Examination of the Release of Heavy Metals from Self-Hardening Slurries with Fly Ash from Municipal Sewage Sludge Incineration, Considering the Character of Its Operation in a Cut-Off Wall
Abstract
:1. Introduction
- -
- By-product utilization. It draws attention to the need for prudent use of waste in construction materials.
- -
- Waste management. It shows the possibility of using the waste produced in massive quantities.
- -
- Environmental protection. It points out the safe possibility of using SSA in the building material from the point of view of groundwater heavy metal contamination.
2. Leaching of Heavy Metals from Cement-Based Materials
- -
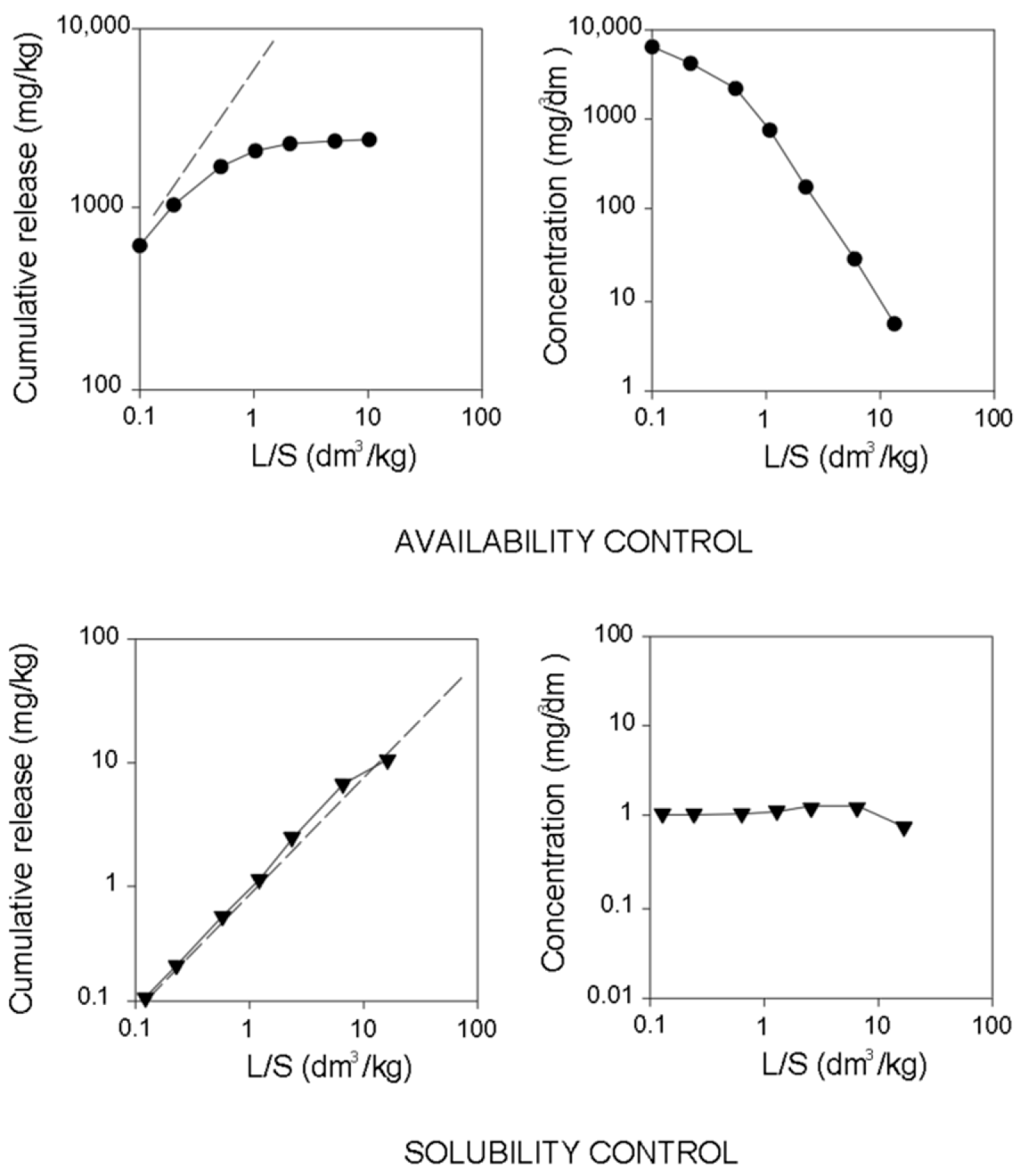
- Solubility control, the process which involves a disintegration of ionic lattices due to a solvent (e.g., ZnO + 2H+ ⇔ Zn2+ + H2O).
- -
- Adsorption processes, in which a layer of adsorbate (metal ions) is formed on the surface of adsorbents and forms a molecular or atomic film [32]. Adsorption is mainly classified into two types: physical adsorption and chemisorption [33]. For example, the major adsorption scheme of Cu2+ on the surface of alumina may be written as Cu2+ + AlOH ⇔ AlOCu+ + H+ [34].
- -
- Ion exchange (availability control, sometimes called wash-out), the process which may be defined as the exchange of ions between the substrate and surrounding medium [35]. Ion exchange can occur in the case of very easily soluble salts (such as NaCl), the release of which is not determined by the process of mineral solubility and adsorption [36]. As a result of contact with water, they are rapidly released in massive quantities.
- -
- Advection process, in which dissolved or precipitated chemicals are dynamically transported by a flowing liquid. The release of heavy metals can be a long-term process due to the material’s exposure to fresh portions of liquid. A special case of advection is percolation, which is when a fluid flows through a porous material. This process mainly applies to materials in a fragmented form. For example, groundwater flows through a porous soil medium.
- -
- Diffusion process, in which mass flow is caused by a concentration gradient generated in an isothermal single-phase system. Dissolved substances in a solvent spontaneously move from areas of higher concentration to regions of lower concentration, striving to reach equilibrium in the lack of fluid flow. The molecular cause of the diffusion phenomenon is the thermal movement of molecules. In this case, the form of the material does not matter.
- -
- Surface wash-off process, in which the release of heavy metals occurs at the initial stage of leaching when the material is exposed to a fresh portion of the liquid. The process applies to materials in a monolithic form. It occurs only when unbound substances are available on the surface and often results in high concentrations of heavy metals in the early fractions of the eluate. Once the surface is depleted, further leaching from the monolithic forms takes place by diffusion.
3. Materials
4. Methods and Procedures
4.1. Filtration Leaching Test
4.2. Release Mechanism
4.3. Determination of Heavy Metal Concentrations in Eluates
4.4. Statistical Analysis
4.5. Immobilization Level
5. Results and Discussion
5.1. Eluate Reaction and Specific Conductance
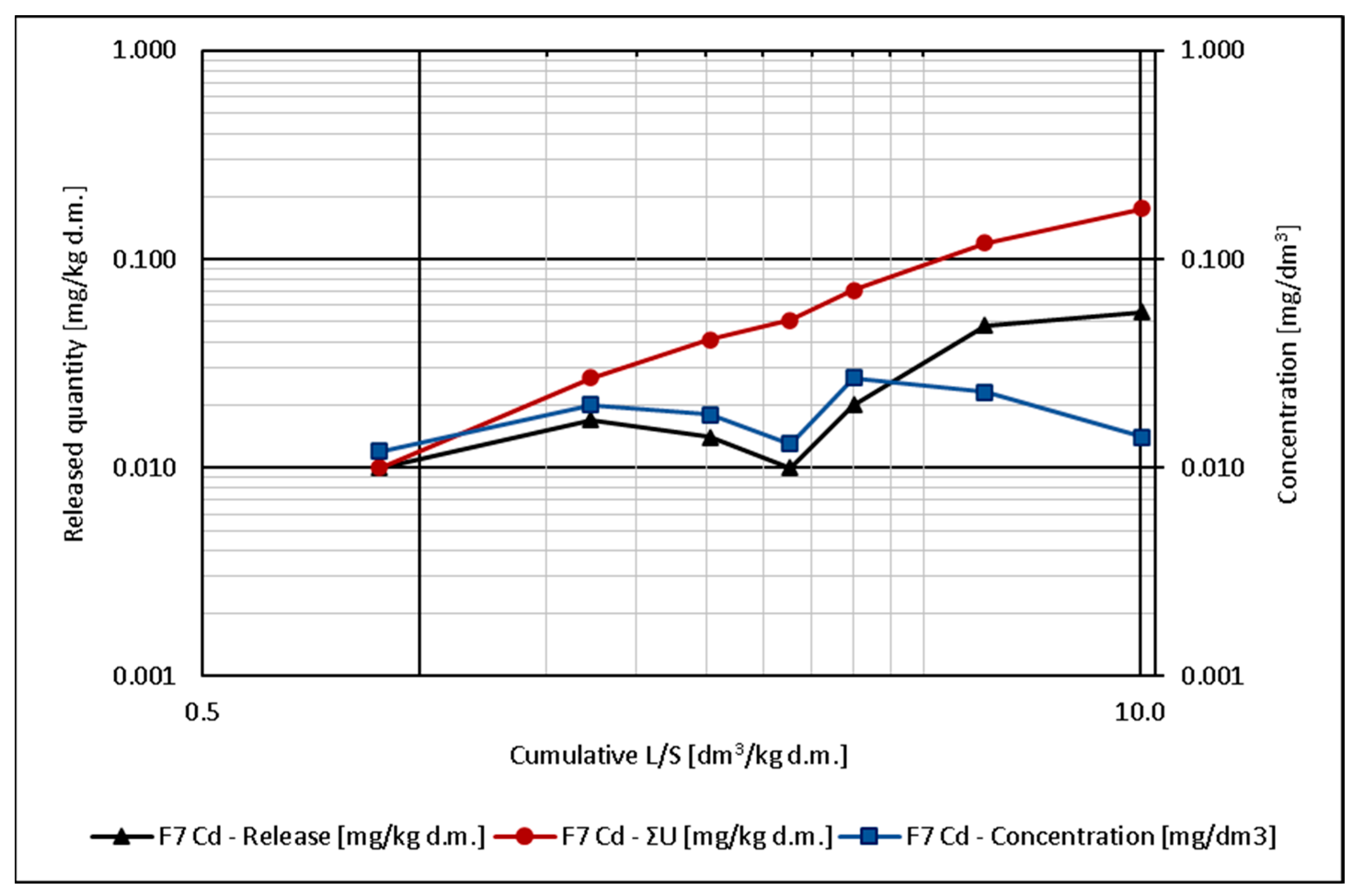
5.2. Heavy Metal Release
6. Conclusions
- (1)
- The filtration leaching test makes it possible to study the immobilization of heavy metals in SHSs in a monolithic form, considering the character of their work. It is a test that combines the features of static and dynamic testing due to the linear flow of the leaching liquid, which depends on the given pressure gradient, the properties of the leaching liquid and the properties of the sample itself.
- (2)
- The reaction of the obtained eluates from SHS samples was alkaline, similar to that of the pore solution in non-carbonated concrete. The reaction and specific conductivity of the obtained eluates depended on the L/S ratio (negative partial correlation and positive partial correlation, respectively) and the duration of the test (negative partial correlation in both cases).
- (3)
- All tested elements were immobilized at high levels. The highest level of immobilization was obtained for zinc (≥99.98%), and the lowest level was obtained for cadmium (≥98.18%).
- (4)
- A significant positive partial correlation was observed between chromium and zinc concentrations and the eluate’s pH, which agrees with the literature data. No significant correlation was observed for the other metals, which may be due to the narrow range of pH values of the obtained eluates. A significant partial negative correlation was observed between the cumulative L/S ratio and the concentration of cadmium and chromium. A significant positive partial correlation was observed between the test duration and cadmium concentration.
- (5)
- Due to the observed low concentrations of heavy metals in the eluates, analysis of the leaching mechanism was difficult. For cadmium, dissolution and wash-out occurred most likely; for chromium, dissolution; for lead, dissolution and wash-out; and for zinc, dissolution.
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Karwowska, E.; Łebkowska, M.; Tabernacka, A.; Andrzejewska, D. Eliminacja Metali Ciężkich z Popiołów z Użyciem Roztworów Ługujących Zawierających Bakterie Utleniające Siarkę Lub Bakterie Produkujące Biologiczne Substancje Powierzchniowo Czynne. Rocz. Ochr. Sr. 2011, 13, 1133–1154. [Google Scholar]
- Czerniak, A.; Poszyler-Adamska, A. Ocena Immobilizacji Metali Ciezkich w Kompozytach Cementowo-Gruntowych Stosowanych Do Budowy Drog Wiejskich. Acta Sci. Pol. Form. Circumiectus 2006, 5, 29–38. [Google Scholar]
- Poluszyńska, J.; Ślęzak, E. Charakterystyka Popiołów Ze Spalania Biomasy i Ocena Możliwości Ich Wykorzystania w Celach Przyrodniczych. Pr. Inst. Ceram. Mater. Bud. 2015, 8, 71–78. [Google Scholar]
- Kalarus, D.; Baran, T.; Ostrowski, M. Wpływ Składników Paliw Wtórnych Stosowanych Do Produkcji Klinkieru Portlandzkiego Na Wartość Emisji Metali Ciężkich z Cementu i Betonu. Pr. Inst. Ceram. Mater. Bud. 2016, 9, 7–17. [Google Scholar]
- Malviya, R.; Chaudhary, R. Leaching Behavior and Immobilization of Heavy Metals in Solidified/Stabilized Products. J. Hazard. Mater. 2006, 137, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, E.; Uliasz-Bocheńczyk, A.; Sarna, M. Use of Alternative Fuels in the Polish Cement Industry. Appl. Energy 2003, 74, 101–111. [Google Scholar] [CrossRef]
- Su, Y.; Yang, J.; Liu, D.; Zhen, S.; Lin, N.; Zhou, Y. Effects of Municipal Solid Waste Incineration Fly Ash on Solidification/Stabilization of Cd and Pb by Magnesium Potassium Phosphate Cement. J. Environ. Chem. Eng. 2016, 4, 259–265. [Google Scholar] [CrossRef]
- Çoruh, S.; Elevli, S.; Geyikçi, F. Statistical Evaluation and Optimization of Factors Affecting the Leaching Performance of Copper Flotation Waste. Sci. World J. 2012, 2012, 758719. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Analysis of Fly Ash Heavy Metal Content and Disposal in Three Thermal Power Plants in India. Fuel 2006, 85, 2676–2679. [Google Scholar] [CrossRef]
- Król, A. Uwalnianie Metali Ciężkich z Kompozytów Mineralnych z Uwzględnieniem Oddziaływania Środowiska; Oficyna Wydawnicza Politechniki Opolskiej: Opole, Poland, 2012; ISBN 8362736593. [Google Scholar]
- Szarek, Ł. Leaching of Heavy Metals from Thermal Treatment Municipal Sewage Sludge Fly Ashes. Arch. Environ. Prot. 2020, 46, 49–59. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage Sludge Combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Eurostat. Eurostat Sewage Sludge Production and Disposal from Urban Wastewater. Available online: https://ec.europa.eu/eurostat/databrowser/product/view/env_ww_spd (accessed on 4 June 2024).
- Commission Regulation Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture. 2006; 1881, 1–5.
- Bianchini, A.; Bonfiglioli, L.; Pellegrini, M.; Saccani, C. Sewage Sludge Management in Europe: A Critical Analysis of Data Quality. Int. J. Environ. Waste Manag. 2016, 18, 226–238. [Google Scholar] [CrossRef]
- Franz, M. Phosphate Fertilizer from Sewage Sludge Ash (SSA). Waste Manag. 2008, 28, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Mattenberger, H.; Fraißler, G.; Brunner, T.; Herk, P.; Hermann, L.; Obernberger, I. Sewage Sludge Ash to Phosphorus Fertiliser: Variables Influencing Heavy Metal Removal during Thermochemical Treatment. Waste Manag. 2008, 28, 2709–2722. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, Q.; Li, J.; Poon, C.S.; Cheeseman, C.R.; Donatello, S.; Tsang, D.C.W. Feasibility of Wet-Extraction of Phosphorus from Incinerated Sewage Sludge Ash (ISSA) for Phosphate Fertilizer Production: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 939–971. [Google Scholar] [CrossRef]
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Sewage Sludge Ash—A Promising Secondary Phosphorus Source for Fertilizer Production. Sci. Total Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Białowiec, A.; Janczukowicz, W.; Krzemieniewski, M. Possibilities of Management of Waste Fly Ashes from Sewage Sludge Thermal Treatment in the Aspect of Legal Regulations. Rocz. Ochr. Sr. 2009, 11, 959–971. [Google Scholar]
- Lam, C.H.; Barford, J.P.; McKay, G. Utilization of Incineration Waste Ash Residues in Portland Cement Clinker. Chem. Eng. 2010, 21, 757–762. [Google Scholar]
- Rutkowska, G.; Fronczyk, J.; Wichowski, P. Research on the Possibility of Using Fly Ashes from Combustion of Municipal Sewage Sludge on Properties of Ordinary Concretes. Rocz. Ochr. Sr. 2018, 20, 1113–1128. [Google Scholar]
- Rutkowska, G.; Chalecki, M.; Żółtowski, M. Fly Ash from Thermal Conversion of Sludge as a Cement Substitute in Concrete Manufacturing. Sustainability 2021, 13, 4182. [Google Scholar] [CrossRef]
- Rutkowska, G.; Żółtowski, M.; Rusakov, K.; Pawluk, K.; Andrzejak, J.; Żółtowski, B. The Influence of Fly Ash from Sewage Sludge on the Concrete Carbonation Course. Buildings 2023, 13, 1838. [Google Scholar] [CrossRef]
- Lin, D.F.; Chang, W.C.; Yuan, C.; Luo, H.L. Production and Characterization of Glazed Tiles Containing Incinerated Sewage Sludge. Waste Manag. 2008, 28, 502–508. [Google Scholar] [CrossRef]
- Wiśniewski, K.; Rutkowska, G.; Jeleniewicz, K.; Dąbkowski, N.; Wójt, J.; Chalecki, M.; Siwiński, J. The Impact of Fly Ashes from Thermal Conversion of Sewage Sludge on Properties of Natural Building Materials on the Example of Clay. Sustainability 2022, 14, 6213. [Google Scholar] [CrossRef]
- Borowski, B. Processing of Ashes from Sewage Sludge Combustion for Building Material. Inżynieria Ekol. 2011, 251–258. [Google Scholar]
- Huang, X.; Li, J.; Xue, Q.; Chen, Z.; Du, Y.; Wan, Y.; Liu, L.; Poon, C.S. Use of Self-Hardening Slurry for Trench Cutoff Wall: A Review. Constr. Build. Mater. 2021, 286, 122959. [Google Scholar] [CrossRef]
- Szarek, Ł.; Krysiak, Ł.; Kledyński, Z.; Machowska, A.; Falaciński, P. Durability, Carbon Footprint and Contaminant Immobilization in Self-Hardening Slurries Applied to Cut-Off Walls: A Review. Arch. Civ. Eng. 2023, 69, 5–29. [Google Scholar]
- Evans, J.C. Vertical Cutoff Walls. In Geotechnical Practice for Waste Disposal; Springer: Berlin/Heidelberg, Germany, 1993; pp. 430–454. [Google Scholar]
- Van der Sloot, H.A.; Dijkstra, J.J. Development of Horizontally Standardized Leaching Tests for Construction Materials: A Material Based or Release Based Approach?: Identical Leaching Mechanisms for Different Materials; ECN: Petten, The Netherlands, 2004. [Google Scholar]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of Heavy Metals on Conventional and Nanostructured Materials for Wastewater Treatment Purposes: A Review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Panday, K.K.; Prasad, G.; Singh, V.N. Copper (II) Removal from Aqueous Solutions by Fly Ash. Water Res. 1985, 19, 869–873. [Google Scholar] [CrossRef]
- Hubicki, Z.; Kołodyńska, D. Selective Removal of Heavy Metal Ions from Waters and Waste Waters Using Ion Exchange Methods. Ion Exch. Technol. 2012, 7, 193–240. [Google Scholar]
- Dijkstra, J.J.; Sloot, H.A.; Spanka, G.; Thielen, G. How to Judge Release of Dangerous Substances from Construction Products to Soil and Groundwater: CPD Topic 1-Soil and Groundwater Impact; ECN report number ECN-C-05; ECN: Petten, The Netherlands, 2005. [Google Scholar]
- Van der Sloot, H.A.; Mulder, E. Test Methods to Assess Environmental Properties of Aggregates in Different Applications: The Role of EN 1744-3. Energieonderzoek Cent. Ned. ECN 2002. [Google Scholar]
- Król, A. Problems of Assessment of Heavy Metals Leaching from Construction Materials to the Environment. Archit. Civ. Eng. Environ. 2011, 4, 71–76. [Google Scholar]
- Mizerna, K.; Król, A. Wpływ Wybranych Czynników Na Wymywalność Metali Ciężkich z Odpadu Hutniczego. Inżynieria Ekol. 2015, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Van der Sloot, H.A. Comparison of the Characteristic Leaching Behavior of Cements Using Standard (EN 196-1) Cement Mortar and an Assessment of Their Long-Term Environmental Behavior in Construction Products during Service Life and Recycling. Cem. Concr. Res. 2000, 30, 1079–1096. [Google Scholar] [CrossRef]
- Vollpracht, A.; Brameshuber, W. Binding and Leaching of Trace Elements in Portland Cement Pastes. Cem. Concr. Res. 2016, 79, 76–92. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mupatsi, N.M. Evaluation of Heavy Metal Leaching from Coal Ash-versus Conventional Concrete Monoliths and Debris. Waste Manag. 2016, 49, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Deja, J. Immobilization of Cr6+, Cd2+, Zn2+ and Pb2+ in Alkali-Activated Slag Binders. Cem. Concr. Res. 2002, 32, 1971–1979. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of Heavy Metal in Cement-Based Solidification/Stabilisation: A Review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef]
- Bańkowski, Z.; Achmatowiez, O.; Michalski, M.; Minczewski, T.; Orszagh, A. Encyklopedia Techniki Chemia; Gajewski, W., Ed.; Wydawnictwo Naukowo-Techniczne: Warsaw, Poland, 1965. [Google Scholar]
- Neville, A.M. Properties of Concrete; Longman: London, UK, 1995; Volume 4. [Google Scholar]
- Jennings, H.M. A Model for the Microstructure of Calcium Silicate Hydrate in Cement Paste. Cem. Concr. Res. 2000, 30, 101–116. [Google Scholar] [CrossRef]
- Kurdowski, W. Chemia Cementu i Betonu; Stowarzyszenie Producentów Cementu: Kraków, Poland, 2010; ISBN 839131524X. [Google Scholar]
- PKN PN-EN 197-1:2012; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2012.
- Kledyński, Z.; Falaciński, P.; Machowska, A.; Szarek, Ł.; Krysiak, Ł. Hardening Slurries with Fluidized-Bed Combustion By-Products and Their Potential Significance in Terms of Circular Economy. Materials 2021, 14, 2104. [Google Scholar] [CrossRef]
- BN-90/1785-01:1990; Płuczka Wiertnicza. Metody Badań w Warunkach Polowych. “Wydawnictwa Normalizacjne” ALFA: Warsaw, Poland, 1990.
- PN-EN 12390-3:2011; Badania Betonu—Część 3: Wytrzymałość Na Ściskanie Próbek Do Badań. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Falaciński, P.; Szarek, Ł. Possible Applications of Hardening Slurries with Fly Ash from Thermal Treatment of Municipal Sewage Sludge in Environmental Protection Structures. Arch. Hydro-Eng. Environ. Mech. 2016, 63, 47–61. [Google Scholar] [CrossRef]
- Szarek, Ł.; Wojtkowska, M. Properties of Fly Ash from Thermal Treatment of Municipal Sewage Sludge in Terms of EN 450-1. Arch. Environ. Prot. 2018, 44, 63–69. [Google Scholar] [CrossRef]
- Łukawska, M. Speciation Analysis of Phosphorus in Sewage Sludge after Thermal Utilization of Sludge. Inżynieria Ochr. Sr. 2014, 17, 433–439. [Google Scholar]
- Wzorek, Z. Odzysk Zwia̜zków Fosforu z Termicznie Przetworzonych Odpadów i Ich Zastosowanie Jako Substytutu Naturalnych Surowców Fosforowych; Politechnika Krakowska: Krakow, Poland, 2008. [Google Scholar]
- Van der Sloot, H.A.; Van Zomeren, A.; Meeussen, J.C.L.; Hoede, D.; Rietra, R.P.J.J.; Stenger, R.; Lang, T.; Schneider, M.; Spanka, G.; Stoltenberg-Hansson, E.; et al. Environmental Criteria for Cement Based Products; ECN: Petten, The Netherlands, 2011. [Google Scholar]
- CEN/TS 14405:2017; Characterization of Waste—Leaching Behaviour Tests—Up-Flow Percolation Test (under Specified Conditions). British Standards Institution: London, UK, 2017.
- EU Council Decision of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC. Available online: https://faolex.fao.org/docs/pdf/eur39228.pdf (accessed on 5 August 2024).
- Falaciński, P. Przepuszczalność Hydrauliczna Zawiesin Twardniejących z Dodatkiem Popiołów Fluidalnych; Politechnika Warszawska: Warsaw, Poland, 2006. [Google Scholar]
- Bourg, A.C.M.; Loch, J.P.G. Mobilization of Heavy Metals as Affected by PH and Redox Conditions. In Biogeodynamics of Pollutants in Soils and Sediments; Springer: Berlin/Heidelberg, Germany, 1995; pp. 87–102. [Google Scholar]
- Delay, M.; Lager, T.; Schulz, H.D.; Frimmel, F.H. Comparison of Leaching Tests to Determine and Quantify the Release of Inorganic Contaminants in Demolition Waste. Waste Manag. 2007, 27, 248–255. [Google Scholar] [CrossRef]
- Cyr, M.; Idir, R.; Escadeillas, G. Use of Metakaolin to Stabilize Sewage Sludge Ash and Municipal Solid Waste Incineration Fly Ash in Cement-Based Materials. J. Hazard. Mater. 2012, 243, 193–203. [Google Scholar] [CrossRef]
- Szarek, Ł.; Kledyński, Z. Censored Random Variable as a Form of Coping with Missing Data in Studying the Leachability of Heavy Metals from Hardening Slurries. Arch. Civ. Eng. 2021, 67, 233–247. [Google Scholar] [CrossRef]
- Chen, M.; Blanc, D.; Gautier, M.; Mehu, J.; Gourdon, R. Environmental and Technical Assessments of the Potential Utilization of Sewage Sludge Ashes (SSAs) as Secondary Raw Materials in Construction. Waste Manag. 2013, 33, 1268–1275. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. EU Landfill Waste Acceptance Criteria and EU Hazardous Waste Directive Compliance Testing of Incinerated Sewage Sludge Ash. Waste Manag. 2010, 30, 63–71. [Google Scholar] [CrossRef]
- Li, J.; Xue, Q.; Fang, L.; Poon, C.S. Characteristics and Metal Leachability of Incinerated Sewage Sludge Ash and Air Pollution Control Residues from Hong Kong Evaluated by Different Methods. Waste Manag. 2017, 64, 161–170. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
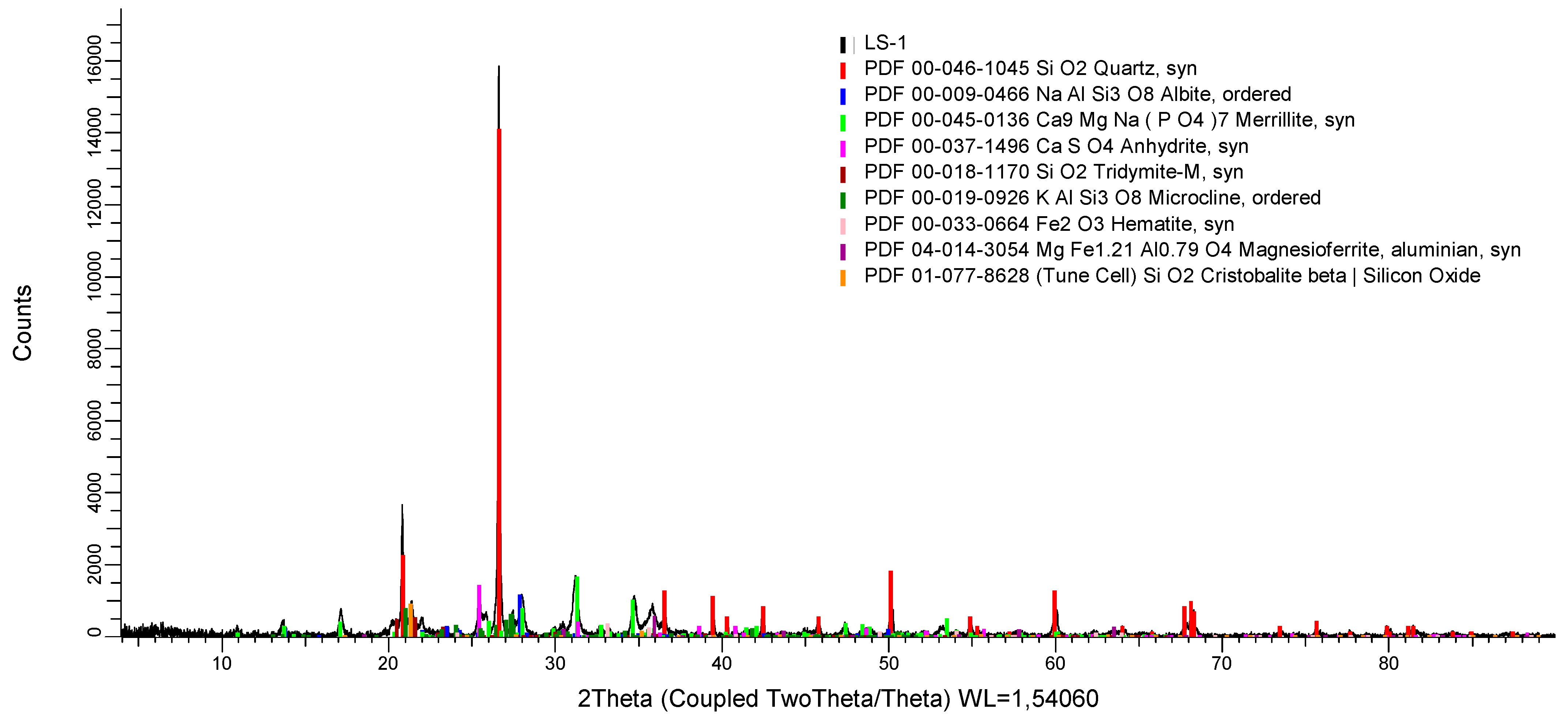

| Heavy Metal | Cement | Tires | CCFA 1 | BA 2 | MSWIFA 3 | SA 4 |
|---|---|---|---|---|---|---|
| Content [mg/kg] | ||||||
| Cd | 0.3–4.0 | 1–20 | 11–299 | 5.3–19.1 | 29.5–199.8 | 5.0–14 |
| Cr | 42–68 | 130–640 | 54–4697 | 44.3–281 | 320.7–598 | 119–179 |
| Cu | 11–68 | 10–300 | 33.4–83 | 133–342 | 480–1925.5 | 808–901 |
| Pb | <10–110 | 3–760 | 10–443 | 42.8–234 | 538.3–1540 | 60.4–83 |
| Zn | 47–615 | 1300–35,000 | 22.6–124 | 121–868 | 2835–11,324 | 2558–33,290 |
| Component | Content | Cd | Cr | Cu | Pb | Zn |
|---|---|---|---|---|---|---|
| [kg/m3 of Slurry] | [mg/kg Dry Matter] | |||||
| Tap water | 841.0 | − 1 | − 1 | − 1 | − 1 | − 1 |
| Sodium bentonite | 21.0 | − 1 | − 1 | − 1 | − 1 | − 1 |
| SSA | 84.0 | 14.0 ± 0.7 | 179 ± 9 | 808 ± 24 | 83.0 ± 6.5 | 3290 ± 83 |
| CEM I 32.5 R 2 | 380.0 | 10.8 ± 0.6 | 64.4 ± 4.2 | 120 ± 4 | 104 ± 7 | 804 ± 24 |
| SHS 3 | 1313 ± 32 | 9.6 ± 0.9 | 71.8 ± 6.8 | 206 ± 14 | 84.5 ± 8.8 | 1057 ± 66 |
| Property | Testing Method | Value |
|---|---|---|
| Conventional viscosity [s] | Marsh funnel viscosity test [51] | 50 ± 2 |
| Bleeding [%] | Bleed water test [51] | 3.9 ± 0.6 |
| Structural (gel) strength [Pa] | Shearometer test [51] | 5.2 ± 1.1 |
| Bulk density (in liquid state) [kg/m3] | Mud balance test [51] | 1332 ± 3 |
| Compressive strength [MPa] | Uniaxial compression strength test [52] | 1.80 ± 0.13 |
| Hydraulic conductivity (filtration coefficient k) [m/s] | Variable hydraulic gradient method [53] | (9.55 ± 7.20)·10−9 |
| Chemical Component/Physical Property | Value |
|---|---|
| % Mass | |
| Al2O3 | 18.1 ± 0.3 |
| Fe2O3 | 5.7 ± 0.3 |
| SiO2 + Al2O3 + Fe2O3 | 60.2 ± 1.3 |
| Total silicon dioxide SiO2 | 36.4 ± 1.2 |
| Sulfuric anhydride as SO3 | 2.78 ± 0.18 |
| Total calcium oxide CaO | 13.2 ± 1.8 |
| MgO | 4.15 ± 0.26 |
| Total phosphate P2O5 | 5.50 ± 1.02 mg/kg |
| Loss on ignition | 2.09 ± 0.07 |
| Fineness | 62.5 ± 4.0 |
| Activity index after 28 days of curing | 54.0 ± 3.4% |
| Water demand | 129 ± 1% |
| Particle density | 2263.7 ± 154.2 mg/m3 |
| Specific surface area by Blaine | 2860 ± 80 cm2/g |
| Heavy Metal | λ | Determination Limit |
|---|---|---|
| [nm] | [mg/dm3] | |
| Cd | 228.8 | 0.01 |
| Cr | 357.9 | 0.03 |
| Cu | 324.8 | 0.02 |
| Pb | 283.3 | 0.03 |
| Zn | 213.9 | 0.01 |
| Sample | Immobilization Level [%] | ||||
|---|---|---|---|---|---|
| Cd | Cr | Cu | Pb | Zn | |
| F1 | >98.95 | 99.30 | >99.90 | 98.31 | >99.99 |
| F2 | >98.85 | >99.25 | >99.90 | 98.38 | >99.99 |
| F3 | >98.93 | >99.47 | >99.90 | 98.57 | >99.99 |
| F4 | >98.91 | >99.43 | >99.90 | 98.47 | >99.99 |
| F5 | >98.92 | 99.36 | >99.90 | >98.62 | >99.99 |
| F6 | >98.90 | 99.44 | >99.90 | 98.61 | >99.99 |
| F7 | 98.18 | 98.93 | >99.90 | 98.56 | 99.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szarek, Ł. Examination of the Release of Heavy Metals from Self-Hardening Slurries with Fly Ash from Municipal Sewage Sludge Incineration, Considering the Character of Its Operation in a Cut-Off Wall. Buildings 2024, 14, 2461. https://doi.org/10.3390/buildings14082461
Szarek Ł. Examination of the Release of Heavy Metals from Self-Hardening Slurries with Fly Ash from Municipal Sewage Sludge Incineration, Considering the Character of Its Operation in a Cut-Off Wall. Buildings. 2024; 14(8):2461. https://doi.org/10.3390/buildings14082461
Chicago/Turabian StyleSzarek, Łukasz. 2024. "Examination of the Release of Heavy Metals from Self-Hardening Slurries with Fly Ash from Municipal Sewage Sludge Incineration, Considering the Character of Its Operation in a Cut-Off Wall" Buildings 14, no. 8: 2461. https://doi.org/10.3390/buildings14082461
APA StyleSzarek, Ł. (2024). Examination of the Release of Heavy Metals from Self-Hardening Slurries with Fly Ash from Municipal Sewage Sludge Incineration, Considering the Character of Its Operation in a Cut-Off Wall. Buildings, 14(8), 2461. https://doi.org/10.3390/buildings14082461






