google Scholar] [CrossRef]
- Huang, L.; Yu, F.; Liu, Y.; Lu, A.; Song, Z.; Liu, W.; Zhu, C. Structural analyses of the bound rubber in silica-filled silicone rubber nanocomposites reveal mechanisms of filler-rubber interaction. Compos. Sci. Technol. 2023, 233, 109905. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Zhang, X.; Li, X.; Bai, Y. Preparation, structure, and properties of solution-polymerized styrene-butadiene rubber with functionalized end-groups and its silica-filled composites. Polymer 2014, 55, 1964–1976. [Google Scholar] [CrossRef]
- Thaiyasuit, P.; Pianthong, K.; Worapun, I. Acid esterification-alkaline transesterification process for methyl ester production from crude rubber seed oil. J. Oleo Sci. 2012, 61, 81–88. [Google Scholar] [CrossRef]
- Yassaroh, Y.; Nurhaini, F.F.; Woortman, A.J.; Loos, K. Physicochemical properties of heat-moisture treated, sodium stearate complexed starch: The effect of sodium stearate concentration. Carbohydr. Polym. 2021, 269, 118263. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zheng, X.; Zhan, S.; Zhou, J.; Liao, S. Study on the ozone aging mechanism of Natural Rubber. Polym. Degrad. Stab. 2021, 186, 109514. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Xu, L.; Zhang, P. Investigation of aging behavior and mechanism of nitrile-butadiene rubber (NBR) in the accelerated thermal aging environment. Polym. Test. 2016, 54, 59–66. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Liang, Q.; Chen, F.; Zhou, X. A state-of-the-art review of rubber modified cement-based materials: Cement stabilized base. J. Clean. Prod. 2023, 392, 136270. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Q.; Chen, J.; Shao, Y.; Wang, Y.; Wang, J. PDMS-OH and nano-SiO2 Modified KH570-TEOS silica-sol coating and protective effect on concrete. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129279. [Google Scholar] [CrossRef]
- Thiedeitz, M.; Kränkel, T.; Kartal, D.; Timothy, J.J. The Slump Flow of Cementitious Pastes: Simulation vs. Experiments. Materials 2024, 17, 532. [Google Scholar] [CrossRef]





| Samples | Methods | Waste Rubber Granules (kg) | Sand (kg) | Stone (kg) | Cement (kg) | Water-Reducing Agent (kg) |
|---|---|---|---|---|---|---|
| 0 | No rubber | - | 672 | 1173 | 420 | 3.7 |
| 1 | No processed | 55 | 537.6 | 1173 | 420 | 3.7 |
| 2 | NaOH | 55 | 537.6 | 1173 | 420 | 3.7 |
| 3 | KH570 | 55 | 537.6 | 1173 | 420 | 3.7 |
| 4 | Stir at 180 °C | 55 | 537.6 | 1173 | 420 | 3.7 |
| Samples | Weight Loss(%) | |||
|---|---|---|---|---|
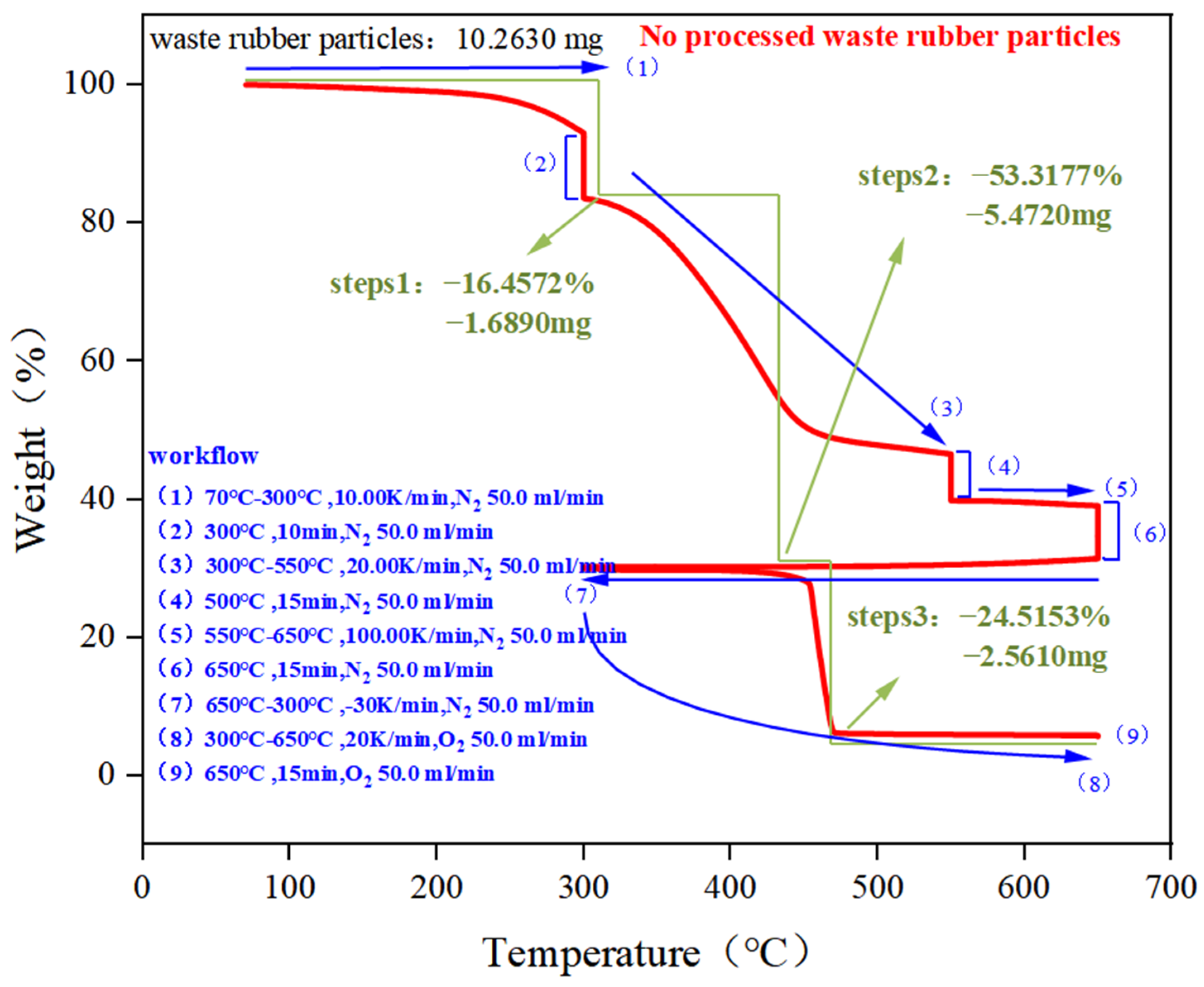
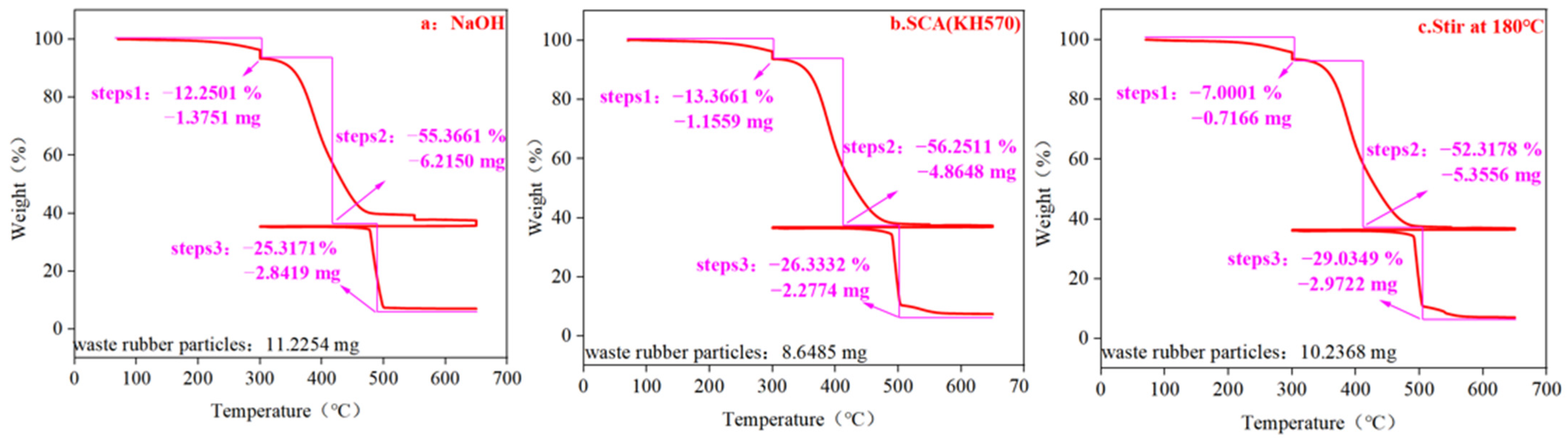
| Component | Weight Loss under N2 Atmosphere (Total Organic Content) | Weight Loss under O2 Atmosphere (Total Carbon Black Content) | Ash Content | |
| Low-Molecular-Weight Organics | Rubber Hydrocarbons | |||
| No processed | 16.4527 | 53.3177 | 24.5153 | 5.7143 |
| NaOH | 12.2501 | 55.3661 | 25.3171 | 7.0667 |
| KH570 | 13.3661 | 56.2511 | 26.331 | 4.0518 |
| Stir at 180 °C | 7.0001 | 52.3178 | 29.0349 | 11.6472 |
| Sample | m1 (g) | m2 (g) | X1 (%) | X (%) |
|---|---|---|---|---|
| No processed | 0.9987 | 0.9239 | 0.0036 | 7.4861 |
| NaOH | 0.9930 | 0.9238 | 0.0037 | 6.9163 |
| KH570 | 0.8981 | 0.8324 | 0.0036 | 7.3118 |
| Stir at 180 °C | 0.9910 | 0.9301 | 0.0034 | 6.1419 |
| Sample | V1 (mL) | V1 (mL) | C (mol/L) | M (g) | Wp (%) |
|---|---|---|---|---|---|
| Not processed | 100 | 88 | 0.05 | 0.2 | 27.24 |
| NaOH | 100 | 87 | 0.05 | 0.2 | 29.51 |
| KH570 | 100 | 88 | 0.05 | 0.2 | 27.24 |
| Stirred at 180 °C | 100 | 89 | 0.05 | 0.2 | 24.97 |
| Sample | Acetone Extract | Polyisoprene | Other Rubber Hydrocarbon | Carbon Black | Ash |
|---|---|---|---|---|---|
| No processed | 7.4861 | 27.24 | 35.0443 | 24.5153 | 5.7143 |
| NaOH | 6.9163 | 29.51 | 31.1899 | 25.3171 | 7.0667 |
| KH570 | 7.3118 | 27.24 | 34.0564 | 26.331 | 4.0518 |
| Stir at 180 °C | 6.1419 | 24.97 | 28.206 | 29.0349 | 11.6472 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Y.; Zhang, C.; Arab, A.; Lin, G.; Zhao, M. High-Temperature Stirring Pretreatment of Waste Rubber Particles Enhances the Interfacial Bonding and Mechanical Properties of Rubberized Concrete. Buildings 2024, 14, 2162. https://doi.org/10.3390/buildings14072162
Jing Y, Zhang C, Arab A, Lin G, Zhao M. High-Temperature Stirring Pretreatment of Waste Rubber Particles Enhances the Interfacial Bonding and Mechanical Properties of Rubberized Concrete. Buildings. 2024; 14(7):2162. https://doi.org/10.3390/buildings14072162
Chicago/Turabian StyleJing, Yuan, Chunwei Zhang, Ali Arab, Guangyi Lin, and Meng Zhao. 2024. "High-Temperature Stirring Pretreatment of Waste Rubber Particles Enhances the Interfacial Bonding and Mechanical Properties of Rubberized Concrete" Buildings 14, no. 7: 2162. https://doi.org/10.3390/buildings14072162






