Protective Effects of Small Molecular Inhibitors on Steel Corrosion: The Generation of a Multi-Electric Layer on Passivation Films
Abstract
1. Introduction
2. Models and Simulation Details
2.1. Modeling Details
2.2. Molecular Dynamics Simulation
3. Results and Discussion
3.1. ATL Inhibiting Effect
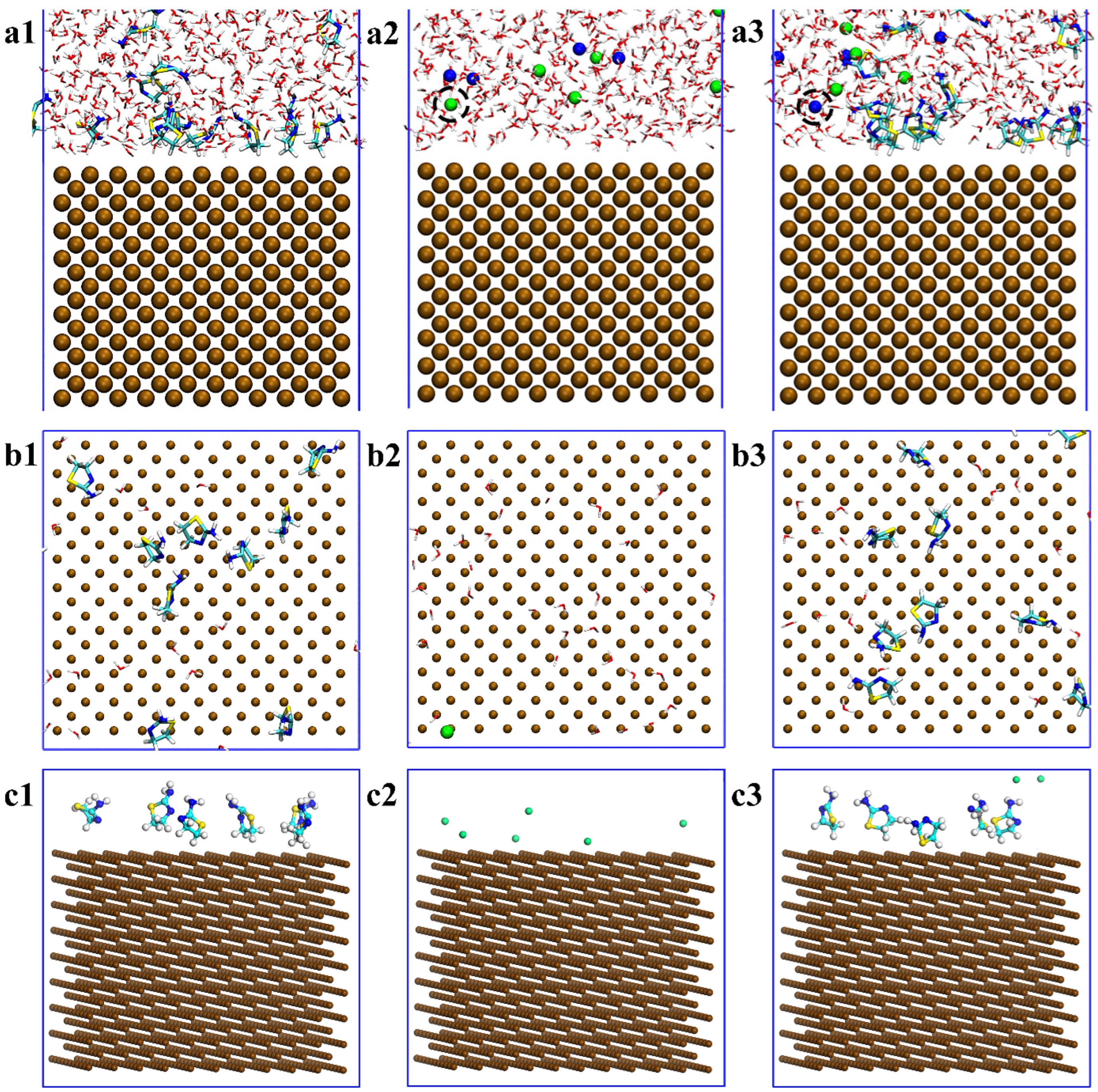
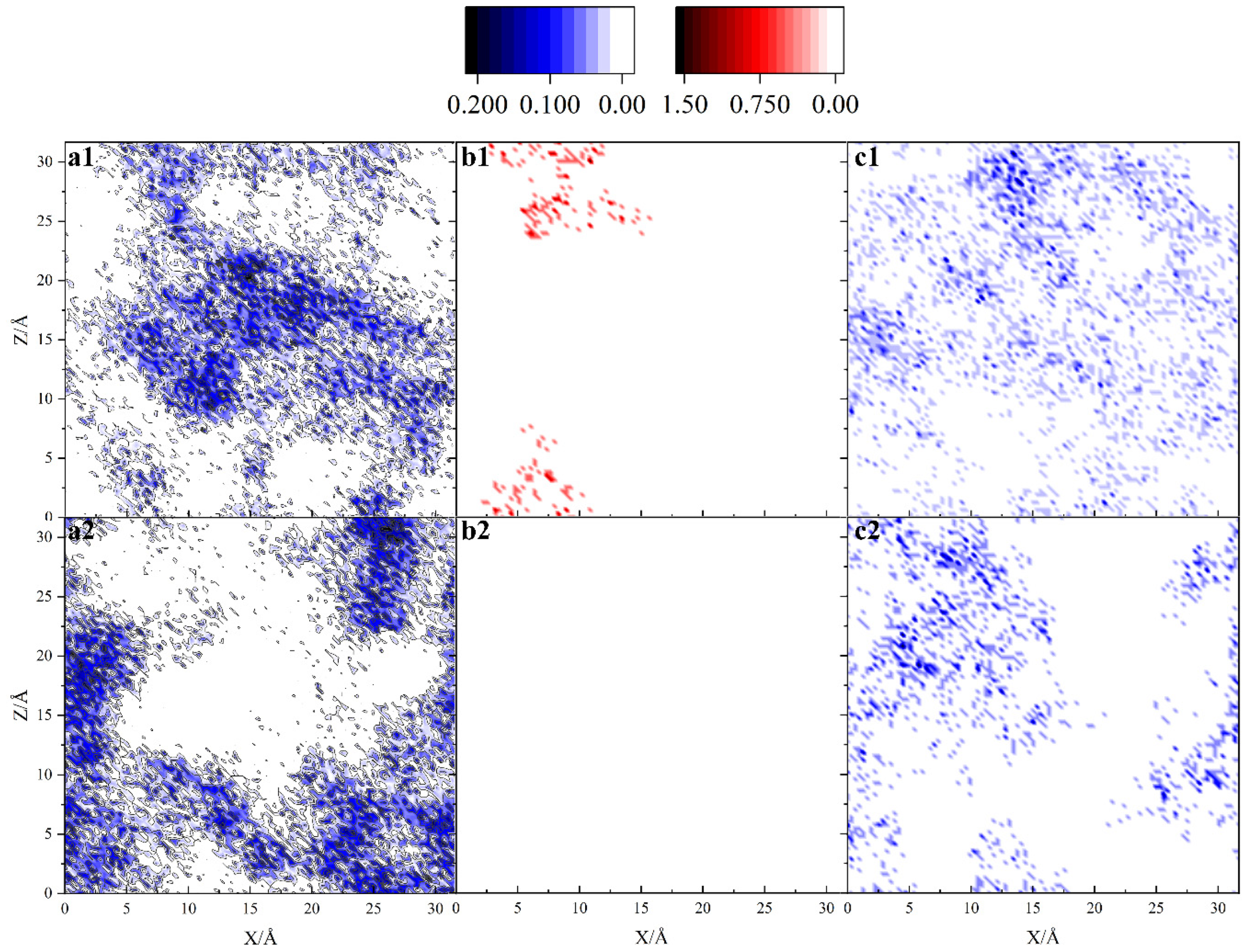
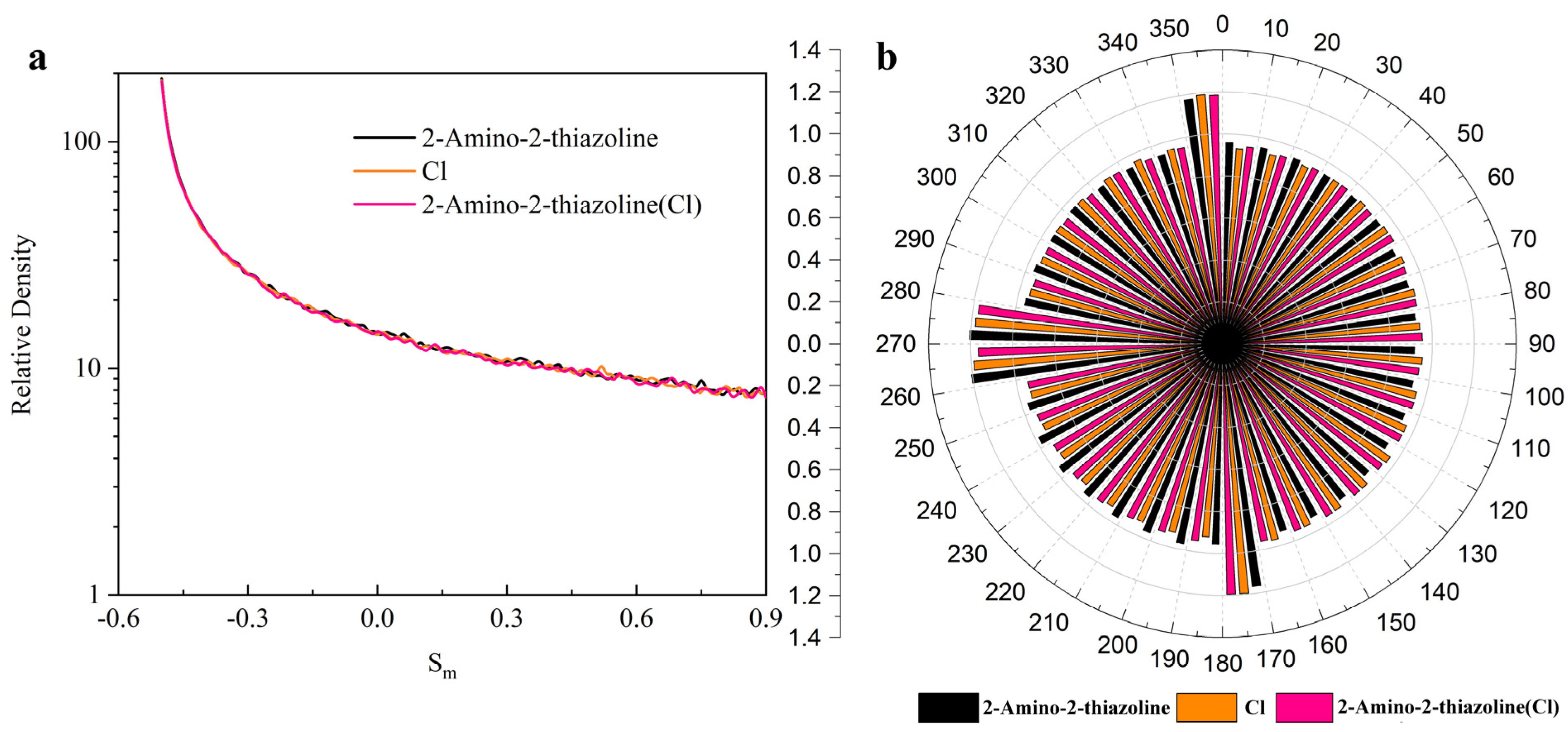
3.2. Density Distribution of Each Part of the System
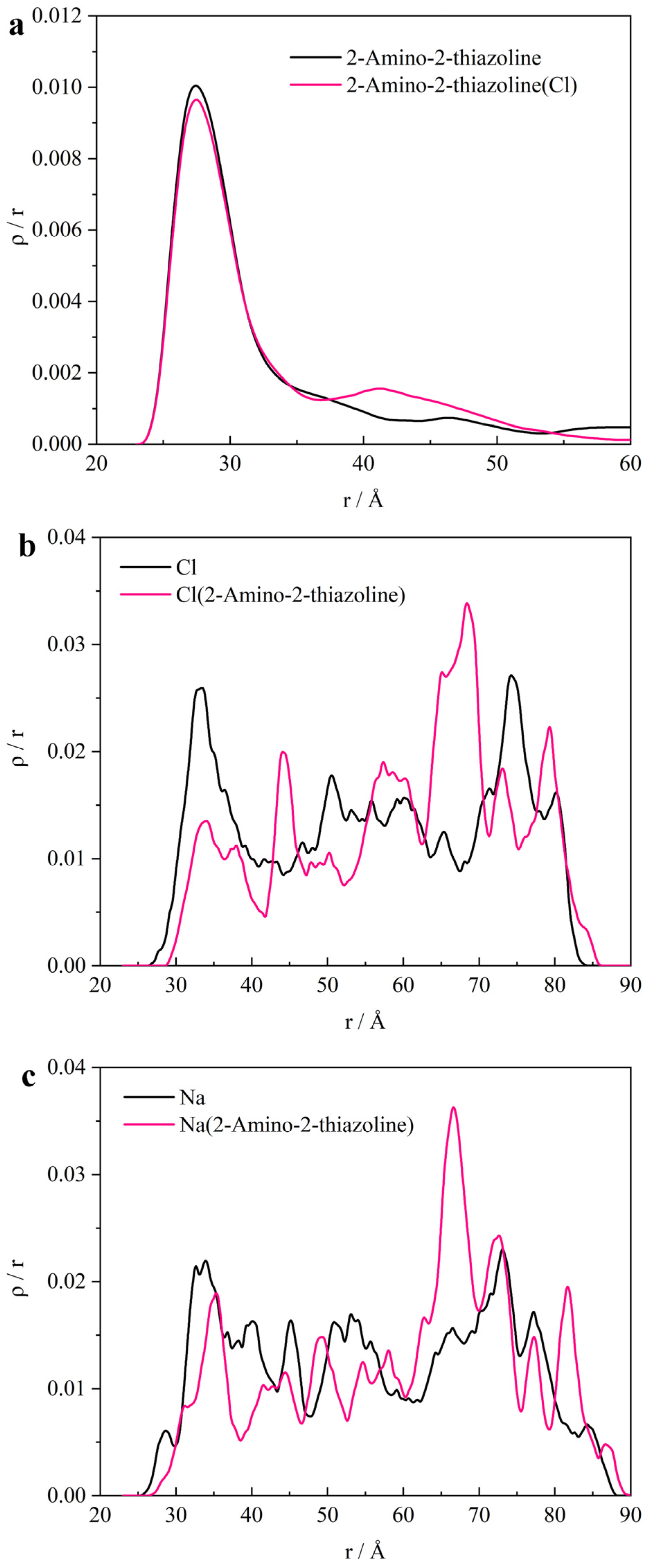
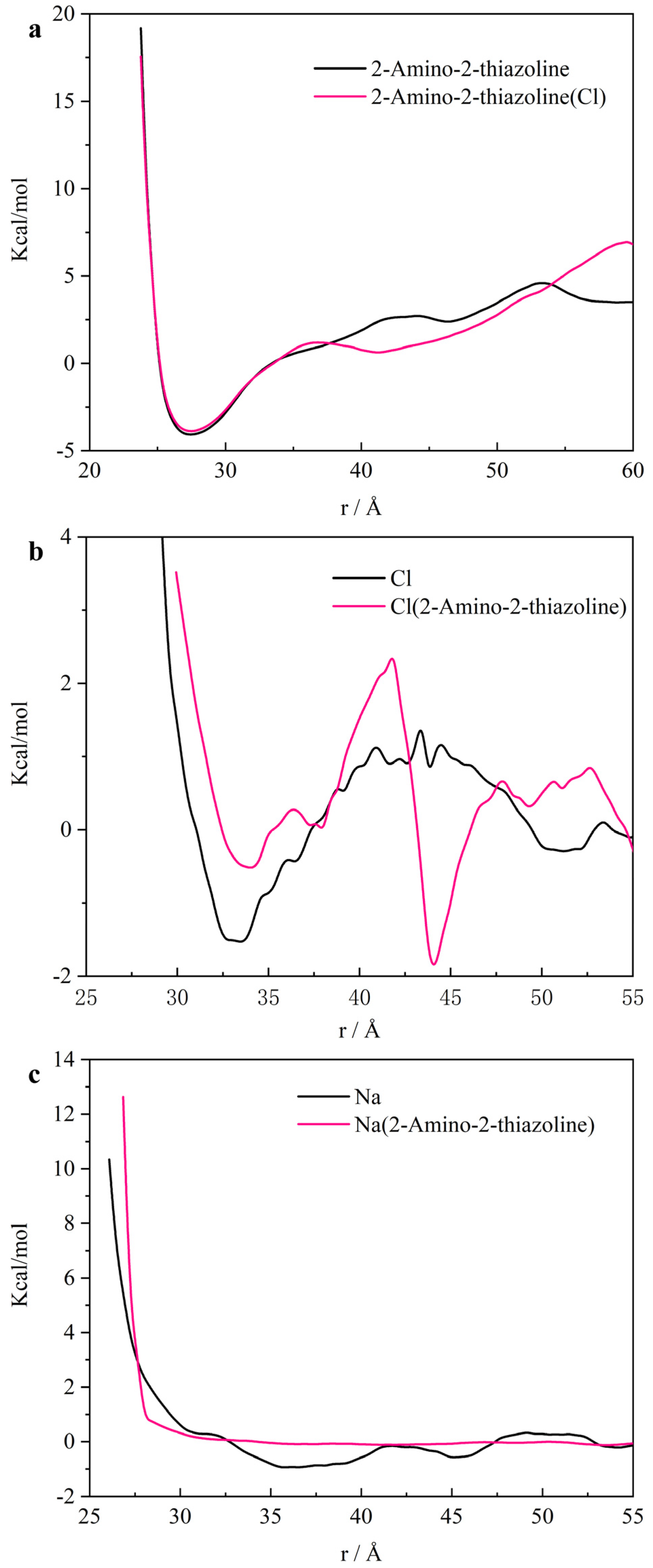
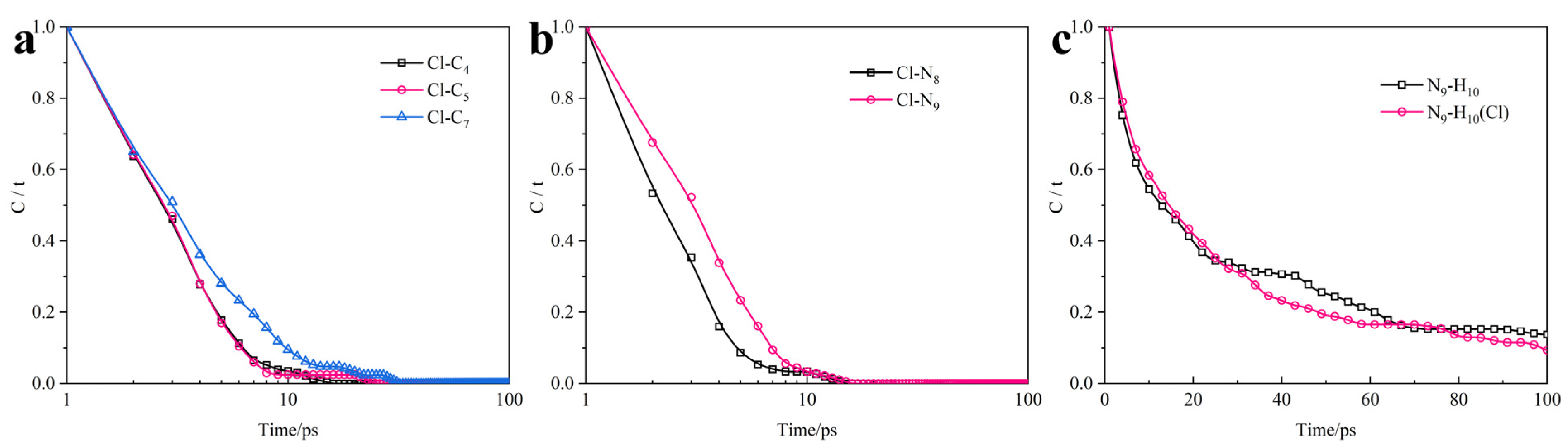
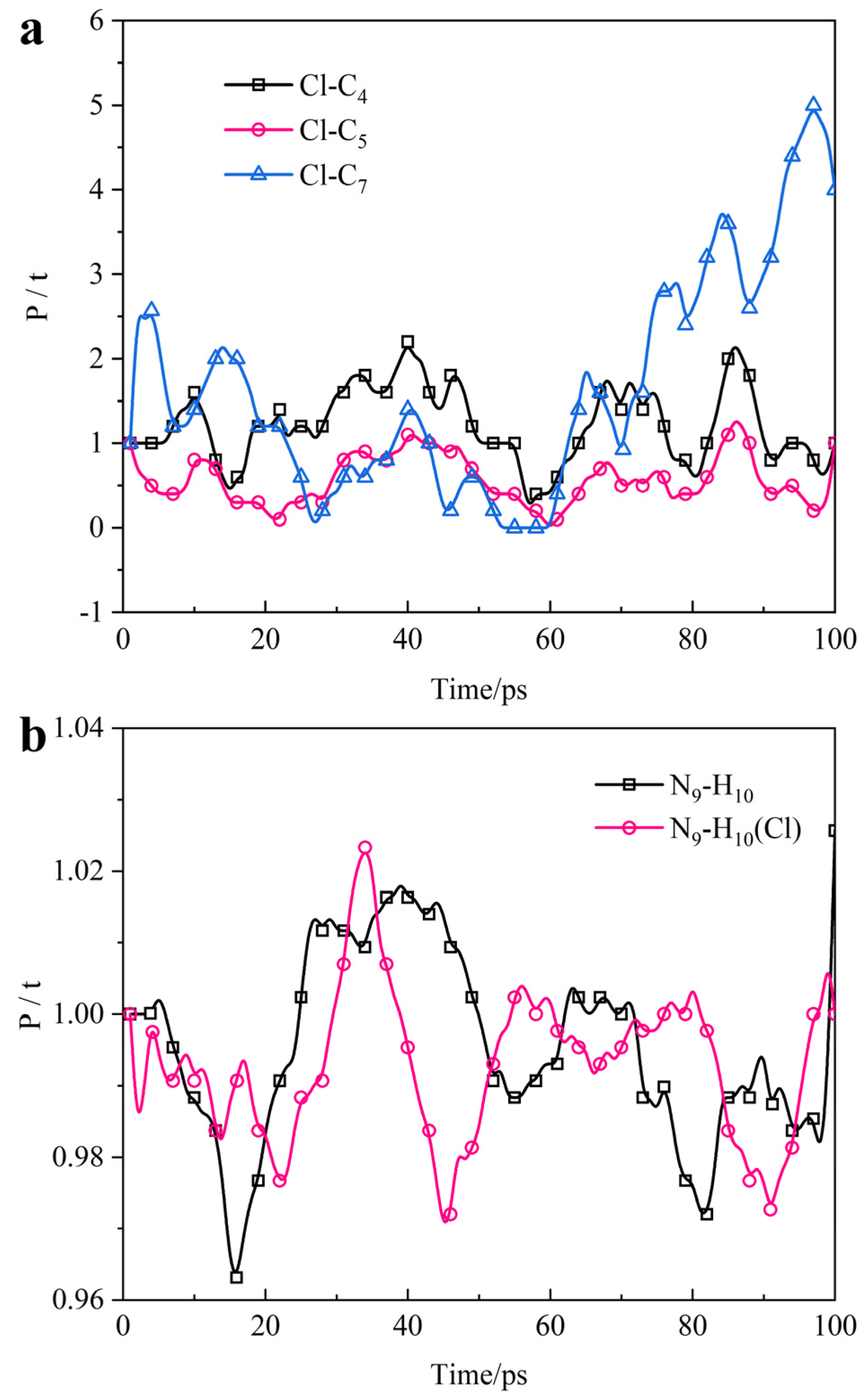
3.3. Micro-Data Analysis
4. Conclusions
- ATL molecules are adsorbed on the iron surface by the Van der Waals force between alkanes and surfaces. It is partially detached under the attack of chloride ions, and the surface coverage of iron decreases from 74% to 64%. But, it still has an overall protective effect on steel. The density distribution of chloride ions shows that there is no chloride distribution below 30 Å.
- ATL molecules expelling chloride ions is an active capture process. (i) Monitor: the adsorption part discovers chloride ions. (ii) Desorption: actively desorbing from the iron surface. (iii) Movement: moving toward chloride ions to approach it. (iv) Control: capturing chloride ions and preventing them from approaching the steel surface.
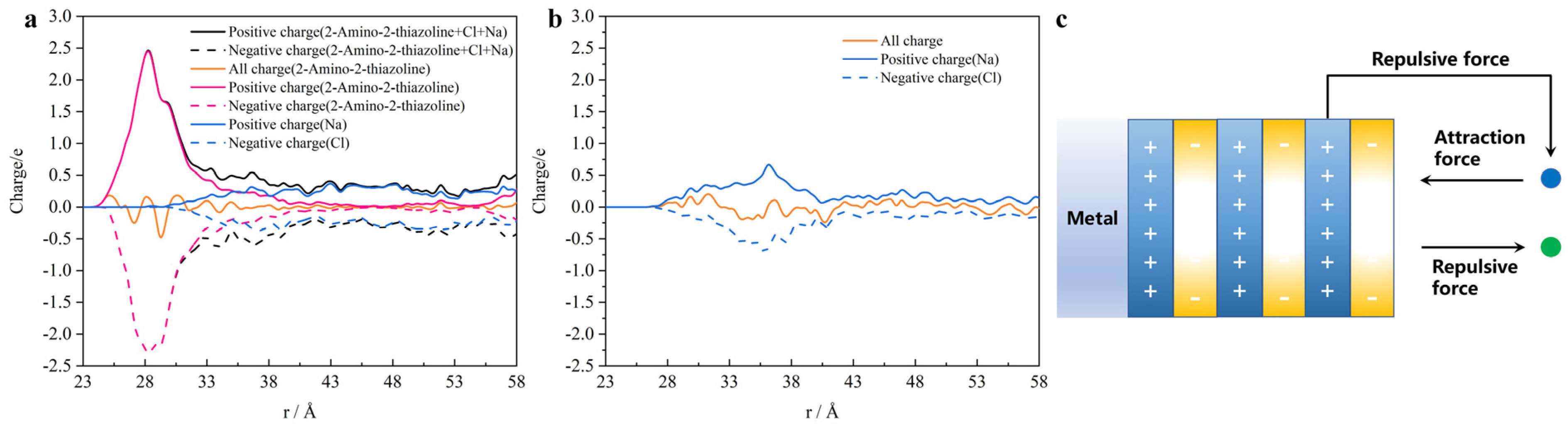
- ATL forms multiple electron layers on the surface of iron in the [010] direction. The outermost layer is negatively charged. It blocks chloride ions passing through the negative electron barrier by the Coulomb force. This causes the free energy curve of the chloride ion barrier to weaken. And it continues to shield the sodium ion in the adjacent positive layer. Therefore, the sodium ion is closer to the iron surface than the chloride ion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alexander, M.; Beushausen, H. Durability, service life prediction, and modelling for reinforced concrete structures—Review and critique. Cem. Concr. Res. 2019, 122, 17–29. [Google Scholar] [CrossRef]
- Alexander, M.G.; Ballim, Y.; Stanish, K. A framework for use of durability indexes in performance-based design and specifications for reinforced concrete structures. Mater. Struct. 2007, 41, 921–936. [Google Scholar] [CrossRef]
- Angst, U.M.; Hooton, R.D.; Marchand, J.; Page, C.L.; Flatt, R.J.; Elsener, B.; Gehlen, C.; Gulikers, J. Present and future durability challenges for reinforced concrete structures. Mater. Corros. 2012, 63, 1047–1051. [Google Scholar] [CrossRef]
- Cheng, A.; Huang, R.; Wu, J.-K.; Chen, C.-H. Influence of GGBS on durability and corrosion behavior of reinforced concrete. Mater. Chem. Phys. 2005, 93, 404–411. [Google Scholar] [CrossRef]
- Deby, F.; Carcassès, M.; Sellier, A. Probabilistic approach for durability design of reinforced concrete in marine environment. Cem. Concr. Res. 2009, 39, 466–471. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Teng, S.; Afroughsabet, V.; Ostertag, C.P. Flexural behavior and durability properties of high performance hybrid-fiber-reinforced concrete. Constr. Build. Mater. 2018, 182, 504–515. [Google Scholar] [CrossRef]
- de Rincón, O.T.; Sánchez, M.; Millano, V.; Fernández, R.; de Partidas, E.A.; Andrade, C.; Martínez, I.; Castellote, M.; Barboza, M.; Irassar, F.; et al. Effect of the marine environment on reinforced concrete durability in Iberoamerican countries: DURACON project/CYTED. Corros. Sci. 2007, 49, 2832–2843. [Google Scholar] [CrossRef]
- Jang, S.; Song, J.; Amalnerkar, D.; Qin, H.; Kim, T. Effect of secondary inhibitors on material removal rate and nano-roughness of Cu chemical mechanical planarization. Mater. Express 2016, 6, 383–393. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, G.; Hu, S.; Yan, Y.; Ren, Z.; Yu, L. Theoretical evaluation of corrosion inhibition performance of imidazoline compounds with different hydrophilic groups. Corros. Sci. 2011, 53, 147–152. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Şafak, S.; Duran, B.; Yurt, A.; Türkoğlu, G. Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros. Sci. 2012, 54, 251–259. [Google Scholar] [CrossRef]
- Branzoi, V.; Golgovici, F.; Branzoi, F. Aluminium corrosion in hydrochloric acid solutions and the effect of some organic inhibitors. Mater. Chem. Phys. 2002, 78, 122–131. [Google Scholar] [CrossRef]
- Salasi, M.; Stachowiak, G.B.; Stachowiak, G.W.; Kilburn, M.R. NanoSIMS investigation of passive oxide films on high-Cr cast iron. Corros. Sci. 2013, 67, 298–303. [Google Scholar] [CrossRef]
- Söylev, T.A.; Richardson, M.G. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Goyal, A.; Pouya, H.S.; Ganjian, E.; Claisse, P. A Review of Corrosion and Protection of Steel in Concrete. Arab. J. Sci. Eng. 2018, 43, 5035–5055. [Google Scholar] [CrossRef]
- Li, C.; Chen, Q.; Wang, R.; Wu, M.; Jiang, Z. Corrosion assessment of reinforced concrete structures exposed to chloride environments in underground tunnels: Theoretical insights and practical data interpretations. Cem. Concr. Compos. 2020, 112, 103652. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, S.; Du, Y.; Lin, Z.; Liang, X.; Liu, J.; Xing, F. Field test of a reinforced concrete bridge under marine environmental corrosion. Eng. Fail. Anal. 2020, 115, 104669. [Google Scholar] [CrossRef]
- Díez-Pérez, I.; Vericat, C.; Gorostiza, P.; Sanz, F. The iron passive film breakdown in chloride media may be mediated by transient chloride-induced surface states located within the band gap. Electrochem. Commun. 2006, 8, 627–632. [Google Scholar] [CrossRef]
- Gaidis, J.M. Chemistry of corrosion inhibitors. Cem. Concr. Compos. 2004, 26, 181–189. [Google Scholar] [CrossRef]
- Ormellese, M.; Berra, M.; Bolzoni, F.; Pastore, T. Corrosion inhibitors for chlorides induced corrosion in reinforced concrete structures. Cem. Concr. Res. 2006, 36, 536–547. [Google Scholar] [CrossRef]
- Palanivel, V.; Huang, Y.; van Ooij, W.J. Effects of addition of corrosion inhibitors to silane films on the performance of AA2024-T3 in a 0.5M NaCl solution. Prog. Org. Coat. 2005, 53, 153–168. [Google Scholar] [CrossRef]
- John, S. Challenges of chromate inhibitor pigments replacement in organic coatings. Prog. Org. Coat. 2001, 42, 267–282. [Google Scholar]
- Nmai, C.K. Multi-functional organic corrosion inhibitor. Cem. Concr. Compos. 2004, 26, 199–207. [Google Scholar] [CrossRef]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on Corrosion Inhibitors: A Short View. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Tang, Z. A review of corrosion inhibitors for rust preventative fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759. [Google Scholar] [CrossRef]
- de Souza, F.S.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Moretti, G.; Guidi, F.; Grion, G. Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros. Sci. 2004, 46, 387–403. [Google Scholar] [CrossRef]
- Hoseinzadeh, A.R.; Javadpour, S. Electrochemical, thermodynamic and theoretical study on anticorrosion performance of a novel organic corrosion inhibitor in 3.5% NaCl solution for carbon steel. Bull. Mater. Sci. 2019, 42, 188. [Google Scholar] [CrossRef]
- Norman, A.B.; Simon, J.C.; Christopher, P.C.; David, E.H.; Michael, B.H.; Rafizah, M.; Eric, S.R.; Chris, S. Complexes of heterocyclic thiones and Group 12 metals Part 5. Reactions of 1,3-thiazolidine-2-thione and benzo-1,3-thiazoline-2-thione with mercury(II) halides in a 2:1 ratio. Crystal structures of bis(1,3-thiazolidine-2-thione) mercury(II) bromide and bis(benzo-1,3-thiazolinato)mercury(II). Inorganica Chim. Acta 2001, 323, 69–77. [Google Scholar]
- Shih, M.H.; Ke, F.Y. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorganic Med. Chem. 2004, 12, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, L.; Han, X.; Yao, L.; Zhao, S.; Sun, J.; Xu, Y.; Li, J.; Xiong, C. Green chemical synthesis of new chelating fiber and its mechanism for recovery gold from aqueous solution. J. Hazard. Mater. 2019, 378, 120674. [Google Scholar] [CrossRef] [PubMed]
- Hekmatifar, M.; Toghraie, D.; Khosravi, A.; Saberi, F.; Soltani, F.; Sabetvand, R.; Goldanlou, A.S. The study of asphaltene desorption from the iron surface with molecular dynamics method. J. Mol. Liq. 2020, 318, 114325. [Google Scholar] [CrossRef]
- Verma, C.; Lgaz, H.; Verma, D.K.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase: A review. J. Mol. Liq. 2018, 260, 99–120. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Ren, H.; Zhao, W.; Zhang, H. Inhibition performance of 2-mercaptobenzothiazole derivatives in CO2 saturated solution and its adsorption behavior at Fe surface. Appl. Surf. Sci. 2007, 253, 7416–7422. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B.; Mahdavian, M.; Motamedi, M. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martinez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Ezquerro, C.S.; Aznar, J.M.G.; Laspalas, M. Prediction of the structure and mechanical properties of polycaprolactone-silica nanocomposites and the interphase region by molecular dynamics simulations: The effect of PEGylation. Soft Matter 2022, 18, 2800–2813. [Google Scholar] [CrossRef] [PubMed]
- Hockney, R.W.; Goel, S.P.; Eastwood, J.W. Quiet high-resolution computer models of a plasma. J. Comput. Phys. 1974, 14, 148–158. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Margenau, H. Van der waals forces. Rev. Mod. Phys. 1939, 11, 1–35. [Google Scholar] [CrossRef]
- Jedicke, R.; Nesvorny, D.; Whiteley, R.; Ivezic, Z.Z.; Juric, M. An age-colour relationship for main-belt S-complex asteroids. Nature 2004, 429, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Niermann, B.; Böke, M.; Sadeghi, N.; Winter, J. Space resolved density measurements of argon and helium metastable atoms in radio-frequency generated He-Ar micro-plasmas. Eur. Phys. J. D 2010, 60, 489–495. [Google Scholar] [CrossRef]
- Harmandaris, V.A.; Daoulas, K.C.; Mavrantzas, V.G. Molecular Dynamics Simulation of a Polymer Melt/Solid Interface: Local Dynamics and Chain Mobility in a Thin Film of Polyethylene Melt Adsorbed on Graphite. Macromolecules 2005, 38, 5796–5809. [Google Scholar] [CrossRef]
- Domińczuk, J.; Krawczuk, A. Comparison of Surface Free Energy Calculation Methods. Appl. Mech. Mater. 2015, 791, 259–265. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, D.; Darve, E.; Pohorille, A. Assessing the efficiency of free energy calculation methods. J. Chem. Phys. 2004, 120, 3563–3578. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, K.; Hong, F.; Wu, S.; Wang, Z.; Li, M.; Wang, M. The corrosion deterioration of reinforced passivation Film: The impact of defects. Appl. Surf. Sci. 2022, 582, 152408. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, K.; Ji, X.; Wang, P.; Ma, H.; Zhang, J.; Hou, D. Molecular insight into the fluidity of cement pastes: Nano-boundary lubrication of cementitious materials. Constr. Build. Mater. 2022, 316, 125800. [Google Scholar] [CrossRef]
- Han, Y.; Elliott, J. Molecular dynamics simulations of the elastic properties of polymer/carbon nanotube composites. Comput. Mater. Sci. 2007, 39, 315–323. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, W.; Sun, M.; Wang, P.; Wang, M.; Zhang, J.; Li, Z. Modified Lucas-Washburn function of capillary transport in the calcium silicate hydrate gel pore: A coarse-grained molecular dynamics study. Cem. Concr. Res. 2020, 136, 106166. [Google Scholar] [CrossRef]
- Wang, M.; Fang, T.; Zhong, H.; Li, J.; Yan, Y.; Zhang, J. Optimal aggregation number of reverse micelles in supercritical carbon dioxide: A theoretical perspective. Soft Matter 2019, 15, 3323–3329. [Google Scholar] [CrossRef]
- Hou, D.; Yu, J.; Liu, Q.-F.; Dong, B.; Wang, X.; Wang, P.; Wang, M. Nanoscale insight on the epoxy-cement interface in salt solution: A molecular dynamics study. Appl. Surf. Sci. 2020, 509, 145322. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, K.; Hou, D.; Wang, P. Microscopic insight into nanodiamond polymer composites: Reinforcement, structural, and interaction properties. Nanoscale 2020, 12, 24107–24118. [Google Scholar] [CrossRef]
- Stalke, D. Meaningful structural descriptors from charge density. Chemistry 2011, 17, 9264–9278. [Google Scholar] [CrossRef]
- Sun, X.D.; Yuan, X.Z.; Jia, Y.; Feng, L.J.; Zhu, F.P.; Dong, S.S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Liu, C.; Xu, H.; Guo, F.; Chen, F.; Li, M.; Wang, P. Protective Effects of Small Molecular Inhibitors on Steel Corrosion: The Generation of a Multi-Electric Layer on Passivation Films. Buildings 2024, 14, 2558. https://doi.org/10.3390/buildings14082558
Wu S, Liu C, Xu H, Guo F, Chen F, Li M, Wang P. Protective Effects of Small Molecular Inhibitors on Steel Corrosion: The Generation of a Multi-Electric Layer on Passivation Films. Buildings. 2024; 14(8):2558. https://doi.org/10.3390/buildings14082558
Chicago/Turabian StyleWu, Shenrong, Chengbo Liu, Hongjian Xu, Feng Guo, Feixiang Chen, Mengmeng Li, and Pan Wang. 2024. "Protective Effects of Small Molecular Inhibitors on Steel Corrosion: The Generation of a Multi-Electric Layer on Passivation Films" Buildings 14, no. 8: 2558. https://doi.org/10.3390/buildings14082558
APA StyleWu, S., Liu, C., Xu, H., Guo, F., Chen, F., Li, M., & Wang, P. (2024). Protective Effects of Small Molecular Inhibitors on Steel Corrosion: The Generation of a Multi-Electric Layer on Passivation Films. Buildings, 14(8), 2558. https://doi.org/10.3390/buildings14082558





