Research Progress on Effects of Antifreeze Components, Nanoparticles and Pre-Curing on the Properties of Low-Temperature Curing Materials
Abstract
1. Introduction
2. Effect of Antifreeze Components on the Mechanical Properties and Microstructure of Different Cement-Based Materials and Alkali-Activated Materials Under Low-Temperature Curing
2.1. Portland Cement
2.1.1. Mechanical Properties
2.1.2. Microscopic Mechanism
2.2. Sulfate Aluminum Cement
2.2.1. Mechanical Properties
2.2.2. Microscopic Mechanism
2.3. Magnesium Phosphate Cement
2.4. Alkali-Activated Cementitious Material
2.4.1. Mechanical Properties
2.4.2. Microscopic Mechanism
3. Effects of Admixtures and Nanoparticles on the Mechanical Properties and Microstructure of Ordinary Cement-Based Materials Under Low-Temperature Curing
3.1. Mineral Admixtures
3.1.1. Mechanical Properties
3.1.2. Microscopic Mechanism
3.2. Nanomaterials
3.2.1. Mechanical Properties
3.2.2. Microscopic Mechanism
3.3. Hydrated Calcium Silicate
3.3.1. Mechanical Properties
3.3.2. Microscopic Mechanism
4. Relationship Between Pre-Curing Time and Frost Resistance Critical Strength
4.1. Determination of Critical Strength of Frost Resistance
| Strength Grade | Curing Temperature (°C) | Pre-Curing Time (h) | Frost Resistance Critical Strength (MPa) | Age (d) | Ref. |
|---|---|---|---|---|---|
| C30 | −25 | 18 | 5 | −1 + 28 | [121] |
| C30 | −10 | 18 | 3.6 | One freeze + 28 | [118] |
| C20 C30 C40 | −19~−2 | 12 | 3.63 | −7 + 28 | [123] |
| C20 | −25 | 16 | 1.5 | −2 + 28 | [126] |
| C25 | Winter natural conservation −32~18 | 16 | 1.5 | 120 | [127] |
| C30 | −15 | - | 1.5 | −28 + 28 | [128] |
| C60 | −15 | 15 | 16.4 | −7 + 28 | [129] |
| C30 | −15 | 8 | 1.9 | −7 + 28 | [130] |
| C60 | −10 | 28 | 3.5 | −7 + 28 | [131] |
| C35 | −15 | 2 | 4.2 | −7 + 28 | [124] |
| C50 | −10 | 28 | 3.5 | −7 + 28 | [132] |
| C35 | −20 | 18 | 3.7 | −3 + 25 | [133] |
| C35 | once freeze under air (−20 °C) | 18 | 3.7 | −3 + 25 | [27] |
| once freeze after soaking (soaking 4 h) | 34 | 7.8 | |||
| repeatedly freeze (Freeze–thaw 6 cycles) | 32 | 7.0 | |||
| C50 | 0 | 16 | 5.2 | −3 + 28 | [134] |
| −5 | 24 | 11.8 | |||
| −10 | 29 | 16.2 | |||
| C30 | −5 | 16 | 5.3 | −4 + 60 | [135] |
| −10 | 20 | 9.5 | |||
| −15 | 20 | 9.5 | |||
| −20 | 24 | 11.2 | |||
| C30 | −5 | 18 | 6.6 | −7 + 28 | [136] |
| −10 | 24 | 8.1 | |||
| −15 | 36 | 12.2 |
4.2. Effect of Porosity and Pre-Curing Time on the Critical Strength of Frost
4.3. The Relationship Between Pre-Curing and Material Properties
5. Discussion and Outlook
5.1. Effect of Additives on Material Properties
5.2. Effect of Admixtures on the Properties of Materials
5.3. Relationship Between Pre-Curing Time and Material Properties
5.4. Outlook
6. Conclusions and Recommendations
- (1)
- Various types of antifreeze early strength components are used with ordinary Portland cement-based materials, including calcium chloride, sodium nitrite, calcium nitrate, ethylene glycol, and triethanolamine, which have shown better effects. It is worth noting that existing data vary in maintenance ages, and the urgency to determine whether the compressive strength measured at the curing age of −7 + 28 days is representative of different materials and field environments as a unified evaluation system. Future research should progressively consider the influence of incorporating single and composite antifreeze components at low temperatures on the performance of new materials, such as ultra-high-performance concrete and alkali-activated materials.
- (2)
- Sulfoaluminate cement and magnesium phosphate cement-based materials strengthen quickly and early. Additionally, their performance at room temperature is already very superior, and the incorporation of antifreeze components can play a strengthening role under low-temperature curing conditions. However, magnesium phosphate cement has a high production cost and is more suitable as a rapid repair material at low temperatures.
- (3)
- Alkali-activated cementitious materials, utilizing industrial solid waste, exhibit superior performance and are considered alternatives to ordinary Portland cement. However, research in this area has started late, and due to the choice of precursor and activator ratios, research findings are limited and scattered, lacking a systematic structural system. Future studies should not only examine the performance of alkali-activated cementitious materials under low-temperature conditions but also focus on their long-term comprehensive performance, establishing a diversified index performance evaluation system. The relevant standards and procedures for alkali-activated materials should be formulated promptly.
- (4)
- The mineral admixtures when studying admixtures are mainly fly ash and silica fume. Silica fume has a better low-temperature improvement effect than fly ash under identical conditions. The incorporation of nano-materials and hydrated calcium silicate seeds has contributed to physically filling and refining the pores of the slurry, reducing material porosity, mitigating the impact of freezing damage at low temperatures, and improving the later strength. The selection of beneficial admixtures should aim to make rational use of industrial solid waste, adhere to the dual-carbon strategy, respond to the United Nations’ call for zero carbon, and promote the green development of the construction industry.
- (5)
- The length of pre-curing time varies according to the different strength grades of concrete; higher strength grades necessitate longer pre-curing times. After experimental research on different materials and strength grades, various pre-curing methods are analyzed, and later evaluation methods are considered to accurately predict the critical strength of concrete frost resistance for different materials. This is combined with pre-curing methods such as temperature storage and greenhouse methods, allowing for more scientific, reasonable, and economical determination of pre-curing time, resource conservation, and environmental protection. The combination of pre-curing and antifreeze component measures indicates a need for further research to explore whether a complementary effect exists between the two under different materials, as well as to determine the optimal pre-curing time, select antifreeze components, and ascertain the appropriate dosages.
- (6)
- For new composite materials, new high-strength cement-based materials, alkali-activated cementitious materials, and geopolymer concrete, the late start of research and the multitude of influencing factors necessitate accelerated research efforts by scholars. Combined with engineering practice, the mechanical properties and durability of materials under low-temperature curing are studied, which can allow high-efficiency materials to be applied in practical engineering as soon as possible. Moreover, based on the test results, combining test data with numerical analysis to establish an intelligent prediction model through artificial neural networks and machine learning methods will guide engineering practices scientifically, efficiently, and conveniently, providing a reference for the safety, durability, and economic design of the structure under low-temperature curing in winter.
Author Contributions
Funding
Conflicts of Interest
References
- Lothenbach, B.; Winnefeld, F.; Alder, C.; Wieland, E. Effect of temperature on the pore solution, microstructure and hydration products of Portland cement pastes. Cem. Concr. Res. 2007, 37, 483–491. [Google Scholar] [CrossRef]
- Alzaza, A.; Ohenoja, K.; Dabbebi, R.; Illikainen, M. Enhancing the hardened properties of blended cement paste cured at 0 °C by using alkali-treated ground granulated blast furnace slag. Cem. Concr. Compos. 2022, 134, 104757. [Google Scholar] [CrossRef]
- Çullu, M.; Arslan, M. The effects of antifreeze use on physical and mechanical properties of concrete produced in cold weather. Compos. Part B Eng. 2013, 50, 202–209. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.H.; Chen, C.B.; Huang, L.M. Variations, controls and predictions of soil saturated hydraulic conductivity under different land use types in the alpine region of Tibet, China. Geoderma Reg. 2023, 35, e00723. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, S.; Chen, H.Y.; Zhou, Y.L. Recent advances in concrete winterisation technology in the last two decades. Concrete 2013, 40–42. (In Chinese) [Google Scholar]
- Lu, J.G.; Liu, J.N.; Yang, H.H.; Gao, J.J.; Wan, X.S. Influence of curing temperatures on the performances of fiber-reinforced concrete. Constr. Build. Mater. 2022, 339, 127640. [Google Scholar] [CrossRef]
- Jiang, Z.W.; He, B.; Zhu, X.P.; Ren, Q.; Zhang, Y. State-of-the-art review on properties evolution and deterioration mec-hanism of concrete at cryogenic temperature. Constr. Build. Mater. 2020, 257, 11945–11946. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Ai, J.H.; Ren, Q.; ZHU, X.P.; He, B.; Jiang, Z.W. Understanding the strength evolution of alkali-activated slag pastes cured at subzero temperature. Cem. Concr. Compos. 2023, 138, 104993. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, H.L.; Cheng, J.; Yang, Y.Z. Novel selection of environment-friendly cementitious materials for winter construction: Alkali-activated slag/Portland cement. J. Clean. Prod. 2020, 258, 120592. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.L.; Li, J.S.; Zhang, Z.R.; Huang, X.; XUE, Q. Strength evolution and microstructure of alkali-activated ultra-fine slag in low-temperature environments. Constr. Build. Mater. 2024, 435, 136852. [Google Scholar] [CrossRef]
- Yang, K.; Yang, C.; Zhang, J. First structural use of site-cast, alkali-activated slag concrete in China. Proc. Inst. Civ. Eng.-Struct. Build. 2018, 171, 800–809. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Q.S.; Yue, X.L.; Sun, Z.L.; Liu, Y.C. Temperature field analysis and prediction of winter construction warm shed method based on hot air heating. Case Stud. Therm. Eng. 2024, 60, 104709. [Google Scholar] [CrossRef]
- Shi, T.; Deng, C.; Zhao, J.; Ding, P.X.; Fan, Z.H. Temperature field of concrete cured in winter conditions using thermal control measures. Adv. Mater. Sci. Eng. 2022, 2022, 7255601. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, H.L.; Sun, X.J.; Zhao, M.; Hui, D. Research on mechanical properties and microscopic mechanism of multi-based geopolymer concrete under combined action of pre-curing and nano-silica. J. Build. Eng. 2024, 97, 110930. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Shang, X.L. Understanding and practice of some concepts of frost critical strength of concrete in winter construction. Constr. Technol. 1995, 30–33. (In Chinese) [Google Scholar]
- Koniorczyk, M.; Bednarska, D.; Omrani, I.A.N.; Wieczorek, A.; Gong, F. On Water Freezing in Slag-Blended Cementitious Materials at Early Ages. J. Build. Eng. 2024, 92, 109778. [Google Scholar] [CrossRef]
- Mobili, A.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate and alkali-activated fly ash cements as alternative to Portland cement: Study on chemical, physical-mechanical, and durability properties of mortars with the same strength class. Constr. Build. Mater. 2020, 246, 118436. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Cao, X.; Ma, R.; Sun, S.; Fang, L.; Lin, J.; Luo, J. Solid waste-based magnesium phosphate cements: Preparation, performance and solidification/stabilization mechanism. Constr. Build. Mater. 2021, 297, 123761. [Google Scholar] [CrossRef]
- Zhang, L.H.; Lu, Z.C.; Zhang, Y.R. New insight into the combined effect of aluminum sulfate and triethanolamine on cement hydration. Cem. Concr. Res. 2024, 181, 107547. [Google Scholar] [CrossRef]
- Liu, Z.; Lou, B.; Barbieri, D.M.; Sha, A.; Ye, T.; Li, Y. Effects of pre-curing treatment and chemical accelerators on Portland cement mortars at low temperature (5 °C). Constr. Build. Mater. 2020, 240, 117893. [Google Scholar] [CrossRef]
- Zhang, L.F.; Ma, R.; Lai, J.Y.; Ruan, S.Q.; Qian, X.Q.; Yan, D.M. Performance buildup of concrete cured under low-temperatures: Use of a new nanocomposite accelerator and its application. Constr. Build. Mater. 2022, 335, 127529. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Zhao, Y.X.; Zhu, Z.H.; Chen, G.F.; Wu, H.X.; Liu, C.; Gao, J.M. Effect of a novel negative temperature early-strength agent in improving concrete performance under varying curing temperatures. Case Stud. Constr. Mater. 2024, 21, e03748. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Feng, P.; Zhang, P. A review on shrinkage-reducing methods and mechanisms of alkaliactivated/geopolymer systems: Effects of chemical additives. J. Build. Eng. 2022, 49, 104056. [Google Scholar] [CrossRef]
- Kothari, A.; Habermehl-Cwirzen, K.; Hedlund, H.; Cwirzen, A. A Review of the Mechanical Properties and Durability of Ecological Concretes in a Cold Climate in Comparison to Standard Ordinary Portland Cement-Based Concrete. Materials 2020, 13, 3467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Lei, L.; Liu, J.H.; Ma, Y.H.; Liu, Y.; Xiao, Z.Q.; Shi, C.J. Accelerators for normal concrete: A critical review on hydration, mic-rostructure and properties of cement-based materials. Cem. Concr. Compos. 2022, 134, 104762. [Google Scholar] [CrossRef]
- JC 475-2004; National Development and Reform Commission of the People’s Republic of China. Antifreeze for Concrete. China Building Material Industry Press: Beijing, China, 2004. (In Chinese)
- Liu, R.Q.; Liu, J.; Liu, Y.; Zhou, H.H. Influence of freezing environment on freezing critical strength of low-temperature concrete. J. Liaoning Tech. Univ. 2011, 30, 92–95. [Google Scholar] [CrossRef]
- Jiang, S.H.; Dong, S.H.; Zhu, W.Z.; Li, H.B.; Yin, S.H. Research on critical strength of negative temperature concrete subjected to freezing based on frost resistance. China Concr. Cem. Prod. 2019, 8–10. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, S.H.; Dong, S.H.; Zhu, W.Z.; Li, H.B.; Yin, S.H. Research on critical strength of negative temperature concrete subjected to freezing based on compressive strength. China Concr. Cem. Prod. 2019, 24–26. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, C.H.; Yang, Y.; Wang, Y.; Zhou, Y.N.; Ma, C.C. Autogenous shrinkage of high performance concrete containing mineral admixtures under different curing temperatures. Constr. Build. Mater. 2014, 61, 260–269. [Google Scholar] [CrossRef]
- Velay-Lizancos, M.; Martinez-Lage, I.; Azenha, M.; Vázquez-Burgo, P. Influence of temperature in the evolution of compressive strength and in its correlations with UPV in eco-concretes with recycled materials. Constr. Build. Mater. 2016, 124, 276–286. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhang, R.L.; Duan, Y.; Guo, H.Z.; Zhang, J.W. Effect of mould entry temperature on the heat of hydration of cement under continuous negative temperature and its prediction model. Acta Mater. Compos. Sin. 2022, 39, 4718–4731. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, W.Y.; Nie, S.; Xu, M.F.; Zhou, J.; Li, H. Progress of activation of high Baileyite sulphoaluminate cement. Bull. Chin. Ceram. Soc. 2022, 41, 2979–2992. (In Chinese) [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, W.; Chen, X.; Li, X.P. Research progress of sulphoaluminate cement modification. Bull. Chin. Ceram. Soc. 2019, 38, 683–687. (In Chinese) [Google Scholar] [CrossRef]
- Liu, J.; Lu, R.H.; Zhang, Z.Q. Research Progress on Properties of Magnesium Phosphate Cement. Mater. Rep. 2021, 35, 23068–23075. (In Chinese) [Google Scholar]
- Haque, M.A.; Chen, B. Research progresses on magnesium phosphate cement: A review. Constr. Build. Mater. 2019, 211, 885–898. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sustain. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Tuyan, M.; Nehdi, M.L. Alkali-activated and geopolymer materials developed using innovative manufacturing techniques: A critical review. Constr. Build. Mater. 2021, 303, 124483. [Google Scholar] [CrossRef]
- Tan, H.B.; Li, M.G.; Ren, J.; Deng, X.F.; Zhang, X.; Nie, K.J.; Zhang, J.J.; Yu, Z.Q. Effect of aluminum sulfate on the hydration of tricalcium silicate. Constr. Build. Mater. 2019, 205, 414–424. [Google Scholar] [CrossRef]
- Hoang, K.; Justnes, H.; Geiker, M. Early age strength increase of fly ash blended cement by a ternary hardening accelerating admixture. Cem. Concr. Res. 2016, 81, 59–69. [Google Scholar] [CrossRef]
- Hirsch, T.; Lu, Z.; Stephan, D. Effect of different sulphate carriers on Portland cement hydration in the presence of triethanolamine. Constr. Build. Mater. 2021, 294, 123528. [Google Scholar] [CrossRef]
- Lu, Z.C.; Kong, X.M.; Jansen, D.; Zhang, C.Y.; Wang, J.; Pang, X.F.; Yin, J.H. Towards a further understanding of cement hydration in the presence of triethanolamine. Cem. Concr. Res. 2020, 132, 106041. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yang, S.; Li, J.H.; Wang, F.Z.; Hu, S.G. Effect of w/c ratio and antifreeze admixture on the frost damage of sulfoaluminate cement concrete at −20 °C. Constr. Build. Mater. 2022, 347, 128457. [Google Scholar] [CrossRef]
- Nmai, C.K. Cold weather concreting admixtures. Cem. Concr. Compos. 1998, 20, 121–128. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cem. Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Marchon, D.; Kawashima, S.; Bessaies-Bey, H.; Mantellato, S.; Ng, S. Hydration and rheology control of concrete for digital fabrication: Potential admixtures and cement chemistry. Cem. Concr. Res. 2018, 112, 96–110. [Google Scholar] [CrossRef]
- Chaves Figueiredo, S.; Çopuroğlu, O.; Schlangen, E. Effect of viscosity modifier admixture on Portland cement paste hydration and microstructure. Constr. Build. Mater. 2019, 212, 818–840. [Google Scholar] [CrossRef]
- Nwankwo, C.O.; Bamigboye, G.O.; Davies, I.E.E.l; Michaels, T.A. High volume Portland cement replacement: A review. Constr. Build. Mater. 2020, 260, 120445. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Gao, X.J.; Deng, H.G.; Ba, H.J. Non-destructive monitoring of internal structural development of negative temperature concrete. J. Harbin Inst. Technol. 2009, 41, 43–47. (In Chinese) [Google Scholar]
- Zhang, L.; Li, X. Research on special antifreeze agent for BFJ polycarboxylic acid high-efficiency water reducing agent. Concrete 2011, 88–89. (In Chinese) [Google Scholar]
- Zhou, M.R.; Zhang, H.J.; Zhang, J.; Long, L.J. Evaluation method and application research of compound antifreeze. Concrete 2012, 78–80. (In Chinese) [Google Scholar]
- Wang, J.L.; Sun, X.B.; Yang, Z.F. Research on the effect of antifreeze components on the properties of cement concrete. Bull. Chin. Ceram. Soc. 2014, 33, 3331–3337. (In Chinese) [Google Scholar] [CrossRef]
- Song, D.S.; Dai, M.; Gai, Y.F.; Zhang, J.L.; Xin, Y.L. Influence of antifreeze components on the properties of negative temperature concrete. Concrete 2011, 55–56+61. (In Chinese) [Google Scholar]
- Yin, M.; Bai, H.T.; Zhou, L. Experimental study on the determination of antifreeze admixture for negative temperature concrete. Sci. Technol. Eng. 2014, 14, 290–294. (In Chinese) [Google Scholar]
- Karagoel, F.; Demirboga, R.; Kaygusuz, M.A.; Yadollahi, M.M.; Polatet, R. The influence of calcium nitrate as antifreeze admixture on the compressive strength of concrete exposed to low temperatures. Cold Reg. Sci. Technol. 2013, 89, 30–35. [Google Scholar] [CrossRef]
- Demirboğa, R.; Karagöl, K.; Polat, R.; Kaygusuzet, M.A. The effects of urea on strength gaining of fresh concrete under the cold weather conditions. Constr. Build. Mater. 2014, 64, 114–120. [Google Scholar] [CrossRef]
- Jia, B.X.; Li, Q.W.; Liang, P.F.; Li, G.X. Research on the effect of antifreeze on the mechanical properties of spontaneous combustion gangue concrete. Non-Met. Mines 2016, 39, 68–71. (In Chinese) [Google Scholar]
- Reddy, P.N.; Naqash, J.A. Effect of Antifreeze Admixtures on Cold Weather Concrete. Int. J. Eng. 2019, 32, 366–372. [Google Scholar]
- Zhang, G.; Yu, H.Y.; Li, H.M.; Yang, Y.Z. Experimental study of deformation of early age concrete suffering from frost damage. Constr. Build. Mater. 2019, 215, 410–421. [Google Scholar] [CrossRef]
- Skripkiunas, G.; Kicaite, A.; Justnes, H.; Pundienė, I. Effect of Calcium Nitrate on the Properties of Portland-Limestone Cement-Based Concrete Cured at Low Temperature. Materials 2021, 14, 71611. [Google Scholar] [CrossRef]
- Karagol, F.; Demirboga, R.; Khushefati, W.H. Behavior of fresh and hardened concretes with antifreeze admixtures in deepfreeze low temperatures and exterior winter conditions. Constr. Build. Mater. 2015, 76, 388–395. [Google Scholar] [CrossRef]
- Khan, J.; Kumar, G.S. Influence of binary antifreeze admixtures on strength performance of concrete under cold weather conditions. J. Build. Eng. 2021, 34, 102055. [Google Scholar] [CrossRef]
- Dong, S.H.; Feng, C.F.; Jiang, S.H.; Zhu, W.Z. Effect of freezing temperature on the microstructure of negative temperature concrete. Adv. Mater. Res. 2013, 663, 343–348. [Google Scholar] [CrossRef]
- Hu, Y.B.; Miao, G.Y.; Yu, X. Research on mechanical properties and hydration characteristics of concrete under negative temperature environment. J. Build. Mater. 2017, 20, 975–980. (In Chinese) [Google Scholar]
- Thomas, J.J.; Allen, A.J.; Jennings, H.M. Hydration Kinetics and Microstructure Development of Normal and CaCl2-Accelerated Tricalcium Silicate Pastes. J. Phys. Chem. C 2009, 113, 19836–19844. [Google Scholar] [CrossRef]
- Tao, Y.X.; Rahul, A.V.; Mohan, M.K.; De, S.G.; Van, T.K. Recent progress and technical challenges in using calcium sulfoaluminate (CSA) cement. Cem. Concr. Compos. 2023, 137, 104908. [Google Scholar] [CrossRef]
- Ke, G.J.; Zhang, J.; Liu, Y. Shrinkage characteristics of calcium sulphoaluminate cement concrete. Constr. Build. Mater. 2022, 337, 127627. [Google Scholar] [CrossRef]
- Li, M. Research on Hydration Characteristics of Sulphoaluminate Cement Under Low Temperature Environment. Master’s Thesis, Beijing Institute of Technology, Beijing, China, 2020. (In Chinese). [Google Scholar]
- Wang, P.M.; Li, N.; Xu, L.L.; Zhang, G.F. Hydration process and strength development of sulphoaluminate cement under low temperature maintenance. J. Chin. Ceram. Soc. 2017, 45, 242–248. (In Chinese) [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.D. Effect of curing temperature on the physical and mechanical properties of butylbenzene emulsion/sulphoaluminate cement mortar. J. Chin. Ceram. Soc. 2017, 45, 227–234. (In Chinese) [Google Scholar] [CrossRef]
- Wang, R.; Zhang, T. Drying shrinkage of styrene-butadiene emulsion/sulphoaluminate cement mortar at different temperature and humidity. J. Build. Mater. 2018, 21, 768–774. (In Chinese) [Google Scholar]
- Wang, J.Y.; Ye, J.Y.; Cheng, H.; Shi, D.; Ren, J.R.; Zhang, W.S. Effect of retarder on hydration and strength of fasthardening sulphoaluminate cement at minus 10 °C. J. Chin. Ceram. Soc. 2020, 48, 1285–1294. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Z.P.; Zhao, Y.T.; Xu, L.L. Hydration characteristics of sulphoaluminate cement under different maintenance temperatures. J. South China Univ. Technol. 2018, 46, 30–38. (In Chinese) [Google Scholar]
- Li, G.X.; Zhang, J.; Song, Z.; Shi, C.; Zhang, A. Improvement of workability and early strength of calcium sulphoal-uminate cement at various temperature by chemical admixtures. Constr. Build. Mater. 2018, 160, 427–439. [Google Scholar] [CrossRef]
- Liu, Y.P.; Li, J.H.; Yang, C.; Liu, Z.C. Influence of antifreeze and early-strengthening agent on the negative temperature hydration property of sulphoaluminate cement. Bull. Chin. Ceram. Soc. 2021, 40, 359–367. (In Chinese) [Google Scholar] [CrossRef]
- Wang, W.B. Effect of lithium carbonate on hydration and properties of sulphoaluminate cement under low temperature environment. New Build. Mater. 2019, 46, 54–57. (In Chinese) [Google Scholar]
- Wang, P.M.; Li, N.; Xu, L.L. Hydration evolution and compressive strength of calcium sulphoaluminate cement constantly cured over the temperature range of 0 to 80 °C. Cem. Concr. Res. 2017, 100, 203–213. [Google Scholar] [CrossRef]
- Zhang, H.F.; Ye, J.Y.; Ren, J.R.; Yang, C.S.; Zhang, W.S.; Shi, D.; Zhang, J.T. Effect of calcium chloride solution on the properties of sulphoaluminate cement at −10 °C. J. Chin. Ceram. Soc. 2022, 50, 2834–2843. (In Chinese) [Google Scholar]
- Jia, X.W.; Luo, J.Y.; Zhang, W.X.; Qian, S.H.; Li, J.M. Preparation and Application of Self-Curing Magnesium Phosphate Cement Concrete with High Early Strength in Severe Cold Environments. Materials 2020, 13, 5587. [Google Scholar] [CrossRef]
- Jia, X.W.; Lian, L.; To, J.; Tu, H.; Hou, T.J.; Wang, P. Effect of magnesium oxide whiskers on the early mechanical properties of magnesium phosphate cement under severe cold environment. J. Funct. Mater. 2021, 52, 12030–12035. (In Chinese) [Google Scholar]
- Yi, H.H.; Zhang, Y.; Lu, J.B.; Li, D.X. Study on the failure of magnesium phosphate cement under low temperature. Bull. Chin. Ceram. Soc. 2014, 33, 197–201. (In Chinese) [Google Scholar]
- Jia, X.W.; Li, J.M.; Wang, P.; Qian, J.S.; Tang, M.H. Preparation and mechanical properties of magnesium phosphate cement for rapid construction repair in ice and snow. Constr. Build. Mater. 2019, 229, 116921–116927. [Google Scholar] [CrossRef]
- Jia, X.W.; Luo, J.Y.; Zhang, W.X.; Tang, M.H.; Qian, J.S. Reaction characteristics and compressive strength of magnesia-phosphate cement at negative temperatures. Constr. Build. Mater. 2021, 305, 124819. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Z.C.; Chen, X.; Pan, Z.; Huang, X. Study on early mechanical properties of icewater blended magnesium phosphate cement in cold environment. Concrete 2022, 8, 10–14. (In Chinese) [Google Scholar]
- Yuan, J.; Huang, X.; Chen, X.; Ge, Q.; Zhang, Z.C. Early-age mechanical properties and hydration degrees of magnesium phosphate cement paste in freezing winter of cold regions. Constr. Build. Mater. 2022, 345, 128337. [Google Scholar] [CrossRef]
- Habert, G.; De Lacaillerie, D.E.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Escalante-García, J.I. Strength and durability in acid media of alkali silicate-activated metakaolin geopolymers. J. Am. Ceram. Soc. 2012, 95, 2307–2313. [Google Scholar] [CrossRef]
- Justnes, H.; Kim, M.O.; Ng, S.; Qian, X. Methodology of calculating required chloride diffusion coefficient for intended service life as function of concrete cover in reinforced marine structures. Cem. Concr. Compos. 2016, 73, 316–323. [Google Scholar] [CrossRef]
- Yang, G.J.; Wang, Z.Y. Durability properties of sustainable alkali-activated cementitious materials as marine engineering material: A review. Mater. Today Sustain. 2022, 17, 100099. [Google Scholar] [CrossRef]
- Akinyemi, B.A.; Alaba, P.A.; Rashedi, A. Selected performance of alkali-activated mine tailings as cementitious composites: A review. J. Build. Eng. 2022, 50, 104154. [Google Scholar] [CrossRef]
- Wei, X.B.; Li, D.Q.; Ming, F.; Yang, C.S.; Chen, L. Influence of Low-Temperature Curing on the Mechanical Strength, Hydration Process, and Microstructure of Alkali-Activated Fly Ash and Ground Granulated Blast Furnace Slag Mortar. Constr. Build. Mater. 2021, 269, 121811. [Google Scholar] [CrossRef]
- Huang, X.; Mao, H.Y.; Yuan, X.F.; Yang, Y. Study on the properties of alkali slag cement at −20 °C. Bulletin of the Chinese Ceramic Society 2014, 33, 2052–2055. (In Chinese) [Google Scholar] [CrossRef]
- Ju, C.; Liu, Y.S.; Jia, M.J.; Yu, K.; Yu, Z.; Yang, Y. Effect of calcium oxide on mechanical properties and microstructure of alkali-activated slag composites at subzero temperature. J. Build. Eng. 2020, 32, 101561. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Y.; Deng, J.X.; Xiong, D.Y.; Zhu, X.H. Using calcium-rich precursors to improve the early-compressive strength of alkali-activated slag cement at low temperature. Struct. Concr. 2022, 23, 2221–2232. [Google Scholar] [CrossRef]
- Chen, T.; Ren, B.; Wang, Z.H.; Meng, X.; Ning, Y.P.; Lv, Y. Effect of early strength agent on the hydration of geopolymer mortar at low temperatures. Case Stud. Constr. Mater. 2022, 17, e01419. [Google Scholar] [CrossRef]
- Alzaza, A.; Ohenoja, K.; Illikainen, M. Onepart alkali-activated blast furnace slag for sustainable const-ruction at subzero temperatures. Constr. Build. Mater. 2021, 276, 122026. [Google Scholar] [CrossRef]
- Bai, S.; Guan, X.C.; Li, G.Y. Effect of the earlyage frost damage and nano-SiO2 modification on the properties of Portland cement paste. Constr. Build. Mater. 2020, 262, 120098. [Google Scholar] [CrossRef]
- Ogawa, Y.; Uji, K.; Ueno, A.; Kawai, K. Contribution of fly ash to the strength development of mortars cured at different temperatures. Constr. Build. Mater. 2021, 276, 122191. [Google Scholar] [CrossRef]
- Alzaza, A.; Ohenoja, K.; Langås, I.; Arntsen, B.; Poikelispää, M.; Illikainen, M. Low-temperature (−10 °C) curing of Portland cement paste—Synergetic effects of chloride-free antifreeze admixture, C–S–H seeds, and room-temperature pre-curing. Cem. Concr. Compos. 2022, 125, 104319. [Google Scholar] [CrossRef]
- Lv, Y.; Bai, E.L.; Wang, Z.H.; Meng, X.; Sun, H.Y. Experimental and microscopic study on the preparation of silica fume-metakaolin-slag base polymer mortar at low temperature. Mater. Rep. 2023, 37, 218–226. (In Chinese) [Google Scholar]
- Yang, Z.B.; Liu, Y.; Chen, Q.R.; Tang, J.S.; Zhu, B.Q.; Zhu, X.K. Mechanism of ultrafine mineral admixture for concrete. Mater. Rep. 2023, 37, 151–155. (In Chinese) [Google Scholar]
- Zhao, Y.M.; Zhang, Z.; Wang, P.; Zhang, M.F. Influence of mineral dopants on the properties of UHPC. Bull. Chin. Ceram. Soc. 2022, 41, 3170–3175. (In Chinese) [Google Scholar]
- Yang, Y.Z.; Ba, H.J. Interfacial microstructure and properties of negative temperature antifreeze concrete. J. Chin. Ceram. Soc. 2007, 1125–1130. (In Chinese) [Google Scholar]
- He, X.X.; Kong, L.Z.; Zhou, S. Experimental study on compressive properties of fly ash concrete prisms with low-temperature curing. Build. Struct. 2020, 50, 38–43. (In Chinese) [Google Scholar]
- Liu, L.; Li, Y.; Ouyang, P.; Yang, Y.Q. Hydration of the silica fume-Portland cement binary system at lower temperature. Constr. Build. Mater. 2015, 93, 919–925. [Google Scholar] [CrossRef]
- Jun, L.; Li, Y.; Yang, Y.Q.; Cui, Y.P. Effect of Low Temperature on Hydration Performance of the Complex Binder of Silica Fume-Portland Cement. J. Wuhan Univ. Technol. 2014, 29, 75–81. [Google Scholar]
- Bai, S.; Yu, L.B.; Guan, X.C.; Li, H.; Ou, J.P. Study on the long-term chloride permeability of nano-silica modified cement pastes cured at negative temperature. J. Build. Eng. 2022, 57, 104854. [Google Scholar] [CrossRef]
- Feng, J.H.; Yang, F.; Qian, S.Z. Improving the bond between polypropylene fiber and cement matrix by nano calcium carbonate modification. Constr. Build. Mater. 2021, 269, 121249. [Google Scholar] [CrossRef]
- Francioso, V.; Moro, C.; Martinez-Lage, I.; Velay-Lizancos, M. Curing temperature: A key factor that changes the effect of TiO2 nanoparticles on mechanical properties, calcium hydroxide formation and pore structure of cement mortars. Cem. Concr. Compos. 2019, 104, 103374. [Google Scholar] [CrossRef]
- Xu, Q.; Meng, T.; Huang, M. Effects of Nano-CaCO3 on the Compressive Strength and Microstructure of High Strength Concrete in Different Curing Temperature. Appl. Mech. Mater. 2011, 121, 126–131. [Google Scholar]
- Skoczylas, K.; Rucińska, T. The effects of low curing temperature on the properties of cement mortars containing nanosilica. Nanotehnologii V Stroit. 2019, 11, 536–544. [Google Scholar] [CrossRef]
- Tang, S.W.; Wang, Y.; Geng, Z.C.; Xu, X.F.; Yu, W.Z.; Hu, B.A.; Chen, J.T. Structure, Fractality, Mechanics and Durability of Calcium Silicate Hydrates. Fractal Fract. 2021, 5, 47. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, X.; Gu, X.; Zhang, Q. Simulation of free water freezing with hydrated calcium silicate pore water based on coarse-grained molecular dynamics. Chin. J. Comput. Mech. 2024, 41, 194–201. (In Chinese) [Google Scholar]
- Zhang, G.; Yang, Y.Z.; Li, H.M. Calcium-silicate-hydrate seeds as an accelerator for saving energy in cold weather concreting. Constr. Build. Mater. 2020, 264, 120191. [Google Scholar] [CrossRef]
- Zheng, X.G.; Yu, P.Y.; Duan, L.F.; Li, S.M.; Zhang, C.; Deng, Q.S. Effect of hydrated calcium silicate crystal species on compressive strength of concrete under negative temperature curing. Railw. Eng. 2022, 62, 150–153. (In Chinese) [Google Scholar]
- Alzaza, A.; Ohenoja, K.; Ahmed Shaikh, F.U.; Illikainen, M. Mechanical and durability properties of C–S–H-seeded cement mortar cured at fluctuating low temperatures with granulated blast furnace slag as fine aggregates. J. Build. Eng. 2022, 57, 104879. [Google Scholar] [CrossRef]
- GBJ 204-1983; Specification for Construction and Acceptance of Reinforced Concrete Works. China Building Industry Press: Beijing, China, 1983. (In Chinese)
- Huang, S.Y.; Wu, C.S. Several controversial issues in winter concrete construction. Low Temp. Archit. Technol. 1990, 38–40. (In Chinese) [Google Scholar]
- Xiang, Z.X. Study on the freezing critical strength of negative temperature concrete. Archit. Technol. 1988, 2–8. (In Chinese) [Google Scholar]
- Song, X.T. Discussion on the critical strength of concrete with antifreeze. Low Temp. Archit. Technol. 1989, 20–22. (In Chinese) [Google Scholar]
- Qu, Z.Z. Critical strength of concrete before freezing. Constr. Technol. 1988, 59–60. (In Chinese) [Google Scholar]
- Zhao, Y.Y. Early allowable freezing critical strength of antifreeze-added concrete. Ind. Constr. 1987, 14–17. (In Chinese) [Google Scholar]
- Sun, M.I. Tests and discussions on the critical strength of concrete subjected to freezing. Low Temp. Archit. Technol. 1992, 2–6. (In Chinese) [Google Scholar]
- Yang, Y.Z.; Wang, Z.; Gao, X.J.; Ba, H.J. Frozen critical strength and durability of negative temperature concrete. J. Wuhan Univ. Technol. 2009, 31, 37–41. (In Chinese) [Google Scholar]
- JGJ/T 104-2011; Winter Construction Regulations for Building Projects. Ministry of Housing and Urban-Rural Development of the People’s Republic of China. China Construction Industry Press: Beijing, China, 2011. (In Chinese)
- Zhu, W.Z.; Gao, Y.B.; Shi, M.X. Influence of one freezing mode on the freezing critical strength of antifreeze concrete. Low Temp. Constr. Technol. 1993, 36–38. (In Chinese) [Google Scholar]
- Zhu, W.Z.; Ma, W.H.; Kang, B.Z. Effect of multiple freezing modes on the freezing critical strength of antifreeze concrete. Low Temp. Archit. Technol. 1993, 37–40. (In Chinese) [Google Scholar]
- Ma, W.H.; Yu, B.Q. Analysis of the role of antifreeze from the theory of freezing critical strength. Low Temp. Archit. Technol. 1995, 41–42. (In Chinese) [Google Scholar]
- Li, J.H.; Wang, Z.; Lu, B.Y. Study on strength development of high strength concrete at negative temperature. J. Harbin Inst. Technol. 2002, 373–375. (In Chinese) [Google Scholar]
- Su, X.N.; Wang, X.; Chi, D.C. Experimental study on early freezing of concrete. Concrete 2005, 87–89. (In Chinese) [Google Scholar]
- Liu, J.; Li, Z.G.; Tian, Y.; Liu, R.Q. Effects of mineral admixtures on strength development and frost critical strength of concrete under low temperature. J. Shenyang Jianzhu Univ. 2006, 22, 415–418+427. (In Chinese) [Google Scholar]
- Liu, J.; Liu, R.Q. Prediction of antifreeze critical strength of infant age concrete. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2008, 23, 272–275. [Google Scholar] [CrossRef]
- Liu, J.; Liu, R.Q. The Antifreeze Critical Strength of Low-temperature Concrete Effected by Index. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2011, 26, 354–359. [Google Scholar] [CrossRef]
- An, G.S.; Liu, Z.Y.; Li, J.X. Effect of fly ash on frost critical strength of concrete. Low Temp. Archit. Technol. 2016, 38, 23–25. (In Chinese) [Google Scholar]
- Chen, S.X. Research on the effect of fly ash on the frost critical strength of concrete. Contemp. Chem. Ind. 2019, 48, 2781–2784. (In Chinese) [Google Scholar]
- Liu, Z.Y.; Fu, S.; Ma, G.W.; Wang, S.; Li, B.Y.; Yang, H.; Cui, S.J.; Zhang, Z. Influence of pre-conditioning with electric trace heat on the critical strength of concrete subjected to freezing. Bull. Chin. Ceram. Soc. 2022, 41, 1990–1997. (In Chinese) [Google Scholar]
- Yi, S.T.; Pae, S.W.; Kim, J.K. Minimum curing time prediction of early-age concrete to prevent frost damage. Constr. Build. Mater. 2011, 25, 1439–1449. [Google Scholar] [CrossRef]
- Liu, D.Y.; Tu, Y.M.; Shi, P.; Sas, G. Mechanical and durability properties of concrete subjected to early-age freeze–thaw cycles. Mater. Struct. 2021, 54, 211. [Google Scholar] [CrossRef]
- Li, Y.; Chai, J.Q.; Wang, R.J.; Zahng, X. Experimental evaluation of the frost resistance durability of RCC layer subjected to early-age freezing. J. Build. Eng. 2023, 76, 107153. [Google Scholar] [CrossRef]

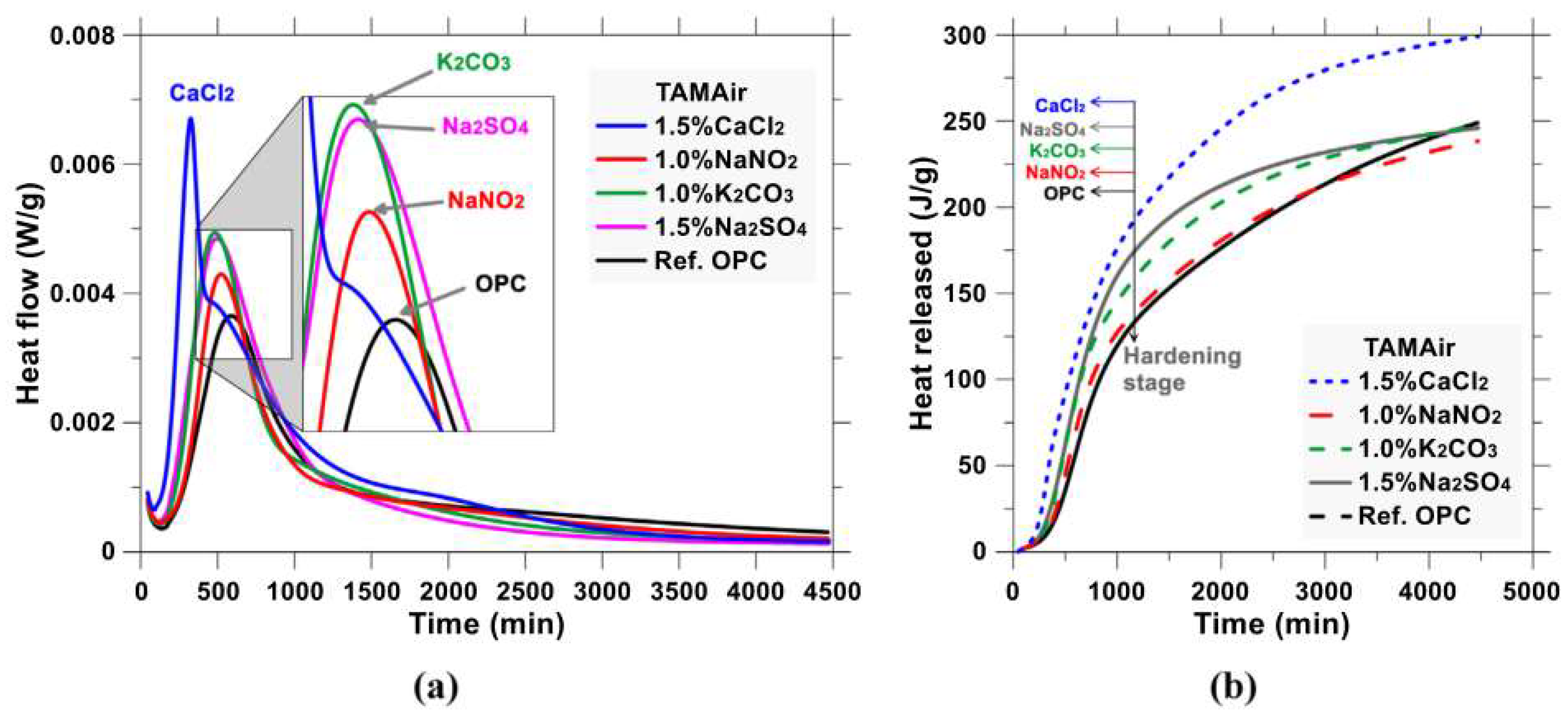
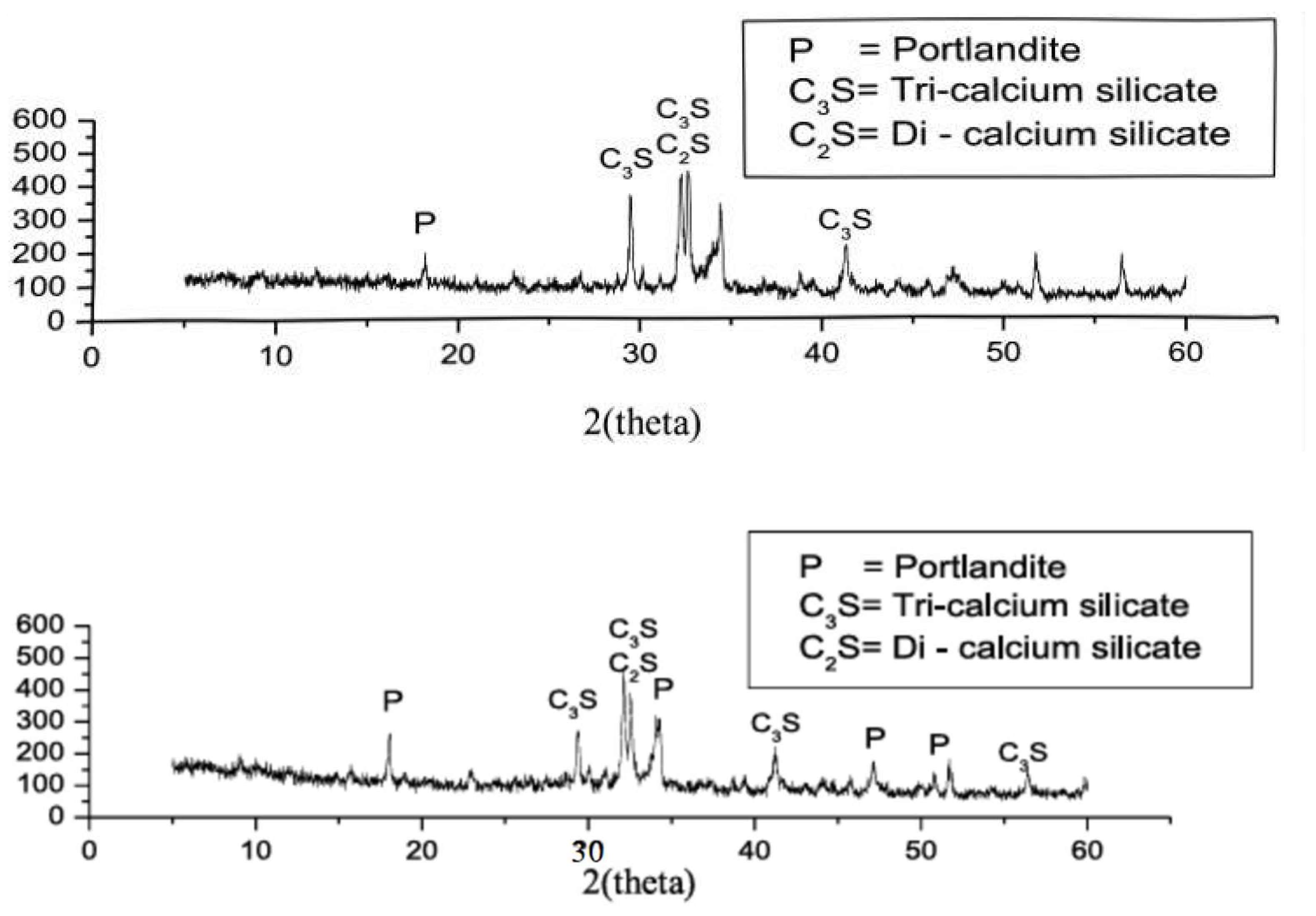

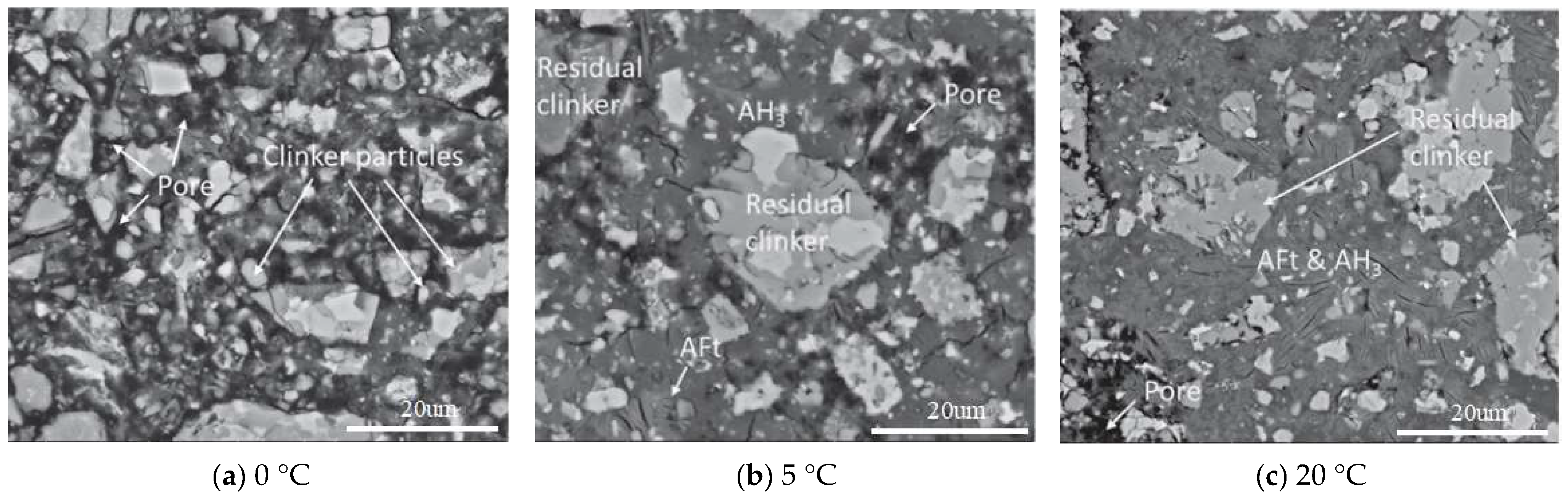
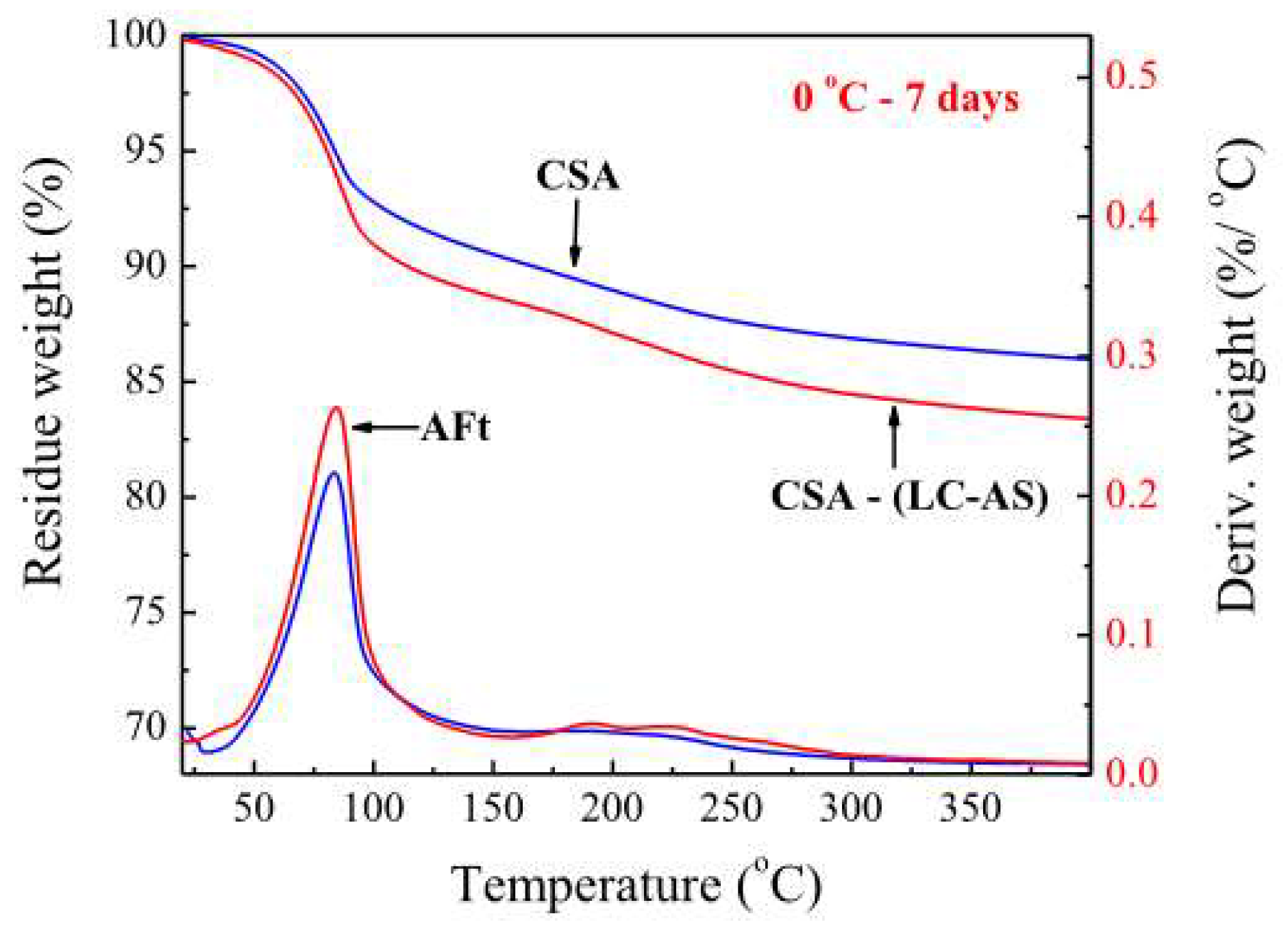
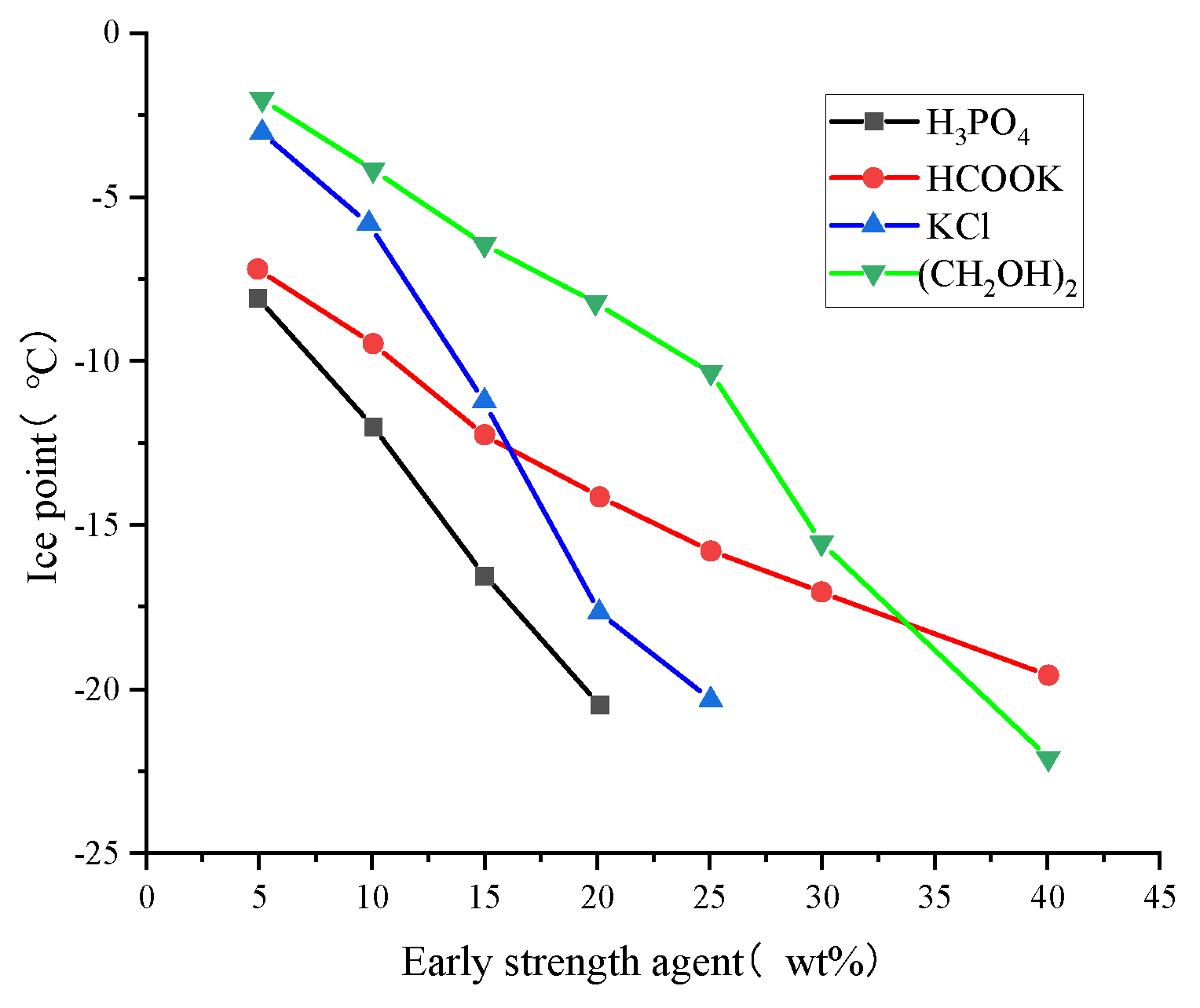
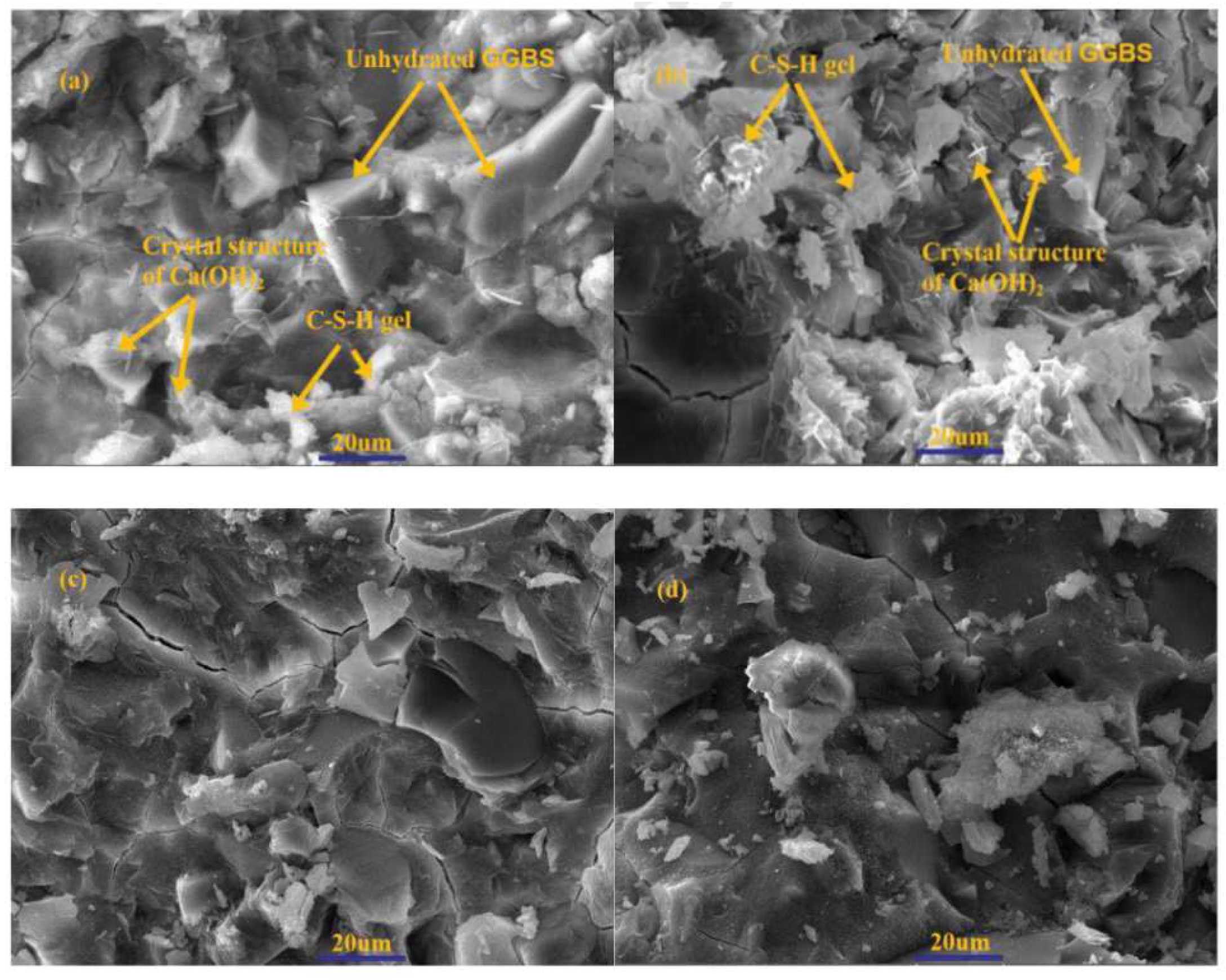
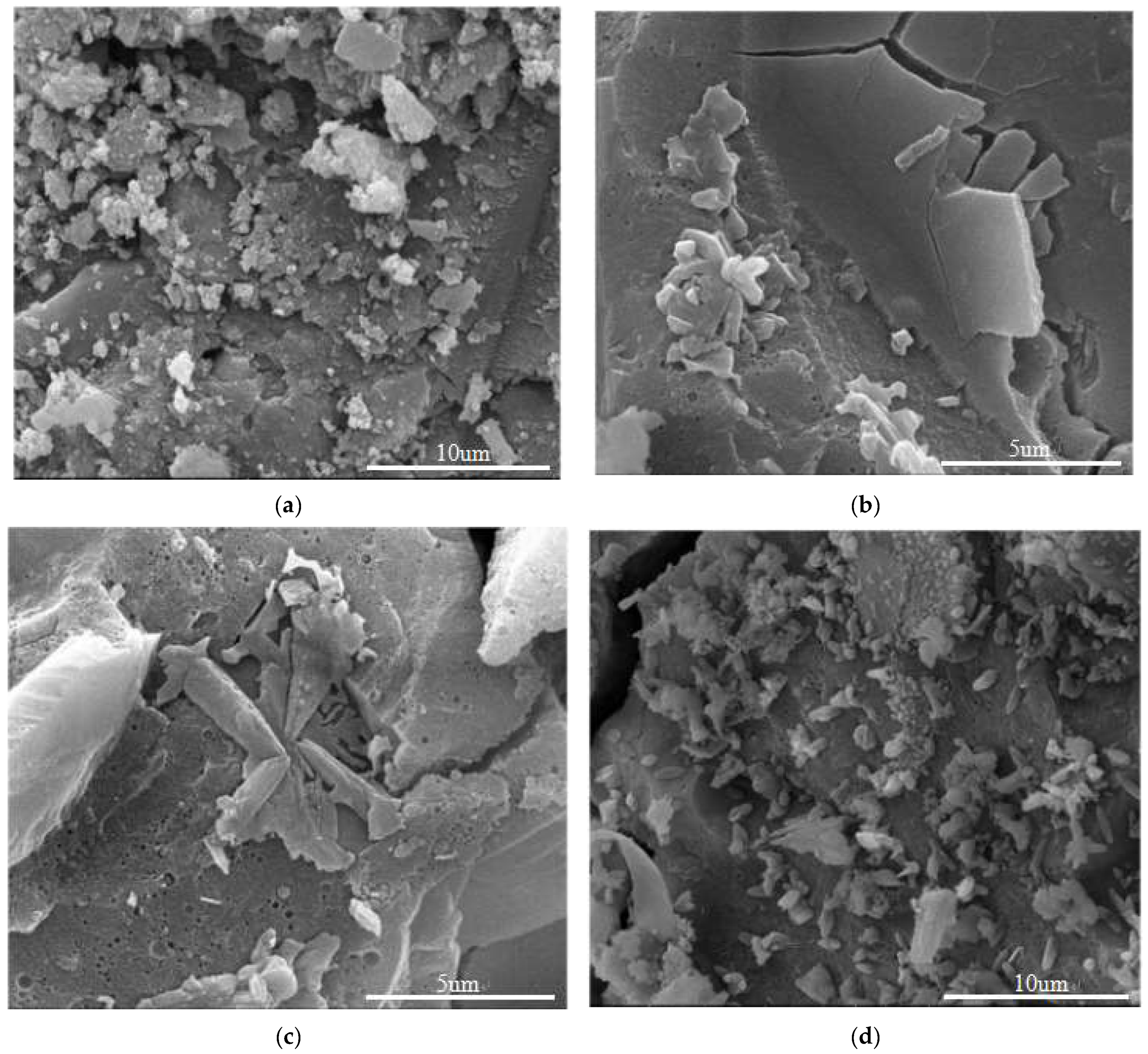
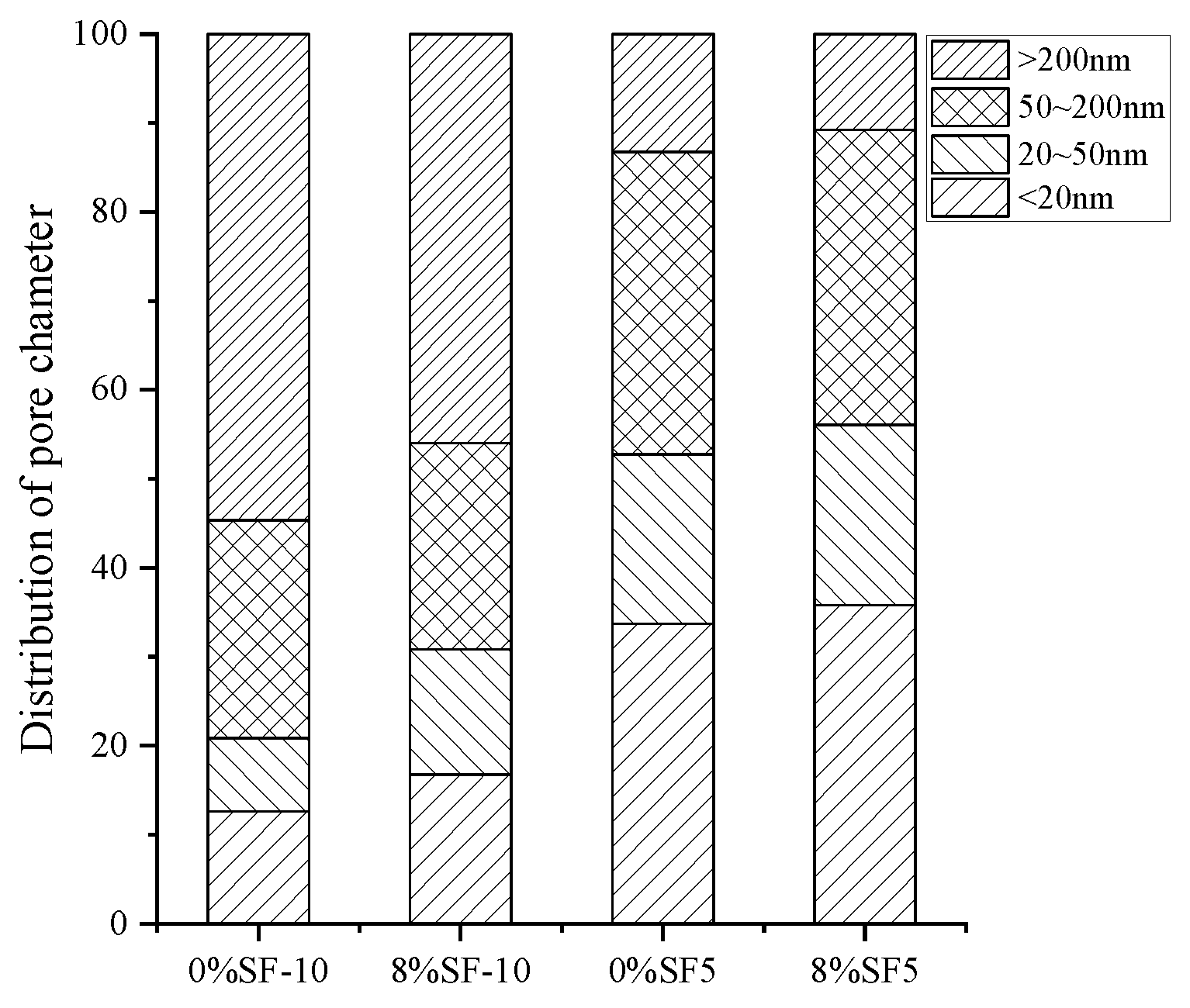

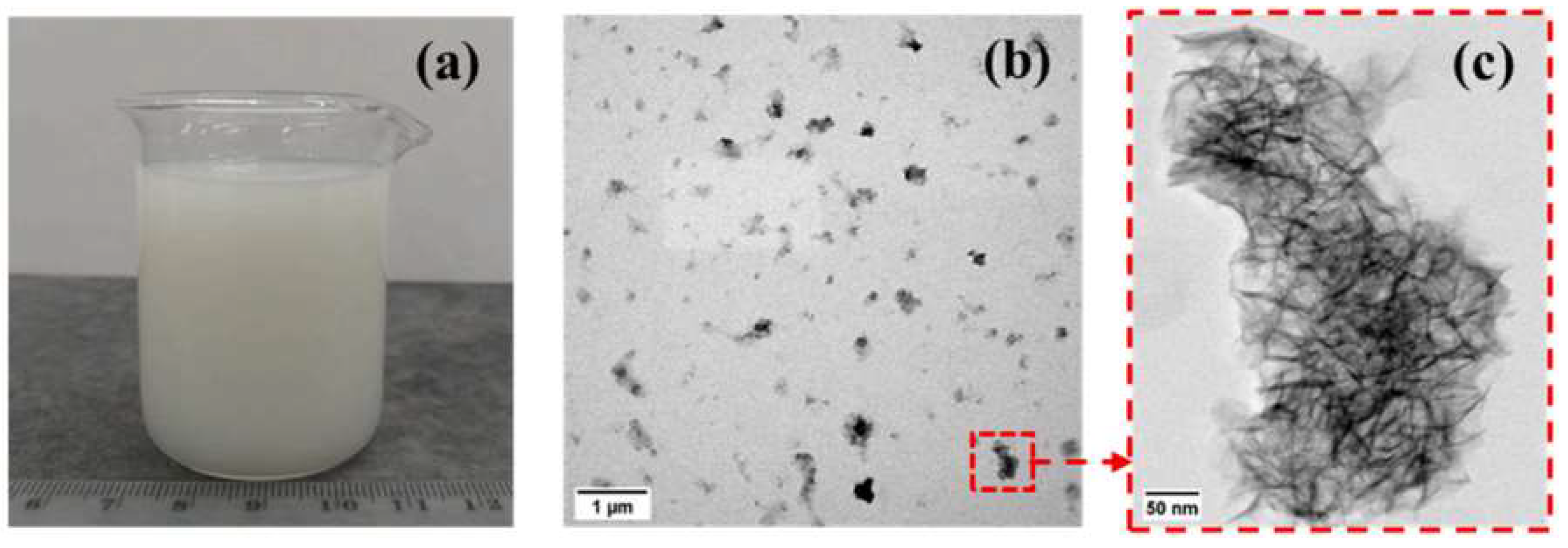
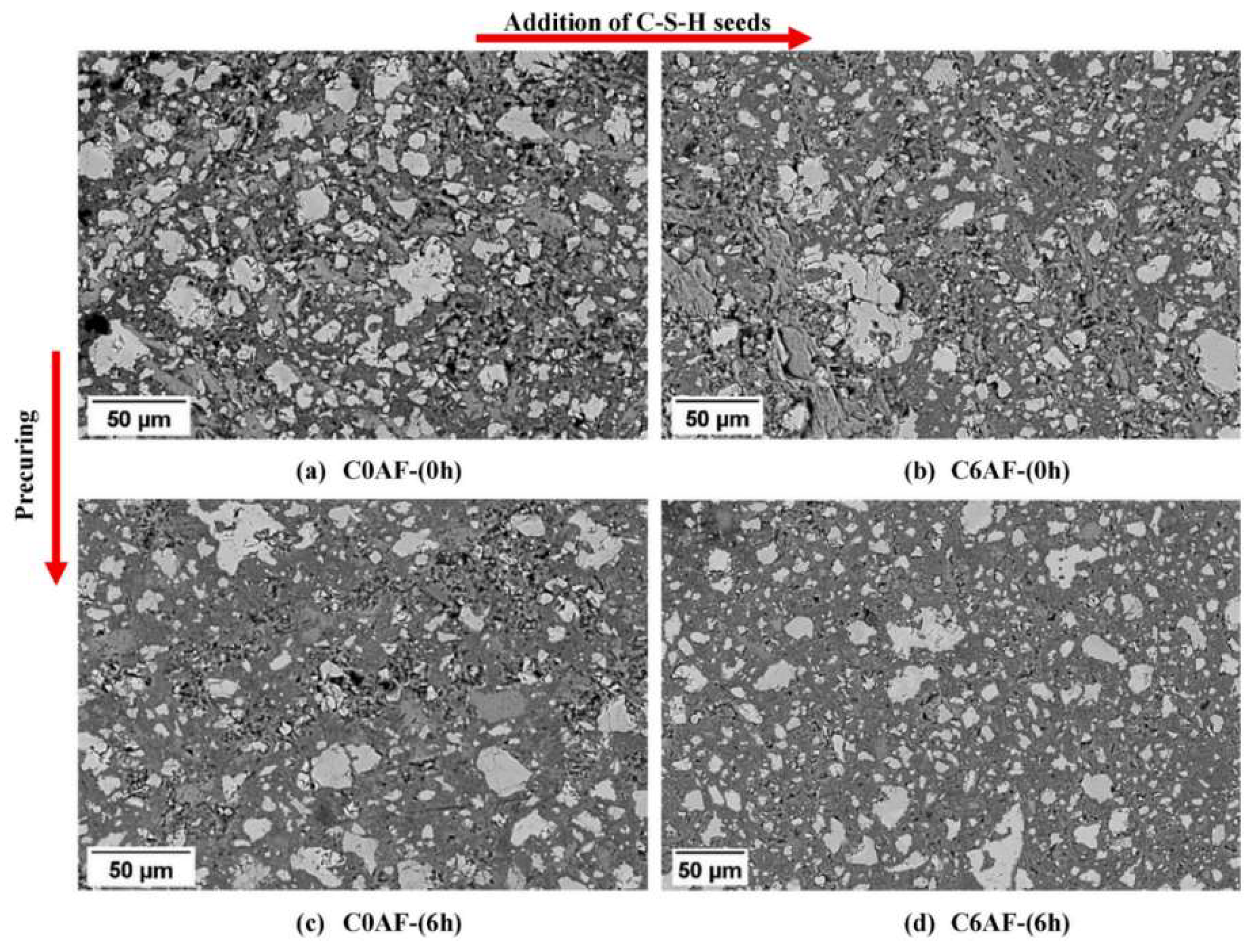
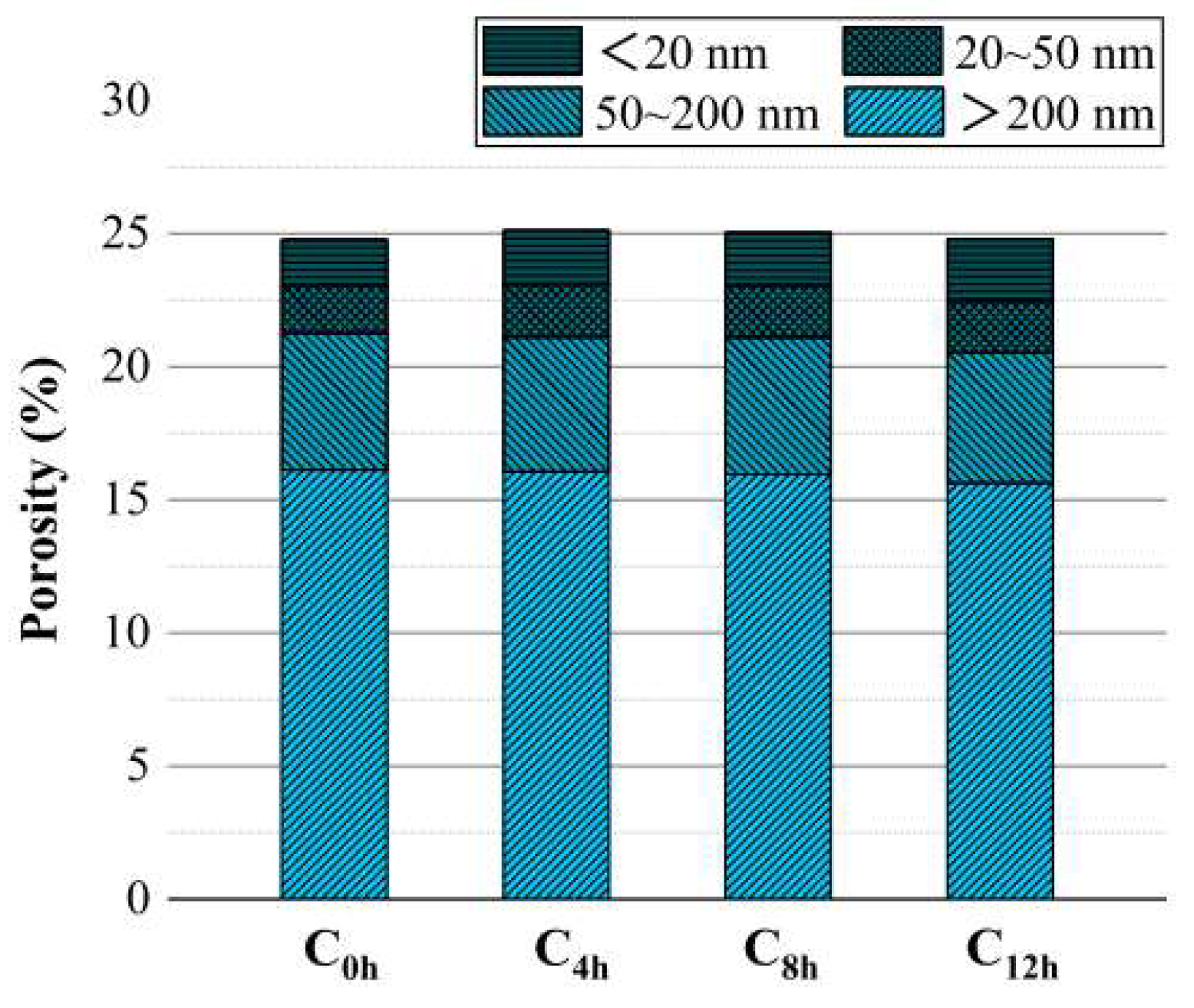
| Serial Number | Curing Temperature (°C) | Antifreeze Component | Optimum Content (%) | Age (d) | IRCS (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | −20 | Antifreeze early strength water reducer | 5 | −7 + 56 | 112 | [49] |
| 2 | −15 | CaCl2 | 7 | −7 + 28 | 72 | [52] |
| 3 | −15 | C2H6O2 | 1.5 | −7 + 56 | 70 | [53] |
| 4 | −15 ± 0.5 | NaNO2 | 2 | −7 + 28 | 100 | [54] |
| 5 | −15 | Ca(NO3)2 | 6 | −28 + 28 | 263 | [55] |
| 6 | −10 | CO(NH2)2 | 6 | −14 + 28 | 45 | [56] |
| 7 | −10 | CaCl2 | 7 | −7 + 28 | 49 | [57] |
| 8 | −5 | K2CO3 | 5 | −28 | 40 | [58] |
| 9 | −20 | NaNO2 | 5 | −7 + 28 | −36 | [59] |
| 10 | −5 | Ca(NO3)2 | 1 | −28 | 92 | [60] |
| 11 | −10 | Ca(NO3)2 + CO(NH2)2 | 4.5 + 4.5 | −90 | 208 | [61] |
| 12 | −10 | Ca(NO3)2 + CNNaS | 8 + 1.5 | −28 + 28 | 427 | [62] |
| 13 | −15 | Non-chloride salt + C6H15NO3 + C2H6O2 | 20 + 1 + 7.5 | −7 + 56 | 26 | [50] |
| Material Type | Curing Temperature (°C) | Antifreeze Component | Optimum Content (%) | Age (d) | IRCS (%) | Ref. |
|---|---|---|---|---|---|---|
| Cement mortar | 5 | Li2CO3 | 20 | 28 | 25 | [70,71] |
| 5 | CaSO4·2H2O | 40 | 28 | −29 | [73] | |
| −10 | Li2CO3 + Ca(NO3)2 | Li2CO3(0.9) + Ca(NO3)2(1) | −7 | 251 | [74] | |
| −20 | Ca(NO3)2 + Al2(SO4)3 + C6H15NO3 | Ca(NO3)2(9) + Al2(SO4)3(0.9) + C6H15NO3(0.04) | −28 | 13 | [75] | |
| −20 | Ca(NO3)2 | 5 | −3 + 28 | 11 | [43] |
| Material Type | Curing Temperature (°C) | Antifreeze Component | Optimum Content (%) | Age (d) | IRCS (%) | Ref. |
|---|---|---|---|---|---|---|
| Concrete | −20 | Light calcined magnesia | 10 | −1 d | 198 | [79] |
| Cement paste | −20 | Magnesia whiskers | 3 | −1 d | 203 | [80] |
| −20 | Light calcined magnesia | 8 | −1 d | 33 | [84] | |
| −10 | Light calcined magnesia | 12 | −2 h | 450 | [82,83] |
| Precursor | Activator | Curing Temperature (°C) | Antifreeze Component | Optimum Content (%) | Age (d) | IRCS (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Slag | NaOH | −20 | NaNO3 | 2 | −7 + 28 | 15 | [92] |
| Slag | K2SiO3 and KOH | −10 | CaO | 3 | −28 | 5 | [93] |
| Slag Portland cement Calcium aluminum sulfate cement | Na2SiO3 and NaOH | 5 | CaO | 5 | 28 | 26 | [94] |
| Slag Silica fume | Na2SiO3 and NaOH | 10 | C6H15NO3 | 0.06 | 7 | 75 | [95] |
| Antifreeze Component | Price (CNY/kg) |
|---|---|
| CaCl2 | 1–1.5 |
| C2H6O2 | 6–8 |
| NaNO2 | 4–6 |
| Ca(NO3)2 | 2–3 |
| CO(NH2)2 | 2–3 |
| K2CO3 | 7–9 |
| CNNaS | 15–20 |
| C6H15NO3 | 10–15 |
| Li2CO3 | 200–250 |
| CaSO4·2H2O | 0.3–0.5 |
| Al2(SO4)3 | 1.5–2.5 |
| CaO | 0.5–0.8 |
| NaNO3 | 3–8 |
| Material Type | Curing Temperature (°C) | Admixture Type | Optimum Content (%) | Age (d) | IRCS (%) | Ref. |
|---|---|---|---|---|---|---|
| Concrete | −5 | Fly ash + Silica fume | 10 + 5 | −7 + 28 | 19 | [21] |
| −10 | Silica fume | 6 | −7 + 56 | 37 | [22] | |
| −20 | Silica fume | 7 | −7 + 56 | 12 | [103] | |
| Winter natural conservation | Fly ash | 25 | 572 | 21 | [104] | |
| Cement paste | −5 | Silica fume | 8 | −14 | 15 | [105] |
| Material Type | Curing Temperature (°C) | Nanomaterial | Optimum Content (%) | Age (d) | IRCS (%) | Ref. |
|---|---|---|---|---|---|---|
| Concrete | 6.5 | CaCO3 | 2 | 28 | 2 | [110] |
| Cement mortar | −5 | SiO2 | 1.2 | −120 | 5 | [107] |
| −15 | SiO2 | 0.9 | −7 + 28 | 23 | [98] | |
| 5 | TiO2 | 1 | 7 | 114 | [109] | |
| 5 | SiO2 | 3 | 28 | 10 | [111] |
| Material Type | Adding Mode | Curing Temperature (°C) | Optimum Content (%) | Age (d) | Control Group (MPa) | Experimental Group (MPa) | IRCS (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cement paste | Liquid reagent with C-S-H seed solid content of about 23.7% | −10 | 6 | −28 | - | 15 | 13 | [99] |
| Liquid reagent with C-S-H seed solid content of 24.46% | −5 | 5 | −28 | 12.21 | 34.3 | 181 | [114] | |
| Concrete | Hydrated calcium silicate suspension | −5 | 1 | −7 | 9.4 | 15.5 | 65 | [115] |
| Type | Frost Resistance | Compressive Strength | |||
|---|---|---|---|---|---|
| Frost Resistance Critical Strength (MPa) | Required Pre-Curing Time (h) | Frost Resistance Critical Strength (MPa) | Required Pre-Curing Time (h) | ||
| C30 | 24.6 | 96 | 13.2 | 48 | |
| Adding antifreeze | 19.6 | 48 | 5.8 | 18 | |
| C40 | 33.6 | 72 | 17.4 | 36 | |
| Adding antifreeze | 27.5 | 48 | 7.8 | 14 | |
| C50 | 40.4 | 72 | 24.1 | 36 | |
| Adding antifreeze | 31.7 | 36 | 23.6 | 24 | |
| C60 | 49.5 | 96 | 33.2 | 36 | |
| Adding antifreeze | 40.5 | 48 | 29.1 | 24 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Wan, M.; Zhu, Y.; Niu, L.; Ji, X.; Chen, S.; He, W.; Han, L. Research Progress on Effects of Antifreeze Components, Nanoparticles and Pre-Curing on the Properties of Low-Temperature Curing Materials. Buildings 2025, 15, 223. https://doi.org/10.3390/buildings15020223
Yao X, Wan M, Zhu Y, Niu L, Ji X, Chen S, He W, Han L. Research Progress on Effects of Antifreeze Components, Nanoparticles and Pre-Curing on the Properties of Low-Temperature Curing Materials. Buildings. 2025; 15(2):223. https://doi.org/10.3390/buildings15020223
Chicago/Turabian StyleYao, Xianhua, Mingduo Wan, Yongsheng Zhu, Lihua Niu, Xiaoxiang Ji, Shengqiang Chen, Wei He, and Linyan Han. 2025. "Research Progress on Effects of Antifreeze Components, Nanoparticles and Pre-Curing on the Properties of Low-Temperature Curing Materials" Buildings 15, no. 2: 223. https://doi.org/10.3390/buildings15020223
APA StyleYao, X., Wan, M., Zhu, Y., Niu, L., Ji, X., Chen, S., He, W., & Han, L. (2025). Research Progress on Effects of Antifreeze Components, Nanoparticles and Pre-Curing on the Properties of Low-Temperature Curing Materials. Buildings, 15(2), 223. https://doi.org/10.3390/buildings15020223









