Evaluating the Impact of CO2 on Calcium SulphoAluminate (CSA) Concrete
Abstract
1. Introduction
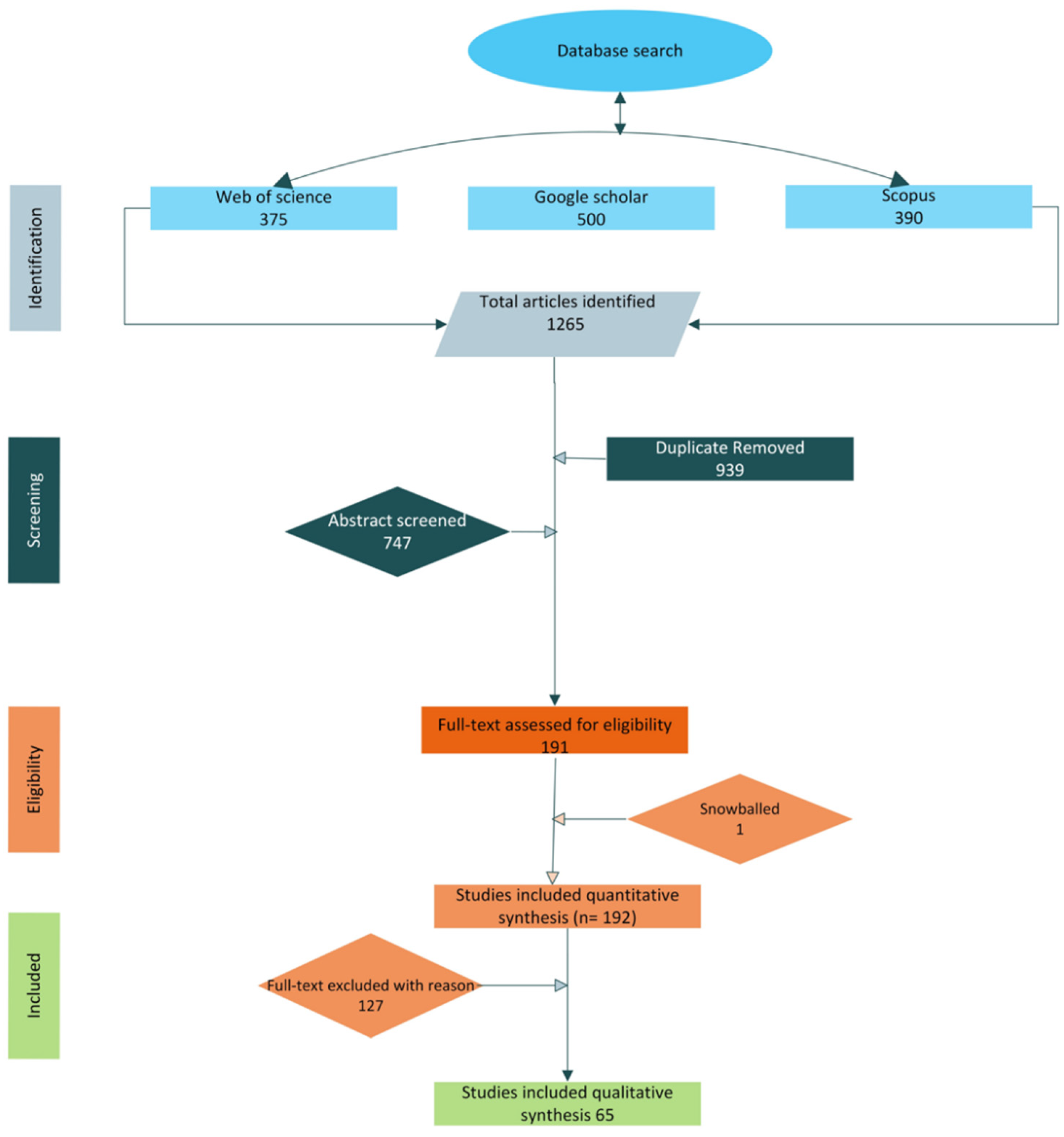
2. Systematic Literature Methodology
3. Results and Discussion
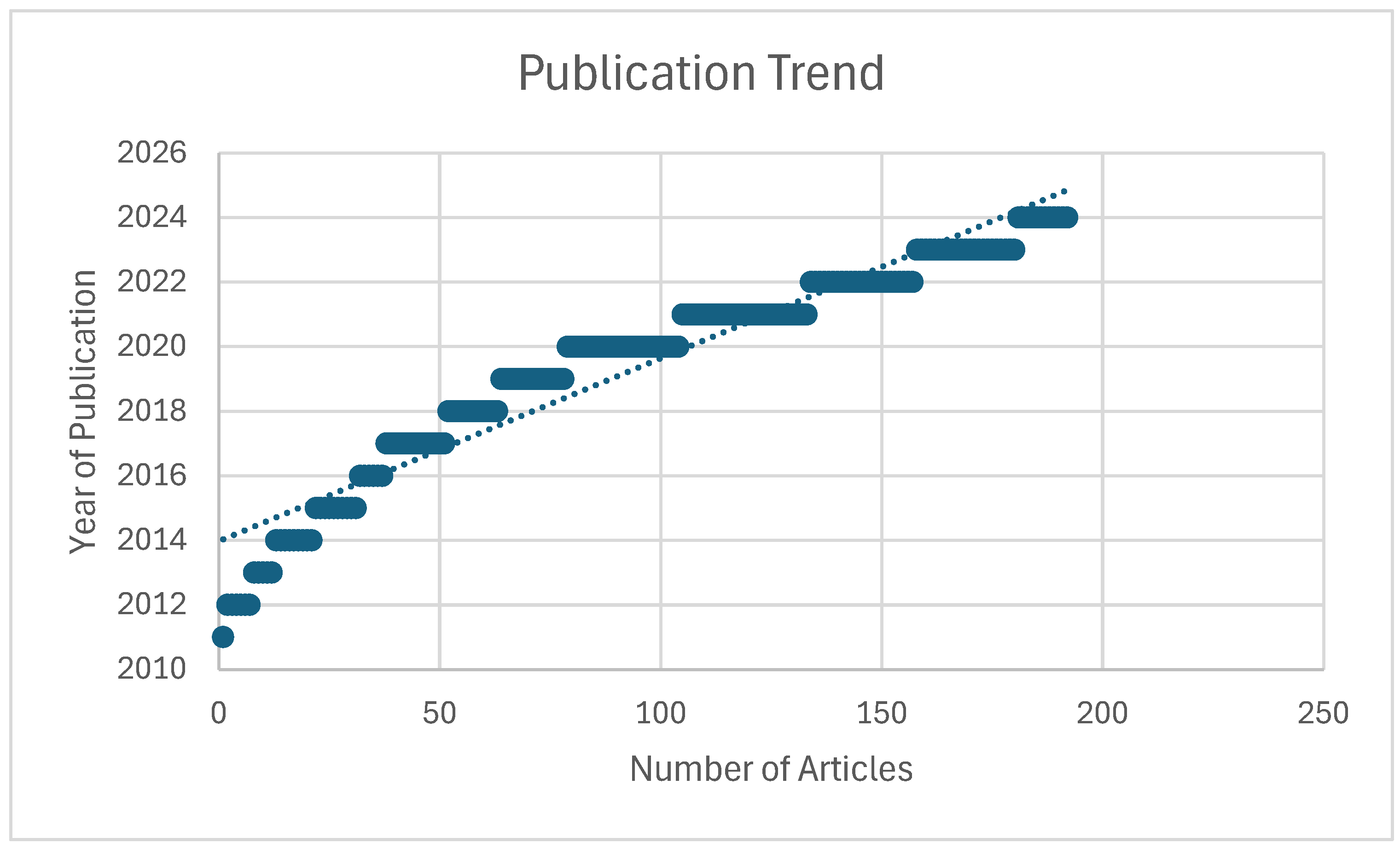
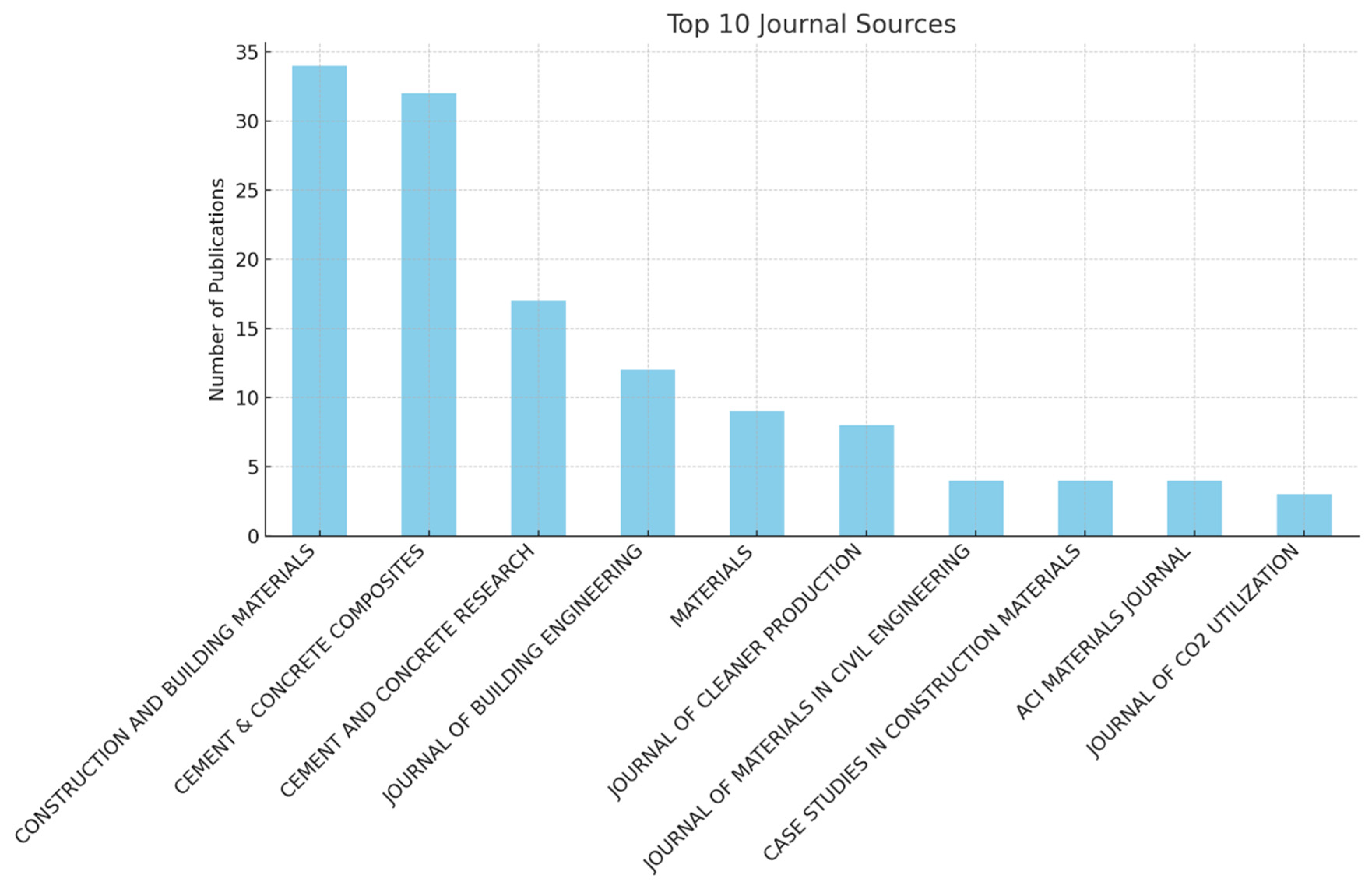
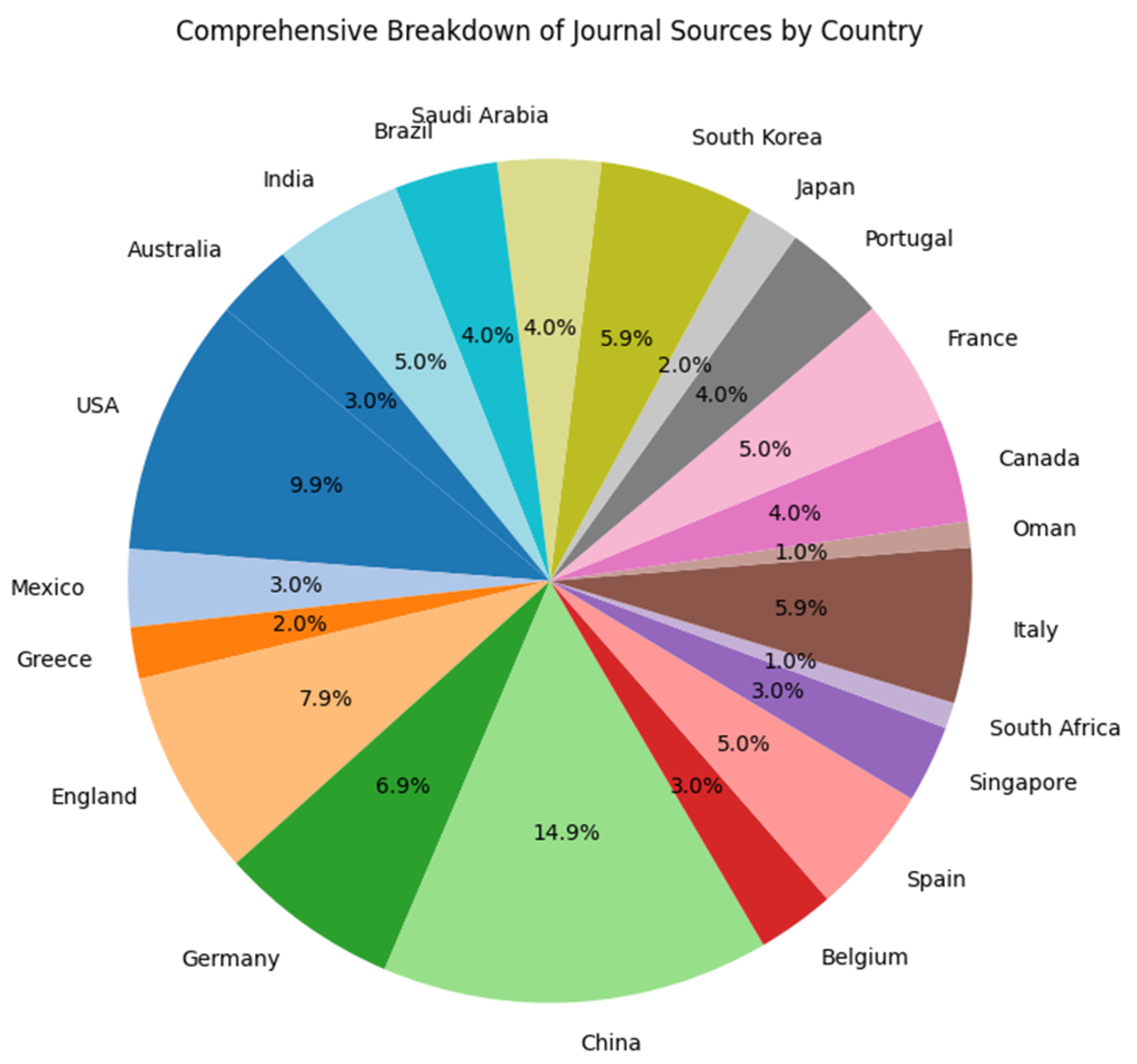
3.1. Results
3.2. Discussion
Bibliometric Analysis
4. CO2 Interactions with Calcium SulphoAluminate Cement
4.1. Carbonation of CSA Binders
4.2. Shrinkage Property of CSA Concrete
- Water Demand and Chemical Binding: CSA cement requires more water than portland cement for proper hydration, but this water is chemically bound, causing no water surplus, and thus preventing shrinkage cracking and warping.
- Chemical and Stable Expansion: During the hardening phase, a chemical and stable expansion occurs that prevents further shrinkage over time.
- Low-Shrinkage Phases Formation: CSA cement forms phases such as calcium sulphoaluminate (C4A3S), ettringite (C6AS3H32), and belite (C2S), which have lower shrinkage potential than phases of portland cement such as alite (C3S) and free lime (CaO) [53].
4.3. Impact on Mechanical Properties
4.4. Impact on Carbonation Depth
4.5. Impact on Chemical Properties of Concrete
4.6. Environmental Impact
4.7. Mitigation Strategies for Carbonation of CSA Concrete
4.8. Summary of Some Studies on CSA Concrete
| Author(s) | Study Title | Findings | Novelty |
| [28] | A novel approach to improve carbonation resistance of Calcium SulfoAluminate cement by assimilating fine cement-sand mix. | CSA could be more resistant to carbonation with partial replacement of CSA cement with fine particles. Modified CSA samples with fine-cement sand mix showed an over 12% increase in compressive strength upon carbonation as compared to OPC and CSA. | The study proposes an enhanced cement formulation (OMCSA) to reduce carbonation of CSA. It also addresses the main barriers of carbonation in CSA cement by partial replacement with CSA < 2.4 μm powder and shows that mixing OPC or conventional CSA with fine CSA < 2.4 μm cement mortar to various proportions is a viable way to mitigate this issue. |
| [24] | SulfoAluminate cement behaviors in carbon dioxide, warm and moist environments | CSA mortar is highly sensitive to carbonation. Increased water content reduces the mechanical properties and dimensional stability of CSA concrete. | The authors propose a set of mortars at 3 w/c ratios to test the material’s performance under different conditions. The study also compares CSA mortars with CEM I portland cement mortars. In addition, the behavior of calcium sulphoaluminate cements in four different environments (ambient air @20 °C and 65% RH, dry air @45 °C and 35% RH, water @45 °C, saturated CO2 @20 °C) were compared. |
| [39] | Effects of Environmental Factors on Concrete Carbonation Depth and Compressive Strength | Found out that concrete has three stages of carbonation, each of which corresponds to the presence of different polymorphs. | The authors propose a relationship between concrete carbonation and temperature, CO2 concentration, and humidity based on XRD, ESEM, and SEM. A novel model to predict carbonation depth and compressive strength in concrete is also proposed. |
| [60] | Carbonation of cement-based materials: Challenges and opportunities | Noticed that concrete using OPC as the primary binder had a stronger carbonation resistance than concrete with SCMs (such as silica fume, slag, or fly ash) or alternative binder ingredients (i.e., C2S, AAM). Carbonation has historically been seen as a negative factor for cement-based materials. However, it can also be seen as a chance to develop a CO2-efficient concrete industry by storing CO2 in cement-based materials and using carbonate binders. | The study reviews progress in carbonation of cement-based materials by extracting concrete highlights from peer-reviewed journals. In addition to the existing knowledge regarding the carbonation of cement-based materials, areas that require further research are also identified. |
| [16] | Durability and microstructure of CSA cement-based materials from MSWI fly ash. | Researched the use of fly ash as a raw material for cement. Used MSWI fly ash as a raw material to prepare new type of cement, which was called CSA cement. Successfully used MSWI fly ash as raw material to prepare lime-free sintering cement. | The study explored MSWI fly ash as cementitious material for sustainable construction. It proposes a novel method of MSWI fly ash being transformed into cement that results in durable and effective material that exceeds current global limits for heavy metal leaching. The study also used MSWI fly ash in cement, with hydrated alkali solutions and biomaterials to produce new mortar and building blocks. |
| [77] | CSA-based portland-free binders to manufacture sustainable concretes for jointless slabs on ground. | The paper explored the possibility of manufacturing eco-friendly shrinkage-compensating mortars by using CSA-based ternary mixtures to replace OPC with SCMs and lime (CH). Replacing OPC with SCM and lime can potentially reduce CO2 emission and energy consumption by up to 60 percent. | The paper evaluates the impact of water/binder ratio, condition of curing, and tartaric acid dosage on elastic, rheological, and physical properties of CSA concretes, SCM, and anhydride concretes that have shrinkage-compensating properties. |
| [17] | Recent progress and technical challenges in using calcium sulfoAluminate (CSA) cement. | Found out that CSA cement can be used as a replacement for portland cement to reduce CO2 emissions. The study introduces the hydration mechanism, long-term durability, and new applications of cement made from the CSA minerals. | Profiles CSA cement with respect to its hydration mechanism, mechanical properties, and long-term durability. The study reviewed the literature on the hydration mechanisms of CSA cement and the properties of its hardened cement paste, including strength, durability, and chemical composition. It discussed recent work and potential challenges associated with producing CSA cement at an industrial scale. The potential applications of CSA cement in developing infrastructure in regions where fresh water is scarce was also explored. |
| [78] | Novel use of calcium sulfoAluminate (CSA) cement for treating problematic soils | The study noticed that using CSA cement in clay-based ground stabilization solutions was more environmentally sustainable than using lime or cement. Identified CSA cement as a sustainable alternative in porous aggregates stabilization. Found out that CSA cement-treated expansive soil exhibited three distinct hydration phases, where the state of hydration was identified through experimentation. | The authors explored a sustainable approach to cement replacement by investigating the effectiveness of CSA cement in ground stabilization. The study proposes CSA cement as an alternative for expansive soil stabilization due to reduced carbon impact. Hydration of CSA cement into expansive soil and its effect on mechanical properties and microstructural change was investigated. The cationic exchange, flocculation, and agglomeration characteristics of CSA cement in expansive soil was also investigated. |
| [79] | Behavior of blends of CSA and portland cements in high chloride environment | The study achieved high early strength of CSA cement composite in high chloride environments. CSA was found to be effective in showing high mechanical performance even in high chloride conditions. CSA binders could also prove resistant to chloride attack. | Describes a novel approach to cement mix design that uses multi-objective optimization to maximize early strengths and durability in marine environments. The study addressed the durability aspects of CSA cement in concrete. Proposes concrete with CSA replacing some of the OPC in different proportions, suitable for marine environments where seawater interacts with concrete. |
| [80] | Decomposition of synthesized ettringite by carbonation | The study showed that the carbonation mechanism is different under wet and dry conditions. This can be explained by the excess or lack of water around or in the ettringite. It was discovered that ettringite can capture CO2 and produce stable solid mineral products. | The authors proposed a theoretical model of carbonation kinetics based on ettringite and its reaction with CO2 gas. The theoretical model was used to predict carbonation rates using the Jander equation. Proposes a realistic and powerful model for long-term geological sequestration of CO2. |
| [81] | Cement-based materials eventually reabsorb much of the CO2 released during creation. | Concrete re-absorbs about 43% of the CO2 emitted during cement production over its life cycle. | Discovered that concrete reabsorbs a significant amount of the CO2 emitted during cement production. |
| [82] | Effects of CSH2 and CH on Strength and Hydration of Calcium SulphoAluminate Cement Prepared from Phosphogypsum | Phosphogypsum increases the strength performance of CSA concrete. The pH of samples increased with the addition of CH. The paper found that adding gypsum dihydrate and calcium hydroxide to cement clinker prepared from phosphogypsum affects the compressive strength and hydration process of the cement. | Investigation of carbonation of CSA with varying w/c ratio. The intervention of optimizing the use of mineral admixtures such as gypsum dihydrate and calcium hydroxide to cement clinker prepared from phosphogypsum. |
| [63] | Research into Carbon Dioxide Curing’s Effects on the Properties of Reactive Powder Concrete with Assembly Unit of SulphoAluminate Cement and Ordinary portland Cement | The findings of the paper are that the addition of sulphoaluminate cement can improve the mechanical strength of RPC at low curing age, but when the curing age is beyond 7 days, the sulphoaluminate cement has a negative effect on the mechanical strength. CO2 curing increases the mechanical strength and the resistance of RPC to sodium chloride (NaCl) freeze–thaw cycles. | The paper examines the effects of carbon dioxide curing on the properties of reactive powder concrete with assembly unit of sulphoaluminate cement and ordinary portland cement. This study uses a combination of rapid-setting sulphoaluminate cement, OPC, and silica fume as one kind of mineral admixture. |
| [76] | Durability of calcium sulfoAluminate cement concrete | The paper suggests that CSA concrete has better performance than portland cement concrete in several aspects, including shrinkage and cracking due to restrained shrinkage, freeze–thaw damage, alkali–silica reaction, and sulfate attack. | Reviewed durability of CSA concretes in general. |
| [45] | Carbonation of concrete: the role of CO2 concentration, relative humidity, and CO2 buffer capacity | The carbonation coefficients determined at the different relative humidities (RH) of 57, 70, and 80% clearly indicate that the response of a concrete to carbonation at increased RH is both dependent on cement type and w/c. The carbonation resistance of concrete in sheltered and with restrictions in unsheltered outdoor exposure can be assessed at 4% CO2 and 57% RH. | The effect of CO2 concentration and ambient relative humidity (RH) on the accelerated and natural carbonation of eighteen concrete mixtures produced with nine different cement types was studied over days. |
| [74] | Pore Solution Chemistry of Calcium Sulfoaluminate Cement and Its Effects on Steel Passivation. | The pore solution chemistry of CSA can affect the passivation of steel reinforcement, which can have implications for the durability and long-term performance of concrete structures. The study identified a novel ion (Al(OH)4−) in CSA pore solution, which is not present in OPC. The study found that CSA pore solution chemistry can promote the formation of a protective layer on the steel surface, which can help to prevent corrosion. However, the study also found that certain ions in the pore solution, such as chloride ions, can negatively impact steel passivation and promote corrosion. This highlights the importance of carefully managing the chemistry of the pore solution in CSA concrete to ensure the long-term durability of concrete structures. | The study investigates the effects of CSA cement on the passivation of steel, which has not been extensively studied in previous research. The study also explores the pore solution chemistry of CSA cement, providing insights into the mechanisms by which the cement impacts steel corrosion. Additionally, the study suggests that the use of CSA cement may offer an eco-friendlier alternative to traditional portland cement due to its lower carbon footprint. |
| [46] | Carbonation of calcium sulfoaluminate mortars | Found that CSA mortars carbonate faster than portland cement mortars and at the same rate as calcium aluminate cement (CAC) mortars. The anhydrite content of the CSA mortars strongly affects the ye’elimite reaction kinetics, which play a vital role in imparting carbonation resistance in CSA mortars. CSA mortars carbonate slower with decreasing water content. Calcium sulfate additions to CSA clinker to produce CSA cement dilutes the clinker content and reduces the amount of CO2 that the CSA cement can bind. Thermodynamic modeling can be used to predict the carbonation process of CSA mortars. | This study provides a comprehensive investigation of the carbonation process in CSA mortars, including the physical and chemical parameters that govern the carbonation rate and the microstructural and chemical factors that affect the rate of carbonation in CSA cement. Additionally, it also presents a detailed analysis of the part anhydrite content and calcium sulfate additions play in the carbonation resistance of CSA mortars. Finally, it provides thermodynamic modeling to support the experimental results and to better understand the carbonation process in CSA cement. The study gives comprehensive insight to the behavior of CSA cement under carbonation and gives suggestions on how to improve the durability of CSA. |
| [67] | Influence of layered double hydroxides on microstructure and carbonation resistance of sulphoAluminate cement concrete | The addition of magnesium aluminate-based layered double hydroxides (LDH) to CSA concrete improves its carbonation resistance significantly. The LDHs modify the pore structure of the concrete, reducing the amount of available space for CO2 to penetrate and react with the cement phases. The LDHs can immobilize carbonate ions, preventing them from reacting with the cement phases. The use of fly ash in the concrete mixture further improves the carbonation resistance of the CSA concrete, resulting in a lesser carbonation depth compared to portland cement concrete. | The use of magnesium aluminate-based layered double hydroxides (LDHs) as an additive to improve the carbonation resistance of sulphoaluminate cement (SAC) concrete. Previous studies have focused on the use of various materials to improve the performance of portland cement-based concrete, but this study specifically focuses on SAC concrete, which has different properties and composition. The study also characterizes the effects of LDHs on the pore structure of the concrete using mercury intrusion porosimetry (MIP), which is a technique not commonly used in studies on concrete carbonation resistance. The finding that the addition of LDHs can significantly improve the carbonation resistance of SAC concrete by modifying its pore structure and immobilizing carbonate ions is also novel and contributes to the development of more sustainable and durable concrete materials. |
| [47] | Carbonation of ternary building cementing materials | The carbonation resistance of ternary building cement materials is higher than that of pure portland cement. The carbonation resistance of the cementing materials increases with the increase in fly ash and limestone powder content. The carbonation depth of the cementing materials decreases as the curing time increases, indicating that the carbonation reaction is a slow process. The C-S-H in the cementing materials are more stable than the CH phase during the carbonation process, which contributes to the carbonation resistance of the materials. The formation of CaCO3 during the carbonation process aids the decrease in porosity and the improvement in the mechanical properties of the cementing materials. | The study investigates the carbonation behavior of ternary cementitious materials, which are composed of different combinations of portland cement, calcium aluminate cement, and calcium sulphoaluminate cement. This study’s objective was to provide a comprehensive understanding of the behavior of ternary cements under carbonation, which can potentially lead to the development of more sustainable and durable building materials. Additionally, the use of different techniques such as XRD and thermogravimetric analysis allowed for a detailed characterization of the reaction products and the carbonation process. |
| [65] | Mechanical and Microstructural Characteristics of Calcium Sulfoaluminate Cement Exposed to Early-Age Carbonation Curing | CSA cement pastes’ compressive strength increased with briquetting pressure. However, the relationship is not linear; the growth rate of the compressive strength slows down with increasing briquetting pressure. At briquetting pressure less than 20 MPa, the compressive strength of the test block at 3 days does not differ much from the strength at 1 day. When the briquetting pressure exceeds 20 MPa, the gap between the strength at 1 day and 3 days gradually increases. The water/binder ratio also impacts the compressive strength of the cement paste. When the water/binder ratio is 0.05, the compressive strength of the test block after carbonation curing becomes very low. The optimal starting point for carbonation curing is 4 h after the start of hydration. The compressive strengths after carbonation curing are all above 50 MPa, while the strength of the specimens only reaches 37 MPa after 3 days of standard curing. This shows that early-age carbonation curing can greatly improve the early mechanical properties of CSA cement paste. As the carbonation curing starting point progresses, the strength of the specimens after carbonation curing showed a tendency to decrease rather than increase. The occurrence of the carbonization reaction can promote the hydration of the cement paste. As the hydration progresses, the paste becomes denser and the porosity becomes lower, so it is increasingly difficult for CO2 to diffuse into the paste. When carbonization is carried out at the early stage of hydration, the CO2 diffuses more easily into the paste, and the hydration and carbonization reactions proceed simultaneously and mutually reinforce each other, thus significantly increasing the compressive strength of the specimen. | The paper discusses the physicochemical reactions that occur during early accelerated carbonization, including phase transition and mass transfer of CO2, dissolution of solid Ca(OH)2 and mass transfer of dissolved Ca(OH)2, hydration of cement compounds, and carbonation of unhydrated cement compounds and hydration products. The authors note that while there are similar studies on other types of cement and concrete materials, there are relatively few studies on the performance of early carbonation curing of CSA cement. The paper also presents a comprehensive analysis of the hydration carbonization reaction of minerals in the CSA cement paste, indicating that the earlier the carbonation starting point is, the more favorable it is for the reaction. It was also noted that different carbonation starting points will not change the types of carbonization reaction and hydration reaction products but will affect the degree of reaction and macroscopic performance. |
5. Analysis of CO2 Impact on CSA Concrete
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nañagas, K.A.; Penfound, S.J.; Kao, L.W. Carbon Monoxide Toxicity. Emerg. Med. Clin. N. Am. 2022, 40, 283–312. [Google Scholar] [CrossRef] [PubMed]
- Rocque, R.J.; Beaudoin, C.; Ndjaboue, R.; Cameron, L.; Poirier-Bergeron, L.; Poulin-Rheault, R.-A.; Fallon, C.; Tricco, A.C.; Witteman, H.O. Health effects of climate change: An overview of systematic reviews. BMJ Open 2021, 11, e046333. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, C.; McGushin, A.; Romanello, M.; Ayeb-Karlsson, S.; Cai, W.; Chambers, J.; Dasgupta, S.; Escobar, L.E.; Kelman, I.; Kjellstrom, T.; et al. Tracking the impacts of climate change on human health via indicators: Lessons from the Lancet Countdown. BMC Public Health 2022, 22, 663. [Google Scholar] [CrossRef] [PubMed]
- How Can We Reduce the Construction Industry’s Carbon Footprint? World Economic Forum. Available online: https://www.weforum.org/agenda/2021/07/construction-industry-doesn-t-know-where-it-stands-when-it-comes-to-carbon-emissions/ (accessed on 24 May 2023).
- Watts, J. Concrete: The Most Destructive Material on Earth. Guardian. 2019. Available online: https://www.theguardian.com/cities/2019/feb/25/concrete-the-most-destructive-material-on-earth (accessed on 16 January 2023).
- Greer, F.; Raftery, P.; Brager, G.; Horvath, A. A perspective on tools for assessing the building sector’s greenhouse gas emissions and beyond. Environ. Res. Infrastruct. Sustain. 2023, 3, 043001. [Google Scholar] [CrossRef]
- Sousa, V.; Bogas, J.A.; Real, S.; Meireles, I. Industrial production of recycled cement: Energy consumption and carbon dioxide emission estimation. Environ. Sci. Pollut. Res. 2023, 30, 8778–8789. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Gao, T.; Zhao, J.; Wang, L.; Wang, L.; Liu, L.; Chen, F.; Xue, J. Factory-level measurements on CO2 emission factors of cement production in China. Renew. Sustain. Energy Rev. 2014, 34, 337–349. [Google Scholar] [CrossRef]
- Torgal, F.P.; Miraldo, S.; Labrincha, J.A.; De Brito, J. An overview on concrete carbonation in the context of eco-efficient construction: Evaluation, use of SCMs and/or RAC. Constr. Build. Mater. 2012, 36, 141–150. [Google Scholar] [CrossRef]
- Concrete needs to lose its colossal carbon footprint. Int. J. Sci. 2021, 597, 593–594. [CrossRef]
- Bishnoi, S. Carbon Emissions and Their Mitigation in the Cement Sector. In Carbon Utilization: Applications for the Energy Industry; Goel, M., Sudhakar, M., Eds.; Springer: Singapore, 2017; pp. 257–268. [Google Scholar] [CrossRef]
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the carbonation of concrete with supplementary cementitious materials: A critical review by RILEM TC 281-CCC. Mater. Struct. 2020, 53, 136. [Google Scholar] [CrossRef]
- Tekin, İ.; Dirikolu, İ.; Gökçe, H.S. A regional supplementary cementitious material for the cement industry: Pistachio shell ash. J. Clean. Prod. 2021, 285, 124810. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Hu, W.; Wu, K. Durability and microstructure of CSA cement-based materials from MSWI fly ash. Cem. Concr. Compos. 2014, 46, 26–31. [Google Scholar] [CrossRef]
- Tao, Y.; Rahul, A.V.; Mohan, M.K.; De Schutter, G.; Van Tittelboom, K. Recent progress and technical challenges in using calcium sulfoaluminate (CSA) cement. Cem. Concr. Compos. 2023, 137, 104908. [Google Scholar] [CrossRef]
- Al Fuhaid, A.F.; Niaz, A. Carbonation and Corrosion Problems in Reinforced Concrete Structures. Buildings 2022, 12, 586. [Google Scholar] [CrossRef]
- Durisety, H.; Palcham, K.; Babu, K.P. The Concrete Incorporated with Zeolite for Reducing Atmospheric Carbon Dioxide. Int. J. Recent Technol. Eng. 2020, 8, 2117–2121. [Google Scholar] [CrossRef]
- Korec, E.; Mingazzi, L.; Freddi, F.; Martínez-Pañeda, E. Predicting the impact of water transport on carbonation-induced corrosion in variably saturated reinforced concrete. Mater. Struct. 2024, 57, 91. [Google Scholar] [CrossRef]
- Sarkis-Onofre, R.; Catalá-López, F.; Aromataris, E.; Lockwood, C. How to properly use the PRISMA Statement. Syst. Rev. 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Suh, C.H.; Woo, S.; Kim, P.H.; Kim, K.W. Quality Reporting of Systematic Review and Meta-Analysis According to PRISMA 2020 Guidelines: Results from Recently Published Papers in the Korean Journal of Radiology. Korean J. Radiol. 2022, 23, 355. [Google Scholar] [CrossRef]
- McCombes, S. How to Write a Literature Review|Guide, Examples, & Templates. Scribbr. Available online: https://www.scribbr.com/dissertation/literature-review/ (accessed on 8 June 2023).
- Mechling, J.-M.; Lecomte, A.; Roux, A.; Le Rolland, B. Sulfoaluminate cement behaviours in carbon dioxide, warm and moist environments. Adv. Cem. Res. 2014, 26, 52–61. [Google Scholar] [CrossRef]
- Moffatt, E.G.; Thomas, M.D.A. Effect of Carbonation on the Durability and Mechanical Performance of Ettringite-Based Binders. ACI Mater. J. 2019, 116, 95–102. [Google Scholar] [CrossRef]
- Alapati, P.; Kurtis, K. Carbonation in Alternative Cementitious Materials: Implications on Durability and Mechanical Properties. 2019. Available online: https://www.semanticscholar.org/paper/Carbonation-in-Alternative-Cementitious-Materials%3A-Alapati-Kurtis/03575798bd8236d3229714af4bf106d68c7b79eb (accessed on 20 February 2023).
- Vu, Q.H.; Pham, G.; Chonier, A.; Brouard, E.; Rathnarajan, S.; Pillai, R.; Gettu, R.; Santhanam, M.; Aguayo, F.; Folliard, K.J.; et al. Impact of different climates on the resistance of concrete to natural carbonation. Constr. Build. Mater. 2019, 216, 450–467. [Google Scholar] [CrossRef]
- Ansari, W.S.; Chang, J.; Rehman, Z.U.; Nawaz, U.; Junaid, M.F. A novel approach to improve carbonation resistance of Calcium Sulfoaluminate cement by assimilating fine cement-sand mix. Constr. Build. Mater. 2022, 317, 125598. [Google Scholar] [CrossRef]
- Sharma, R.; Kim, H.; Lee, N.K.; Park, J.-J.; Jang, J.G. Microstructural characteristics and CO2 uptake of calcium sulfoaluminate cement by carbonation curing at different water-to-cement ratios. Cem. Concr. Res. 2023, 163, 107012. [Google Scholar] [CrossRef]
- Cagno, E.; Neri, A.; Marta, N.; Bassani, C.A.; Lampertico, T. Applied Sciences | Free Full-Text | The Role of Digital Technologies in Operationalizing the Circular Economy Transition: A Systematic Literature Review. J. Appl. Sci. 2021, 11, 3328. [Google Scholar] [CrossRef]
- Bertola, F.; Gastaldi, D.; Irico, S.; Paul, G.; Canonico, F. Influence of the amount of calcium sulfate on physical/mineralogical p roperties and carbonation resistance of CSA-based cements. Cem. Concr. Res. 2022, 151, 106634. [Google Scholar] [CrossRef]
- Monkman, S.; Grandfield, K.; Langelier, B. On the Mechanism of Using Carbon Dioxide as a Beneficial Concrete Admixture. In SP-329: Superplasticizers and Other Chemical Admixtures in Concrete Proceedings Twelfth International Conference, Beijing, China; American Concrete Institute: Farmington Hills, MI, USA, 2018. [Google Scholar] [CrossRef]
- Shenbagam, V.K.; Shaji, P.; Eswita, Y.; Cepuritis, R.; Chaunsali, P. Carbonation of calcium sulfoaluminate belite binder: Mechanism and its implication on properties. J. Sustain. Cem.-Based Mater. 2024, 13, 938–950. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X.; Gao, X. Use of carbonation curing to improve mechanical strength and durability of pervious concrete. ACS Sustain. Chem. Eng. 2020, 8, 3872–3884. [Google Scholar] [CrossRef]
- Wang, L.; Ma, H.; Li, Z.; Ma, G.; Guan, J. Cementitious composites blending with high belite sulfoaluminate and medium-heat Portland cements for largescale 3D printing. Addit. Manuf. 2021, 46, 102189. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, D.; Cheng, X.; Chen, W. Carbonation resistance of sulphoaluminate cement-based high performance concrete. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2009, 24, 663–666. [Google Scholar] [CrossRef]
- Kamal, N.L.M.; Itam, Z.; Sivaganese, Y.; Beddu, S. Carbon dioxide sequestration in concrete and its effects on concrete compressive strength. Mater. Today Proc. 2020, 31, A18–A21. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A. A review of microbial precipitation for sustainable construction. Constr. Build. Mater. 2015, 93, 1224–1235. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Yu, Z. Effects of Environmental Factors on Concrete Carbonation Depth and Compressive Strength. Materials 2018, 11, 2167. [Google Scholar] [CrossRef]
- Sirtoli, D.; Wyrzykowski, M.; Riva, P.; Tortelli, S.; Marchi, M.; Lura, P. Shrinkage and creep of high-performance concrete based on calcium sulfoaluminate cement. Cem. Concr. Compos. 2019, 98, 61–73. [Google Scholar] [CrossRef]
- Mi, T.; Li, Y.; Liu, W.; Dong, Z.; Gong, Q.; Min, C.; Xing, F.; Wang, Y.; Chu, S. The effect of carbonation on chloride redistribution and corrosion of steel reinforcement. Constr. Build. Mater. 2023, 363, 129641. [Google Scholar] [CrossRef]
- Lin, X. Effect of Early Age Carbonation on Strength and pH of Concrete. Master’s Thesis, McGill University, Montréal, QC, Canada, 2007. [Google Scholar]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction––A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Zhou, Q.; Glasser, F.P. Kinetics and mechanism of the carbonation of ettringite. Adv. Cem. Res. 2000, 12, 131–136. [Google Scholar] [CrossRef]
- Leemann, A.; Moro, F. Carbonation of concrete: The role of CO2 concentration, relative humidity and CO2 buffer capacity. Mater. Struct. 2017, 50, 30. [Google Scholar] [CrossRef]
- Hargis, C.W.; Lothenbach, B.; Müller, C.J.; Winnefeld, F. Carbonation of calcium sulfoaluminate mortars. Cem. Concr. Compos. 2017, 80, 123–134. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torréns-Martín, D.; Martínez-Ramírez, S. Carbonation of ternary building cementing materials. Cem. Concr. Compos. 2012, 34, 1180–1186. [Google Scholar] [CrossRef]
- Geng, H.; Duan, P.; Chen, W.; Shui, Z. Carbonation of sulphoaluminate cement with layered double hydroxides. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2014, 29, 97–101. [Google Scholar] [CrossRef]
- Lai, M.H.; Binhowimal, S.A.M.; Griffith, A.M.; Hanzic, L.; Chen, Z.; Wang, Q.; Ho, J.C.M. Shrinkage, cementitious paste volume, and wet packing density of concrete|Semantic Scholar. Struct. Concr. 2022, 23, 488–504. [Google Scholar] [CrossRef]
- Ke, G.; Zhang, J.; Liu, Y. Shrinkage characteristics of calcium sulphoaluminate cement concrete. Constr. Build. Mater. 2022, 337, 127627. [Google Scholar] [CrossRef]
- Concrete Counter Top Institute. CSA Cements in Concrete Countertops: Rapid Strength with a Low Carbon Footprint. Concrete Countertop Institute. Available online: https://concretecountertopinstitute.com/free-training/csa-cements-in-concrete-countertops-rapid-strength-with-a-low-carbon-footprint/ (accessed on 6 November 2023).
- Tam, V.W.Y.; Butera, A.; Le, K.N. An investigation of the shrinkage, concrete shrinkage reversibility and permeability of CO2-treated concrete. Constr. Build. Mater. 2023, 365, 130120. [Google Scholar] [CrossRef]
- Chaunsali, P.; Mondal, P. Influence of Calcium Sulfoaluminate (CSA) Cement Content on Expansion and Hydration Behavior of Various Ordinary Portland Cement-CSA Blends. J. Am. Ceram. Soc. 2015, 98, 2617–2624. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of early carbonation curing on chloride penetration and weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A.; Neves, J. Drying and carbonation shrinkage of cement paste containing alkalis. Mater. Struct. 2017, 50, 132. [Google Scholar] [CrossRef]
- Suda, Y.; Tomiyama, J.; Saito, T.; Saeki, T. Phase Assemblage, Microstructure and Shrinkage of Cement Paste during Carbonation at Different Relative Humidities. J. Adv. Concr. Technol. 2021, 19, 687–699. [Google Scholar] [CrossRef]
- Lootens, D.; Bentz, D.P. On the relation of setting and early-age strength development to porosity and hydration in cement-based materials. Cem. Concr. Compos. 2016, 68, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Markosian, N.; Thomas, R.; Maguire, M.; Sorensen, A. Calcium Sulfoaluminate Cement Concrete for Prestressed Bridge Girders: Prestressing Losses, Bond, and Strength Behavior. Utah State University, MPC-560. Available online: https://digitalcommons.usu.edu/etd/7474/ (accessed on 23 April 2024).
- Wang, J.; Lord, T.; Wang, Y.; Black, L.; Li, Q. Effects of carbonation on mechanical properties of two types of concrete under extreme loadings of high temperature and impact. Proc. Inst. Civ. Eng. 2022, 175, 44–56. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Zhang, Q.; Zhang, C.; Wu, K.; Shen, P. Microstructural Evolution of Calcium Sulfoaluminate Cement during the Wet-Carbonation Process. Buildings 2024, 14, 343. [Google Scholar] [CrossRef]
- Sevelsted, T.F.; Skibsted, J. Carbonation of C–S–H and C–A–S–H samples studied by 13C, 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2015, 71, 56–65. [Google Scholar] [CrossRef]
- Cao, H.; Liang, Z.; Peng, X.; Cai, X.; Wang, K.; Wang, H.; Lyu, Z. Research into Carbon Dioxide Curing’s Effects on the Properties of Reactive Powder Concrete with Assembly Unit of Sulphoaluminate Cement and Ordinary Portland Cement. Coatings 2022, 12, 209. [Google Scholar] [CrossRef]
- Groves, G.W.; Brough, A.; Richardson, I.G.; Dobson, C.M. Progressive Changes in the Structure of Hardened C3S Cement Pastes due to Carbonation. J. Am. Ceram. Soc. 1991, 74, 2891–2896. [Google Scholar] [CrossRef]
- Wang, W.; Wei, X.; Cai, X.; Deng, H.; Li, B. Mechanical and Microstructural Characteristics of Calcium Sulfoaluminate Cement Exposed to Early-Age Carbonation Curing. Materials 2021, 14, 3515. [Google Scholar] [CrossRef]
- Carbonation—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/carbonation (accessed on 8 June 2023).
- Duan, P.; Chen, W.; Ma, J.; Shui, Z. Influence of layered double hydroxides on microstructure and carbonation resistance of sulphoaluminate cement concrete. Constr. Build. Mater. 2013, 48, 601–609. [Google Scholar] [CrossRef]
- Aguayo, F.M.; Drimalas, T.; Folliard, K.J. Natural Carbonation of Concrete. Spec. Publ. 2015, 305, 2.1–2.12. [Google Scholar] [CrossRef]
- Tang, J.; Wu, J.; Zou, Z.; Yue, A.; Mueller, A. Influence of axial loading and carbonation age on the carbonation resistance of recycled aggregate concrete. Constr. Build. Mater. 2018, 173, 707–717. [Google Scholar] [CrossRef]
- Taylor, H.F.W.; Famy, C.; Scrivener, K.L. Delayed ettringite formation. Cem. Concr. Res. 2001, 31, 683–693. [Google Scholar] [CrossRef]
- Cho, B.S.; Lee, H.H.; Choi, Y.C. Effects of aluminate rich slag on compressive strength, drying shrinkage and microstructure of blast furnace slag cement. Constr. Build. Mater. 2017, 140, 293–300. [Google Scholar] [CrossRef]
- Gastaldi, D.; Bertola, F.; Canonico, F.; Buzzi, L.; Mutke, S.; Irico, S.; Paul, G.; Marchese, L.; Boccaleri, E. A chemical/mineralogical investigation of the behavior of sulfoaluminate binders submitted to accelerated carbonation. Cem. Concr. Res. 2018, 109, 30–41. [Google Scholar] [CrossRef]
- Quillin, K. Performance of belite–sulfoaluminate cements. Cem. Concr. Res. 2001, 31, 1341–1349. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, S.; Tang, X.; Xu, Q.; Qian, K. Pore Solution Chemistry of Calcium Sulfoaluminate Cement and Its Effects on Steel Passivation. Appl. Sci. 2019, 9, 1092. [Google Scholar] [CrossRef]
- Jain, J.; Seth, A.; Decristofaro, N. Environmental impact and durability of carbonated calcium silicate concrete. Proc. Inst. Civil. Eng. Constr. Mater. 2019, 172, 179–191. [Google Scholar] [CrossRef]
- Tan, B.; Okoronkwo, M.U.; Kumar, A.; Ma, H. Durability of calcium sulfoaluminate cement concrete. J. Zhejiang Univ. Sci. A 2020, 21, 118–128. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Pastore, T. CSA-based Portland-free binders to manufacture sustainable concretes for jointless slabs on ground. Constr. Build. Mater. 2018, 187, 691–698. [Google Scholar] [CrossRef]
- Pooni, J.; Robert, D.; Giustozzi, F.; Setunge, S.; Xie, Y.M.; Xia, J. Novel use of calcium sulfoaluminate (CSA) cement for treating problematic soils. Constr. Build. Mater. 2020, 260, 120433. [Google Scholar] [CrossRef]
- Bertola, F.; Gastaldi, D.; Irico, S.; Paul, G.; Canonico, F. Behavior of blends of CSA and Portland cements in high chloride environment. Constr. Build. Mater. 2020, 262, 120852. [Google Scholar] [CrossRef]
- Nishikawa, T.; Suzuki, K.; Ito, S.; Sato, K.; Takebe, T. Decomposition of synthesized ettringite by carbonation. Cem. Concr. Res. 1992, 22, 6–14. [Google Scholar] [CrossRef]
- Concrete Jungle Functions as Carbon Sink, UCI and Other Researchers Find. UCI News. Available online: https://news.uci.edu/2016/11/21/concrete-jungle-functions-as-carbon-sink-uci-and-other-researchers-find/ (accessed on 26 February 2023).
- Zhang, J.; Chang, J.; Zhang, P.; Wang, T. Effects of C$H2 and CH on Strength and Hydration of Calcium Sulphoaluminate Cement Prepared from Phosphogypsum. Buildings 2022, 12, 1692. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Zhou, W.; Lu, Y.; Liu, X.; Chang, X. A fast-setting and eco-friendly superhydrophobic high belite sulphoaluminate cement mortar. J. Mater. Res. Technol. 2023, 23, 2690–2702. [Google Scholar] [CrossRef]
- Sirtoli, D.; Tortelli, S.; Riva, P.; Marchi, M.; Cucitore, R.; Nangah, M. Mechanical and Environmental Performances of Sulpho-Based Rapid Hardening Concrete. SP-305 Durab. Sustain. Concr. Struct. 2015, 305, 47.1–47.8. [Google Scholar]
- Shenbagam, V.K.; Chaunsali, P. Influence of calcium hydroxide and calcium sulfate on early-age proper ties of non-expansive calcium sulfoaluminate belite cement. Cem. Concr. Compos. 2022, 128, 104444. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akerele, D.D.; Aguayo, F. Evaluating the Impact of CO2 on Calcium SulphoAluminate (CSA) Concrete. Buildings 2024, 14, 2462. https://doi.org/10.3390/buildings14082462
Akerele DD, Aguayo F. Evaluating the Impact of CO2 on Calcium SulphoAluminate (CSA) Concrete. Buildings. 2024; 14(8):2462. https://doi.org/10.3390/buildings14082462
Chicago/Turabian StyleAkerele, Daniel D., and Federico Aguayo. 2024. "Evaluating the Impact of CO2 on Calcium SulphoAluminate (CSA) Concrete" Buildings 14, no. 8: 2462. https://doi.org/10.3390/buildings14082462
APA StyleAkerele, D. D., & Aguayo, F. (2024). Evaluating the Impact of CO2 on Calcium SulphoAluminate (CSA) Concrete. Buildings, 14(8), 2462. https://doi.org/10.3390/buildings14082462






