Enhancing the Surface Structure of Public Filler and Macroscopic Properties of Recycled Cement Mortar Using Polyethyleneimine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation Process
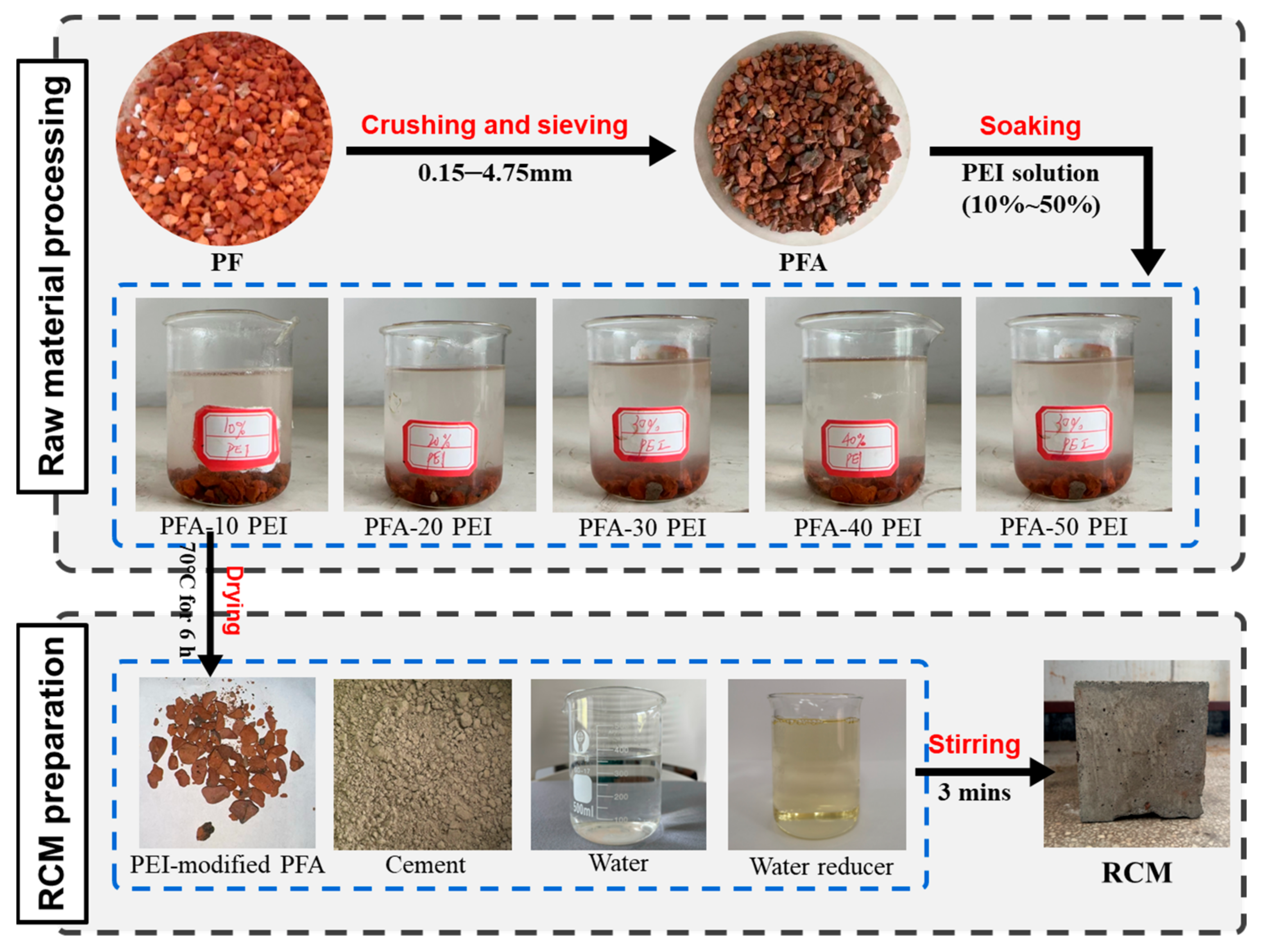
2.2.1. Pretreatment of PFA
2.2.2. Mix Proportion and Preparation of RCM
2.3. Evaluation Methods
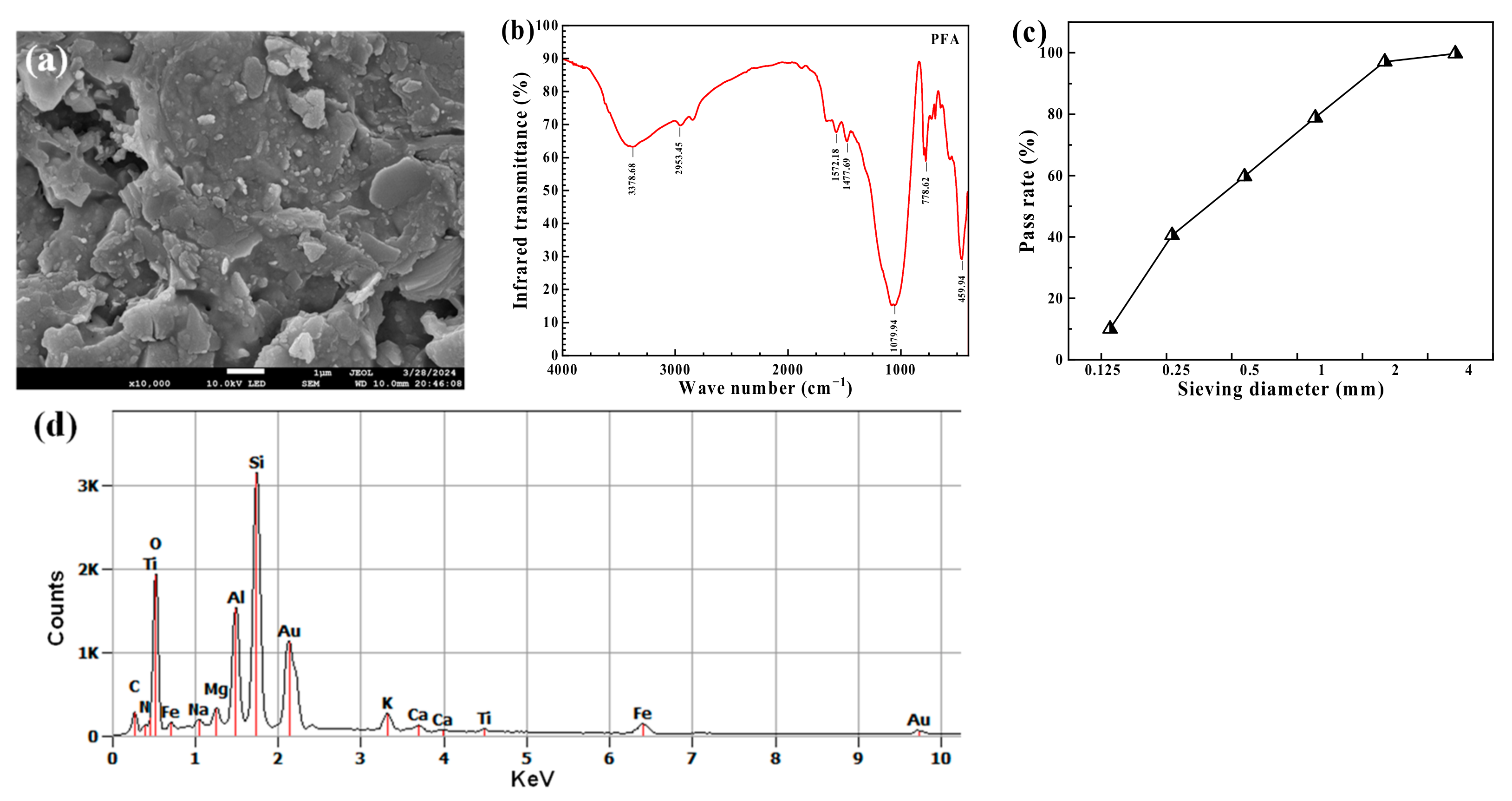
2.3.1. Microstructures of PFA
2.3.2. Mineral Composition
2.3.3. Physical Properties
2.3.4. Capillary Water Absorption (CWA) Test
2.3.5. Electric Flux
3. Results and Discussion
3.1. Influence of PEI on the Structural Densification of PFA
3.1.1. Pore Structure
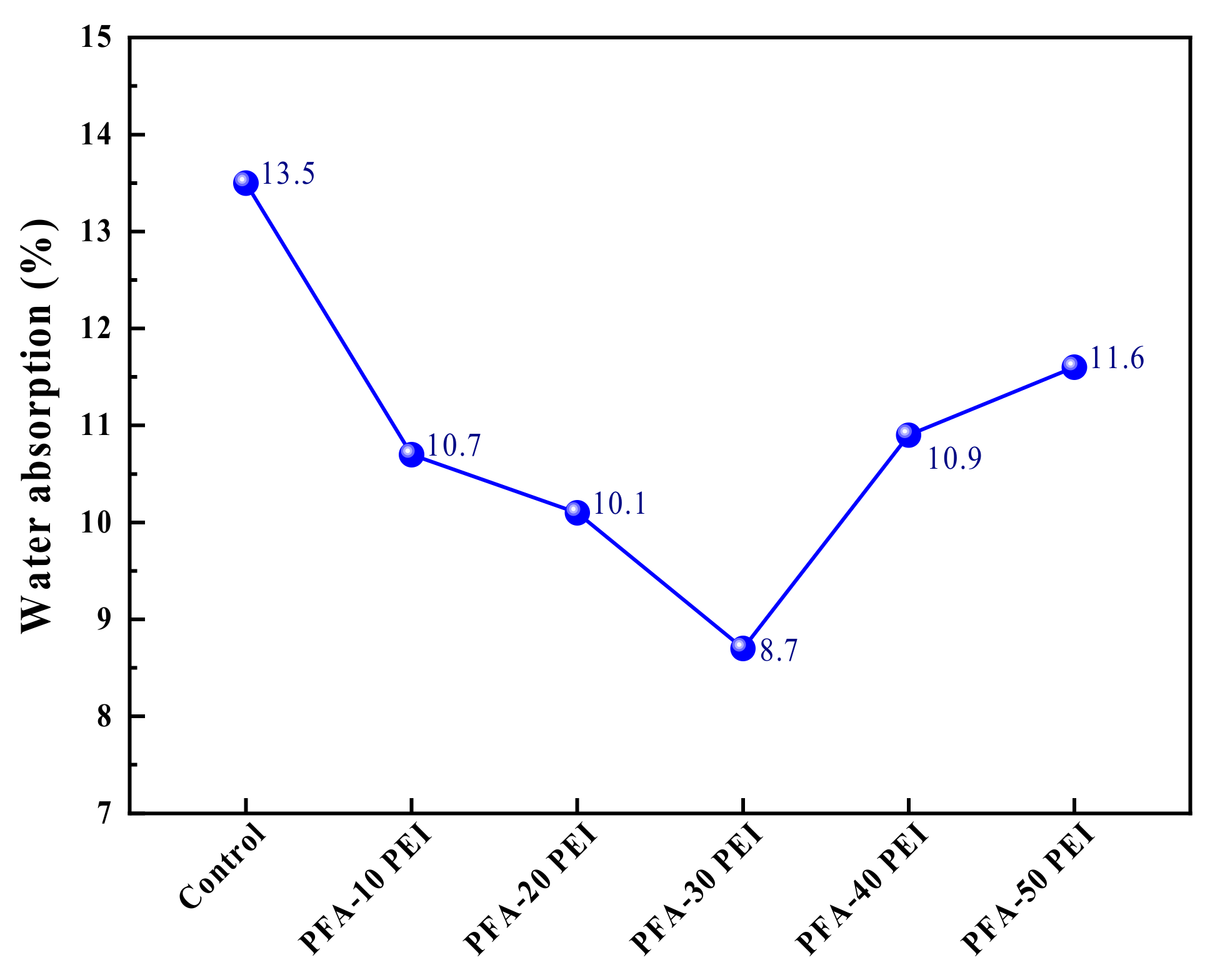
3.1.2. Water Absorption
3.2. Influence of PEI on the Texture of PFA
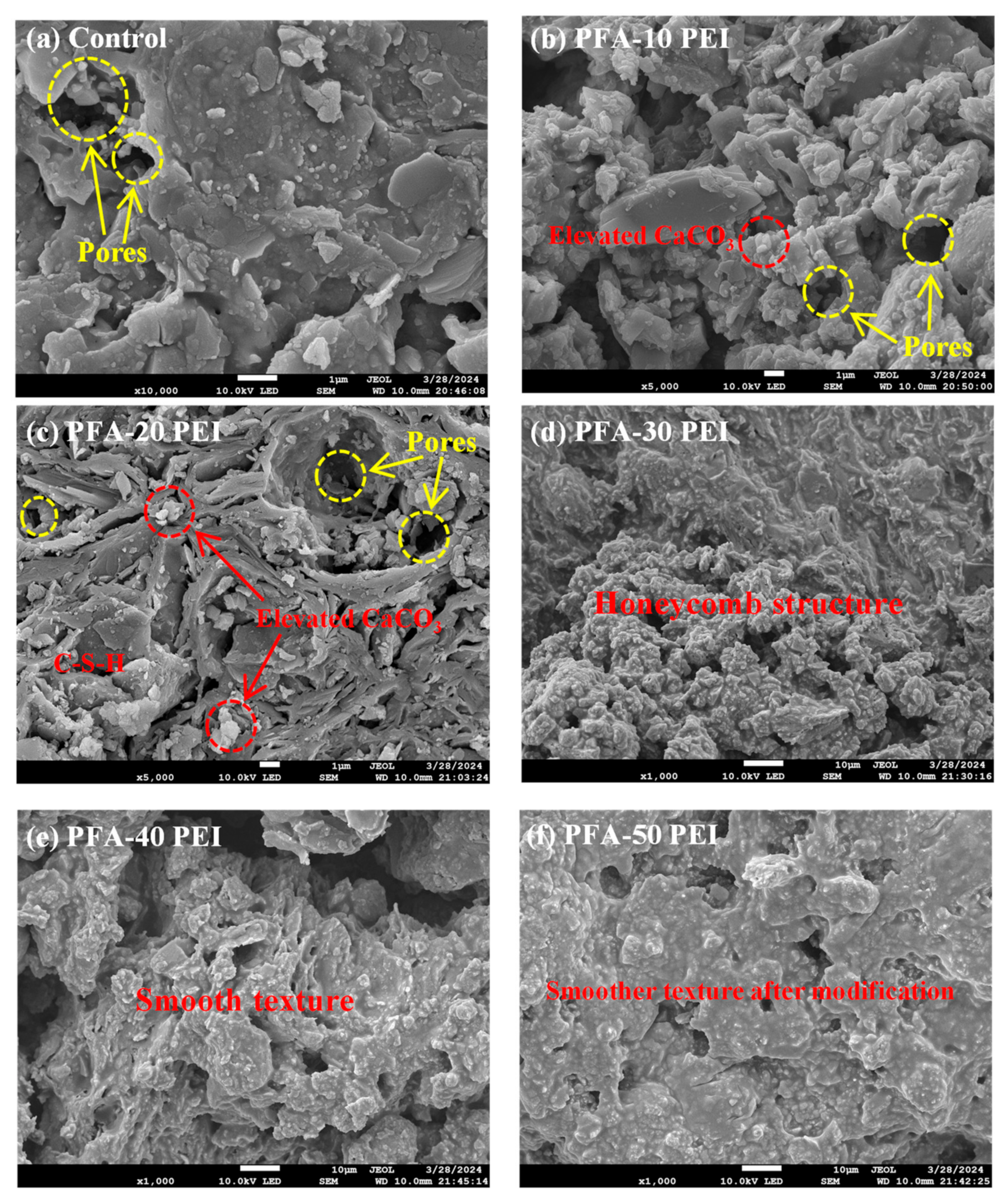
3.2.1. SEM
3.2.2. XRD
3.2.3. FTIR
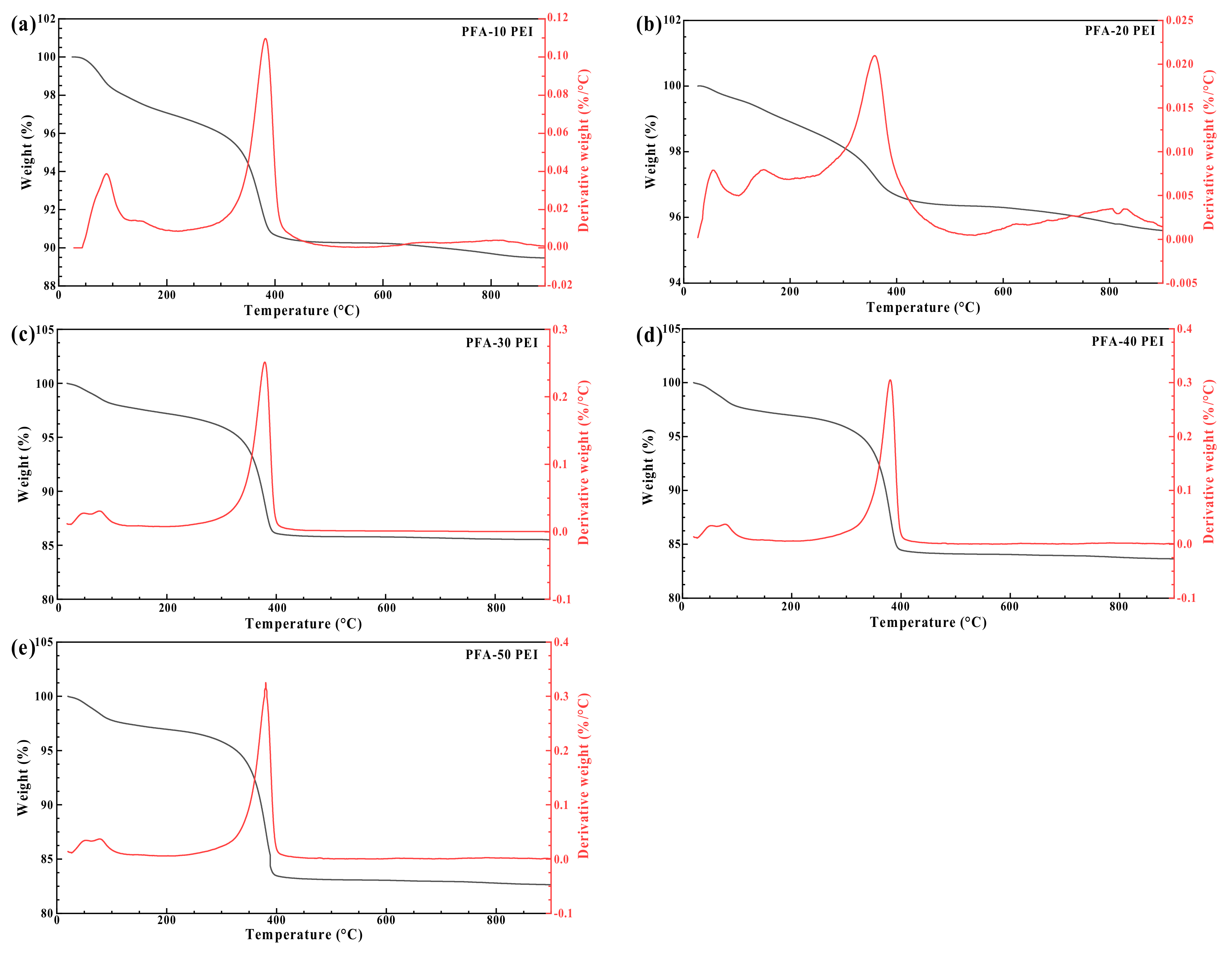
3.2.4. Thermogravimetric Analysis (TGA)
3.3. Influence of PEI-Modified PFA Incorporation on the Properties of Fresh Mortar
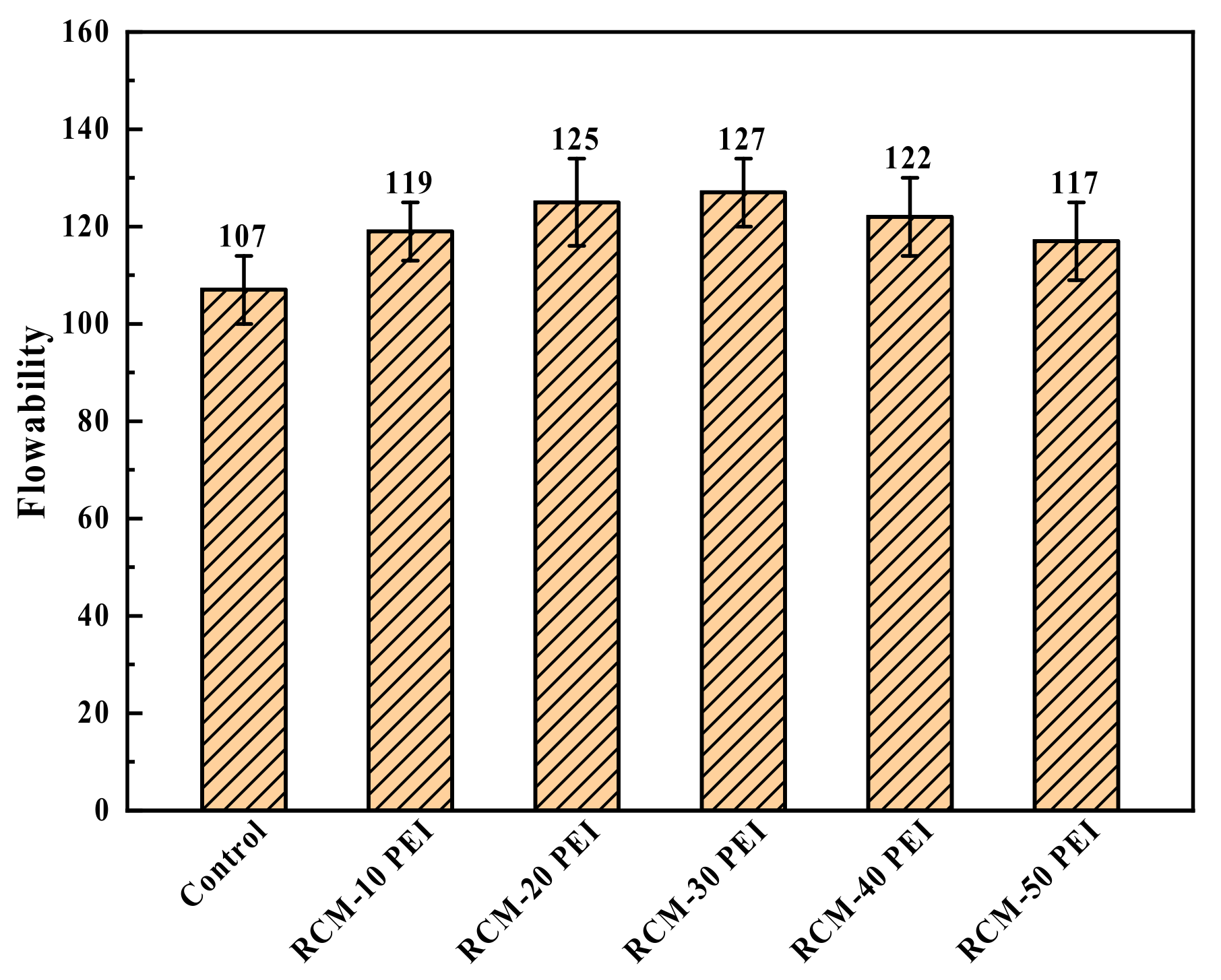
3.3.1. Flowability
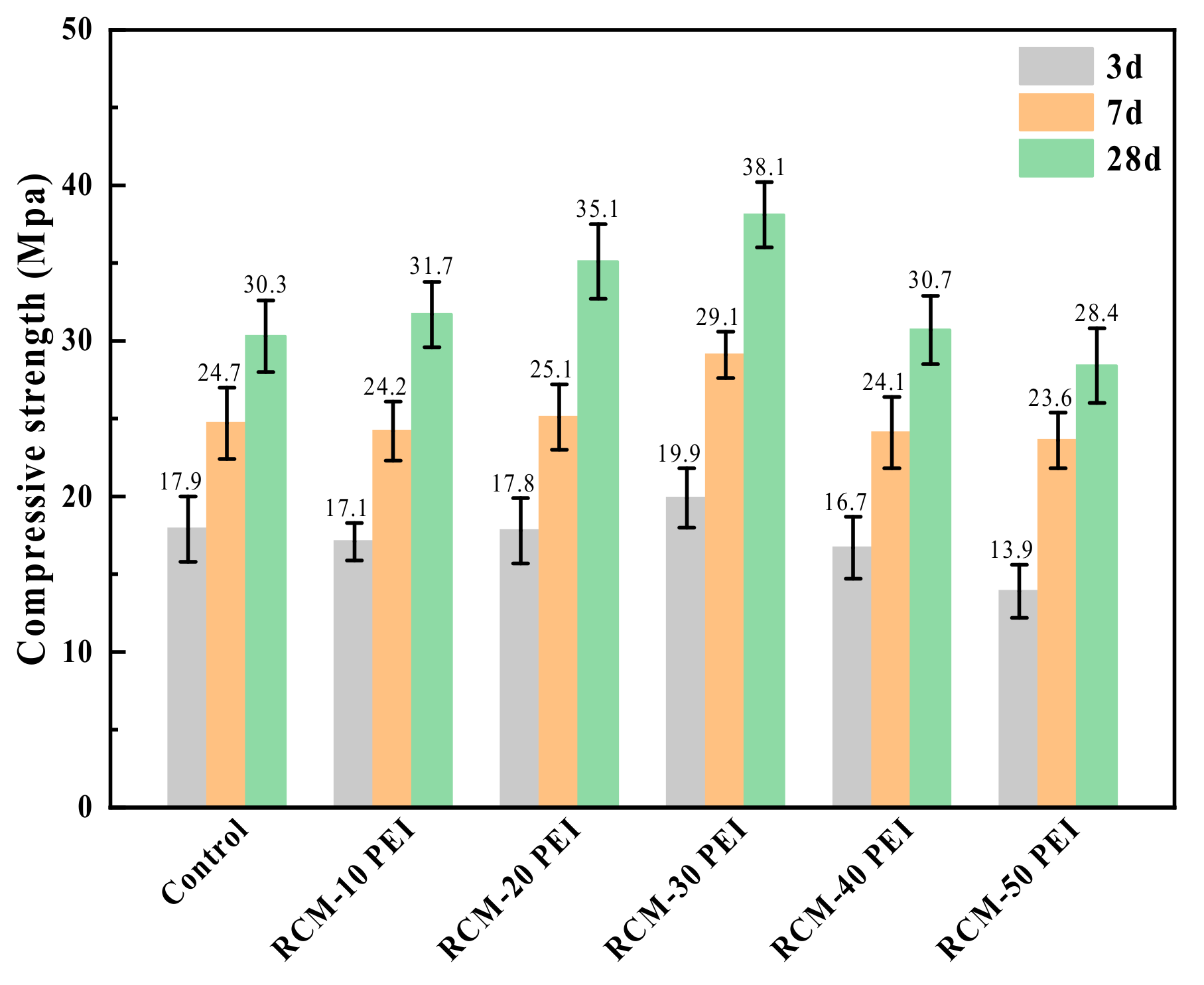
3.3.2. Compressive Strength
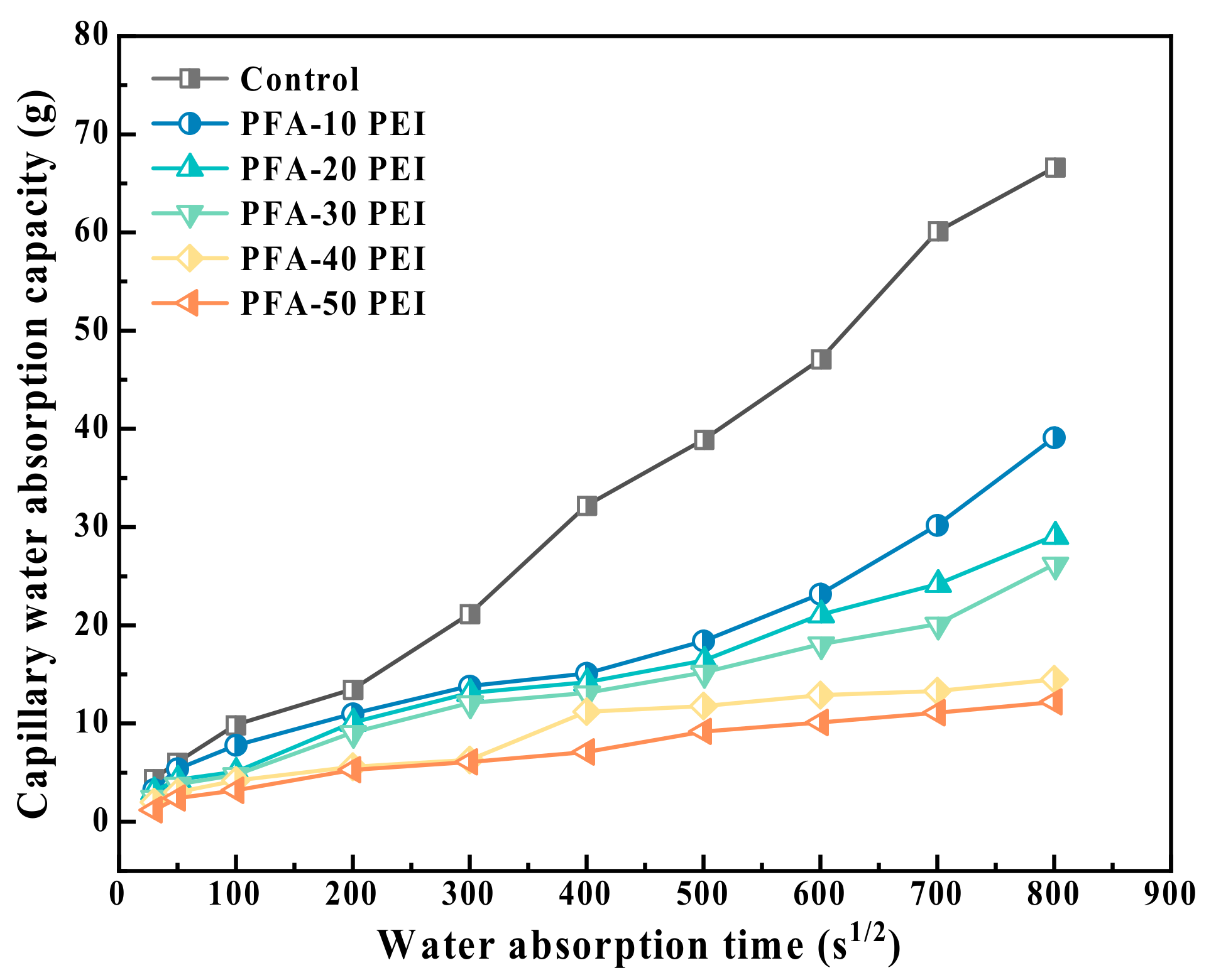
3.3.3. Capillary Water Absorption
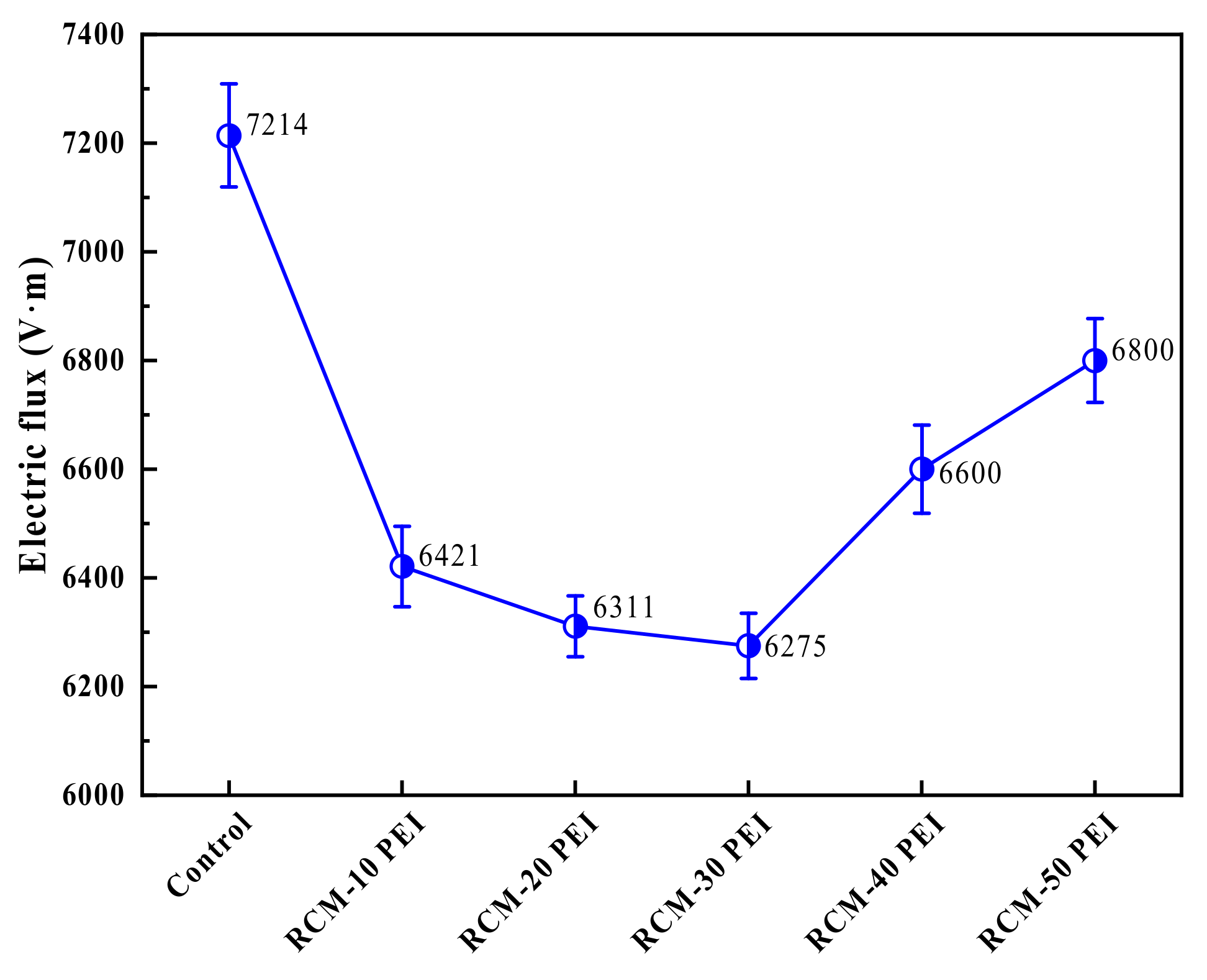
3.3.4. Electric Flux
3.3.5. BET
4. Conclusions
- (1)
- Increasing the PEI concentration to 40% and 50% decreased the pore volume of the PFA. However, the effect diminished at higher concentrations, with total pore volumes of 0.0258 cm3/g for PFA-40 PEI and 0.0281 cm3/g for PFA-50 PEI. This suggests that while higher PEI concentrations do reduce pore volume, the benefit levels off, indicating a saturation point in the modification of PFA’s porosity.
- (2)
- The water absorption rates for PFA-10 PEI, PFA-20 PEI, and PFA-30 PEI were 10.7%, 10.1%, and 8.7%, respectively. These observations demonstrate the effectiveness of PEI in enhancing the performance of PFA by refining its pore structure and reducing its water absorption capacity.
- (3)
- The compressive strength of the RCM improved from 30.3 MPa to 38.1 MPa with the use of the PEI-modified PFA. This enhancement originates from the increased density of the pore structure and the rougher surface texture of the modified PFA. The optimal values for electric flux and capillary water absorption were achieved with a 20% PEI concentration, demonstrating the optimal balance between performance and modification level.
- (4)
- The study suggests that PEI is a promising agent for improving the carbonation efficiency of PFA, refining its microstructure, and improving its surface characteristics. The development of PEI-based CO2 capture technologies can significantly reduce CO2 emissions, contributing positively to climate change mitigation efforts.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| TGA | Thermogravimetric analysis |
| PFA | Pulverized fly ash |
| PEI | Polyethyleneimine |
| PF | Public filler |
| RCM | Recycled concrete mortar |
| PFA | Public fine aggregate |
| ITZ | Interface transition zone |
| XRD | X-ray diffraction |
| ATR | Attenuated total reflectance |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| RAC | Recycled aggregate concrete |
| RCBA | Recycled clay brick aggregate |
| SEM | Scanning electron microscopy |
| CDW | Construction and demolition waste |
| DTG | Derivative thermogravimetry |
| EDS | Energy-dispersive spectroscopy |
| CWAM | Capillary water absorption mass |
| ICSD | International Crystal Structure Database |
| FTIR | Fourier transform infrared |
| BCRA | Brick–concrete recycled aggregate |
References
- Xu, K.; Shen, G.Q.; Liu, G.; Martek, I. Demolition of existing buildings in urban renewal projects: A decision support system in the China context. Sustainability 2019, 11, 491. [Google Scholar] [CrossRef]
- Liu, J.; Yi, Y.; Wang, X. Exploring factors influencing construction waste reduction: A structural equation modeling approach. J. Clean. Prod. 2020, 276, 123185. [Google Scholar] [CrossRef]
- Yu, B.; Wang, J.; Li, J.; Zhang, J.; Lai, Y.; Xu, X. Prediction of large-scale demolition waste generation during urban renewal: A hybrid trilogy method. Waste Manag. 2019, 89, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ogunmakinde, O.E. A review of circular economy development models in China, Germany and Japan. Recycling 2019, 4, 27. [Google Scholar] [CrossRef]
- Tam, V.W.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Akhtar, A.; Sarmah, A.K. Construction and demolition waste generation and properties of recycled aggregate concrete: A global perspective. J. Clean. Prod. 2018, 186, 262–281. [Google Scholar] [CrossRef]
- Whittaker, M.J.; Grigoriadis, K.; Soutsos, M.; Sha, W.; Klinge, A.; Paganoni, S.; Casado, M.; Brander, L.; Mousavi, M.; Scullin, M. Novel construction and demolition waste (CDW) treatment and uses to maximize reuse and recycling. Adv. Build. Energy Res. 2021, 15, 253–269. [Google Scholar] [CrossRef]
- Sormunen, P.; Kärki, T. Recycled construction and demolition waste as a possible source of materials for composite manufacturing. J. Build. Eng. 2019, 24, 100742. [Google Scholar] [CrossRef]
- Nautiyal, H.; Shree, V.; Khurana, S.; Kumar, N.; Varun. Recycling potential of building materials: A review. In Environmental Implications of Recycling and Recycled Products; Springer: Berlin/Heidelberg, Germany, 2015; pp. 31–50. [Google Scholar] [CrossRef]
- Islam, R.; Nazifa, T.H.; Yuniarto, A.; Uddin, A.S.; Salmiati, S.; Shahid, S. An empirical study of construction and demolition waste generation and implication of recycling. Waste Manag. 2019, 95, 10–21. [Google Scholar] [CrossRef]
- Kujawa, W.; Olewnik-Kruszkowska, E.; Nowaczyk, J. Concrete strengthening by introducing polymer-based additives into the cement matrix—A mini review. Materials 2021, 14, 6071. [Google Scholar] [CrossRef]
- Knapen, E.; Beeldens, A.; Van Gemert, D.; Van Rickstal, F. Modification of cement concrete by means of polymers in solution. In Proceedings of the 11th Congress on Polymers in Concrete, Berlin, Germany, 2–4 June 2004; pp. 83–90. [Google Scholar]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent progress in silane coupling agent with its emerging applications. J. Polym. Environ. 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
- Gao, C.; Huang, L.; Yan, L.; Jin, R.; Chen, H. Mechanical properties of recycled aggregate concrete modified by nano-particles. Constr. Build. Mater. 2020, 241, 118030. [Google Scholar] [CrossRef]
- He, Z.; Shen, A.; Wu, H.; Wang, W.; Wang, L.; Guo, Y. Properties and mechanisms of brick-concrete recycled aggregate strengthened by compound modification treatment. Constr. Build. Mater. 2022, 315, 125678. [Google Scholar] [CrossRef]
- Feng, Y.; Qin, D.; Zhao, C. The synergistic strengthening effect of silane coupling agent on the interface between PVA/EPS and cement: Experiment and molecular simulation. Compos. Interfaces 2023, 30, 21–41. [Google Scholar] [CrossRef]
- You, J.; Wang, L.; Zhao, Y.; Bao, W. A review of amino-functionalized magnetic nanoparticles for water treatment: Features and prospects. J. Clean. Prod. 2021, 281, 124668. [Google Scholar] [CrossRef]
- Singh, R.; Nayak, D.; Pandey, A.; Kumar, R.; Kumar, V. Effects of recycled fine aggregates on properties of concrete containing natural or recycled coarse aggregates: A comparative study. J. Build. Eng. 2022, 45, 103442. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.; Zhao, L. Application of magnetic chitosan nanocomposites modified by graphene oxide and polyethyleneimine for removal of toxic heavy metals and dyes from water. Int. J. Biol. Macromol. 2021, 192, 118–125. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, T.; Xue, Q.; Zhou, Y.; Wang, H.; Bolan, N.S.; Jiang, R.; Tsang, D.C. Enhanced adsorption of Cu (II) and Zn (II) from aqueous solution by polyethyleneimine modified straw hydrochar. Sci. Total Environ. 2021, 778, 146116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lv, Z.; Sun, Y.; Chi, Z.; Qing, G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J. Mater. Chem. B 2020, 8, 2951–2973. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Punta, C. Synthesis and application of cellulose-polyethyleneimine composites and nanocomposites: A concise review. Materials 2021, 14, 473. [Google Scholar] [CrossRef]
- Neelamegam, P.; Muthusubramanian, B. Influence of Polyethylenimine (PEI) in enhancement of microstructure and surface morphology of recycled construction and demolition waste aggregate in concrete by carbonation. Constr. Build. Mater. 2023, 405, 133342. [Google Scholar] [CrossRef]
- Junak, J.; Sicakova, A. Effect of surface modifications of recycled concrete aggregate on concrete properties. Buildings 2017, 8, 2. [Google Scholar] [CrossRef]
- GB/T 25176-2010; Recycled Fine Aggregate for Concrete and Mortar. Standard Press of China: Beijing, China, 2011.
- GB/T 14685-2011; Pebble and Crushed Stone for Construction. China National Standardization Management Committee: Beijing, China, 2005.
- JGJ/T 70-2009; Standard for Test Method of Basic Properties of Construction Mortar. Standard Press of China: Beijing, China, 2009.
- ASTM C1585; Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. ASTM International: West Conshohocken, PA, USA, 2004.
- Da Rocha Gomes, S.; Ferrara, L.; Sánchez, L.; Moreno, M.S. A comprehensive review of cementitious grouts: Composition, properties, requirements and advanced performance. Constr. Build. Mater. 2023, 375, 130991. [Google Scholar] [CrossRef]
- Pontes, J.; Bogas, J.A.; Real, S.; Silva, A. The rapid chloride migration test in assessing the chloride penetration resistance of normal and lightweight concrete. Appl. Sci. 2021, 11, 7251. [Google Scholar] [CrossRef]
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Architecture & Building Press: Beijing, China, 2009.
- Ouyang, K.; Shi, C.; Chu, H.; Guo, H.; Song, B.; Ding, Y.; Guan, X.; Zhu, J.; Zhang, H.; Wang, Y. An overview on the efficiency of different pretreatment techniques for recycled concrete aggregate. J. Clean. Prod. 2020, 263, 121264. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; Lu, J.-x.; Shen, P.; Ling, T.-C.; Poon, C.S. Enhancing the microstructure and surface texture of recycled concrete fine aggregate via magnesium-modified carbonation. Cem. Concr. Res. 2022, 162, 106967. [Google Scholar] [CrossRef]
- Meng, T.; Lai, Z.; Yang, X.; Dai, D.; Jia, Y.; Yu, H. An approach to effectively improve the properties of recycled concrete aggregate and recycled brick aggregate by micro-nano particle reconstruction. Constr. Build. Mater. 2024, 421, 135669. [Google Scholar] [CrossRef]
- Wu, K.; Hu, Y.; Zhang, L.; Su, Y.; Han, J.; Yang, Z.; De Schutter, G. Quantitative evaluation of interfacial transition zone of sustainable concrete with recycled and steel slag as aggregate. Struct. Concr. 2021, 22, 926–938. [Google Scholar] [CrossRef]
- Gholizadeh-Vayghan, A.; Bellinkx, A.; Snellings, R.; Vandoren, B.; Quaghebeur, M. The effects of carbonation conditions on the physical and microstructural properties of recycled concrete coarse aggregates. Constr. Build. Mater. 2020, 257, 119486. [Google Scholar] [CrossRef]
- Pandurangan, K.; Dayanithy, A.; Prakash, S.O. Influence of treatment methods on the bond strength of recycled aggregate concrete. Constr. Build. Mater. 2016, 120, 212–221. [Google Scholar] [CrossRef]
- Verian, K.P.; Ashraf, W.; Cao, Y. Properties of recycled concrete aggregate and their influence in new concrete production. Resour. Conserv. Recycl. 2018, 133, 30–49. [Google Scholar] [CrossRef]
- Zhao, Z.; Remond, S.; Damidot, D.; Courard, L.; Michel, F. Improving the properties of recycled concrete aggregates by accelerated carbonation. Proc. Inst. Civ. Eng. Constr. Mater. 2018, 171, 126–132. [Google Scholar] [CrossRef]
- Eguchi, K.; Teranishi, K.; Nakagome, A.; Kishimoto, H.; Shinozaki, K.; Narikawa, M. Application of recycled coarse aggregate by mixture to concrete construction. Constr. Build. Mater. 2007, 21, 1542–1551. [Google Scholar] [CrossRef]
- Gonzalez-Fonteboa, B.; Martinez-Abella, F.; Eiras-Lopez, J.; Seara-Paz, S. Effect of recycled coarse aggregate on damage of recycled concrete. Mater. Struct. 2011, 44, 1759–1771. [Google Scholar] [CrossRef]
- Etxeberria, M.; Vázquez, E.; Marí, A.; Barra, M. Influence of amount of recycled coarse aggregates and production process on properties of recycled aggregate concrete. Cem. Concr. Res. 2007, 37, 735–742. [Google Scholar] [CrossRef]
- Ji, Y.; Pei, Z. Investigation of Mechanical Properties of Ultra-High-Performance Polyethylene-Fiber-Reinforced Recycled-Brick-Aggregate Concrete. Polymers 2023, 15, 4573. [Google Scholar] [CrossRef]



| Specimen | Initial Water Content (%) | Treatment | Mass Change (%) after Treatment |
|---|---|---|---|
| PFA-10 PEI | 1.7 | 10% PEI solution | 1.77 |
| PFA-20 PEI | 20% PEI solution | 2.01 | |
| PFA-30 PEI | 30% PEI solution | 2.31 | |
| PFA-40 PEI | 40% PEI solution | 2.61 | |
| PFA-50 PEI | 50% PEI solution | 2.79 |
| Notation | BET Specific Surface Area (m2/g) | BJH Pore Volume (mL/g) |
|---|---|---|
| PFA-10 PEI | 0.8550 | 0.001893 |
| PFA-20 PEI | 1.8830 | 0.008557 |
| PFA-30 PEI | 0.5753 | 0.001471 |
| PFA-40 PEI | 0.7269 | 0.000875 |
| PFA-50 PEI | 0.3276 | 0.000763 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Chiang, K.; Wang, X.; Qu, X.; Zhu, Y.; Luo, H. Enhancing the Surface Structure of Public Filler and Macroscopic Properties of Recycled Cement Mortar Using Polyethyleneimine. Buildings 2024, 14, 2856. https://doi.org/10.3390/buildings14092856
Cheng C, Chiang K, Wang X, Qu X, Zhu Y, Luo H. Enhancing the Surface Structure of Public Filler and Macroscopic Properties of Recycled Cement Mortar Using Polyethyleneimine. Buildings. 2024; 14(9):2856. https://doi.org/10.3390/buildings14092856
Chicago/Turabian StyleCheng, Chen, Kingsley Chiang, Xinxin Wang, Xiaoyang Qu, Yazhi Zhu, and Hui Luo. 2024. "Enhancing the Surface Structure of Public Filler and Macroscopic Properties of Recycled Cement Mortar Using Polyethyleneimine" Buildings 14, no. 9: 2856. https://doi.org/10.3390/buildings14092856





