Assessing the Suitability of Phosphate Waste Rock as a Construction Aggregate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
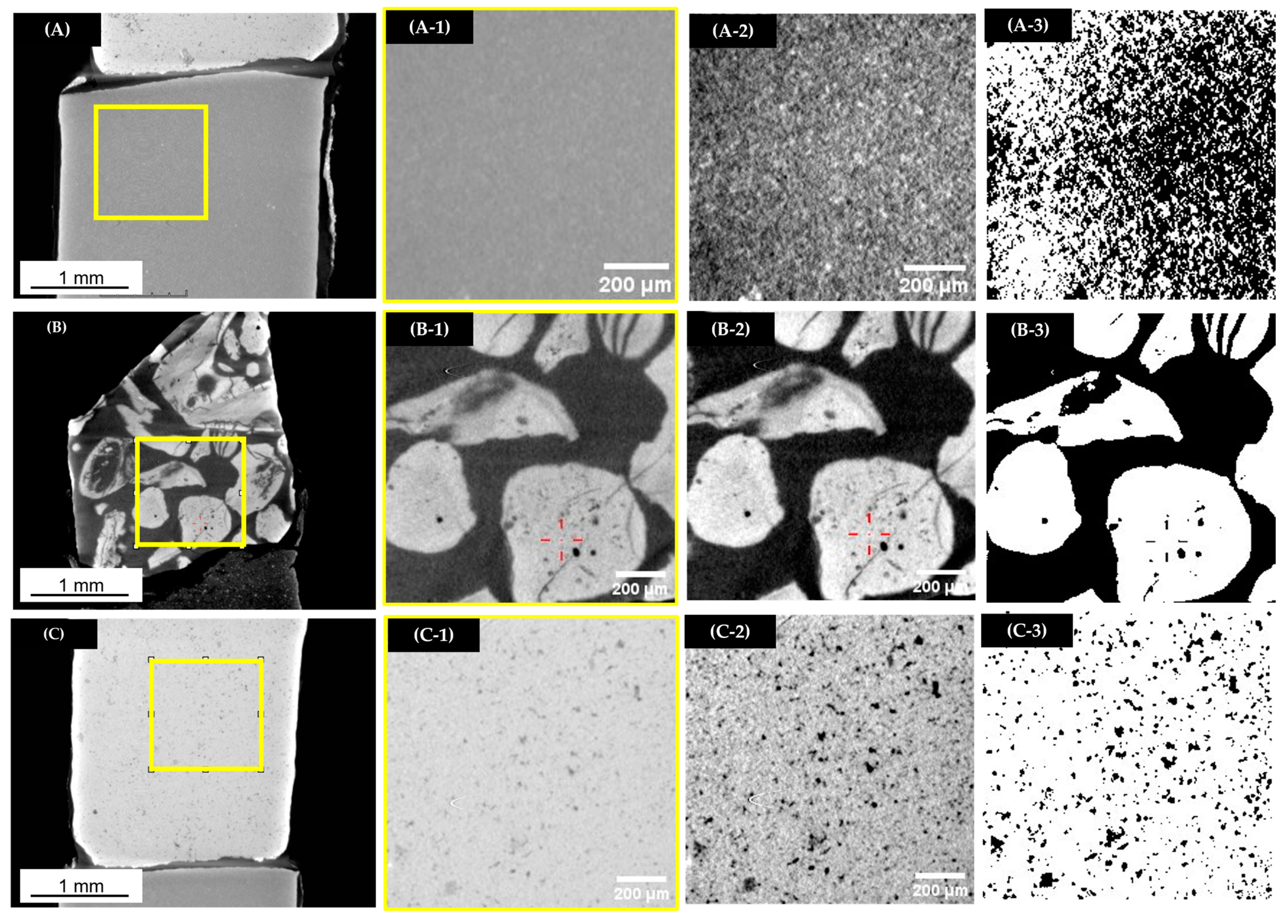
2.2.1. Microscopic Observations
2.2.2. Chemical and Mineralogical Compositions
2.2.3. Geotechnical Properties
2.2.4. X-ray Computed Tomography
| Properties | Test | Method | Equations | Testing measures | |
|---|---|---|---|---|---|
| Geometric properties | Flakiness coefficient | NF EN 933-3 [26] | (1) | A: global coefficient of flakiness (%). M2: sum of weight of aggregates passing through grids of Di/2 (g). M1: sum of weight of fraction of aggregates di/Di (g). | |
| Intergranular porosity | NF EN 1097-3 [27] | (2) | : bulk density (t/m3). M1: weight of empty container (kg); M2: weight of container and sample (kg). V: volume of container (liter). v: intergranular porosity. : bulk density (Mg/m3). : real density (Mg/m3) according to NF EN 1097-6. | ||
| (3) | |||||
| Physical properties | Real dry density | NF EN 1097-6 [28] | (4) | : real dry density (t/m3). M1: weight of saturated surface dry aggregates in air (g). M2: weight of pycnometer with sample of saturated aggregates (g). M3: weight of pycnometer full of water only (g). M4: weight of sample of oven-dried aggregates in air (g). | |
| Coefficient of water absorption 1 | NF EN 1097-6 [28] | (5) | : coefficient of water absorption after imbibtion for 24 h (%). M1: weight of saturated surface dry aggregates in air (g). M4: weight of sample of aggregates dried in oven in air (g). | ||
| Absolute density | Helium pycnometer | (6) | : absolute density (t/m3). m: weight of sample (g); V: volume of sample (cm3). | ||
| Total porosity | (7) | p: total porosity. | |||
| Mechanical properties | Los Angeles resistance to fragmentation | NF EN 1097-2 [29] | (8) | LA: Los Angeles coefficient executed on G1 fraction (%). m: weight of sample retained by the 1.6 mm sieve after testing (g). | |
| Micro-Deval resistance to wear | NF EN 1097-1 [30] | (9) | MDE: micro-Deval coefficient with water executed on G1 fraction (%). m: weight of sample retained by the 1.6 mm sieve after testing (g). | ||
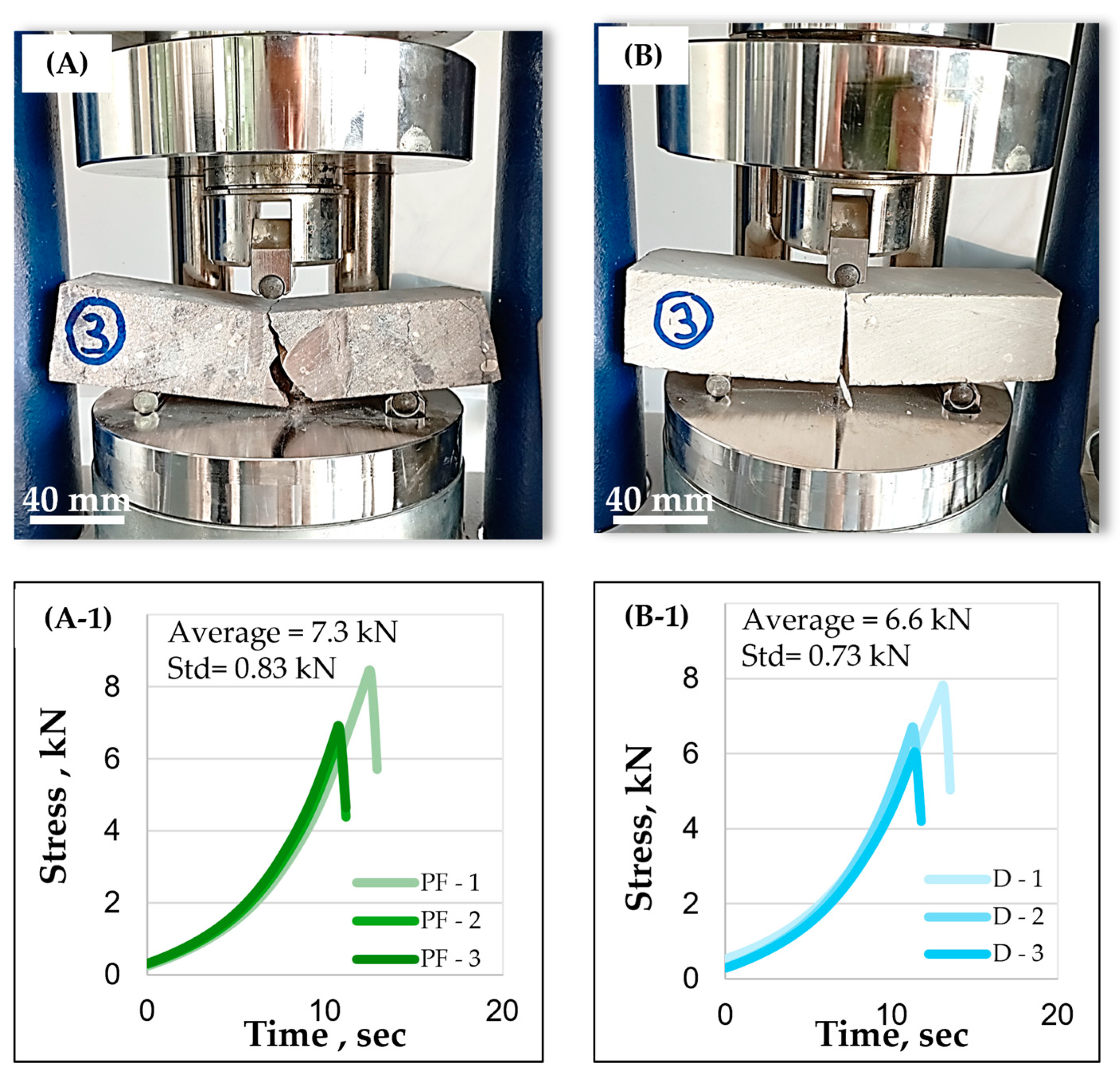
| Flexural strength | Prismatic rock specimen | (10) | : flexural strength (MPa). F: maximum force applied during the bending test (N). b: width of the specimen (m); h: height of the specimen (m). | ||
| Sand cleanliness | Sand equivalent | NF EN 933-8 [31] | (11) | h2: total height of suspension (mm). h1: height of sand sample (mm). | |
| Methylene blue test | NF EN 933-9 [32] | (12) | MB: value of methylene blue (g/kg). V1: total volume of injected solution (mL). M1: weight of test sample (g). | ||
3. Results
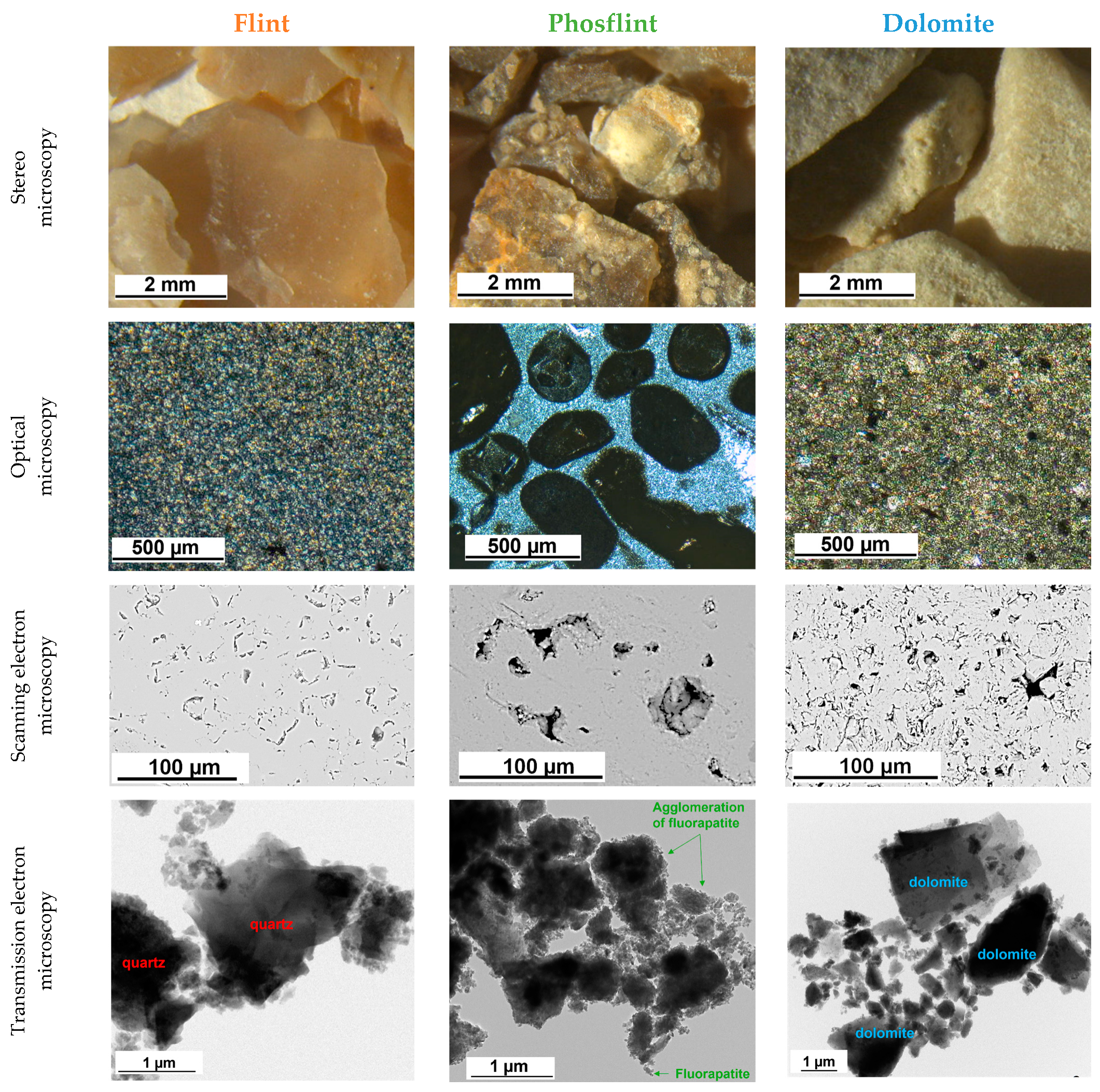
3.1. Microstructural Observations
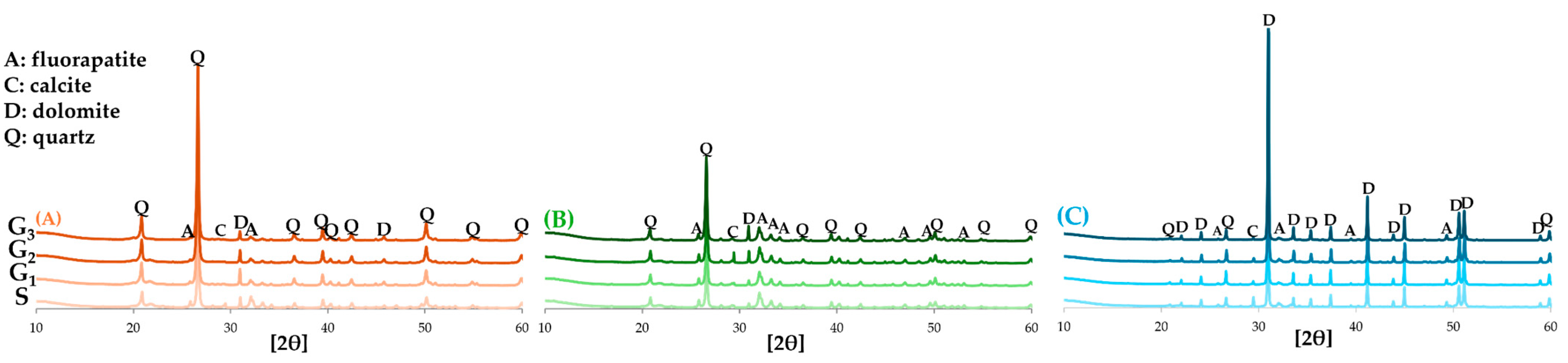
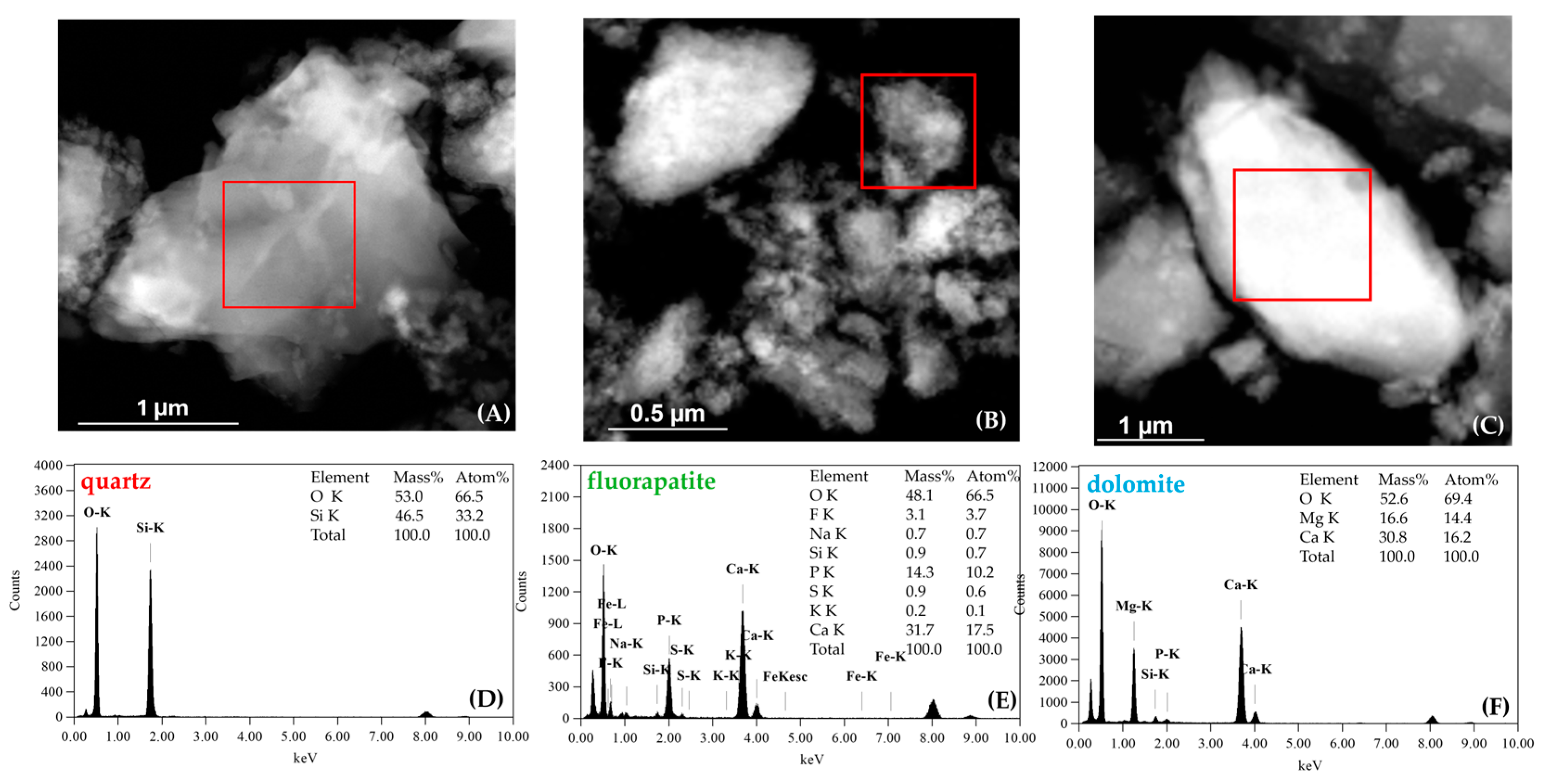
3.2. Mineralogical and Chemical Compositions
3.3. Geotechnical Properties
3.4. X-ray Computed Tomography
4. Discussion
5. Conclusions
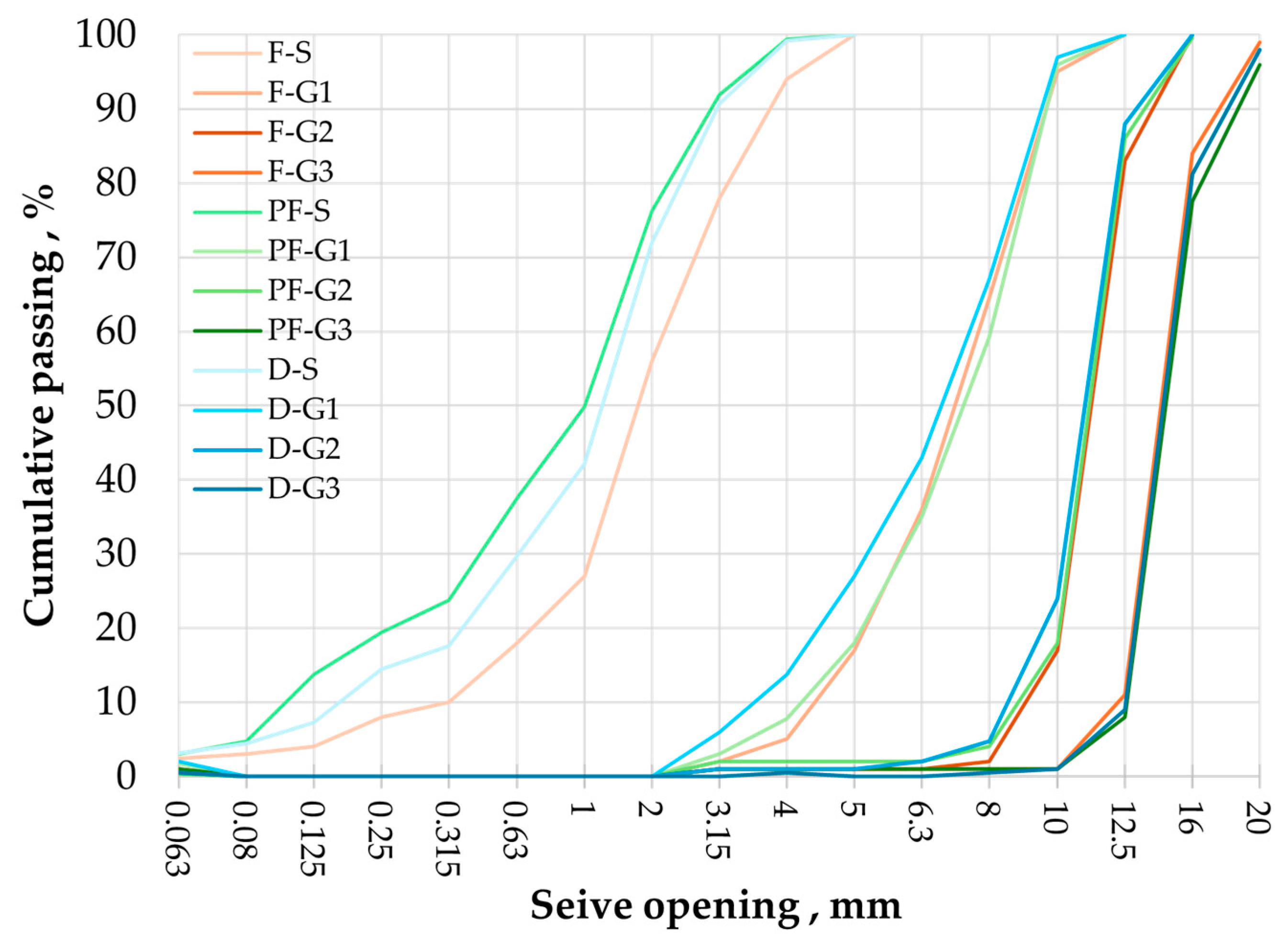
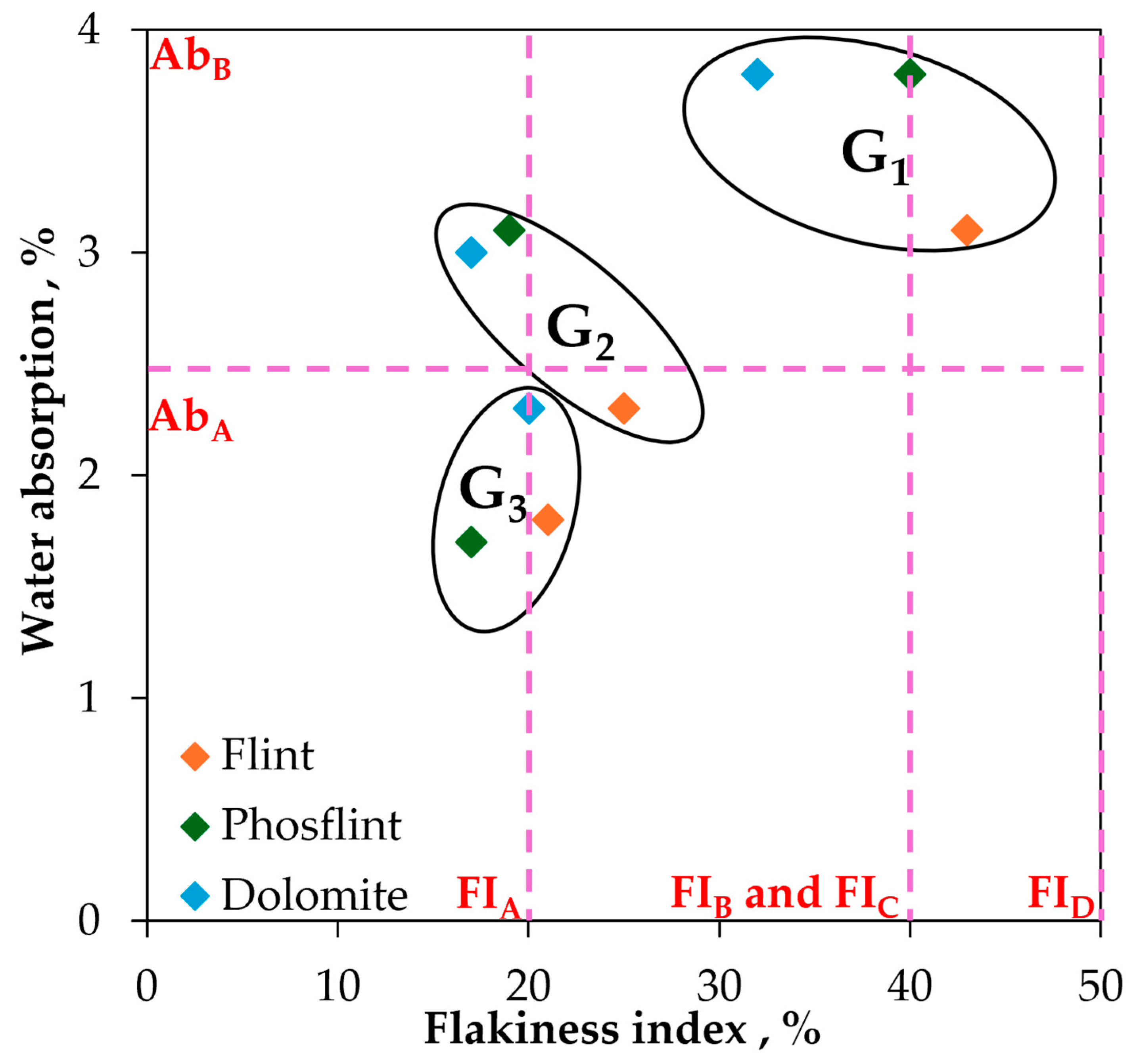
- Flint aggregates break into a conchoidal shape, which causes a very high flakiness index in smaller fractions (equal to 43% in the G1 fraction). Their irregular shape causes a very high intergranular porosity equal to 0.526. The total porosity is equal to 5.8% for the G3 fraction, which is similar to natural Flint aggregates. It is caused by very fine and vein-like pores that can be observed under SEM. Flint aggregates are very strong, as attested with an LA and MDE equal to 25% and 11%, respectively, placing them in code A for both these properties; they are suitable for the production of concrete with higher compressive strength. The surface texture is smooth and glassy, and the water absorption of the G1 fraction is quite high (equal to 3.1%); this potentially causes adherence issues and the high water demand for concrete.
- Phosflint aggregates are a virgin aggregate type constituted of phosphatic particles and encapsulated in silica-rich cement. They have received little interest for use as concrete aggregates because the P2O5 content is superior to 12%. However, the use of Phosflint in concrete is possible considering its geotechnical properties: the G3 fraction is represented by code A for water absorption, the flakiness index, and LA and MDE values. The flakiness index is high for the G1 fraction because of the brittleness of the rock. Even though it contains cryptocrystalline silica, the SiO2 content is inferior to Flint, and the surface texture is rougher than Flint, making it a better candidate.
- Dolomite aggregates have a higher real dry density equal to 2611 kg/m3 for the G3 fraction. They also possess important intrinsic strength with resistance to fragmentation LA that is equal to 32% and flexural strength on rock specimens equal to 16.1 MPa because they have a compact arrangement of Dolomite rhombs. Dolomite aggregates showcase a more regular shape and a lower flakiness index compared to other aggregates (flakiness index equal to 32% for the G1 fraction) and a rough surface texture. These properties are more favorable for concrete aggregates because they improve adherence with the cement paste and packing density. However, Dolomite possesses an important porosity, up to 12% of the total volume caused by closed pores between Dolomite rhombs, with an average surface area equal to 135 µm2. Dolomite aggregates possess the most favorable properties for use as concrete aggregates compared to Flint and to Phosflint.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, M.; Mindess, S. Aggregates in Concrete; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-203-96369-2. [Google Scholar]
- Smith, M.R.; Collis, L. (Eds.) 9. Aggregates for Mortar. In Aggregates: Sand, Gravel and Crushed Rock Aggregates for Construction Purposes; Geological Society of London: London, UK, 2001; Volume 17, ISBN 978-1-86239-079-9. [Google Scholar]
- Lavin, P. Asphalt Pavements: A Practical Guide to Design, Production and Maintenance for Engineers and Architects; CRC Press: London, UK, 2014; ISBN 978-0-429-17525-1. [Google Scholar]
- Přikryl, R. Constructional Geomaterials: Versatile Earth Resources in the Service of Humankind—Introduction to the Thematic Set of Papers on: Challenges to Supply and Quality of Geomaterials Used in Construction. Bull. Eng. Geol. Environ. 2017, 76, 1–9. [Google Scholar] [CrossRef]
- Přikryl, R. Geomaterials as Construction Aggregates: A State-of-the-Art. Bull. Eng. Geol. Environ. 2021, 80, 8831–8845. [Google Scholar] [CrossRef]
- Wang, B.; Yan, L.; Fu, Q.; Kasal, B. A Comprehensive Review on Recycled Aggregate and Recycled Aggregate Concrete. Resour. Conserv. Recycl. 2021, 171, 105565. [Google Scholar] [CrossRef]
- El Machi, A.; El Berdai, Y.; Mabroum, S.; Safhi, A.E.M.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Recycling of Mine Wastes in the Concrete Industry: A Review. Buildings 2024, 14, 1508. [Google Scholar] [CrossRef]
- Evangelista, L.; Guedes, M.; de Brito, J.; Ferro, A.C.; Pereira, M.F. Physical, Chemical and Mineralogical Properties of Fine Recycled Aggregates Made from Concrete Waste. Constr. Build. Mater. 2015, 86, 178–188. [Google Scholar] [CrossRef]
- Sánchez-Cotte, E.H.; Pacheco-Bustos, C.A.; Fonseca, A.; Triana, Y.P.; Mercado, R.; Yepes-Martínez, J.; Lagares Espinoza, R.G. The Chemical-Mineralogical Characterization of Recycled Concrete Aggregates from Different Sources and Their Potential Reactions in Asphalt Mixtures. Materials 2020, 13, 5592. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, A.; Ramalingam, M.; Kathirvel, P.; Murali, G.; Vatin, N.I. Mechanical, Physico-Chemical and Morphological Characterization of Energy Optimised Furnace (EOF) Steel Slag as Coarse Aggregate in Concrete. Materials 2022, 15, 3079. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, N.R.; Murmu, M. Alternative Coarse Aggregate for Sustainable and Eco-Friendly Concrete—A Review. J. Build. Eng. 2022, 59, 105079. [Google Scholar] [CrossRef]
- Taha, Y.; Elghali, A.; Hakkou, R.; Benzaazoua, M. Towards Zero Solid Waste in the Sedimentary Phosphate Industry: Challenges and Opportunities. Minerals 2021, 11, 1250. [Google Scholar] [CrossRef]
- Almeida, J.; Ribeiro, A.B.; Silva, A.S.; Faria, P. Overview of Mining Residues Incorporation in Construction Materials and Barriers for Full-Scale Application. J. Build. Eng. 2020, 29, 101215. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Abouzeid, A.-Z.M. An Environmental Solution for Phosphate Coarse Waste Reject-Using Them as Concrete Mix Aggregates. JES J. Eng. Sci. 2011, 39, 207–218. [Google Scholar] [CrossRef]
- Eljufout, T.; Alhomaidat, F. Utilizing Waste Rocks from Phosphate Mining in Jordan as Concrete Aggregates. Results Eng. 2024, 22, 102350. [Google Scholar] [CrossRef]
- El Machi, A.; Mabroum, S.; Taha, Y.; Tagnit-Hamou, A.; Benzaazoua, M.; Hakkou, R. Use of Flint from Phosphate Mine Waste Rocks as an Alternative Aggregates for Concrete. Constr. Build. Mater. 2021, 271, 121886. [Google Scholar] [CrossRef]
- Chlahbi, S.; Elghali, A.; Inabi, O.; Belem, T.; Zerouali, E.; Benzaazoua, M. Integrated Approach to Sustainable Utilization of Phosphate Waste Rock in Road Embankments: Experimental Insights, Stability Analysis, and Preliminary Economic Evaluation. Case Stud. Constr. Mater. 2024, 20, e03222. [Google Scholar] [CrossRef]
- Amar, H.; Benzaazoua, M.; Elghali, A.; Taha, Y.; El Ghorfi, M.; Krause, A.; Hakkou, R. Mine Waste Rock Reprocessing Using Sensor-Based Sorting (SBS): Novel Approach toward Circular Economy in Phosphate Mining. Miner. Eng. 2023, 204, 108415. [Google Scholar] [CrossRef]
- Safhi, A.e.M.; Amar, H.; El Berdai, Y.; El Ghorfi, M.; Taha, Y.; Hakkou, R.; Al-Dahhan, M.; Benzaazoua, M. Characterizations and Potential Recovery Pathways of Phosphate Mines Waste Rocks. J. Clean. Prod. 2022, 374, 134034. [Google Scholar] [CrossRef]
- NF EN 932-2; Essais Pour Déterminer les Propriétés Générales des Granulats—Partie 2: Méthodes de Réduction d’un Échantillon de Laboratoire. AFNOR: Paris, France, 1999; p. 16.
- NF P 18-545; Granulats, Éléments de Définition, Conformité et Codification. AFNOR: Paris, France, 2004; p. 59.
- Bruker X-ray Diffraction (XRD) DIFFRAC.EVA. Available online: https://www.bruker.com/en/products-and-solutions/diffractometers-and-x-ray-microscopes/x-ray-diffractometers/diffrac-suite-software/diffrac-eva.html (accessed on 3 July 2024).
- NF EN 12620; Granulats Pour Béton. AFNOR: Paris, France, 2003; p. 54.
- Zhang, C.; Jia, S.; Huang, X.; Shi, X.; Zhang, T.; Zhang, L.; Wang, F. Accurate Characterization Method of Pores and Various Minerals in Coal Based on CT Scanning. Fuel 2024, 358, 130128. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- NF EN 933-3; Essais Pour Déterminer les Caractéristiques Géométriques Des Granulats. Partie 3: Détermination de La Forme Des Granulats—Coefficient d’aplatissement. AFNOR: Paris, France, 1997.
- NF EN 1097-3; Essais Pour Déterminer les Caractéristiques Mécaniques et Physiques des Granulats—Partie 3: Méthode Pour la Détermination de la Masse Volumique en vrac et de la Porosité Intergranulaire. AFNOR: Paris, France, 1998.
- NF EN 1097-6; Essais Pour Déterminer les Caractéristiques Mécaniques et Physiques des Granulats—Partie 6: Détermination de la masse Volumique et du Coefficient d’absorption d’eau. AFNOR: Paris, France, 2022.
- NF EN 1097-2; Essais Pour Déterminer les Caractéristiques Mécaniques et Physiques des Granulats—Partie 2: Méthodes Pour la Détermination de la Résistance à la Fragmentation. AFNOR: Paris, France, 2010.
- NF EN 1097-1; Essais Pour Déterminer les Caractéristiques Mécaniques et Physiques Des Granulats. Partie 1: Détermination de La Résistance à l’usure (Micro-Deval). AFNOR: Paris, France, 1996.
- NF EN 933-8; Essais Pour Déterminer les Caractéristiques Géométriques des Granulats—Partie 8: Évaluation des Fines—Équivalent de Sable. AFNOR: Paris, France, 2015.
- NF EN 933-9; Essais Pour Déterminer les Caractéristiques Géométriques des Granulats—Partie 9: Qualification des Fines—Essais au Bleu de Méthylène. AFNOR: Paris, France, 2011.
- Boujou, A. Contribution à l’étude Géologique Du Gisement de Phosphate Crétacé-Éocène Des Ganntour (Maroc Occidental). Ph.D. Thesis, Université Louis-Pasteur, Strasbourg, France, 1976. [Google Scholar]
- El Haddi, H. The Silicification of Phosphate Series of Ouled Abdoun (Maastrichtian Lutetian-Morocco): Sedimentology, Mineralogy, Geochemistry and Genetic Context. Ph.D. Thesis, Faculté des Sciences Ben M’Sik, Université Hassan II de Casablanca, Casablanca, Morocco, 2014. [Google Scholar]
- Perkins, L.; Royal, A.C.D.; Jefferson, I.; Hills, C.D. The Use of Recycled and Secondary Aggregates to Achieve a Circular Economy within Geotechnical Engineering. Geotechnics 2021, 1, 416–438. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Tsikouras, B.; Pomonis, P.; Hatzipanagiotou, K. Determination of the Interrelations between the Engineering Parameters of Construction Aggregates from Ophiolite Complexes of Greece Using Factor Analysis. Constr. Build. Mater. 2013, 49, 747–757. [Google Scholar] [CrossRef]
- Teymen, A. Estimations of Los Angeles Abrasion Resistance of Igneous Rocks from Mechanical Aggregate Properties. Bull. Eng. Geol. Environ. 2019, 78, 837–846. [Google Scholar] [CrossRef]
- Jakobsen, F.; Lindgreen, H.; Nytoft, H.P. Oil-Impregnated Flint in Danian Chalk in the Tyra Field, North Sea Central Graben. J. Pet. Geol. 2014, 37, 43–53. [Google Scholar] [CrossRef]
- Aliyu, M.; Shang, J.; Murphy, W.; Lawrence, J.; Collier, R.; Kong, F.; Zhao, Z. Assessing the Uniaxial Compressive Strength of Extremely Hard Cryptocrystalline Flint. Int. J. Rock Mech. Min. Sci. 2019, 113, 310–321. [Google Scholar] [CrossRef]
- Rifa, A.; Subhani, S.M.; Bahurudeen, A.; Santhosh, K.G. A Systematic Comparison of Performance of Recycled Concrete Fine Aggregates with Other Alternative Fine Aggregates: An Approach to Find a Sustainable Alternative to River Sand. J. Build. Eng. 2023, 78, 107695. [Google Scholar] [CrossRef]
- Tanesi, J.; Bentz, D.P.; Jones, S.Z.; Beyene, M.; Kim, M.; Ardani, A.; Arnold, J.; Stutzman, P.E. Influence of Aggregate Properties on Concrete Mechanical Performance. In Proceedings of the Transportation Research Board 96th Annual Meeting, Washington, DC, USA, 8–12 January 2017; pp. 8–12. [Google Scholar]
- de Larrard, F.; Belloc, A. The Influence of Aggregate on the Compressive Strength of Normal and High-Strength Concrete. ACI Mater. J. 1997, 94, 417–426. [Google Scholar] [CrossRef]
- Kamani, M.; Ajalloeian, R. The Effect of Rock Crusher and Rock Type on the Aggregate Shape. Constr. Build. Mater. 2020, 230, 117016. [Google Scholar] [CrossRef]
- Hafeez, I.; Juniad, F.; Kamal, M.A.; Hussain, J. Influence of Single- and Two-Stage Aggregate Manufacturing Mechanisms on Asphalt Mixture Performance. J. Mater. Civ. Eng. 2016, 28, 04015180. [Google Scholar] [CrossRef]
- Fernandes, I.; Broekmans, M.A.T.M. Alkali–Silica Reactions: An Overview. Part I. Metallogr. Microstruct. Anal. 2013, 2, 257–267. [Google Scholar] [CrossRef]
- Sims, I.; Nixon, P. RILEM Recommended Test Method AAR0: Detection of Alkali-Reactivity Potential in Concrete—Outline Guide to the Use of RILEM Methods in Assessments of Aggregates for Potential Alkali-Reactivity. Mater. Struct. 2003, 36, 472–479. [Google Scholar] [CrossRef]






| Aggregates | SiO2 | CaO | MgO | P2O5 | Na2O | K2O | Al2O3 | Fe2O3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| F-S | 63.2 | 18.5 | 1.7 | 10.1 | 0.5 | 0.1 | 0.5 | 0.5 | 4.9 |
| F-G1 | 79.7 | 8.0 | 2.0 | 4.1 | 0.3 | 0.1 | 0.3 | 0.8 | 4.6 |
| F-G2 | 82.6 | 6.6 | 1.6 | 3.3 | 0.3 | 0.1 | 0.3 | 1.2 | 4.1 |
| F-G3 | 83.1 | 6.3 | 1.7 | 3.3 | 0.3 | 0.1 | 0.2 | 1.2 | 3.8 |
| PF-S | 44.7 | 29.6 | 1.4 | 16.7 | 0.6 | 0.1 | 0.3 | 0.4 | 6.3 |
| PF-G1 | 49.4 | 26.4 | 1.3 | 14.2 | 0.5 | 0.1 | 0.3 | 0.4 | 7.4 |
| PF-G2 | 49.4 | 26.2 | 1.7 | 14.1 | 0.5 | 0.1 | 0.2 | 0.3 | 7.5 |
| PF-G3 | 51.9 | 24.5 | 2.1 | 12.8 | 0.5 | 0.1 | 0.2 | 0.6 | 7.2 |
| D-S | 7.3 | 33.1 | 18.5 | 3.7 | 0.3 | 0.1 | 0.4 | 0.2 | 36.4 |
| D-G1 | 6.7 | 33.0 | 18.4 | 2.1 | 0.2 | 0.1 | 0.4 | 0.2 | 38.9 |
| D-G2 | 7.3 | 33.1 | 18.3 | 3.5 | 0.3 | 0.1 | 0.4 | 0.2 | 36.8 |
| D-G3 | 6.9 | 32.3 | 18.2 | 2.2 | 0.3 | 0.1 | 0.3 | 0.2 | 39.5 |
| Aggregates Fraction | Geometric Properties | Physical Properties | Mechanical Properties | Cleanliness Properties | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flakiness Index, % | Intergranular Porosity | Absolute Density, t/m3 | Real Dry Density, kg/m3 | Water Absorption, % | Total Porosity, % | Los Angeles, % | Micro-Deval, % | Flexural Strength, MPa | Sand Equivalent, % | Methylene Blue Value, g/kg | |
| F-S | – | 0.526 | 2.71 | 2440 | 4.8 | 10.0 | – | 83 | 0.75 | ||
| F-G1 | 43 | 0.573 | 2.66 | 2423 | 3.1 | 8.9 | 25 | 11 | |||
| F-G2 | 25 | 0.534 | 2.67 | 2467 | 2.3 | 7.6 | |||||
| F-G3 | 21 | 0.493 | 2.68 | 2525 | 1.8 | 5.8 | |||||
| PF-S | – | 0.484 | 2.80 | 2347 | 6.1 | 16.2 | 17.1 | 83 | 0.75 | ||
| PF-G1 | 40 | 0.579 | 2.78 | 2467 | 3.8 | 11.3 | 28 | 15 | |||
| PF-G2 | 19 | 0.544 | 2.78 | 2511 | 3.1 | 9.7 | |||||
| PF-G3 | 17 | 0.496 | 2.78 | 2586 | 1.7 | 7.0 | |||||
| D-S | – | 0.429 | 2.89 | 2157 | 7.1 | 25.4 | 16.1 | 78 | 1.25 | ||
| D-G1 | 32 | 0.541 | 2.89 | 2539 | 3.8 | 12.1 | 32 | 23 | |||
| D-G2 | 17 | 0.530 | 2.89 | 2570 | 3.0 | 11.1 | |||||
| D-G3 | 20 | 0.524 | 2.89 | 2611 | 2.3 | 9.7 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Berdai, Y.; Trauchessec, R.; Taha, Y.; Safhi, A.e.M.; Hakkou, R.; Benzaazoua, M. Assessing the Suitability of Phosphate Waste Rock as a Construction Aggregate. Buildings 2024, 14, 2375. https://doi.org/10.3390/buildings14082375
El Berdai Y, Trauchessec R, Taha Y, Safhi AeM, Hakkou R, Benzaazoua M. Assessing the Suitability of Phosphate Waste Rock as a Construction Aggregate. Buildings. 2024; 14(8):2375. https://doi.org/10.3390/buildings14082375
Chicago/Turabian StyleEl Berdai, Yahya, Romain Trauchessec, Yassine Taha, Amine el Mahdi Safhi, Rachid Hakkou, and Mostafa Benzaazoua. 2024. "Assessing the Suitability of Phosphate Waste Rock as a Construction Aggregate" Buildings 14, no. 8: 2375. https://doi.org/10.3390/buildings14082375
APA StyleEl Berdai, Y., Trauchessec, R., Taha, Y., Safhi, A. e. M., Hakkou, R., & Benzaazoua, M. (2024). Assessing the Suitability of Phosphate Waste Rock as a Construction Aggregate. Buildings, 14(8), 2375. https://doi.org/10.3390/buildings14082375








