Abstract
Microbial-induced calcium carbonate precipitation (MICP) can enhance the physical properties of recycled aggregates. Compared to traditional technologies, MICP offers environmental benefits and produces no pollution. However, its mineralization efficacy is significantly influenced by the process parameters. To investigate this, an MICP mineralization test was conducted by manipulating various process parameters throughout the mineralization process. The water absorption rate, apparent density, and calcium carbonate content of the mineralized recycled aggregates were assessed to discern the impact of these parameters on the mineralization outcome. Further analysis using techniques such as thermogravimetric analysis (TG), X-ray diffraction (XRD), nuclear magnetic resonance (NMR), and scanning electron microscopy (SEM) were employed to elucidate the mineralization mechanism of the recycled aggregates at a micro-level. The findings indicated that the MICP treatment induced bacteria to precipitate CaCO3, forming calcite crystalline CaCO3 within the pores and microcracks. This led to a denser interfacial transition zone and, consequently, improved the physical properties of the recycled aggregates. Optimal mineralization was achieved when the bacterial solution concentration was 1.4, the temperature and pH were 35 °C and 9, respectively, and the urea concentration, Ca+ concentration, and mineralization time were 0.5 mol/L, 0.5 mol/L, and 7 days, respectively. Under these conditions, the mineralized recycled aggregate exhibited a 16.07% reduction in water absorption, a 1.07% increase in apparent density, and a 2.28% change in mass.
1. Introduction
As one of the most commonly used building materials, reducing the carbon emissions of concrete is considered a core task in the construction materials industry’s transition to low carbon [1]. The construction industry is a major consumer of natural resources, and according to statistics, total global production of waste concrete nearly doubled from 21 billion tons in 2007 to 40 billion tons in 2014 [2]. Recycled aggregate (RA) is obtained by recycling, crushing, and mixing waste concrete, and it can partially or completely replace natural aggregate to produce concrete [3]. This significantly improves the recycling rate of solid waste. It also helps reduce carbon emissions during the production process of concrete raw materials [4], which is of extremely important strategic significance for global energy conservation, emission reduction, and green production.
In comparison with natural aggregates, the adherent mortar on the surface of recycled aggregates contributes to their high porosity, high water absorption, and low strength. The inclusion of recycled aggregates in concrete mixtures leads to an increased water absorption rate, which complicates the control of the effective water–cement ratio. This not only diminishes the workability of fresh concrete, resulting in significant loss of slump, but also impacts the strength and durability of the hardened concrete [5,6,7,8]. Studies have demonstrated that the thin layer of old mortar adhering to natural aggregates (NAs) is primarily responsible for the high water absorption of recycled aggregates (RAs) and the weak bond with the new substrate. Upon incorporating RA into new concrete, the old interfacial transition zones (ITZs) merge with the new ITZs between RA and the new substrate, creating an extended weak zone that compromises the concrete’s strength [5,9]. Consequently, it is imperative to employ specific technical measures to enhance the physical properties of recycled aggregates.
Currently, scholars have conducted a comprehensive study on the enhancement of recycled aggregates, focusing on two primary strategies to improve their performance: the removal of bonded mortar and the reinforcement of bonded mortar. These strategies represent the two main categories of treatment. The methods for removing adherent mortar primarily involve heating and grinding, as well as acid treatment [10]. Techniques for reinforcing adherent mortar include soaking the recycled aggregate with various substances, such as water glass or polymer emulsion [11,12,13]. Of these methods, heating and grinding are straightforward to execute but require substantial energy and mechanical resources. This process not only tends to cause internal damage to the recycled aggregates, creating additional microcracks, but also leads to significant energy consumption [8,14]. In contrast, chemical treatments like acid solutions, water glass solutions, and polymer emulsions are effective in enhancing the performance of recycled aggregates. However, these treatments may generate corrosive byproducts, which can lead to the corrosion of reinforcing bars, thereby reducing structural durability. Moreover, the corrosive substances produced during this process can result in significant environmental pollution, conflicting with the aims of sustainable development [15,16].
To avoid these disadvantages, many environmentally friendly methods have been proposed. Microbial mineralization deposition technology, in particular, has found application in the construction sector as a sustainable and eco-friendly approach. This biomineralization process offers benefits such as ease of control, user-friendly operation, high mineralization efficiency, and the production of environmentally friendly, non-polluting products that exhibit excellent ecological compatibility [17]. It has also demonstrated promising application potential in engineering practice. Furthermore, building upon carbonate precipitation technology, several researchers have explored the use of micro-organisms to enhance the properties of recycled aggregates (RAs). Qiu et al. [18], Grabiec et al. [19], and Wang et al. [20] have investigated the impact of microbial carbonate precipitation (MICP) on the modification of RAs, affirming the feasibility and efficacy of microbial modification. However, current research lacks in-depth investigation into achieving profound mineralization of RA and significantly enhancing its physical properties. Therefore, a systematic inquiry into the mineralization effects of RA under varying process parameters is required. By optimizing these parameters, the efficiency of MICP technology can be enhanced, the consumption of energy and resources can be decreased, thereby reducing costs and rendering MICP technology more cost-effective, which in turn boosts its competitiveness within the construction industry.
In this study, recycled aggregate (RA) derived from the repurposing of waste concrete served as the primary research subject. The investigation focused on the enhancing effects of various mineralization process parameters, including bacterial solution concentration, temperature, pH, urea concentration, Ca+ concentration, and reaction time, on the physical properties of RA and its Ca+ curing capacity. Additionally, the study utilized microanalytical techniques to examine the microscopic characteristics of RA post-mineralization, assessing aspects such as physical phase composition, pore structure, and micromorphology.
2. Materials and Methods
2.1. Recycled Aggregates
Recycled aggregate was used in the test, which was obtained from the waste concrete of a commercial mixing station after crushing and air-selecting treatment. A 20 mm sieve was used to screen out any large particles, and RA with particle sizes ranging from 5 to 20 mm was obtained after cleaning. The screening and cleaning process of RA is shown in Figure 1. The properties of RA used in this study were determined according to GB/T 14685-2022 [21], and the water absorption rate of RA was measured to be 9.1%, and the apparent density was 2650 kg/m3. The grading curve of RA is shown in Figure 2.

Figure 1.
The screening and cleaning process of recycled aggregate: (a) Screening of RA; (b) cleaning of RA; (c) air-drying of RA; (d) drying of RA.
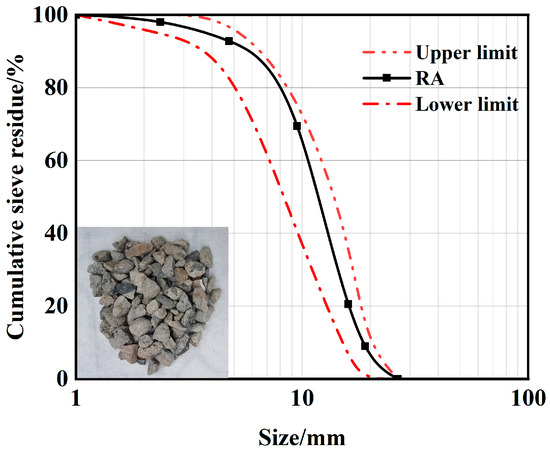
Figure 2.
Gradation curve of recycled aggregate.
2.2. Bacteria and Culture Media
Sporosarcina pasteurii (S. pasteurii, DSM No. 33), formerly known as Bacillus pasteurii. The strain was purchased from the American Mycobacterial Treasure Center under the number ATCC11859.
The micro-organisms were activated, and one ultra-pure water was used as the solvent to configure the proliferation medium. The medium was autoclaved at 121 °C (100 kPa pressure) for 20 min according to the Chinese standard GB/T 15981-2021 [22], so that all bacteria and spores were killed and a completely sterile medium was obtained, and Bacillus pasteurii was inoculated in the sterilized liquid medium. It was incubated in a constant temperature shaking chamber for 24 h to obtain sufficient bacterial growth. After 24 h, the bacterial concentration was determined by spectrophotometer using uninoculated enrichment medium as a control. The bacterial solution and medium are shown in Figure 3.

Figure 3.
Liquid and plate of Bacillus pasteurii: (a) aseptic operating table; (b) bacteriophage and media of Bacillus pasteurii; (c) plates of Bacillus pasteurii; (d) spectrophotometer.
2.3. MICP Modified Recycled Aggregate
Figure 4 illustrates the appearance of RA after MICP treatment. Compared with the spraying method adopted by many scholars, the main features of the soaking method [23] are simple operation and the ability to treat RA in large quantities, which improve the utilization rate of the bacterial liquid and the collodion, and contribute to the practical application of the microbial mineralization technology in engineering. The bacterial count in the bacterial solution can be quantified by measuring the optical density of the solution (also known as the turbidimetric method). The core idea of this method is that the concentration of cells is positively correlated with the turbidity of the solution, and further with the optical density. This method is simple and efficient, making it one of the common techniques for detecting microbial concentration [24]. RA is placed inside the MICP reactor. The study explored how various variables such as bacterial liquid concentration, urea concentration, Ca+ concentration, and time affect the impact of the MICP mineralization reaction on the physical properties of RA. The experimental conditions are shown in Table 1.
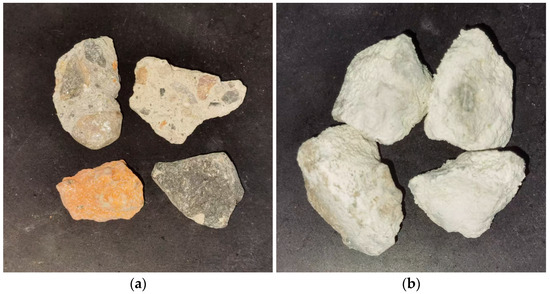
Figure 4.
Comparison of the appearance of RA before and after MICP treatment: (a) appearance of RA before MICP treatment; (b) appearance of RA after MICP treatment.

Table 1.
Culture conditions for bacteria.
2.4. Methods
As shown in Figure 5, the experimental methods employed in this study are detailed as follows:
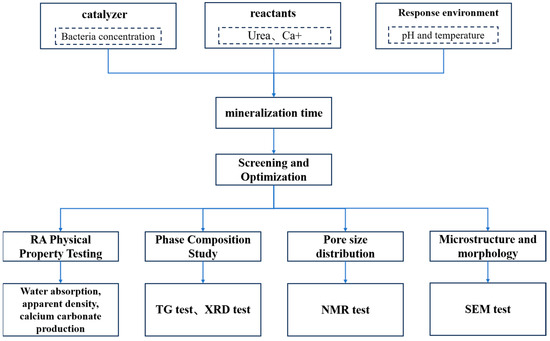
Figure 5.
Testing program of this study.
(1) RA physical property test: RA physical properties were tested for water absorption and apparent density of RA before and after carbonization according to GB/T 14685-2022 [21].
(2) RA phase composition test: The RA samples before and after mineralization were crushed, and after crushing, the primary aggregate was removed and the attached mortar was retained. After that, the samples were placed in a mortar and ground to powder with a particle size of ≤75 μm. The powder samples were then dried in a vacuum oven at a temperature of 35 °C for 72 h, and then taken out and cooled down to room temperature, and the RA powder samples obtained were subjected to thermo-gravimetric analysis (TGA) and X-ray diffraction (XRD) tests. TGA was used to quantitatively analyze the CaCO3 production of RA, and in the TG curve, the mass loss from 550 °C to 950 °C was due to the CO2 released from CaCO3 decomposition [25]. The CaCO3 content on the RA surface was calculated by determining the difference in the relative content of CaCO3 in the RA before and after mineralization, and the physical phase composition of the RA before and after carbonization was studied by XRD [26].
(3) RA pore structure test: The pore structure test of the mortar attached with RA was carried out by low-field nuclear magnetic method. Firstly, the samples modified by MICP technology were saturated with water in vacuum for 24 h. Then, the volume of saturated samples was determined according to the floating weight method and the saturated surface dry mass was determined, and finally, the porosity and pore size distribution of the samples were calculated by using the low-field NMR testing equipment.
(4) RA microstructure and morphology testing: use scanning electron microscope to observe the microstructure and morphology of untreated RAs and RAs modified by MICP technology.
3. Results and Discussion
3.1. Analysis of Factors Affecting RA Mineralization
3.1.1. The Effect of Bacterial Suspension Concentration on RA Mineralization Reaction
Figure 6 illustrates the trend in water absorption and apparent density of RA after being modified for 7 days with varying concentrations of bacterial solution. Microbial mineralization treatment had a positive impact on the water absorption and apparent density of RA, and the degree of improvement in these properties varied with different concentrations of the bacterial solution. There is an optimal range of bacterial solution concentration to achieve the maximum rate of CaCO3 formation, meaning that excessively high or low concentrations are not conducive to CaCO3 production. When the bacterial solution’s OD600 value is 1.4 or 1.8, the water absorption of the RA increases with the concentration of the bacterial solution. Compared to the control group, they decreased by 9.90% and 3.70%, respectively. However, when the OD600 value of the bacterial solution is less than 1.4, the improvement effect of microbial mineralization treatment on the water absorption of RA increases with the increase in the bacterial solution concentration. When the OD600 values are 0.2, 0.6, and 1.0, the water absorption rates of the aggregate decrease by 2.20%, 4.60%, and 6.51%, respectively, indicating that the improvement effect of the bacterial solution concentration on the water absorption performance of the RA increases first and then decreases with the increase in the bacterial solution concentration. The apparent density of RA was also improved by MICP technology, and the modification effect was affected by the concentration of bacterial liquid. When the OD600 value of the bacterial liquid was 1.4, the apparent density of the regenerated aggregate reached a maximum of 2673 kg/m3, which increased by 0.83% compared with the baseline group. When the OD600 value of the bacterial solution was less than 1.4, the apparent density of the regenerated aggregate increased with the increase in the concentration of the bacterial solution, and the apparent densities of the aggregates under the conditions of OD600 value of 0.2, 0.6, and 1.0 increased by 0.23%, 0.38%, and 0.42%, respectively. The apparent density of the RA increased by 0.42% when the OD600 value of the bacterial solution was 1.8. This indicates that the effect of the concentration of the bacterial solution on the apparent density of the aggregate increases and then decreases when microbial mineralization is used to treat the RA. The generation of CaCO3 reaches the highest at an OD600 value of 1.4, with a growth rate of 2.22% by weight, and exhibits a similar trend with the physical properties of the RA.
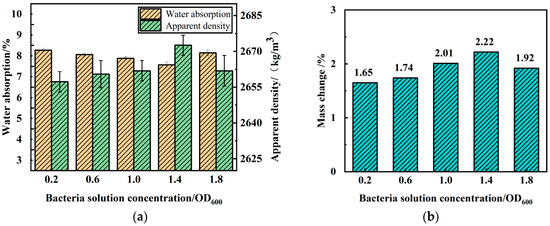
Figure 6.
Effects of different bacterial solution concentrations on RA physical properties and calcium carbonate production: (a) water absorption and apparent density; (b) mass change.
The performance enhancement of regenerated aggregate improved by microbial mineralization is restricted by the concentration level of the bacterial solution. For ureolytic micro-organisms, urease production was positively correlated with the number of bacteria, and the process of urea hydrolysis was closely related to the number of bacteria. Within a specific range of bacterial concentration, an increase in bacterial concentration often signifies an enhancement in MICP mineralization efficiency [18]. The increase in the number of bacteria not only decomposes more urea but also creates more nucleation sites for the deposition of CaCO3 crystals, thereby increasing the amount of CaCO3 produced. However, such performance enhancement also has certain limitations, as the number of bacteria is closely linked to the number of nucleation sites for CaCO3 formation. When the concentration of the bacterial solution is relatively low, the number of CaCO3 crystals formed is limited, which constrains the further improvement of the basic physical properties of the RA. When the concentration of the bacterial solution is relatively high, the microbial mineralization activity may be negatively affected by the bacteria entering the logarithmic and death phases prematurely. However, the large amount of CaCO3 produced by the micro-organisms in a short period may cover the surface of the RA, which is detrimental to microbial activity, thus inhibiting the urea hydrolysis process [27]. Thus, the CaCO3 filling the pores and cracks of the RA is reduced.
3.1.2. Effect of Temperature on RA Mineralization Reaction
Figure 7 illustrates the trend in water absorption and apparent density of RA after 7 days of mineralization under different temperature conditions. When the mineralization temperature increased from 15 °C to 35 °C, the physical properties of RA significantly improved, and the production efficiency of CaCO3 also enhanced, leading to a gradual increase in the amount of CaCO3 precipitate. However, as the temperature continued to rise, both the improvement in RA’s physical properties and the amount of CaCO3 precipitate showed a gradual decline.
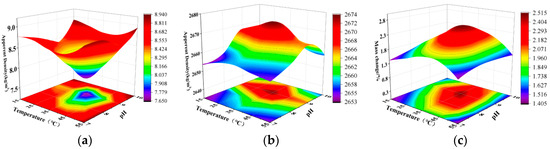
Figure 7.
Effect of temperature and pH on MICP reaction: (a) water absorption; (b) apparent density; (c) mass change.
The mechanism of action of the MICP technique [28] is the hydrolysis of urea by bacterially produced urease to produce carbonates; these carbonates subsequently combine with Ca+ in solution to form a precipitate. Kumar et al. [29] found a link between enzyme synthesis and energy metabolism in Bacillus, a process that is controlled by temperature and oxygen uptake. For Bacillus, the suitable temperature range for proteases is suggested to be 30 to 40 °C. Omoregie et al. [30] studied different species of urease and found that urease activity was highest at temperatures ranging from warm 25 to 30 °C. The mineralization efficiency of MICP was increased at the higher temperature of 35 °C compared to 15 °C. This may be attributed to the enhanced enzyme activity and urea hydrolysis rate at higher temperatures [31], which in turn increased the rate of mineralization and total CaCO3 production by MICP. When the temperature exceeds 45 °C, the number of bacteria is lower than that under other temperature conditions, and the reason for this phenomenon may be that high temperature inhibits the growth and reproduction of bacteria. With further increase in temperature, the effect of 55 °C on bacteria is more obvious; high temperature not only inhibits the growth and reproduction of bacteria, but even may lead to denaturation and inactivation of enzymes. This observation suggests that tropical climatic regions have a great potential to participate in microbial CaCO3 precipitation due to the hot and humid environment throughout the year. Therefore, based on the experimental results and other studies, 35 °C was selected as the optimal temperature for subsequent experiments.
3.1.3. Effect of pH on the RA Mineralization Reaction
Figure 7 illustrates the impact of varying pH levels on the mineralization reaction of recycled aggregate (RA) using microbial-induced carbonate precipitation (MICP). The figure reveals that the mineralization efficiency of MICP progressively intensifies with a rise in pH, culminating in an optimal performance at pH 9. Subsequently, at higher pH values, the enhancement in RA’s physical properties diminishes.
As with temperature, the initial pH of the medium also has an effect on enzyme catalysis. Mycobacteria can fully develop their catalytic activity in the optimal pH range, and when deviating from this range, pH affects the enzyme activity by influencing the dissociation state of the carboxyl and amino groups in the enzyme and substrate molecules. According to previous studies, the optimal pH range for bacterial and spore growth is between 7 and 9 [32]; when using the MICP method, the ideal initial pH range is from 6.5 to 9.3 [33]. In acidic environments (below pH 6) and alkaline environments (above pH 9), the activity of urease significantly decreases; under extremely acidic or alkaline conditions (pH ranging from 5 to 10), urease activity is notably reduced [34,35]. This finding indicates that a suitable pH value has a more positive effect on bacteria, thereby enhancing urease activity and accelerating the urease decomposition process. This method not only aids in the formation of CaCO3 precipitates but also improves the enhancement effect of MICP technology on the physical properties of RA. Therefore, when using ureolytic bacteria in MICP, both bacterial growth and urease activity should be considered.
3.1.4. Effect of Urea Concentration on RA Mineralization Reaction
Figure 8 exhibits the trend of the effect of different urea concentrations on water absorption and apparent density of RAs under microbial mineralization treatment. The results showed that urea concentrations of 0.25, 0.50, 0.75, 1.00, and 1.25 reduced the water absorption of RA by 5.29%, 8.34%, 11.23%, 13.68%, and 14.36%, respectively, compared to untreated RA. The data indicated that the modification effect of microbial mineralization treatment on water absorption of RAs increased with increasing urea concentration at urea concentration less than 1.0 mol/L. However, when the urea concentration increased from 1.0 mol/L to 1.25 mol/L, the growth rate of water absorption rate slowed down, and the overall trend showed an increase and then stabilization. For apparent density, the modification effect of urea concentration showed a similar pattern, i.e., with the increase in urea concentration, the modification effect of microbial mineralization treatment on the apparent density of RAs firstly increased and then stabilized. Compared with the untreated RA, the apparent density of the RA increased by 0.29%, 0.53%, 0.86%, 0.98%, and 1.07% when the urea concentration was 0.25 mol/L, 0.50 mol/L, 0.75 mol/L, 1.00 mol/L, and 1.2 mol/L, respectively. In addition, CaCO3 production increased with increasing urea concentration, reaching a maximum at a urea concentration of 1.25 mol/L, at which time the mass of RA increased by 2.76%.
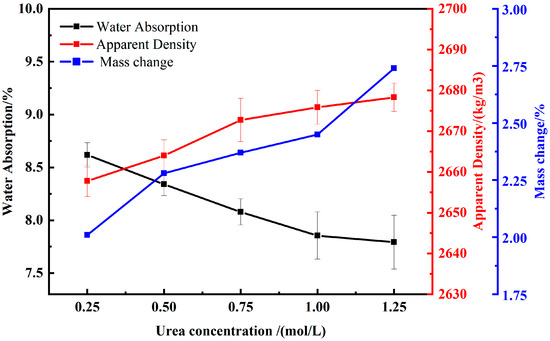
Figure 8.
Effects of different urea concentrations on RA physical properties and calcium carbonate production.
Urea is regarded as a crucial raw material in the MICP process and is one of the key factors affecting the MICP effectiveness. Research indicates that the presence of urea can induce bacteria to produce urease [36,37]. The method of modifying RAs based on microbial mineralization deposition technology is influenced by urea concentration. When the concentration of urea exceeds the limit, the rate of microbial urea decomposition reaches its maximum, and the accumulation of excessive enzymatic products may reduce the bacteria’s enzymatic capability, adversely affecting the microbial mineralization activity. Therefore, when using MICP technology to modify RAs, consider appropriately increasing the urea concentration to enhance microbial activity.
3.1.5. Effect of Ca+ Concentration on RA Mineralization Reaction
Figure 9 shows the trend in water absorption rate of RA before and after microbial mineralization modification under different Ca+ concentrations. Within the Ca+ concentration range of 0.25 to 0.50 mol/L, the effect of microbial mineralization on the water absorption rate of RA increases with the rise in Ca+ concentration. Specifically, when the Ca+ concentrations are 0.25 mol/L and 0.5 mol/L, the water absorption rate of RA decreases by 7.30% and 11.87%, respectively. However, when the Ca+ concentration exceeds 0.5 mol/L, the mineralization improvement effect of micro-organisms gradually decreases with the increase in Ca+ concentration. When the Ca+ concentrations are 0.75 mol/L, 1.0 mol/L, and 1.25 mol/L, the water absorption rates of the RA decrease by 9.56%, 5.71%, and 2.97%, respectively.
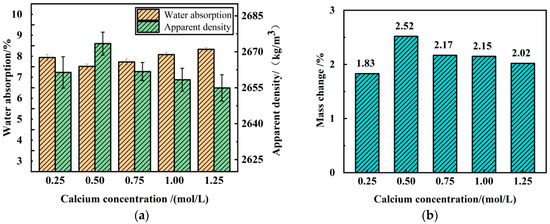
Figure 9.
Effects of different Ca+ concentrations on physical properties and calcium carbonate production of RA: (a) water absorption and apparent density; (b) mass change.
Similarly, the modification effect of microbial mineralization treatment on the apparent density of RA under different Ca+ concentrations follows a similar pattern, i.e., when the Ca+ concentration is greater than 0.5 mol/L, this improvement effect gradually weakens with the increase in Ca+ concentration.
When the Ca+ concentration exceeded 0.5 mol/L, the decreasing trend of the microbial mineralization effect became obvious and almost linear, which indicated that the high concentration of calcium chloride had an obvious inhibitory effect on the microbial mineralization under this condition, and this phenomenon was in agreement with the results of the study by Whiffin et al. [38]. There are two main reasons for this phenomenon. First, bacteria act as nucleation sites for MICP, and positively charged Ca+ are attracted to the cell wall due to the negatively charged cell wall of bacteria. In the lower Ca+ concentration range, the number of Ca+ attached to the bacterial cell wall increases as the Ca+ concentration increases, leading to the generation of more CaCO3 precipitates. However, as the calcium chloride concentration continues to increase, the Ca+ concentration supersaturates, resulting in the number of Ca+ attached to the bacterial cell wall not increasing proportionally. Therefore, the insufficient number of bacteria at higher Ca+ concentrations limits the MICP mineralization process [18]. Secondly, the precipitation of large amounts of CaCO3 is detrimental to microbial activity, thus inhibiting urea hydrolysis [29]. This result suggests that there exists an optimal calcium content for MICP technology that ensures cost-effective treatment of recycled aggregates. When increasing CaCO3 production by increasing the amount of calcium chloride, care should be taken to control the calcium chloride concentration in order to avoid inhibiting microbial activity and thus reducing the rate of urea hydrolysis. Under the condition of low Ca+ concentration, less than 0.5 mol/L, the CaCO3 crystals deposited by microbial mineralization are limited; while when the Ca+ concentration is high, the microbial mineralization activity will be inhibited, thus affecting the modification effect of the recycled aggregate.
3.1.6. Effect of Time on RA Mineralization Reaction
The effect pattern of mineralization time on the physical properties of RA is shown in Figure 10. The mineralization time was 7, 14, and 21 days at a mineralization temperature of 25 °C. The water absorption of RA was reduced by 16.07%, 13.68%, and 11.23%, while the apparent density of RA was enhanced by 1.07%, 0.98%, and 0.86%, respectively. The CaCO3 generation rate increased with the extension of the mineralization time, and reached 2.74% at 21 days. From the figure, it can be observed that the reduction in water absorption of RA was most significant when the mineralization time was 7 days, and this effect was weakened with the extension of the modification time. The apparent density of RA showed the same trend with the water absorption, which reached the highest when the mineralization time was 7 days, and then gradually decreased with the increase in the mineralization time.
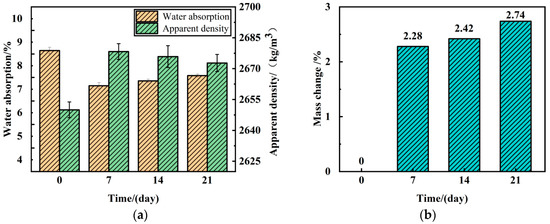
Figure 10.
Effects of different mineralization times on RA physical properties and calcium carbonate production: (a) water absorption and apparent density; (b) mass change.
After a period of MICP treatment, the quality of RA improved, further proving that the CaCO3 produced by MICP technology accumulates on the surface of RA. Additionally, this indicates that the improved method based on Bacillus pasteurii mineralization deposition can significantly reduce the water absorption of RA. However, with the gradual extension of the modification process time, the water absorption rate of the aggregate shows a trend of first decreasing and then increasing. When the mineralization time is 7 days, the change in water absorption rate is most significant compared to the untreated group. However, after 7 days, the effect does not change significantly, indicating that from the perspective of aggregate water absorption rate, there is an optimal modification time, which is consistent with the conclusions of Feng et al. [39]. Within the scope of this experimental study, the optimal value is determined to be 7 days.
3.2. Transformation and Microstructural Evolution of RA Phases
The most significant improvement in physical properties of RA was achieved at a bacterial solution OD600 of 1.4, a temperature of 35 °C, a pH of 9, a concentration of urea, and Ca+ of 0.5 mol/L, and a mineralization time of 7 d. The microanalysis of the untreated RA and the RA with the best mineralization effect was carried out to illustrate the effect of mineralization treatment on the physical phase composition and microstructure of RA.
3.2.1. TG and XRD Analysis
The TG results of RA before and after mineralization are shown in Figure 11. Calcium carbonate thermally decomposes into calcium oxide and carbon dioxide in the temperature range of 650–800 °C [40], and thus CaCO3 can be identified from the characteristic heat-absorbing peaks in Figure 10. In addition, significant exothermic peaks were observed in the temperature interval of 35–200 °C, corresponding to the removal of bound water in C-S-H gels and AFt as well as in Mc, respectively. In the temperature range of 650–800 °C, the weight loss rate of RA is 19.99%, while the weight loss rate of treated RA is 27.07%. Based on the weight loss rate within the decomposition temperature range of CaCO3, the content of CaCO3 can be calculated. The CaCO3 content in the RA group is 45.46%, and after MICP mineralization, the CaCO3 content in the RA group increased to 61.56%. The significant increase in CaCO3 content indicates that a large amount of CaCO3 was generated in the RA after mineralization treatment. Furthermore, thermogravimetric analysis revealed that the decomposition temperature of biogenic calcite is higher than that of chemical calcite intrinsic to RA, indicating that, besides chemical bonds, another special bond must be broken. Qian et al. [41] suggested through a series of studies that this is due to the formation of hydrogen bonds between hydroxyl groups and oxygen atoms in the polypeptides of the biogenic calcite’s organic matter. This also demonstrates the advantage of MICP mineralization treatment for RA and explains the improvement in RA’s physical properties.
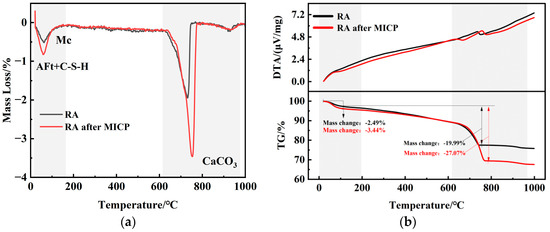
Figure 11.
TG analysis of RA before and after MICP: (a) curve DTG; (b) curve DTA and TG.
In order to further confirm the physical phase changes of RA after mineralization, XRD analysis was performed. Figure 12 illustrates the X-ray diffractograms of MICP before and after mineralization, which were mainly used to qualitatively detect their mineral composition. Calcite and calcite are the two most common crystalline forms of CaCO3 [40], with calcite being the most stable crystalline form. From the Figure 12, it can be seen that the characteristic peaks are mainly distributed near 29° for calcite crystal diffraction peaks, in addition to a small amount of calcite alumina, hydromagnesite, and hydroxycalcite. Specifically, the area of the peaks indicates the crystal content, and the larger the area of the peaks, the higher the crystal phase content. Under the treatment of MICP, the diffraction peaks of calcite-type CaCO3 become stronger and stronger, which confirms the generation of calcium carbonate on the surface of RA.
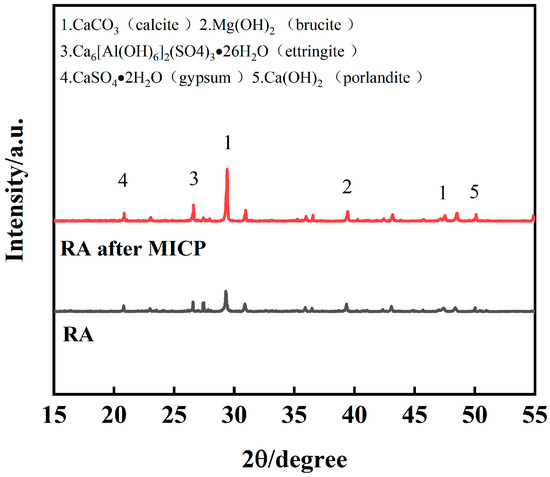
Figure 12.
XRD analysis of RA before and after MICP mineralization.
According to the above TG and XRD analysis results, the main components of RA include AFt, C-S-H gel, and CaCO3. By analyzing the physical phase of RA, the relationship between the amount of calcium carbonate generation and the physical properties of RA can be determined. Within a certain range, the amount of calcium carbonate generation showed a positive correlation with the physical properties of RA [42,43], which further proved that the MICP mineralization reaction increased the compactness of RA, thus improving the physical properties of RA.
3.2.2. NMR Analysis
Microbial mineralization technology involves a wide range of areas, complex processes, and numerous influencing factors. During the mineralization process, the reduction in aggregate porosity is directly related to the amount of CaCO3 precipitation generated during microbial solidification [44]. Using NMR, the impact of pore changes on the performance of RAs can be analyzed from a micro perspective. The NMR spectrum includes two time variables, T1 and T2, which are two independent frequency variables obtained after two Fourier transformations. Among them, T2 is the sampling time during the detection period, and T1 is an independent variable during the evolution period that is unrelated to T2. T2 is related to the composition and type of the material; even for the same material, slight differences in composition can ultimately lead to different T2 values. The transverse relaxation time (T2) is a key parameter commonly used in nuclear magnetic resonance experiments, and it involves time variables. The distribution of the T2 spectrum reflects the distribution of pores within the specimen, with different peaks corresponding to different pore size categories. The number of peaks can indicate the connectivity of various pore sizes. In the spectrum, the first peak represents the porosity of small pores in the RA, while the second peak represents the porosity of large pores in the RA.
When the porous medium is in a saturated state, T2 is proportional to S/V and can be used as a measure of pore size. Therefore, the inverted T2 spectrum reflects the pore size distribution curve of the sample. Due to layout limitations, this study compares the RA with the best MICP treatment effect and the untreated RA, and the inversion results are shown in Figure 13. The inverted graph after the NMR test was processed, and the results were compared and analyzed. By comparing the changes in the T2 relaxation peaks of the NMR test spectra before and after MICP treatment (including the range of relaxation times, the peak maxima, and the changes in peak areas), and combining these with the amount of CaCO3 deposited on the RA surface, the effect of MICP treatment on the changes in RA porosity and its impact on physical properties was determined.
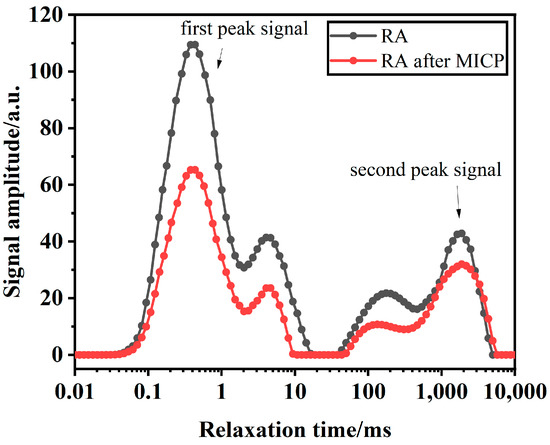
Figure 13.
NMR differential maps of RA before and after MICP mineralization.
It can be observed from Figure 13 that the T2 spectral values of RA treated with MICP were significantly reduced compared with those of untreated RA. In the test, the chirp times of the first and second peaks were 0.01~10 ms and 100~10,000 ms, respectively. In addition, the second peak of the T2 spectrum of the RA and its peak area also had a decreasing trend after MICP treatment, which indicated that the macroporous porosity of the RA also showed a decreasing trend. According to the positive relationship between T2 value and pore size, the larger T2 value indicates that the signal value corresponds to a higher proportion of pore size. The dominant peak of the specimen in Figure 13 has not changed, and the amplitude of the first peak is always larger than that of the second peak, which indicates that the pore size distribution inside the specimen is relatively uniform, and the proportion of large pores is higher than that of small pores.
From the analysis of the signal amplitude integration graphs of samples in Figure 14a,b, it can be observed that the internal porosity of RA treated with MICP is lower. Combined with the XRD results, it was found that the amount of CaCO3 on the surface of RA increased gradually after MICP treatment. Since micro-organisms need space to reproduce, biocrystals tend to form around and inside open pores, including surface pores and microcracks [45]. This may be because large pores and microcracks are more easily occupied by bacteria, serving as nucleation sites. Thus, it is repaired by biological CaCO3 [46]. When the pores on the surface of RA are filled with CaCO3, micro-organisms form a dense layer of CaCO3 on the RA surface, thereby affecting the porosity of RA. As a result, the physical properties of RAs treated with MICP are improved compared to untreated RA, with an overall reduction in porosity and effective filling of various types of pores.
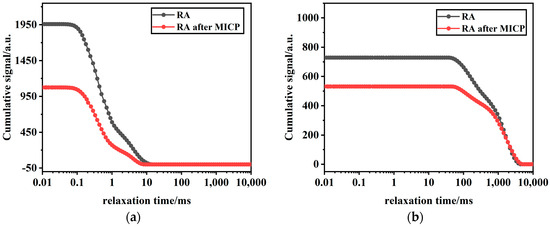
Figure 14.
NMR integrals of RA before and after MICP mineralization: (a) integration of the amplitude of the first peak signal; (b) integration of the amplitude of the second peak signal.
3.2.3. SEM Analysis
Scanning electron microscope was used to observe the micromorphology of the RA surface treated by the MICP technique, as shown in Figure 15 and Figure 16. It was observed that the CaCO3 particles on the RA surface showed regular spherical and square shapes, with diameters ranging from 1 μm to 10 μm, with a relatively uniform distribution. In addition, pores with a diameter of about 0.5 μm were observed on the surface of the spherical particles, which were traces formed by the bacterium during the mineralization process [43]. This indicates that the bacterial strain not only promotes the crystallization formation of calcite through its enzymatic action, but also plays a nucleation role itself, acting as a nucleation site at the RA pores and microcracks to form a crystal protective layer, which can effectively cover the defects such as cracks and pores on the surface of RA. Combined with the analysis of XRD results, the deposition product was determined to be CaCO3.
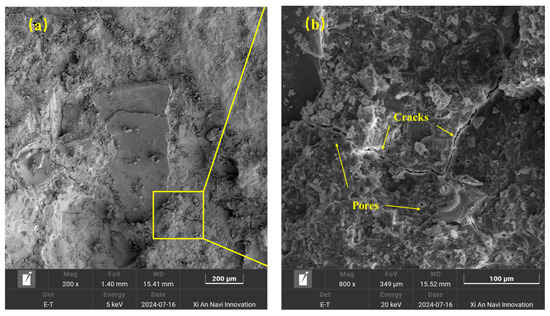
Figure 15.
SEM images of unprocessed RA: (a) magnification 200×; (b) magnification 800×.
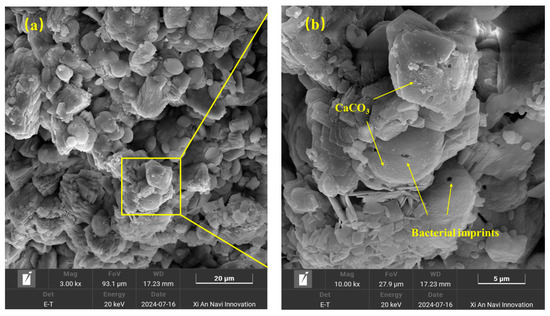
Figure 16.
SEM image of RA after MICP treatment: (a) magnification 3000×; (b) magnification 10,000×.
4. Conclusions and Future Research
4.1. Conclusions
This study conducted MICP mineralization treatment on high-porosity RA made from construction demolition materials, and tested its physical properties and CaCO3 generation rate, drawing the following conclusions:
- (1)
- The enhancement of physical properties is more pronounced as the generation of calcium carbonate in recycled aggregates increases. Following treatment with MICP, the precipitated calcium carbonate fills microcracks and pores within the recycled aggregate, resulting in a reduction in porosity and a densification of the interfacial transition zone between the aggregate and adherent mortar. This process contributes to the overall improvement in the physical properties of the recycled aggregate.
- (2)
- Proper control of incubation and precipitation conditions significantly influences the rate and quantity of CaCO3 produced on recycled aggregates (RAs). The bacteriophage and urease present in the system each have their optimal temperature and pH ranges for activity. There exists an optimal temperature interval and pH level for maximizing CaCO3 production; conditions that are either too low or too high in temperature and pH are detrimental to the MICP process.
- (3)
- Upon considering several key factors in the microbial-induced carbonate precipitation (MICP) technique, including bacterial solution concentration, temperature, pH, urea concentration, Ca+ concentration, and mineralization time, it was determined that optimal physical properties of recycled aggregate (RA) were achieved under specific conditions. When the bacterial solution had an optical density (OD600) of 1.4, with a temperature of 35 °C, a pH of 9, and concentrations of urea and Ca+ at 0.5 mol/L, along with a mineralization time of 7 days, the best physical properties of RA were obtained. Notably, the water absorption rate was reduced by 16.07%, the apparent density increased by 1.07%, and the mass change was recorded at 2.28%.
4.2. Future Research
Microbial mineralization deposition technology, a burgeoning technique for modifying recycled aggregates, has recently emerged. This technology enhances the quality of recycled aggregates by precipitating calcium carbonate to fill pores and microcracks through microbial reactions. Although this method has received extensive attention from scholars, there are few research reports and the technology is not yet mature, which needs to be further explored in future research. In addition, the application of microbial mineralization deposition technology is affected by many factors, resulting in the application of this technology still having some problems that need to be solved:
- (1)
- Alkaliphilic micro-organisms exhibit a greater capacity to thrive in alkaline conditions, although they tend to display diminished enzyme activity in highly alkaline settings. Buffers are commonly introduced during microbial mineralization deposition processes to counteract pH extremes, thereby mitigating the detrimental effects of an alkaline environment on microbial activity. However, the disruption of the highly alkaline milieu within the cement system can compromise the durability of reinforced concrete structures. Consequently, it is crucial to undertake research into the development of alkaline-tolerant micro-organisms to enhance their adaptability in alkaline environments.
- (2)
- Currently, research on the modification of recycled aggregates using microbial mineralization deposition technology is in its nascent phase, and the durability performance of recycled concrete is an area with limited investigation. Further research is warranted to assess the resistance of microbial mineralization-treated recycled aggregates to processes such as carbonation, freeze–thaw cycles, and erosion.
Author Contributions
Conceptualization, J.Z. and Z.W.; methodology, C.W. and Z.W.; software, J.Z., C.W. and Z.W.; validation, J.Z. and C.W.; writing—original draft preparation, J.Z. and C.W.; writing—review and editing, C.W.; visualization, J.Z.; supervision, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zheng, C.; Zhang, H.; Cai, X.; Chen, L.; Liu, M.; Lin, H.; Wang, X. Characteristics of CO2 and atmospheric pollutant emissions from China’s cement industry: A life-cycle perspective. J. Clean. Prod. 2021, 282, 124533. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Reddy, N.S.; Lahoti, M. A succinct review on the durability of treated recycled concrete aggregates. Environ. Sci. Pollut. Res. 2023, 30, 25356–25366. [Google Scholar] [CrossRef]
- Safiuddin, M.; Alengaram, U.J.; Rahman, M.; Salam, A.; Jumaat, M.Z. Use of Recycled Concrete Aggregate in Concrete: A Review. J. Civ. Eng. Manag. 2013, 19, 796–810. [Google Scholar] [CrossRef]
- Poon, C.; Shui, Z.; Lam, L. Effect of microstructure of ITZ on compressive strength of concrete prepared with recycled aggregates. Constr. Build. Mater. 2004, 18, 461–468. [Google Scholar] [CrossRef]
- Djerbi, A. Effect of recycled coarse aggregate on the new interfacial transition zone concrete. Constr. Build. Mater. 2018, 190, 1023–1033. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W.; Sun, Z.; Lange, D.A.; Shah, S.P. Properties of interfacial transition zones in recycled aggregate concrete tested by nanoindentation. Cem. Concr. Compos. 2013, 37, 276–292. [Google Scholar] [CrossRef]
- Dilbas, H.; Çakır, Ö.; Atiş, C.D. Experimental investigation on properties of recycled aggregate concrete with optimized Ball Milling Method. Constr. Build. Mater. 2019, 212, 716–726. [Google Scholar] [CrossRef]
- Otsuki, N.; Miyazato, S.-I.; Yodsudjai, W. Influence of recycled aggregate on interfacial transition zone, strength, chloride penetration and carbonation of concrete. J. Mater. Civ. Eng. 2003, 15, 443–451. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Tam, C.M.; Le, K.N. Removal of cement mortar remains from recycled aggregate using pre-soaking approaches. Resour. Conserv. Recycl. 2007, 50, 82–101. [Google Scholar] [CrossRef]
- Li, J.; Xiao, H.; Zhou, Y. Influence of coating recycled aggregate surface with pozzolanic powder on properties of recycled aggregate concrete. Constr. Build. Mater. 2009, 23, 1287–1291. [Google Scholar] [CrossRef]
- Kou, S.-C.; Poon, C.-S. Properties of concrete prepared with PVA-impregnated recycled concrete aggregates. Cem. Concr. Compos. 2010, 32, 649–654. [Google Scholar] [CrossRef]
- Spaeth, V.; Tegguer, A.D. Improvement of recycled concrete aggregate properties by polymer treatments. Int. J. Sustain. Built Environ. 2013, 2, 143–152. [Google Scholar] [CrossRef]
- Sui, Y.; Mueller, A. Development of thermo-mechanical treatment for recycling of used concrete. Mater. Struct. 2012, 45, 1487–1495. [Google Scholar] [CrossRef]
- Ismail, S.; Ramli, M. Engineering properties of treated recycled concrete aggregate(RCA) for structural applications. Constr. Build. Mater. 2013, 44, 464–476. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Kou, S.-C.; Poon, C.-S.; Dai, J.-G.; Li, Q.-Y. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Jia, Q.; Chen, X.H.; Sun, Z.B. The raising calcite yield experiment for bacteria induced precipitation. J. Shandong Jianzhu Univ. 2015, 30, 423–428. [Google Scholar]
- Qiu, J.; Tng, D.Q.S.; Yang, E.-H. Surface treatment of recycled concrete aggregates through microbial carbonate precipitation. Constr. Build. Mater. 2014, 57, 144–150. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Klama, J.; Zawal, D.; Krupa, D. Modification of Recycled Concrete Aggregate by Calcium Carbonate Biodeposition. Constr. Build. Mater. 2012, 34, 145–150. [Google Scholar] [CrossRef]
- Wang, J.; Vandevyvere, B.; Vanhessche, S.; Schoon, J.; Boon, N.; De Belie, N. Microbial carbonate precipitation for the improvement of quality of recycled aggregates. J. Clean. Prod. 2017, 156, 355–366. [Google Scholar] [CrossRef]
- Construction Pebbles, Crushed Stone. GB/T 14685-2022. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=26E33B40996B9CB1DED15E514B34E7F8 (accessed on 7 September 2024).
- Evaluating Method for the Efficacy of Sterilization for Disinfection Equipment. GB/T 15981-2021. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=C98AC5FACDC10DAE52C1961445F63129 (accessed on 7 September 2024).
- Singh, L.P.; Bisht, V.; Aswathy, M.S.; Chaurasia, L.; Gupta, S. Studies on performance enhancement of recycled aggregate by incorporating bio and nano materials. Constr. Build. Mater. 2018, 181, 217–226. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using microorganisms. Mater. J. 2001, 98, 3–9. [Google Scholar] [CrossRef]
- Lu, Z.; Tan, Q.; Lin, J.; Wang, D. Properties investigation of recycled aggregates and concrete modified by accelerated carbonation through increased temperature. Constr. Build. Mater. 2022, 341, 127813. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, Q.; Chen, L.; Zheng, K.; Wei, S.; Iftikhar, Y. Sulfate resistance of carbonated basic oxygen furnace slag-metakaolin-Portland cement blends. J. Build. Eng. 2024, 87, 109098. [Google Scholar] [CrossRef]
- De Muynck, W.; Verbeken, K.; De Belie, N.; Verstraete, W. Influence of urea and calcium dosage on the effectiveness of bacterially induced carbonate precipitation on limestone. Ecol. Eng. 2010, 36, 99–111. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Kumar, C.; Takagi, H. Microbial alkaline proteases: From a bioindustrial viewpoint. Biotechnol. Adv. 1999, 17, 561–594. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Khoshdelnezamiha, G.; Senian, N.; Ong, D.E.L.; Nissom, P.M. Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol. Eng. 2017, 109, 65–75. [Google Scholar] [CrossRef]
- De Muynck, W.; Verbeken, K.; De Belie, N.; Verstraete, W. Influence of temperature on the effectiveness of a biogenic carbonate surface treatment for limestone conservation. Appl. Microbiol. Biotechnol. 2013, 97, 1335–1347. [Google Scholar] [CrossRef]
- Wang, J.; Jonkers, H.M.; Boon, N.; De Belie, N. Bacillus sphaericus LMG 22257 is physiologically suitable for self-healing concrete. Appl. Microbiol. Biotechnol. 2017, 101, 5101–5114. [Google Scholar] [CrossRef]
- Imran, A.; Kimura, S.; Nakashima, K.; Evelpidou, N.; Kawasaki, S. Factors affecting the urease activity of native ureolytic bacteria isolated from coastal areas. Geomech. Eng. 2019, 17, 421–427. [Google Scholar] [CrossRef]
- Fujita, M.; Nakashima, K.; Achal, V.; Kawasaki, S. Whole-cell evaluation of urease activity of Pararhodobacter sp. isolated from peripheral beachrock. Biochem. Eng. J. 2017, 124, 1–5. [Google Scholar] [CrossRef]
- Cuzman, O.A.; Rescic, S.; Richter, K.; Wittig, L.; Tiano, P. Sporosarcina pasteurii use in extreme alkaline conditions for recycling solid industrial wastes. J. Biotechnol. 2015, 214, 49–56. [Google Scholar] [CrossRef]
- Kaltwasser, H.; Conger, W.R.; Krämer, J. Control of urease formation in certain aerobic bacteria. Arch. Microbiol. 1972, 81, 178–196. [Google Scholar] [CrossRef]
- Friedrich, B.; Magasanik, B. Utilization of arginine by Klebsiella aerogenes. J. Bacteriol. 1978, 133, 680–685. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the production of Biocement. Doctoral Dissertation, Murdoch University, Murdoch, WA, Australia, 2004. [Google Scholar] [CrossRef]
- Feng, Z.; Zhao, Y.; Zeng, W.; Lu, Z.; Shah, S.P. Using microbial carbonate precipitation to improve the properties of recycled fine aggregate and mortar. Constr. Build. Mater. 2020, 230, 116949. [Google Scholar] [CrossRef]
- Ali, A.; Li, M.; Su, J.; Li, Y.; Wang, Z.; Bai, Y.; Ali, E.F.; Shaheen, S.M. Brevundimonas diminuta isolated from mines polluted soil immobilized cadmium (Cd2+) and zinc (Zn2+) through calcium carbonate precipitation: Microscopic and spectroscopic investigations. Sci. Total. Environ. 2022, 813, 152668. [Google Scholar] [CrossRef]
- Qian, C.; Yu, X.; Wang, X. A study on the cementation interface of bio-cement. Mater. Charact. 2018, 136, 122–127. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Naeimi, M.; Stabnikov, V.; Liu, H.-L. Optimization of calcium-based bioclogging and biocementation of sand. Acta Geotech. 2014, 9, 277–285. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, D.; Zhang, J. Affecting Factors for Calcareous Sand Reinforcement Based on Microbial Induced Mineralization. Bull. Geol. Sci. Technol. 2019, 38, 206–211. [Google Scholar] [CrossRef]
- Liu, J.; Tong, H.; Zhao, J.; Yuan, J. Experimental Study on Strengthening Calcareous Sand with Microbial Solidification Technology in Salt Solution Environment. Sci. Technol. Eng. 2021, 21, 5046–5053. [Google Scholar]
- Zamarreno, D.V.; Inkpen, R.; May, E. Carbonate crystals precipitated by freshwater bacteria and their use as a limestone consolidant. Appl. Environ. Microbiol. 2009, 75, 5981–5990. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, R.; Hou, F.; Ye, G. Influence of pore property and alkalinity on the bio-deposition treatment efficiency of recycled aggregates. J. Build. Eng. 2024, 89, 109381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).