Efficacy of Accelerated Carbonation Curing and Its Influence on the Strength Development of Concrete
Abstract
1. Introduction
2. Materials and Methods
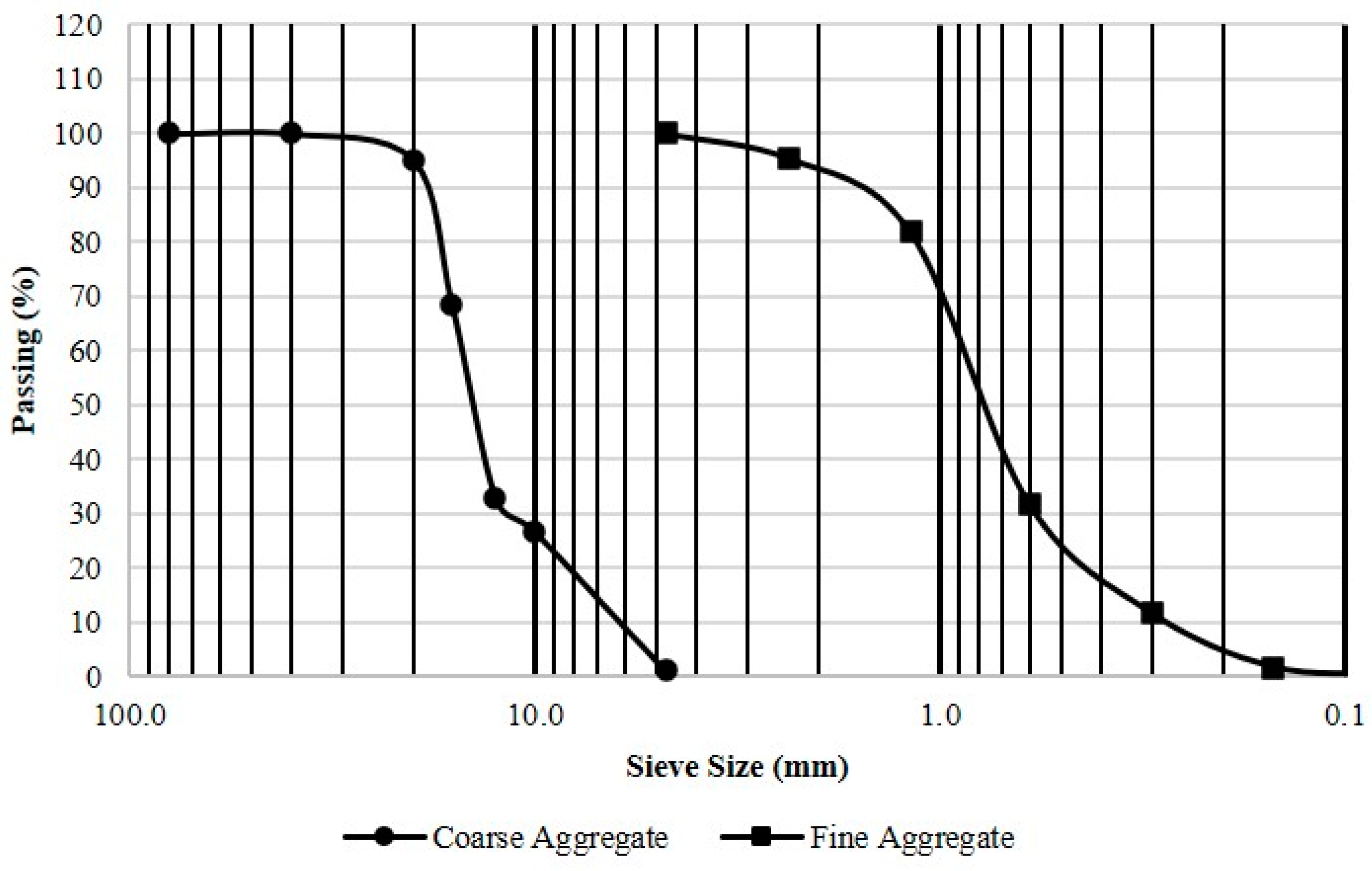
2.1. Material
2.2. Concrete Mixtures
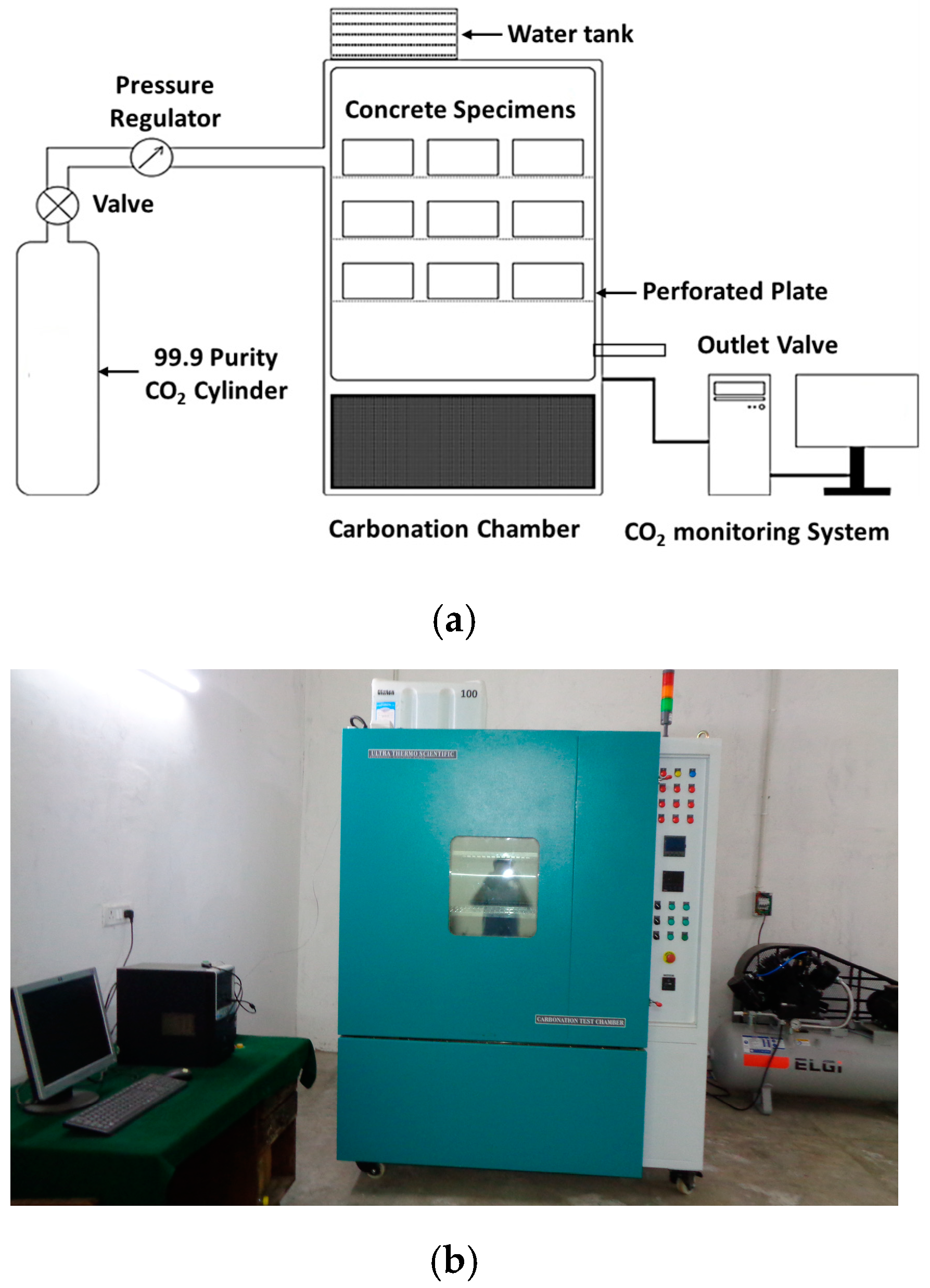
2.3. Experimental Setup for Carbonation
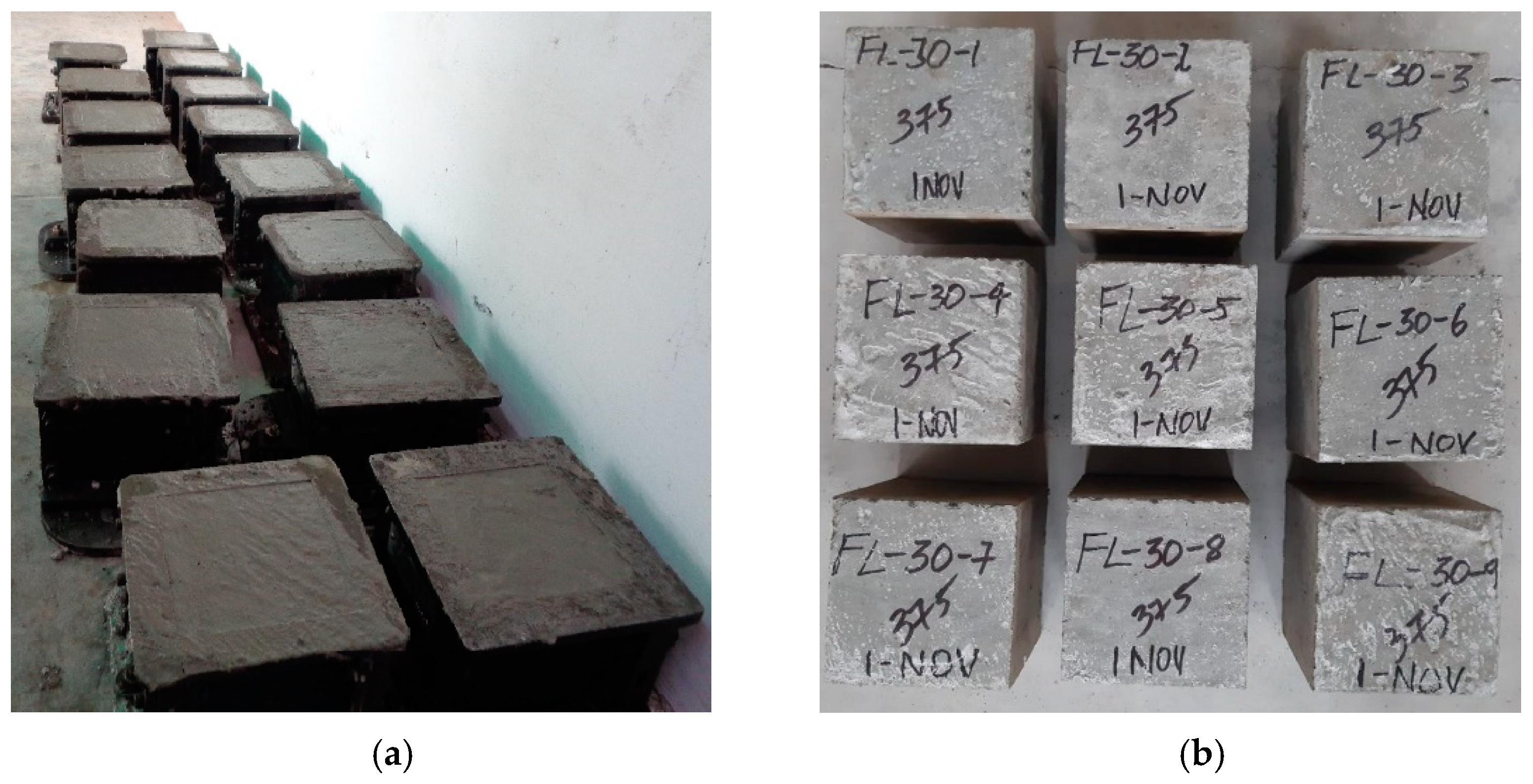
2.4. Preparation and Curing of Sample
2.5. Testing of Specimens
3. Results and Discussion
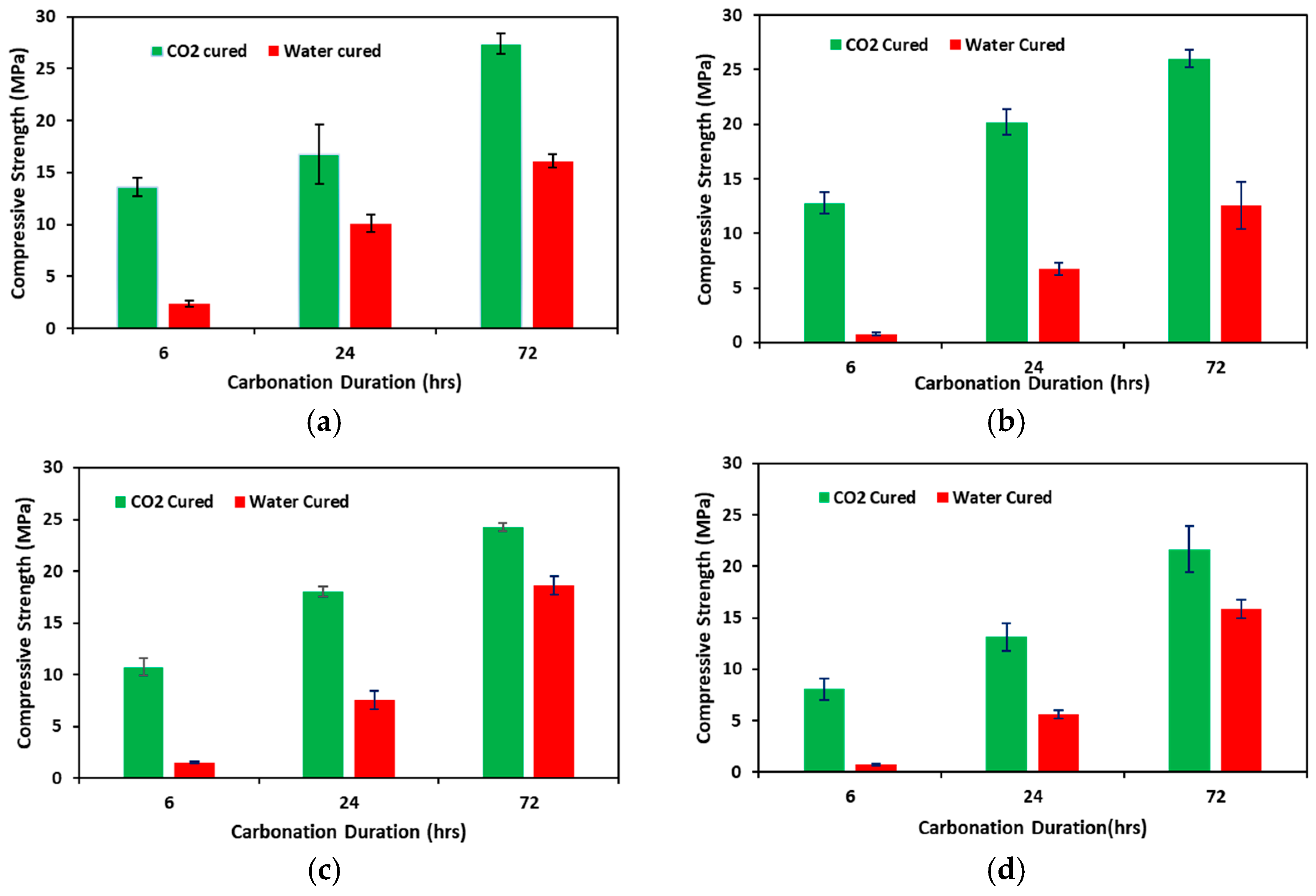
3.1. Compressive Strength
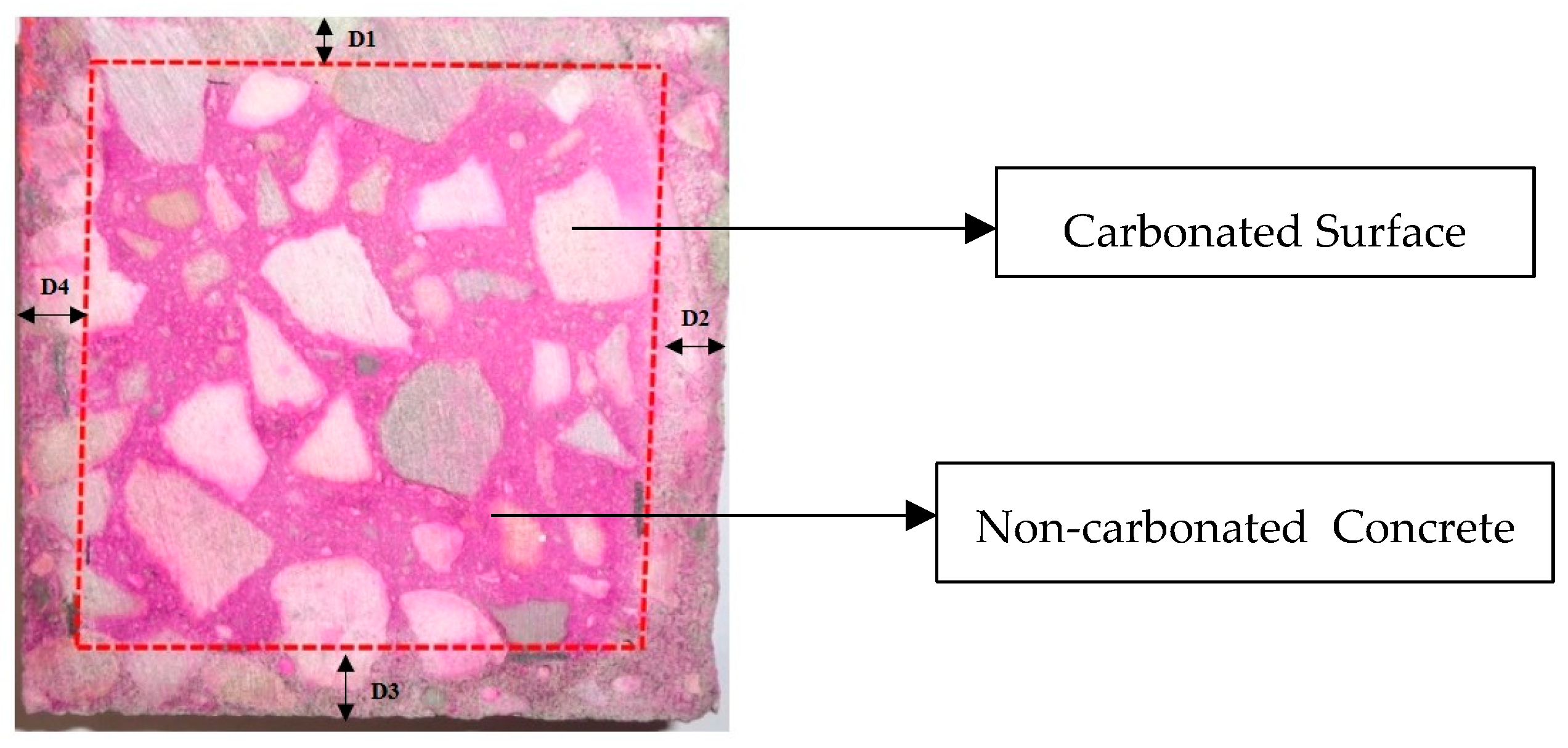
3.2. Carbonation Depth and pH Analysis
3.3. Microstructural Study
3.3.1. XRD Analysis
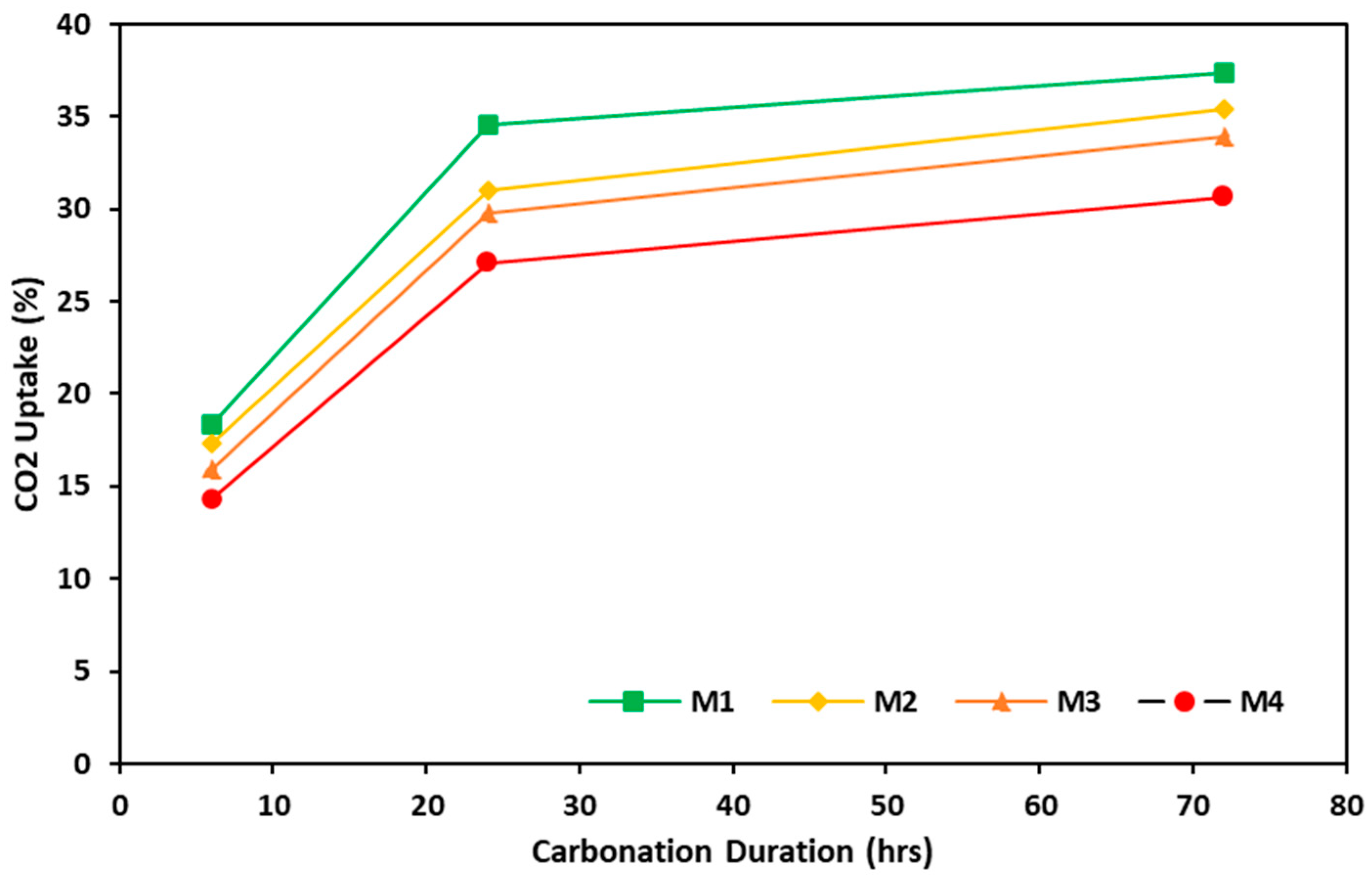
3.3.2. TGA and CO2 Uptake
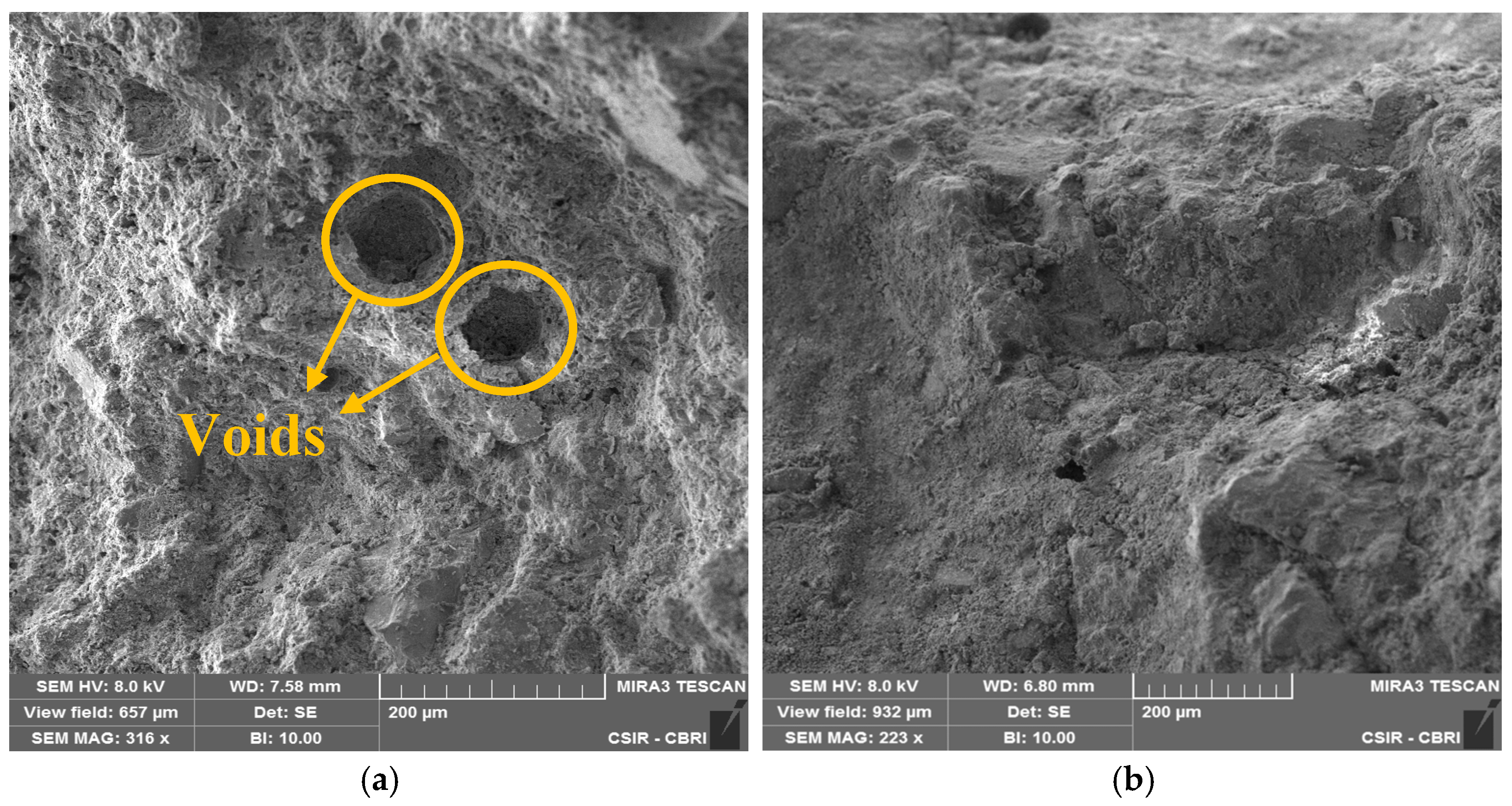
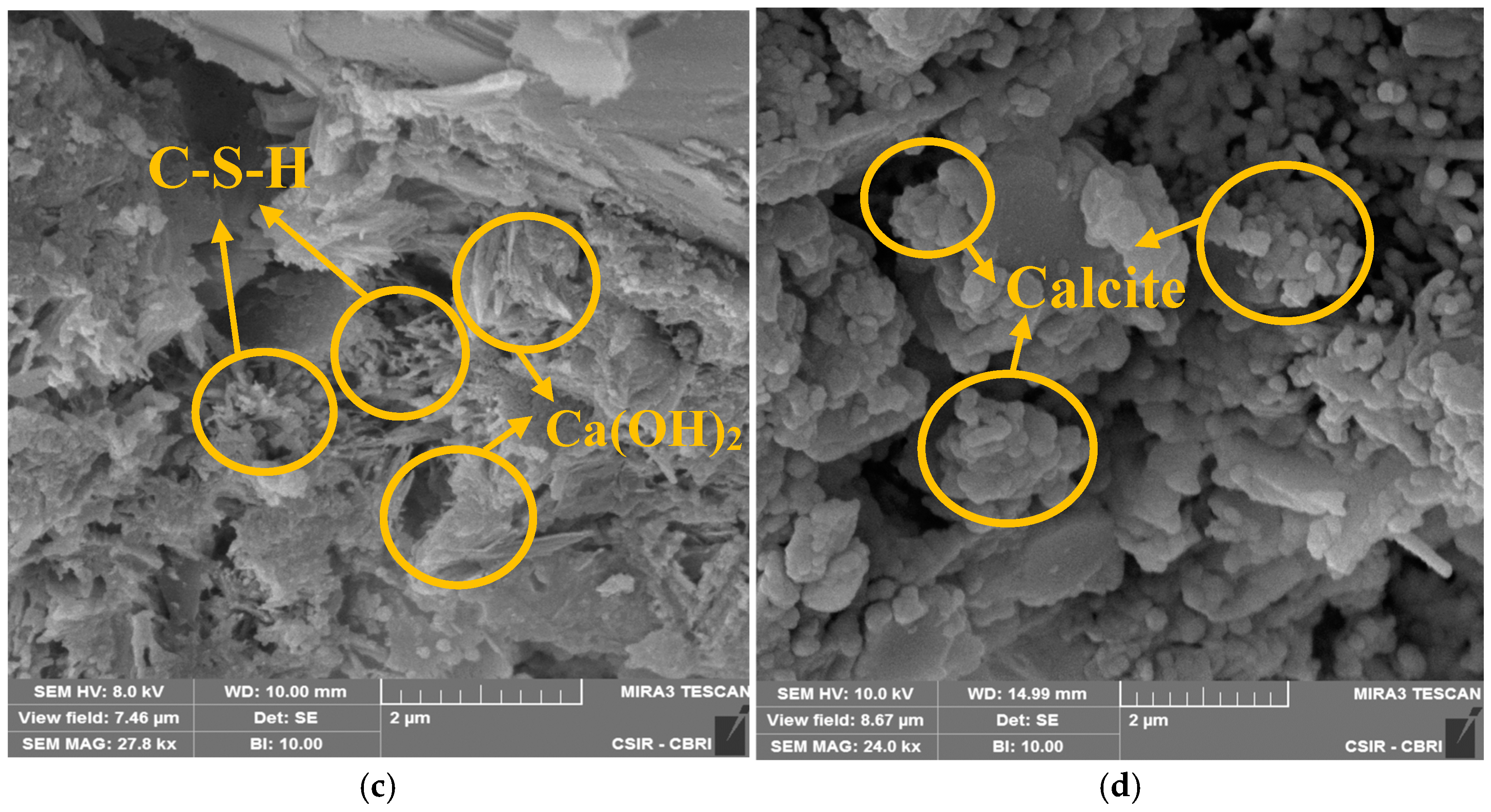
3.4. SEM
3.5. Statistical Analysis
4. Conclusions
- (1)
- Carbonation curing leads to accelerated strength development and densification of the concrete surface, resulting in improved durability over conventional water-curing methods.
- (2)
- The process of carbonation curing allows concrete to absorb CO2, offering potential benefits for reducing greenhouse gas emissions and mitigating global warming.
- (3)
- There is a significant improvement in the compressive strength with a percentage increase of 70.46%, 111.28%, 30.36%, and 36.69%, respectively, for 0%, 10%, 20%, and 30% fly ash contents in concrete samples subjected to 72 h of accelerated carbonation curing.
- (4)
- Longer durations of carbonation result in higher compressive strength gains, with 72-h durations showing the most significant improvement. Additionally, substituting fly ash with 10% in the mix can achieve comparable strength to conventional water-cured concrete.
- (5)
- While the pH of carbonated concrete is lower than water-cured concrete, it remains within safe limits to prevent reinforcement corrosion. Additionally, the average carbonation depth is minimal, reducing the risk of CO2-induced corrosion.
- (6)
- The use of carbonation curing with industrial waste fly ash not only creates eco-friendly concrete but also contributes to CO2 sequestration efforts, promoting sustainability in construction practices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Huang, L.; Krigsvoll, G.; Johansen, F.; Liu, Y.; Zhang, X. Carbon emission of global construction sector. Renew. Sustain. Energy Rev. 2018, 81, 1906–1916. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Shi, Q.; Peng, X.; Zheng, J. An international comparison analysis of CO2 emissions in the construction industry. Sustain. Dev. 2021, 29, 754–767. [Google Scholar] [CrossRef]
- Ye, J.; Liu, S.; Zhao, Y.; Li, Y.; Fang, J.; Zhang, H.; Guan, X. Development of Ultrafine Mineral Admixture from Magnesium Slag and Sequestration of CO2. Buildings 2023, 13, 204. [Google Scholar] [CrossRef]
- Davidovits, J. Global Warming Impact on the Cement and Aggregates Industries. World Resour. Rev. 1994, 6, 263–278. [Google Scholar]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Meyer, C. The greening of the concrete industry. Cem. Concr. Compos. 2009, 31, 601–605. [Google Scholar] [CrossRef]
- Pasupathy, K.; Sanjayan, J.; Rajeev, P. Evaluation of alkalinity changes and carbonation of geopolymer concrete exposed to wetting and drying. J. Build. Eng. 2021, 35, 102029. [Google Scholar] [CrossRef]
- Guleria, H.; Sharma, D.; Goyal, S. Long term performance of concrete using accelerated carbonation curing: An effective CCUS technique. Eur. J. Environ. Civ. Eng. 2023, 28, 2138–2159. [Google Scholar] [CrossRef]
- Goyal, S.; Sharma, D. CO2 Sequestration on Cement; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation Curing versus Steam Curing for Precast Concrete Production. J. Mater. Civ. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of Hydration of Calcium Silicates by Carbon Dioxide Treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Berger, R.L. Stabilization of silicate structures by carbonation. Cem. Concr. Res. 1979, 9, 649–651. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J.; He, Z. Microstructure of cement paste subject to early carbonation curing. Cem. Concr. Res. 2012, 42, 186–193. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuge, Y.; Chow, C.W.; Keegan, A.; Li, D.; Pham, P.N.; Huang, J.; Siddique, R. Properties and microstructure of concrete blocks incorporating drinking water treatment sludge exposed to early-age carbonation curing. J. Clean. Prod. 2020, 261, 121257. [Google Scholar] [CrossRef]
- Jang, J.; Kim, G.; Kim, H.; Lee, H. Review on recent advances in CO2 utilization and sequestration technologies in cement-based materials. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Sharma, D.; Goyal, S. Effect of accelerated carbonation curing on near surface properties of concrete. Eur. J. Environ. Civ. Eng. 2020, 26, 1300–1321. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. A reaction engineering approach to the problem of concrete carbonation. AIChE J. 1989, 35, 1639–1650. [Google Scholar] [CrossRef]
- Zhan, B.J.; Poon, C.S.; Shi, C.J. Materials characteristics affecting CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 67, 50–59. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Development of a new generation of eco-friendly concrete blocks by accelerated mineral carbonation. J. Clean. Prod. 2016, 133, 1235–1241. [Google Scholar] [CrossRef]
- IS: 8112—2013; Specification for 43 Grade Ordinary Portland Cement. Bureau of Indian Standard (BIS): Delhi, Indian, 2013; p. 17.
- IS:3812 (Part-1); Pulverized Fuel Ash—Specification. Part 1: For use as Pozzolana in cement, Cement Mortar and Concrete (Second Revision). Bureau of Indian Standards: New Delhi, India, 2013; pp. 1–14.
- IS 383 (2016); Coarse and Fine Aggregate for Concrete-Specification. Bureau of Indian Standard: New Delhi, India, 2016; p. 110002.
- Assaggaf, R.; Adekunle, S.K.; Ahmad, S.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Ali, S. Mechanical properties, durability characteristics and shrinkage of plain cement and fly ash concretes subjected to accelerated carbonation curing. J. S. Afr. Inst. Civ. Eng. 2019, 61, 73–81. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Durability of concrete pipes subjected to combined steam and carbonation curing. Constr. Build. Mater. 2011, 25, 3345–3355. [Google Scholar] [CrossRef]
- Reddy, P.N.; Naqash, J.A. Experimental study on TGA, XRD and SEM analysis of concrete with ultra-fine slag. Int. J. Eng. Trans. B Appl. 2019, 32, 679–684. [Google Scholar] [CrossRef]
- Xian, X.; Zhang, D.; Lin, H.; Shao, Y. Ambient pressure carbonation curing of reinforced concrete for CO2 utilization and corrosion resistance. J. CO2 Util. 2022, 56, 101861. [Google Scholar] [CrossRef]
- Horn, L. Targeted/Emerging Therapies for Metastatic Non–Small Cell Lung Cancer. J. Natl. Compr. Cancer Netw. 2015, 13, 676–678. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Kirkpatrick, R.J. Innovation in use and research on cementitious material. Cem. Concr. Res. 2008, 38, 128–136. [Google Scholar] [CrossRef]
- Douglas, C.M. Design and Analysis of Experiments; John Wiley & Sons, Inc.: New York, NY, USA, 2012. [Google Scholar]




| Oxide | CaO | SiO2 | Al2O3 | Fe2O3 | MgO |
|---|---|---|---|---|---|
| Cement | 63.50 | 21.70 | 6.60 | 4.60 | 2.40 |
| Fly ash | 3.13 | 62.57 | 27.40 | 5.92 | 0.98 |
| Materials | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| OPC | 375 | 337.5 | 300 | 262.5 |
| Fly ash | - | 37.5 | 75 | 112.5 |
| Fine Aggregate | 672.06 | 672.06 | 672.06 | 672.06 |
| Coarse Aggregate | 1157.57 | 1157.57 | 1157.57 | 1157.57 |
| Water | 168.75 | 168.75 | 168.75 | 168.75 |
| Response Factor | Control Factor | DF | SS | MS | F-Level | Contribution (%) | p-Level |
|---|---|---|---|---|---|---|---|
| Compressive Strength | FAC | 3 | 53.6 | 17.87 | 11.95 | 12.4 | 0.006 |
| CD | 2 | 369.37 | 184.69 | 123.47 | 85.5 | 0 | |
| Error | 6 | 8.98 | 22.55 | 2.1 | |||
| pH | FAC | 3 | 0.43 | 0.14 | 10.7 | 59.72 | 0.008 |
| CD | 2 | 0.21 | 0.1 | 7.72 | 29.16 | 0.022 | |
| Error | 6 | 0.08 | 0.01 | ||||
| CO2 uptake | FAC | 3 | 4.47 | 1.49 | 12.5 | 21.3 | 0.005 |
| CD | 2 | 15.29 | 7.89 | 66.23 | 75.26 | 0 | |
| Error | 6 | 0.72 | 0.012 | 3.44 |
| Input Term | Output Parameters | |||||
|---|---|---|---|---|---|---|
| Compressive Strength | CO2 Uptake | |||||
| Coefficient | T | p | Coefficient | T | p | |
| Constant | 10.5182 | 8.373 | 0.000 | 2.91796 | 7.725 | 0.000 |
| FAC | 0.1227 | 1.045 | 0.336 | −0.11938 | −3.382 | 0.015 |
| CD | 0.3916 | 5.121 | 0.002 | 0.18037 | 7.845 | 0.000 |
| FAC × FAC | −0.0094 | −2.656 | 0.038 | 0.00185 | 1.738 | 0.133 |
| CD × CD | −0.0023 | −2.600 | 0.041 | −0.0019 | −7.029 | 0.000 |
| FAC × CD | −0.0003 | −0.248 | 0.813 | 0.00040 | 1.183 | 0.282 |
| Standard Deviation | 1.22673 | 0.36883 | ||||
| R Sq. (%) | 97.9 | 96.11 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padmalal, A.; Kulkarni, K.S.; Rawat, P.; Sugandhini, H.K. Efficacy of Accelerated Carbonation Curing and Its Influence on the Strength Development of Concrete. Buildings 2024, 14, 2573. https://doi.org/10.3390/buildings14082573
Padmalal A, Kulkarni KS, Rawat P, Sugandhini HK. Efficacy of Accelerated Carbonation Curing and Its Influence on the Strength Development of Concrete. Buildings. 2024; 14(8):2573. https://doi.org/10.3390/buildings14082573
Chicago/Turabian StylePadmalal, Akarsh, Kishor S. Kulkarni, Pradeep Rawat, and H. K. Sugandhini. 2024. "Efficacy of Accelerated Carbonation Curing and Its Influence on the Strength Development of Concrete" Buildings 14, no. 8: 2573. https://doi.org/10.3390/buildings14082573
APA StylePadmalal, A., Kulkarni, K. S., Rawat, P., & Sugandhini, H. K. (2024). Efficacy of Accelerated Carbonation Curing and Its Influence on the Strength Development of Concrete. Buildings, 14(8), 2573. https://doi.org/10.3390/buildings14082573






