Effect of Aggregate-to-Binders Ratio on Water Resistance of Red-Mud-Modified Magnesium Phosphate Repair Mortar
Abstract
:1. Introduction
2. Experimental Materials and Methods
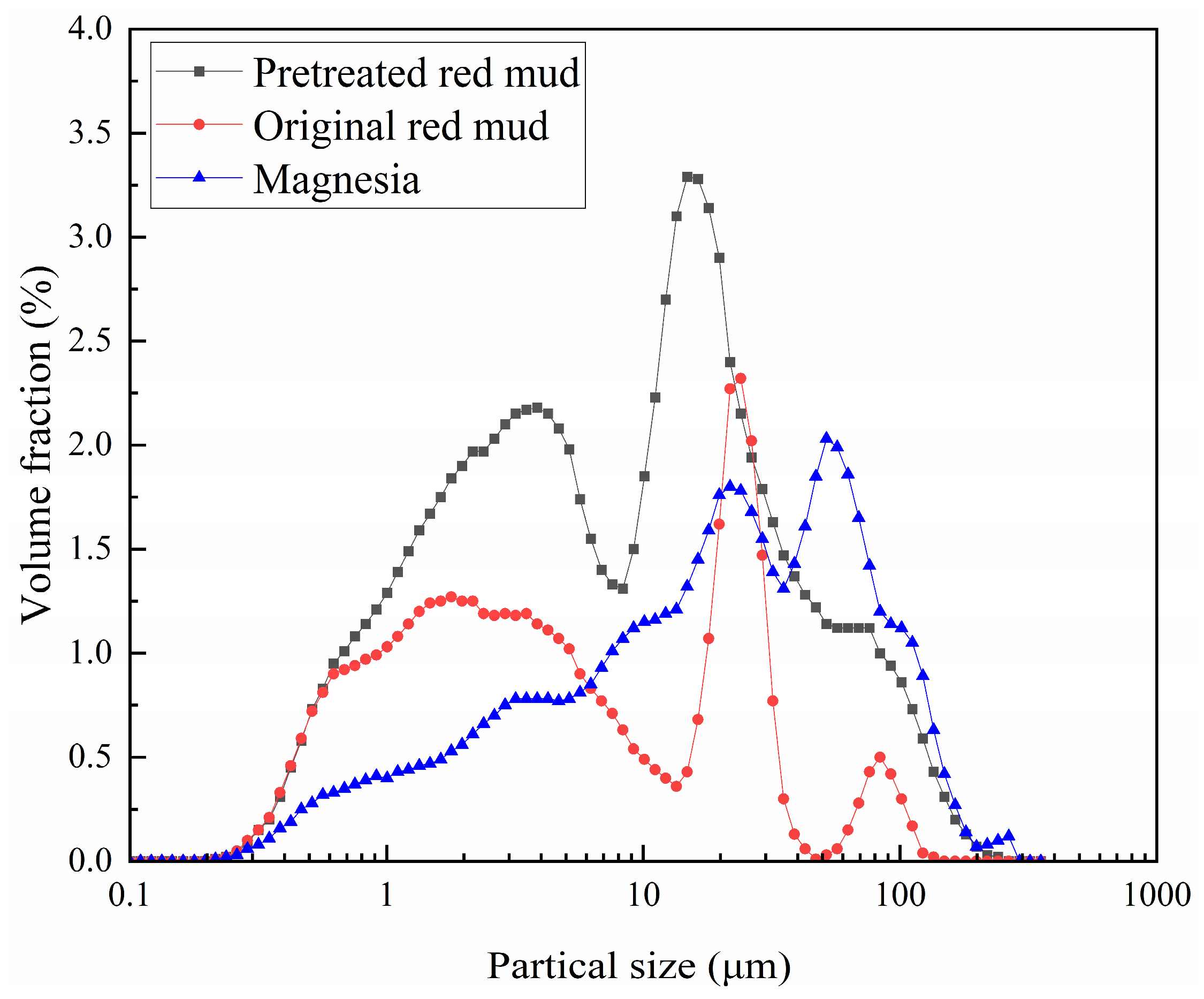
2.1. Materials
2.2. Preparation of RMPM
2.3. Physical and Mechanical Properties Test
- (1)
- Setting time and fluidity
- (2)
- Mechanical properties
2.4. Capillary Water Absorption Test
2.5. Capillary Porosity Test
3. Results and Discussion
3.1. Setting Time and Fluidity
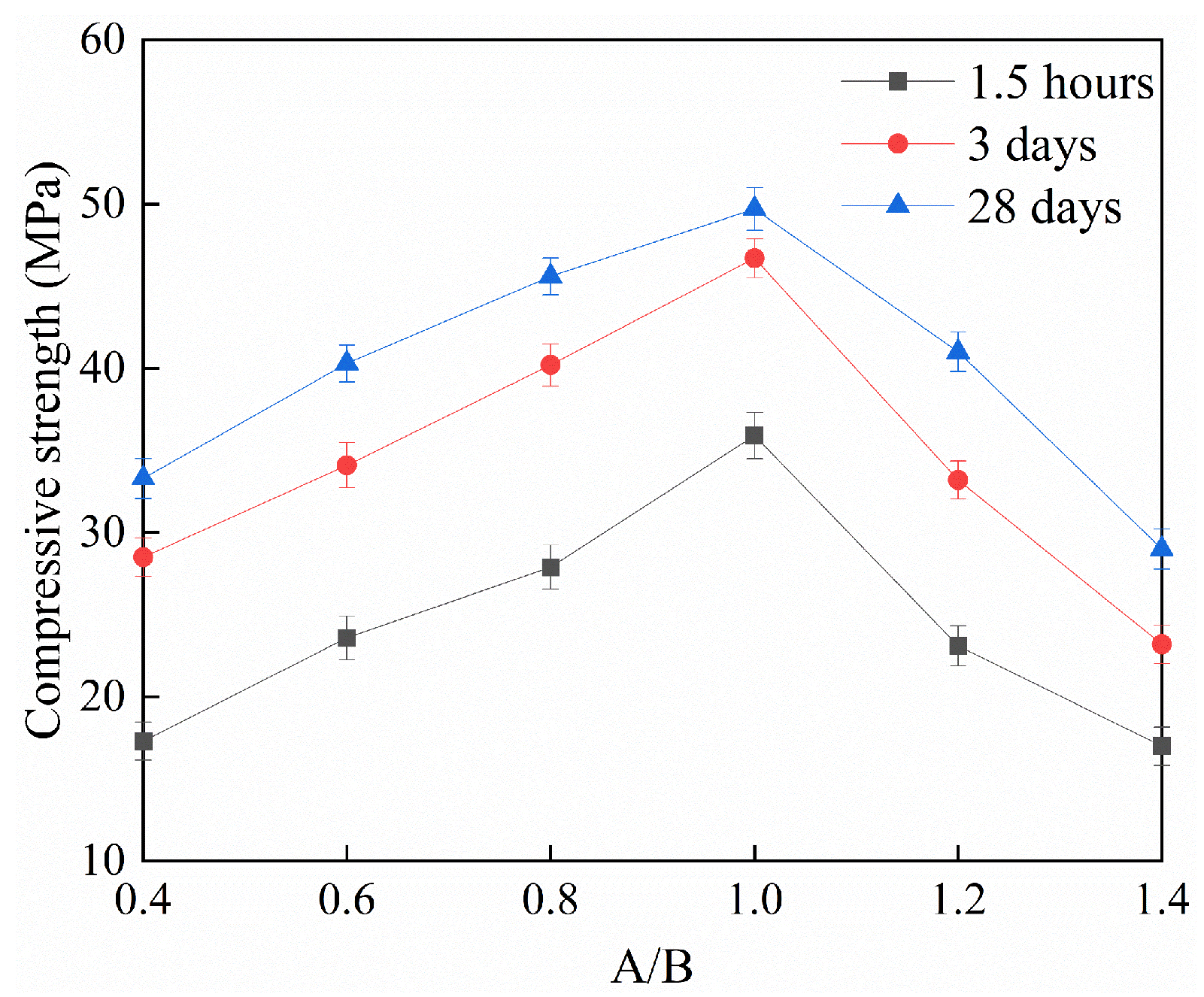
3.2. Mechanical Properties
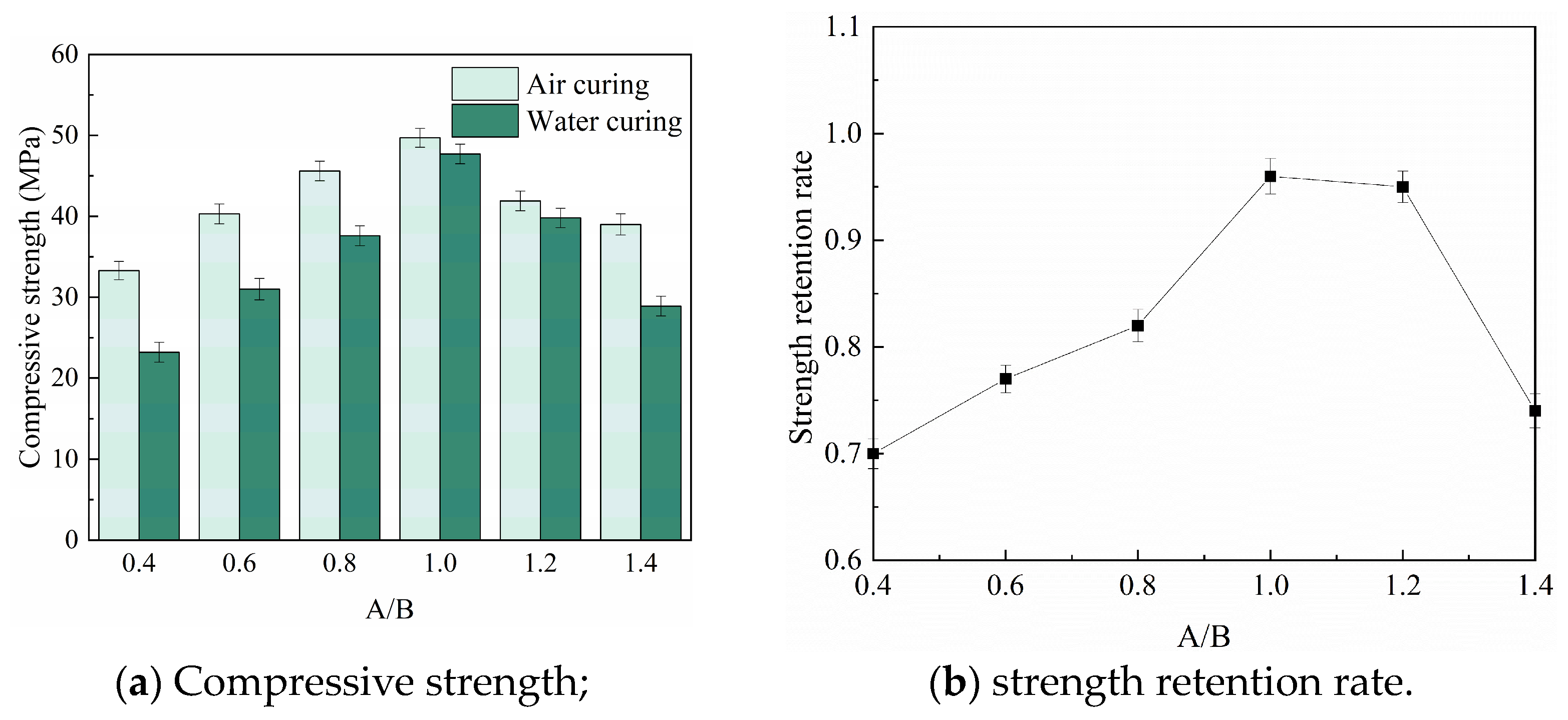
3.3. Water Resistance
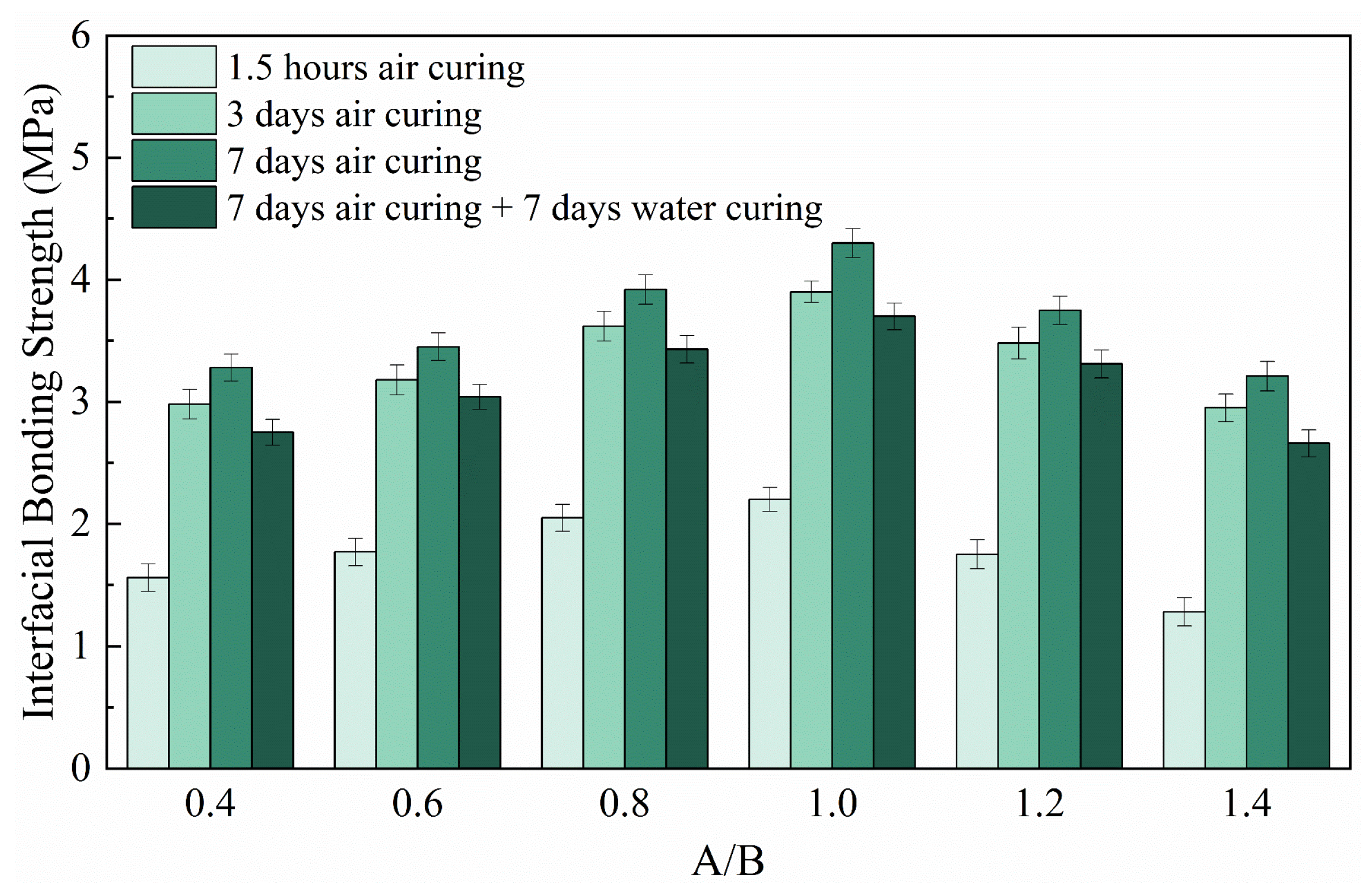
3.4. Interfacial Bonding Strength
3.5. Moisture Transportation Characteristics
3.6. Influence Mechanism of Water Resistance
4. Conclusions
- (1)
- A/Bs significantly affect the physical and mechanical properties of RMPM. With the increase in A/Bs, the fluidity of RMPM decreases significantly, and the setting time extends first and then shortens. The compressive and flexural strength and the interfacial bonding strength increase first and then decrease with the increase in A/Bs. When the A/Bs is 1.0, the compressive strength, flexural strength, and interfacial bonding strength of RMPM cured for 1.5 h reaches 35.9 MPa, 9.8 MPa, and 2.2 MPa, respectively, which indicates that it provides a good capability for emergency repairs.
- (2)
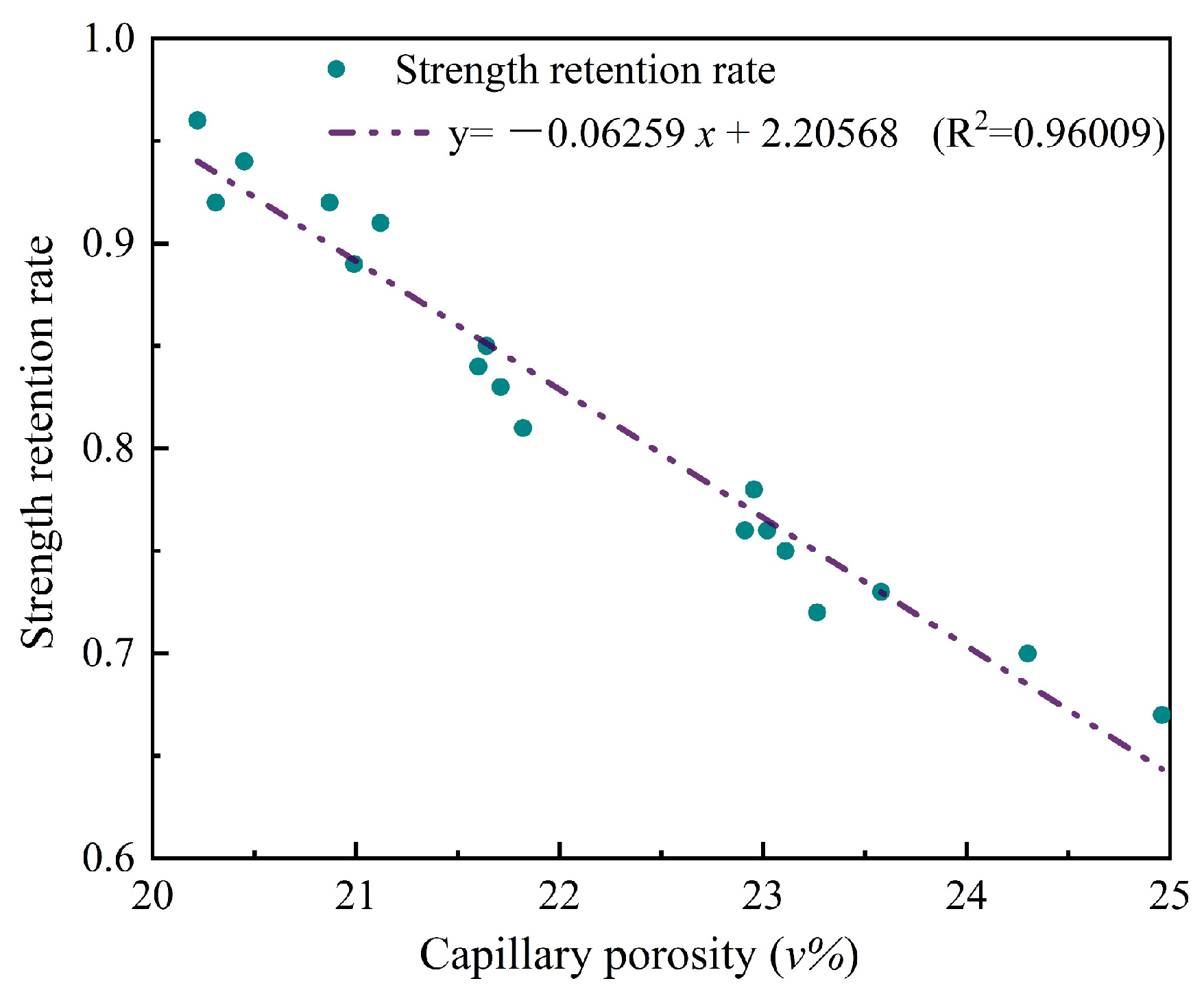
- RMPM with suitable A/Bs has good water resistance. The compressive strength retention rate of RMPM increases first and then decreases with the increase in A/Bs. When A/Bs is 1.0, the water resistance of RMPM reaches 0.96. However, as A/Bs is 0.4 and 1.2, its water resistance is 0.70 and 0.74, respectively. In addition, water curing also deteriorates the interfacial bonding strength of RMPM.
- (3)
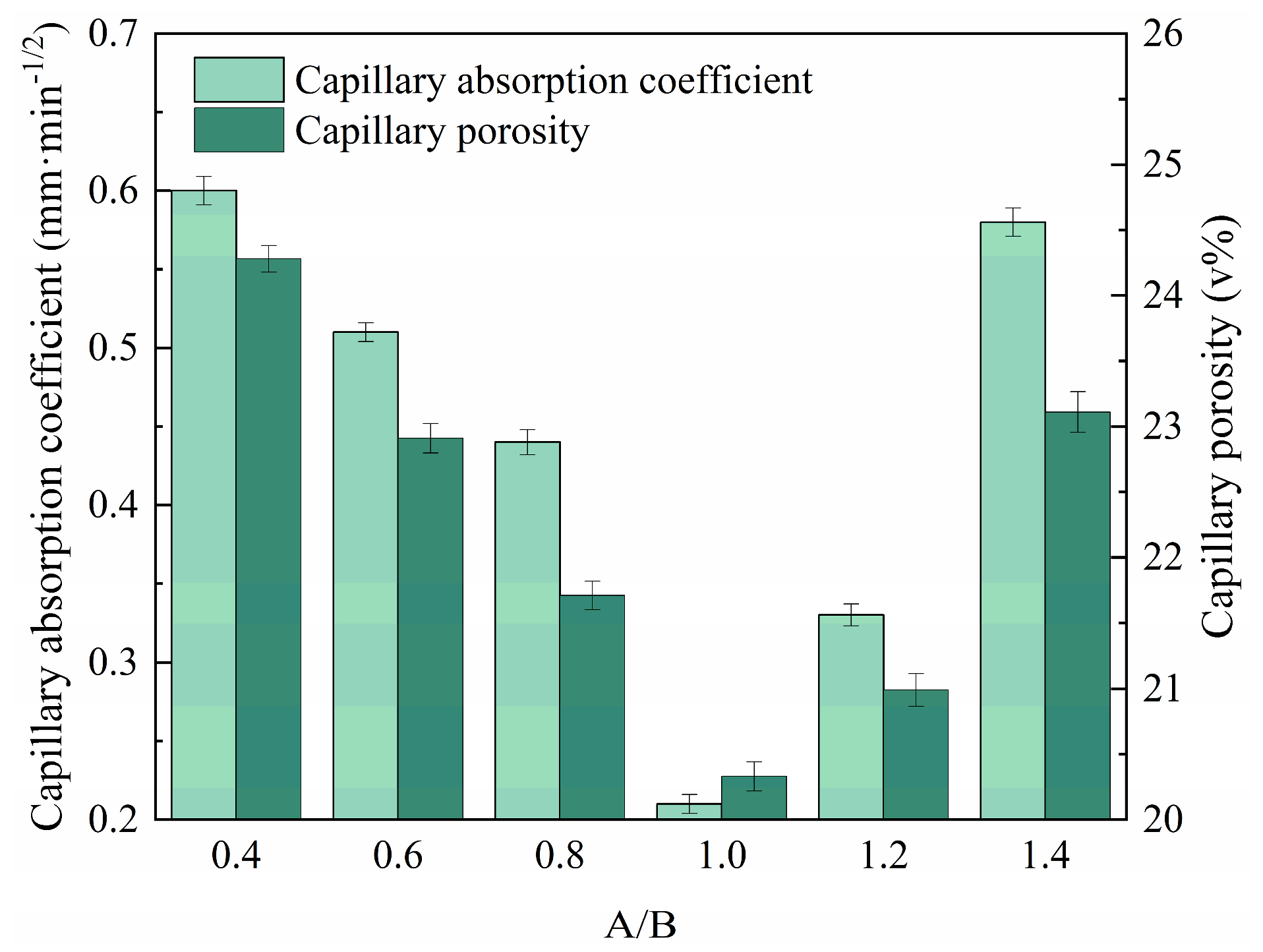
- An appropriate A/Bs reduces the capillary absorption coefficient and capillary porosity of RMPM, which in turn improves its water resistance. When the immersing time is no longer than 3600 min, the capillary absorption of RMPM has a linear relationship with the square root of time. The capillary porosity and capillary absorption coefficient of RMPM decreases first and then increases with the rise in A/Bs, and reduces to the lowest when A/Bs is 1.0, at which the water resistance, mechanical properties, and interfacial bonding properties of RMPM also achieve the optimal level.
5. Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, B.; Lothenbach, B.; Winnefeld, F. Influence of wollastonite on hydration and properties of magnesium potassium phosphate cements. Cem. Concr. Res. 2020, 131, 106012. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Z. Effect of aggregates and water contents on the properties of magnesium phospho-silicate cement. Cem. Concr. Compos. 2005, 27, 11–18. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Cao, Y. Laboratory evaluation of magnesium phosphate cement paste and mortar for rapid repair of cement concrete pavement. Constr. Build. Mater. 2014, 58, 122–128. [Google Scholar] [CrossRef]
- Lee, K.H.; Yoon, H.S.; Yang, K.H. Tests on magnesium potassium phosphate composite mortars with different water-to-binder ratios and molar ratios of magnesium-to-phosphate. Constr. Build. Mater. 2017, 146, 303–311. [Google Scholar] [CrossRef]
- Lai, Z.; Lai, X.; Shi, J.; Lu, Z. Effect of Zn2+ on the early hydration behavior of potassium phosphate based magnesium phosphate cement. Constr. Build. Mater. 2016, 129, 70–78. [Google Scholar] [CrossRef]
- Coumes, C.C.D.; Lambertin, D.; Lahalle, H.; Antonucci, P.; Cannes, C.; Delpech, S. Selection of a mineral binder with potentialities for the stabilization/solidification of aluminum metal. J. Nucl. Mater. 2014, 453, 31–40. [Google Scholar] [CrossRef]
- Wei, M.-L.; Du, Y.-J.; Reddy, K.R.; Wu, H.-L. Effects of freeze-thaw on characteristics of new KMP binder stabilized Zn- and Pb-contaminated soils. Environ. Sci. Pollut. Res. 2015, 22, 19473–19484. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, I.K.M.; Tsang, D.C.W.; Li, S.; Poon, C.S. Mixture design and reaction sequence for recycling construction wood waste into rapid-shaping magnesia–phosphate cement particleboard. Ind. Eng. Chem. Res. 2017, 56, 6645–6654. [Google Scholar] [CrossRef]
- Weng, Y.; Ruan, S.; Li, M.; Mo, L.; Unluer, C.; Tan, M.J.; Qian, S. Feasibility study on sustainable magnesium potassium phosphate cement paste for 3D printing. Constr. Build. Mater. 2019, 221, 595–603. [Google Scholar] [CrossRef]
- Wang, A.-J.; Zhang, J.; Li, J.-M.; Ma, A.-B.; Liu, L.-T. Effect of liquid-to-solid ratios on the properties of magnesium phosphate chemically bonded ceramics. Mater. Sci. Eng. C 2013, 33, 2508–2512. [Google Scholar] [CrossRef]
- Tang, H.; Qian, J.; Ji, Z.; Dai, X.; Li, Z. The protective effect of magnesium phosphate cement on steel corrosion. Constr. Build. Mater. 2020, 255, 119422. [Google Scholar] [CrossRef]
- Qin, J.; Qian, J.; You, C.; Fan, Y.; Li, Z.; Wang, H. Bond behavior and interfacial micro-characteristics of magnesium phosphate cement onto old concrete substrate. Constr. Build. Mater. 2018, 167, 166–176. [Google Scholar] [CrossRef]
- Hou, D.; Yan, H.; Zhang, J.; Wang, P.; Li, Z. Experimental and computational investigation of magnesium phosphate cement mortar. Constr. Build. Mater. 2016, 112, 331–342. [Google Scholar] [CrossRef]
- Wu, B.; Jiao, Y.; Cao, R.; Zhai, J.; Zhang, Q. Effect of Metakaolin on the Water Resistance of Magnesium Phosphate Cement Mortar. Coatings 2023, 13, 1664. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, B.; Wu, X. Characteristics and durability test of magnesium phosphate cement-based material for rapid repair of concrete. Mater. Struct. 2000, 33, 229–234. [Google Scholar] [CrossRef]
- Fan, S.; Chen, B. Experimental research of water stability of magnesium alumina phosphate cements mortar. Constr. Build. Mater. 2015, 94, 164–171. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Stefan, L.; Saillio, M. On the influence of Mg/P ratio on the properties and durability of magnesium potassium phosphate cement pastes. Cem. Concr. Res. 2017, 96, 27–41. [Google Scholar] [CrossRef]
- Pang, B.; Liu, J.; Wang, B.; Liu, R.; Yang, Y. Effects of K-struvite on hydration behavior of magnesium potassium phosphate cement. Constr. Build. Mater. 2021, 275, 121741. [Google Scholar] [CrossRef]
- Zhang, F.; Poh, L.H.; Zhang, M.H. Effect of bauxite aggregate in cement composites on mechanical properties and resistance against high-velocity projectile impact. Cem. Concr. Compos. 2021, 118, 103915. [Google Scholar] [CrossRef]
- Menéndez, E.; Sanjuán, M.; García-Roves, R.; Argiz, C.; Recino, H. Durability of blended cements made with reactive aggregates. Materials 2021, 14, 2948. [Google Scholar] [CrossRef]
- Diliberto, C.; Lecomte, A.; Aissaoui, C.; Mechling, J.-M.; Izoret, L. The incorporation of fine recycled concrete aggregates as a main constituent of cement. Mater. Struct. 2021, 54, 197. [Google Scholar] [CrossRef]
- Zhang, G.; Li, G.; He, T. Effects of sulphoaluminate cement on the strength and water stability of magnesium potassium phosphate cement. Constr. Build. Mater. 2017, 132, 335–342. [Google Scholar] [CrossRef]
- Ruan, W.; Liao, J.; Li, F.; Gu, X.; Zhou, A.; Xie, Y. Effects of water purifying material waste and red mud on performance of magnesium phosphate cement. Constr. Build. Mater. 2021, 303, 124563. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Chen, B. Experimental research on magnesium phosphate cements modified by red mud. Constr. Build. Mater. 2020, 231, 117131. [Google Scholar] [CrossRef]
- Ma, S.; Cao, Z.; Wei, C.; Shao, Y.; Wu, P.; Zhang, Z.; Liu, X. Red mud-modified magnesium potassium phosphate cement used as rapid repair materials: Durability, bonding property, volume stability and environment performance optimization. Constr. Build. Mater. 2024, 415, 135144. [Google Scholar] [CrossRef]
- Ruan, W.; Liao, J.; Mo, J.; Li, F.; Gu, X.; Ma, Y.; Zhu, Y.; Ma, X. Effects of red mud on properties of magnesium phosphate cement-based grouting material and its bonding mechanism with coal rock. Ceram. Int. 2023, 49, 2015–2025. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Y.; Li, Z.; Yang, F.; Hai, R. Hardening and microstructural properties of red mud modified magnesium ammonium phosphate cements. J. Build. Eng. 2023, 66, 105849. [Google Scholar] [CrossRef]
- ASTM C1585-13; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2013.
- Hall, C.; Yau, M.H.R. Water movement in porous building materials—IX. The water absorption and sorptivity of concretes. Build. Environ. 1987, 22, 77–82. [Google Scholar] [CrossRef]
- Debnath, S.; Saha, A.K.; Mazumder, B.; Roy, A.K. Hydrodynamic dispersion of reactive solute in a Hagen–Poiseuille flow of a layered liquid. Chin. J. Chem. Eng. 2017, 25, 862–873. [Google Scholar] [CrossRef]
- ASTM C642-13; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2013.
- Feng, X.; Yao, J.; Wu, P.; Zhang, S.; Sunahara, G.; Ni, W. Effect of water quenched silicomanganese slag as fine aggregate on mechanical properties and microstructure characteristics of solid waste-based mortar and concrete. J. Build. Eng. 2024, 88, 109115. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Xue, K.; Zhang, Y.; Xu, L. Effects of spodumene flotation tailings as aggregates on mechanical properties of cement mortar. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128346. [Google Scholar] [CrossRef]
- Ganesan, K.; Kanagarajan, V.; Dominic, J.R.J. Influence of marine sand as fine aggregate on mechanical and durability properties of cement mortar and concrete. Mater. Res. Express 2022, 9, 035504. [Google Scholar] [CrossRef]
- Li, X.; Xu, C.; Du, P. The Bond Strength of a Calcium Sulfoaluminate Cement-based Repair Mortar. Ceram. Silik. 2020, 64, 100–106. [Google Scholar] [CrossRef]
- Hall, M.; Allinson, D. Influence of cementitious binder content on moisture transport in stabilized earth materials analyzed using 1-dimensional sharp wet front theory. Build. Environ. 2009, 44, 688–693. [Google Scholar] [CrossRef]






| Materials | MgO | Al2O3 | SiO2 | CaO | Fe2O3 | K2O | Na2O | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| Dead-burned MgO | 92.28 | 1.14 | 1.96 | 1.36 | 1.45 | - | - | - | 0.32 |
| Red mud | 0.67 | 24.86 | 17.62 | 12.73 | 18.65 | 2.38 | 9.65 | 4.78 | 1.34 |
| Batch | M + P (w%) | M/P | W/B | A/Bs | N/M (w%) |
|---|---|---|---|---|---|
| RA1 | 85 | 3/1 | 0.20 | 0.4 | 12 |
| RA2 | 85 | 3/1 | 0.20 | 0.6 | 12 |
| RA3 | 85 | 3/1 | 0.20 | 0.8 | 12 |
| RA4 | 85 | 3/1 | 0.20 | 1.0 | 12 |
| RA5 | 85 | 3/1 | 0.20 | 1.2 | 12 |
| RA6 | 85 | 3/1 | 0.20 | 1.4 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, X.; Li, J.; Ma, Y.; Zhu, Z.; Liu, J. Effect of Aggregate-to-Binders Ratio on Water Resistance of Red-Mud-Modified Magnesium Phosphate Repair Mortar. Buildings 2024, 14, 2174. https://doi.org/10.3390/buildings14072174
Zhang M, Zhang X, Li J, Ma Y, Zhu Z, Liu J. Effect of Aggregate-to-Binders Ratio on Water Resistance of Red-Mud-Modified Magnesium Phosphate Repair Mortar. Buildings. 2024; 14(7):2174. https://doi.org/10.3390/buildings14072174
Chicago/Turabian StyleZhang, Maoliang, Xiaorong Zhang, Jianwei Li, Yan Ma, Zheyu Zhu, and Junxia Liu. 2024. "Effect of Aggregate-to-Binders Ratio on Water Resistance of Red-Mud-Modified Magnesium Phosphate Repair Mortar" Buildings 14, no. 7: 2174. https://doi.org/10.3390/buildings14072174





