Research Review of Reaction Mechanism and Mechanical Properties of Chemically Solidified Silt
Abstract
1. Introduction
2. Reaction Mechanisms of Silty Soil Stabilization Using Different Types of Curing Agents
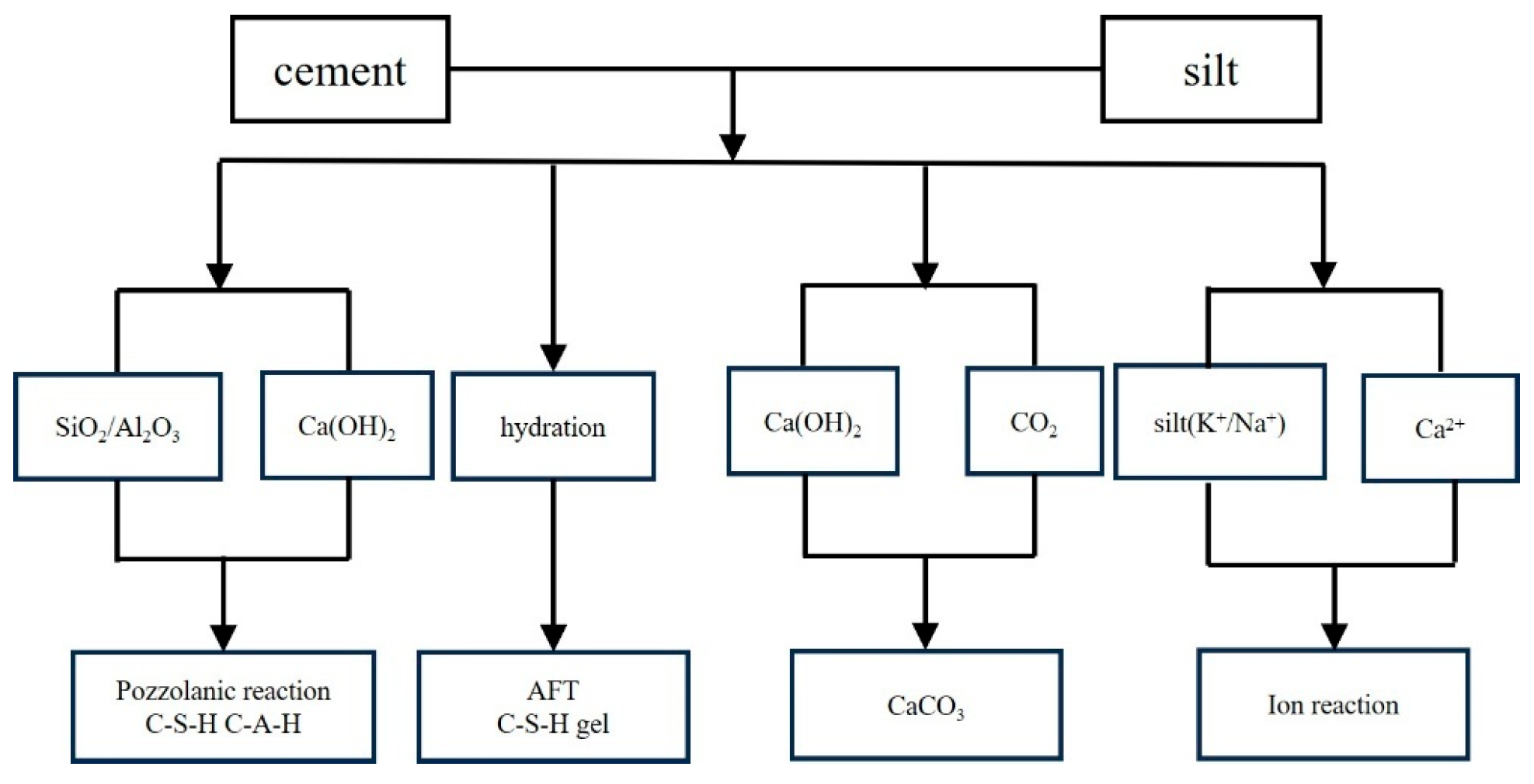
2.1. Reaction Mechanism of Cement-Stabilized Silty Soil
2.2. Mechanism of Silty Soil Stabilization Using Industrial Waste-Based Curing Agents
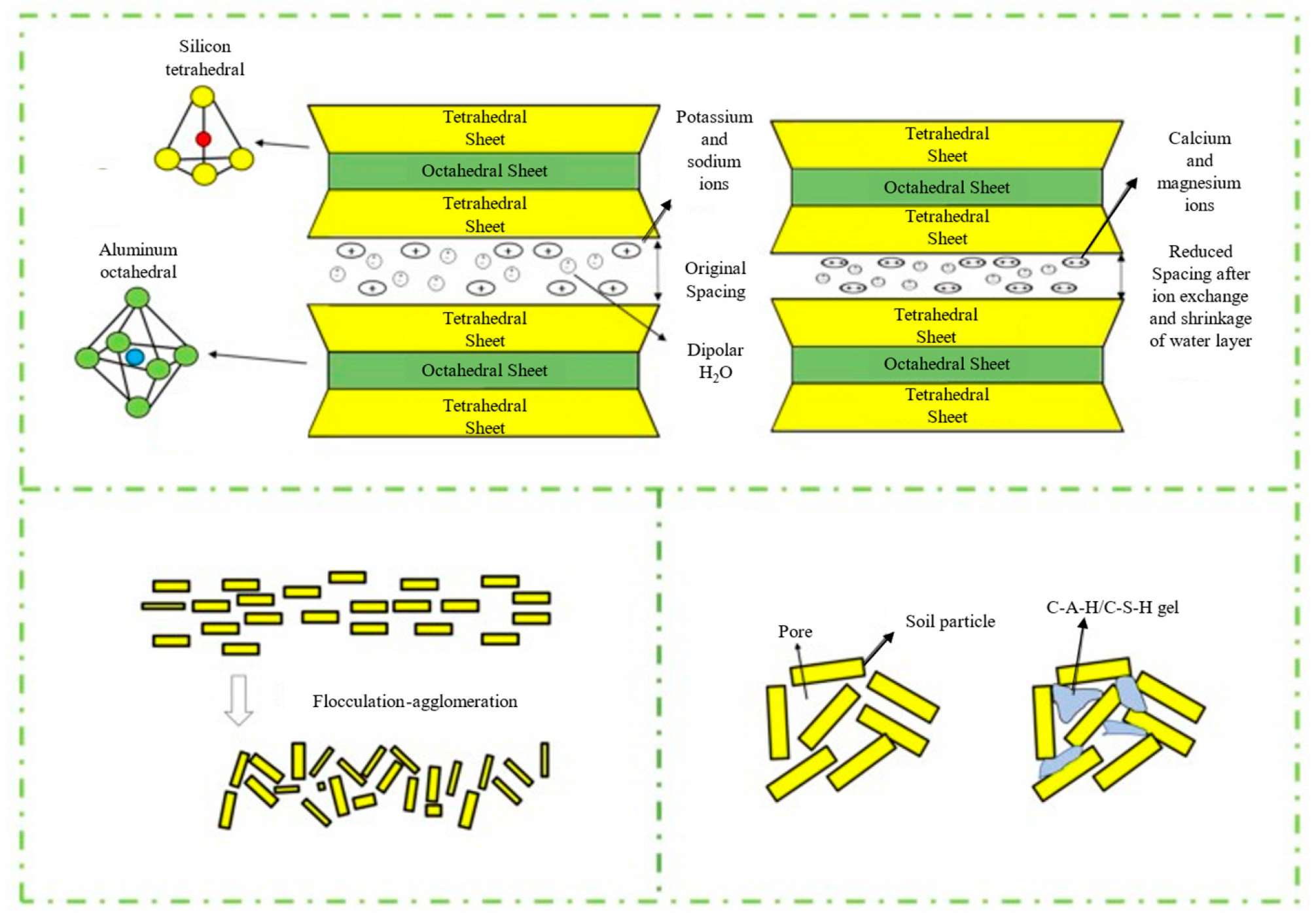
2.3. Mechanism of Polymer-Based Stabilization of Silty Soils
- Thinning of the double layerWhen polymer materials are mixed into the soil, they adsorb onto the surfaces of soil particles. This adsorption shields the binding of water molecules, reducing their adsorption on particle surfaces. Concurrently, the polymer increases the ion concentration and ionic valence in the pore water, thereby decreasing the degree of ion hydration. These combined effects reduce the thickness of the double electric layer. A thinner double layer weakens the soil’s hygroscopicity. Since water absorption typically induces significant soil expansion, reduced hygroscopicity minimizes volumetric swelling, leading to improved soil strength.
- Enhancing interparticle bondingThe adsorption of abundant polymer materials onto soil particle surfaces lowers the surface free energy of the particles while amplifying intermolecular attractive forces. This promotes stronger aggregation of soil particles, resulting in enhanced soil strength and structural integrity.
- Optimization of pore distributionThe incorporation of polymer curing agents reduces the proportion of large-diameter pores within the soil matrix. This modification enhances the structural stability of the soil under load and promotes a more uniform pore size distribution. These changes collectively improve the soil’s bearing capacity and resistance to deformation.
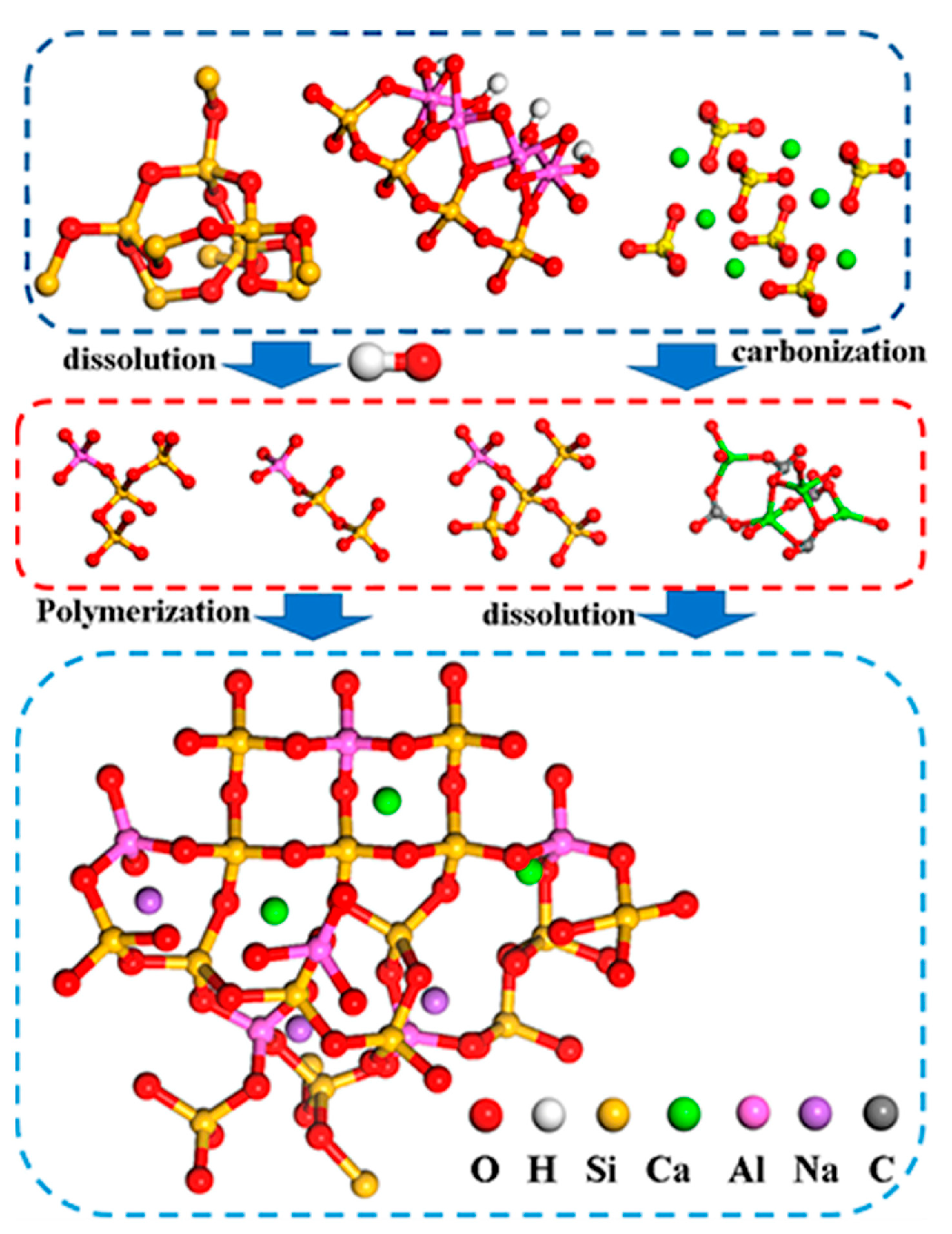
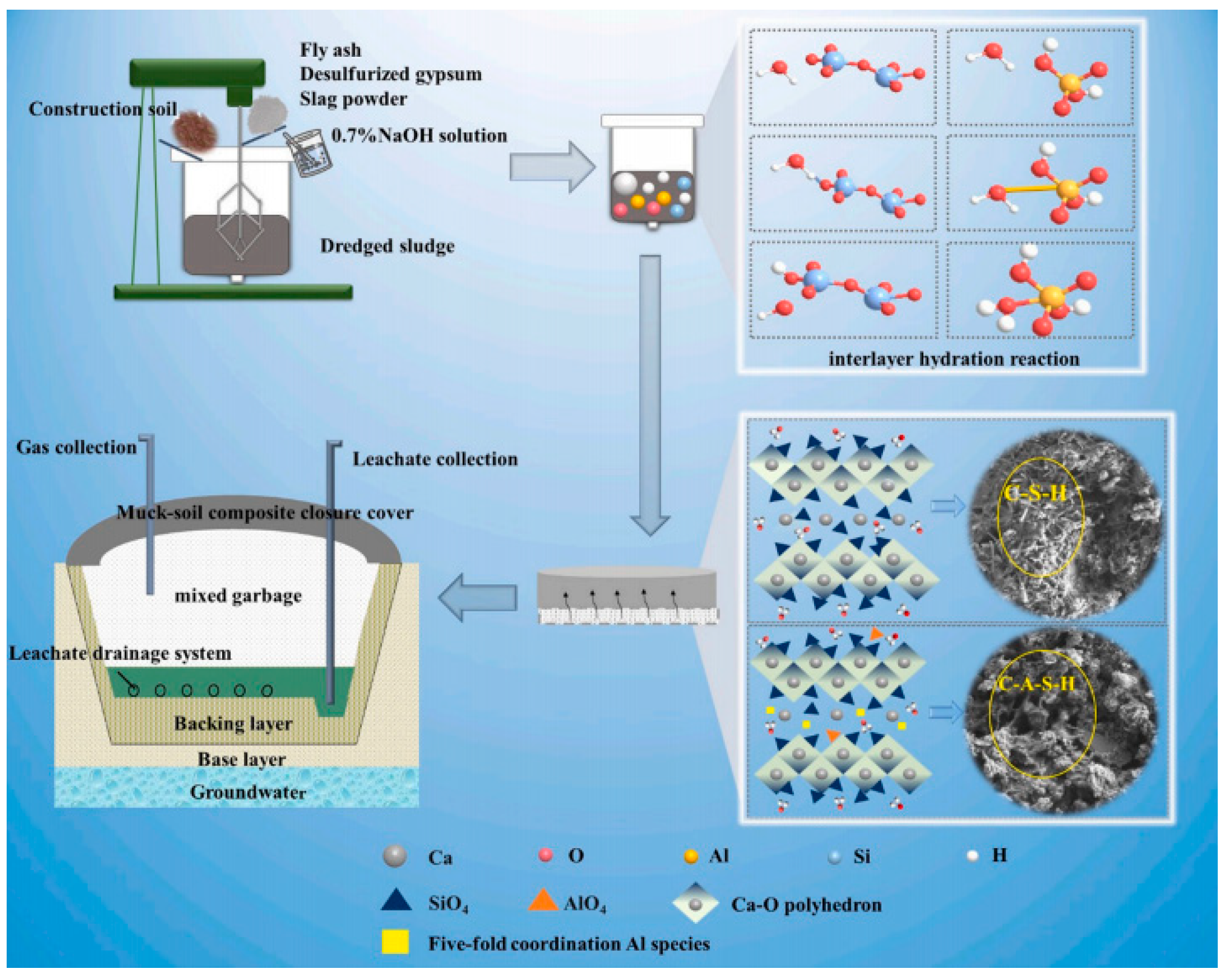
2.4. Mechanism of Silty Soil Stabilization Using Geopolymer-Based Curing Agents
3. Mechanical Properties of Silty Soils Stabilized with Different Types of Curing Agents
3.1. Unconfined Compression Strength
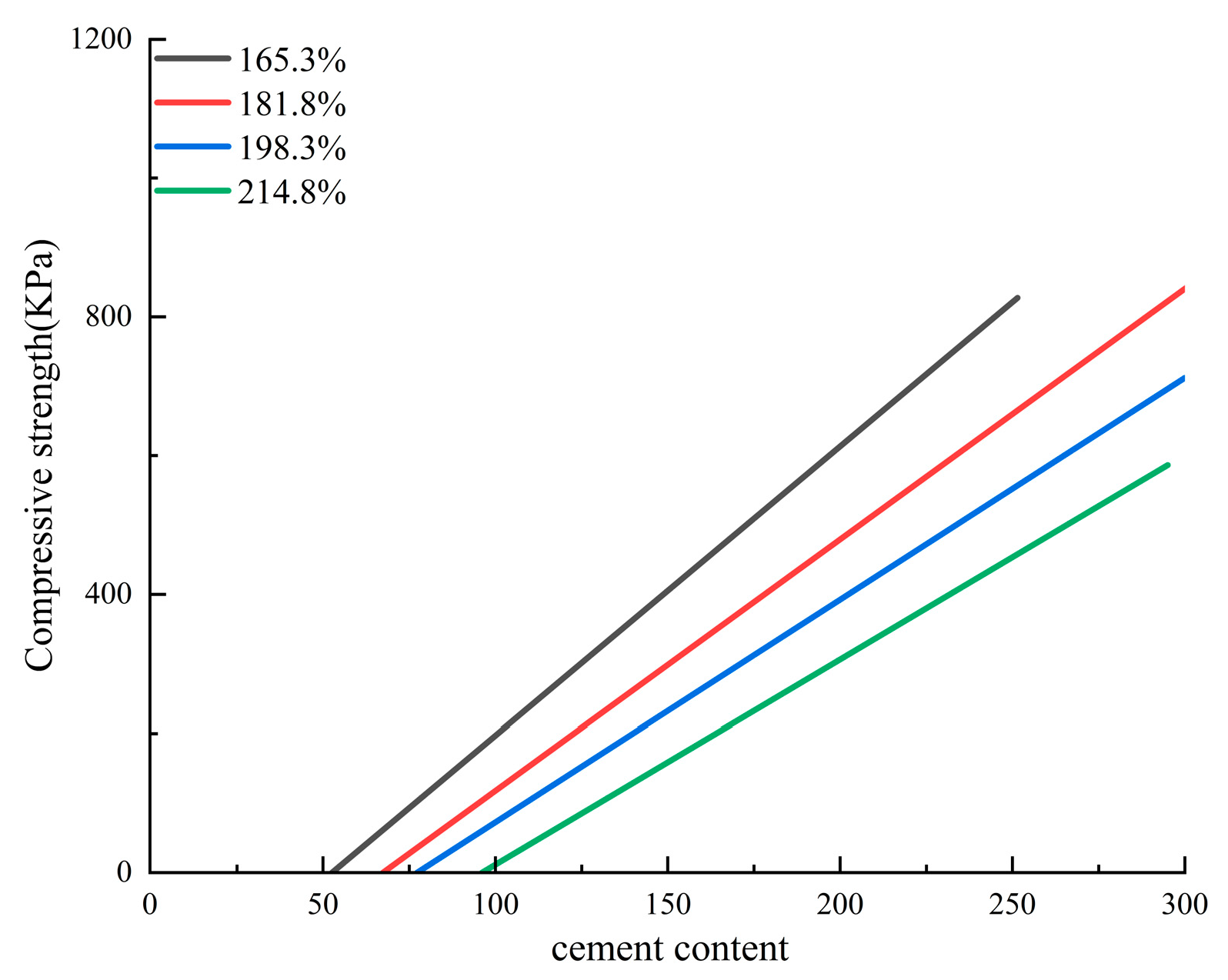
3.1.1. Traditional Inorganic-Based Silty Soil Curing Agent
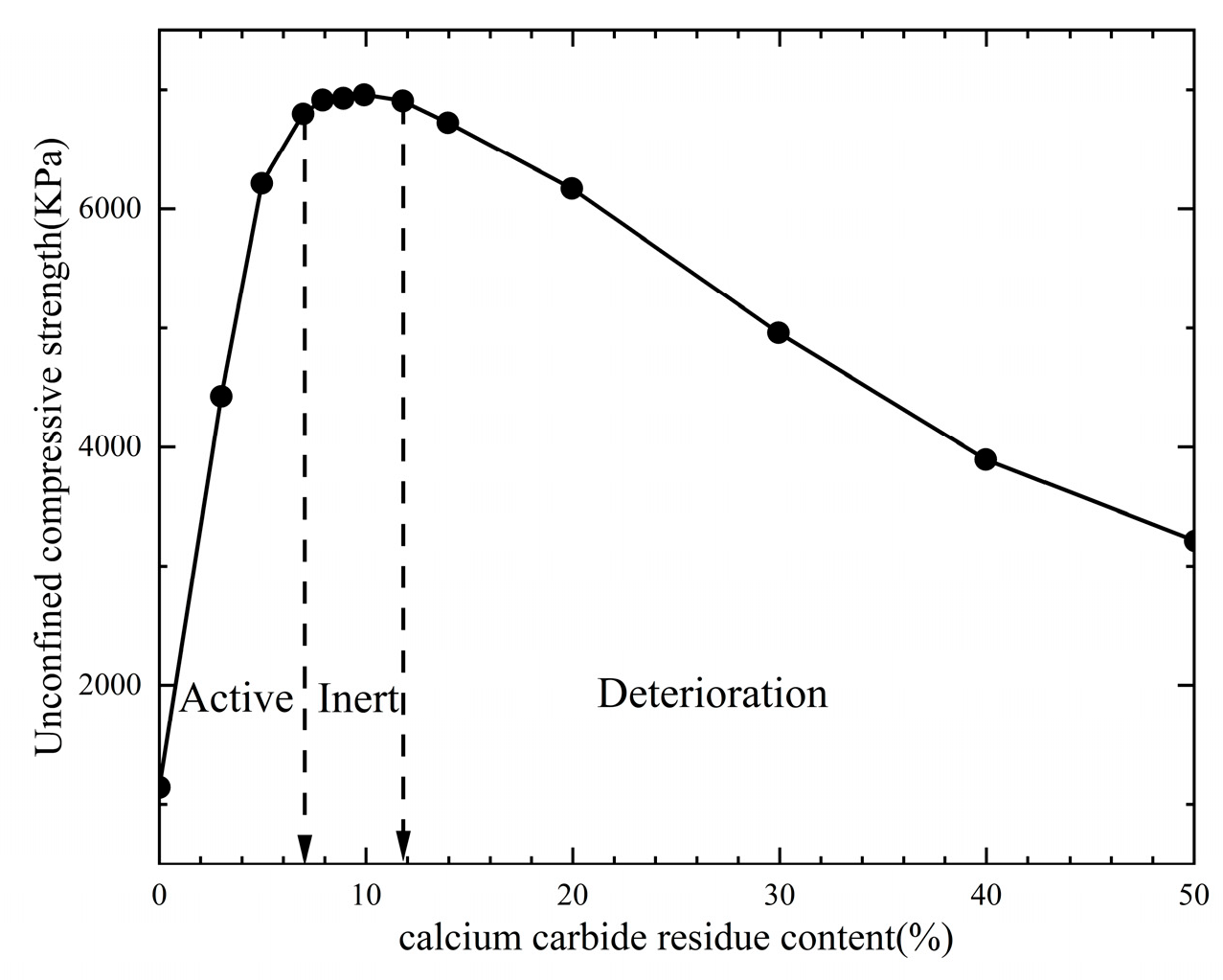
3.1.2. Industrial Waste-Based Silty Soil Curing Agent
3.1.3. Polymer-Based Silty Soil Curing Agent
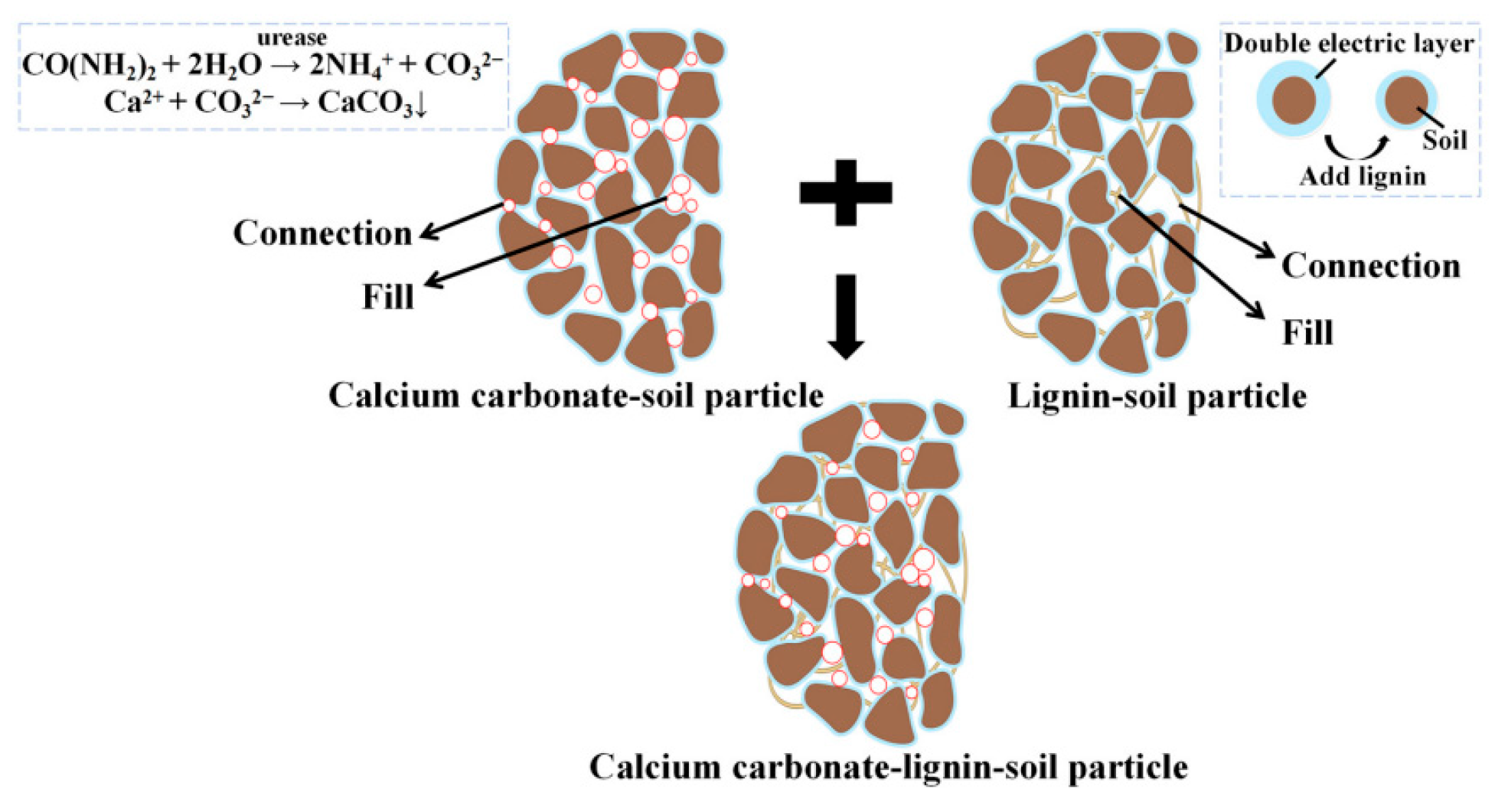
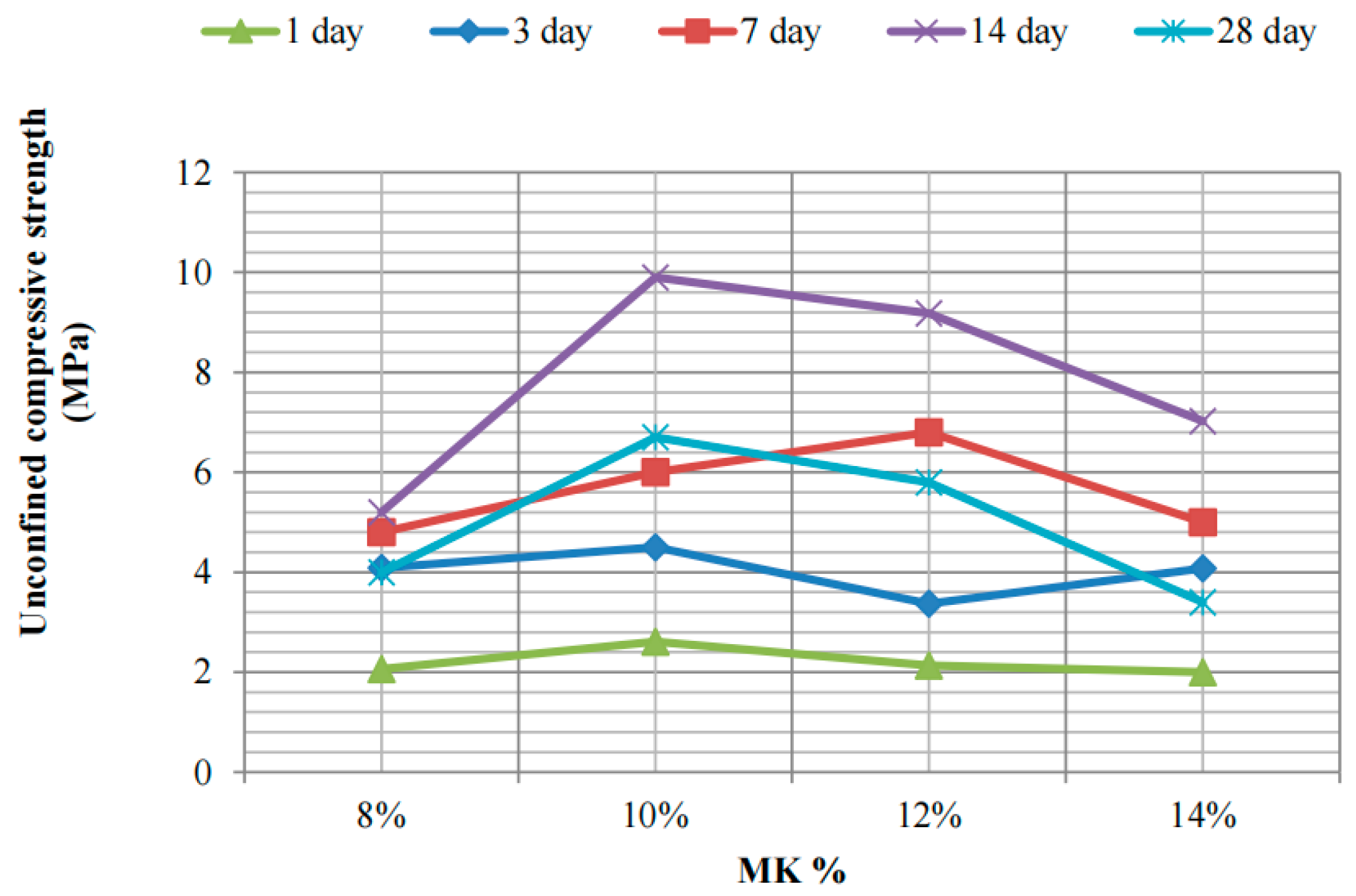
3.1.4. Geopolymer-Based Silty Soil Curing Agent


3.2. Shear Strength
4. Discussion
4.1. Analysis of Various Curing Agents
4.2. Challenges of Geopolymer Stabilization
4.3. Future Research Priorities
4.3.1. Fundamental Mechanisms of Geopolymer Stabilization
4.3.2. Field Performance Challenges
| Raw Materials or Precursor | Alkali Activators | Alkali Binder Ratio | Curing Age | UCS(MPa) | References |
|---|---|---|---|---|---|
| OPC | - | - | 28 | 1.27 | [35] |
| MK | Na2SiO3 + NaOH | - | 28 | 31.22 | [104] |
| OPC | Na2SiO3 + NaOH + CaCl2 | 0.6 | 7 | 0.124 | [105] |
| FA | Na2SiO3 + NaOH | 0.8 | 28 | 4.08 | [106] |
| FA and GGBS | Na2SiO3 + NaOH | 0.35 | 28 | 1.53 | [107] |
| FA and GBFS | NaOH | - | 7 | 3.32 | [108] |
| POFA | KOH | - | 7 | 0.59 | [109] |
| FA | OPC | 0.5 | 120 | 6.84 | [110] |
| CMK | Na2SiO3 + NaOH | 1.5 | 90 | 0.317 | [111] |
| MK | Na2SiO3 + NaOH | 5.13 | 1 | 2 | [78] |
| FA | Na2SiO3 + NaOH | 1 | 14 | 0.649 | [112] |
| SF | - | - | 7 | 0.054633 | [113] |
| FA and Cement | SR | 2.14 | 7 | 0.41 | [114] |
| FA(F) and GGBFS | Na2SiO3 + NaOH | 0.5 | 28 | 0.574 | [115] |
| Palm oil fuel ash | Na2SiO3 + NaOH | 0.76 | 28 | 4.18 | [116] |
| FA and Slag | Na2SiO3 + NaOH | 1 | 28 | 1.034 | [81] |
| FA and GGBS | Na2SiO3 + NaOH | 0.67 | 7 | 2.27 | [117] |
| MK | Na2SiO3 + NaOH | 1.0 | 14 | 4.928 | [118] |
| MK | Na2SiO3 + NaOH | 3.1 | 28 | 5.6 | [119] |
| FA and GGBS | Na2SiO3 + NaOH | 0.32 | 28 | 0.86 | [120] |
| FA and GGBS | Na2SiO3 + NaOH | - | 28 | 8.5 | [74] |
| FA and Slag | Na2SiO3 + NaOH | 0.4 | 28 | 0.4 | [121] |
| GGBS | Na2SiO3 + NaOH | 17.4 | 28 | - | [122] |
| MK | NaHCO3 + CaO | 0.5 | 7 | 0.15 | [85] |
| MK | NaOH | 0.7 | 28 | 2.09 | [123] |
| POFA | Na2SiO3 + NaOH | 1.0 | 7 | 0.294 | [124] |
| GP | CaO + MgO | - | 7 | 1.2 | [125] |
| GGBS | CS | 0.25 | 28 | 0.22 | [126] |
| WGP | OPC | 1 | 28 | 0.18 | [127] |
| POFA | KOH | - | 28 | 1.48 | [128] |
| FA(F) and PP | KOH | 0.91 | 28 | 0.645 | [129] |
5. Conclusions and Future Perspectives
- Sustainable Development Trends
- 2.
- Industrial Waste Valorization
- 3.
- Geopolymer System Optimization
- 4.
- Curing Agents’ Diversity Gap
- 5.
- Region-Specific Optimization
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
- Cement Hydrolysis/Hydration Reactions
- 2.
- Interfacial Reactions Between Soil Particles and Cement Hydrates
- (1) Ion exchange and flocculation:
- (2) Pozzolanic reaction:
- 3.
- Carbonation
References
- Ji, F.L.; Zhu, W.; Zhang, C.L. Study of treatment technology of dredging sludge with geosynthetizing method. Rock Soil Mech. 2004, 25, 1999–2002. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, J.; Liu, L. Advancements in the evolution of engineering characteristics and reinforcement technologies for subgrade silt. Materials 2023, 16, 6965. [Google Scholar] [CrossRef]
- Fratini, C.; Anselmi, S.; Renzi, M. Dredge sediment as an opportunity: A comprehensive and updated review of beneficial uses in marine, river, and lagoon eco-systems. Environments 2025, 12, 200. [Google Scholar] [CrossRef]
- Carreira, C.; Bollwerk, S.M.; Lønborg, C. A review on beneficial use of dredged marine sediment. Anthr. Coasts 2025, 8, 12. [Google Scholar] [CrossRef]
- Chan, C.-M. Geo-parametric study of dredged marine clay with solidification for potential reuse as good engineering soil. Environ. Earth Sci. 2016, 75, 941. [Google Scholar] [CrossRef]
- Hao, N.; Song, Y.; Wang, Z.; He, C.; Ruan, S. Utilization of silt, sludge, and industrial waste residues in building materials: A review. J. Appl. Biomater. Funct. Mater. 2022, 20, 22808000221114709. [Google Scholar] [CrossRef]
- Long, K.q.; Fang, X.w.; Shen, C.n.; Zhang, X.c.; Wang, M.m. Strength characteristics of sludge solidified by composite rapid soil stabilizer. Rock Soil Mech. 2023, 44, 309–317. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, Z.; Ma, F.; Luo, X. Analysis of strength development and soil–water characteristics of rice husk ash–lime stabilized soft soil. Materials 2019, 12, 3873. [Google Scholar] [CrossRef]
- Ye, G.b.; Chen, W.c.; Yang, X. Lab study on early strength of cement- stabilized soil. Geotech. Eng. Tech. 2003, 17, 346–348. [Google Scholar]
- Wang, H.S.; Tang, C.S.; Gu, K.; Shi, B.; Inyang, H.I. Mechanical behavior of fiber-reinforced, chemically stabilized dredged sludge. Bull. Eng. Geol. Environ. 2019, 79, 629–643. [Google Scholar] [CrossRef]
- Croft, J.B. The Influence of soil mineralogical composition on cement stabilization. Géotechnique 1967, 17, 119–135. [Google Scholar] [CrossRef]
- Kamon, M.; Nontananandh, S. Combining industrial wastes with lime for soil stabilization. J. Geotech. Eng. 1991, 117, 1–17. [Google Scholar] [CrossRef]
- Winterkorn, H.F.; Pamukcu, S. Soil stabilization and grouting. Found. Eng. Handb. 1991, 9, 317–378. [Google Scholar]
- Cai, L. Experimental Study on Mechanical Properties of Silty Soft Soil Modified by Industrial Solid Waste and Residual Soil. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2021. [Google Scholar]
- Luo, Z.; Zhang, B.; Su, Y.; Jiang, B. Research status and prospect of geopolymer solidified soil. J. Civ. Environ. Eng. 2022, 46, 31–43. [Google Scholar] [CrossRef]
- Yao, J.; Qiu, H.; He, H.; Chen, X.; Hao, G. Application of a soft soil stabilized by composite geopolymer. J. Perform. Constr. Facil 2021, 35, 04021018. [Google Scholar] [CrossRef]
- Liu, J.J.; Luo, H.P.; Lei, H.Y.; Zhen, G. Study on the compressive strength and curing mechanism of alkali-activated geopolymer curing marine silty soft soil. J. Railw. Sci. Eng. 2023, 21, 2745–2754. [Google Scholar] [CrossRef]
- Farid, S.; Balasingam, M. Effect of cement treatment on geotechnical properties of some Washington state soils. Eng. Geol. 2009, 104, 119–125. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Q. The behaviour of organic matter in the process of soft soil stabilization using cement. Bull. Eng. Geol. Environ. 2005, 65, 445–448. [Google Scholar] [CrossRef]
- Tang, Y.X.; Miyazaki, Y.; Tsuchida, T. Practices of reused dredgings by cement treatment. Soils Found. 2001, 41, 129–143. [Google Scholar] [CrossRef]
- Boutouil, M.; Levacher, D. Effect of high initial water content on cement based sludge solidification. Ground Improv. 2005, 9, 169–174. [Google Scholar] [CrossRef]
- Tang, Y.X.; Liu, H.L.; Zhu, W. Study on engineering properties of cement-stabilized soil. Chin. J. Geotech. Eng. 2000, 22, 549–554. [Google Scholar]
- Horpibulsuk, S.; Miura, N.; Nagaraj, T.S. Assessment of strength development in cement-admixed high water content clays with Abrams’ law as a basis. Géotechnique 2003, 53, 439–444. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, C.L.; Gao, Y.F.; Fan, Z.P. Fundamental mechanical properties of solidified dredged marine sediment. J. Zhejiang Univ.-Sci. A 2005, 39, 1561–1565. [Google Scholar]
- Liu, S.Y.; Zhang, D.W.; Liu, Z.B.; Deng, Y.F. Assessment of unconfined compressive strength of cement stabilized marine clay. Mar. Georesour. Geotechnol. 2008, 26, 19–35. [Google Scholar] [CrossRef]
- Ding, J.W.; Liu, T.P.; Cao, Y.P.; Yang, R.M. Unconfined compression tests and strength prediction method for solidified soils of dredged clays with high water content. Chin. J. Geotech. Eng. 2013, 35, 55–60. [Google Scholar]
- Zhu, F.; Li, Z.; Dong, W.; Ou, Y. Geotechnical properties and microstructure of lime-stabilized silt clay. Bull. Eng. Geol. Environ. 2018, 78, 2345–2354. [Google Scholar] [CrossRef]
- Consoli, N.C.; da Silva Lopes, L., Jr.; Heineck, K.S. Key parameters for the strength control of lime stabilized soil. J. Mater. Civ. Eng. 2009, 21, 210–216. [Google Scholar] [CrossRef]
- Lin, Z.S.; Huang, Y.; Liu, S.N.; Han, B.Q. Study on the high-strength lime stabilizer. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2000, 22, 4–5+24. [Google Scholar]
- Wang, D.X.; Abriak, N.E.; Zentar, R.; Xu, W. Solidification/stabilization of dredged marine sediments for road construction. Environ. Technol. 2012, 33, 95–101. [Google Scholar] [CrossRef]
- Jauberthie, R.; Rendell, F.; Rangeard, D.; Molez, L. Stabilisation of estuarine silt with lime and/or cement. Appl. Clay Sci. 2010, 50, 395–400. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Rachan, R.; Chinkulkijniwat, A.; Raksachon, Y.; Suddeepong, A. Analysis of strength development in cement-stabilized silty clay from microstructural considerations. Constr. Build. Mater. 2010, 24, 2011–2021. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Env. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Yu, J.R.; Chen, Y.H.; Chen, G.; Tang, T.H. Mechanical behaviour of geopolymer stabilized clay and its mechanism. J. Build. Mater. 2020, 23, 364–371. [Google Scholar]
- Sun, J.; Zhang, C.L.; Zhang, Z.T.; Wang, X.T. Present situation of comprehensive utilization technology of industrial solid waste. Mater. Rev. 2012, 26, 105–109. [Google Scholar]
- Xun, Y. Test on strengthening soft soil with cementatory solidifying agent containing industrial waste. Chin. J. Geotech. Eng. 2000, 22, 210–213. [Google Scholar]
- Huang, X.; Hu, T.G. On stabilization of soft soil with waste gypsum and cement. Chin. J. Geotech. Eng. 1998, 20, 72–76. [Google Scholar]
- Meng, Q.S.; Yang, C.; Lei, X.W.; Sun, S.L. Experimental study of early solidification of sludge in East Lake, Wuhan. Rock Soil Mech. 2010, 31, 701–712. [Google Scholar] [CrossRef]
- Yu, Z.H.; Gui, Y.; Zhang, Q.; Kong, X.Y. Experimental study on the stabilization effects of dredged sludge by fly ash or phosphogypsum. Adv. Mater. Res. 2013, 689, 342–347. [Google Scholar] [CrossRef]
- Chu, C.F.; Li, X.C.; Deng, Y.F.; Tang, J.W. Influence of metakaolin on mechanical properties of cement-modified marine soft soil. Chin. J. Geotech. Eng. 2013, 35, 170–174. [Google Scholar]
- Zhang, T.; Yue, X.; Deng, Y.; Zhang, D.; Liu, S. Mechanical behaviour and micro-structure of cement-stabilised marine clay with a metakaolin agent. Constr. Build. Mater. 2014, 73, 51–57. [Google Scholar] [CrossRef]
- Cai, G.; Zhang, T.; Liu, S.; Li, J.; Jie, D. Stabilization mechanism and effect evaluation of stabilized silt with lignin based on laboratory data. Mar. Georesour. Geotechnol. 2014, 34, 331–340. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, S.; Cai, G.; Puppala, A.J. Experimental investigation of thermal and mechanical properties of lignin treated silt. Eng. Geol. 2015, 196, 1–11. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S.; Puppala, A.J. Engineering properties and microstructural characteristics of foundation silt stabilized by lignin-based industrial by-product. KSCE J. Civ. Eng. 2016, 20, 2725–2736. [Google Scholar] [CrossRef]
- Gao, Q.; Ge, J.; Zhang, J.; Ren, Z.; Wu, D.; Cheng, G.; Zhang, K. Experimental study on the engineering characteristics of modified silt in the Yellow River alluvial plain. Constr. Build. Mater. 2023, 398, 132491. [Google Scholar] [CrossRef]
- Zou, B.; Shen, R.; Qi, L.L. Research on stabilizing the railway roadbed with improved urea-formaldehyde-resin. J. Southw. Jiaotong Univ. 2001, 36, 33–36. [Google Scholar]
- Li, F.; Wang, C.; Xia, Y.; Hao, Y.; Zhao, P.; Shi, M. Strength and solidification mechanism of silt solidified bypolyurethane. Adv. Civ. Eng. 2020, 2020, 8824524. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S. Application of lignin-stabilized silty soil in highway subgrade: A macroscale laboratory study. J. Mater. Civ. Eng. 2018, 30, 04018034. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Sekkal, W.; Zaoui, A. Thermal and acoustic insulation properties in nanoporous geopolymer nanocomposite. Cem. Concr. Compos. 2023, 138, 104955. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Damtie Yehualaw, M.; Vo, D.-H.; Huynh, T.-P. Development of high-strength alkali-activated pastes containing high volumes of waste brick and ceramic powders. Constr. Build. Mater. 2019, 218, 519–529. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V.; Petrović, R. Physical–mechanical and microstructural properties of alkali-activated fly ash–blast furnace slag blends. Ceram. Int. 2015, 41, 1421–1435. [Google Scholar] [CrossRef]
- Barbhuiya, S.A.; Gbagbo, J.K.; Russell, M.I.; Basheer, P.A.M. Properties of fly ash concrete modified with hydrated lime and silica fume. Constr. Build. Mater. 2009, 23, 3233–3239. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Guo, Q.; Wei, M.; Wu, H.; Gu, Y. Strength and micro-mechanism of MK-blended alkaline cement treated high plasticity clay. Constr. Build. Mater. 2020, 236, 117567. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Fernandes, L.; Pinto, A.T. Effect of calcium content on soil stabilisation with alkaline activation. Constr. Build. Mater. 2012, 29, 167–174. [Google Scholar] [CrossRef]
- Bhavita Chowdary, V.; Ramanamurty, V.; Pillai, R.J. Experimental evaluation of strength and durability characteristics of geopolymer stabilised soft soil for deep mixing applications. Innov. Infrastruct. Solut. 2020, 6, 40. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S. Alkali-activated ground-granulated blast furnace slag for stabilization of mrine soft clay. J. Mater. Civ. Eng. 2015, 27, 04014146. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Chen, G.; Wang, L. Experimental study of the feasibility of using anhydrous sodium metasilicate as a geopolymer activator for soil stabilization. Eng. Geol. 2020, 264, 105316. [Google Scholar] [CrossRef]
- Wang, D.; Wang, R.; Benzerzour, M.; Wang, H.; Abriak, N.-E. Comparison between reactive MgO- and Na2SO4-activated low-calcium fly ash-solidified soils dredged from East Lake, China. Mar. Georesour. Geotechnol. 2019, 38, 1046–1055. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, L.; Lu, C.; Liu, Y.; Yu, Q.; Chen, S. Investigation on the effect of calcium on the properties of geopolymer prepared from uncalcined coal gangue. Polymers 2023, 15, 1241. [Google Scholar] [CrossRef]
- Xu, G.; Shi, X. Characteristics and applications of fly ash as a sustainable construction material: A state-of-the-art review. Resour. Conserv. Recycl. 2018, 136, 95–109. [Google Scholar] [CrossRef]
- Li, Z.; Fei, M.-E.; Huyan, C.; Shi, X. Nano-engineered, fly ash-based geopolymer composites: An overview. Resour. Conserv. Recycl. 2021, 168, 105334. [Google Scholar] [CrossRef]
- Li, Z.; Xu, G.; Shi, X. Reactivity of coal fly ash used in cementitious binder systems: A state-of-the-art overview. Fuel 2021, 301, 121031. [Google Scholar] [CrossRef]
- Zhu, Z.; Pu, S.; Zhang, J.; Wan, Y.; Song, W.; Wang, H. Water resistance and compressibility of silt solidified with lime and fly-ash mixtures. Environ. Earth Sci. 2021, 80, 103. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Teixeira Pinto, A. Deep soft soil improvement by alkaline activation. Proc. Inst. Civ. Eng. 2011, 164, 73–82. [Google Scholar] [CrossRef]
- Hwang, K.; Noguchi, T.; Tomosawa, F. Prediction model of compressive strength development of fly-ash concrete. Cem. Concr. Res. 2004, 34, 2269–2276. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, F.; Zhu, R.; Song, Z.; Hua, S.; Ma, Y. Strength performance of mucky silty clay modified using early-age fly ash-based curing agent. Case Stud. Constr. Mater. 2022, 17, e01595. [Google Scholar] [CrossRef]
- Mozumder, R.A.; Laskar, A.I. Prediction of unconfined compressive strength of geopolymer stabilized clayey soil using artificial neural network. Comput. Geotech. 2015, 69, 291–300. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Arulrajah, A.; Disfani, M.M.; Horpibulsuk, S.; Bo, M.W.; Darmawan, S. Effects of industrial by-product based geopolymers on the strength development of a soft soil. Soils Found. 2018, 58, 716–728. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Arulrajah, A.; Disfani, M.M.; Horpibulsuk, S.; Darmawan, S.; Wang, J. Impact of field conditions on the strength development of a geopolymer stabilized marine clay. Appl. Clay Sci. 2019, 167, 33–42. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.Y.; Yang, A.W.; Li, Y.B. Experimental study on the compressive strength of muddy clay solidified by the one-part slag-fly ash based geopolymer. Rock Soil Mech. 2021, 42, 647–655. [Google Scholar] [CrossRef]
- Palomoa, A.; Grutzeckb, M.W.; Blancoa, M.T. Alkali activated fly ashs a cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Othman, S.; Abbas, J.M. Stabilization soft clay soil using metakaolin based geopolymer. Diyala J. Eng. Sci. 2021, 14, 131–140. [Google Scholar] [CrossRef]
- Wang, D.X.; Wang, H.W.; Zou, W.L.; Wang, X.Q. Research on micro-mechanisms of dredged sludge solidified with alkali-activated fly ash. Chin. J. Rock Mech. Eng. 2019, 38, 3197–3205. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Phetchuay, C.; Chinkulkijniwat, A. Soil Stabilization by calcium carbide residue and fly ash. J. Mater. Civ. Eng. 2012, 24, 184–193. [Google Scholar] [CrossRef]
- Arulrajah, A.; Yaghoubi, M.; Disfani, M.M.; Horpibulsuk, S.; Bo, M.W.; Leong, M. Evaluation of fly ash- and slag-based geopolymers for the improvement of a soft marine clay by deep soil mixing. Soils Found. 2018, 58, 1358–1370. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Le, V.Q.; Do, M.Q.; Hoang, M.D.; Pham, V.T.H.Q.; Bui, T.H.; Nguyen, H.T. Effect of alkaline activators to engineering properties of geopolymer-based materials synthesized from red mud. Key Eng. Mater. 2018, 777, 508–512. [Google Scholar] [CrossRef]
- Chimoye, W. Strength of soft bangkok clay improved by geopolymer from palm fuel ash. Int. J. Eng. Technol. Res. 2014, 2, 1–10. [Google Scholar]
- Wang, S.; Xue, Q.; Zhu, Y.; Li, G.; Wu, Z.; Zhao, K. Experimental study on material ratio and strength performance of geopolymer-improved soil. Constr. Build. Mater. 2021, 267, 120469. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Liu, M.; Cai, L.; Wei, N.; Liu, Y. Microanalytical characterizations, mechanical strength and water resistance performance of solidified dredged sludge with industrial solid waste and architecture residue soil. Case Stud. Constr. Mater. 2022, 17, e01492. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, C.; Yang, K.; Ni, J.; Hai, J.; Gao, S. Effects of fly ash and slag content on the solidification of river-dredged sludge. Mar. Georesour. Geotechnol. 2019, 39, 65–73. [Google Scholar] [CrossRef]
- Wang, H.; Yao, J.; Lin, Y.; He, H. Research of geopolymer deal with the strength of soft soil and microstructure test. In Civil Infrastructures Confronting Severe Weathers and Climate Changes Conference, HangZhou, China, 23–25 July 2018; Springer: Cham, Switzerland, 2019; pp. 204–214. [Google Scholar] [CrossRef]
- Li, G.X.; Chen, L.; Zhen, J.Q.; Jie, Y.X. Experimental study on fiber- reinforced cohesive soil. J. Hydraul. Eng. 1995, 6, 31–36. [Google Scholar] [CrossRef]
- Tang, C.; Shi, B.; Gao, W.; Chen, F.; Cai, Y. Strength and mechanical behavior of short polypropylene fiber reinforced and cement stabilized clayey soil. Geotext. Geomembr. 2007, 25, 194–202. [Google Scholar] [CrossRef]
- Prabakar, J.; Sridhar, R.S. Effect of random inclusion of sisal fiber on strength behaviour of soil. Constr. Build. Mater. 2002, 16, 123–131. [Google Scholar] [CrossRef]
- Jiang, H.T.; Cai, Y.; Liu, J. Engineering properties of soils reinforced by short discrete polypropylene fiber. J. Mater. Civ. Eng. 2020, 22, 1313–1322. [Google Scholar] [CrossRef]
- An, N.; Yan, C.G.; Wang, Y.C.; Lan, H.X. Experimental study on anti-erosion performance of polypropylene fiber-reinforced loess. Rock Soil Mech. 2021, 42, 501–510. [Google Scholar] [CrossRef]
- Chen, R.M.; Jian, W.B.; Zhang, X.F.; Fang, Z.H. Experimental study on performance of sludge stabilized by CSFG-FR synergy. Rock Soil Mech. 2022, 43, 1020–1030. [Google Scholar] [CrossRef]
- Afrin, H. A review on different types soil stabilization techniques. Int. J. Transp. Eng. Technol. 2017, 3, 19–24. [Google Scholar] [CrossRef]
- Huang, J.; Kogbara, R.B.; Hariharan, N.; Masad, E.A.; Little, D.N. A state-of-the-art review of polymers used in soil stabilization. Constr. Build. Mater. 2021, 305, 124685. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Cho, G.-C. Global CO2 emission-related geotechnical engineering hazards and the mission for sustainable geotechnical engineering. Energies 2019, 12, 2567. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Vizureanu, P.; Tahir, M.F.M. Geopolymers and their Uses: Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012019. [Google Scholar] [CrossRef]
- Jeremiah, J.J.; Abbey, S.J.; Booth, C.A.; Kashyap, A. Geopolymers as alternative sustainable binders for stabilisation of clays—A review. Geotechnics 2021, 1, 439–459. [Google Scholar] [CrossRef]
- Santoni, R.L.; Tingle, J.S.; Webster, S.L. Stabilization of silty sand with nontraditional additives. Transp. Res. Rec. 2002, 1787, 61–70. [Google Scholar] [CrossRef]
- Onyejekwe, S.; Ghataora, G.S. Soil stabilization using proprietary liquid chemical stabilizers: Sulphonated oil and a polymer. Bull. Eng. Geol. Environ. 2014, 74, 651–665. [Google Scholar] [CrossRef]
- Petry, T.M.; Little, D.N. Review of stabilization of clays and expansive soils in pavements and lightly loaded structures—History, practice, and future. J. Mater. Civ. Eng. 2002, 14, 447–460. [Google Scholar] [CrossRef]
- Ayub, F.; Khan, S.A. An overview of geopolymer composites for stabilization of soft soils. Constr. Build. Mater. 2023, 404, 133195. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, H.; El-Korchi, T.; Zhang, G.; Tao, M. Experimental feasibility study of geopolymer as the next-generation soil stabilizer. Constr. Build. Mater. 2013, 47, 1468–1478. [Google Scholar] [CrossRef]
- Ma, C.; Chen, B.; Chen, L. Experimental feasibility research on a high-efficiency cement-based clay stabilizer. KSCE J. Civ. Eng. 2018, 22, 62–72. [Google Scholar] [CrossRef]
- Odeh, N.A.; Al-Rkaby, A.H.J. Strength, durability, and microstructures characterization of sustainable geopolymer improved clayey soil. Case Stud. Constr. Mater. 2022, 16, e00988. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Z.; Chen, K.; Xia, L. Experimental Investigation and mechanism of fly ash/slag-based geopolymer-stabilized soft soil. Appl. Sci. 2022, 12, 7438. [Google Scholar] [CrossRef]
- Hamed, E.; Demiröz, A. Optimization of geotechnical characteristics of clayey soils using fly ash and granulated blast furnace slag-based geopolymer. Constr. Build. Mater. 2024, 441, 137488. [Google Scholar] [CrossRef]
- Abdeldjouad, L.; Asadi, A.; Nahazanan, H.; Huat, B.B.K.; Dheyab, W.; Elkhebu, A.G. Effect of clay content on soil stabilization with alkaline activation. Int. J. Geosynth. Ground Eng. 2019, 5, 4–11. [Google Scholar] [CrossRef]
- Jamsawang, P.; Charoensil, S.; Namjan, T.; Jongpradist, P.; Likitlersuang, S. Mechanical and microstructural properties of dredged sediments treated with cement and fly ash for use as road materials. Road Mater. Pavement Des. 2020, 22, 2498–2522. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Wu, Y.; Zhang, X. Mechanical properties and micro-mechanisms of marine soft soil stabilized by different calcium content precursors based geopolymers. Constr. Build. Mater. 2021, 305, 124722. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Arulrajah, A.; Suksiripattanapong, C.; Udomchai, A. Strength development in soft marine clay stabilized by fly ash and calcium carbide residue based geopolymer. Appl. Clay Sci. 2016, 127, 134–142. [Google Scholar] [CrossRef]
- Zhu, J.F.; Zheng, Q.Q.; Tao, Y.L.; Ju, L.Y.; Yang, H.; Gong, X.N.; Pan, B.J.; Wang, Z.Q. The curing mechanism and empirical model for the marine organic soft clay stabilized with calcium carbide residue and silica fume under the optimal ratio. Acta Geotech. 2024, 20, 683–706. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, T.; Zong, Z.; Jiang, C.; Xie, Q.; Zhou, Z. Study on mechanical properties and mechanism of soda residue-fly ash stabilised marine clay based on orthogonal matrix method. Road Mater. Pavement Des. 2024, 26, 2262–2283. [Google Scholar] [CrossRef]
- Abdullah, H.H.; Shahin, M.A. Geomechanical behaviour of clay stabilised with fly-ash-based geopolymer for deep mixing. Geosciences 2022, 12, 207. [Google Scholar] [CrossRef]
- Khasib, I.A.; Daud, N.N.N.; Nasir, N.A.M. Strength development and microstructural behavior of soils stabilized with palm oil fuel ash (POFA)-based geopolymer. Appl. Sci. 2021, 11, 3572. [Google Scholar] [CrossRef]
- Abdila, S.R.; Abdullah, M.M.A.B.; Ahmad, R.; Rahim, S.Z.A.; Rychta, M.; Wnuk, I.; Nabiałek, M.; Muskalski, K.; Tahir, M.F.M.; Syafwandi; et al. Evaluation on the mechanical properties of ground granulated blast slag (GGBS) and fly ash stabilized soil via geopolymer process. Materials 2021, 14, 2851. [Google Scholar] [CrossRef]
- Luo, Y.; Meng, J.; Wang, D.; Jiao, L.; Xue, G. Experimental study on mechanical properties and microstructure of metakaolin based geopolymer stabilized silty clay. Constr. Build. Mater. 2022, 316, 125662. [Google Scholar] [CrossRef]
- Abdulkareem, S.O.; Abbas, J.M. Effect of adding metakaolin based geopolymer to Improve soft clay under different conditions. IOP Conf. Ser. Earth Environ. Sci 2021, 856, 012011. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Zhang, Z.; Pan, C.; Shen, X.; Xia, L.; Zang, J. Modeling and optimization of fly ash–slag-based geopolymer using response surface method and its application in soft soil stabilization. Constr. Build. Mater. 2022, 315, 125723. [Google Scholar] [CrossRef]
- Abdullah, H.H.; Shahin, M.A.; Walske, M.L. Geo-mechanical behavior of clay soils stabilized at ambient temperature with fly-ash geopolymer-incorporated granulated slag. Soils Found. 2019, 59, 1906–1920. [Google Scholar] [CrossRef]
- Lang, L.; Chen, B.; Chen, B. Strength evolutions of varying water content-dredged sludge stabilized with alkali-activated ground granulated blast-furnace slag. Constr. Build. Mater. 2021, 275, 122111. [Google Scholar] [CrossRef]
- Shi, X.; Zha, Q.; Li, S.; Cai, G.; Wu, D.; Zhai, C. Experimental study on the mechanical properties and microstructure of metakaolin-based geopolymer modified clay. Molecules 2022, 27, 4805. [Google Scholar] [CrossRef]
- Sukmak, P.; Sukmak, G.; Horpibulsuk, S.; Setkit, M.; Kassawat, S.; Arulrajah, A. Palm oil fuel ash-soft soil geopolymer for subgrade applications: Strength and microstructural evaluation. Road Mater. Pavement Des. 2017, 20, 110–131. [Google Scholar] [CrossRef]
- Baldovino, J.J.A.; Izzo, R.L.S.; Rose, J.L.; Domingos, M.D.I. Strength, durability, and microstructure of geopolymers based on recycled-glass powder waste and dolomitic lime for soil stabilization. Constr. Build. Mater. 2021, 271, 121874. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y.; Puppala, A.J. Comparing carbide sludge-ground granulated blastfurnace slag and ordinary portland cement: Different findings from binder paste and stabilized clay slurry. Constr. Build. Mater. 2022, 321, 126382. [Google Scholar] [CrossRef]
- Ordoñez Muñoz, Y.; Villota-Mora, A.J.E.; Perretto, F.; dos Santos Izzo, R.L. Eco-friendly stabilization of clayey soil with waste glass powder-based geopolymer. Geomech. Geoeng. 2024, 20, 557–586. [Google Scholar] [CrossRef]
- Abdeldjouad, L. Effect of curing temperature on the development of hard structure of alkali-activated soil. Int. J. GEOMATE 2019, 17, 117–123. [Google Scholar] [CrossRef]
- Elkhebu, A.; Zainorabidin, A.; Asadi, A.; Bakar, I.H.; Huat, B.B.K.; Abdeldjouad, L.; Dheyab, W. Effect of incorporating multifilament polypropylene fibers into alkaline activated fly ash soil mixtures. Soils Found. 2019, 59, 2144–2154. [Google Scholar] [CrossRef]
- Brunauer, S.; Copeland, L.E. The chemistry of concrete. Sci. Am. 1964, 210, 80–93. [Google Scholar] [CrossRef]
- Tong, X.D. Experimental Research on the Additive and Damage Model of Cement-Stabilized Soil. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 1998. [Google Scholar]
- Boardman, D.I.; Glendinning, S.; Rogers, C.D.F. Development of stabilisation and solidification in lime-clay mixes. Géotechnique 2001, 50, 533–543. [Google Scholar] [CrossRef]
- Suzuk, K.; Nishikawa, T.; Ito, S. Formation and carbonation of C-S-H in water. Cem. Concr. Res. 1984, 15, 213–224. [Google Scholar] [CrossRef]


| Type | Name | Main Components |
|---|---|---|
| Inorganic curing materials | Cement | 3CaO·SiO2, 2CaO·SiO2, 3CaO·Al2O3, 4CaO·Al2O3·Fe2O3 |
| Lime | CaO | |
| Fly ash | SiO2, Al2O3, Fe2O3, CaO, TiO2, MgO, K2O, Na2O, SO3, MnO2, etc. | |
| Waste gypsum | CaSO4 | |
| Phosphogypsum | CaSO4·2H2O | |
| Slag | Ca, Mg, Fe, Si and their oxides | |
| Alkaline residue | CaSO3, CaCO3, CaCl2, CaO, etc. | |
| Silicon powder | SiO2 | |
| Coal gangue | Al2O3, SiO2 | |
| Organic curing materials | Epoxy resin | Organic polymer compounds containing two or more epoxy groups in their molecules |
| Polymer materials | Polymer-based materials, including rubber, fibers, adhesives, asphalt, etc. | |
| Sodium silicate | Na2SiO3 | |
| Composite curing materials | Composite curing agent | Formulated by compounding two or more types of inorganic and organic curing materials |
| Parameter | Geopolymer | OPC |
|---|---|---|
| Energy consumption (calcination and crushing) | 990 × 106 J/ton | 3430 × 106 J/ton |
| Carbon emission | Low (169 kg CO2/m3) | High (306 kg CO2/m3) |
| Environmental impact | Alternative waste management solution | Release of cement kiln dust (CKD) |
| Major raw material | Industrial and agricultural wastes | Limestone, shale, rocks etc. |
| Thermal characteristics | Higher resistance to high temperatures | Lower resistance to high temperatures |
| Advantages | Strong, durable, sufficient, systematic case studies | Strong, durable, Low carbon footprint |
| Disadvantages | High carbon footprint, disposal issues, high alkalinity | limited application, high alkalinity, heat treatment, high dosages |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Xie, X.; He, M.; Luo, Z.; Wu, J.; Bin, J.; Yang, L.; Zhang, B. Research Review of Reaction Mechanism and Mechanical Properties of Chemically Solidified Silt. Buildings 2025, 15, 3431. https://doi.org/10.3390/buildings15183431
Xu Z, Xie X, He M, Luo Z, Wu J, Bin J, Yang L, Zhang B. Research Review of Reaction Mechanism and Mechanical Properties of Chemically Solidified Silt. Buildings. 2025; 15(18):3431. https://doi.org/10.3390/buildings15183431
Chicago/Turabian StyleXu, Zhuojun, Xiaolong Xie, Min He, Zhengdong Luo, Jingjing Wu, Jia Bin, Liuyiyi Yang, and Benben Zhang. 2025. "Research Review of Reaction Mechanism and Mechanical Properties of Chemically Solidified Silt" Buildings 15, no. 18: 3431. https://doi.org/10.3390/buildings15183431
APA StyleXu, Z., Xie, X., He, M., Luo, Z., Wu, J., Bin, J., Yang, L., & Zhang, B. (2025). Research Review of Reaction Mechanism and Mechanical Properties of Chemically Solidified Silt. Buildings, 15(18), 3431. https://doi.org/10.3390/buildings15183431





