Long-Term Performance of Mortars with Combined Incorporation of Ladle Furnace Slag and Metakaolin
Abstract
:1. Introduction
2. Materials and Methods: Description and Analysis
2.1. Characterization of the Materials
2.2. Analysis of the Mortars
| Test | No. of Specimens | Dimension (*) | Standard | |
|---|---|---|---|---|
| Fresh state | Consistence | NBR 13276 [40] | ||
| Water retention | NBR 13277 [43] | |||
| Entrained air content | NBR 13278 [44] | |||
| Density | NBR 13278 [44] | |||
| Hardened State | Flexural strength (Fs) | 3 | 40 × 40 × 160 | NBR 13279 [45] |
| Compressive strength (Cs) | 3 | 40 × 40 × 160 | NBR 13279 [45] | |
| Apparent density | 3 | 40 × 40 × 160 | NBR 13280 [46] | |
| Water absorption by capillarity | 3 | 40 × 40 × 160 | NBR 15259 [47] | |
| Dynamic modulus of elasticity | 3 | 40 × 40 × 160 | NBR 15630 [48] | |
| Volumetric stability | 3 | 25 × 25 × 285 | NBR 15261 [49] |
3. Results and Discussion
3.1. Performance Properties of Mortars in the Fresh State
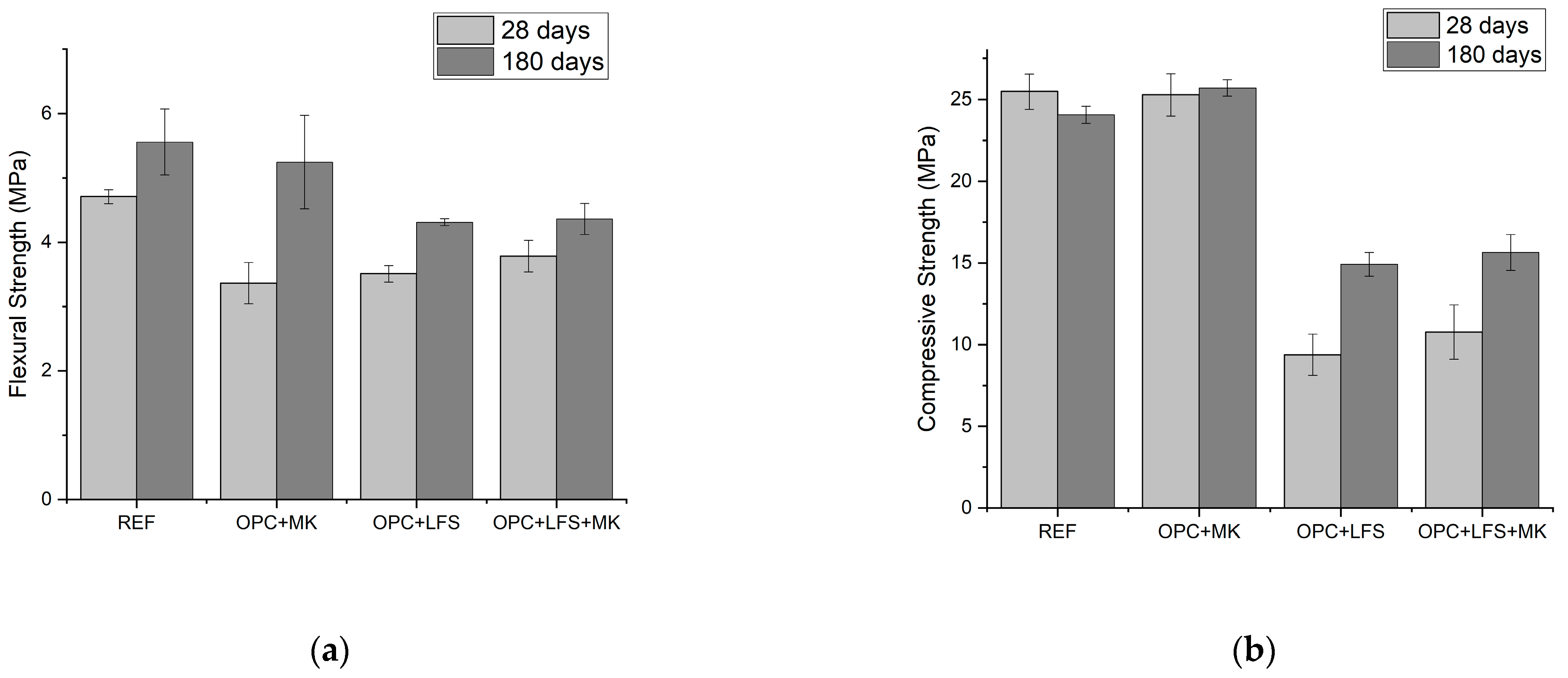
3.2. Mechanical Properties of Mortars
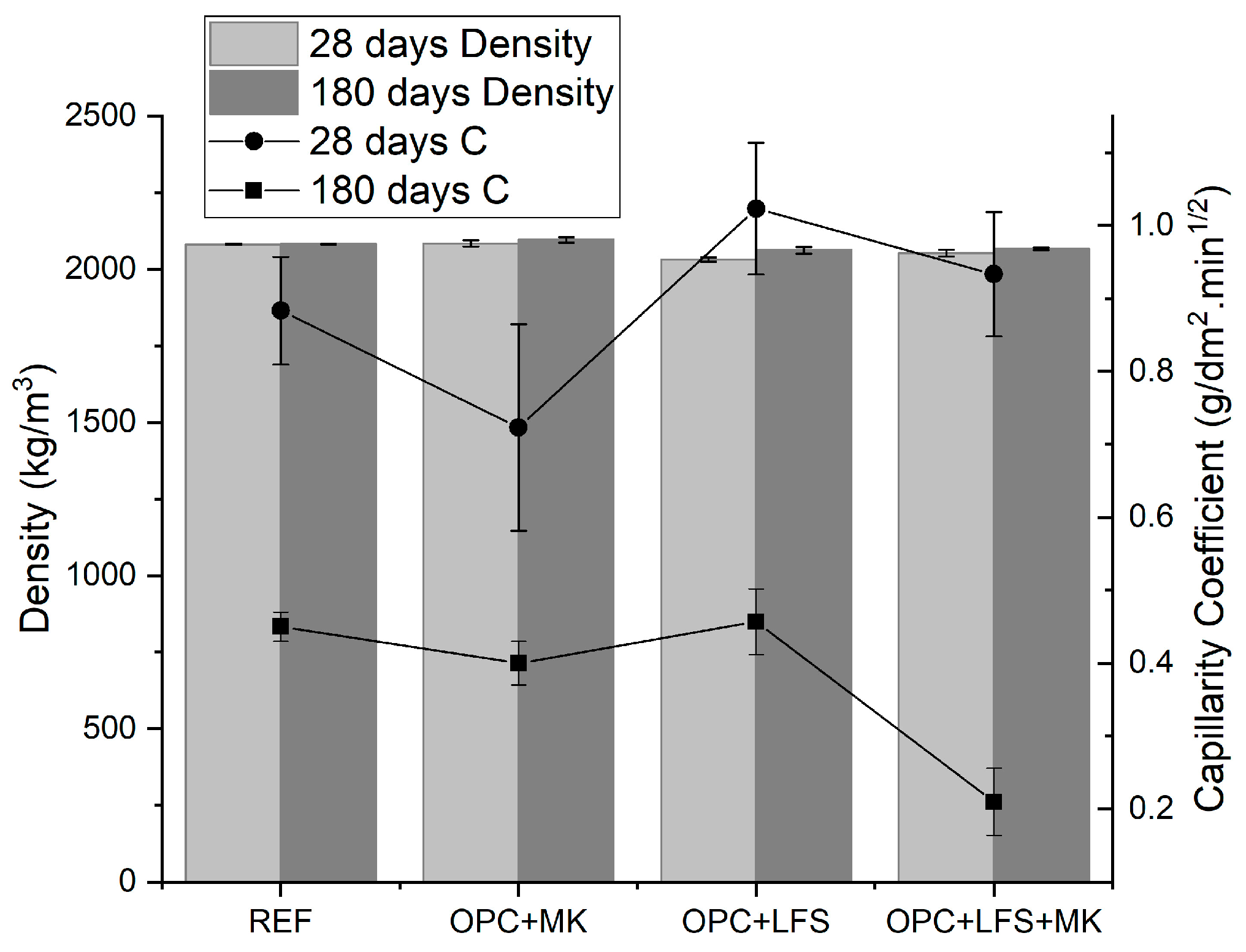
3.3. Performance Properties of Mortars in the Hardened State
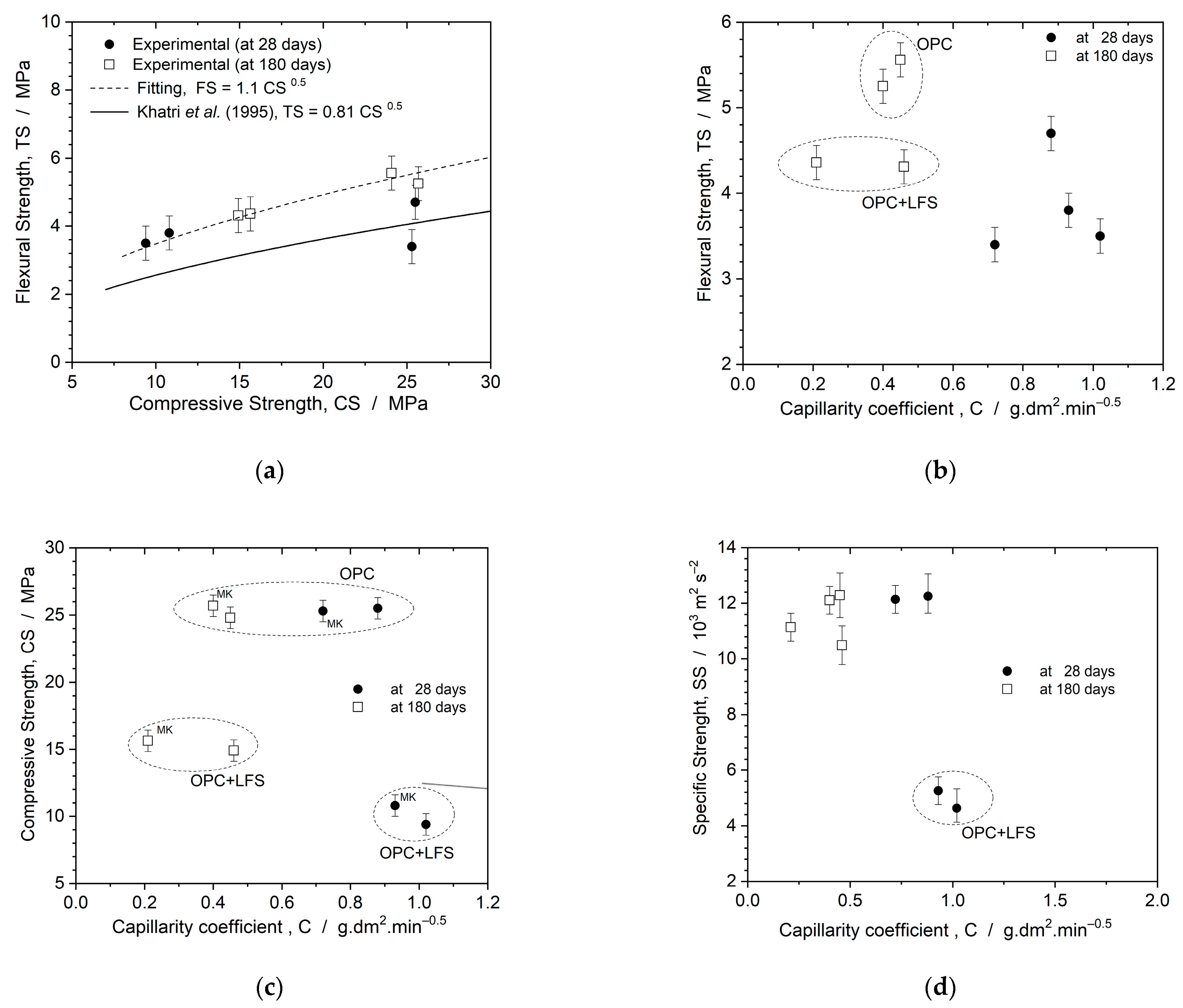
3.4. Correlation between Mechanical Properties and Capillarity Coefficient of Mortars
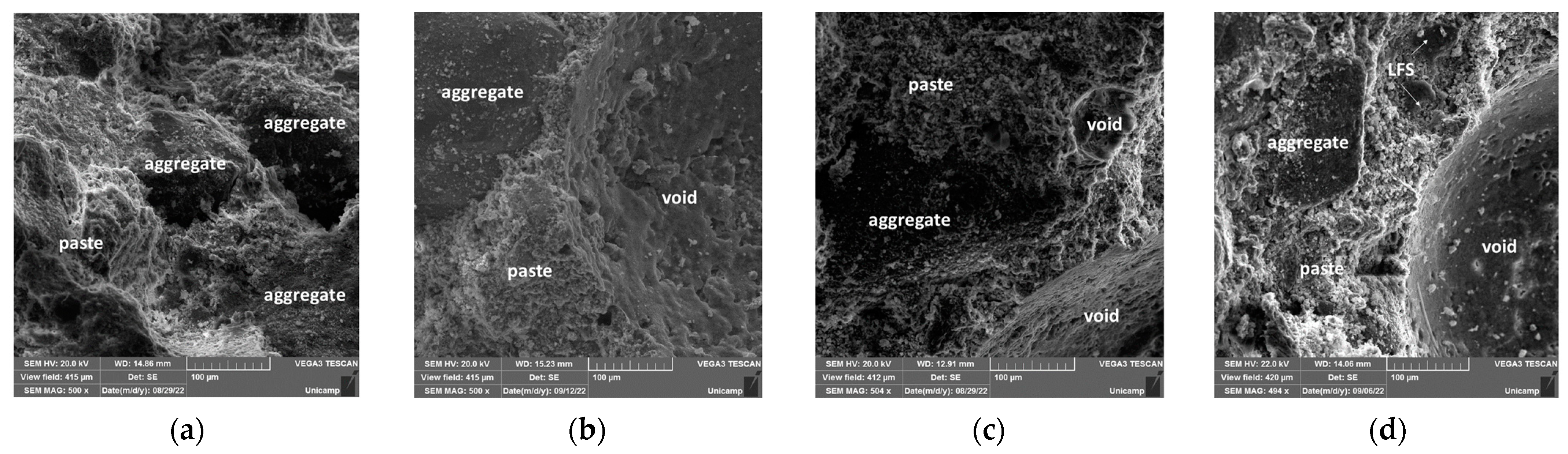
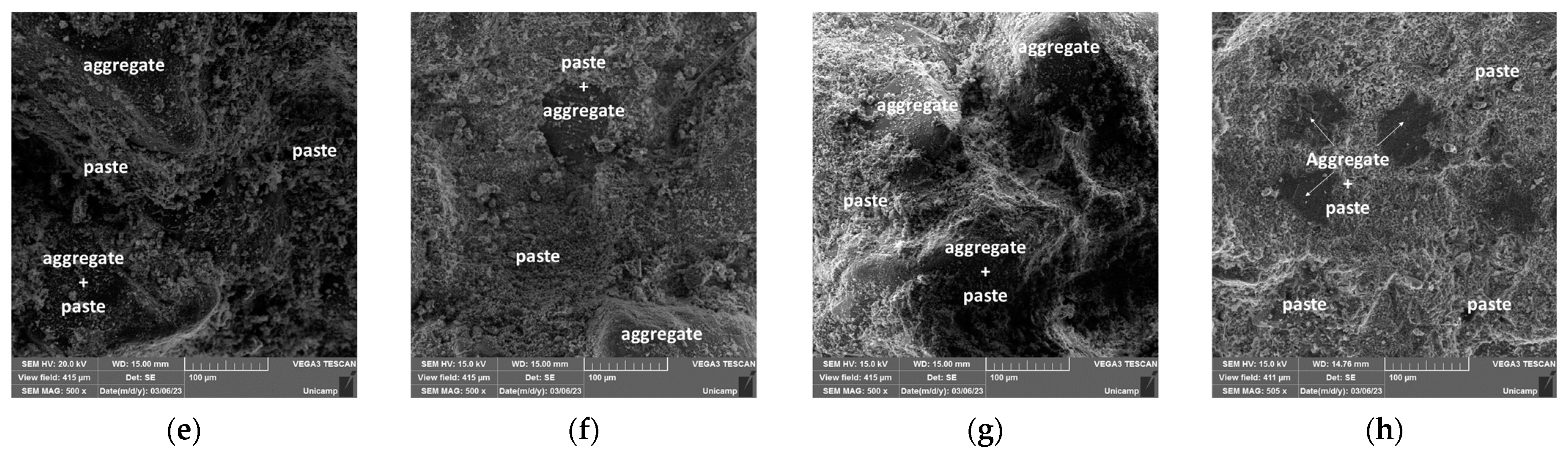
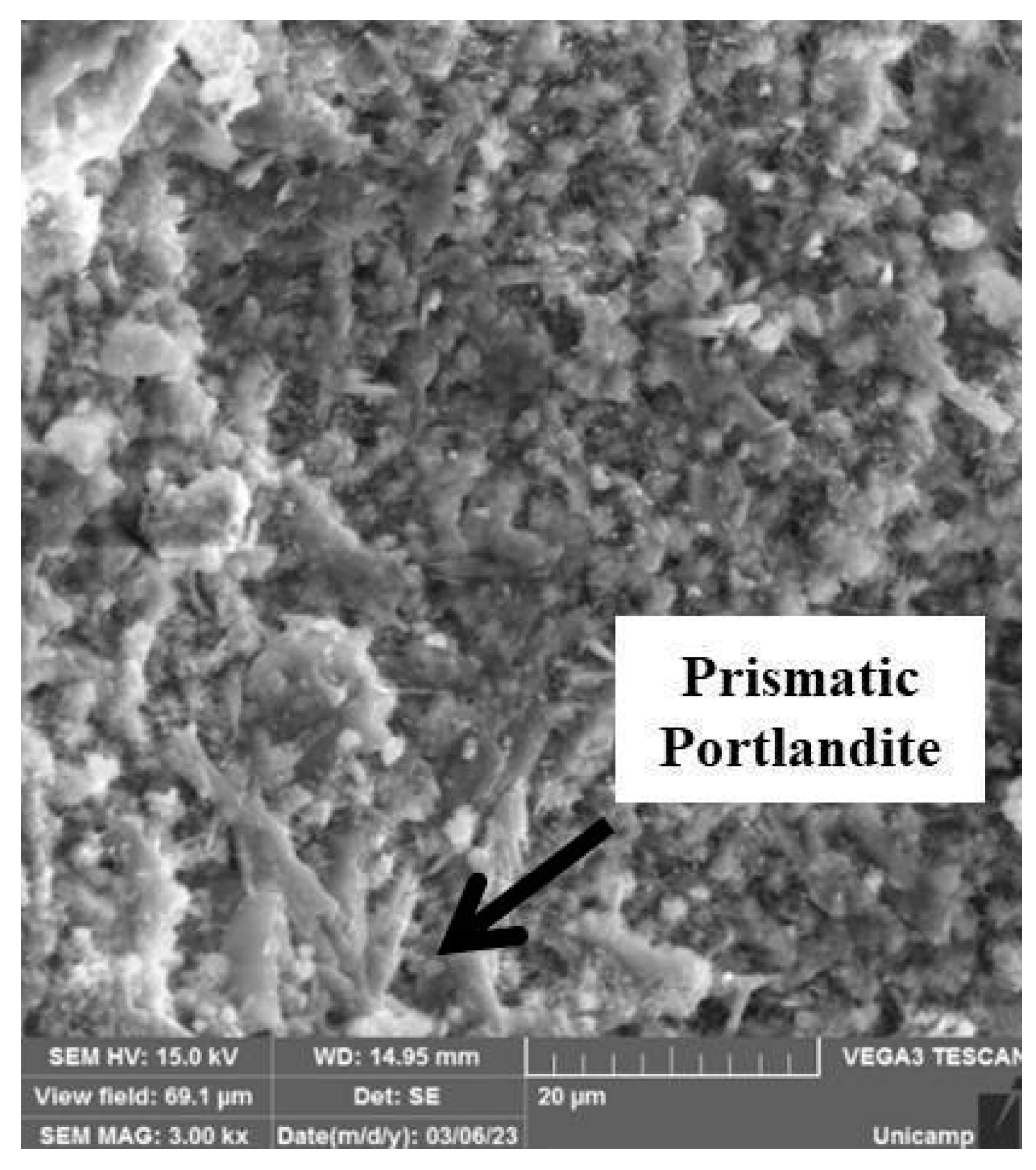
3.5. Microstructural and Mechanical Behavior Correlations
4. Conclusions
- Comparing the mechanical properties of the mortars with respect to curing time, it is evident that the extended curing period (180 days) significantly influenced the mixtures containing LFS (OPC + LFS and OPC + LFS + MK). After 180 days, the compressive strength of the OPC + LFS + MK mixture showed a substantial 44% increase. Additionally, the longer curing time had a greater impact on the compressive strength and dynamic modulus of elasticity (Ed) than on the flexural strength, suggesting that MK was more effective in improving the microstructure rather than the mechanical behavior of the mortars. Prolonged curing is essential for the full development of belite hydration (LFS) and pozzolanic reactions (MK), which are key to realizing the enhanced properties of OPC + LFS + MK blends.
- Analysis of the chemical compounds in the mortar samples using X-ray diffraction (XRD) showed that the extended curing time (180 days) resulted in a substantial reduction in Ca(OH)2 and the formation of anorthite. This reduction is attributed to the consumption of Ca(OH)2 by pozzolanic reactions, which also leads to the formation of C-S-H (calcium silicate hydrate) compounds.
- Scanning electron microscopy (SEM) images confirmed the XRD findings, showing a significant reduction in the porosity of the mortars when comparing 28 and 180 days of curing. This effect was most pronounced in the OPC + LFS and OPC + LFS + MK mortars. The improved microstructure is due to the filler effect and the formation of additional chemical compounds (C-S-H and C-A-S-H) from the combined presence of LFS and MK, as evidenced by the increased encapsulation of sand grains by the cement paste. This behavior is also indicated by the lower capillarity coefficients and higher stiffness (>Ed) at 180 days. The filling effect provided by both LFS and MK contributes to better microstructural stability.
- The combined effect of the OPC + LFS + MK blend not only enhances the mechanical properties and microstructure of the mortars after 180 days of curing but also reduces OPC consumption by 42% compared to the reference mixture. This reduction leads to significant cost savings, as cement is a major expense in construction. Environmentally, using MK and LFS reduces the demand for OPC, thus lowering the carbon footprint and supporting circular economy principles. By incorporating industrial by-products and reducing waste, this approach promotes more sustainable and efficient resource use in construction.
- For future studies, it is recommended to explore the effects of combining LFS with other pozzolans and to conduct durability analyses. Additionally, evaluating the environmental and economic parameters of using LFS as a supplementary cementitious material in mortars should be considered.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WorldSteel Association. Total Production of Crude Steel. 2023. Available online: https://worldsteel.org/data/annual-production-steel-data/ (accessed on 20 July 2024).
- Maghool, F.; Arulrajah, A.; Du, Y.J.; Horpibulsuk, S.; Chinkulkijniwat, A. Environmental impacts of utilizing waste steel slag aggregates as recycled road construction materials. Clean. Technol. Environ. Policy 2017, 19, 949–958. [Google Scholar] [CrossRef]
- Jin, F. Sustainable utilization of slags. In Low Carbon Stabilization and Solidification of Hazardous Wastes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 321–341. [Google Scholar]
- Martins, A.C.P.; Franco de Carvalho, J.M.; Costa, L.C.B.; Andrade, H.D.; de Melo, T.V.; Ribeiro, J.C.L.; Pedroti, L.G.; Peixoto, R.A.F. Steel slags in cement-based composites: An ultimate review on characterization, applications and performance. Constr. Build. Mater. 2021, 291, 123265. [Google Scholar] [CrossRef]
- López-Ausín, V.; Revilla-Cuesta, V.; Skaf, M.; Ortega-López, V. Mechanical properties of sustainable concrete containing powdery ladle furnace slag from different sources. Powder Technol. 2024, 434, 119396. [Google Scholar] [CrossRef]
- Sideris, K.K.; Tassos, C.; Chatzopoulos, A. Production of durable self-compacting concrete using ladle furnace slag (LFS) as filler material. Procedia Eng. 2015, 108, 592–597. [Google Scholar] [CrossRef]
- Najm, O.; El-Hassan, H.; El-Dieb, A. Ladle slag characteristics and use in mortar and concrete: A comprehensive review. J. Clean. Prod. 2021, 288, 125584. [Google Scholar] [CrossRef]
- Herrero, T.; Vegas, I.J.; Santamaría, A.; San-José, J.T.; Skaf, M. Effect of high-alumina ladle furnace slag as cement substitution in masonry mortars. Constr. Build. Mater. 2016, 123, 404–413. [Google Scholar] [CrossRef]
- Manso, J.M.; Rodriguez, Á.; Aragón, Á.; Gonzalez, J.J. The durability of masonry mortars made with ladle furnace slag. Constr. Build. Mater. 2011, 25, 3508–3519. [Google Scholar] [CrossRef]
- Borges Marinho, A.L.; Mol Santos, C.M.; de Carvalho, J.M.F.; Mendes, J.C.; Brigolini, G.J.; André Fiorotti Peixoto, R. Ladle Furnace Slag as Binder for Cement-Based Composites. J. Mater. Civ. Eng. 2017, 29, 04017207. [Google Scholar] [CrossRef]
- de Freitas Cordova de Souza, E.; da Silva, T.F.; de Castro, M.A.; dos Santos Ferreira, G.C. Combined Use of Ladle Furnace Slag and Rice Husk Ash as a Supplementary Cementitious Material. Mater. Res. 2023, 26 (Suppl. S1), e20230069. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J. Hydration reactivity of calcium-aluminate-based ladle furnace slag powder according to various cooling conditions. Cem. Concr. Compos. 2020, 114, 103734. [Google Scholar] [CrossRef]
- da Silva, T.F.; Moura, M.A.D.N.; de Souza, E.d.F.C.; Ferreira, G.C.D.S.; Pereira, V.F.R. Influence of the cooling process on the physicochemical properties of ladle furnace slag, used in the replacement of Portland cement. Rev. Mater. 2022, 27, e20220089. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Akter, S.; Reza, T.B. Sustainable high-performance, self-compacting concrete using ladle slag. Clean. Eng. Technol. 2022, 7, 100439. [Google Scholar] [CrossRef]
- Jo, B.W.; Kim, C.H.; Tae, G.h.; Park, J.B. Characteristics of cement mortar with nano-SiO2 particles. Constr. Build. Mater. 2007, 21, 1351–1355. [Google Scholar] [CrossRef]
- Barbero-Barrera, M.M.; Gomez-Villalba, L.S.; Ergenç, D.; Sierra-Fernández, A.; Fort, R. Influence of curing conditions on the mechanical and hydric performance of air-lime mortars with nano-Ca(OH)2 and nano-SiO2 additions. Cem. Concr. Compos. 2022, 132, 104631. [Google Scholar] [CrossRef]
- Singh, S.K.; Tiwari, N.; Jyoti. Development of sustainable ternary cementitious binder with OPC integrating calcined clay and LF slag. J. Build. Eng. 2023, 75, 107025. [Google Scholar] [CrossRef]
- Tebbal, N.; El Abidine Rahmouni, Z. Rheological and Mechanical Behavior of Mortars with Metakaolin Formulation. Procedia Comput. Sci. 2019, 158, 45–50. [Google Scholar] [CrossRef]
- Abrão, P.C.R.A.; Cardoso, F.A.; John, V.M. Efficiency of Portland-pozzolana cements: Water demand, chemical reactivity and environmental impact. Constr. Build. Mater. 2020, 247, 118546. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.; Patel, M. Effect of metakaolin on mechanical characteristics of the mortar and concrete: A critical review. Mater. Today Proc. 2023, 93, 315–319. [Google Scholar] [CrossRef]
- Nadeem, A.; Memon, S.A.; Lo, T.Y. Mechanical performance, durability, qualitative and quantitative analysis of microstructure of fly ash and Metakaolin mortar at elevated temperatures. Constr. Build. Mater. 2013, 38, 338–347. [Google Scholar] [CrossRef]
- Hossain, M.U.; Poon, C.S.; Dong, Y.; Xuan, D. Evaluation of environmental impact distribution methods for supplementary cementitious materials. Renew. Sustain. Energy Rev. 2018, 82, 597–608. [Google Scholar] [CrossRef]
- Jameel, G.S.; Ipek, S.; Ahmed, A.D.; Guneyisi, E.; Guneyisi, E.M. Rheological behavior and key properties of metakaolin and nano-SiO2 blended fibrous self-compacting concretes. Constr. Build. Mater. 2023, 368, 130372. [Google Scholar] [CrossRef]
- Grilo, J.; Santos Silva, A.; Faria, P.; Gameiro, A.; Veiga, R.; Velosa, A. Mechanical and mineralogical properties of natural hydraulic lime-metakaolin mortars in different curing conditions. Constr. Build. Mater. 2014, 51, 287–294. [Google Scholar] [CrossRef]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Manzi, S.; Lancellotti, I.; Kamseu, E.; Barbieri, L.; Leonelli, C. Mix-design and characterization of alkali activated materials based on metakaolin and ladle slag. Appl. Clay Sci. 2013, 73, 78–85. [Google Scholar] [CrossRef]
- Natali Murri, A.; Rickard, W.D.A.; Bignozzi, M.C.; Van Riessen, A. High temperature behaviour of ambient cured alkali-activated materials based on ladle slag. Cem. Concr. Res. 2013, 43, 51–61. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Alkali Activation of Ladle Slag from Steel-Making Process. J. Sustain. Metall. 2017, 3, 300–310. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J. A new supplementary cementitious material: Walnut shell ash. Constr. Build. Mater. 2023, 408, 133852. [Google Scholar] [CrossRef]
- Fang, K.; Zhao, J.; Wang, D.; Wang, H.; Dong, Z. Use of ladle furnace slag as supplementary cementitious material before and after modification by rapid air cooling: A comparative study of influence on the properties of blended cement paste. Constr. Build. Mater. 2022, 314, 125434. [Google Scholar] [CrossRef]
- NBR 16697; Portland Cement—Requirements. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2018.
- ASTM C595; Standard Specification for Blended Hydraulic Cements. American Society of Testing and Materials: West Conshohocken, PA, USA, 2010.
- Araos Henríquez, P.; Aponte, D.; Ibáñez-Insa, J.; Barra Bizinotto, M. Ladle furnace slag as a partial replacement of Portland cement. Constr. Build. Mater. 2021, 289, 123106. [Google Scholar] [CrossRef]
- Xu, B.; Yi, Y. Treatment of ladle furnace slag by carbonation: Carbon dioxide sequestration, heavy metal immobilization, and strength enhancement. Chemosphere 2022, 287, 132274. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, H.; Mei, H.; Rao, L.; Wang, H.; Lv, N. Generation, utilization, and environmental impact of ladle furnace slag: A minor review. Sci. Total Environ. 2023, 895, 165070. [Google Scholar] [CrossRef] [PubMed]
- Goli, V.S.N.S.; Yadav, R.; Singh, M.R. Forensic investigations on 1900 years old brick and mortar samples from Buddhist stupa located at Nalasopara, India. Constr. Build. Mater. 2023, 367, 130281. [Google Scholar] [CrossRef]
- Araos, P.; Montaño, T.; Valls, S.; Barra, M.; Aponte, D. Effects of the mineralogical composition and particle size distribution of ladle furnace slag as a cement/fine aggregate replacement in concrete. Mater. Constr. 2023, 73, e304. [Google Scholar] [CrossRef]
- Fang, K.; Wang, D.; Zhao, J.; Zhang, M. Utilization of ladle furnace slag as cement partial replacement: Influences on the hydration and hardening properties of cement. Constr. Build. Mater. 2021, 299, 124265. [Google Scholar] [CrossRef]
- NBR 13276; Mortars Applied on Walls and Ceilings—Determination of the Consistence Index. Brazilian Association of Technical Standard: Rio de Janeiro, Brazil, 2016.
- ASTM C109/C109M-20; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). American Society of Testing and Materials: West Conshohocken, PA, USA, 2020.
- Václavík, V.; Eštoková, A.; Papesch, R.; Dvorský, T.; Fabianova, M.; Halik, M.; Hološová, M. Investigation of the engineering and environmental properties of cement mortars incorporating ladle furnace steel slag. Case Stud. Constr. Mater. 2024, 20, e02876. [Google Scholar] [CrossRef]
- NBR 13277; Mortars Applied on Walls and Ceilings—Determination of the Water Retentivity. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2005.
- NBR 13278; Mortars Applied on Walls and Ceilings—Determination of the Specific Gravity and the Air Entrained Content in the Fresh Stage. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2005.
- NBR 13279; Mortars Applied on Walls and Ceilings—Determination of the Flexural and the Compressive Strength in the Hardened Stage. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2005.
- NBR 13280; Mortars Applied on Walls and Ceilings—Determination of The Specific Gravity in the Hardened Stage. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2005.
- NBR 15259; Mortars Applied on Walls and Ceilings—Determination of Water Absorption Coefficient Due to Capilary Action. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2005.
- NBR 15630; Mortars Applied on Walls and Ceilings—Determination of Elasticity Modulus by the Ultrasonic Wave Propagation. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2005.
- NBR 15261; Mortars Applied on Walls and Ceilings—Determination of Dimensional Charges (Shrinkage). Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2005.
- ASTM C110-20; Standart Test Methods for Physical Testing of Quicklime, Hydrated Lime, and Limestone. Annual Book of ASTM Standarts. American Society for Testing Materials (ASTM): West Conshohocken, PA, USA, 2024.
- ASTM C349-14; Standard Test Method for Compressive Strength of Hydraulic-Cement Mortars (Using Portions of Prisms Broken in Flexure). Annual Book of ASTM Standarts. American Society for Testing Materials (ASTM): West Conshohocken, PA, USA, 2018.
- ASTM C215-14; Standard Test Method for Fundamental Transverse, Longitudinal, and Torsional Resonant Frequencies of Concrete Specimens. American Society of Testing and Materials: West Conshohocken, PA, USA, 2016.
- Xu, B.; Yi, Y. Use of ladle furnace slag containing heavy metals as a binding material in civil engineering. Sci. Total Environ. 2020, 705, 135854. [Google Scholar] [CrossRef]
- Marques, A.I.; Morais, J.; Morais, P.; do Rosário Veiga, M.; Santos, C.; Candeias, P.; Ferreira, J.G. Modulus of elasticity of mortars: Static and dynamic analyses. Constr. Build. Mater. 2020, 232, 117216. [Google Scholar] [CrossRef]
- Han, T.; Wang, X.; Li, D.; Li, D.; Han, N.; Xing, F. Damage and degradation mechanism for single intermittent cracked mortar specimens under a combination of chemical solutions and dry-wet cycles. Constr. Build. Mater. 2019, 213, 567–581. [Google Scholar] [CrossRef]
- Chu, S.H.; Chen, J.J.; Li, L.G.; Ng, P.L.; Kwan, A.K.H. Roles of packing density and slurry film thickness in synergistic effects of metakaolin and silica fume. Powder Technol. 2021, 387, 575–583. [Google Scholar] [CrossRef]
- Khatri, R.P.; Sirivivatnanon, V.; Gross, W. Effect of different supplementary cementitious materials on mechanical properties of high performance concrete. Cem. Concr. Res. 1995, 25, 209–220. [Google Scholar] [CrossRef]
- Angelin, A.F.; Andrade, M.F.F.; Bonatti, R.; Cecche Lintz, R.C.; Gachet-Barbosa, L.A.; Osório, W.R. Effects of spheroid and fiber-like waste-tire rubbers on interrelation of strength-to-porosity in rubberized cement and mortars. Constr. Build. Mater. 2015, 95, 525–536. [Google Scholar] [CrossRef]
- Xavier, B.C.; Verzegnassi, E.; Bortolozo, A.D.; Alves, S.M.; Cecche Lintz, R.C.; Andreia Gachet, L.; Osório, W.R. Fresh and Hardened States of Distinctive Self-Compacting Concrete with Marble- and Phyllite-Powder Aggregate Contents. J. Mater. Civ. Eng. 2020, 32, 04020065. [Google Scholar] [CrossRef]
- Ramli, M.; Dawood, E.T. Comparative Study between Flowable High Strength Mortar and Flowing High Strength Concrete [Internet]. 2011, Volume 2. Available online: https://www.researchgate.net/publication/283408541_Comparative_study_between_flowable_high_strength_mortar_and_flowing_high_strength_concrete (accessed on 13 June 2024).
- Zhou, Y.; Pu, S.; Han, F.; Zhang, H.; Zhang, Z. Effect of ultrafine slag on hydration heat and rheology properties of Portland cement paste. Powder Technol. 2022, 405, 117549. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Q.; Yu, Q. The effect of size distribution of slag particles obtained in dry granulation on blast furnace slag cement strength. Powder Technol. 2020, 362, 32–36. [Google Scholar] [CrossRef]

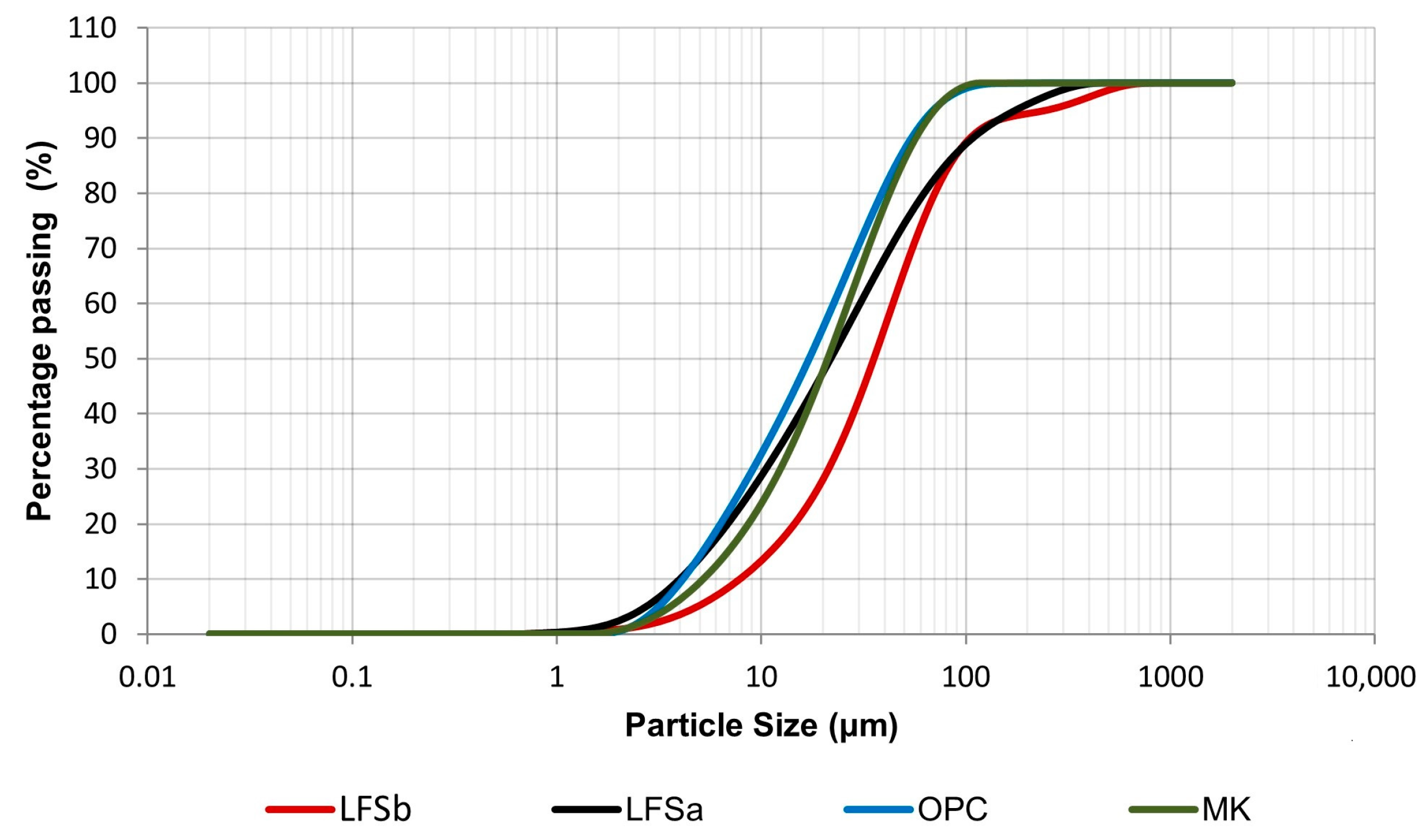
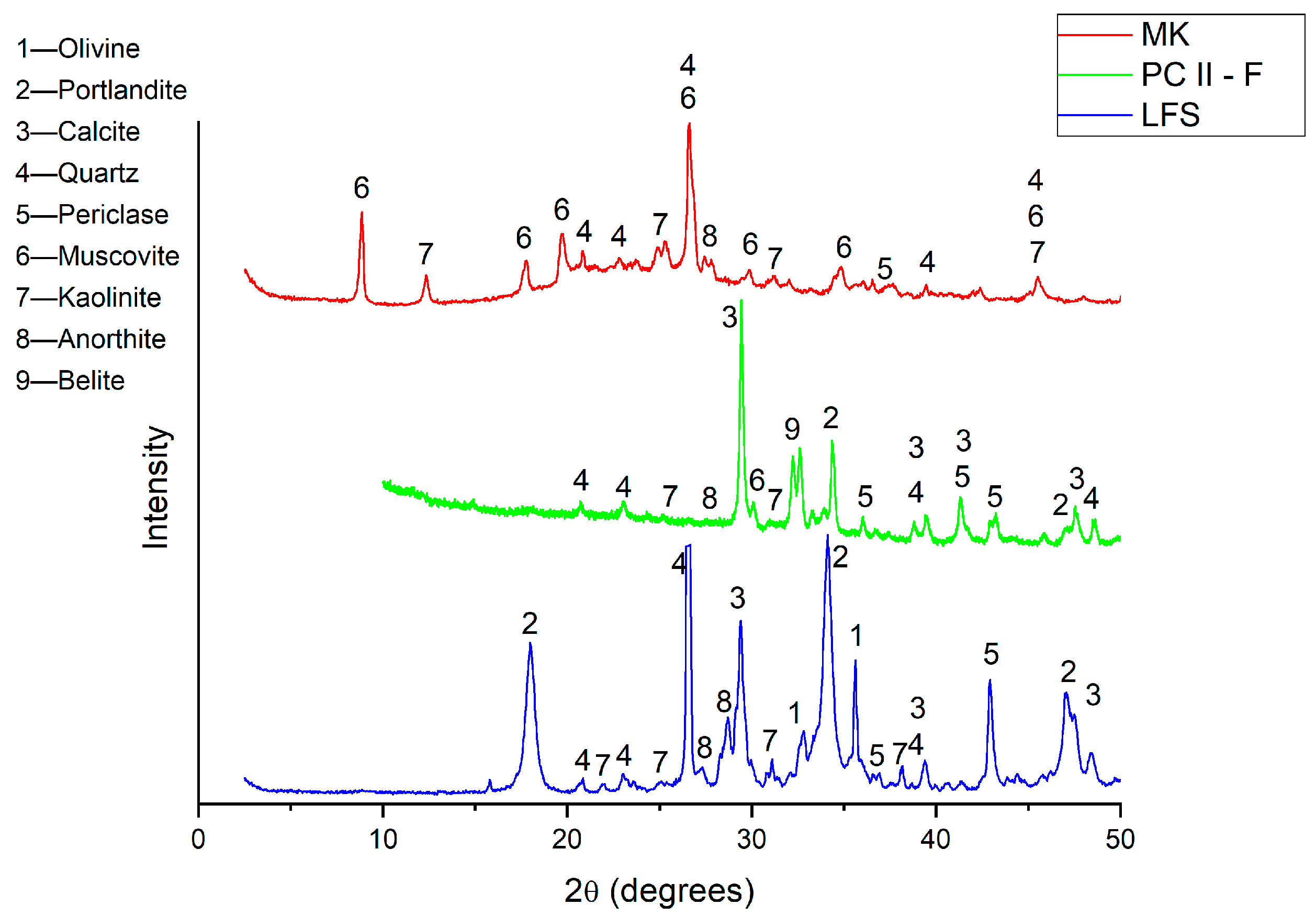
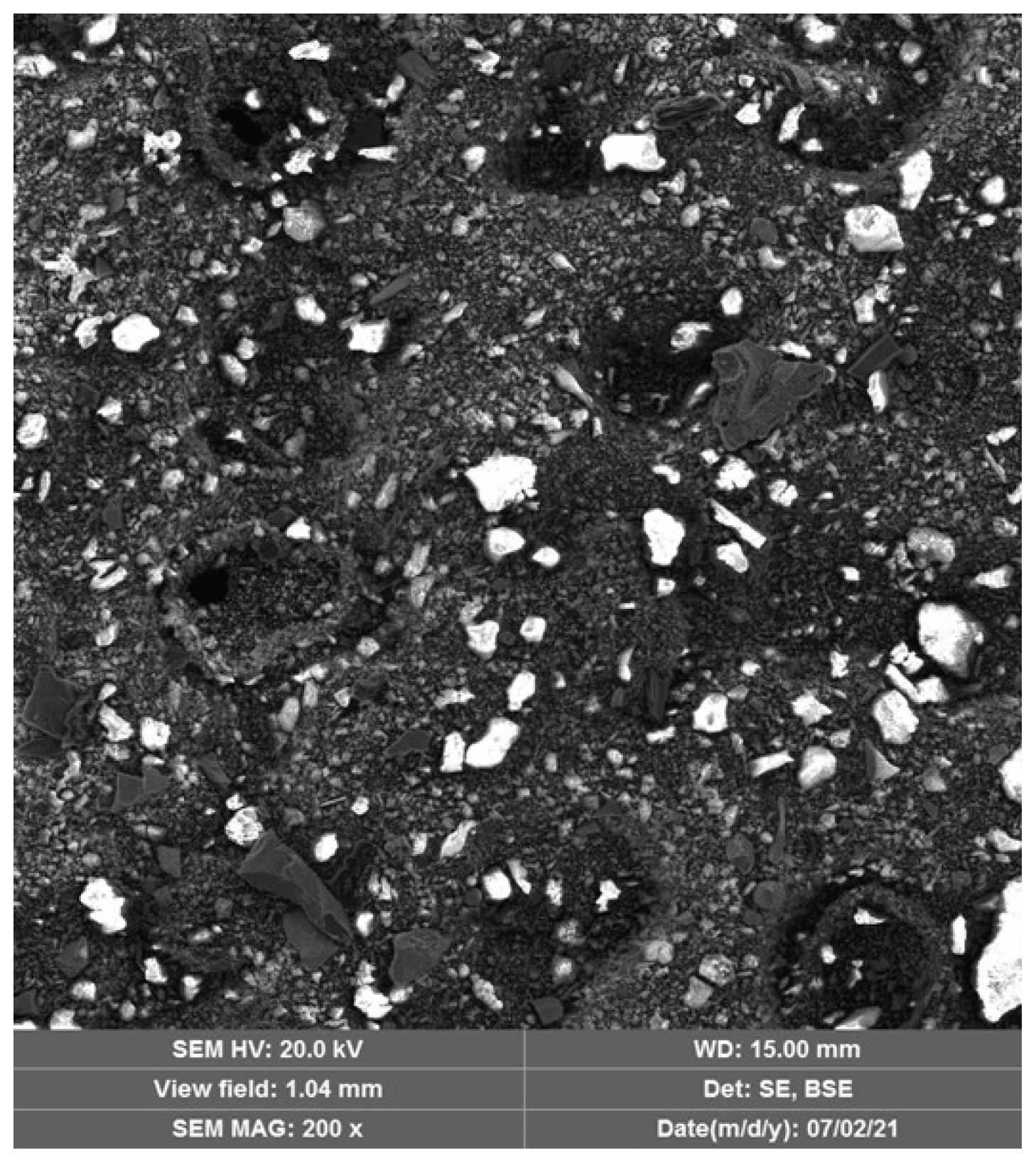

| Contents (wt.%) * | CaO | Fe2O3 | SiO2 | MgO | MnO | Al2O3 | SO3 | LOI (%) |
|---|---|---|---|---|---|---|---|---|
| LFS | 54.60 | 7.59 | 9.78 | 5.79 | 7.29 | 0.80 | 0.60 | 12.00 |
| OPC | 63.40 | 3.86 | 12.40 | 2.83 | 0.19 | 2.52 | 2.76 | 10.80 |
| MK | 0.13 | 3.43 | 50.80 | 0.70 | - | 35.90 | 0.40 | 3.36 |
| Mixtures (*) | Indexes | Binder (kg/m3) | Sand (kg/m3) | w/b Ratio | ||
|---|---|---|---|---|---|---|
| OPC | LFS | MK | ||||
| REF | 1:3 | 477.23 | 0.00 | 0.00 | 1431.69 | 0.63 |
| OPC + MK | 0.9:0.1:3 | 420.15 | 0.00 | 46.68 | 1400.52 | 0.67 |
| OPC + LFS | 0.7:0.3:3 | 331.05 | 141.88 | 0.00 | 1418.79 | 0.64 |
| OPC + LFS + MK | 0.6:0.3:0.1:3 | 277.63 | 138.81 | 46.27 | 1388.13 | 0.68 |
| Mixtures | Density (kg/m3) | Entrained Air Content (%) | Water Retention (%) |
|---|---|---|---|
| REF | 2153 | 3 | 83 |
| OPC + MK | 2106 | 3 | 82 |
| OPC + LFS | 2083 | 5 | 82 |
| OPC + LFS + MK | 2097 | 3 | 84 |
| Mixtures | Flexural Strength (MPa) | SD 1 | CV 2 | HG 3 | Compressive Strength (MPa) | SD 1 | CV 2 | HG 3 |
|---|---|---|---|---|---|---|---|---|
| At 28 days | ||||||||
| REF | 4.70 | 0.11 | 2.30 | A | 25.50 | 1.07 | 4.21 | A |
| OPC + MK | 3.40 | 0.32 | 9.51 | B | 25.30 | 1.29 | 5.09 | A |
| OPC + LFS | 3.50 | 0.13 | 3.70 | B | 9.40 | 1.26 | 13.39 | E |
| OPC + LFS + MK | 3.80 | 0.25 | 6.49 | C | 10.80 | 1.66 | 15.41 | B |
| At 180 days | ||||||||
| REF | 5.56 | 0.51 | 9.21 | D | 24.08 | 0.52 | 2.18 | C |
| OPC + MK | 5.25 | 0.73 | 13.82 | D | 25.69 | 0.50 | 1.95 | D |
| OPC + LFS | 4.31 | 0.06 | 1.28 | E | 14.92 | 0.73 | 4.91 | E |
| OPC + LFS + MK | 4.36 | 0.24 | 5.54 | E | 15.64 | 1.11 | 7.06 | E |
| Mixtures | Ed (GPa) | SD 1 | CV 2 | HG 3 | Density (kg/m3) 2 | SD | CV | HG | C (g/dm2·min1/2) | SD 1 | CV 2 | HG 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At 28 days | ||||||||||||
| REF | 25.68 | 0.07 | 0.27 | A | 2081 | 2.05 | 0.10 | A | 0.88 | 0.07 | 8.34 | AB |
| OPC + MK | 25.11 | 1.04 | 4.13 | A | 2084 | 10.45 | 0.50 | A | 0.72 | 0.14 | 19.57 | A |
| OPC + LFS | 16.72 | 0.29 | 1.71 | B | 2031 | 6.89 | 0.34 | B | 1.02 | 0.09 | 8.81 | B |
| OPC + LFS + MK | 19.55 | 0.17 | 0.85 | B | 2053 | 10.73 | 0.52 | B | 0.93 | 0.09 | 9.11 | B |
| At 180 days | ||||||||||||
| REF | 25.60 | 0.07 | 0.27 | C | 2082 | 1.10 | 0.05 | CE | 0.45 | 0.02 | 4.44 | C |
| OPC + MK | 25.40 | 0.42 | 1.66 | C | 2096 | 8.92 | 0.43 | C | 0.40 | 0.03 | 7.50 | C |
| OPC + LFS | 21.63 | 0.23 | 1.07 | D | 2062 | 10.24 | 0.50 | D | 0.46 | 0.05 | 9.87 | C |
| OPC + LFS + MK | 23.02 | 0.12 | 0.50 | E | 2067 | 4.09 | 0.20 | DE | 0.21 | 0.05 | 23.81 | D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.; Souza, E.; Mariano, E.; Ferreira, G.; Osório, W.R. Long-Term Performance of Mortars with Combined Incorporation of Ladle Furnace Slag and Metakaolin. Buildings 2024, 14, 2762. https://doi.org/10.3390/buildings14092762
Silva T, Souza E, Mariano E, Ferreira G, Osório WR. Long-Term Performance of Mortars with Combined Incorporation of Ladle Furnace Slag and Metakaolin. Buildings. 2024; 14(9):2762. https://doi.org/10.3390/buildings14092762
Chicago/Turabian StyleSilva, Tayná, Everton Souza, Eduardo Mariano, Gisleiva Ferreira, and Wislei R. Osório. 2024. "Long-Term Performance of Mortars with Combined Incorporation of Ladle Furnace Slag and Metakaolin" Buildings 14, no. 9: 2762. https://doi.org/10.3390/buildings14092762





