Enhancement of Compressive Strength and Durability of Sulfate-Attacked Concrete
Abstract
1. Introduction
2. Experimental Details
3. Experiment Results and Discussion
3.1. The Compressive Strength
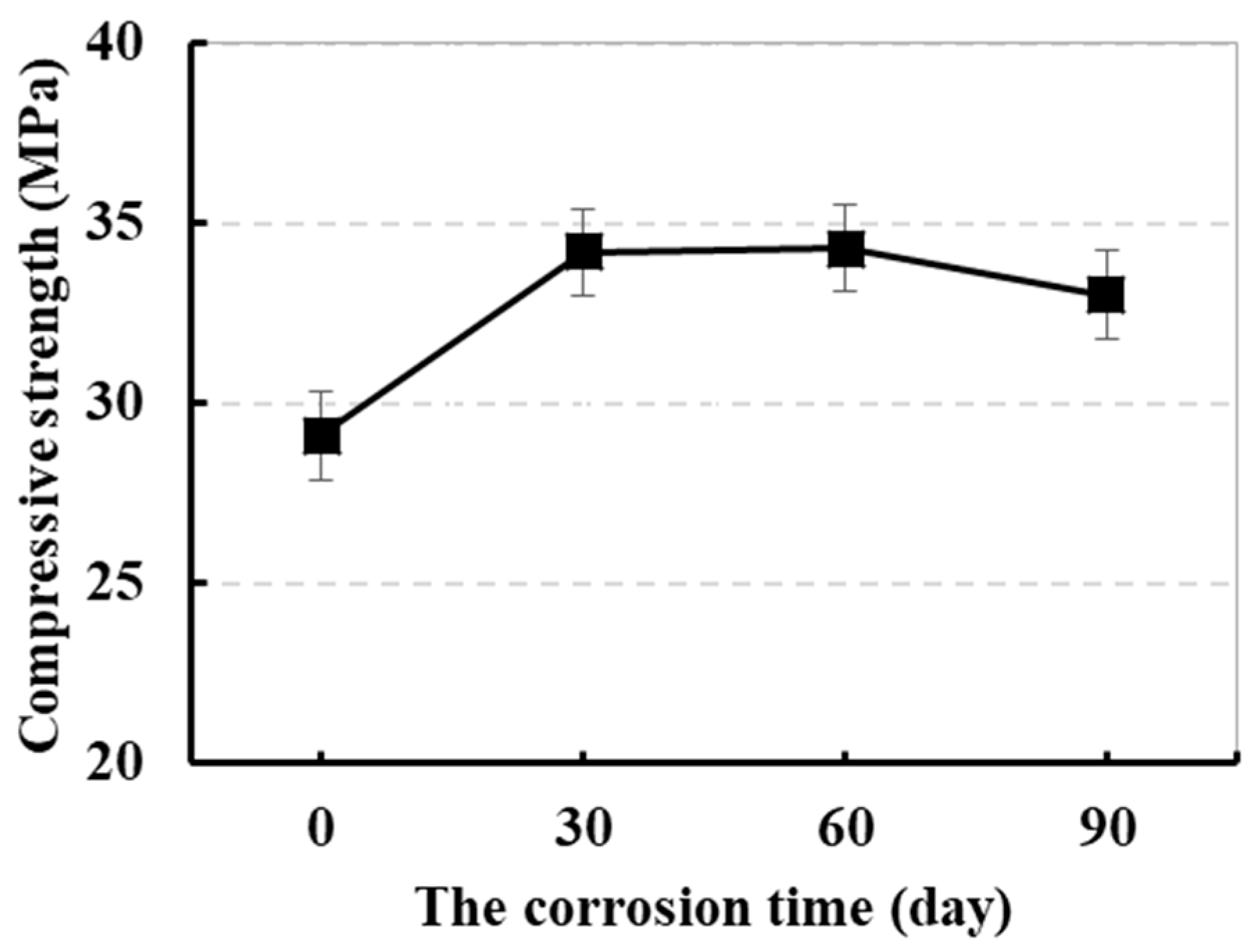
3.1.1. Effect of Sulfate Corrosion Time on the Strength
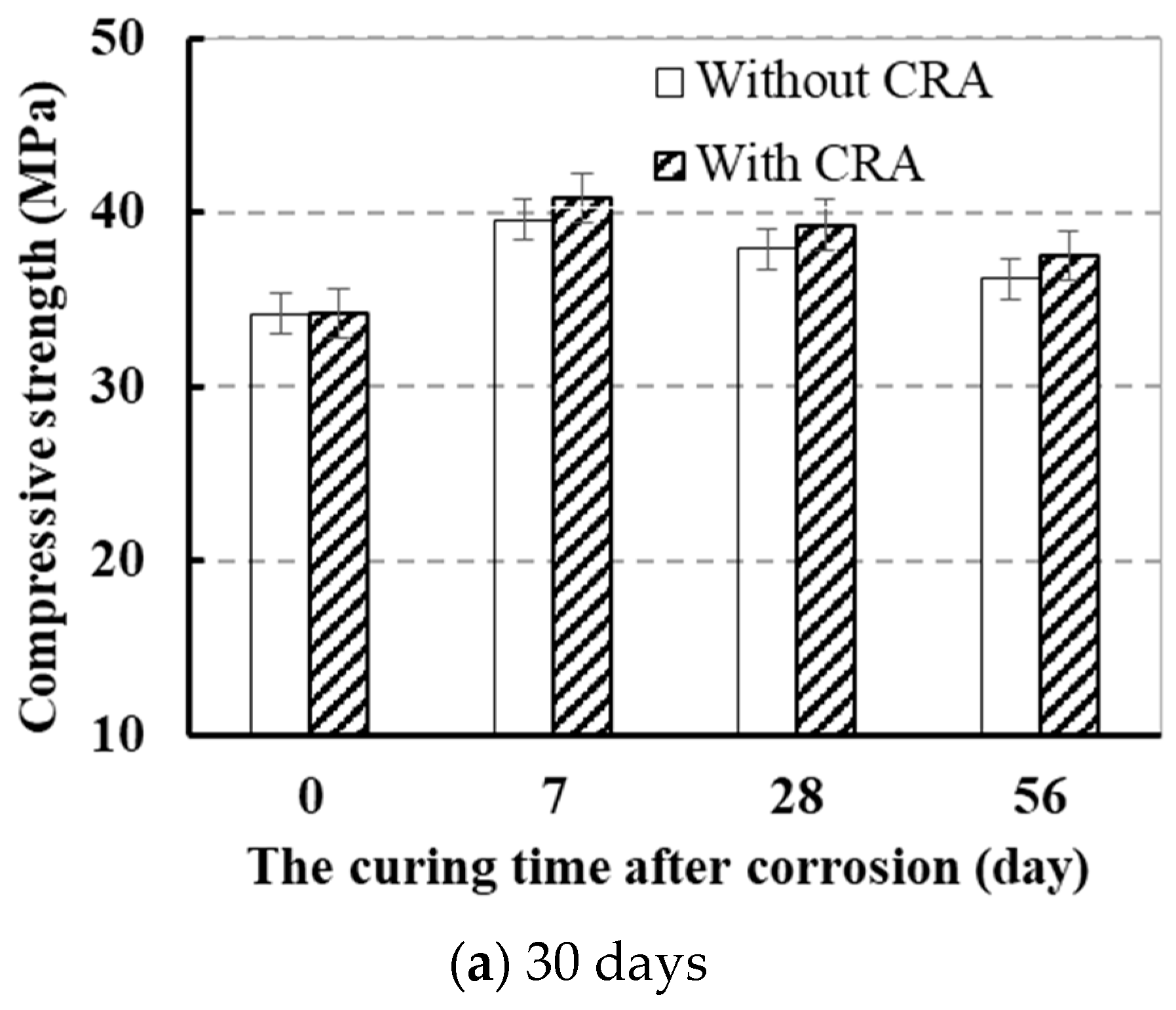
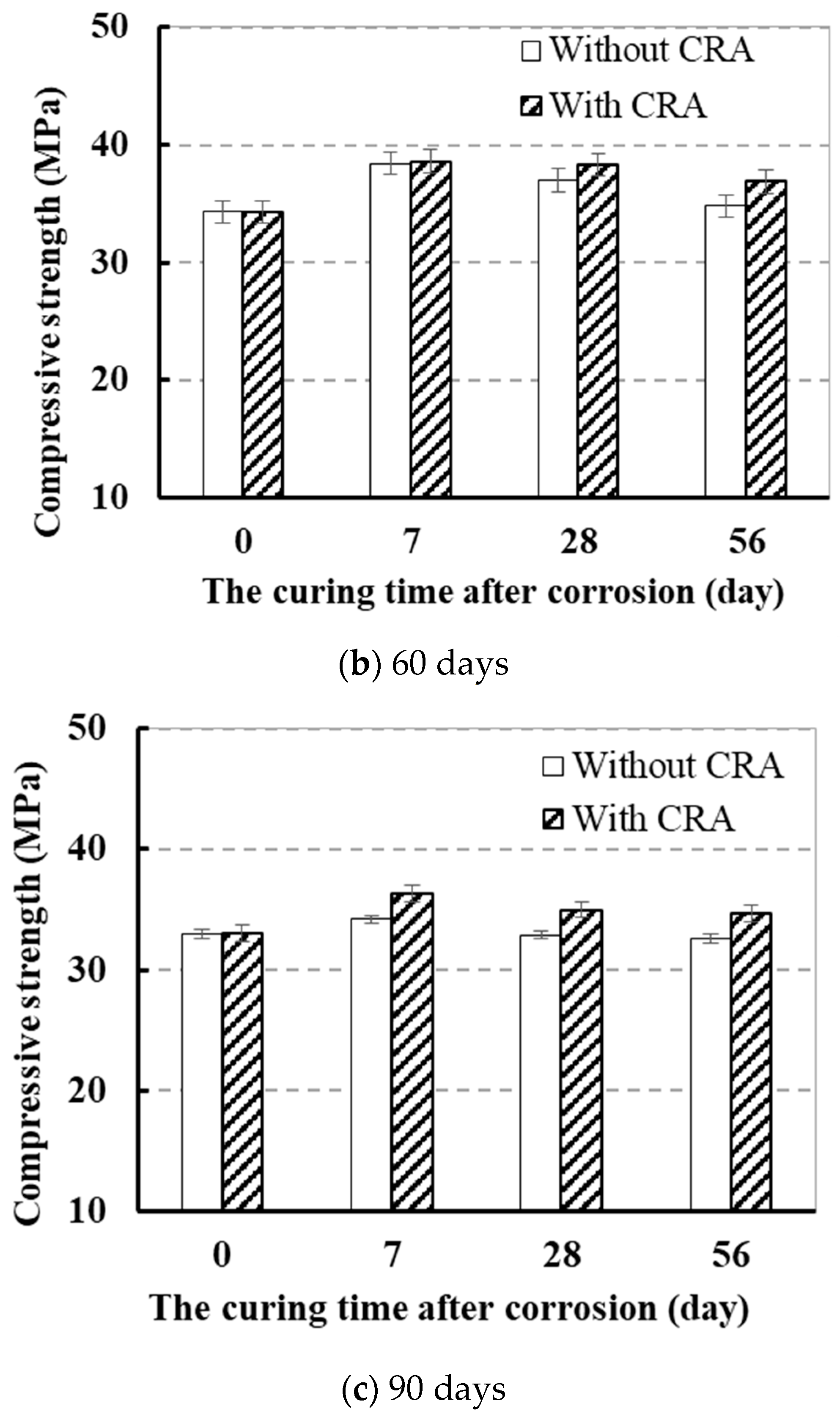
3.1.2. Effect of CRA on the Strength
3.1.3. Effect of Curing Time after Being Attacked in Sulfate
3.2. Anti-Carbonization Performance of Concrete
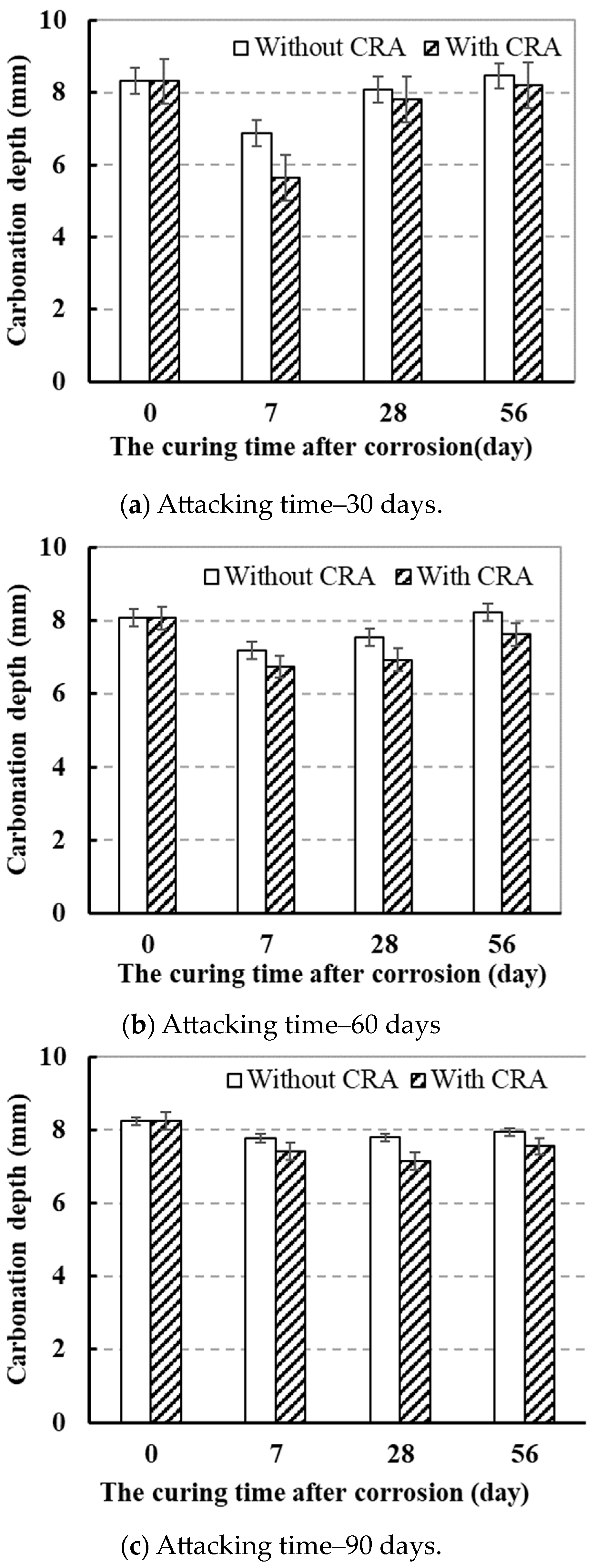
3.2.1. Effect of Sulfate Corrosion Time on Carbonation Resistance of Concrete
3.2.2. Effect of CRA on Carbonation Resistance of Concrete
3.3. Chloride Ion Permeation Resistance of Concrete
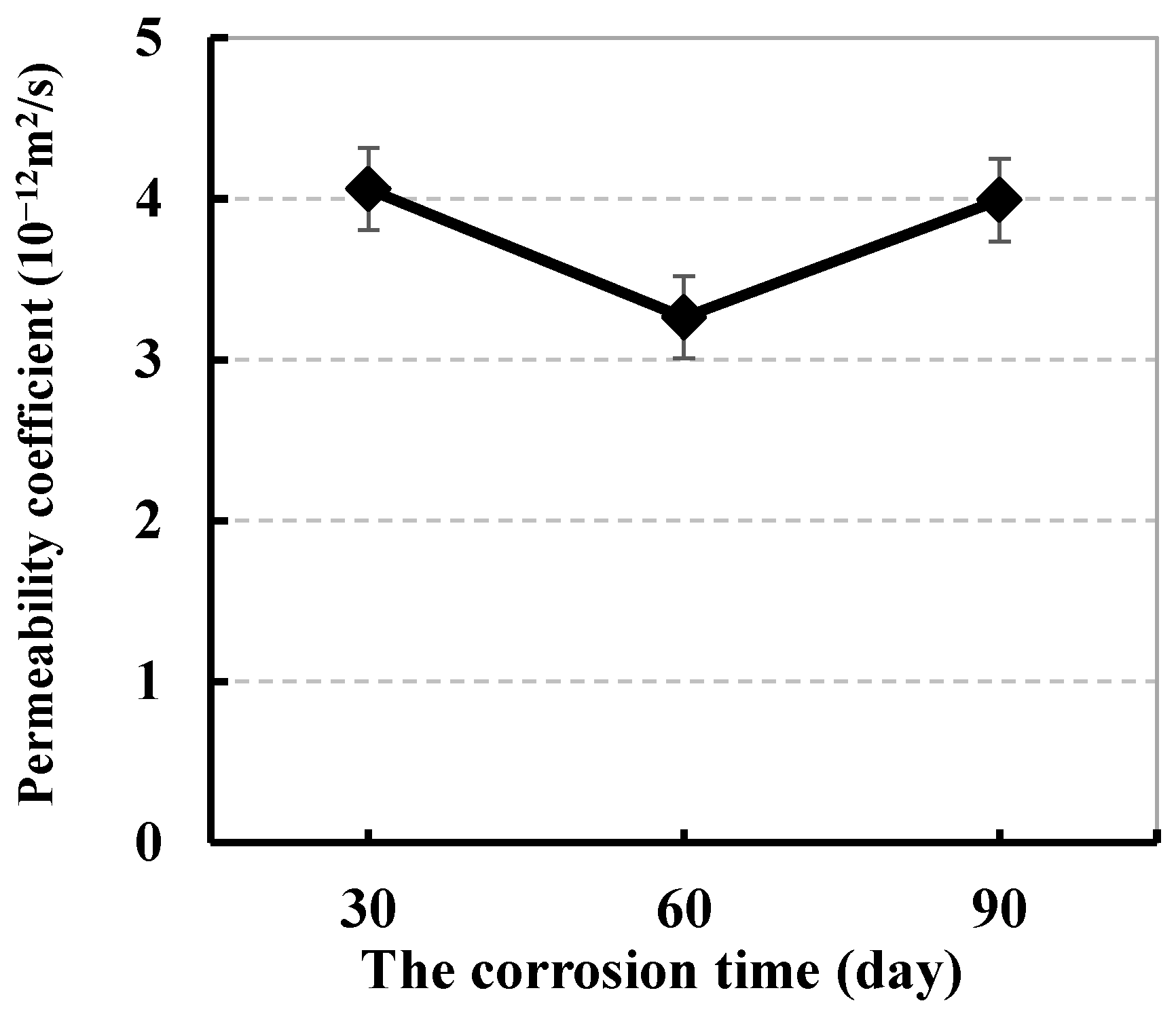
3.3.1. Effect of Sulfate Corrosion Time on Chloride Ion Permeation Resistance
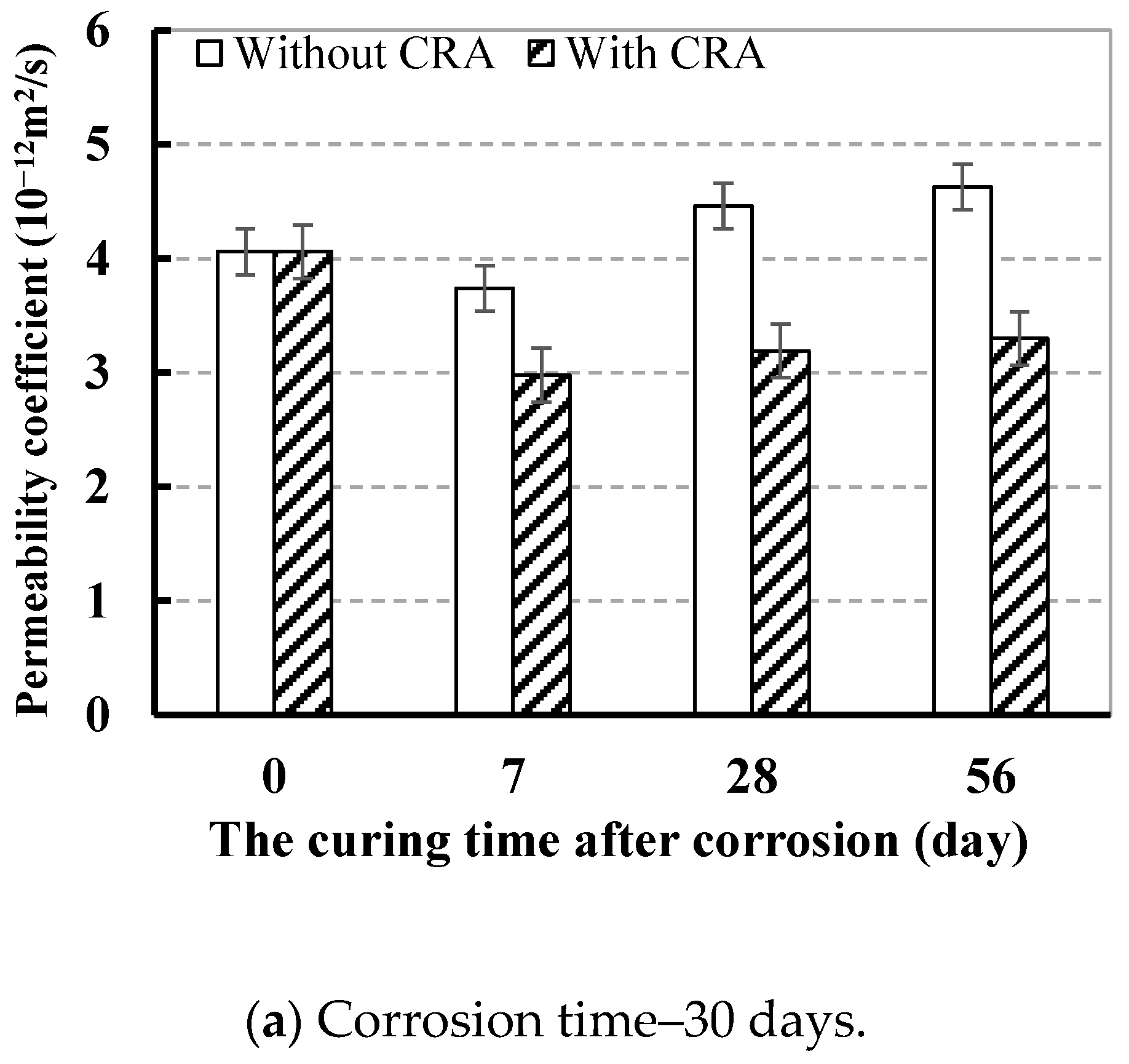
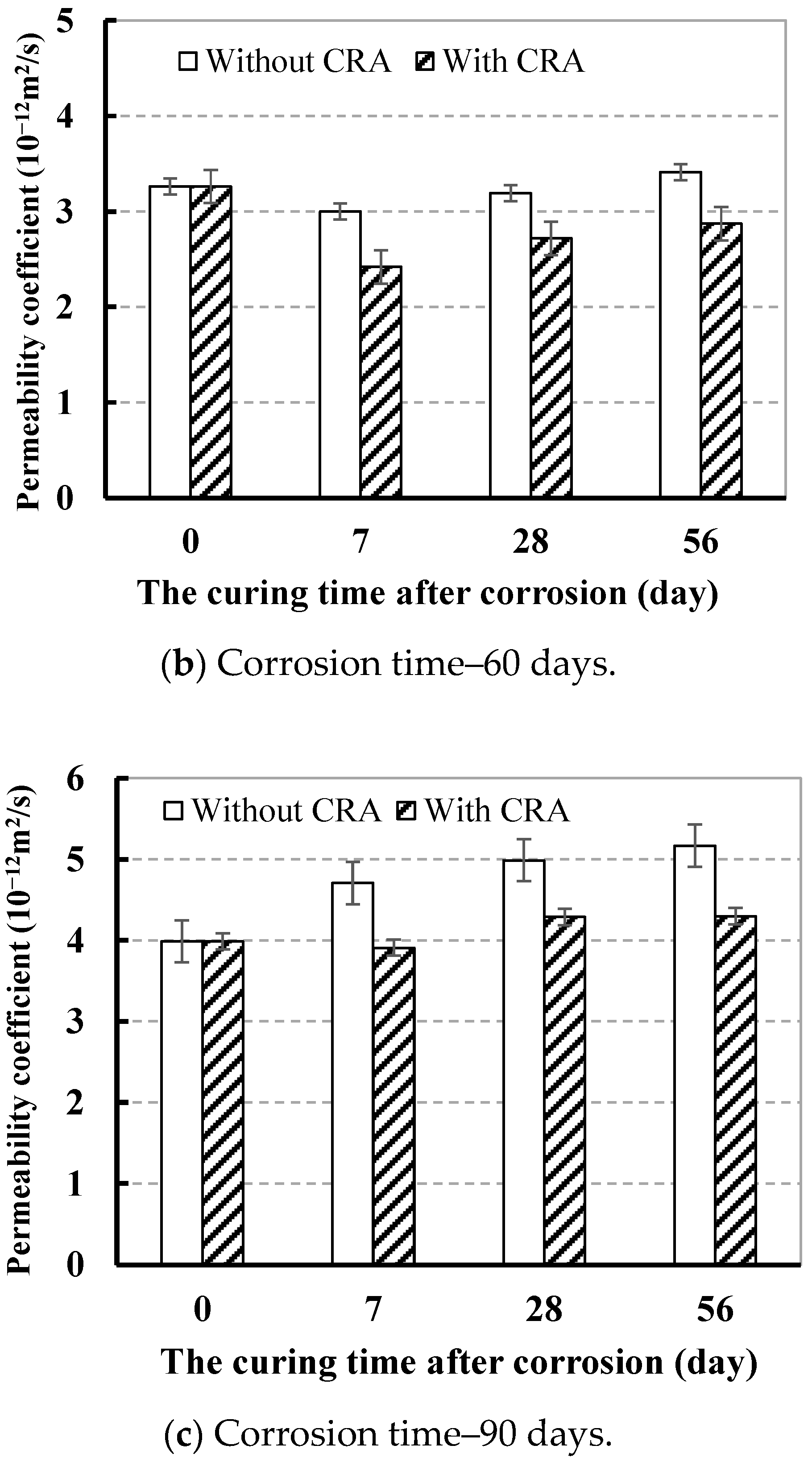
3.3.2. Effect of CRA on Chloride Ion Permeation Resistance
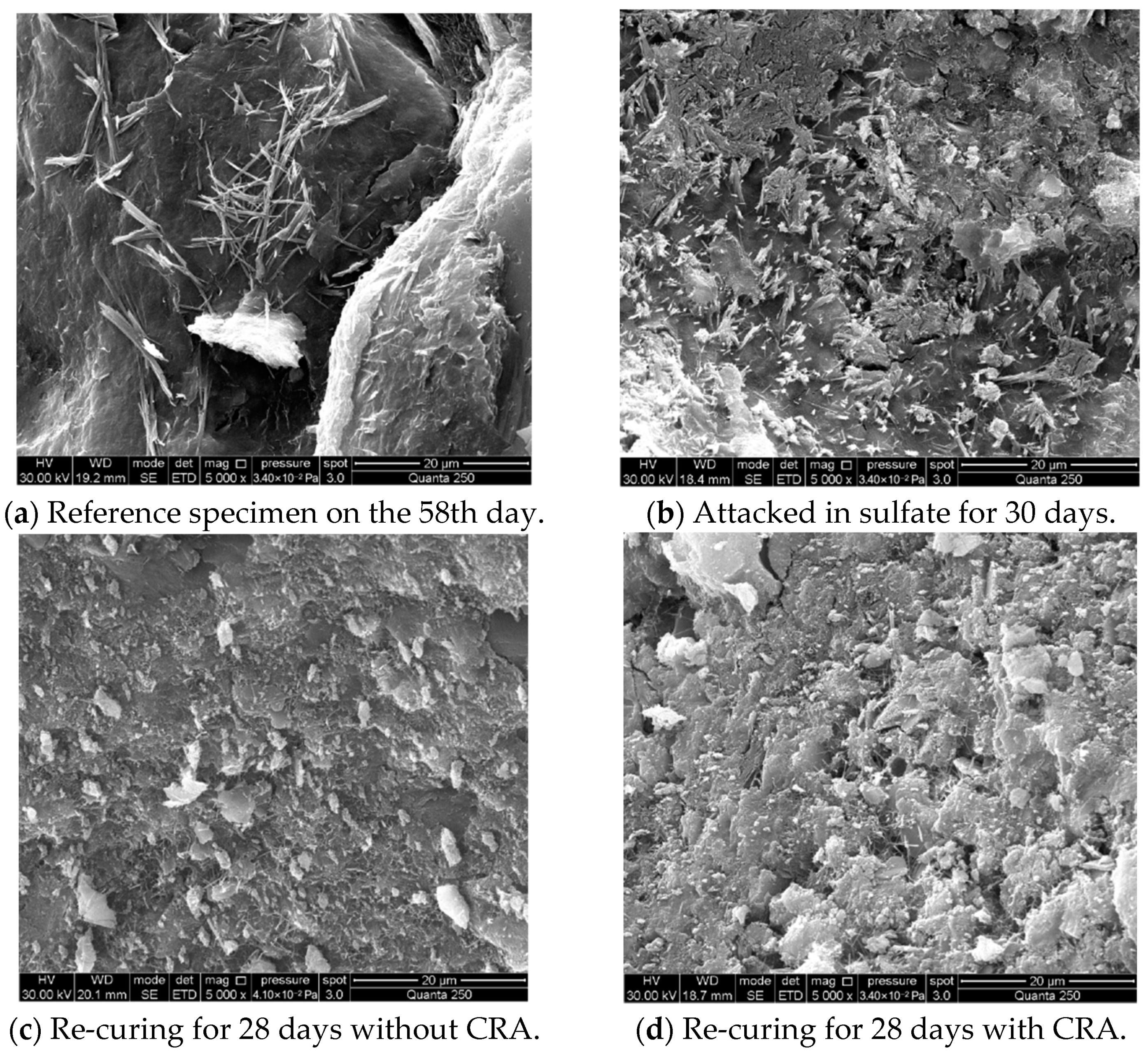
3.4. SEM Analysis
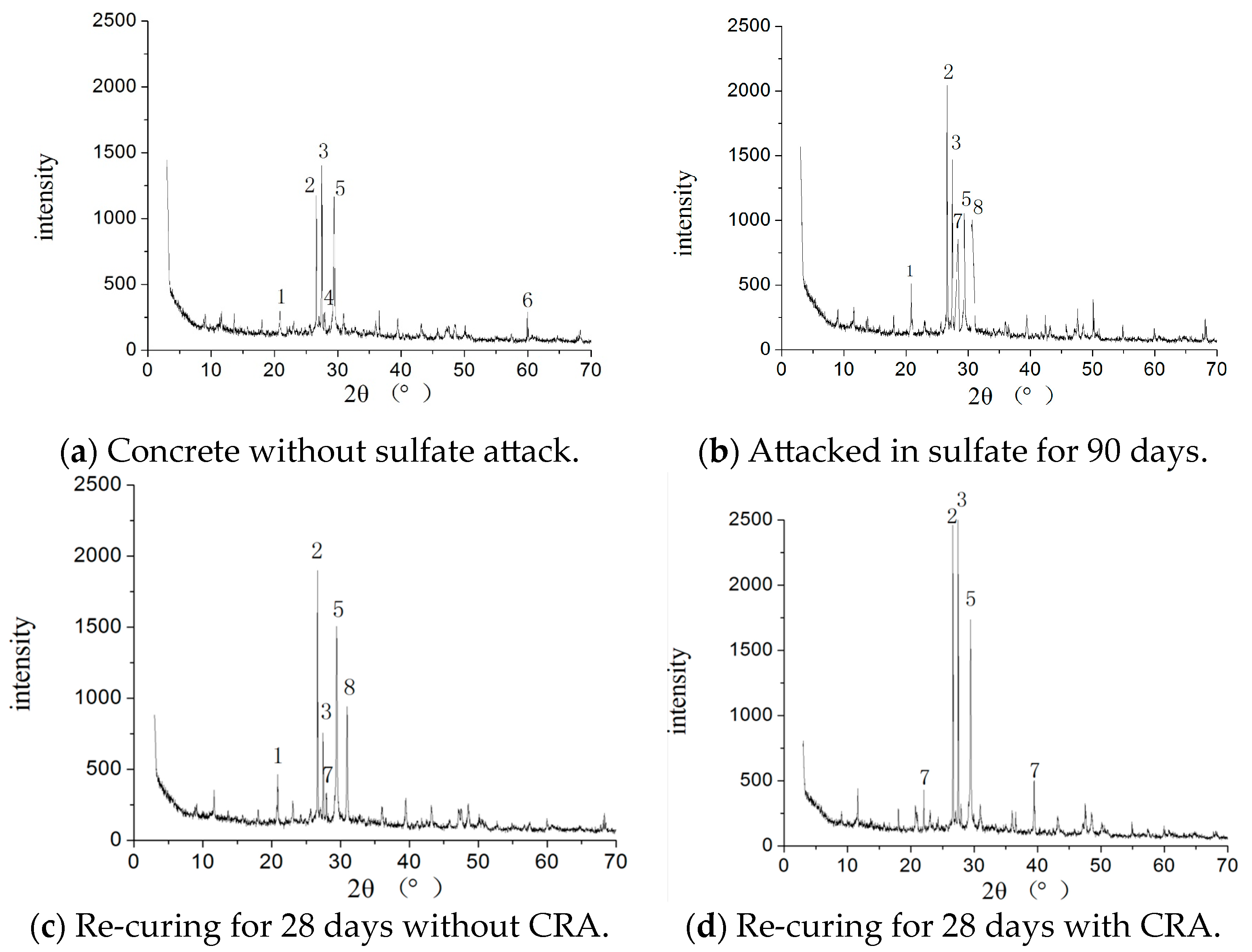
3.5. XRD Analysis
4. Conclusions
- (1)
- The strength and durability of concrete attacked for 60 days were higher than that of the concrete attacked for 30 days. While the sulfate corrosion time increased to 90 days, the strength of sulfate-attacked concrete decreased significantly.
- (2)
- CRA could effectively improve the compressive strength, enhance the carbonation resistance, and reduce the chloride diffusion coefficient of sulfate-attacked concrete.
- (3)
- The strength of attacked concrete coated with CRA increased by 6.1% more than that of the concrete without CRA.
- (4)
- The carbonation depth of concrete specimens with CRA decreased by 4.6% compared to concrete specimens without CRA.
- (5)
- The relative chloride ion permeation coefficient of concrete with CRA decreased by 14.0% more than that of the concrete without CRA.
- (6)
- Even if the concrete was removed from the sulfate environment, the compressive strength and durability of sulfate-attacked concrete were still affected by sulfate ions and continued to decrease along with the increase in re-curing time.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, B.; Gulzar, M.A.; Raza, A. Effect of sulfate activation of fly ash on mechanical and durability properties of recycled aggregate concrete. Constr. Build. Mater. 2021, 277, 122329. [Google Scholar] [CrossRef]
- Stroh, J.; Schlegel, M.-C.; Irassar, E.; Meng, B.; Emmerling, F. Applying high resolution SyXRD analysis on sulfate attacked concrete field samples. Cem. Concr. Res. 2014, 66, 19–26. [Google Scholar] [CrossRef]
- Campos, A.; López, C.M.; Aguado, A. Diffusion–reaction model for the internal sulfate attack in concrete. Constr. Build. Mater. 2016, 102, 531–540. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Y.; Tan, Z.; Zhang, B. Investigating the failure process of concrete under the coupled actions between sulfate attack and drying–wetting cycles by using X-ray CT. Constr. Build. Mater. 2016, 108, 129–138. [Google Scholar] [CrossRef]
- Martins, M.C.; Langaro, E.A.; Macioski, G.; Medeiros, M.H.F. External ammonium sulfate attack in concrete: Analysis of the current methodology. Constr. Build. Mater. 2021, 277, 122252. [Google Scholar] [CrossRef]
- Maes, M.; Belie, N.D. Resistance of concrete and mortar against combined attack of chloride and sodium sulphate. Cem. Concr. Compos. 2014, 53, 59–72. [Google Scholar] [CrossRef]
- Ouyang, W.Y.; Chen, J.K.; Jiang, M.Q. Evolution of surface hardness of concrete under sulfate attack. Constr. Build. Mater. 2014, 53, 419–424. [Google Scholar] [CrossRef]
- Jiang, L.; Niu, D.T. Study of deterioration of concrete exposed to different types of sulfate solutions under drying-wetting cycles. Constr. Build. Mater. 2016, 117, 88–98. [Google Scholar] [CrossRef]
- Jiang, L.; Niu, D.; Yuan, L.; Fei, Q. Durability of concrete under sulfate attack exposed to freeze–thaw cycles. Cold Reg. Sci. Technol. 2015, 112, 112–117. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Nikolopoulou, E.; Tsivilis, S. Sulfate resistance of limestone cement concrete exposed to combined chloride and sulfate environment at low temperature. Cem. Concr. Compos. 2012, 34, 903–910. [Google Scholar] [CrossRef]
- Skaropoulou, A.; Sotiriadis, K.; Kakali, G.; Tsivilis, S. Use of mineral admixtures to improve the resistance of limestone cement concrete against thaumasite form of sulfate attack. Cem. Concr. Compos. 2013, 37, 267–275. [Google Scholar] [CrossRef]
- Sotiriadis, K.; Nikolopoulou, E.; Tsivilis, S.; Pavlou, A.; Chaniotakis, E.; Swamy, R.N. The effect of chlorides on the thaumasite form of sulfate attack of limestone cement concrete containing mineral admixtures at low temperature. Constr. Build. Mater. 2013, 43, 156–164. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Yuan, G.; Shu, Q. The effect of a proprietary inorganic coating on compressive strength and carbonation depth of simulated fire-damaged concrete. Mag. Concr. Res. 2013, 65, 651–659. [Google Scholar] [CrossRef]
- Li, Q.T.; Liu, L.; Yuan, G. Improvement of bond behaviour between steel bar and concrete subjected to elevated temperature at early age. Mag. Concr. Res. 2018, 70, 885–893. [Google Scholar] [CrossRef]
- Scarfato, P.; Di Maio, L.; Fariello, M.L.; Russo, P.; Incarnato, L. Preparation and evaluation of polymer/clay nanocomposite surface treatments for concrete durability enhancement. Cem. Concr. Compos. 2012, 34, 297–305. [Google Scholar] [CrossRef]
- Nosouhian, F.; Mostofinejad, D.; Hasheminejad, H. Influence of biodeposition treatment on concrete durability in a sulphate environment. Biosyst. Eng. 2015, 133, 141–152. [Google Scholar] [CrossRef]
- Suleiman, A.R.; Soliman, A.M.; Nehdi, M.L. Effect of surface treatment on durability of concrete exposed to physical sulfate attack. Constr. Build. Mater. 2014, 73, 674–681. [Google Scholar] [CrossRef]
- Joshi, S.; Goyal, S.; Mukherjee, A.; Sudhakara Reddy, M. Protection of concrete structures under sulfate environments by using calcifying bacteria. Constr. Build. Mater. 2019, 209, 156–166. [Google Scholar] [CrossRef]
- GB/T 50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. MOC: Beijing, China, 2019.
- GB/T 50082-2009; Standard for Test Method of Long-Term Performance and Furability of Ordinary Concrete. MOC: Beijing, China, 2009.
- Haufe, J.; Vollpracht, A. Tensile strength of concrete exposed to sulfate attack. Cem. Concr. Res. 2019, 116, 81–88. [Google Scholar] [CrossRef]
- Ragoug, R.; Metalssi, O.O.; Barberon, F.; Torrenti, J.M.; Roussel, N.; Divet, L.; Lacaillerie, J.B.E. Durability of cement pastes exposed to external sulfate attack and leaching: Physical and chemical aspects. Cem. Concr. Res. 2019, 116, 134–145. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, T.; Zou, D.; Zhou, A. Compressive strength assessment of sulfate-attacked concrete by using sulfate ions distributions. Constr. Build. Mater. 2021, 293, 123550. [Google Scholar] [CrossRef]
- Burgos, D.M.; Guzmán, Á; Delvasto, S. Assessment of the performance of SCC incorporating volcanic materials in a sodium sulfate environment. Constr. Build. Mater. 2019, 195, 52–65. [Google Scholar] [CrossRef]
- Boudali, S.; Kerdal, D.; Ayed, K.; Abdulsalam, B.; Soliman, A. Performance of self-compacting concrete incorporating recycled concrete fines and aggregate exposed to sulphate attack. Constr. Build. Mater. 2016, 124, 705–713. [Google Scholar] [CrossRef]
- Schmidt, T.; Lothenbach, B.; Romer, M.; Neuenschwander, J.; Scrivener, K. Physical and microstructural aspects of sulfate attack on ordinary and limestone blended Portland cements. Cem. Concr. Res. 2009, 39, 1111–1121. [Google Scholar] [CrossRef]
- Wei, L.; Guang, J.X.; Ya, Z. Triaxial test on concrete material containing accelerators under physical sulphate attack. Constr. Build. Mater. 2019, 206, 641–654. [Google Scholar] [CrossRef]
- Liu, Z.; Heede, P.V.; Zhang, C.; Shi, X.; Wang, L.; Li, J.; Yao, Y.; Lothenbach, B.; Belie, N.D. Carbonation of blast furnace slag concrete at different CO2 concentrations: Carbonation rate, phase assemblage, microstructure and thermodynamic modelling. Cem. Concr. Res. 2023, 169, 107161. [Google Scholar] [CrossRef]
- Oliveira, R.L.N.; Bragança, M.O.G.; Junior, R.A.M. Effect of coarse aggregate size on corrosion of reinforced concrete exposed to carbonation and chloride ingress by electrochemical measurements. Constr. Build. Mater. 2022, 361, 129665. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Z.; Xiong, Y.; Liu, Z.; Zhang, W. Effect of imitation fair-faced curing and protective coating on the durability of concrete. J. Build. Eng. 2023, 63, 10554. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, H.; Zeze, A.L.P.; Liu, X.; Tao, M. Coating performance, durability and anti-corrosion mechanism of organic modified geopolymer composite for marine concrete protection. Cem. Concr. Compos. 2022, 129, 104495. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Zeng, Q.; Yang, C.; Luo, C.; Yang, K. Deterioration of mortars exposed to sulfate attack under electrical field. Constr. Build. Mater. 2016, 117, 121–128. [Google Scholar] [CrossRef]
- Brekailo, F.; Pereira, E.; Medeiros-Junior, R.A. Migration test as an accelerated methodology in concrete for evaluating sulfate attack by Na2SO4 and MgSO4. Constr. Build. Mater. 2023, 388, 131681. [Google Scholar] [CrossRef]
- Gurumoorthy, N.; Arunachalam, K. Micro and mechanical behaviour of treated used foundry sand. Constr. Build. Mater. 2016, 123, 184–190. [Google Scholar] [CrossRef]
- Gurumoorthy, N.; Arunachalam, K. Durability studies on concrete containing treated used foundry sand. Constr. Build. Mater. 2019, 201, 651–661. [Google Scholar] [CrossRef]



| Components/Property | Value |
|---|---|
| SiO2 | 22.86% |
| Al2O3 | 7.34% |
| Fe2O3 | 3.24% |
| CaO | 58.64% |
| MgO | 1.94% |
| SO3 | 2.02% |
| Na2O | 0.86% |
| K2O | 0.76% |
| MnO | 0.07% |
| P2O5 | 0.06% |
| Cl | 0.029% |
| Ignition loss | 2.14% |
| Specific gravity | 3.14 |
| Specific surface (cm2/g) | 3280 |
| Content | Property |
|---|---|
| Main components | Propylene glycol (1.0%) Aluminate solution (0.3%) Na2O·nSiO2 (30%) Water (68%) |
| Appearance | Clear transparent liquid |
| Odors | Inodorous |
| Specific gravity | 1.23 |
| Solid content | 31.1% |
| pH | 11.27 |
| Kinematic viscosity | <5.70 cSt |
| w/c Ratio | Mixture Proportion (kg/m3) | ||||
|---|---|---|---|---|---|
| Water | Cement | Sand | Crushed Stone | Superplasticizer | |
| 0.593 | 172 | 290 | 735 | 1102 | 2.65 |
| Sulfate Corrosion Time (Day) | Dosage of CRA (kg/m2) | Curing Time after Being Coated CRA (Day) |
|---|---|---|
| 30 | 0 | 0 |
| 30 | 0 | 7 |
| 30 | 0 | 28 |
| 30 | 0 | 56 |
| 30 | 0.6 | 7 |
| 30 | 0.6 | 28 |
| 30 | 0.6 | 56 |
| 60 | 0 | 0 |
| 60 | 0 | 7 |
| 60 | 0 | 28 |
| 60 | 0 | 56 |
| 60 | 0.6 | 7 |
| 60 | 0.6 | 28 |
| 60 | 0.6 | 56 |
| 90 | 0 | 0 |
| 90 | 0 | 7 |
| 90 | 0 | 28 |
| 90 | 0 | 56 |
| 90 | 0.6 | 7 |
| 90 | 0.6 | 28 |
| 90 | 0.6 | 56 |
| Corrosion Time (Day) | Dosage of CRA (kg/m2) | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|---|
| Without Sulfate | Curing Time after Being Attacked (Day) | |||||
| 0 | 7 | 28 | 56 | |||
| 30 | 0 | 33.2 | 34.2 | 39.6 | 37.9 | 36.2 |
| 60 | 33.8 | 34.3 | 38.4 | 37.0 | 34.8 | |
| 90 | 34.2 | 33.0 | 34.2 | 32.9 | 32.6 | |
| 30 | 0.6 | 33.2 | 34.2 | 40.8 | 39.3 | 37.5 |
| 60 | 33.8 | 34.3 | 38.6 | 38.3 | 36.9 | |
| 90 | 34.2 | 33.0 | 36.3 | 35.0 | 34.7 | |
| Corrosion Time (Day) | Dosage of CRA (kg/m2) | Carbonization Depth (mm) | ||||
|---|---|---|---|---|---|---|
| Without Sulfate | Curing Time after Being Attacked (Day) | |||||
| 0 | 7 | 28 | 56 | |||
| 30 | 0 | 10.02 | 8.31 | 6.88 | 8.09 | 8.46 |
| 60 | 9.74 | 8.07 | 7.19 | 7.55 | 8.23 | |
| 90 | 9.37 | 8.24 | 7.77 | 7.79 | 7.94 | |
| 30 | 0.6 | 10.02 | 8.31 | 5.63 | 7.82 | 8.20 |
| 60 | 9.74 | 8.07 | 6.74 | 6.93 | 7.62 | |
| 90 | 9.37 | 8.24 | 7.41 | 7.14 | 7.55 | |
| Corrosion Time (Day) | Dosage of CRA (kg/m2) | Chloride Ion Permeation Coefficient (10–12 m2/s) | ||||
|---|---|---|---|---|---|---|
| Without Sulfate | Curing Time after Being Attacked (Day) | |||||
| 0 | 7 | 28 | 56 | |||
| 30 | 0 | 4.75 | 4.06 | 3.74 | 4.46 | 4.63 |
| 60 | 3.82 | 3.26 | 3.00 | 3.19 | 3.41 | |
| 90 | 3.49 | 3.99 | 4.71 | 4.99 | 5.17 | |
| 30 | 0.6 | 4.75 | 4.06 | 2.98 | 3.19 | 3.30 |
| 60 | 3.82 | 3.26 | 2.42 | 2.72 | 2.87 | |
| 90 | 3.49 | 3.99 | 3.91 | 4.29 | 4.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Li, J. Enhancement of Compressive Strength and Durability of Sulfate-Attacked Concrete. Buildings 2024, 14, 2187. https://doi.org/10.3390/buildings14072187
Han M, Li J. Enhancement of Compressive Strength and Durability of Sulfate-Attacked Concrete. Buildings. 2024; 14(7):2187. https://doi.org/10.3390/buildings14072187
Chicago/Turabian StyleHan, Meiqin, and Jianguo Li. 2024. "Enhancement of Compressive Strength and Durability of Sulfate-Attacked Concrete" Buildings 14, no. 7: 2187. https://doi.org/10.3390/buildings14072187
APA StyleHan, M., & Li, J. (2024). Enhancement of Compressive Strength and Durability of Sulfate-Attacked Concrete. Buildings, 14(7), 2187. https://doi.org/10.3390/buildings14072187





